The effect of temperature on experimental and natural chemical weathering rates of granitoid rocks

PII S0016-7037(99)00250-1
The effect of temperature on experimental and natural
chemical weathering rates of granitoid rocks
A RT F.W HITE ,*A LEX E.
B LUM ,T HOMAS D.B ULLEN ,D AVISON V.V IVIT ,M ARJORIE S CHULZ ,and J OHN F ITZPATRICK
U.S.Geological Survey,Menlo Park,CA 94025,USA
(Received October 15,1998;accepted in revised form April 29,1999)
Abstract —The effects of climatic temperature variations (5–35°C)on chemical weathering are investigated both experimentally using ?ow-through columns containing fresh and weathered granitoid rocks and for natural granitoid weathering in watersheds based on annual solute discharge.Although experimental Na and Si ef?uent concentrations are signi?cantly higher in the fresh relative to the weathered granitoids,the proportional increases in concentration with increasing temperature are similar.Si and Na exhibit comparable average apparent activation energies (E a )of 56and 61kJ/mol,respectively,which are similar to those reported for experimental feldspar dissolution measured over larger temperature ranges.A coupled temperature–precipitation model,using an expanded database for solute discharge ?uxes from a global distribution of 86granitoid watersheds,produces an apparent activation energy for Si (51kJ/mol),which is also comparable to those derived from the experimental study.This correlation reinforces evidence that temperature does signi?cantly impact natural silicate weathering rates.
Ef?uent K concentrations in the column study are elevated with respect to other cations compared to watershed discharge due to the rapid oxidation/dissolution of biotite.K concentrations are less sensitive to temperature,resulting in a lower average E a value (27kJ/mol)indicative of K loss from lower energy interlayer sites in biotite.At lower temperatures,initial cation release from biotite is signi?cantly faster than cation release from plagioclase.This agrees with reported higher K/Na ratios in cold glacial watersheds relative to warmer temperate environments.Increased release of less radiogenic Sr from plagioclase relative to biotite at increasing temperature produces corresponding decreases in 87Sr/86Sr ratios in the column ef?uents.A simple mixing calculation using ef?uent K/Na ratios,Sr concentrations and 87Sr/86Sr ratios for biotite and plagioclase approximates stoichiometric cation ratios from biotite/plagioclase dissolution at warmer temperatures (35°C),but progressively overestimates the relative proportion of biotite with decreasing temperature.Ca,Mg,and Sr concentrations closely correlate,exhibit no consistent trends with temperature,and are controlled by trace amounts of calcite or exchange within weathered biotite.The inability of the watershed model to differentiate a climate signal for such species correlates with the lower temperature dependence observed in the experimental studies.Copyright ?1999Elsevier Science Ltd
1.INTRODUCTION
Climate,principally temperature and precipitation,has been proposed as a linkage by which the rates of sur?cial weathering of silicates closely balance rates of atmospheric CO 2produc-tion,thus promoting stable climatic conditions that permit life on earth (Walker et al.,1981;Berner et al.,1983;Berner and Berner,1997).In theory,any increase in atmospheric CO 2from sources such as volcanism is counterbalanced by increased CO 2consumption by more rapid silicate weathering under increased greenhouse temperatures.In contrast,diminished atmospheric CO 2is counterbalanced by decreased weathering rates caused by lower temperatures.
This linkage between climate,continental silicate weather-ing,and atmospheric CO 2is not universally accepted.Staudigel et al.(1989)and Francois and Walker (1992)suggested that low temperature sea?oor-basalt alteration and not terrestrial silicate weathering exerts the dominant long-term control on atmospheric CO 2.Bickle (1996),Edmond and Huh (1997),and Huh et al.(1998a)concluded that atmospheric CO 2is princi-pally in?uenced by weathering rates controlled by tectonics
rather than climate.These workers concluded that increases in physical weathering and exposure of fresh and rapidly weath-erable mineral phases dominate changes in silicate-weathering processes.
An important issue in this ongoing controversy is the quan-titative impact of temperature on chemical weathering of sili-cate rocks (Brady and Carroll,1994;Lasaga et al.,1994).The effects of temperature on weathering rates are experimentally well established for silicate minerals such as feldspars and quartz (for reviews,see Blum and Stillings,1995;Dove,1995).Average experimental activation energies of between 50and 80kJ/mol predict that a temperature increase from 0to 25°C increases weathering rates by about an order of magnitude.Such an effect should be observable in the natural environment.Temperature effects on weathering rates have not been exper-imentally established for other minerals such as biotite and hornblende,which are important in weathering of common silicate rocks such as granitoids.In addition,complexities associated with de?ning temperature effects on such common multimineralic silicate rocks have not been investigated.
Direct observations of temperature impacts on natural weath-ering processes have proven to be elusive.The most relevant study to the climate issue would be of a natural weathering system that has undergone sustained temperature change.How-
*Author to whom correspondence should be addressed (afwhite@
https://www.360docs.net/doc/1d17931011.html,).
Pergamon
Geochimica et Cosmochimica Acta,Vol.63,No.19/20,pp.3277–3291,1999
Copyright ?1999Elsevier Science Ltd Printed in the USA.All rights reserved
0016-7037/99$20.00?.00
3277
ever,long-term data required for such a study are not available, and surrogate weathering studies comparing spatially separated climatic regimes are used.The utility of such comparisons depends on the ability to isolate the effect of temperature from other variables in?uencing chemical weathering including pre-cipitation,geomorphology,vegetation,and lithology.This abil-ity decreases as the scale of the weathering process increases. This explains why comparison of solute concentrations and?uxes in large-scale river systems most often fail to detect a temperature effect(Edmond et al.,1995;Huh et al.,1998b).
Although limited in number,comparison of smaller scale weathering environments have been more successful in docu-menting temperature impacts due to an increased ability to separate out other nonweathering variables.Velbel(1993)es-timated elevation-dependent temperature differences in the Coweeta watershed in North Carolina(10.6–11.7°C)and cal-culated an activation energy of77kJ/mol for plagioclase weathering.Dorn and Brady(1995)used plagioclase porosity formed by etch pitting in Hawaiian basalt?ows at different elevations and temperatures(12.5–23.3°C)to calculate an ac-tivation energy of109kJ/mol.Recently,Louvat(1997)calcu-lated activation energies ranging between38and43kJ/mol for Si and Na released from basalt weathering based on tempera-ture differences(5–30°C)in small river basins on the islands of Iceland,Reunion,Sao Miguel,and Java.
In an investigation of the climate effects on small water-sheds,White and Blum(1995)tabulated chemical?ux data from a global distribution of68watersheds underlain by gran-itoid rocks.These workers delineated the impact of mean annual air temperature(0–22°C)on weathering rates.A rein-forcing effect of high precipitation and temperature was pro-posed to explain rapid weathering rates such as in tropical mountainous regions.On the basis of this coupled model, White and Blum(1995)calculated average apparent activation energies of59and62kJ/mol,respectively,for Si and Na?uxes, values that are very similar to experimental values for plagio-clase(Blum and Stillings,1995).This calculation was heavily dependent on the extreme weathering rates observed for the Rio Icacos watershed in Puerto Rico(White et al.,1998)with a meager amount of published collaborative data for other upland tropical watersheds.
The present paper further investigates the temperature effects on granitoid weathering undertaken by the study of White and Blum(1995).The study investigates long-term experimental dissolution of granitoid rock samples from several of the wa-tersheds included in the original study over an environmentally relevant temperature range(5–35°C).Issues related to temper-ature effects on multimineralic rocks are addressed.Experi-mental activation energies are compared to watershed activa-tion energies based on the coupled temperature–precipitation model,which contains an expanded watershed database includ-ing additional tropical watersheds.These results further support the linkage between climate and silicate weathering on the earth’s surface.
