USP38-NF33_C71_无菌检查法-中英对照

USP38 NF33
<71> STERILITY TESTS
<71>无菌检查法
◆Portions of this general chapter have been harmonized with the corresponding texts of the European Pharmacopeia and/or the Japanese Pharmacopeia. Those portions that are not harmonized are marked with symbols (◆◆) to specify this fact.◆
◆本检查法已与《欧洲药典》和《日本药局方》对应部分进行了协调,不一致的
部分用符号()标注。◆
These Pharmacopeial procedures are not by themselves designed to ensure that a batch of product is sterile or has been sterilized. This is accomplished primarily by validation of the sterilization process or of the aseptic processing procedures.
按药典规定的无菌检验本身并不能确保一批产品无菌或已经灭菌,产品的无菌性主要通过对灭菌工艺或者无菌保障程序的验证来完成。
The test is applied to substances, preparations, or articles which, according to the Pharmacopeia, are required to be sterile. However, a satisfactory result only indicates that no contaminating microorganism has been found in the sample examined under the conditions of the test.
无菌检查法系用于检查药典要求无菌的药物、制剂产品和其他物品是否无菌的一种方法。若供试品符合无菌检查法的规定,仅表明供试品在该检验条件下未发现微生物污染。
PRECAUTIONS AGAINST MICROBIAL CONTAMINATION
预防微生物污染
The test for sterility is carried out under aseptic conditions. In order to achieve such conditions, the test environment has to be adapted to the way in which the sterility test is performed. The precautions taken to avoid contamination are such that they do not affect any microorganisms that are to be revealed in the test. The working conditions in which the tests are performed are monitored regularly by appropriate sampling of the working area and by carrying out appropriate controls.
无菌检查应在无菌条件下进行,为了达到该条件,检测环境应符合无菌检查的规定。防止污染的措施不得影响供试品中微生物的检出。检测环境应定期抽样监测并进行适当的控制。
CULTURE MEDIA AND INCUBATION TEMPERATURES
培养基和培养温度
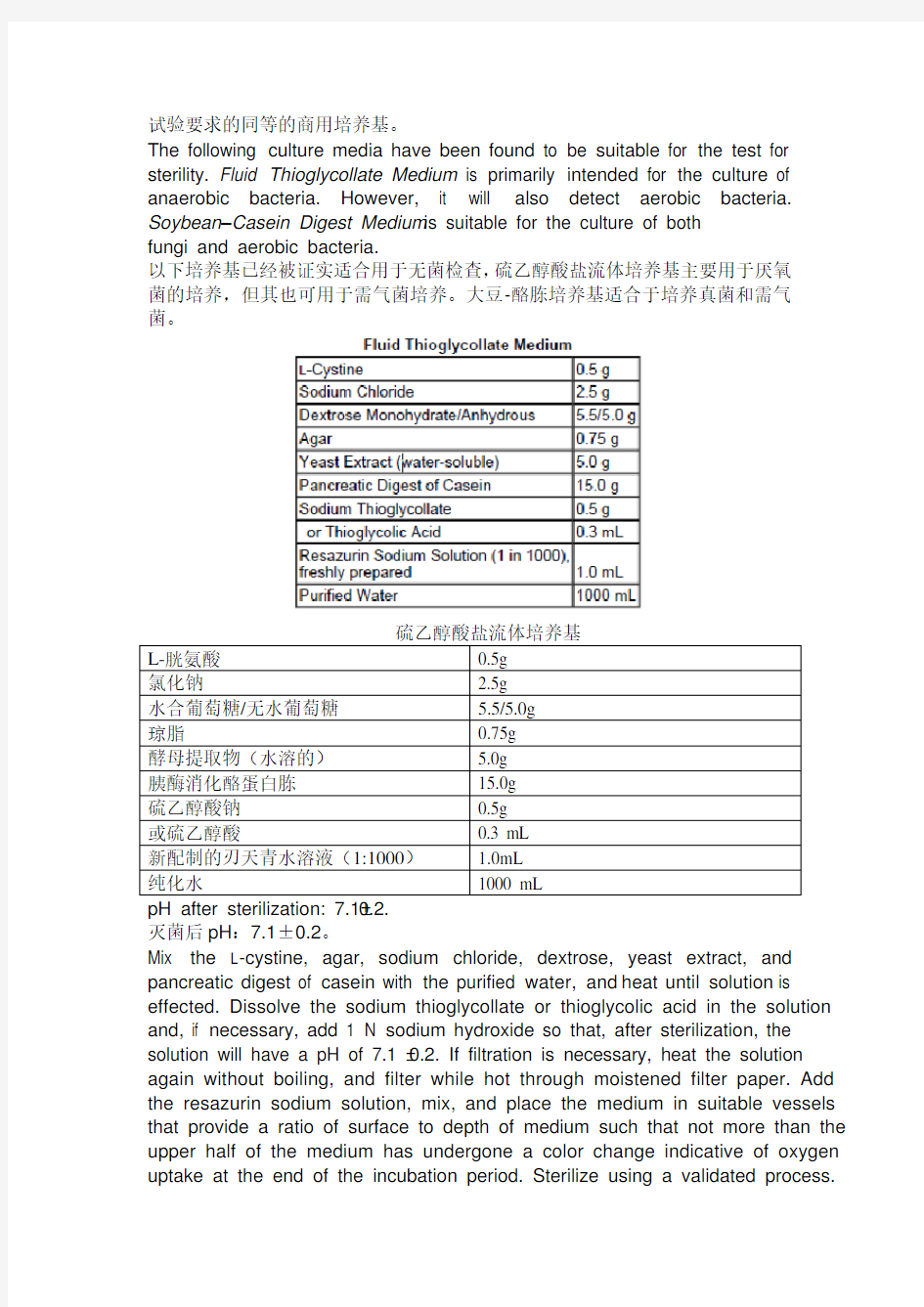
