LSPR biosensor

Effects of nanoparticle size and cell type on high sensitivity cell
detection using a localized surface plasmon resonance biosensor
Fei Liu a,b,e,1,Matthew Man-Kin Wong c,b,1,Sung-Kay Chiu c,b,Hao Lin d,b,
Johnny C.Ho d,b,Stella W.Pang a,b,n
a Department of Electronic Engineering,City University of Hong Kong,Kowloon,Hong Kong
b Center for Biosystems,Neuroscience,and Nanotechnology,City University of Hong Kong,Kowloon,Hong Kong
c Department of Biology an
d Chemistry,City University of Hong Kong,Kowloon,Hong Kong
d Department of Physics and Materials Science,City University of Hong Kong,Kowloon,Hong Kong
e Department o
f Electronic Information Engineering,Tianjin University,Tianjin300072,China
a r t i c l e i n f o
Article history:
Received20September2013
Received in revised form
28November2013
Accepted29November2013
Available online10December2013
Keywords:
Au nanoparticles
Localized surface plasmon resonance(LSPR)
Cell concentration detection
Resonance peak shift
Human-derived retinal pigment epithelial
RPE-1cell
Breast cancer MCF-7cell
a b s t r a c t
A localized surface plasmon resonance(LSPR)effect was used to distinguish cell concentration on
ordered arrays of Au nanoparticles(NPs)on glass substrates.Human-derived retinal pigment epithelial
RPE-1cells with?atter bodies and higher con?uency were compared with breast cancer MCF-7cells.
Nanosphere lithography was used to form Au NPs with average diameters of500and60nm in order to
compare cell detection range,resonance peak shift,and cell concentration sensitivity.A larger cell
concentration range was detected on the larger500nm Au NPs compared to60nm Au NPs(8.56?103–
1.09?106vs.3.43?104–
2.73?105cells/ml).Resonance peak shift could distinguish RPE-1from MCF-7
cells on both Au NPs.RPE-1cells consistently displayed larger resonance peak shifts compared to MCF-7
cells until the detection became saturated at higher concentration.For both types of cells,higher
concentration sensitivity in the range of$104–106cells/ml was observed on500nm compared to60nm
Au NPs.Our results show that cells on Au NPs can be detected in a large range and at low concentration.
Optimal cell sensing can be achieved by altering the dimensions of Au NPs according to different cell
characteristics and concentrations.
&2013Elsevier B.V.All rights reserved.
1.Introduction
Localized surface plasmon resonance(LSPR)spectroscopy of
metallic nanoparticles(NPs)is a widely studied technique for
ef?cient biosensor applications.Incident light on NPs induces
collective oscillation of electrons at speci?c resonance wave-
lengths,resulting in enhanced intensity of localized electromag-
netic(EM)?eld.Fabrication techniques such as chemical synthesis
(Joshi et al.,2012),electron beam lithography(EBL;Cinel et al.,
2012),and nanosphere lithography(NSL;Huang et al.,2012)have
been adopted for NP preparation.The NSL method can provide a
uniform template for fabricating long-range ordered NP arrays
with low cost and high throughput,while the size and shape of the
NPs can be adjusted in a controllable way(Jung and Byun,2011).
The LSPR of Au NPs provides high sensitivity to short-range
refractive index change(Δn,in refractive index unit(RIU)),and can
be used for adsorbate concentration detection.The analyte con-
centration can be distinguished by corresponding resonance
wavelength shifts(ΔλR,in nm),which depend on the material,
size,shape,and distribution of NPs.Refractive index sensitivity
(RIS)is de?ned asΔλR/Δn;therefore high RIS is preferable for
measuring low cell concentration.The commercial LightPath TM S4
system applies the LSPR principle,and uses the resonance peak
shift of Au NPs to detect human IgG in the range of0.1m g/ml–
5.0mg/ml(LamdaGen,2012).Higher detection sensitivity has also
been demonstrated,with detection limits up to0.1ng/ml for
thrombin and1.6nM for anti-human IgG(Cao et al.,2013;Guo
and Kim,2012).In addition,detection limits up to8pM have been
demonstrated on120nm diameter Au NPs for extracellular adher-
ence proteins found on the outer surface of the Staphylococcus
(Chen et al.,2009).Nanoparticles have also been utilized to detect
resonance peak shifts of Escherichia coli(E.coli)and Salmonella.For
instance,60nm diameter Ag NPs(fabricated by EBL)could detect
E.coli at a concentration of$107cfu/ml(Cinel et al.,2012),and
synthesized Au nanorods with different aspect ratios could simul-
taneously detect Salmonella and E.coli at concentrations of
1012cfu/ml(Wang and Irudayaraj,2008).Using antibody-
conjugated Au NPs to enhance intensity of localized EM?elds,
Contents lists available at ScienceDirect
journal homepage:https://www.360docs.net/doc/169424523.html,/locate/bios
Biosensors and Bioelectronics
0956-5663/$-see front matter&2013Elsevier B.V.All rights reserved.
https://www.360docs.net/doc/169424523.html,/10.1016/j.bios.2013.11.075
n Corresponding author at:City University of Hong Kong,Department of
Electronic Engineering,G6419,83Tat Chee Avenue,Kowloon,Hong Kong.
Tel.:t852********;fax:t852********.
E-mail address:pang@https://www.360docs.net/doc/169424523.html,.hk(S.W.Pang).
1These authors contributed equally to this work.
Biosensors and Bioelectronics55(2014)141–148
dark ?eld optical microscopy can detect E.coli at concentrations of 2?104–6?104cfu/ml (Xu et al.,2012).All these studies demon-strate cell detection methods that involve immobilizing corre-sponding antibodies on NPs.However,the concentration of Salmonella could not be distinguished by 30nm diameter Au NPs.This limitation has been explained by the small contact area between the Au NPs and the rigid bodies of Salmonella ,which leads to a small modi ?cation of the local electric ?eld,and thus a plasmon peak shift that is always $2–4nm regardless of cell concentration (Fu et al.,2009).Therefore,it can be inferred that cell sensing performance depends on the dimensions of the NPs and the physical characteristics of the cell.
In addition to detecting biomolecules and bacteria,Au NPs have shown advantages for targeted diagnosis of cancer biomarkers and cancer cells (Perfézou et al.,2012),such as breast cancer cells (Lu et al.,2010)and oral epithelial cancer cells (EI-Sayed et al.,2005).Aptamers (nucleic acid ligand)conjugated Au NPs (Apt –Au NPs)can speci ?cally bind with platelet-derived growth factor which is over-expressed in certain breast cancer cells.Thus the bound Apt –Au NPs in the breast cancer MDA-MB-231,Hs578T,and MCF-7cells resulted in enhanced intensity of localized EM ?eld,and this can be used to distinguish breast cancer cells from normal cells by dark ?eld optical microscopy (Huang et al.,2009).In addition,electrochemical techniques based on Au NPs have been used for cell concentration detection (Costa et al.,2012;Arya et al.,2013);for instance the electrocatalytic method has been used for the quanti ?cation of human cancer HMy2cells (Escosura-Mu?iz et al.,2009).MCF-7cancer cells can be detected in the range of 104–107cells/ml by the electrochemical method (Li et al.,2010).
Here,we use the LSPR effect to distinguish the cell concentra-tion of MCF-7cancer cells on ordered arrays of Au NPs.The NSL method was used to form Au NPs on glass substrates with average diameters of 500and 60nm to compare cell detection range,resonance peak shift,and cell concentration sensitivity.Human-derived retinal pigmented epithelium RPE-1cells that are ?atter and exhibit contact inhibition were used for comparison with the smaller individual MCF-7cells.Optimal cell sensing can be achieved by altering the dimensions of Au NPs according to different cell characteristics and concentrations.While small size (60nm)NPs have been widely used for LSPR-based sensing of small size biomolecules,larger size NPs (500nm)could be better
in detecting larger size cells due to the longer EM ?eld decay length and enhanced near-?eld electric ?eld intensity due to the coupling between LSPR of NPs and the diffracted wave of the periodic NP https://www.360docs.net/doc/169424523.html,ing this LSPR sensor for cell concentration measurement has advantages over traditional cell counting meth-ods in that it can monitor changes in concentration of cells adhered on a solid surface in real time without any staining or cell removal from the surface as required by a hemocytometer.Time-lapse monitoring of the extinction spectra similar to the cell migration study could be utilized (Tang et al.,2013).In most applications,the use of a single cell type instead of multiple cell types is preferred as this can be used to ?gure out the direct effect of a drug or chemical on a single cell type such as liver cancer cell.Our results show that RPE-1and MCF-7cells can be detected in a large range and at low concentration based on the LSPR effect of Au NPs with de ?ned diameters.To the best of our knowledge,this is the ?rst study on applying the LSPR effect related to the shift of the resonance peak of NPs with various dimensions for detecting cell concentration without attachment of antibodies to NPs.