2.METHODS
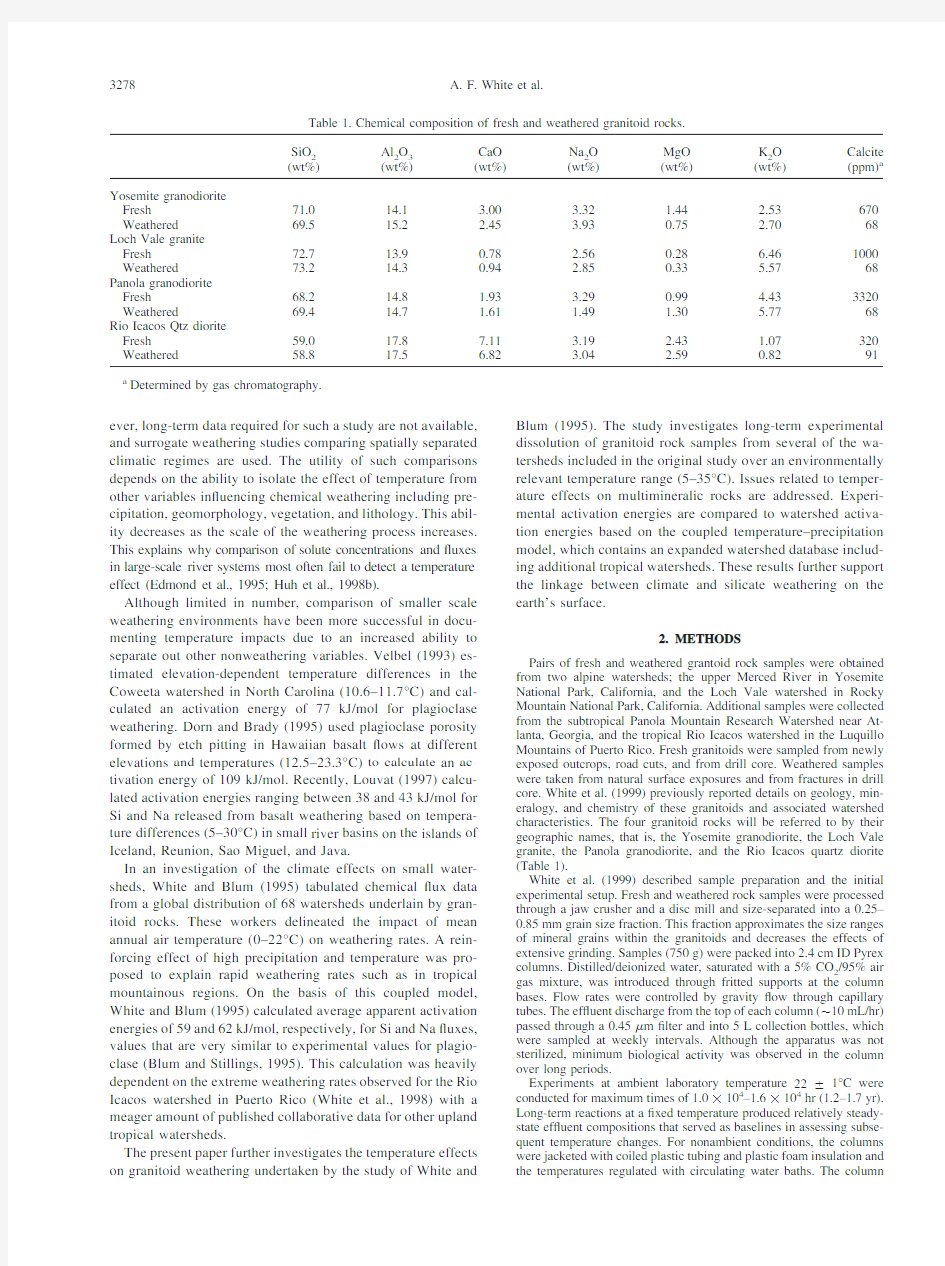
Pairs of fresh and weathered grantoid rock samples were obtained from two alpine watersheds;the upper Merced River in Yosemite National Park,California,and the Loch Vale watershed in Rocky Mountain National Park,California.Additional samples were collected from the subtropical Panola Mountain Research Watershed near At-lanta,Georgia,and the tropical Rio Icacos watershed in the Luquillo Mountains of Puerto Rico.Fresh granitoids were sampled from newly exposed outcrops,road cuts,and from drill core.Weathered samples were taken from natural surface exposures and from fractures in drill core.White et al.(1999)previously reported details on geology,min-eralogy,and chemistry of these granitoids and associated watershed characteristics.The four granitoid rocks will be referred to by their geographic names,that is,the Yosemite granodiorite,the Loch Vale granite,the Panola granodiorite,and the Rio Icacos quartz diorite (Table1).
White et al.(1999)described sample preparation and the initial experimental setup.Fresh and weathered rock samples were processed through a jaw crusher and a disc mill and size-separated into a0.25–0.85mm grain size fraction.This fraction approximates the size ranges of mineral grains within the granitoids and decreases the effects of extensive grinding.Samples(750g)were packed into2.4cm ID Pyrex columns.Distilled/deionized water,saturated with a5%CO
2
/95%air gas mixture,was introduced through fritted supports at the column bases.Flow rates were controlled by gravity?ow through capillary tubes.The ef?uent discharge from the top of each column(?10mL/hr) passed through a0.45?m?lter and into5L collection bottles,which were sampled at weekly intervals.Although the apparatus was not sterilized,minimum biological activity was observed in the column over long periods.
Experiments at ambient laboratory temperature22?1°C were conducted for maximum times of1.0?104–1.6?104hr(1.2–1.7yr). Long-term reactions at a?xed temperature produced relatively steady-state ef?uent compositions that served as baselines in assessing subse-quent temperature changes.For nonambient conditions,the columns were jacketed with coiled plastic tubing and plastic foam insulation and the temperatures regulated with circulating water baths.The column
Table1.Chemical composition of fresh and weathered granitoid rocks.
SiO
2 (wt%)Al
2
O
3
(wt%)
CaO
(wt%)
Na
2
O
(wt%)
MgO
(wt%)
K
2
O
(wt%)
Calcite
(ppm)a
Yosemite granodiorite
Fresh71.014.1 3.00 3.32 1.44 2.53670 Weathered69.515.2 2.45 3.930.75 2.7068 Loch Vale granite
Fresh72.713.90.78 2.560.28 6.461000 Weathered73.214.30.94 2.850.33 5.5768 Panola granodiorite
Fresh68.214.8 1.93 3.290.99 4.433320 Weathered69.414.7 1.61 1.49 1.30 5.7768 Rio Icacos Qtz diorite
Fresh59.017.87.11 3.19 2.43 1.07320 Weathered58.817.5 6.82 3.04 2.590.8291 a Determined by gas chromatography.
3278 A.F.White et al.
temperatures were ?rst decreased to a temperature range of 5?1°C for ?2500hr,subsequently increased to 17?1°C for ?2500hr,and ?nally increased to 35?1°C for ?1500hr.The range of experimental temperatures used for the columns is less than that previously em-ployed in most temperature investigations of silicate dissolution and more accurately simulates natural weathering temperatures.
Cation concentrations in the experimental ef?uents and mineral separates were determined by inductively coupled plasma–mass spec-trometry using a Perkin-Elmer Elan 6000.Analytical methods are similar to those described in Bullen et al.(1997).Alkalinity and pH were measured on collected ef?uents.87Sr/86Sr ratios were determined by thermal ionization using a Finnigan-MAT 261mass spectrometer and are precise to better than 0.00004at the 95%con?dence level.
3.EXPERIMENTAL RESULTS
3.1.Mineralogy and Rock Chemistry
Bulk compositions for the fresh and weathered granitoid samples are summarized in Table 1(White et al.,1999).Se-lected mineral abundances,Na,K,and Sr concentrations,and 87
Sr/86Sr isotopic ratios as summarized in Table 2(Bullen et al.1998).The plagioclase compositions of the Yosemite granodi-orite (An 0.33),Loch Vale granite (An 0.21),and Panola grano-diorite (An 0.25),correspond to Na-rich oligioclase,whereas plagioclase in the Rio Icacos quartz diorite (An 0.50)is a more calcic andesine.All the granitoids contain biotite.The Panola granodiorite also contains muscovite.The low K contents of the Rio Icacos quartz diorite is re?ected in a lack of signi?cant K-feldspar.The higher Mg contents of the Yosemite granodi-orite and Rio Icacos quartz diorite correlate with abundant hornblende,which is a minor component in the Panola grano-diorite and is absent in the Loch Vale granite.
Variations in composition between the fresh and weathered granitoid pairs (Table 1)are attributable to natural variations in rock samples,which were collected from different locations.The lack of any consistent losses in major cations in the weathered element compositions relative to their fresh coun-terparts indicates that these rocks have been exposed to only the initial phases of natural weathering and have not undergone signi?cant loss of primary minerals (except calcite)or major element mobilization.