Media for the test may be prepared as described below or equivalent commercial media may be used provided that they comply with the requirements of the Growth Promotion Test of Aerobes, Anaerobes, and Fungi.
无菌检查需制备下表所述培养基,或者是能够符合需气菌、厌氧菌、真菌促生长
试验要求的同等的商用培养基。
The following culture media have been found to be suitable for the test for sterility. Fluid Thioglycollate Medium is primarily intended for the culture of anaerobic bacteria. However, it will also detect aerobic bacteria. Soybean–Casein Digest Medium is suitable for the culture of both
fungi and aerobic bacteria.
以下培养基已经被证实适合用于无菌检查,硫乙醇酸盐流体培养基主要用于厌氧菌的培养,但其也可用于需气菌培养。大豆-酪胨培养基适合于培养真菌和需气菌。
灭菌后pH:7.1±0.2。
Mix the L-cystine, agar, sodium chloride, dextrose, yeast extract, and pancreatic digest of casein with the purified water, and heat until solution is effected. Dissolve the sodium thioglycollate or thioglycolic acid in the solution and, if necessary, add 1 N sodium hydroxide so that, after sterilization, the solution will have a pH of 7.1 ± 0.2. If filtration is necessary, heat the solution again without boiling, and filter while hot through moistened filter paper. Add the resazurin sodium solution, mix, and place the medium in suitable vessels that provide a ratio of surface to depth of medium such that not more than the upper half of the medium has undergone a color change indicative of oxygen uptake at the end of the incubation period. Sterilize using a validated process.
If the medium is stored, store at a temperature between 2 and 25 in a sterile, airtight container. If more than the upper one-third of the medium has acquired a pink color, the medium may be restored once by heating the containers in a water-bath or in free-flowing steam until the pink color disappears and by cooling quickly, taking care to prevent the introduction of nonsterile air into the container. Do not use the medium for a longer storage period than has been validated.
