SAT2_Physics_08_Thermodynamics_01_with_Chinese

SAT2 Physics (by: Dr. Yu) Thermodynamics (Review) 热力学
? Conversion between Fahrenheit 华氏 and Celsius 摄氏: 9532F C T T =+ ? Conversion between Celsius and Kelvin 绝对温度K: 273K C T T =+
? Relationship between Heat 热能 and Temperature 温度: Q C T ?=? Q mc T ?=?
C: heat capacity /J C O
or /J K
c : specific heat capacity 比热容 /()J kg C O
?or
/()J kg K ?
Calories: 1 1 /cal g C ο
== 4.19 J Specific Heat of Water:
water c =34.1910?J/kg ·
oC or 1 cal/g · oC
? The specific heat capacity 比热容 of a substance is the heat energy required to change the mass of one gram of a substance by one degree Celsius.
? There are no temperature changes during a phase change 相变. Heat and Phase Change:
Latent Heat 潜热of Transformation 变换: latent heat of fusion f L ,
latent heat of vaporization 蒸发v L , Q mL = Latent heat of fusion f L for water:
433.510f L =?J/kg
Latent heat of vaporization v L for water:
522.610v L =?J/kg
? Most substances expand when they are heated and contract when they cool. Water is a notable exception.
? The Kinetic Theory of Ideal Gas: 32
K k T
=
;
rms v ?=
? The Ideal Gas Law: PV nRT = or PV NkT = P : in Pascal (a P ), 2a 1P 1N/m =
Avogadro’s constant:236.0210A N =?particles/mole R : gas constant: o
8.31 J/mol K R =? k : Boltzmann constant,
o 23-1
8.31 J/mol K
/ 6.0210 mol A k R N ?==?, 23
o 1.3810 J/k K -=?
? The combined gas law is
11
2212
PV PV T T = ? Boyle’s Law: 1122PV PV = (at a constant
temperature) ? Charles ’s Law:
12
12
V V T T = (at a constant pressure) ? The first law of thermodynamics (the law of conservation of heat energy):
Q U W ?=?+? (or Q U W =?+) heat flow = change in internal energy + workdone by the system
? The second law of thermodynamics states that no
heat engine can have efficiency equal to 100%. ? An alternate statement of the second law is that an ordered system tends to become disordered.
? The Entropy:
Q
S T
??=
S : Entropy (State function)
? The work done by a heat engine is the area under its P – V curve.
W p V =?
? A heat engine operating in reverse produces cooling (Refrigerator). Heat engine: Efficiency: hot W
e Q =
= hot cold hot
Q Q Q - Refrigerator:
Coefficient of performance:
cold cold cold hot cold
hot cold
Q Q T k W
Q Q T T =
=
=
--
? Calorimetry热量测定学is the study of heat transfer between objects.
? A su bstance that loses heat has a negative change of heat (?ΔQ) .
? A substance that gains heat has a positive change of heat (+ΔQ) .
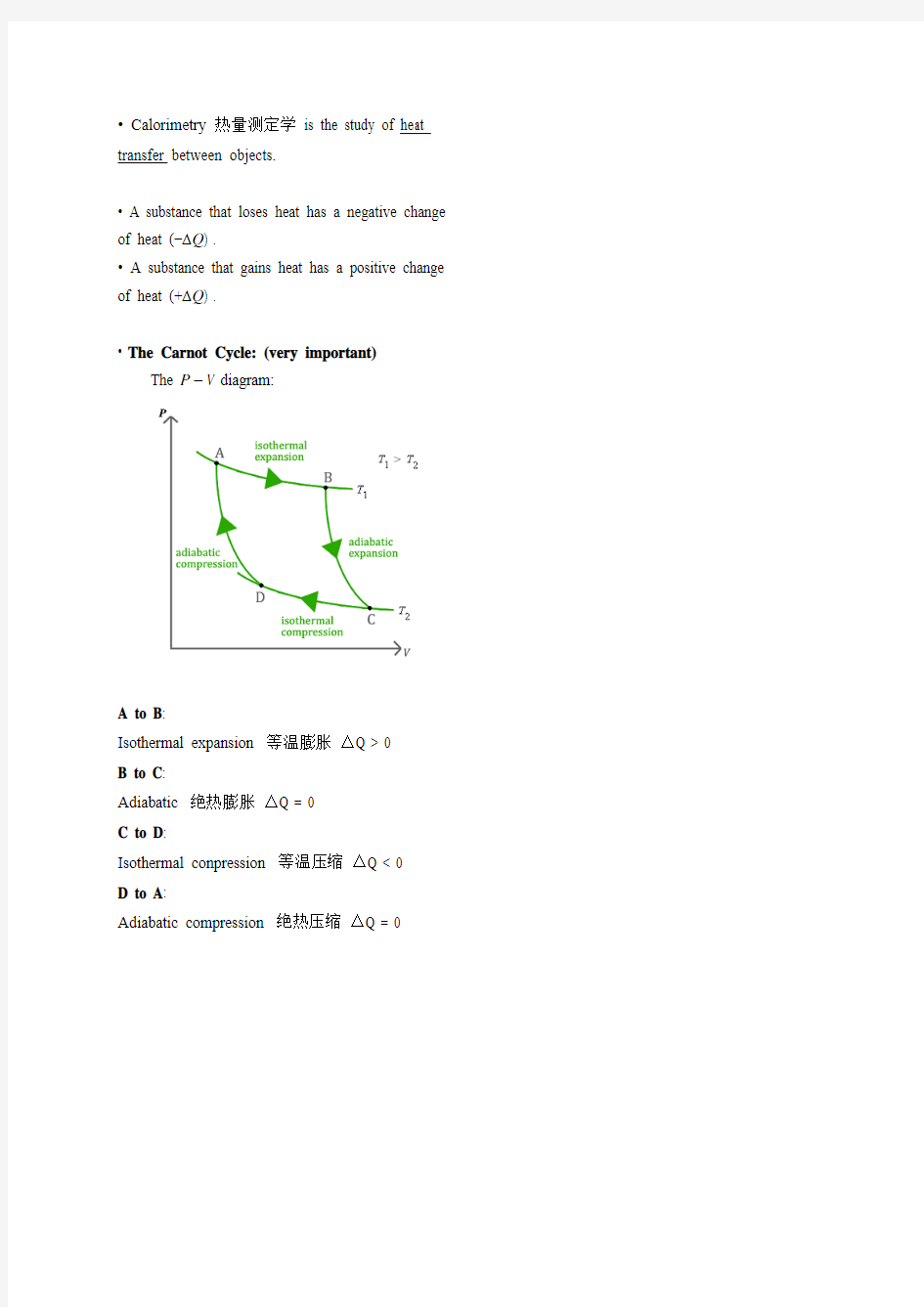
? The Carnot Cycle: (very important)
The P–V diagram:
A to B:
Isothermal expansion 等温膨胀△Q > 0
B to C:
Adiabatic 绝热膨胀△Q = 0
C to D:
Isothermal conpression 等温压缩△Q < 0
D to A:
Adiabatic compression 绝热压缩△Q = 0