3.2.Ef?uent Compositions and Temperature Effects The total reaction times,ef?uent volumes,and concentra-tions at the end of successive temperature runs are tabulated in Table https://www.360docs.net/doc/1d17931011.html,plete ef?uent Si,Na,K,Ca,and Mg data from the fresh and weathered Yosemite granodiorites are plotted in Fig.1.These plots are representative of time and temperature sequences in all the experiments.The horizontal scale refers to the time elapsed since the initiation of the column experiments
with only the ef?uent data from the last four months of ambient temperature experiments (22°C)included.The plots show con-centration responses to subsequent temperature adjustments to 5°,17°,and 35°C.Ef?uent concentrations of Si,Na,K,Ca,and Mg derived from the weathered granitoids are signi?cantly lower relative to their fresh counterparts.This implies that crushing of weathered samples to a size fraction comparable to mineral grains within the granitoids did not produce signi?cant fresh reactive surfaces.
After thermal reequilibrium to a different column tempera-ture,relatively short times (?500hr)were required to reestab-lish steady-state ef?uent Si,Na,and K concentrations.This delayed temperature response,shown in more detail for Na concentrations after the initial temperature decrease from 22°to 5°C (Fig.2),partly re?ects hydrodynamic dispersion,in which a ?nite time is required to ?ush out pore water that had reacted at the previous temperature.After this initial ?ushing,Na concentrations approach steady-state conditions as described by mean average ef?uent concentrations (Fig.2,dashed lines).Similar relatively rapid temperature adjustments are observed for K and Si (Fig.1A).In contrast,Ca,Mg,and Sr require much longer equilibration times in response to experimental temperature changes.In some cases,as shown by the example of ef?uent Mg after adjustment from 22°to 5°C,steady-state conditions are never acheived.
Arrhenius-type plots (Figs.3–5)are used to characterize the effects of column temperature on steady state Si,Na,and K ef?uent compositions.Ef?uent concentrations in log units from fresh and weathered granitoids are plotted against the recipro-cal of absolute temperature (1000/°K)on the bottom axis and the equivalent temperature in degree Celsius on the top axis of each ?gure.Data,which re?ect residual effects during temper-ature transitions,are excluded from the plots.In most cases,these excluded data correspond to the ?rst one to three ef?uent samples collected after the change in column temperature.The dependencies of the ef?uent Si,Na,and K concentra-tions (C;in micromoles per liter)on column temperature are determined by least square ?ts to a semi-log linear regression relationship of the form
Log C ?a o ?a 1/T(°K),
(1)
where a o is the intercept and a 1is the slope de?ned in terms of the inverse of absolute temperature °K.Ef?uent data and re-gression ?ts are plotted as functions of temperature in Figs.3–5for the fresh (solid lines)and weathered (dashed lines)grani-toids.Fitting parameters are tabulated in Table 4.The temper-ature sensitivity of ef?uent concentrations is inversely depen-dent on the slope a 1in Eqn.1,which is greater for Si and Na
Table 2.Mineral abundances (wt%),Sr,Na,and K concentrations (mg/kg)and Sr isotopic ratios for minerals in fresh granitoid rocks.
Plagioclase
K-feldspar
Biotite
Hornblende
wt%
Sr Na 87
Sr/86Sr
wt%Sr 87
Sr/86Sr
wt%Sr K 87
Sr/86Sr
wt%Sr 87
Sr/86Sr
Yosemite 361100556000.7065157500.7075811376000.87535750.7069Loch Vale 20175653000.7422451900.86624841200 4.83390na na Panola 35750638000.7081206000.7160101179000 1.27302550.7098Rio Icacos
55
560
40800
0.70410
na
na 10
17
75200
0.782714
80
0.7042
na,not available.
3279
Effect of temperature on chemical weathering rates of granitoid rocks
than for K(Table4).Releases of Si and Na from the granitoids are,therefore,more temperature dependent than is K.Correlation coef?cients(r2)in all but four experiments exceeded0.80for Eqn. 1,indicating that the ef?uent concentrations increase exponen-tially with temperature.Fits for Si ef?uents from the weathered Yosemite granodiorite did not include data at5°C,which were at or below detection limits(Fig.3A).
In contrast to Si,Na,and K,the Ca,Mg,and Sr concentrations from the fresh granitoid rocks generally exhibit negative correla-tions with temperature as is shown for the Yosemite granodiorite and Rio Icacos quartz diorite(Fig.6).Ef?uent Ca,Mg,and Sr concentrations from the weathered samples are signi?cantly lower than for their fresh counterparts(Table3)and do not show consistent concentration trends with temperature.
3.3.Effect of Natural Weathering on Temperature
Sensitivity
The temperature trends for ef?uent Si,Na,and K,de?ned by
the slope a
1in Eqn.1,are parallel to subparallel for fresh and
weathered paired granitoids(Figs.3–5).Therefore,the sensi-
tivities of ef?uent concentrations to temperature changes are
similar whether or not the granitoids have undergone natural
weathering.This is an important observation because it implies
that the intrinsic nature of the weathering reactions is also
comparable.At any given temperature,however,the ef?uent
concentrations from the weathered rocks are lower than from
the fresh rocks.Natural weathering has decreased Si,Na,and K
concentrations by a factor of approximately?ve for the Yo-
semite and Panola granitoids(Figs.3–5)and a factor of ap-
proximately two for the Loch Vale and Rio Icacos granitoids.
These ratios between fresh and weathered ef?uents are rela-
tively consistent over the entire temperature range.
No systematic differences in bulk chemistries are apparent
between any of the fresh and weathered granitoid pairs(Table
1).Therefore,the signi?cant temperature-independent de-
creases in ef?uent Si,Na,and K in the weathered granitoids
cannot be explained by decreases in primary mineral contents.
There is some physical evidence,based on greater friability and Table3.Experimental conditions and ef?uent compositions at the end of successive temperature runs(?M).
Sample no.
Time
h(102)a
Vol
(L)b
T
(°C)pH Alk Na Mg Si K Ca Sr86Sr/87Sr
Yosemite Fresh
CA5414615122 6.337.5 2.59 3.1924.511.47.880.01290.71998 CA641711715 6.231.00.62 3.24 5.4 5.287.520.01210.72301 CA7319118716 6.229.0 1.21 2.2611.8 6.67 6.370.01030.72151 CA7919619734 6.431.07.60 1.3548.77.60 4.320.00990.71582 Yosemite Weathered
CE5013313522 5.9 5.50.400.189.0 1.230.450.00230.71180 CE611561606 5.77.00.120.11 3.10.48?0.200.00050.71550 CE7218018716 5.6 5.00.220.13 4.50.57?0.300.00110.71140 CE821952635 5.6 6.5 1.000.2813.3 1.330.610.00440.70932 Loch Vale Fresh
CC5414615122 6.022.5 2.290.9612.5 1.847.910.00400.84125 CC641701806 5.67.50.380.05 2.30.63 3.850.00320.84919 CC7319120616 5.6 6.50.820.27 6.7 1.03 3.290.00280.85409 CC7920122234 5.811.0 5.750.3625.9 2.47 2.510.00220.83821 Loch Vale Weathered
CG5013313322 5.7 4.0 1.330.1111.6 1.010.500.00130.82610 CG611561596 5.78.50.240.07 3.00.30?0.200.00020.89300 CG7218017816 5.7 3.50.840.11 6.10.760.280.00090.85950 CG8220219534 5.77.57.190.2833.2 1.990.770.00220.79483 Panola Fresh
CD5414615222 6.457.5 1.76 1.2324.3 5.2033.70.01980.72766 CD641701776 6.227.50.370.34 5.8 3.0012.40.00870.73062 CD7319019916 6.221.00.680.7712.0 2.7113.70.00930.73095 CD7920321335 6.430.5 4.93 1.2240.7 3.9119.30.02040.71991 Panola Weathered
CF5013314622 5.69.5?0.200.09 6.80.860.300.00110.73210 CF611561706 5.711.0?0.100.07 3.50.37 1.010.00050.73700 CF7218019516 5.6 6.00.130.12 5.00.690.490.00080.73030 CF8220221734 5.7 5.5 1.250.3318.0 1.36 1.070.00450.71830 Rio Icacos Fresh
CB5414616522 6.440.0 3.69 2.2129.311.511.00.01230.70655 CB641711845 6.431.5 1.46 1.9111.9 6.979.570.00900.70691 CB7319119816 6.431.0 2.26 1.5224.48.347.770.00900.70680 CB7920320734 6.633.59.000.9071.814.2 6.550.01010.70660 Rio Icacos Weathered
CK3810510922 6.280.0 2.97 1.6927.511.4 6.050.02100.70564 CK491281386 6.123.00.700.817.1 4.79 5.550.01470.70638 CK6015216816 6.026.0 1.47 1.0313.7 6.38 4.450.01280.70615 CK7017419734 6.037.0 5.96 1.5945.49.998.000.02560.70554
a Total elapsed time since initiation of experiment.
b Cumulative ef?uent output.