将L-胱氨酸、氯化钠、葡萄糖、酵母提取物、酪蛋白胰酶消化物与纯化水混合,并加热至溶解。将硫乙醇酸钠或硫乙醇酸溶解于该溶液,如果需要可加入1mol/L 氢氧化钠溶液,以便在灭菌后该溶液呈pH值7.1±0.2。如需要过滤,再次加热该溶液但不得煮沸,并趁热以湿润滤纸将该溶液过滤。加入刃天青钠溶液,混匀,并将该培养基置于适当容器中,该容器应为培养基提供特定的面积/深度比,以使在培养期结束后能明确显示氧气摄入的变色部分不超过培养基的上半部分。使用经过验证的工艺进行灭菌。如果需要贮存该培养基,应将其置于无菌、气密容器中,在2~25℃贮藏。如果超过三分之一的培养基已经呈粉红色,可以用以下方法恢复该培养基功能,但每批培养基仅能恢复一次:在水浴锅中或者自由流动蒸汽中加热该容器,直至粉色消失,并迅速放凉,须小心防止非无菌空气进入到容器中。灭菌后培养基存放时间超过验证期限时,不得使用。
Fluid Thioglycollate Medium is to be incubated at 30°–35° . For products containing a mercurial preservative that cannot be tested by the membrane filtration method, Fluid Thioglycollate Medium incubated at 20°–25° may be used instead of Soybean–Casein Digest Medium provided that it has been validated as described in Growth Promotion Test of Aerobes, Anaerobes, and Fungi. Where prescribed or justified and authorized, the following alternative thioglycollate medium might be used. Prepare a mixture having the same composition as that of the Fluid Thioglycollate Medium, but omitting the agar and the resazurin sodium solution. Sterilize as directed above. The pH after sterilization is 7.1 ± 0.2. Heat in a water bath prior to use and incubate at 30 –35 under anaerobic conditions.
硫乙醇酸盐流体培养基应在30~35℃条件下进行培养。含有汞制剂防腐剂的产品不能使用膜过滤方法检测。经需气菌、厌氧菌、真菌促生长试验验证,在20~25℃培养时,大豆酪蛋白消化培养基可以替代硫乙醇酸盐流体培养基。经合理授权,配制的与硫乙醇酸盐流体培养基成分相同,但省略了琼脂和刃天青溶液的培养基,可以替代硫乙醇酸盐流体培养基使用。按上述方法灭菌,灭菌后pH值为pH:7.1±0.2。使用前用水浴加热,置于30~35℃厌氧条件下培养。
pH after sterilization: 7.3±0.2.
Dissolve the solids in the Purified Water, heating slightly to effect a solution. Cool the solution to room temperature, and adjust the pH with 1 N sodium hydroxide so that, after sterilization, it will have a pH of 7.3 ±0.2. Filter, if necessary to clarify, dispense into suitable containers, and sterilize using a validated procedure. Store at a temperature between 2° and 25°in a sterile well-closed container, unless it is intended for immediate use. Do not use the medium for a longer storage period than has been validated.
将固体物质置纯化水中,轻微加热使其溶解。溶液放凉至室温,并用1mol/L氢氧化钠溶液调整pH值,使其灭菌后pH值为7.1±0.2。如需要使之澄清,则过滤,分装入适合的容器,并用经过验证的程序灭菌。如果不立刻使用,则保存在2~25℃无菌且密闭良好的容器中。灭菌后培养基存放时间超过验证期限时,不得使用。
Soybean–Casein Digest Medium is to be incubated at 22.5° ± 2.5°.
大豆-酪胨消化物培养基在22.5℃±2.5℃条件下培养。
◆Media for Penicillins or Cephalosporins
用于青霉素和头孢菌素的培养基
Where sterility test media are to be used in the Direct Inoculation of the Culture Medium method under Test for Sterility of the Product to be Examined, modify the preparation of Fluid Pancreatic Digest of Casein 17.0 g Papaic Digest of Soybean Meal 3.0 g Sodium Chloride 5.0 g Dibasic Potassium Phosphate 2.5 g Dextrose Monohydrate/Anhydrous 2.5/2.3 g Purified Water 1000 mL Thioglycollate Medium and the Soybean–Casein Digest Medium as follows. To the containers of each medium, transfer aseptically a quantity of -lactamase sufficient to inactivate the amount of antibiotic in the specimen under test. Determine the quantity of -lactamase required to inactivate the antibiotic by using a -lactamase preparation that has been assayed previously for its penicillin- or cephalosporin-inactivating power. [NOTE—Supplemented -lactamase media can also be used in the membrane filtration test. ]
当无菌检查培养基用于供试产品无菌检查项下的直接接种法检验时,按如下内容变更硫乙醇酸盐流体培养基和大豆-酪胨培养基的制备方法。向每一种培养基的容器中,以无菌操作转移足够量能灭活供试样品中抗生素的β-内酰胺酶(之前已经对β-内酰胺酶制品灭活青霉素或头孢菌素的能力进行过测定,据此来确定灭活抗生素所需的β-内酰胺酶量)。(注:添加β-内酰胺酶的培养基也可以用于膜过滤试验)。
Alternatively (in an area completely separate from that used for sterility testing),
confirm that an appropriate amount of -lactamase is incorporated into the medium, following either method under Method Suitability Test, using less than 100 colony-forming units (cfu) of Staphylococcus aureus (see Table 1) as the challenge. Typical microbial growth of the inoculated culture must be observed as a confirmation that the -lactamase concentration is appropriate.◆
或者(在与无菌试验完全隔离的区域),按照验证试验项下的任意一种方法,使用少于100cfu的金黄色葡萄球菌(表1)作为验证菌,来确认该培养基中含有的β-内酰胺酶量是否适宜。必须观测到接种后培养物中出现典型的微生物生长,才能确认β-内酰胺酶量是适宜的。
生物是普通拟杆菌。
The media used comply with the following tests, carried out before, or in parallel, with the test on the product to be examined.