2.Experiment and methods
2.1.Fabrication of Au nanoparticles on glass substrates
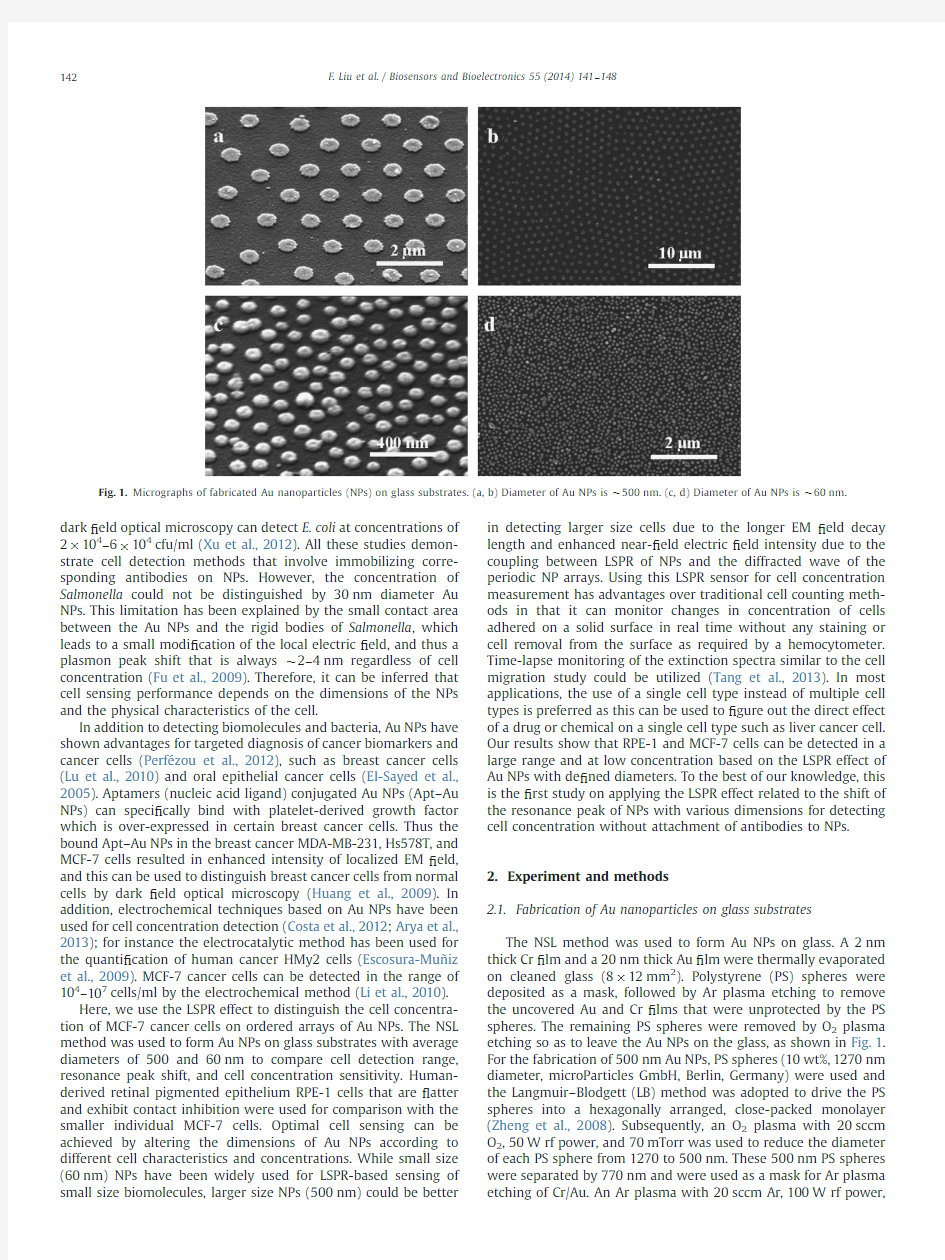
The NSL method was used to form Au NPs on glass.A 2nm thick Cr ?lm and a 20nm thick Au ?lm were thermally evaporated on cleaned glass (8?12mm 2).Polystyrene (PS)spheres were deposited as a mask,followed by Ar plasma etching to remove the uncovered Au and Cr ?lms that were unprotected by the PS spheres.The remaining PS spheres were removed by O 2plasma etching so as to leave the Au NPs on the glass,as shown in Fig.1.For the fabrication of 500nm Au NPs,PS spheres (10wt%,1270nm diameter,microParticles GmbH,Berlin,Germany)were used and the Langmuir –Blodgett (LB)method was adopted to drive the PS spheres into a hexagonally arranged,close-packed monolayer (Zheng et al.,2008).Subsequently,an O 2plasma with 20sccm O 2,50W rf power,and 70mTorr was used to reduce the diameter of each PS sphere from 1270to 500nm.These 500nm PS spheres were separated by 770nm and were used as a mask for Ar plasma etching of Cr/Au.An Ar plasma with 20sccm Ar,100W rf
power,
Fig.1.Micrographs of fabricated Au nanoparticles (NPs)on glass substrates.(a,b)Diameter of Au NPs is $500nm.(c,d)Diameter of Au NPs is $60nm.
F.Liu et al./Biosensors and Bioelectronics 55(2014)141–148
142
and 70mTorr pressure was used to remove the Cr/Au ?lms.For the fabrication of 60nm Au NPs,PS spheres (sulfate-modi ?ed latex,8%w/v 60nm diameter,Life Technologies Limited,NY,USA)were deposited uniformly by electrostatic self-assembly on Au ?lm treated with 5%w/v aluminum chlorohydrate (Adamas,CA,USA)as a monolayer etch mask (Andersson et al.,2007).O 2plasma was not used to reduce NP size,but the rest of the fabrication process for forming the Au NPs using 60nm diameter PS spheres was similar as described above.
2.2.Refractive index sensitivity tests
Extinction spectra with wavelengths ranging from 400to 3200nm were collected from Au NP coated glass substrates with an area of 12.6mm 2.A spectrophotometer (PE Lambda 19,PerkinElmer,MA,USA)was used for the optical measurements.Light generated by a tungsten –halogen lamp was illuminated perpendicularly to the glass substrate,and the intensity of trans-mitted light was measured by a photomultiplier tube for visible light and a lead-sul ?de cell for near infrared light.The extinction in %was calculated by subtracting the measured %transmission from 100.
The RIS of the 500nm Au NPs was measured by submerging the glass substrates in the following media (with n being different refractive indices):air (n ?1.00),n-hexane (n ?1.37),and toluene (n ?1.50).The RIS of 60nm Au NPs was measured by submerging the glass substrates in the following media:air (n ?1.00),water (n ?1.33),acetone (n ?1.36),and 2-propanol (n ?1.38).Different media had to be used because water,acetone,and 2-propanol all have strong light absorption in the range of $1300–3200nm,and therefore cannot be used for 500nm Au NPs (with major reso-nance peak occurring at 1900nm).
2.3.Biosensing protocol
2.3.1.Sterilization of Au nanoparticles and cell culture preparation
Au NPs on glass substrates were rinsed with Milli-Q water,sterilized with 70%ethanol for 10min,and air-dried in tissue culture hood before putting into 35mm sterile tissue culture dishes.
RPE-1and MCF-7cells (American type culture collection,MD,USA)were routinely maintained in Dulbecco's modi ?ed eagle medium (DMEM)supplemented with 10%fetal bovine serum (FBS;Invitrogen,CA,USA),in a humidi ?ed incubator at 371C and 5%CO 2.Cell suspensions of 120m l at different concentrations in DMEM and 10%FBS were loaded onto the surface of glass substrates with Au NPs,incubated at 371C for 2h for cell attachment.Subsequently,2ml of DMEM with 10%FBS was added and the cells were incubated at 371C for 12h before chemical ?xation.
2.3.2.Cell ?xing and extinction measurements
The cells on the surface of the glass substrates with Au NPs were rinsed twice with phosphate buffered saline (PBS;Invitrogen,CA,USA)and were ?xed with freshly prepared 3%formaldehyde (Sigma,MO,USA)in PBS for 10min at room temperature.Extinc-tion spectrum measurements were conducted after the cells were air-dried on the 8?12mm 2glass substrate surface with Au NPs.The reference spectrum for extinction spectra without cells was collected using a sample consisted of a glass substrate without Au NPs,while the reference spectra for extinction spectra with cells at different concentrations were measured using samples consisted of glass substrates with the same corresponding cell concentra-tions but without Au NPs.
600
7008009001000110012001300
1400
R e s o n a n c e W a v e l e n g t h (n m )
Refractive index (RIU)
10
20
3040506070
8090E x t i n c t i o n (%)
Wavelength (nm)
Air (n=1.00)Water (n=1.33)Acetone (n=1.36)2-Propanol (n=1.38)
60 nm NPs
400450500550600650700750800850
400
800
1200
1600
2000
2400
2800
3200
01020304050607080
90100E x t i n c t i o n (%)
Wavelength (nm)Air (n=1.00)
n-hexane (n=1.37)Toluene (n=1.50)
500 nm NPs
Fig.2.(a)Extinction spectra of 500nm Au NPs in different media:air with n ?1.00,n-hexane with n ?1.37,and toluene with n ?1.50.(b)Extinction spectra of 60nm Au NPs in different media:air with n ?1.00,water with n ?1.33,acetone with n ?1.36,and 2-propanol with n ?1.38.(c)Linear relationship between the LSPR wavelength and the refractive index of the 1175and 750nm resonance peaks of 500nm Au NPs and 622nm resonance peak of 60nm Au NPs.
F.Liu et al./Biosensors and Bioelectronics 55(2014)141–148143
2.4.Scanning electron and confocal microscopy measurements Au NPs on glass substrates were observed under an environ-mental scanning electron microscope (XL30ESEM-FEG,Philips Electronics,Netherlands),after coating with Au –Pd using a sputter-deposition coater (SCD005,Leica Microsystems,Wetzlar,Germany).To measure the surface area and thickness of the cells on the glass surface,the cells were ?xed with 3%formaldehyde in PBS for 10min,permeabilized in 0.2%Triton X-100(Sigma,MO,USA)in PBS for 10min,and stained with 2m g/ml propidium iodide (Sigma,MO,USA)for 20min at room temperature.The cells were mounted on 24?50mm 2cover glasses of thickness no.1(Marienfeld-SupeRior,Lauda-K?nigshofen,Germany)using a Vectashield mounting med-ium (Vector Laboratories,CA,USA).Fluorescence signals from the cells were photographed using a confocal laser microscope (TCS-SPE,Leica Microsystems,Wetzlar,Germany)and the cells were scanned using a step size of 250nm.The micrographs from all scanned layers were compiled,and the maximum projections of these images were generated by ImageJ version 1.47v (NIH,MD,USA)to compute the surface https://www.360docs.net/doc/169424523.html,ing the Volume Viewer plugin,the average distances
from the bottom of the cells to the peak (thicknesses;n ?10)were measured on the cells of different concentrations.
3.Results and discussion
3.1.High refractive index sensitivity of Au nanoparticles
Extinction spectra of both 500and 60nm Au NPs are measured in media with different refractive indices as shown in Fig.2(a)and (b).The ratio of the surface area occupied by the 500and 60nm Au NPs was 15.5%and 27.7%,respectively.The measured extinction is close to the simulated results for the 60nm Au NPs and the enhanced near-?eld electric ?eld intensity effect related to the coupling between the LSPR of NP and the diffracted wave of the periodic 500nm NP array (Chu et al.,2008).The dipole plasmon resonance peak was at 1900nm for the 500nm Au NPs measured in air (n ?1.00),and it blue-shifted to 622nm when the diameter of the Au NPs was reduced to 60nm.Also,the corre-sponding full width at half maximum (FWHM)was narrowed
from
Fig.3.Maximum projections of 30?uorescent confocal micrographs of the X –Y plane of RPE-1(a)–(c)and MCF-7(d)–(f)cells at different con ?uencies seeded on 60nm Au NP coated glass substrates.RPE-1cells with (a)8.56?103cells/ml,(b) 1.37?105cells/ml,and (c) 1.09?106cells/ml.MCF-7cells with (d)8.56?103cells/ml,(e)1.37?105cells/ml,and (f)1.09?106cells/ml.The ?gure below each micrograph is the Z-stack of micrographs to show the thickness of the cell https://www.360docs.net/doc/169424523.html,rmation on cell surface area,percentage con ?uency,and maximum cell thickness of RPE-1and MCF-7cells at different concentrations are summarized below each set of micrographs.
F.Liu et al./Biosensors and Bioelectronics 55(2014)141–148
144
400to 120nm.For 500nm Au NPs,two weak resonance peaks at shorter wavelengths of 750and 1175nm were also present,which has been explained by the different plasmon modes related to non-uniform fabrication (Cai et al.,2012;Ding et al.,2011).
The RIS of Au NPs was measured by submerging the glass substrates in media with different refractive indices.The reso-nance peaks red-shifted linearly with increasing refractive index of surrounding medium,which is shown in Fig.2.The measured RIS was 107nm/RIU for the 60nm Au NPs.For the 500nm Au NPs,the RIS was 116nm/RIU for the 750nm resonance peak and 412nm/RIU for the 1175nm resonance peak.This shows that the RIS is higher when the resonance peak at longer wavelength is used for the same Au nanostructure,a phenomenon that has been pre-viously observed (Larsson et al.,2007).Due to the limitation of the solutions'light absorption in the near infrared region,only the resonance peaks in air and n-hexane were obtained for the 1900nm peak.However,this peak has much larger RIS,because when the refractive index was changed from 1.00to 1.37in different media,the corresponding 200nm peak shift was almost 5?larger than the 622nm peak shift for 60nm NPs.For 500nm Au NPs,the 1900nm dipole resonance peak had a larger extinction and RIS compared to 750and 1175nm resonance peaks,and was therefore adopted for cell sensing measurements.