3280 A.F.White et al.
more extensive Fe staining,that the Yosemite and Panola samples have been subjected to more natural weathering than the Loch Vale and Rio Icacos samples.The speci?c surface areas of the minerals tend to increase during alteration by development of internal porosity,favoring increased overall rates (White et al.,1996).Thus,the observed decreases in reaction rates as a result of this initial weathering must correlate with properties of the mineral surfaces,such as decreases in the reactivity or density of high energy sites or reduced surface free energies (White et al.,1996).3.4.Temperature Effects on
87
Sr/86Sr Ratios
Sr isotopes are particularly useful for mineral weathering studies because different granitoid minerals typically have sig-ni?cantly and systematically different 87Sr/86Sr ratios that are not modi?ed during mineral dissolution and subsequent cation exchange reactions (Bullen et al.,1997).The 87Sr/86Sr ratio provides an additional constraint on de?ning the effects of temperature on granitoid weathering.The Sr concentrations and 87
Sr/86Sr ratios for mineral phases contained in the fresh gran-itoid rocks used for this study are given in Table 2.Plagioclase typically has the highest Sr concentrations and the lowest 87
Sr/86Sr ratios,whereas biotite has the lowest Sr concentra-tions and the highest 87Sr/86Sr ratios.
Sr concentrations and 87Sr/86Sr ratios of column ef?uents at the end of each temperature experiment run are given in Table 3.As shown in Fig.7,87Sr/86Sr ratios of both fresh and weathered granitoid column ef?uents are typically highest for the low temperature runs and lowest for the high temperature runs.As indicated by the slopes of the linear regression lines in Fig.7,87Sr/86Sr ratios from the weathered granitoids exhibit a slightly stronger retrograde temperature dependency than
do
Fig.1.Concentrations of Si and major cations in ef?uents from (A)fresh and (B)weathered Yosemite granodiorite plotted as a function of time.Vertical dashed lines correspond to changes in temperature.Silica concentrations for 35°C experiments are plotted on separate higher concentration
scale.
Fig.2.Changes in Na and Mg ef?uent concentrations in fresh granitoids after temperature reequilibration from 22°to 5°C.Dashed lines correspond to linear regression ?ts for Na data after initial temperature equilibration.
3281
Effect of temperature on chemical weathering rates of granitoid rocks
the fresh granitoid ef?uents.Qualitatively,the data are consis-tent with a greater contribution of Sr from biotite at low temperatures and a greater contribution from plagioclase (and hornblende,when present)at high temperatures.
4.DISCUSSION
4.1.Activation Energies
The temperature effect on weathering is commonly charac-terized by the Arrhenius relationship,which describes the change in the ratio of reaction rates r and r o (in moles per meter per second)over a temperature range T to T o (°K)such that (Brady and Carroll,1994)
r 0?exp ?E a ?10?1
??
(2)
E a is the activation energy (in kilojoule per mole)and R is the
molar gas constant (in kilojoule per mole per degree Kelvin).
The ratios of steady-state ef?uent concentrations C and C o (in micromoles)characterized by Eqn.1in the present study are equivalent to the ratio of reaction rate ratios in Eqn.2(i.e.,r /r o ?C/C o )if the mineral surface areas (in square meters)and ?uid ?ow rates (in liters per second)are assumed constant.However,the resulting values for E a ,determined for a multi-component rock,do not necessarily de?ne the temperature-dependent rate constants for unique phases or reactions as required by the Arrhenius expression.In addition,recent stud-ies have found that experimental activation energies for silicate dissolution are dependent on solution composition,in particular pH and Al concentrations (Brady and Walter,1992;Casey and Sposito,1992;Chen and Brantley,1996).The effects of such variables are not considered in the present study.Consequently,temperature dependencies in the present work are discussed in terms of apparent activation energies.
Apparent activation energies calculated from Eqn.2are tabulated in Table 4.The mean average activation energies
for
Fig.3.Arrhenius plots of the log Si ef?uent concentrations from fresh and weathered granitoid rocks.Lower horizontal scale corresponds to the inverse of absolute temperature and upper nonlinear scale to the temperature increase in °C.Solid and dashed lines correspond to linear ?ts to the ef?uents from fresh and weathered granitoids (see text,Eqn.1).
3282 A.F.White et al.
Si release from the fresh and weathered granitoids are,respec-tively 53?5kJ/mol and 57?5kJ/mol (Table 4).Average activation energies for Na are only slightly higher for the fresh and weathered granitoids (57?15kJ/mol and 65?8kJ/mol).The magnitude of experimental activation energies has been traditionally related to the nature of the reaction mechanisms.E a values on the order of 50–80kJ/mol have been commonly ascribed to enthalpies for metal cation detachment from silicate surfaces (Lasaga,1984).The average respective E a values for K release from the fresh and weathered granitoids columns are 22?9kJ/mol and 32?11kJ/mol.These values are signi?-cantly lower than expected for dissolution of structural cations but are higher than E a values determined for reactions con-trolled by solute diffusion during relatively rapid dissolution processes (10–20kJ/mol;Lasaga,1984).
4.2.Experimental Temperature Effects for Granitoid
Weathering
Experimental weathering of a multimineralic rock,such as a granitoid,adds complexities to any thermodynamic interpreta-
tion of activation energies.The effect of temperature on ele-mental release,such as monitored by ef?uent concentrations in the present study,re?ect the stoichiometries,masses,and re-action rates of various primary minerals present,as well as the precipitation of any secondary phases.4.2.1.Si and Na release
If an element in a granitoid is unique to a single dissolving mineral,the resulting temperature dependency may be com-pared to the activation energy for that phase (Eqn.2).Such is the case for Na,which in granitoids is contained predominantly in plagioclase,and is not incorporated to any extent into sec-ondary phases.Activation energies must be interpreted within the context of variable plagioclase stoichiometry,which in the present study ranges from between An 0.21for the Loch Vale granite to An 0.50for the Rio Icacos quartz diorite.Experimental E a values for plagioclases over a comparable composition range are not strongly composition dependent,averaging 60kJ/mol (Blum and Stillings,1995).This activation energy
for
Fig.4.Arrhenius plots of the log Na ef?uent concentrations from fresh and weathered granitoid rocks.Lower horizontal scale corresponds to the inverse of absolute temperature and upper nonlinear scale to the temperature increase in °C.Solid and dashed lines correspond to linear ?ts to the ef?uents from fresh and weathered granitoids (see text,Eqn.1).
3283
Effect of temperature on chemical weathering rates of granitoid rocks
plagioclase is very similar to the averages of 57and 65kJ/mol determined for ef?uent Na released from fresh and weathered rocks used in this study.
In contrast to Na,the temperature effect on Si ef?uent concentrations cannot be ascribed with certainty to a unique phase.The similarities in Na and Si activation energies are in the accordance with plagioclase reactivity.However,Si could also be contributed from other phases including K-feldspar,biotite,and hornblende,or by changes in the stoichiometry of Si-containing secondary phases.The activation energies in-volving the release of tetrahedral Si from various silicate min-erals may be similar as suggested by a number of experimental studies (White and Brantley,1995,and references therein).4.2.2.K release
Signi?cant K is contained in both alkali feldspar and biotite.As a result of the generally slower weathering rates of K-feldspar relative to plagioclase,high K/Na ratios in ef?uents (Table 2)indicate that biotite is the principal source of elevated K concentrations.High Rb/K ratios in the ef?uents are also indicative of biotite dissolution compared to low Rb/K ratios for K feldspar dissolution (Bullen et al.,1998).The average activation energies for K release from the fresh and weathered granitoid columns (22?7kJ/mol and 32?11kJ/mol,respec-tively)are signi?cantly lower than for experimental K-feldspar (60kJ/mol;Blum and Stillings,1995).Although activation data are not available for biotite,these low E a values are suggestive of K release from interlayer sites.At near neutral pH,K is preferentially released from such sites during the oxidation of biotite to form hydrobiotite (Acker and Bricker,1992;Kali-nowski and Schweda,1996).