所使用的培养基须符合下列试验,这些试验应在供试产品检验前完成或者同时进行。
Sterility
培养基无菌性
Incubate portions of the media for 14 days. No growth of microorganisms occurs.
取部分检验用培养基,连续培养14天,应不得出现微生物生长。
Growth Promotion Test of Aerobes, Anaerobes, and Fungi
需气菌、厌氧菌和真菌的促生长试验
Test each lot of ready-prepared medium and each batch of medium prepared either from dehydrated medium or from ingredients. Suitable strains of microorganisms are indicated in Table 1.
每一批配制好的培养基(采用脱水培养基或按配方制备的培养基)均需进行检查,使用的微生物菌株见表1。
Inoculate portions of Fluid Thioglycollate Medium with a small number (not more than 100 cfu) of the following microorganisms, using a separate portion of medium for each of the following species of microorganism: Clostridium sporogenes, Pseudomonas aeruginosa, and Staphylococcus aureus. Inoculate portions of alternative thioglycollate medium with a small number (not more than 100 cfu) of Clostridium sporogenes. Inoculate portions of Soybean–Casein Digest Medium with a small number (not more than 100 cfu) of the following microorganisms, using a separate portion of medium for each of the following species of microorganism: Aspergillus brasiliensis, Bacillus subtilis, and Candida albicans. Incubate for not more than 3 days in the case of bacteria and not more than 5 days in the case of fungi. Seed lot culture maintenance techniques (seed-lot systems) are used so that the viable microorganisms used for inoculation are not more than five passages removed from the original master seed-lot.
去取部分硫乙醇酸盐流体培养基,接种少量(不超过100cfu)下列微生物,每一种微生物均分别接种于单独培养基中:生孢梭菌、铜绿假单胞菌、金黄色葡萄球菌(在替代硫乙醇酸盐的流体培养基中接种少量(不超过100cfu)生孢梭菌)。在大豆-酪胨消化物培养基中接种少量(不超过100cfu)下列微生物,每一种微生物均接种于单独的培养基:黑曲霉、枯草芽孢杆菌、白色念珠菌。细菌培养时间不超过3天,真菌培养时间不超过5天。采用适宜的菌种保藏技术,以确保用于接种的微生物的传代次数不超过5代。
The media are suitable if a clearly visible growth of the microorganisms occurs.如果可见清晰的微生物生长,则该培养基是符合要求的。
DILUTING AND RINSING FLUIDS FOR MEMBRANE FILTRATION
用于膜过滤的稀释剂和冲洗液
Fluid A
稀释剂A
PREPARATION
制备
Dissolve 1 g of peptic digest of animal tissue in water to make 1 L, filter or centrifuge to clarify, if necessary, and adjust to a pH of 7.1 ± 0.2. Dispense into containers, and sterilize using a validated process.
将1g动物组织胃蛋白酶消化物溶于1L水中,如果需要则通过过滤或离心使其澄清,再调节pH值至7.1±0.2。分装入容器中,并用经过验证的工艺灭菌。
PREPARATION FOR PENICILLINS OR CEPHALOSPORINS
用于青霉素或头孢菌素的稀释剂制备
Aseptically add to the above Preparation, if necessary, a quantity of sterile -lactamase sufficient to inactivate any residual antibiotic activity on the membranes after the solution of the test specimen has been filtered (see Media for Penicillins or Cephalosporins).