3.2.RPE-1and MCF-7cells immobilized on Au nanoparticles RPE-1and MCF-7cells were both tested to study the relation-ship between the LSPR shift and cell characteristics.The typical diameter of an individual RPE-1cell (after trypsinization and resuspension in PBS)was measured to be $20m m by a Coulter counter,which was slightly larger than that of MCF-7cells with diameter $18m m.The percentage of area covered by the cells on substrates,de ?ned as %con ?uency,was higher for RPE-1cells than that for MCF-7cells at the same cell concentration.At low
con ?uency (o 50%),the surface area covered by each RPE-1cell (2449–3951μm 2)was much larger than that of an MCF-7cell (545–682μm 2)when seeded onto the Au NP coated glass sub-strates (Fig.3(a),(b),(d),and (e)).In contrast,when the cells were grown to con ?uence,the average cell surface area of MCF-7cells was larger (928μm 2)than that of RPE-1cells (609μm 2).This observation is likely due to the fact that the larger cell surface area of RPE-1cells enabled full cell –cell contact (con ?uence)when 1.09?106cells/ml cells were seeded onto the glass surface.
Typically,the center of mammalian cells contains the nucleus and is the thickest part with thickness gradually decreasing along the cell edges.Overall,cell –cell contact was not observed at low concentration as shown in Fig.3(a),(b),(d),and (e).As cell concentration increased,cells formed a monolayer cover on the Au NP coated glass surfaces.At high concentration,cells were in close proximity with each other as shown in Fig.3(c)and (f).At concentra-tions of 1.09?106cells/ml,the larger RPE-1cells completely covered the entire Au NP coated glass surface,and a few cells started to stack on top of the others as shown in Fig.3(c).The maximum thickness of RPE-1cells at high con ?uency was 17.7m m,with some cells forming the second layer,while MCF-7cells were only 7.1m m thick with the same number of cells seeded on the surface (Fig.3(c)and (f)).For RPE-1cells,the thickness of the cell layers decreased with lower cell con ?uence,while the cell layer of MCF-7increased in less crowded conditions.We found similar cell adsorption behavior and no statis-tical differences of both types of the adhered cells on arrays of Au NPs of different sizes and on bare glass substrates.
3.3.Dependence of resonance peak shift on con ?uency of cells on 500nm Au nanoparticles
The extinction spectra of the 500nm Au NPs with RPE-1and MCF-7cells at concentrations of 8.56?103and 1.09?106cells/ml were measured after the cells were ?xed and air dried on the
Au
Fig.4.(a,b)Extinction spectra of 500nm Au NPs without cells and with cells of concentration 8.56?103and 1.09?106cells/ml.(a)RPE-1cells and (b)MCF-7cells.(c)For 500nm Au NPs,LSPR peak shift as a function of RPE-1and MCF-7cell concentration in the range 8.56?103–1.09?106cells/ml.
F.Liu et al./Biosensors and Bioelectronics 55(2014)141–148145
NP coated glass substrates,as shown in Fig.4(a)and(b).For both types of cells,there was a red-shift of the resonance wavelength after the cells were?xed(due to the increased refractive index caused by the cell layer on the Au NPs),and this shift increased with increasing cell concentration.The width of the resonance peak increased with the radiative and non-radiative damping (Wokaun et al.,1982;Zori?et al.,2011).The spectra were broadened with higher cell concentration on the Au NPs because both the radiative and non-radiative damping increased related to the larger refractive index at higher cell concentration(Maier, 2007;Mortazavi et al.,2012).The resonance peak shift(ΔλR)in response to a change of refractive index due to the presence of cells on the Au NP coated glass surface can be described as(Jung et al.,1998)follows:
ΔλR%men adsorbateàn mediumTe1àeeà2d=l dTTe1Twhere m is the RIS of the Au NPs(nm/RIU),n medium is the refractive index of the surrounding medium,n adsorbate is the refractive index of the adsorbate in a form of uniform?lm with thickness d(in nm),and l d(in nm)is the EM?eld decay length of the Au NPs.
Mammalian cells contain numerous organelles with different refractive indices.Therefore the protein concentration within the cells mainly determines the effective refractive index of the cells because of its higher refractive index of1.50–1.58.Cells with more proteins such as cancer cells have a relatively larger refractive index.The refractive index of immobilized MCF-7cancer cells ranges from1.39to1.40,which is slightly larger than the typical value of1.35–1.37for a normal cell(Liang et al.,2007).Since the immobilized cells form the adsorbate islands or closely packed layers on the Au NP surface,the effective thickness d of the cell layer should be properly weighted by the surface area,con?uency, actual thickness,and concentration of the cells.The EM?eld decays exponentially with the decay length l d,which depends on the size and shape of the Au NPs.Previous studies have shown that EM?eld decay length l d is$52nm for Au NPs with70nm widths (Haes et al.,2004).Au crescent nanostructures with410nm diameter show a692nm EM?eld decay length for the$2300nm long-axis dipole resonance peak(Bukasov et al.,2010).The EM?eld decay length increases with the size of Au NPs(Kedem et al.,2011).This gives us a general idea of the decay lengths of the500and60nm Au NPs used in our work.
Fig.4(c)shows the resonance peak shifts of the500nm Au NPs for the cell concentrations within a range of8.56?103–1.09?106cells/ml.All the results on resonance peak shifts were obtained from at least three sets of separately prepared sensors at each cell concentration.To demonstrate reproducibility,an addi-tional three sets of sensors at all seven different cell concentra-tions were measured for the MCF-7cells,providing a total of six different sets of samples shown in Fig.4(c)for the MCF-7cells. As expected,the resonance peak shift and sensing ef?ciency greatly depend on the surface area,con?uency,thickness,and concentration of the cells.At each concentration,RPE-1cells covered a higher con?uency on the surface with Au NPs compared to MCF-7cells,which resulted in a larger value of effective thickness d.Therefore,resonance peak shift of RPE-1cells was larger than that of MCF-7cells.With increasing cell concentration, the separation between two cells became smaller,and the cells were closely packed and reached con?uence at the concentration of1.09?106cells/ml for RPE-1cells,as shown in Fig.3(c).The thickness d increases with cell concentration and becomes com-parable with or even thicker than the EM?eld decay length l d. Therefore,at higher concentration,the EM?eld of Au NPs is not sensitive to the increasing cell concentration,or the effective thickness d.Accordingly,APRE-19cells at higher con?uency became saturated at lower concentration compared with MCF-7cells.The slopes of the linear?tting curves describe the cell sensing sensitivity(resonance peak shift/cell concentration,nm/ (cells/ml)).Since the slope is60for RPE-1cells and38for MCF-7 cells for concentrations below4.1?105cells/ml,this indicates that the500nm Au NPs are more sensitive to the change in concentra-tion of RPE-1cells in this range.This is due to the larger surface area of RPE-1cells which causes a more rapid increase of cell con?uency and thickness,and hence corresponding effective thickness d.By taking difference of peak shift between two cell concentrations and standard error of mean(SEM)for three to six sets of sensors into account,the detection ranges for RPE-1 and MCF-7cells are2.28?104–8.20?105and8.56?103–1.09?106cells/ml,respectively.
We have analyzed the SEM among each three sets of data. Within the detection limits,the SEM at a given concentration is always smaller than the difference in resonance peak shift when the cell concentration is changed.In addition,we further tested the reproducibility using three additional sets of500nm Au NP sensors(total of six sets)for MCF-7cells as shown in Fig.4(c). Again,the results are reproducible among six sets of separately prepared sensors at various concentrations with SEMs smaller than the differences of resonance peak shifts.For example,for MCF-7cells at the lowest two concentrations,the difference of average resonance peak shift is 6.7nm while the SEM is only 1.5nm.Therefore,we can conclude that the results from multiple sets of sensors at various concentrations,as well as the depen-dence of the resonance peak shift on cell concentration,are reproducible.
3.4.Dependence of resonance peak shift on con?uency of cells on 60nm Au nanoparticles
60nm Au NPs were also studied for RPE-1and MCF-7 cell concentration detection.For60nm Au NPs,the RIS is only107 nm/RIU,which is much smaller than the535nm/RIU measured for 500nm Au NPs.On the other hand,since the resonance peak is in the visible light region,the60nm Au NPs have the advantage of being able to detect living cells in solution without light absorption by the aqueous solution.
The extinction spectra of60nm Au NPs with RPE-1and MCF-7 cells at concentrations of8.56?103and 5.45?105cells/ml are shown in Fig.5(a)and(b).For60nm Au NPs,the resonance wavelength was$622nm(vs.$1900nm for500nm Au NPs), and also the extinction increased with increasing cell concentra-tion for both cell types(unlike500nm Au NPs,as shown in Fig.4(a)and(b)).A probable explanation for this difference is that the extinction is affected by both the refractive index of the surrounding adsorbate and medium(n(adsorbatetmedium)),and the radiative damping and non-radiative damping,and is approxi-mately inversely proportional to the imaginary part of the Au dielectric constant(ε2)(Moores and Goettmann,2006;Mortazavi et al.,2012;Zhang et al.,2011).For resonance peaks around 622nm,the change ofε2due to the red-shifted peak at higher cell concentration is much smaller than that of resonance peaks around1900nm(Palik,1985).Therefore,for60nm Au NPs,larger extinction was obtained for cells at higher concentration,com-pared to the500nm Au NPs.
Fig.5(c)shows the resonance wavelength peak shifts of the 60nm Au NPs for cell concentrations within a range of8.56?103–5.45?105cells/ml.The relationship between cell concentration and resonance peak shift shows similar trends compared to 500nm Au NPs,as shown in Fig.4(c).For instance,RPE-1cells at higher con?uency became saturated at lower concentration (1.37?105cells/ml)compared with MCF-7cells.On the other hand,MCF-7cells began to show a larger peak shift compared to RPE-1cells for cell concentration above2.73?105cells/ml,which
F.Liu et al./Biosensors and Bioelectronics55(2014)141–148 146
could imply that MCF-7cells have a larger refractive index since the resonance peak shift depends mainly on the refractive index of the cells at high concentrations.
Since the slope of the linear ?tting curves is 20for RPE-1cells and 22for MCF-7cells for concentrations between 3.43?104and 1.37?105cells/ml,this indicates that 60nm Au NPs have a similar sensitivity for both cell types in this range of cell concentration.The similarity in sensitivity is mainly due to the local EM ?eld with short decay length l d (in the order of tens of nm)for small size NPs (Haes et al.,2004),which is much smaller compared to the effective thickness d (in the order of a few m m).Therefore,the cell concentration detection sensitivity for 60nm Au NPs will be similar for different cell types due to the exponential dependence of d/l d in Eq.(1)when l d is much smaller than d .Overall,by taking difference of peak shift and SEM into account,the detection ranges for RPE-1and MCF-7cells are 3.43?104–1.37?105and 3.43?104–2.73?105cells/ml,respectively,for 60nm sensors.
3.5.Cell sensing comparison of 500and 60nm Au nanoparticles 500nm Au NPs show a much larger RIS and a longer EM ?eld decay length compared to 60nm Au NPs.The sensing performance of 500and 60nm Au NPs for RPE-1cells is compared in Fig.6.Due to the larger RIS,the saturated resonance peak shift of 500nm Au NPs is almost 6?larger than that of 60nm Au NPs ($300vs.$50nm).A similar peak shift ratio was also obtained with the extinction spectra of Au NPs measured in media with different refractive indices,as shown in Fig.2.Due to the longer EM ?eld decay length l d for 500nm Au NPs,a larger cell concentration range was detected on 500nm compared to 60nm Au NPs (8.56?103–1.09?106vs.3.43?104–2.73?105cells/ml).
