JonesCapsicumChlorosis-sec

CSIRO PUBLISHING
www.publish.csiro.au/journals/app Australasian Plant Pathology,2005,34,397–399
SHORT RESEARCH NOTES
Capsicum chlorosis virus infecting Capsicum annuum in the East Kimberley region of Western Australia
R.A.C.Jones A,B,D and M.Sharman C
A Department of Agriculture,Locked Bag No.4,Bentley Delivery Centre,Bentley,W A6983,Australia.
B School of Biological Sciences and Biotechnology,Murdoch University,Murdoch,W A6150,Australia.
C Department of Primary Industries and Fisheries,80Meiers Road,Indooroopilly,Qld4068,Australia.
D Corresponding author.Email:rjones@https://www.360docs.net/doc/284661427.html,.au
Abstract.Capsicum chlorosis virus(CaCV)was detected in?eld grown Capsicum annuum from Kununurra in north-east Western Australia.Identi?cation of the Kununurra isolate(W A-99)was con?rmed using sap transmission to indicator hosts,positive reactions with tospovirus serogroup IV-speci?c antibodies and CaCV-speci?c primers,and amino acid sequence comparisons that showed>97%identity with published CaCV nucleocapsid gene sequences.The reactions
of indicator hosts to infection with W A-99often differed from those of the type isolate from Queensland.The virus multiplied best when test plants were grown at warm temperatures.CaCV was not detected in samples collected in a survey of C.annuum crops planted in the Perth Metropolitan area.
Additional keywords:tospovirus,identi?cation,host range,symptomatology,sequence.
Capsicum chlorosis virus(CaCV;Family Bunyaviridae, Genus Tospovirus)causes a serious disease of capsicum(bell pepper)and sweet chilli(Capsicum annuum)in Queensland, where it also infects tomato(Lycopersicon esculentum) (McMichael et al.2002).In July2004,plants with symptoms of chlorotic mottle and deformation in young leaves and plant stunting were observed in a sweet chilli crop in the Ord river irrigation area at Kununurra in the East Kimberley region in north-east Western Australia.T wo symptomatic plants were transported to Perth and transplanted into pots.Leaf sample extracts from each plant were tested by ELISA using antibodies speci?c to tospovirus serogroups I–III,Tomato spotted wilt virus(TSWV;Family Bunyaviridae,Genus Tospovirus)and Cucumber mosaic virus(CMV,Family Bromoviridae,Genus Cucumovirus).Although the symptoms present in both plants resembled those caused by TSWV, sample extracts from one of them failed to react with any of the antibodies while sample extracts from the other reacted only with antibodies to CMV.When leaf sap from the former was inoculated to plants of Nicotiana benthamiana and capsicum cv.Rialto,symptoms resembling those caused by CaCV developed.In N.benthamiana,symptoms consisted of chlorotic blotches in inoculated leaves and systemic chlorotic mottle,which was soon followed by wilting and plant death.In capsicum,they were chlorotic ringspots in inoculated leaves followed by systemic chlorotic mottle,interveinal chlorosis,leaf deformation and plant stunting.Leaf extracts from the?eld-infected sweet chilli plant were positive with tospovirus serogroup IV antibodies in ELISA,suggesting presence of a member of this serogroup,to which CaCV belongs(McMichael et al.2002).
An828bp PCR product from the nucleocapsid(N) gene of W A-99was ampli?ed using primers CaCV.NPR (ATG TCT AAC GTT AGG CAA CTT A)and CaCV.NPF (TTA CAC TTC TAT AGA AGT ACT A),cloned using the pGEM-T Easy Vector System(Promega)and sequenced with an Applied Biosystems Inc.(ABI)automated sequencing system.After removal of the primers,this sequence (GenBank accession number A Y839642)was compared with those from two already characterised isolates of CaCV from Queensland(GenBank accessions A Y036057 and A Y036058)using CLUSTAL W(Thompson et al. 1994)and found to be96%identical at the nucleotide level,and>97%identical at the amino acid level,over a 784nt overlap.