在供试样品溶液已经过滤之后,如果需要,向上述制备的稀释剂中,以无菌操作加入数量足够灭活滤膜上残余抗生素的β-内酰胺酶(见用于青霉素或头孢菌素的培养基)。
Fluid D
稀释剂D
To each L of Fluid A add 1 mL of polysorbate 80, adjust to a pH of 7.1 ± 0.2, dispense into containers, and sterilize using a validated process. Use this fluid for articles containing lecithin or oil, or for devices labeled as ―sterilepathway.‖向每升稀释剂A中,加入1mL聚山梨酯80,调节pH值至7.1±0.2,分装入容器中,并使用经过验证的工艺灭菌。此液体用于含有卵磷脂或油脂的样品,或用于标为“无菌通道”的器械。
Fluid K
稀释剂K
Dissolve 5.0 g of peptic digest of animal tissue, 3.0 g of beef extract, and 10.0 g of polysorbate 80 in water to make 1 L. Adjust the pH to obtain, after sterilization, a pH of 6.9 ± 0.2. Dispense into containers, and sterilize using a validated process.
将5.0g动物组织胃蛋白酶消化物、3.0牛肉提取物、10.0g聚山梨酯80溶解于1L水中。调节pH值,使其灭菌后pH值为6.9±0.2。分装入容器中,并使用经过验证的工艺灭菌。
METHOD SUITABILITY TEST
方法适用性试验
Carry out a test as described below under Test for Sterility of the Product to be Examined using exactly the same methods, except for the following
modifications.
严格按照供试产品无菌检查项下的方法进行无菌检查。当使用到以下方法并需要进行方法调整时,需重新进行方法适用性实验。
Membrane Filtration
膜过滤法
After transferring the content of the container or containers to be tested to the membrane, add an inoculum of a small number of viable microorganisms (not more than 100 cfu) to the final portion of sterile diluent used to rinse the filter. 在将一个或多个供试容器中的内容物转移到滤膜之后,在最后一次的冲洗液中加入少量(不超过100cfu)测试菌株。
Direct Inoculation
直接接种法
After transferring the contents of the container or containers to be tested (for catgut and other surgical sutures for veterinary use: strands) to the culture medium, add an inoculum of a small number of viable microorganisms (not more than 100 cfu) to the medium.
在将一个或多个供试容器(对于兽医用的肠线和其他外科缝合用线:若干股线)中的内容物转移至培养基之后,将少量试验菌(不超过100cfu)加入培养基中。In both cases use the same microorganisms as those described above under Growth Promotion Test of Aerobes, Anaerobes, and Fungi. Perform a growth promotion test as a positive control. Incubate all the containers containing medium for not more than 5 days.
以上两种情况,均使用上述需气菌、厌氧菌、真菌促生长试验项下规定的菌株。同时设置促生长试验作为阳性对照。微生物在相应培养基中的培养时间不超过5天。
If clearly visible growth of microorganisms is obtained after the incubation, visually comparable to that in the control vessel without product, either the product possesses no antimicrobial activity under the conditions of the test or such activity has been satisfactorily eliminated. The test for sterility may then be carried out without further modification.
若培养后可见清晰的微生物生长,外观与未接种样品的对照菌生长类似,则认为该产品在此试验条件下没有抑菌作用,或者认为可能存在的抑菌作用已经被较完全的消除。此时可以认为该方法无需进一步的变更,无菌试验依法检查即可。
If clearly visible growth is not obtained in the presence of the product to be tested, visually comparable to that in the control vessels without product, the product possesses antimicrobial activity that has not been satisfactorily eliminated under the conditions of the test. Modify the conditions in order to eliminate the antimicrobial activity, and repeat the Method Suitability Test.
与未接种样品的对照相比,若在供试品存在条件下不能观察到肉眼可见的混浊,则认为该试验条件下不能消除供试品的抑菌作用。需要对实验条件进行调整以消除抗菌活性,并重新进行方法适用性试验。
This method suitability is performed (a) when the test for sterility has to be
carried out on a new product; and (b) whenever there is a change in the experimental conditions of the test. The method suitability may be performed simultaneously with the Test for Sterility of the Product to be Examined.