Optimal cell sensing can be achieved by altering the dimen-sions of Au NPs according to different cell characteristics and concentrations.For the detection of the ?atter MCF-7cells,
500nm Au NPs show more ef ?cient sensing than 60nm Au NPs in the concentration range of 8.56?103–1.09?106cells/ml.Therefore,500nm Au NPs should be used for optimal cell sensing of larger cells at higher concentrations.On the other hand,with resonance peaks in the visible light region,the 60nm Au NPs are preferable for the detection of living cells in aqueous https://www.360docs.net/doc/169424523.html,ing the 60nm Au NPs,we can measure dynamic,live cell concentration using time-lapse monitoring of extinction spectra such as:(1)detecting decrease in the number of cells present on the sensors with time when the adhered cancer cells are subjected to cancer therapeutic drugs that can induce apoptosis and (2)measuring the dynamic increase in cell concentration across the NP array platform placed next to a con ?uent monolayer of epithelial cells and measure the rate of migration onto the array —an assay for cancer cell metastasis or wound healing.Chemical reagents can also be used with this sensor for screening therapeutics in inhibiting metastasis and wound
healing.
Fig.5.(a,b)Extinction spectra of 60nm Au NPs without cells and with cells of concentration 8.56?103and 5.45?105cells/ml.(a)RPE-1cells and (b)MCF-7cells.(c)For 60nm Au NPs,LSPR peak shift as a function of RPE-1and MCF-7cell concentration in the range 8.56?103–5.45?105
cells/ml.
https://www.360docs.net/doc/169424523.html,parison of RPE-1cell concentration detections using 500and 60nm Au NPs.
F.Liu et al./Biosensors and Bioelectronics 55(2014)141–148147
4.Conclusions
The LSPR effect has been used to distinguish RPE-1and MCF-7 cell concentration on ordered arrays of Au NPs on glass substrates formed by the NSL method.Sensing ef?ciency depended on the size,shape,and distribution of the Au NPs as well as the con-?uency and concentration of RPE-1and https://www.360docs.net/doc/169424523.html,rger size (500nm)NPs have longer EM?eld decay length,and they provide a larger detection range than that of smaller size(60nm)NPs. For MCF-7cells,the detection range for500nm NPs is8.56?103–1.09?106cells/ml compared to3.43?104–2.73?105cells/ml for 60nm NPs.On the other hand,?atter cells that spread out on a larger area(RPE-1)displayed larger resonance peak shifts than those of small size cells(MCF-7).These characteristics can be used to distinguish RPE-1from MCF-7cells.Our results show that cells on LPSR-based sensor that consisted of Au NPs can be detected in a large range and at low concentration.Therefore,optimal cell sensing can be achieved by altering the dimensions of Au NPs according to different cell characteristics and concentrations. Acknowledgments
This work was supported by the Center for Biosystems, Neuroscience,and Nanotechnology of City University of Hong Kong under Project number9360148.We gratefully acknowledge Dr.Qing Yuan Tang,Miss Tsing Chung,Dr.Polis Wong,Mr.Robust Lai,Dr.Payton Lin,Dr.Shang Xin Lin,Mr.Bing Zou,and Mr.Michael Chiang for their technical support and helpful discussions. References
Andersson,A-S.,Glasm?star,K.,Hanarp,P.,Seantier, B.,Sutherland,D.S.,2007.
Nanotechnology18,205303.
Arya,S.K.,Wang,Y.P.,Wong,C.C.,Rahman,A.R.A.,2013.Biosens.Bioelectron.41, 446–451.
Bukasov,R.,Ali,T.A.,Nordlander,P.,Shumaker-Parry,J.S.,2010.ACS Nano4(11), 6639–6650.
Cai,Y.J.,Li,Y.,Nordlander,P.,Cremer,P.S.,2012.Nano Lett.12,4881–4888.
Cao,J.,Tu,M.H.,Sun,T.,Grattan,K.T.V.,2013.Sens.Actuators B181,611–619.Chen,S.,Svedendahl,M.,K?ll,M.,Gunnarsson,L.,Dmitriev,A.,2009.Nanotechnology 20,434015.
Chu,Y.Z.,Schonbrun,E.,Yang,T.,Crozier,K.B.,2008.Appl.Phys.Lett.93,181108. Cinel,N.A.,Bütün,S.,?zbay,E.,2012.Opt.Express20(3),2587–2597.
Costa,M.M.D.,Escosura-Mu?iz,A.D.L.,Nogués,C.,Barrios,L,Ibá?ez,E.,Merko?i,A.A., 2012.Small8(23),3605–3612.
Ding,P.,Liang,E.J.,Hu,W.Q.,Cai,G.W.,Xue,Q.Z.,2011.Photon.Nanostruct.9,42–48. EI-Sayed,I.H.,Huang,X.H.,EI-Sayed,M.A.,2005.Nano Lett.5(5),829–834. Escosura-Mu?iz,A.D.L.,Sánchez-Espinel,C.,Díaz-Freitas,B.,González-Fernández,á.,Costa,M.M.,Merko?i,A.,2009.Anal.Chem.81,10268–10274.
Fu,J.X.,Park,B.,Zhao,Y.P.,2009.Sens.Actuators B141,276–283.
Guo,L.H.,Kim,D.H.,2012.Biosens.Bioelectron.31,567–570.
Haes, A.J.,Zou,S.L.,Schatz,G.C.,Van Duyne,R.P.,2004.J.Phys.Chem.B108, 109–116.
Huang,C.J.,Ye,J.,Wang,S.,Stakenborg,T.,Lagae,L.,2012.Appl.Phys.Lett.100, 173114.
Huang,Y.F.,Lin,Y.W.,Lin,Z.H.,Chang,H.T.,2009.J.Nanopart.Res.11,775–783. Joshi,G.K.,McClory,P.J.,Dolai,S.,Sardar,R.,2012.J.Mater.Chem.22,923–931. Jung,L.S.,Campbell,C.T.,Chinowsky,T.M.,Mar,M.N.,Yee,S.S.,https://www.360docs.net/doc/169424523.html,ngmuir14, 5636–5648.
Jung,W.K.,Byun,K.M.,2011.Biomed.Eng.Lett.1,153–162.
Kedem,O.,Tesler,A.B.,Vaskevich,A.,Rubinstein,I.,2011.ACS Nano5(2),748–760. LamdaGen,2012.Available online:?https://www.360docs.net/doc/169424523.html,/lspr-label-free-detection?. Larsson, E.M.,Alegret,J.,K?ll,M.,Sutherland, D.S.,2007.Nano Lett.7(5), 1256–1263.
Li,T.,Fan,Q.,Liu,T.,Zhu,X.L.,Zhao,J.,Li,G.X.,2010.Biosens.Bioelectron.25, 2686–2689.
Liang,X.J.,Liu, A.Q.,Lim,C.S.,Ayi,T.C.,Yap,P.H.,2007.Sens.Actuators A133, 349–354.
Lu,W.T.,Arumugam,S.R.,Senapati,D.,Singh,A.K.,Arbneshi,T.,Khan,S.A.,Yu,H.T., Ray,P.C.,2010.ACS Nano4(3),1739–1749.
Maier,S.A.,2007.Plasmonics:Fundamentals and Applications.Springer SciencetBusiness Media LLC,New York.
Moores,A.,Goettmann,F.,2006.New J.Chem.30,1121–1132.
Mortazavi, D.,Kouzani, A.Z.,Kaynak, A.,Duan,W.,2012.Prog.Electromagn.
Res.126,203–235.
Palik,E.D.,1985.Handbook of Optical Constants of Solids.Academic Press,San Diego.
Perfézou,M.,Turner,A.,Merko?i,A.,2012.Chem.Soc.Rev.41,2606–2622.
Tang Q.Y.,Tong Y.,Lam Y.W.,Shi P.,Pang,S.W.,2013.Proceeding of the International Conference on Electron,Ion,and Photon Beam Technology and Nanofabrication, Tennessee,USA.
Wang,C.G.,Irudayaraj,J.,2008.Small4(12),2204–2208.
Wokaun,A.,Gordon,J.P.,Liao,P.F.,1982.Phys.Rev.Lett.48(14),957–960.
Xu,X.,Chen,Y.,Wei,H.J.,Xia,B.,Liu,F.,Li,N.,2012.Anal.Chem.84,9721–9728. Zhang,S.P.,Bao,K.,Halas,N.J.,Xu,H.X.,Nordlander,P.,2011.Nano Lett.11, 1657–1663.
Zheng,Y.B.,Juluri,B.K.,Mao,X.L.,Walker,T.R.,Huang,T.J.,2008.J.Appl.Phys.103, 014308.
Zori?,I.,Z?ch,M.,Kasemo,B.,Langhammer,C.,2011.ACS Nano5(4),2535–2546.