Using infective N.benthamiana sap extracted in0.05M phosphate buffer,pH7.2,containing0.01M sodium sulphite as inoculum and the abrasive‘celite’,isolate WA-99was
?Australasian Plant Pathology Society200510.1071/AP050260815-3191/05/030397
398Australasian Plant Pathology R.A.C.Jones and M.Sharman
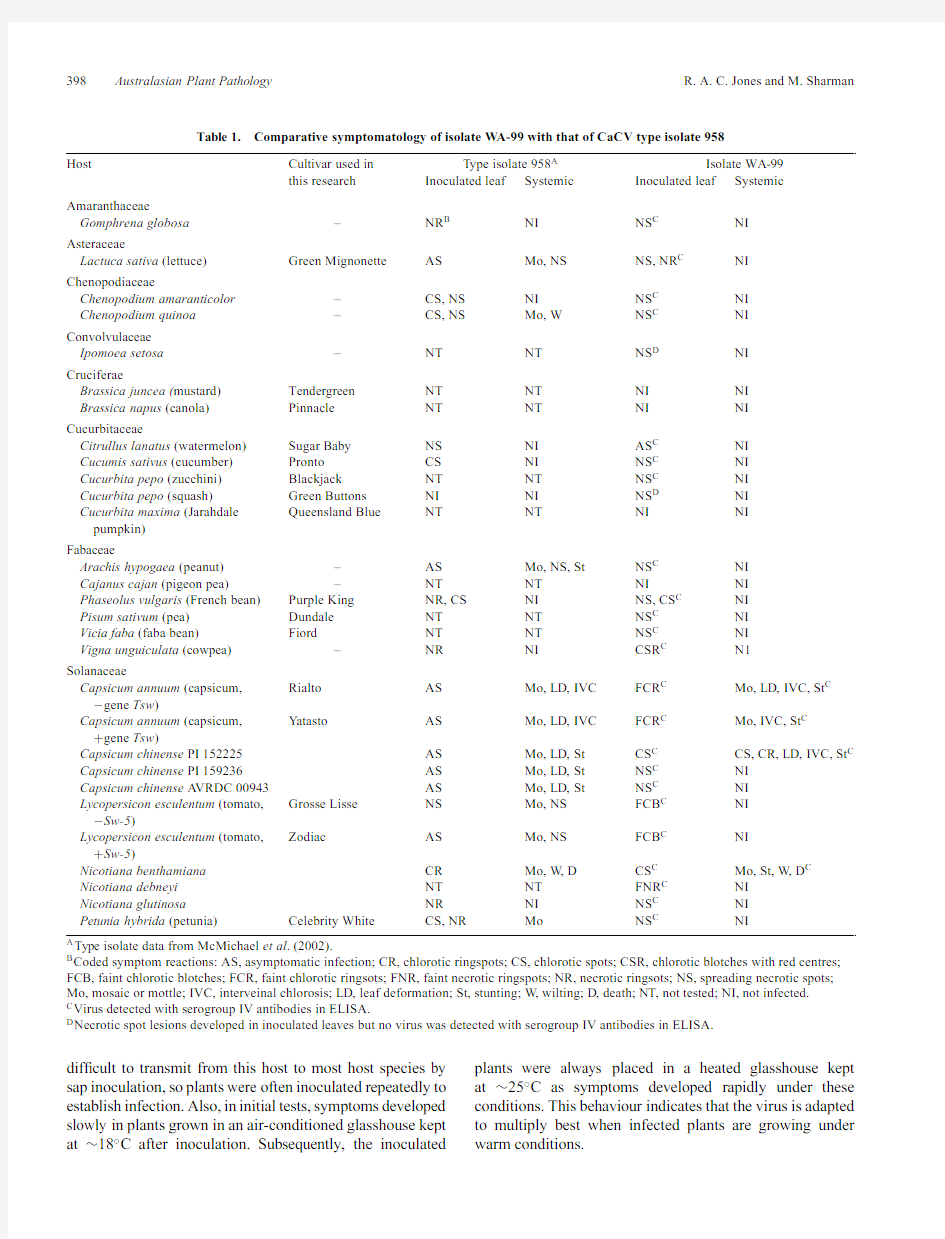
https://www.360docs.net/doc/284661427.html,parative symptomatology of isolate W A-99with that of CaCV type isolate958
Host Cultivar used in T ype isolate958A Isolate WA-99
this research Inoculated leaf Systemic Inoculated leaf Systemic Amaranthaceae
Gomphrena globosa–NR B NI NS C NI
Asteraceae
Lactuca sativa(lettuce)Green Mignonette AS Mo,NS NS,NR C NI Chenopodiaceae
Chenopodium amaranticolor–CS,NS NI NS C NI
Chenopodium quinoa–CS,NS Mo,W NS C NI Convolvulaceae
Ipomoea setosa–NT NT NS D NI
Cruciferae
Brassica juncea(mustard)Tendergreen NT NT NI NI
Brassica napus(canola)Pinnacle NT NT NI NI
Cucurbitaceae
Citrullus lanatus(watermelon)Sugar Baby NS NI AS C NI
Cucumis sativus(cucumber)Pronto CS NI NS C NI
Cucurbita pepo(zucchini)Blackjack NT NT NS C NI
Cucurbita pepo(squash)Green Buttons NI NI NS D NI
Cucurbita maxima(Jarahdale Queensland Blue NT NT NI NI
pumpkin)
Fabaceae
Arachis hypogaea(peanut)–AS Mo,NS,St NS C NI
Cajanus cajan(pigeon pea)–NT NT NI NI
Phaseolus vulgaris(French bean)Purple King NR,CS NI NS,CS C NI
Pisum sativum(pea)Dundale NT NT NS C NI
Vicia faba(faba bean)Fiord NT NT NS C NI
Vigna unguiculata(cowpea)–NR NI CSR C N1
Solanaceae
Capsicum annuum(capsicum,Rialto AS Mo,LD,IVC FCR C Mo,LD,IVC,St C ?gene Tsw)
Capsicum annuum(capsicum,Y atasto AS Mo,LD,IVC FCR C Mo,IVC,St C +gene Tsw)