当一个新产品进行无菌试验时和无论何时无菌试验的试验条件发生改变时,则需进行此验证试验。该验证可以与供试产品无菌检查同时进行。
TEST FOR STERILITY OF THE PRODUCT TO BE EXAMINED
供试产品无菌检查
Number of Articles to Be Tested
供试品数量
Unless otherwise specified elsewhere in this chapter or in the individual monograph, test the number of articles specified in Table 3. If the contents of each article are of sufficient quantity (see Table 2), they may be divided so that equal appropriate portions are added to each of the specified media. [NOTE—Perform sterility testing employing two or more of the specified media. ] If each article does not contain sufficient quantities for each medium, use twice the number of articles indicated in Table 3.
除非在本章节的其他部分或在具体的各论中另有规定,供试物品的数量遵照表3中的规定。如果每个被检验物品的内容物有足够数量(见表2),可以将其分成若干等份,将适当的等份加入到每个指定的培养基。(注:使用两个或更多指定培养基,来进行无菌试验。)如果每个被检验物品的内容物规格不能满足单个培养基的接种量要求时,检验量扩大为表3所规定的检验数量的2倍。
②如果一个容器的内容物足够接种2个培养基,则此表格给出的容器数量为用于全部2个培养基的数量。
The test may be carried out using the technique of Membrane Filtration or by Direct Inoculation of the Culture Medium with the product to be examined. Appropriate negative controls are included. The technique of membrane filtration is used whenever the nature of the product permits; that is, for filterable aqueous preparations, for alcoholic or oily preparations, and for preparations miscible with, or soluble in, aqueous or oily solvents, provided these solvents do not have an antimicrobial effect in the conditions of the test.无菌检验可以使用膜过滤法或培养基直接接种法进行,应设置适宜的阴性对照。但只要该产品的性质许可,就应使用膜过滤法,也就是说,对水溶性制剂、酒精或油性制剂、易混合或溶解于水或油性溶剂的制剂,即使没有抑菌作用,也均应尽量采用薄膜过滤法进行无菌检查。
Membrane Filtration
膜过滤法
Use membrane filters having a nominal pore size not greater than 0.45 μm, in which the effectiveness to retain microorganisms has been established. Cellulose nitrate filters, for example, are used for aqueous, oily, and weakly alcoholic solutions; and cellulose acetate filters, for example, are used for strongly alcoholic solutions. Specially adapted filters may be needed for certain products (e.g., for antibiotics).
使用标称孔径不大于0.45μm的膜过滤器,此孔径已知能够有效截留微生物。例如,硝酸纤维素过滤器可用于水、油、烯醇溶液;而醋酸纤维素可用于浓醇溶液。特定产品(例如抗生素)可能需要特别改造过的特殊过滤器。
The technique described below assumes that membranes about 50 mm in diameter will be used. If filters of a different diameter are used, the volumes of the dilutions and the washings should be adjusted accordingly. The filtration apparatus and membrane are sterilized by appropriate means. The apparatus is designed so that the solution to be examined can be introduced and filtered under aseptic conditions: it permits the aseptic removal of the membrane for transfer to the medium, or it is suitable for carrying out the incubation after adding the medium to the apparatus itself.
以下涉及的方法中所使用的均为直径约50mm的滤膜。如果使用不同直径的过滤器,稀释液和冲洗液的体积应当做相应调节。过滤器和滤膜都应该经过灭菌处理。过滤器应该满足在无菌条件下可以灌注并过滤供试溶液的要求:使得在无菌状态下摘掉滤膜,并将其转移至培养基中成为可能;或者应满足将培养基灌注至该设备中,进行培养的要求。
AQUEOUS SOLUTIONS
水溶性溶液
If appropriate, transfer a small quantity of a suitable, sterile diluent such as Fluid A (see Diluting and Rinsing Fluids for Membrane Filtration) onto the membrane in the apparatus and filter. The diluent may contain suitable neutralizing substances and/or appropriate inactivating substances, for example, in the case of antibiotics.
应当尽量减少转移至过滤器滤膜上的无菌稀释剂,例如稀释剂A(见用于膜过滤的稀释剂和冲洗液)。稀释剂中可能会含有一定量的中和物质或灭活物质,例如对抗生素的检测。
Transfer the contents of the container or containers to be tested to the membrane or membranes, if necessary, after diluting to the volume used in the Method Suitability Test with the chosen sterile diluent, but using not less than the quantities of the product to be examined prescribed in Tables 2 and 3. Filter immediately. If the product has antimicrobial properties, wash the membrane not less than three times by filtering through it each time the volume of the chosen sterile diluent used in the Method Suitability Test. Do not exceed a washing cycle of five times 100 mL per filter, even if during method suitability it has been demonstrated that such a cycle does not fully eliminate the antimicrobial activity. Transfer the whole membrane to the culture medium or cut it aseptically into two equal parts, and transfer one half to each of two suitable media. Use the same volume of each medium as in the Method Suitability Test. Alternatively, transfer the medium onto the membrane in the apparatus. Incubate the media for not less than 14 days.