The lower bonding energy for interlayer K in biotite relative to tetrahedral Na in plagioclase results in faster K release rates as evidenced by the high K/Na ef?uent ratios (Table 3and Fig.8).However,the lower activation energy of K relative to Na is expected to diminish this selective loss of K from granitoid weathering with increasing temperature.This effect is shown by the progressive decreases in the K/Na ratios in ef?uents from the fresh and weathered granitoids (Fig.
8).
Fig.5.Arrhenius plots of the log K ef?uent concentrations from fresh and weathered granitoid rocks.Lower horizontal scale corresponds to the inverse of absolute temperature and upper nonlinear scale to the temperature increase in °C.Solid and dashed lines correspond to linear ?ts to the ef?uents from fresh and weathered granitoids (Eqn.1).
3284 A.F.White et al.
The above temperature discordance,due to differences in activation energies between feldspars and biotite,has interest-ing rami?cations in terms of the natural weathering environ-ment.Colder climates are predicted to promote K release during biotite weathering relative to plagioclase,whereas trop-ical climates should favor plagioclase relative to biotite.On the basis of experimental activation data (Table 4),a temperature increase from 0°to 25°C should decrease the weathering rate of biotite by about a factor of three relative to plagioclase.
This predicted temperature trend is consistent with observed differences in the K/Na ratios in watershed discharge.In a compilation of discharge chemical composition data from gla-cial versus nonglacial catchments,Anderson et al.(1997)ob-served that on a molar basis,K concentrations are about equal to Na in glacial runoff,whereas in nonglacial waters K is rarely greater than one-third of the Na concentration.High K/Na ratios in glacial runoff is commonly ascribed to the relatively rapid initial weathering of biotite induced by exposure of fresh surfaces through grinding and physical erosion (Blum and Erel,1997).However,the lower activation energy of biotite relative to plagioclase produces concentration differences that are ex-pected to be of comparable importance in explaining higher K/Na solute ratios in colder versus warmer enviroments.4.2.3.Ca,Mg,and Sr release
During the initial ambient temperature phases of the exper-iments,ef?uent Ca and alkalinity from fresh granitoid rocks were dominated by dissolution of trace amounts of dissemi-nated calcite (Table 1;White et al.,1999).Ef?uents from the fresh Panola granodiorite,which initially contained the highest
calcite concentration,continue to be dominated by Ca during the subsequent temperature experiments (Table 3).Although lower than Si in the other fresh granitoid ef?uents,Ca continues to exceed concentrations expected from stoichiometric disso-lution of Ca-containing silicates such as plagioclase.Ca con-centrations generally decreased with time regardless of the imposed temperatures (Table 3and Fig.1),suggesting dimin-ishing contributions from calcite,although it continues to dom-inate Ca release.Ca concentrations in weathered granitoid ef?uents were much lower (Table 1)due to the previous re-moval of calcite during natural weathering.
The negative temperature correlation for ef?uent Ca from the fresh granitoids (Fig.6)is counter to that predicted by rela-tively large activation energies for plagioclase (?60kJ/mol;Blum and Stillings,1995)and calcite (?55kJ/mol;Sjoberg and Rickard,1984;MacInnis and Brantley,1992),the two principal sources of Ca in the fresh granitoid rocks used in the study.Part of this apparent decrease in ef?uent Ca with temperature may be attributed to temporal effects in which the highest temper-ature experiments at 17°and 35°C were run last in the exper-imental sequence.Unlike for primary silicates,continued reac-
Table 4.Linear correlation coef?cients to experimental data (Eqn.1)and apparent activation energies (Eqn.2,kJ/mol).
a 0
a 1
r 2
E a
Si
Yosemite (F)11.04?28610.8654.8Yosemite (W)10.42?28460.9054.5Loch Vale (F)11.65?31100.9059.5Loch Vale (W)11.90?32080.9761.4Panola (F)10.78?27880.5353.4Panola (W)9.07?26650.9351.0Rio Icacos (F)9.90?24540.9147.0Rio Icacos (W)11.91?31280.9159.9Na
Yosemite (F)10.85?30850.9859.1Yosemite (W)8.99?27700.9753.0Loch Vale (F)13.69?39400.9675.4Loch Vale (W)10.26?29070.9279.6Panola (F)9.91?28000.9053.6Panola (W)12.25?37640.8872.1Rio Icacos (F)7.55?20600.9639.4Rio Icacos (W)11.02?28600.7154.8K
Yosemite (F) 4.0?9160.6817.5Yosemite (W) 4.9?14550.8427.9Loch Vale (F)7.3?20720.9039.7Loch Vale (W)8.5?24990.9747.8Panola (F) 2.0?4130.287.9Panola (W) 5.9?17350.9333.2Rio Icacos (F) 4.9?11430.9121.9
Rio Icacos (W)
4.6
?1079
0.91
20.7
Fig.6.Log ef?uent concentrations of Ca,Mg,and Sr plotted as functions of experimental temperature.Lower horizontal scale corre-sponds to the inverse of absolute temperature and upper nonlinear scale to the temperature increase in °C.
3285
Effect of temperature on chemical weathering rates of granitoid rocks
tion removes signi?cant proportions of residual calcite resulting in progressively lower Ca concentrations with time (Fig.1).
An inverse correlation between ef?uent Ca and temperature may also be related to the retrograde solubility of calcite for the fresh granitoids.Calcite saturation persisted in the fresh gran-itoid ef?uents only during the initial phases of the ambient temperature experiments (White et al.,1999).Ef?uents are signi?cantly undersaturated during the subsequent temperature experiments described in the present study.However,with continued reaction,residual calcite may only persist in micro-fractures and pores with limited contact with the bulk column ef?uent.Under such transported-limited conditions,retrograde calcite solubility may control diffusion gradients from such isolated environments.
Ef?uent Mg and Sr concentrations vary with temperature in a manner very similar to that of Ca (Table 3and Fig.6).This close correlation between these alkaline earth cations is unex-pected because of the very different source minerals.Micro-probe data indicated only trace amounts of Mg and Sr in calcite in granitoid rocks (White et al.,1999).Biotite is expected to be the principal source of Mg in both fresh and weathered grani-toids with additional contributions from hornblende in the Mg-rich Yosemite and Rio Icacos granitoids.Although ef?uent K,derived principally from biotite,exhibits a strong tempera-ture dependence,Mg does not.Plagioclase feldspar is the principal source of Sr in the granitoids.Likewise,although Na concentrations derived from plagioclase are strongly tempera-ture dependent,corresponding Sr concentrations are not.
The strong correlations between solute Ca,Mg,and Sr suggest that ion exchange reactions control these ef?uent con-centrations and the associated temperature effects.Relatively slow diffusion-controlled ion exchange is compatible with the nonsteady-state temperature effects observed for Ca,Mg,and Sr in the column ef?uents (Figs.1and 2).Initial products of biotite weathering are substrates on which such exchange re-actions can occur.Farmer and Wilson (1970)demonstrated that exchange of aqueous Mg with interlayer K facilitates the ver-miculitization of biotite.Studies of natural biotite weathering have also documented the exchange of Ca and Sr in pore waters for interlayer K during the formation of hydrobiotite (Velbel,1985;Bullen et al.,
1997).
Fig.7.Relationship of temperature and 87Sr/86Sr ratios in ef?uents from fresh and weathered granitoid rocks.Solid symbols correspond to ef?uent data with regression ?ts.Open symbols correspond to predicted 87Sr/86Sr ratios based on congruent dissolution of biotite and plagioclase.