Capsicum chinense PI152225AS Mo,LD,St CS C CS,CR,LD,IVC,St C Capsicum chinense PI159236AS Mo,LD,St NS C NI
Capsicum chinense A VRDC00943AS Mo,LD,St NS C NI
Lycopersicon esculentum(tomato,Grosse Lisse NS Mo,NS FCB C NI
?Sw-5)
Lycopersicon esculentum(tomato,Zodiac AS Mo,NS FCB C NI
+Sw-5)
Nicotiana benthamiana CR Mo,W,D CS C Mo,St,W,D C Nicotiana debneyi NT NT FNR C NI
Nicotiana glutinosa NR NI NS C NI
Petunia hybrida(petunia)Celebrity White CS,NR Mo NS C NI
A Type isolate data from McMichael et al.(2002).
B Coded symptom reactions:AS,asymptomatic infection;CR,chlorotic ringspots;CS,chlorotic spots;CSR,chlorotic blotches with red centres; FCB,faint chlorotic blotches;FCR,faint chlorotic ringsots;FNR,faint necrotic ringspots;NR,necrotic ringsots;NS,spreading necrotic spots; Mo,mosaic or mottle;IVC,interveinal chlorosis;LD,leaf deformation;St,stunting;W,wilting;D,death;NT,not tested;NI,not infected.
C Virus detected with serogroup IV antibodies in ELISA.
D Necrotic spot lesions developed in inoculated leaves but no virus was detected with serogroup IV antibodies in ELISA.
dif?cult to transmit from this host to most host species by sap inoculation,so plants were often inoculated repeatedly to establish infection.Also,in initial tests,symptoms developed slowly in plants grown in an air-conditioned glasshouse kept at~18?C after inoculation.Subsequently,the inoculated plants were always placed in a heated glasshouse kept at~25?C as symptoms developed rapidly under these conditions.This behaviour indicates that the virus is adapted to multiply best when infected plants are growing under warm conditions.