F.Liu et al./Biosensors and Bioelectronics55(2014)141–148 148
生物医学传感器与检测技术教学
《生物医学传感器与检测技术实验》教案大纲 张日欣李元斌 一、课程名称:生物医学传感器与检测技术实验 Experiments in Biomedical Sensor & Detecting Techniques 二、课程编码:0702831 三、学时与学分:24/1.5 四、先修课程:数字电子技术,模拟电子技术,项目生理学,电子测试与实验,生物医学测量与仪器实验。 五、课程教案目标 1.本课程是生物医学项目专业的一门专业课,它应用电子技术,传感器测量技术和计算机技术,解决生物医学领域中的信号提取,检测和处理以及生物医学仪器的设计等问题; 2.使学生了解典型医学仪器的原理、特点和性能指标,学习正确使用传感器,设计检测电路,掌握基本测量技术; 3.为医学仪器设计奠定基础。 六、适用学科专业 生物医学项目 七、基本教案内容与学时安排 ●热敏器件及温度传感器特性实验<4学时) ●压力传感器性能实验<4学时) ●气敏传感器特性实验<4学时) ●光电式脉搏探测器<4学时) ● ECG前置放大器<4学时) ●陷波器仿真、制作与调试<4学时) ●安全隔离设计与调试<4学时) ● ECG放大器的整体调试<4学时) ● 12导联心电工作站的原理及使用<4学时) 八、教材及参考书: 教材:生物医学电子技术与信号处理实验指导书,张日欣、李元斌、邹昂等自编教材,武汉:华中科技大学教材科,2004年9月 参考文献: 1.生物医学检测技术讲义,杨玉星自编教材,1998年 2.生物医学电子学,蔡建新,张唯真,北京大学出版社,1997年 3.传感器原理与应用,黄贤钨,电子科技大学出版社,1999年 4.生物医学测量,陈延航,人民卫生出版社,1986年 5.医学物理,刘普和,人民卫生出版社,1986年 6.医学仪器-应用与设计,约翰G.韦伯斯特,新时代出版社,1985年 7.Protel 98 for windows 电路设计应用指南,程凡等,人民邮电出版社,1999年 九、考核方式 实验报告+实践表现 《生物医学测量与仪器实验》教案大纲
纳米材料的表面界面问题
纳米材料的表面、界面问题 目录 摘要 (2) 1 纳米粒子和纳米固体的表面、界面问题 (3) 纳米微粒的表面效应 (3) 纳米固体的界面效应 (3) 纳米材料尺度效应导致的热学性能问题 (4) 纳米材料尺度效应导致的力学性能问题 (4) 纳米材料尺度效应导致的相变问题 (4) 2. 金属纳米材料的表面、界面问题 (5) 高性能铜(银)合金中的高强高导机理问题 (5) 金属复合材料的强化模型和物理机制问题 (5) 原子尺度上的Cu/X界面研究 (6) 3 纳米材料表面、界面效应的研究成果综述 (9) 参考文献 (11)
摘要 纳米材料包含纳米微粒和纳米固体两部分,纳米微粒的粒子直径与电子的德布罗意波长相当,并且具有巨大的比表面;由纳米微粒构成的纳米固体又存在庞大的界面成分。强大的表面和界面效应使纳米材料体现出许多异常的特性和新的规律,这些特性和规律使其展现出广阔的应用前景。其中,在宏观尺度上制造出具有纳米结构和纳米效应的高性能金属材料,并揭示这些材料的组织演化特征以实现功能调控,是金属材料学科面临的重大科学问题和需要解决的核心关键技术。本文将对纳米材料的表面、界面效应进行介绍并重点阐述金属纳米材料界面、尺度与材料塑变、强化关系的研究进展。 关键词:纳米材料;表面效应;复合材料 、
1 纳米粒子和纳米固体的表面、界面问题 纳米粒子是指颗粒尺度在范围的超细粒子,它的尺度小于通常的微粉,接近于原子簇。是肉眼和一般显微镜看不见的微小粒子[1]。只能用高倍的电子显微镜进行观察。最早日本名古屋大学上田良二教授给纳米微粒下了一个定义:用电子显微镜能看到的微粒被称为纳米微粒[2]。 纳米固体是由纳米微粒压制活特殊加工而成的新型固体材料,它可以是单一材料,也可以是复合材料。纳米固体最早是由联邦德国萨尔兰大学格莱特等人在80年代初首先制成的。他们用气相冷凝发制得具有清洁表面的纳米级超级微粒子,在超高真空下加压形成固体材料。 纳米微粒的表面效应 随着微粒粒径的减小,其比表面积大大增加,位于表面的原子数目将占相当大的比例。例如粒径为5nm时,表面原子的比例达到50%;粒径为2nm时,表面原子的比例数猛增到80%;粒径为1nm时,表面原子比例数达到99%,几乎所有原子都处于表面状态。庞大的表面使纳米微粒的表面自由能,剩余价和剩余键力大大增加。键态严重失配、出现了许多活性中心,表面台阶和粗糙度增加,表面出现非化学平衡、非整数配位的化学价,导致了纳米微粒的化学性质与化学平衡体系有很大差别,我们把这些差别及其作用叫做纳米微粒的表面效应[3]。 从电镜研究中也可以看出,由于强烈的表面效应使得纳米微粒的微观结构处于不断地变化之中。 纳米固体的界面效应 由纳米微粒制成的纳米固体,不同于长程有序的晶态固体,也不同于长程无序短程有序的非晶态固体,而是处于一种无序状态更高的状态。格莱特认为,这类固体的晶界有“类气体”的结构,具有很高的活性和可移动性。从结构组成上看它是由两种组元构成,一是具有不同取向的晶粒构成的颗粒组元,二是完全无序结构各不相同的晶界构成的界面组元。由于颗粒尺寸小,界面组元占据了可以与颗粒组元相比拟的体积百分数。例如当颗粒粒径为5-50nm时构成的纳米固体,
纳米尺寸效应
纳米尺寸效应 纳米是长度单位,原称毫微米,就是10^-9米(10亿分之一米)。纳米科学与技术,有时简称为纳米技术,是研究结构尺寸在1至100纳米范围内材料的性质和应用。纳米效应就是指纳米材料具有传统材料所不具备的奇异或反常的物理、化学特性,如原本导电的铜到某一纳米级界限就不导电,原来绝缘的二氧化硅、晶体等,在某一纳米级界限时开始导电。这是由于纳米材料具有颗粒尺寸小、比表面积大、表面能高、表面原子所占比例大等特点,以及其特有的三大效应:表面效应、小尺寸效应和宏观量子隧道效应。 表面效应 球形颗粒的表面积与直径的平方成正比,其体积与直径的立方成正比,故其比表面积(表面积/体积)与直径成反比。随着颗粒直径变小,比表面积将会显著增大,说明表面原子所占的百分数将会显著地增加。对直径大于0.1微米的颗粒表面效应可忽略不计,当尺寸小于0.1微米时,其表面原子百分数激剧增长,甚至1克超微颗粒表面积的总和可高达100平方米,这时的表面效应将不容忽略。 超微颗粒的表面与大块物体的表面是十分不同的,若用高倍率电子显微镜对金超微颗粒(直径为2*10^-3微米)进行电视摄像,实时观察发现这些颗粒没有固定的形态,随着时间的变化会自动形成各种形状(如立方八面体,十面体,二十面体多李晶等),它既不同于一般固体,又不同于液体,是一种准固体。在电子显微镜的电子束照射下,表面原子仿佛进入了“沸腾”状态,尺寸大于10纳米后才看不到这种颗粒结构的不稳定性,这时微颗粒具有稳定的结构状态。超微颗粒的表面具有很高的活性,在空气中金属颗粒会迅速氧化而燃烧。如要防止自燃,可采用表面包覆或有意识地控制氧化速率,使其缓慢氧化生成一层极薄而致密的氧化层,确保表面稳定化。利用表面活性,金属超微颗粒可望成为新一代的高效催化剂和贮气材料以及低熔点材料。 小尺寸效应 随着颗粒尺寸的量变,在一定条件下会引起颗粒性质的质变。由于颗粒尺寸变小所引起的宏观物理性质的变化称为小尺寸效应。对超微颗粒而言,尺寸变小,同时其比表面积亦显著增加,从而产生如下一系列新奇的性质。 (1)特殊的光学性质当黄金被细分到小于光波波长的尺寸时,即失去了原有的富贵光泽而呈黑色。事实上,所有的金属在超微颗粒状态都呈现为黑色。尺寸越小,颜色愈黑,银白色的铂(白金)变成铂黑,金属铬变成铬黑。由此可见,金属超微颗粒对光的反射率很低,通常可低于l%,大约几微米的厚度就能完全消光。利用这个特性可以作为高效率的光热、光电等转换材料,可以高效率地将太阳能转变为热能、电能。此外又有可能应用于红外敏感元件、红外隐身技术等。 (2)特殊的热学性质固态物质在其形态为大尺寸时,其熔点是固定的,超细微化后却发现其熔点将显著降低,当颗粒小于10纳米量级时尤为显著。例如,金的常规熔点为1064C℃,当颗粒尺寸减小到10纳米尺寸时,则降低27℃,2纳米尺寸时的熔点仅为327℃左右;银的常规熔点为670℃,而超微银颗粒的熔点可低于100℃。因此,超细银粉制成的导电浆料可以进行低温烧结,此时元件的基片不必采用耐高温的陶瓷材料,甚至可用塑料。采用超细银粉浆料,可使膜厚均匀,覆盖面积大,既省料又具高质量。日本川崎制铁公司采用0.1~
最新重庆大学《生物医学传感器原理与应用》第二章--传感器基础
第二章 传感器基础 §2-1 传感器的静态特性 医用传感器的输入量可以分为静态量与动态量两大类。 静态量:是指固定状态的信号或变化极其缓慢的信号(准静态量)。 动态量:通常是指周期信号、瞬变信号或随机信号。 无论对动态量或静态量,传感器输出量都应不失真地复现输入生理量的变化,其关健决定于传感器的静态特性与动态特性。 一.传感器的静态特性 传感器的静特性—表示传感器在被测量处于稳定状态,输入量为恒定值而不随时间变化时,其相应输出量亦不随时间变化,这时输出量与输入量之间的关系称为静态特性。 这种关系一般根据物理、化学、生物学的“效应”和“反应定律”得到,具有各种函数关系。 传感器的输出输入关系或多或少的存在非线性问题。在不考虑迟滞蠕变不稳定性等因素的情况下,其静态特性可用下列多项式代数方程表示: n n x a x a x a x a a y +++++= 332210 (2-1) 式中 y — 输出量; x — 输入量; 0a — 零位输出(零偏); 1a — 传感器的灵敏度,常用K 表示; n a a a ,,,32 — 非线性项系数 各项系数不同,决定了特性曲线的具体形式。 由式(2—1)可知,如果0a =0,表示静态特性通过原点,这时静态特性是由线性项和非线性的高次项迭加而成。这种多项式代数方程可能有四种情况,表现了传感器的四种静态特性,如图2-1所示。 1.线性特性 在理想情况下,式(2—1)中的零偏0a 被校准(0a =0).且x 的高次项为零。