3286 A.F.White et al.
4.2.4.87
Sr/86Sr ratios
As demonstrated by White et al.(1999),the Sr concentra-tions in disseminated calcite in the fresh grantioids used in this study are low (average,330ppm).Therefore,the relative rate of biotite to plagioclase weathering,as documented above by the Na/K ef?uent,has important implications for Sr release from granitoid rocks and accompanying 87Sr/86Sr ratios in watershed discharge (Blum et al.,1994,;Bullen et al.,1996;Blum and Erel,1997;Clow et al.,1997).The experimental results shown in Fig.7indicate that 87Sr/86Sr ratios associated with natural granitoid weathering are expected to be tempera-ture dependent,with lower ratios produced in warmer climates and higher ratios in colder climates.On the basis of linear regression ?ts to the data in Fig.7,a temperature increase from 0°to 25°C decreases the 87Sr/86Sr ratio by an average factor of 0.988for ef?uents from the fresh granitoids and a factor of 0.955in the weathered granitoids.
Much of the utility of the Sr isotopes in delineating weath-ering reactions is based on the assumption that Sr is released from the reacting minerals congruently with respect to major cations,although the validity of this assumption has been questioned by some workers (Bullen et al.,1997).A simple mixing calculation involving 87Sr/86Sr ratios can be used to verify the extent of congruent mineral dissolution in column ef?uents.First,the respective weight proportions of biotite and plagioclase that must dissolve to provide K and Na in the column ef?uents at different temperatures (Table 3)are calcu-lated using the major element compositions of the minerals (Table 2).Next,the relative amounts of Sr that would be provided by each mineral are calculated assuming congruent cation release according to the data in Table 2.Finally,the 87
Sr/86Sr ratio of the mixture of Sr is calculated based on simple isotope mass balance.
There are two striking features in the comparison of mea-sured and predicted 87Sr/86Sr trends with temperature (Fig.7).First,the calculated ratios generally show a strong temperature dependence that accentuates that shown by the measured ef?u-ent data.Second,the calculated 87Sr/86Sr ratios are typically greater than those of the column ef?uents,with the greatest discrepancy at lower temperatures and convergence at high
temperatures.The mismatch of the calculated and measured Sr isotope ratios at low temperatures indicates either that Sr is not released congruently from plagioclase and biotite,at least with respect to Na and K,or that another mineral provides signi?-cant relatively unradiogenic Sr to the column ef?uents.This observation is consistent with our previous conclusion that the relative rate of biotite to plagioclase weathering calculated using K and Na mass balance at the ambient laboratory tem-perature (22°C)generally overestimates that calculated using Sr isotope mass balance (Bullen et al.,1998).
Therefore,it appears that Sr isotopes cannot be used to determine the relative rate of biotite to plagioclase weathering at cooler temperatures.The possible mechanisms for the in-creasing divergence at decreasing temperatures are speculative,but may include:(1)preferential retention of radiogenic Sr relative to K in biotite by an exchange mechanism as proposed above;and (2)preferential leaching of relatively unradiogenic Sr from plagioclase or K-feldspar.Either mechanism is consis-tent with the expected differences in activation energies asso-ciated with the different sites occupied by the trace element Sr and the major stoichiometric constituents K and Na in the biotite and feldspar structures.Brantley et al.(1998)previously noted preferential release of Sr having incongruent 87Sr/86Sr ratios relative to bulk sample compositions in the early stages of experimental dissolution of bytownite,albite,and micro-cline.On the other hand,retention of radiogenic Sr as well as Mg and Ca in biotite by an exchange mechanism provides an explanation for the similar behavior of these alkaline earths (Fig.6).In any case,the use of Sr isotopes to assess relative mineral weathering rates,particularly in colder alpine environ-ments,is clearly problematic.
4.3.Activation Energies in Natural Weathering
In a detailed investigation of the effect of climate on chem-ical weathering (White and Blum,1995)tabulated average chemical ?ux data from a worldwide distribution of 68small upland watersheds underlain by granitoid rocks.This data set overcame inherent problems related to climate interpretations of larger scale river systems,which have much more variable lithology,geomorphology,and vegetation.Average annual
wa-
Fig.8.Average K/Na ratios in ef?uents from fresh and weathered granitoid rocks as functions of temperature.
3287
Effect of temperature on chemical weathering rates of granitoid rocks
tershed temperatures varied between0°and22°C.Included in this database were?uxes from the Loch Vale,Panola,and Rio Icacos watersheds from which granitoid rocks were sampled for the experimental study.
White and Blum(1995)observed positive correlations be-tween increasing Si and Na?uxes and increasing temperature and precipitation based on the expression
Q i,w??a i?P?exp?E a?10?1??(3)
where Q
i,w
is the average?ux of species i(mol/ha/yr),P is
average annual precipitation(mm/yr),and a
i
is the?tted pre-cipitation dependence.The exponential temperature function is essentially the Arrhenius expression(Eqn.2)in which T rep-
resents a speci?c watershed temperature and T
o
is a reference temperature taken to be5°C,the mean annual temperature of the watershed database.Equation3implies a climatic reinforc-ing effect for watersheds in which weathering rates at both high temperatures and high precipitation,such as in the tropics,were very rapid compared to watersheds in more temperate climates. The above approach provided a statistically valid?t to the?ux data resulting in respective activation energies of59.4kJ for Si and62.5kJ for Na.
The database used in the calculations by White and Blum (1995)was weighted heavily in favor of temperate climates based on extensive watershed research conducted principally in North American and Europe.The extremes in chemical weath-ering represented by upland watersheds in tropical environ-ments was constrained only by the Rio Icacos watershed in the Luquillo Mountains of Puerto Rico(White et al.,1998).The Rio Icacos had the highest temperature and nearly the highest precipitation in the database,and a Si?ux over three times faster than any other watershed.It also has An
0.50
plagioclase, which is higher than most granitoid rocks,and could potentially increase its relative reactivity.
Since the work of White and Blum(1995),additional wa-tershed Si?ux data have become available.Climate,hydro-logic,and Si?ux data for20of these watersheds,including the Upper Merced River in Yosemite,are tabulated in Table5. Also included for comparison are previously reported data for the Loch Vale,Panola,and Rio Icacos watersheds(White and Blum,1995)from which granitoid samples were obtained for the present study.There is an obvious relationship between climate and weathering rates for these four watersheds.Silica ?uxes range from154mol/ha/yr from the alpine Loch Vale watershed with an average temperature of0°C to8060mol/ ha/yr for the tropical Rio Icacos watershed(22°C).
Of particular signi?cance in Table5are?uxes for a number of tropical granitoid watersheds in two mountainous areas of Malaysia,which climatically fall in the high temperature–high precipitation regime.These watersheds experience approxi-mately half the annual precipitation as the Rio Icacos watershed (2300mm versus4200mm)but are slightly warmer(25°C versus22°C).Annual Si?uxes from the Malaysian watersheds range from one-half to one-third that of the Rio Icacos water-shed.Sodium discharge?uxes were not used in the present calculations because of inadequate data on precipitation input to these watersheds.Each Malaysian watershed is treated sta-tistically as independent.However,the Malaysian watersheds are from a geographically and climatically restricted region. Thus,there may be systematic biases,which might make the measurements not completely independent,effectively over-weighting the Malaysian watersheds relative to the Rio Icacos. The new temperature,precipitation,and Si?ux data(Table
Table5.Data for watersheds included in this study and for additional watersheds not included in the database of White and Blum(1995).
Watershed name Si?ux Temp
(°C)
Precip
(mm)
Runoff
(mm)
Area
(ha)Location Reference
Gem Lake61 5.018501430123California Stoddard,1987
Loch Vale1540.01100604860Colorado Mast et al.,1990
Fall River2040.07482676820Colorado Sueker,1996
Big Thompson River2240.092542610440Colorado Sueker,1996
Spruce Creek2800.0840*******Colorado Sueker,1996
Merced River/Yosemite320 5.0120064046500California White,unpub.