Capsicum chlorosis virus infecting C.annuum Australasian Plant Pathology399
Using infective N.benthamiana sap,isolate W A-99was inoculated to a range of indicator and crop species belonging to eight different plant families,most of which were tested previously by McMichael et al.(2002)in inoculations with CaCV type isolate958(Table1).Symptom development was monitored and extracts from inoculated and tip leaves of each host were tested separately by ELISA using antibodies to tospovirus serogroup IV.Like isolate958,W A-99infected inoculated leaves of(i)a capsicum cultivar and three C.chinense accessions with TSWV resistance gene Tsw, and(ii)a tomato cultivar with TSWV resistance gene Sw-5.However,unlike isolate958,W A-99infected only two(C.chinense PI152225and capsicum cv.Y atasto) of these four TSWV-resistant genotypes systemically and did not spread out of inoculated leaves of the TSWV-susceptible tomato cultivar used.In addition,unlike isolate 958,isolate W A-99caused only necrotic spots,which tended to enlarge considerably over time,in inoculated leaves of Arachis hypogaea,C.chinense PI159236and A VRDC00943,C.amaranticolor,Chenopodium quinoa, Cucumis sativus,Gomphrena globosa,N.glutinosa and Petunia hybrida.The results with C.chinense PI159236 and A VRDC00943suggest that they may posses strain speci?c hypersensitive resistance to isolate99but not to isolate958,possibly caused by an as yet unidenti?ed strain speci?c,CaCV hypersensitivity gene.Isolate W A-99also failed to infect A.hypogaea, C.quinoa, Lactuca sativa or P.hybrida systemically.Moreover, unlike isolate958,it caused only asymptomatic infection, chlorotic spots or chlorotic blotches in inoculated leaves of Citrullus lanatus,tomato and Vigna unguiculata.In two instances when necrotic spots developed in inoculated leaves,serological con?rmation of virus presence by ELISA was lacking.This was presumably because the virus broke down rapidly within the necrotic tissue.Necrotic local lesion development in Ipomoea setosa suggests that the Convolvulaceae may constitute an additional plant family that CaCV infects.
In November1999,commercial capsicum plantings north and south of the Swan River in the Perth Metropolitan area were surveyed for tospovirus infection.For this,100leaf samples were collected at random from each of11capsicum crops and extracts from them tested by ELISA using antibodies speci?c to TSWV and tospovirus serogroup IV. TSWV was detected in six of the crops with individual infection incidences up to40%.Extracts from freeze-dried material infected with CaCV(supplied by Dr Denis Persley,DPI&F,Brisbane),used as a control in the serogroup IV tests, reacted positively,but none of those from the capsicum survey samples did so.
This study con?rms the presence of CaCV in the East Kimberley region of Western Australia based on symptomatology in capsicum,reactions in indicator plants, serology,RT–PCR ampli?cation and cDNA sequencing.To establish its importance to the Western Australian vegetable industry,surveys are needed to determine its occurrence within the major vegetable growing areas outside the Perth Metropolitan area,especially those in the Ord river irrigation area,Broome and Carnarvon districts.CaCV is the third member of the genus Tospovirus to be found in Western Australia,the others being TSWV(Latham and Jones1997) and Iris yellow spot virus(IYSV;Family Bunyaviridae, Genus Tospovirus)(Coutts et al.2003). Acknowledgements
Eva Gajda,Tracey Smith and Monica Thomas-Carroll provided technical support.Funding for travel to the Kimberley region was provided by Murdoch University, Perth.
References
Coutts BA,McMichael LA,Tesoriero L,Rodoni BC,Wilson CR, Wilson AJ,Persley DM,Jones RAC(2003)Iris yellow spot virus found infecting onions in three Australian states.Australasian Plant Pathology32,555–557.doi:10.1071/AP03060
Latham LJ,Jones RAC(1997)Occurrence of tomato spotted wilt tospovirus in weeds,native?ora and horticultural crops.
Australian Journal of Agricultural Research48,359–369.
doi:10.1071/A96084
McMichael LA,Persley DM,Thomas JE(2002)A new tospovirus serogroup IV species infecting capsicum and tomato in Queensland,Australia.Australasian Plant Pathology31,231–239.
doi:10.1071/AP02016
Thompson JD,Higgins DG,Gibson TJ(1994)CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting,position-speci?c gap penalties and weight matrix choice.Nucleic Acids Research22, 4673–4680.
Note added in proof:CaCV is now known to occur in Thailand as well as Australia.Reference:Premachandra W.T.S.D.et al.(2005) Ceratothripoides claratris,a new vector of a Capsicum chlorosis virus isolate infecting tomato in Thailand.Phytopathology95,659–663. Received1December2004,accepted31January2005
http://www.publish.csiro.au/journals/app