0,,,32=n a a a 线性方程为: x a y 1= 如图2—1(a )所示。 此时, K x y a ==/1 K 称为传感器的灵敏度。 2.非线性项仅有奇次项的特性 当式(2—1)中只有x 的奇次项,即: +++=5 53 31x a x a x a y 时,特性如图2—1(b )所示。在这种情况下,在原点附近相当范围内输出、输入特性基本成线性,对应的曲线有如下特性: y (x )=-y(-x ) 3.非线性项仅有偶次项的特性 当式(2—1)中只有x 的偶次非线性项时.所得曲线不对称,如图2-1(c )所示。 4.一般情况 对应的曲线如2—1(d )所示。在实际应用中,如果非线性项的x 方次不高,则在输入量变化不大的范围内,可以用切线或割线来代替实际静态特性的某一段,使得传感器的静态特性近于线性,称之为传感器静态特性的线性化。只要传感器非线性系数较小,测量范围又不大时,即可这样处理。当没计传感器时,把测量范围选择在最接近直线的那一小段,可 使传感器的静态特性近于线性。 传感器的静态特性实际上是非线性的,所以它的输出不可能丝毫不差地反映被测量的变化,对动态特性也会有一定的影响。 传感器的静态特性是在静态标准条件下进行校准的。静态标准条件是指没有加速度、振动、冲击,环境温度一般在室温20℃±5 ℃,相对湿度不大于85%,大气压为101.3士8 kPa 。在这种标准工作条件下,利用一定等级的校准设备,对传感器进行反复的测试,将得到的输出-输入数据列成表格或画成曲线。把被测量值的正行程输出值和反行程输出值的平均值连接起来的曲线称为传感器的静态校准曲线。 二.传感器的静态特性指标 1.线性度 传感器的线性度也叫作传感器特性曲线的非线性误差。 它是用传感器校准曲线与拟合直线之间的最大偏差与传感器满量程输出平均值之比的百分数来表示的(如图2—2所示): δL =士(ΔL max / Y FS )×100% (2-2) 式中δL 为线性度; ΔL max 为校准曲线与拟合直线之间的最大偏差; Y FS 为传感器满量程输出(平均值),Y FS =Y max -Y 。 常用的拟合直线的方法: ⑴.采用理论直线作为拟合直线来确定传感器的线性度。 所谓理论直线即式(2-1)静态方程式的第一种情况:Y =α1X ,由此式求得的线性度称为理论线性度。拟合直线为传感器的理论特性,与实际测试值无关。该方法十分简单,但ΔL max 较大。图2—3为理论线性度的示意图。 ⑵.采用最小二乘法拟合
生物传感器基本原理与应用
生物传感器基本原理与应用 生物传感器,是一种对生物物质敏感并将其浓度转换为电信号进行检测的仪器。是由固定化的生物敏感材料作识别元件(包括酶、抗体、抗原、微生物、细胞、组织、核酸等生物活性物质)、适当的理化换能器(如氧电极、光敏管、场效应管、压电晶体等等)及信号放大装置构成的分析工具或系统。 生物传感器由分子识别部分(敏感元件)和转换部分(换能器)构成。以分子识别部分去识别被测目标,是可以引起某种物理变化或化学变化的主要功能元件。分子识别部分是生物传感器选择性测定的基础;而换能部分是把生物活性表达的信号转换为电信号的物理或化学换能器(传感器)。 各种生物传感器有以下共同的结构:包括一种或数种相关生物活性材料(生物膜)及能把生物活性表达的信号转换为电信号的物理或化学换能器(传感器),二者组合在一起,用现代微电子和自动化仪表技术进行生物信号的再加工,构成各种可以使用的生物传感器分析装置、仪器和系统。 生物传感器能够选择性地分辩特定物质的物质有酶、结构抗体、组织、细胞等。这些分子识别功能物质通过识别过程可与被测目标结合成复合物,如抗体和抗原的结合,酶与基质的结合。 主要应用: 1.食品工业。生物传感器在食品分析中的应用包括食品成分、食品添加剂、有害毒物及食品鲜度等的测定分析。 2.环境监测。环境污染问题日益严重,人们迫切希望拥有一种能对污染物进行连续、快速、在线监测的仪器,生物传感器满足了人们的要求。目前,在包括水环境监测、大气环境监测等方面,生物传感器已经有了较为广泛的应用和良好的前景。 3.发酵工业。在各种生物传感器中,微生物传感器具有成本低、设备简单、不受发酵液混浊程度的限制、可能消除发酵过程中干扰物质的干扰等特点。因此,在发酵工业中广泛地采用微生物传感器作为一种有效的测量工具。 目前主要的应用方向为:原材料及代谢产物的测定、微生物细胞数目的测定等。 4.医学。医学领域的生物传感器发挥着越来越大的作用。生物传感技术不仅为基础医学研究及临床诊断提供了一种快速简便的新型方法,而且因为其专一、灵敏、响应快等特点,在军事医学方面,也具有广的应用前景。目前主要的应用方向有:临床医学(主要是酶电极)、军事医学等。此外,在法医学中,生物传感器还可用作DNA鉴定和亲子认证等。
金属纳米晶体的表面与其催化效应
金属纳米晶体的表面与其催化效应 沈正阳 (浙大材料系1104 3110103281) 摘要:概括纳米材料的表面与界面特性,从金属纳米晶体表面活性与结构介绍其的催化性能,简要概述金属纳米晶体形状与晶面的关系以及金属纳米晶体的成核与生长。 关键词:纳米金属;表面活性;催化;高指数晶面 1.纳米材料的表面与界面 纳米微粒尺寸小,表面能高,位于表面的原子占相当大的比例。由于表面原子数增多,原子配位不足及高的表面能,使这些表面原子具有高的活性,极不稳定,很容易与其他原子结合。强烈的表面效应,使超微粒子具有高度的活性。如将刚制成的金属超微粒子暴露在大气中,瞬时就会氧化,若在非超高真空环境,则不断吸附气体并发生反应。[1] 纳米晶体是至少有一个维度介于1到100纳米之间的晶体。纳米材料主要由晶粒和晶粒界面2部分组成,二者对纳米材料的性能有重要影响。纳米材料微观结构与传统晶体结构基本一致,但因每个晶粒仅包含着有限个晶胞,晶格点阵必然会发生一定程度的弹性畸变,其内部同样会存在各种缺陷,如点缺陷、位错、孪晶界等。纳米金属粒子的形状、粒径、颗粒间界、晶面间界、杂质原子、结构缺陷等是影响其催化性能的重要因素。纳米材料中,晶界原子质量分数达15%~50%,晶界上的原子排列极为复杂,尤其三相或更多相交叉区,原子几乎是自由的、孤立的,其量子力学状态和原子、电子结构已非传统固体物理、晶体理论所能解释。金属纳米晶体研究中,发现面心立方结构纳米金属如 Al、Ni、Cu 和密排六方结构Co都存在孪晶和层错缺陷,Cu纳米金属中存在晶界滑移。 2.金属纳米晶体的催化性能 近年来,关于纳米微粒催化剂的大量研究表明,纳米粒子作为催化剂,表现出非常高的催化活性和选择性。这是因为纳米微粒尺寸小,位于表面的原子或分子所占的比例非常大,并随纳米粒子尺寸的减小而急剧增大,同时微粒的比表面积及表面结合能迅速增大。纳米颗粒表面原子数的增加、原子配位的不足必然导致了纳米结构表面存在许多缺陷。从化学角度看,表面原子所处的键合状态或键
(完整)量子尺寸效应
(完整)量子尺寸效应 编辑整理: 尊敬的读者朋友们: 这里是精品文档编辑中心,本文档内容是由我和我的同事精心编辑整理后发布的,发布之前我们对文中内容进行仔细校对,但是难免会有疏漏的地方,但是任然希望((完整)量子尺寸效应)的内容能够给您的工作和学习带来便利。同时也真诚的希望收到您的建议和反馈,这将是我们进步的源泉,前进的动力。 本文可编辑可修改,如果觉得对您有帮助请收藏以便随时查阅,最后祝您生活愉快业绩进步,以下为(完整)量子尺寸效应的全部内容。
1.1.1量子尺寸效应 所谓的量子尺寸效应是指粒子尺寸下降到某一值时,金属费米能级附近的电子能级 由准连续变为离散的现象,纳米半导体粒子存在不连续的最高被占据的分子轨道和最低未 被占据的分子轨道能级,能隙变宽,由此导致纳米微粒的光、电、磁、热、催化和超导性等 特性与宏观性存在着显著的差异。如金属纳米材料的电阻随着尺寸下降而增大,电阻温度 系数下降甚至变成负值;相反,原是绝缘体的氧化物达到纳米级时,电阻反而下降;10~ 25nm的铁磁金属微粒矫顽力比同种宏观材料大1000倍,而当颗粒尺寸小于10nm时矫顽力 变为零,表现为超顺磁性。 1。1。2小尺寸效应 当超细微粒的尺寸与光波波长、德布罗意波长以及超导态的相干长度或透射深度等 物理特征尺寸相当或更小时,晶体周期性的边界条件将被破坏;非晶态纳米微粒的颗粒表面 层附近原子密度减小,导致声、光、电、滋、热、力学等特性呈现新的小尺寸效应.例如: 光吸收显著增加,吸收峰的等离子共振频移,磁有序态向磁无序态转变,超导相向正常相 的转变,声子谱发生改变等,这种现象称为小尺寸效应。 1。1.3表面与界面效应 纳米材料的另一个重要特性是表面与界面效应.由于表面原子与内部原子所处的环境 不同,当粒子直径比原子直径大时(如大于0。01时),表面原子可以忽略,但当粒子直径 逐渐接近原子直径时,表面原子的数目及作用就不能忽略,而且这时粒子的比表面积、表 面能和表面结合能都发生很大变化.人们把由此引起的种种特殊效应统称表面效应[8,9]。 随着粒径的减小,比表面迅速增大.当粒径为5nm时,表面原子数比例达到约50%以上,当 粒径为2nm时,表面原子数达到80%,原子几乎全部集中到纳米粒子的表面.庞大的表面原 子的存在导致键态严重失配,表面出现非化学平衡、非整数配位的化学键,产生许多活性中心,从而导致纳米微粒的化学活性大大增强,主要表现在:(1)熔点降低.就熔点来说,纳 米颗粒中由于每一粒子组成原子少,表面原子处于不安定状态,使其表面晶格震动的振幅 较大,所以具有较高的表面能量,造成超微粒子特有的热性质,也就是造成熔点下降,同时 纳米粉末将比传统粉末容易在较低温度烧结,而成为良好的烧结促进材料。如金的常规熔 点是1064℃当颗粒尺寸减小到10nm时,降低了270℃,当金纳米粒子尺寸为2 nm时,熔点 仅为327℃;银的常规熔点为961℃,而超微银颗粒的熔点可低于100℃等。(2)比热增大。粒径越小,比热越大.(3)化学活性增加,有利于催化反应等。 1.1。4宏观量子隧道效应 微观粒子具有贯穿势垒的能力称为隧道效应。近年来,人们发现一些宏观量,如超微 粒的磁化强度和量子相干器件中的磁通量等也具有隧道效应,称为宏观量子隧道效应,利 用它可以解释纳米镍粒子在低温下继续保持超顺磁性的现象。宏观量子隧道效应的研究对 基础研究及实用都具有重要的意义,它确立了现存微电子器件进一步微型化的极限,是未来 微电子器件的基础. 上述的小尺寸效应、表面界面效应、量子尺寸效应及量子隧道效应都是纳米微粒与 纳米固体的基本特性。它使纳米微粒和纳米固体呈现许多奇异的物理、化学性质,出现一 些“反常现象”。例如金属纳米材料的电阻随尺寸下降而增大,电阻温度系数下降甚至变 成负值;相反,原是绝缘体的氧化物达到纳米级时,电阻反而下降;10nm-25nm的铁磁金属
重庆大学《生物医学传感器原理与应用》第三章--敏感元件
第三章 敏感元件 作用:把物理量转换为电量,是传感器中的主要元件。 必备两个基本功能: ①敏感被测量(物理量、化学量)②对应产生输出量(电量)。 §3-1 变换力和压力的弹性敏感元件 一、弹性敏感元件的作用 非电量—→弹性元件—→应变量—→换能元件—→电量 弹性元件两种类型: ①弹性敏感元件:感受力、压力、力矩等-→变换为元件本身的应变、位移等; ②弹性支承:起支承导向作用,不作为测量敏感元件。 二、弹性特性: 作用在弹性元件上的外力与其相应变形间的关系。 1.刚度:弹性元件受外力作用下变形大小的量度。 dx dF k = F —作用外力 x —变形 弹性特性曲线上某点切线水平线夹角的正切为该点处的刚度。 dx dF tg k = =θ 2.灵敏度:单位力产生变形的大小,是刚度的倒数。 dF dx K = 并联时,系统的灵敏度:∑== n i i K K 111 灵敏度低,刚度大 串联时,系统的灵敏度: 1 n i i K K ==∑ 灵敏度高,刚度小 三、弹性滞后和弹性后效 1.弹性滞后——弹性特性曲线的加载曲线与去载曲线不重合现象。 滞后误差:弹性变形之差,直接产生测量误差。 2.弹性后效——当载荷改变后,在一定时间间隔逐渐完成变形的现象。