Fern Creek3330.010********Colorado Sueker,1996
North St.Vrain Creek3410.010*********Colorado Sueker,1996
Boulder Brook3840.0605251990Colorado Sueker,1996
Mont Lozere B573 6.01720130019France Bonneau et al.,1977
Mont Lozere C595 6.01800138081France Bonneau et al.,1977
Mont Lozere A634 6.01810163054France Bonneau et al.,1977 Panola67615.3115033841Georgia Peters,1994a
White Lagan1000 6.0282021905100Scotland Farley and Werritty,1989 https://www.360docs.net/doc/1d17931011.html,wing231025.02250——Malaysia Vegas-Vilarrubia et al.,1994 Sg.Lui249025.624108146800Malaysia Vegas-Vilarrubia et al.,1994 Sg.Batu291024.324109245500Malaysia Nelson,1995b
Sg.Selangor@R.P.293025.624101180145000Malaysia Nelson,1995b
Sg.Batamgso312025.02250——Malaysia Vegas-Vilarrubia et al.,1994 Sg.Selangor@Rasa359025.62410118032100Malaysia Nelson,1995b
Sg.Changkak611025.02250——Malaysia Vegas-Vilarrubia et al.,1994 Rio Icacos806022.043003680326Puerto Rico McDowell and Asbury,1994
a Written communication,N.Peters,U.S.Geological Survey,1994.
b Written communication,B.W.Nelson,University of Virginia,1995.
Average annual Si?ux reported as mol?ha?1?yr?1.
3288 A.F.White et al.
5)are combined with the previous data (Table 1;White and Blum,1995).Eqn.3is numerically optimized based on the expanded data set (Tables 1and 5;White and Blum,1995)for values of a 1and E a .The apparent activation energy for Si discharge ?uxes from granitoid watersheds is calculated to be 51kJ/mol,a value that is only slightly less than the average activation energy for experimental weathering of granitoid rocks reported here.This implies that the temperature sensitiv-ity for Si,which is derived from multimineralic silicate rocks,is comparable under both laboratory and natural weathering conditions.
A useful way to evaluate the effect of temperature on wa-tershed weathering is to correct the measured Si solute ?ux to a constant value of watershed precipitation by use of Eqn.3(White and Blum,1995).A reference precipitation value of 1000mm/yr is used,which approximates the mean for the complete watershed database.The precipitation-corrected Si ?ux data are shown in the Arrhenius plot in Fig.9.The slope of the linear regression ?t to the data is correlated with the activation energy calculated in Eqn.3(51kJ/mol).The tem-perature ranges used in the experiments bracket the natural watershed temperatures,which ranges between 0°and 26°C.The distribution of watershed Si ?uxes clearly increases with increasing temperature and shows that a strong correlation exists between climate and silicate weathering.The Si ?uxes uncorrected for differences in precipitation have an even stron-ger correlation with temperature on an Arrhenius plot (r 2?0.70and apparent E a ?62kJ/mol).We believe this is a result of the data set having a internal correlation between tempera-ture and precipitation (r 2?0.28),largely resulting from the lack of warm,arid watersheds).This enhances the apparent importance of the temperature signal in the uncorrected Si ?uxes.It also demonstrates the dif?culties in separating vari-ables while trying to determine the effects of temperature on chemical weathering within natural watersheds.
The inability to detect climate sensitivity in many other weathering environments is,in part,related to scale.For ex-
ample,the lack of apparent climate correlations with chemical concentrations in large river systems is clearly related to com-plex weathering regimes resulting from variable rock types and geomorphic regimes.However,even in relatively simple wa-tershed settings,attempts to ?t Eqn.3to K,Ca,and Mg ?uxes produced statistically meaningless results.White and Blum (1995)ascribed the lack of a climate correlation for these elements to nonweathering variables such as deforestation and soil acidi?cation.However,these new experimental results indicate that much of this variability is also related to intrinsic properties of granitoid rocks themselves including the persis-tence of trace amounts of disseminated calcite,and ion ex-change properties associated with the weathering of biotite.
5.CONCLUSIONS
Results of the present study provide strong support for sig-ni?cant and selective temperature effects on rates of chemical weathering of granitoid rocks both under experimental and natural conditions.Si and Na concentrations in experimental column ef?uents from weathered granitoids were much lower than for their fresh counterparts but exhibited comparable tem-perature effects,increasing in concentration by approximately an order of magnitude over a temperature range of 5–35°C.Apparent activation energies for Si and Na from all the grani-toids averaged 58kJ/mol.This value is comparable to those previously determined for plagioclase feldspar and re?ects re-actions involving the structural release of Si and Na from tetrahedral and octahedral structural positions in the silicates.The magnitude of this increase is observable for natural weath-ering associated with annual discharge ?uxes from watersheds underlain by granitoid rocks.The Arrhenius temperature ?t to a coupled temperature–precipitation model produces activation energies for Si (51kJ/mol)that are similar to experimental weathering of the fresh and weathered granitoids.
Experimental K release is rapid but less temperature sensi-tive than Na and Si.Lower activation energies for weathered and fresh granitoids (22and 32kJ/mol)indicate that both these effects are attributed to K loss from biotite interlayers.Differ-ences in mineral temperature sensitivities are re?ected in de-creases in ef?uent K/Na ratios with temperature,an effect that has been previously observed in comparisons between glacial vs.more temperate watersheds.Differences in temperature sensitivities of biotite and plagioclase are also re?ected in decreasing 87Sr/86Sr ratios with increasing temperature.Con-gruent reaction of biotite and plagioclase approximates 87Sr/86
Sr ratios at warmer temperatures.With decreasing tempera-ture,87Sr/86Sr ratios become lower than predicted suggesting progressively more incongruent biotite or feldspar dissolution.Ef?uent Ca,Mg,and Sr concentrations do not exhibit posi-tive correlations with temperature.Ca concentrations are con-trolled in part by diminishing trace amounts of calcite and possible retrograde calcite solubility.The close correlation between Ca,Mg,and Sr concentrations implies additional control by ion exchange,possibly during the initial stages of biotite vermiculization.The lack of a temperature correlation for these species in natural watershed weathering is in part related to additional variability instilled by competing pro-cesses such as nutrient cycling and acidi?cation.However,experimental results demonstrate inherent complexities
intrin-
Fig.9.Arrhenius plot of precipitation-corrected average annual Si ?uxes from granitoid watersheds.The solid diagonal line corresponds to the activation energy Ea calculated from Eqn.3(see text).
3289
Effect of temperature on chemical weathering rates of granitoid rocks
sic to granitoid weathering of Ca-and Mg-containing minerals. The persistence of these nonsteady weathering reactions is a fruitful avenue of future research because weathering of these minerals is proposed as a major sink for long-term atmospheric CO
2
draw down.
Acknowledgments—The authors thank David Clow,Tom Huntington, and Matt Larsen of the U.S.Geological Survey for collecting the granitoid samples.The work was supported by the Water Energy and Biogeochemical Budget(WEBB)project of the U.S.Geological Sur-vey Program on Global Change.
REFERENCES
Acker J.G.and Bricker O.P.(1992)The in?uence of pH on biotite dissolution and alteration kinetics at low temperature.Geochim. Cosmochim.Acta56,3073–3092.
Anderson S.P.,Drever J.I.,and Humphrey N.F.(1997)Chemical weathering in glacial enviroments.Geology25,399–402.
Berner R.A.and Berner E.K.(1997)Silicate weathering and climate. In Tectonic Uplift and Climate Change(ed.W.F.Ruddiman),pp. 353–364.Plenum Press.
Berner R.A.,Lasaga A.C.,and Garrels R.M.(1983)The carbonate–silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past100million years.Amer.J.Sci.283,641–683. Bickle M.J.(1996)Metamorphic decarbonation,silicate weathering and the long term carbon cycle.Terra Nova8,270–276.
Blum A.E.and Stillings L.L.(1995)Feldspar dissolution kinetics.In Chemical Weathering Rates of Silicate Minerals(ed.A.F.White and S.L.Brantley),Vol.31.Mineralogical Society of America.
Blum J.D.and Erel Y.(1997)Rb–Sr isotope systematics of a granitic soil chronosequence:The importance of biotite weathering. Geochim.Cosmochim.Acta61,3193–3204.
Blum J.D.,Erel Y.,and Brown K.(1994)87Sr/86Sr ratios of Sierra Nevada stream waters:Implications for relative mineral weathering rates.Geochim.Cosmochim.Acta58,5019–5024.
Bonneau M.,Brethes A.,Lelong F.,Levy G.,Nys C.,and Souchier B. (1977)Effets de boisements resineau purs sur l’evolution de la fertilite du sol.Rev.Forestiere Francaise31,98–207.
Brady P.V.and Carroll S.A.(1994)Direct effects of CO
2and
temperature on silicate weathering:Possible implications for climate control.Geochim.Cosmochim.Acta58,1853–1863.