使弹性敏感元件的变形始终不能迅速跟随作用力的改变而改变,造成测量误差,尤其在动态测量中影响较大。 4.固有振动频率:——由振动质量和材料刚度综合表征的弹性元件特征。 决定弹性元件的动态特性和变换被测参数的滞后作用,希望0f (或0ω)高。 因 e m k = 0ω e m k f π 210= , k — 弹簧刚度,m e — 等效振动质量 所以 提高灵敏度K ,会使线性变差,固有振动频率 0ω、0f ↓。 k K 1= Θ 提高0ω、0f ↑,灵敏度K 会降低,需综合考虑。 5.固有频率f 0与弹性元件的变形dx 以及材料性能的关系 ρ??=l S m , S —截面积,l —长度,ρ—密度 弹性元件相对变形:E l dx σδ== ,式中 E —弹性摸数,σ—应力,∴dx l E ?=σ () 2 02 1 1 1/1 1 222221122S E dx dx k dF dx dx dx l f m Sl Sl l l dx E E dx σσσσπ πρπρπ ρπ ρ σσπ πρ ρ??====== = 最后可得: ρπσ ?= ?E dx f 20 可知弹性元件dx f ?0的乘积对于特定材料是有一个极限值的,σ达到许用应力时, dx 大,f 0就只能小,反之亦然。 6.弹性敏感元件的形式及其应用范围。 力、压力——→弹性敏感元件——→ 输入 输出 应变—各种应变传感器 位移—电感式、电容式、电阻式等传感器
传感器及其工作原理 说课稿 教案
传感器及其工作原理 【三维目标】 1.知识与技能: (1)、了解什么是传感器,知道非电学量转化为电学量的技术意义; (2)、知道传感器中常见的三种敏感元件光敏电阻、热敏电阻和霍尔元件及其它们的工作原理。 (3)、了解传感器的应用。 2.过程与方法: 通过对实验的观察、思考和探究,让学生在了解传感器、熟悉传感器工作原理的同时,经历科学探究过程,学习科学研究方法,培养学生的观察能力、实践 能力和创新思维能力。 3.情感、态度与价值观 (1)、体会传感器在生活、生产、科技领域的种种益处,激发学生的学习兴趣,拓展学生的知识视野,并加强物理与STS的联系。 (2)、通过动手实验,培养学生实事求是的科学态度、团队合作精神和创新意识。【教学重点】:理解并掌握传感器的三种常见敏感元件的工作原理。 【教学难点】:分析并设计传感器的应用电路。 【教学方法】:实验、探究、讨论 【教学用具】:干簧管,磁铁,光敏电阻、热敏电阻演示仪、传感器简单应用实验盒、万用表。 【教学过程】 一、引入新课 准备知识:从上世纪八十年代起,国际上出现了“传感器热”,传感器在当今科技发展中有着十分重要的地位。本课的设计思路是通过对实验的观察、思考和探究,了解什么是传感器,传感器是如何将非电学量转换成电学量的,传感器在生产、生活中有哪些具体应用,为学生利用传感器制作简单的自控装置作一铺垫。教学时力避深奥的理论,侧重于联系实际,让学生感受传感器的巨大作用,进而提高学生的学习兴趣,培养学生热爱科学的情感和崇尚科学的精神。 今天我们生活中常用的电视、空调的遥控器是如何实现远距离操纵的?楼梯上的电灯如何能人来就开,人走就熄的?工业生产中所用的自动报警器、恒温烘箱是如何工作的?“非典”病毒肆虐华夏大地时,机场、车站、港口又是如何实现快速而准确的体温检测的?所有这些,都离不开一个核心,那就是本堂课将要学习的传感器。 二、新课教学 1.什么是传感器 演示实验1:如图1所示,小盒子的侧面露出一个小灯泡,盒外没有开关,当把磁铁放到盒子上面,灯泡就会发光,把磁铁移开,灯泡熄灭。
纳米材料四大效应
1.小尺寸效应:当纳米粒子尺寸与德布罗意波以及超导态的相干长度或透射深度等物理特征尺寸相当或更小时,对于晶体其周期性的边界条件将被破坏,对于非晶态纳米粒子其表面层附近原子密度减小,这些都会导致电、磁、光、声、热力学等性质的变化,这称为小尺寸效应 我的理解是尺寸小了就会出现一些新的现象、新的特性。从理论层面讲主要是由于尺寸变小导致了比表面的急剧增大。由此很好地揭示了纳米材料良好的催化活性。 2.表面效应:是指纳米粒子表面原子数与总原子数之比随粒径的变小而急剧增大后引起的性质上的变化。 其实质就是小尺寸效应。球形颗粒的表面积与直径的平方成正比,其体积与直径的立方成正比,故其比表面积(表面积/体积)与直径成反比。随着颗粒直径变小,比表面积将会显著增大,说明表面原子所占的百分数将会显著地增加。当尺寸小于0.1微米时,其表面原子百分数激剧增长,甚至1克超微颗粒表面积的总和可高达100平方米,这时的表面效应将不容忽略。 3. 量子尺寸效应:当粒子尺寸降低到某一值时,金属费米能级附近的电子能级由准连续变为分立能级和纳米半导体微粒的能隙变宽的现象均称为量子尺寸效应。 可否直接说连续的能带变成能级。 宏观量子隧道效应:微观粒子具有穿越势垒的能力称为隧道效应。近年来,人们发现一些宏观量,例如微粒的磁化强度、量子相干器件中的磁通量等亦具有隧道效应,它们可以穿越宏观系统的势垒而产生变化,故称为宏观量子隧道效应。 表面与界面效应 这是指纳米晶体粒表面原子数与总原子数之比随粒径变小而急剧增大后所引起的性质上的变化。例如粒子直径为10纳米时,微粒包含4000个原子,表面原子占40%;粒子直径为1纳米时,微粒包含有30个原子,表面原子占99%。主要原因就在于直径减少,表面原子数量增多。再例如,粒子直径为10纳米和5纳米时,比表面积分别为90米2/克和180米2/克。因为表面原子数目增多,比表面积大,原子配位不足,表面原子的配位不饱和性导致大量的悬空键和不饱和键,表面能高,因而导致这些表面原子具有高的活性,极不稳定,很容易与其他原子结合。这种表面原子的活性不但易引起纳米粒子表面原子输运和构型的变化,同时也会引起表面电子自旋构象和电子能谱的变化。纳米材料由此具有了较高的化学活性,使得纳米材料的扩散系数大,大量的界面为原子扩散提供了高密度的短程快扩散路径,如金属纳米粒子在空中会燃烧,无机纳米粒子会吸附气体等等。(2)小尺寸效应 当纳米微粒尺寸与光波波长,传导电子的德布罗意波长及超导态的相干长度、透射深度等物理特征尺寸相当或更小时,它的周期性边界被破坏,非晶态纳米粒子的颗粒表面层附近的原子密度减少,从而使其声、光、电、磁,热力学等性能呈现出新的物理性质的变化称为小尺寸效应。例如,铜颗粒达到纳米尺寸时就变得不能导电;绝缘的二氧化硅颗粒在20纳米时却开始导电。再譬如,高分子材料加纳米材料制成的刀具比金钢石制品还要坚硬。利用这些特性,可以高效率地将
传感器及其工作原理教案
江苏省淮阴中学06-07年度优秀教学案例 《传感器及其工作原理》的创新教学设计 王刚 教学依据 ①物理(新人教版)选修3-2第六章第1节《传感器及其工作原理》(P56-P60); ②新物理课程标准(实验). 教学流程图
教学目标1.知识与技能:①知道非电学量转换成电学量的技术意义;②通过实验,知道常见传感器的工作原理;③初步探究利用和设计简单的传感器. 2.过程与方法:①通过对实验的观察、思考和探究,让学生了解传感器、熟悉传感器工作原理;②让学生自己设计简单的传感器,经历科学探究过程,学习科学研究方法,培养学生的实践能力和创新思维能力. 3.情感态度与价值观:在理解传感器工作原理的基础上,通过自己设计简单的传感器,体验科技创新的乐趣,激发学习物理的兴趣. 重、难点 1.几种常见传感器的工作原理(演示实验);2.学生自己设计简单的传感器. 教学策略 用几个有趣的传感器实验引入课题,激发学生探究传感器原理的兴趣.给出“传感器就是把非电学量转换为电学量”的概念之后,重点介绍光敏电阻、金属热电阻、热敏电阻.安排音乐茶杯和火警装置两个设计性问题让学生体会传感器的简单应用.结合电容、霍尔效应、电阻定律等知识让学生设计传感器,进一步深化传感器的工作原理.最后在对本节课总结的基础上,结合《思考与讨论》进行教学反馈. 教学程序 教学环节教学内容及师生互动设计情感与方法 一.课题的引入 二.什么是传感器?【演示实验1】干簧管控制电路的通断 如图,小盒子A的侧面露出一个小灯泡,盒外没有开 关,但是把磁铁B放到盒子上面,灯泡就会发光,把磁铁移 走,灯泡熄灭. 师问:盒子里有怎样的装置,才能实现这样的控制? 生猜:(可以自由讨论,也可以请学生回答) 师生探究:打开盒子,用实物投影仪展示盒内的电路 图,了解元件“干簧管”的结构。探明原因:玻璃管内封入 两个软磁性材料制成的簧片。当磁铁靠近干簧管时,两个簧 片被磁化而接通,电路导通。所以,干簧管能起到开关的作 用。 师点拨:这个装置反过来还可以让我们通过灯泡的发 光情况,感知干簧管周围是否存在着磁场。 【演示实验2】声光控开关控制电路的通断 ①先在普通光照条件下, ②在把开关置于黑暗环境中。 师生总结:声光控开关 师:刚才的两个实验,都用了一种元件,这些元件能够 感受某些信息,通过它能实现电路的自动控制,这种元件有 一个专门的名称:传感器。什么是传感器呢?它能够感受诸 如力、温度、光、声、化学成分等非电学量,并能把它们按 照一定的规律转换为电压、电流等电学量,或转换为电路的 通断。我们把这种元件叫做传感器。它的优点是:把非电学 量转换为电学量以后,就可以很方便地进行测量、传输、处 理和控制了。 其实,传感器并不神秘。你家里可能就有很多的传感 器。请大家相互说说看,你家里,或者在你的生活当中,都 (演示实验1: 干簧管传感器) (干簧管的实 物及原理图) 学生对干簧 管并不熟悉,因 此才有了好奇。 声光控开关在 生活中很普及, 所以又有亲切 感
最新电化学生物传感器
电化学生物传感器 生物分子的分析检测对获取生命过程中的化学与生物信息、了解生物分子及其结构与功能的关系、阐述生命活动的机理以及对疾病的有效诊断与治疗都具有十分重要的意义。如何高效、快速、灵敏地检测这些生物分子,是当前生命科学领域中面临的一个十分重要的问题。解决这些问题的关键就在于发展各种新型的分析检测技术。生物传感器的出现为有效地解决这些问题提供了新的工具,为生命科学及其相关领域的研究提供了许多新的方法 1电化学生物传感器的基本结构及工作原理 1.1 基本结构 通常情况下,生物传感器由两个主要部分组成即生物识别元件和信号转换器。生物识别元件是指具有分子识别能力,能与待测物质发生特异性反应的生物活性物质,如酶、抗原、抗体、核酸、细胞、组织等。信号转换器主要功能是将生物识别作用转换为可以检测的信号,目前常用的有电化学、光学、热和质量分析几种方法[1]。其中,电化学方法就是一种最为理想的检测方法。 图1 电化学生物传感器的基本结构 1.2 工作原理 电化学生物传感器采用固体电极作基础电极,将生物敏感分子固定在电极表面,然后通过生物分子间的特异性识别作用,生物敏感分子能选择性地识别目标分子并将目标分子捕获到电极表面,基础电极作为信号传导器将电极表面发生的识别反应信号导出,变成可以测量的电信号,从面实现对分析目标物进行定量或定性分析的目的。 2电化学生物传感器的分类
由各种生物分子(抗体、DNA、酶、微生物或全细胞)与电化学转换器(电流型、电位型、电容型和电导型)组合可构成多种类型的电化学生物传感器,根据固定在电极表面的生物敏感分子的不同,电化学生物传感器可分为电化学免疫传感器、电化学DNA传感器、电化学酶传感器、电化学微生物传感器和电化学组织细胞传感器等。 2.1 电化学免疫传感器 电化学免疫传感器是一种将免疫技术与电化学检测相结合的标记免疫分析方法。它是以抗原.抗体特异性反应为基础,将抗原/抗体反应达到平衡状态后的生物反应信号转换成可测量的电信号并通过基础电极将其导出。当采用电化学检测方法测量时,其信号大小与目标分析物在一定浓度范围内成线性关系,从而实现对目标检测物的分析测定。 根据抗原-抗体间的免疫反应的类型,电化学免疫传感器可分为两种:竞争法和夹心法。竞争法的分析原理是基于标记抗原和非标记抗原共同竞争与抗体的反应[2]。而夹心法则是将捕获抗体、抗原和检测抗体结合在一起,形成一种捕获抗体/抗原/检测抗体的夹心式复合物,也称“三明治”式结合物[3]。 图2 竞争法 图3 夹心法 2.2 DNA生物传感器 DNA生物传感器主要检测的是核酸的杂交反应。电化学DNA传感器的工作原理如图所示,即将单链DNA(ssDNA)探针,固定在电极上,在适当的温度、pH、离子
生物传感器原理及应用
Chapter 1生物传感器 (Biosensors) ? 1.1 Generalization(概述)? 1.2 Principle (基本原理)? 1.3 Classification(分类)? 1.4 Application(应用)
1.2 生物传感器工作原理 被测对象生物敏 感膜 (分子 识别感 受器) 电 信 号 换 能 器 物理、化学反应 化学物质 力 热 光 声 . . . 图16-1 生物传感器原理图
BIOSENSORS 1.2 生物传感器原理 无论是基于电化学、光学、热学或压电 晶体等不同类型的生物传感器,其探头均由 两个主要部分组成,一是感应器,它是由对 被测定的物质(底物)具有高选择性分子识 别功能的膜构成。二是转换器,它能把膜上 进行的生化反应中消耗或生成的化学物质, 或产生的光、热等转变成电信号,最后把所 得的电信号经过电子技术的处理后,在仪器 上显示或记录下来。
换能器(T r a n s d u c e r )感受器(R e c e p t o r )= 分析物(Analyte ) 溶液(Solution )选择性膜(Thin selective membrane ) 识别元件(Recognition )生物传感器工作机理 测量信号(Measurable Signal ) BIOSENSORS
(1)将化学变化转变成电信号 酶传感器为例,酶催化特定底物发生化学反应,从而使特定生成物的量有所增减。用能把这类物质的量的改变转换为电信号的装置和固定化酶耦合,即组成酶传感器.常用转换装置有氧电极、过氧化氢。
(完整版)纳米材料四大效应及相关解释
纳米材料四大效应及相关解释 四大效应基本释义及内容: 量子尺寸效应:是指当粒子尺寸下降到某一数值时,费米能级附近的电子能级由准连续变为离散能级或者能隙变宽的现象。当能级的变化程度大于热能、光能、电磁能的变化时,导致了纳米微粒磁、光、声、热、电及超导特性与常规材料有显著的不同。 小尺寸效应:当颗粒的尺寸与光波波长、德布罗意波长以及超导态的相干长度或透射深度等物理特征尺寸相当或更小时,晶体周期性的边界条件将被破坏,非晶态纳米粒子的颗粒表面层附近的原子密度减少,导致声、光、电、磁、热、力学等特性呈现新的物理性质的变化称为小尺寸效应。对超微颗粒而言,尺寸变小,同时其比表面积亦显著增加,从而产生如下一系列新奇的性质。 表面效应:球形颗粒的表面积与直径的平方成正比,其体积与直径的立方成正比,故其比表面积(表面积/体积)与直径成反比。随着颗粒直径的变小,比表面积将会显著地增加,颗粒表面原子数相对增多,从而使这些表面原子具有很高的活性且极不稳定,致使颗粒表现出不一样的特性,这就是表面效应。 宏观量子隧道效应:当微观粒子的总能量小于势垒高度时,该粒子仍能穿越这一势垒。近年来,人们发现一些宏观量,例如微颗粒的磁化强度,量子相干器件中的磁通量等亦有隧道效应,称为宏观的量子隧道效应。 四大效应相关解释及应用: 表面效应 球形颗粒的表面积与直径的平方成正比,其体积与直径的立方成正比,故其比表面积(表面积/体积)与直径成反比。随着颗粒直径的变小比表面积将会显著地增加。例如粒径为10nm时,比表面积为90m2/g;粒径为5nm时,比表面积为180m2/g;粒径下降到2nm时,比表面积猛增到450m2/g。粒子直径减小到纳米级,不仅引起表面原子数的迅速增加,而且纳米粒子的表面积、表面能都会迅速增加。这主要是因为处于表面的原子数较多,表面原子的晶场环境和结合能与内部原子不同所引起的。表面原子周围缺少相邻的原子,有许多悬空键,具有不饱
传感器及工作原理
2012学年高二物理导学案编号____使用时间____ 班级:__小组:____姓名:____组内评价:____教师评价:____ 《传感器及其工作原理》导学案一 编制人:陈昌林审核人:____领导签字:____ 【教学目标】 1.知道什么是传感器 2.了解传感器的常用元件的特征 【重点与难点】 1.传感器的原理 【自主预习】 一.传感器: 1.传感器是指这样一类元件:它能够感受诸如力、温度、光、声、化学成分等_____量,并能把它们按照一定的规律转换为电压、电流等____量,或转换为电路的通断。把非电学量转换为电学量以后,就可以很方便地进行测量、传输、处理和控制了。 2.传感器一般由敏感元件和输出部分组成,通过敏感元件获取外界信息并转换____信号,通过输出部分输出,然后经控制器分析处理。 3.常见的传感器有:_____、_____、_____、_____、力传感器、气敏传感器、超声波传感器、磁敏传感器等。 二.常见传感器元件: 1.光敏电阻:光敏电阻的材料是一种半导体,无光照时,载流子极少,导电性能不好;随着光照的增强,载流子增多,导电性能变好,光敏电阻能够把_____这个光学量转换为电阻这个电学量。它就象人的眼睛,可以看到光线的强弱。 2、金属热电阻和热敏电阻:金属热电阻的电阻率随温度的升高而____,用金属丝可以制作____传感器,称为_____。它能把____这个热学量转换为____这个电学量。 3.热敏电阻的电阻率则可以随温度的升高而____或____。与热敏电阻相比,金属热电阻的_____好,测温范围___,但____较差。 4.电容式位移传感器能够把物体的____这个力学量转换为___这个电学量。 5.霍尔元件能够把______这个磁学量转换为电压这个电学量。 【典型例题】 【例1】如图所示,将万用表的选择开关置于“欧姆”挡, 再将电表的两支表笔与一热敏电阻R t的两端相连,这时表针 恰好指在刻度盘的正中间。若往R t上擦一些酒精,表针将向 ____(填“左”或“右”)移动;若用吹风机将热风吹向 电阻,表针将向____(填“左”或“右”)移动。 【例2】传感器是一种采集信息的重要器件。如图所示是一种测定压力的电容式传感器。当待测压力F作用于可动膜片电极时,可使膜片产生形变, 引起电容的变化,将电容器、灵敏电流计和电源串联成 闭合电路,那么() A.当F向上压膜片电极时,电容将减小 B.当F向上压膜片电极时,电容将增大 C.若电流计有示数,则压力F发生变化 D.若电流计有示数,则压力F不发生变化
纳米材料表面效应
纳米材料的表面效应 材料0701 李愿 学号:1002070101 参考文献: 1、卢柯、卢磊金属纳米材料力学性能的研究进展 金属学报 2000年8月第36卷第8期:785—789 摘要 金属纳米按体材料具有独特的力学性能如高强度、超高延展性等。近年来得到广泛深入的研究。在对其新进展进行简要评述的基础上,讨论了它的强度、塑性、弹性模量、应变强化、超塑性、蠕变及变形机理等相关问题。 2、吴锦雷纳米材料的电学、光学和光电性能及应用前景 真空电子学术 2002年第4期:23—27 摘要: 简要介绍了纳米材料的电学性能以及单电子器件的基本原理和应用;纳米材料的光学性能和光电性能,高的光吸收系数和光致荧光现象可使其应用于敏感元件,由于其光电特性具有超快响应速度,可望在超快光电子器件中得到应用。 3、齐卫宏、汪明朴纳米金属微粒表征量的基本关系 材料导报 2002年9月第16卷第9期:76—77 摘要: 在假定纳米微粒近似成球形的前提下,推导出了粒径、微粒原子数、表面原子百分数及比表面积之间的相互关系式,这些关系式对实验将会有一些指导作用。 4、梁海弋、倪向贵、王秀喜表面效应对纳米铜杆拉伸性能影响的原子模拟 金属学报 2001年8月第37卷第8期 833—836 摘要: 采用EAM势对纳米铜杆的拉伸力学性能进行零温分子动力学模拟。研究表面效应对原子能量、截面应力分布的影响模拟结果表明,表面原子弛豫降低了纳米杆初始阶段的拉伸弹性模量。表面效应明显影响截面应力的发展与分布。 5、黄丹、陶伟明、郭乙木分子动力学模拟纳米镍单晶的表面效应 固体力学学报 2005年6月第26卷第2期:241—244 摘要: 对单晶镍纳米丝、纳米薄膜零温准静态拉伸破坏过程进行了分子动力学模拟。模拟表明表面效应对单晶纳米材料的原子运动及整体力学行为有显著影响。自由表面增加纳米材料的塑
生物传感器的原理及应用
生物传感器的原理及应用 摘要: 随着信息技术与生物工程技术的发展,生物传感器得到了极为迅速的发展,当今各发达国家都把生物传感器列为21世纪的关键技术,给予高度的重视。生物传感器不仅广泛用于传统医学领域,推动医学发展,而且还在空间生命科学、食品工业、环境监测和军事等领域广泛应用。 关键词:生物传感器;原理;应用;发展 Abstract: As information technology and biological engineering technology, bio-sensors has been very rapid development,today's developed countries regard the biosensor technology as the key to the 21st century, given a high priority. Biosensors are widely used in traditional medicine not only to promote the development of medicine, but also in space life science, food industry, environmental monitoring and widely used in military and other fields. Keyword s: biosensor; principle; application; development
目录 一. 引言 (4) 二. 生物传感器的原理 (4) 三. 生物传感器的应用 (5) 3.1.生物传感器在医学领域的应用 (5) 3.1.1. 基于中医针灸针的传感针 (5) 3.1.2.生物芯片 (5) 3.1.3.生物传感器的临床应用 (5) 3.2.生物传感器在非传统医学领域的应用 (6) 3.2.1.在空间生命科学发展中的应用 (6) 3.2.2.在环境监测中的应用 (6) 3.2.3.在食品工程中的应用 (6) 3.2.4.在军事领域的应用 (6) 四. 生物传感器的未来 (7) 五. 结束语 (7) 六. 参考文献 (7)