Brady P.V.and Walter J.V.(1992)Surface chemistry and silicate dissolution at elevated temperatures.Amer.J.Sci.292,639–658. Brantley S.L.,Chesley J.T.,and Stillings L.L.(1998)Isotopic ratios and release rates of strontium measured from weathering of feldspars.Geochim.Cosmochim.Acta62,1493–1500.
Bullen T.D.,Krabbenhoft D.P.,and Kendall C.(1996)Kinetic and mineralogic controls on the evolution of groundwater chemistry and 87Sr/86Sr in a sandy silicate aquifer,northern Wisconsin,USA.
Geochim.Cosmochim.Acta60,1807–1821.
Bullen T.,White,A.F.,Blum,A.Harden,J.and Schulz,M.(1997) Chemical weathering of a soil chronosequence on granitoid allu-vium:II.Mineralogic and isotopic constraints on the behavior of strontium.Geochim.Cosmochim.Acta61,291–306.
Bullen T.D.,White A.F.,Vivit D.V.,and Schulz M.S.(1998) Granitoid weathering in the laboratory:Chemical and Sr isotopic perspectives on mineral dissolution rates.Proceedings of9th Intl. Symp.Water Rock Interaction,pp.383–386.A.A.Balkeme Press. Casey W.H.and Sposito G.(1992)On the temperature dependence of mineral dissolution rates.Geochim.Cosmochim.Acta56,3825–3830.
Chen Y.and Brantley S.L.(1996)Temperature and pH-dependence of albite dissolution rate at acid pH.Chem.Geol.135,275–290. Clow D.W.,Mast,M.A.,Bullen T.D.,and Turk J.T.(1997)Strontium 87/strontium86as a tracer of mineral weathering reactions and calcium sources in an alpine/subalpine watershed,Loch Vale,Col-orado.Water Resources Res.33,1335–1351.
Dorn R.I.and Brady P.V.(1995)Rock-based measurement of tem-perature-dependent plagioclase weathering.Geochim.Cosmochim. Acta59,2847–2852.Dove P.M.(1995)Kinetic and thermodynamic controls on silica reactivity in weathering environments.In Chemical Weathering of Silicate Minerals(ed.White,A.F.and Brantley,S.L.),Vol.31,pp. 235–290.Mineralogoical Society of America.
Edmond J.M.and Huh Y.(1997)Chemical weathering yields from basement and orogenic terrains in hot and cold climates.In Tectonic Uplift and Climate Change(ed.W.F.Ruddiman),pp.329–351. Plenum Press.
Edmond J.M.,Palmer M.R.,Measures C.I.,Grant B.,and Stallard R.F.(1995)The?uvial geochemistry and denudation rate of the Guyana Shield in Venezuela,Colombia,and Brazil.Geochim.Cos-mochim.Acta59,3301–3325.
Farley D.A.and Werritty A.(1989)Hydrochemical budgets for the Loch Dee experimental catchments,southwest Scotland (1981–1985).J.Hydrology109,351–368.
Farmer V.C.and Wilson M.J.(1970)Experimental conversion of biotite to hydrobiotite.Nature226,841–842.
Francois L.M.and Walker J.C.G.(1992)Modeling of the Phanero-zoic carbon cycle and climate:Constraints from the87Sr/86Sr isoto-pic ratio of seawater.Amer.J.Sci.292,81–135.
Huh Y.,Tsol M.,Zaitsev A.,and Edmond J.(1998a)The?uvial geochemistry of the rivers of Eastern Siberia:I.Tributaries of the Lena River draining the sedimentary platform of the Siberian Craton. Geochim.Cosmochim.Acta62,1657–1676.
Huh Y.,Panteleyev G.,Babich D.,Zaitsev A.,and Edmond J.(1998b) The?uvial geochemistry of the rivers of Eastern Siberia.II.Tribu-taries of the Lena,Omloy,Yana,Indigirka,Kolyma,and Anadyr draining the collisional/accretionary zone of the Verkhoyansk and Cherskiy ranges.Geochim.Cosmochim.Acta62,2053–2075. Kalinowski B. E.and Schweda P.(1996)Kinetics of muscovite, phlogopite and biotite dissolution and alteration at pH1-4,room temperature.Geochim.Cosmochim.Acta60,367–385.
Lasaga A.C.(1984)Chemical kinetics of water–rock interaction.J. Geophys.Res.89,4009–4025.
Lasaga A.C.,Soler J.M.,Ganor J.,Burch T.E.,and Nagy K.L.(1994) Chemical weathering and global chemical cycles.Geochim.Cosmo-chim.Acta58,2361–2386.
Louvat,P.(1997)Etude geochimique de l’erosion?uviale d’iles vol-caniques a l’aide des bilans d’elements majeurs et traces.PhD thesis, Institut de Physique du Globe de Paris.
MacInnis I.N.and Brantley S.L.(1992)The role of dislocations and surface morphology in calcite dissolution.Geochim.Cosmochim. Acta56,1113–1126.
Mast M.A.,Drever J.I.,and Barron J.(1990)Chemical weathering in the Loch Vale watershed,Rocky Mountain National Park,Colorado. Water Resources Res.26,2971–2978.
McDowell W.H.and Asbury C.E.(1994)Export of carbon,nitrogen, and major ions from three tropical montane watersheds.Limnol. Oceanogr.39,111–125.
Sjoberg E.L.and Rickard D.T.(1984)Temperature dependence of calcite dissolution kinetics between1and62°C at pH2.7to8.4in aqueous solutions.Geochim.Cosmochim.Acta48,485–493. Staudigel H.,Hart S.R.,Schmincke H.U.,and Smith B.M.(1989) Cretaceous ocean crust at DSDP sites417and418:Carbon uptake from weathering versus loss by magmatic outgassing.Geochim. Cosmochim.Acta53,3091–3094.
Stoddard J.L.(1987)Alkalininty dynamics in an unacidi?ed alpine lake,Sierra Nevada,California.Limnol.Oceonogr.32,825–839. Sueker J.K.(1996)Isotopic and chemical?owpath separation of stream?ow during snowmelt and hydrogeochemical controls of surface water in six alpine–subalpine basins,Rocky Mountain Na-tional Park.PhD thesis,Univ.Colorado.
Vegas-Vilarrubia T.,Maass M.,Rull V.,Ellias V.,Ovalle A.,Lopez D., Depetris J.,and Douglas I.(1994)Small catchment studies in the tropical zone.In Biogeochemistry of Small Catchments(ed. B. Moldan),pp.343–360.John Wiley and Sons.
Velbel M.A.(1985)Geochemical mass balances and weathering rates in forested watersheds of the southern Blue Ridge.Amer.J.Sci.285, 904–930.
Velbel M.C.(1993)Temperature dependence of silicate weathering in nature:How strong a feedback on long-term accumulation of atmo-spheric CO
2
and global greenhouse warming.Geology21,1059–1062.
3290 A.F.White et al.
Walker J.C.G.,Hays P.B.,and Kasting J.F.(1981)A negative feedback mechanism for the long-term stabilization of earth’s tem-perature.J.Geophys.Res.86,9776–9782.
White A.F.and Blum A.E.(1995)Effects of climate on chemical weathering rates in watersheds.Geochim.Cosmochim.Acta59, 1729–1747.
White A.F.and Brantley S.L.(1995)Chemical weathering rates of silicate minerals.In Reviews in Mineralogy(ed.White,A.F.and Brantley,S.L.),Vol.31.Mineralogical Society of America. White A.F.,Blum A.E.,Schulz M.S.,Bullen T.D.,Harden J.W.,and
Peterson M.L.(1996)Chemical weathering of a soil chronose-qunece on granitic alluvium.1.Reaction rates based on changes in soil mineralogy.Geochim.Cosmochim.Acta60,2533–2550. White A.F.,Blum A.E.,Schulz M.S.,Vivit D.V.,Larsen M.,and Murphy S.F.(1998)Chemical weathering in a tropical watershed, Luquillo Mountains,Puerto Rico:Weathering rates based on mineral and solute mass balances.Geochim.Cosmochim.Acta62,209–226. White A.F.,Bullen T.D.,Vivit D.V.,and Schulz M.S.(1999)The role of disseminated calcite in the chemical weathering of granitoid rocks.Geochim.Cosmochim.Acta(in press).
3291
Effect of temperature on chemical weathering rates of granitoid rocks