将一个或多个供试容器的内容物转移到滤膜,并立即滤过。如需要可先用选定的无菌稀释剂稀释至方法适用性试验中所用的体积,但须使用不少于表2和表3中规定的供试品数量。如果该产品具有抗菌活性,滤膜至少冲洗3次,每次冲洗量均按照方法适用性试验中确定的无菌稀释剂体积冲洗滤膜。冲洗过程通常不应该超过“5次,100mL/次”的冲洗循环,即便该冲洗循环不能完全消除抗菌活性。将整个滤膜转移至培养基,或以无菌操作将滤膜切至相等的2部分,并将每
一部分转移至适当的培养基中。每个培养基的体积按照方法适用性试验所确定的用量。或者,将培养基转移至带有滤膜的滤器中。培养该培养基,不少于14天。
SOLUBLE SOLIDS
可溶性固体
Use for each medium not less than the quantity prescribed in Tables 2 and 3 of the product dissolved in a suitable solvent, such as the solvent provided with the preparation, Sterile Water for Injection, sterile saline, or a suitable sterile solution such as Fluid A (Diluting and Rinsing Fluids for Membrane Filtration), and proceed with the test as described above for Aqueous Solutions using a membrane appropriate to the chosen solvent.
在每个培养基中,使用不少于表2和表3规定的产品数量溶于适当溶剂,例如稀释剂A(用于膜过滤的稀释剂和冲洗液),并按照上述关于水性溶液样品实验操作的要求,使用适合所选溶液的滤膜,进行试验。
OILS AND OILY SOLUTIONS
油和油性溶液
Use for each medium not less than the quantity of the product prescribed in Tables 2 and 3. Oils and oily solutions of sufficiently low viscosity may be filtered without dilution through a dry membrane. Viscous oils may be diluted as necessary with a suitable sterile diluent such as isopropyl myristate shown not to have antimicrobial activity in the conditions of the test. Allow the oil to penetrate the membrane by its own weight, and then filter, applying the pressure or suction gradually. Wash the membrane at least three times by filtering through it each time about 100 mL of a suitable sterile solution such as Fluid A (see Diluting and Rinsing Fluids for Membrane Filtration) containing a suitable emulsifying agent at a concentration shown to be appropriate in
the Method Suitability Test, for example polysorbate 80 at a concentration of 10 g per L (Fluid K) . Transfer the membrane or membranes to the culture medium or media, or vice versa, as described above for Aqueous Solutions, and incubate at the same temperatures and for the same times.
在每个培养基中,使用不少于表2和表3中规定的检验量。黏性较低的油和油性溶液可在不经稀释的情况下滤过干燥滤膜。黏稠油质可以用适合的无菌稀释剂进行稀释,如已证实在该试验条件下不具有抗菌活性的豆蔻酸异丙酯。油质依靠其自身的重量穿过滤膜,然后逐渐加压或抽吸过滤。使用适宜的无菌冲洗液冲洗滤膜,至少冲洗3次,约100mL/次,例如使用一定浓度乳化剂的稀释剂A(参见用于膜过滤的稀释剂和冲洗液)。通过方法适用性试验确定乳化剂的适宜浓度,例如浓度为每升10克的聚山梨酯80(稀释剂K)。将一个或多个滤膜转移到一个或多个培养基;或者也可以参照关于水性溶液的相关描述进行操作,将培养基在上述规定的温度下培养相同时间。
OINTMENTS AND CREAMS
油膏和乳膏
Use for each medium not less than the quantities of the product prescribed in
Tables 2 and 3. Ointments in a fatty base and emulsions of the water-in-oil type may be diluted to 1% in isopropyl myristate as described above, by heating, if necessary, to not more than 40°. In exceptional cases it may be necessary to heat to not more than 44°. Filter as rapidly as possible, and proceed as described above for Oils and Oily Solutions.
在每个培养基中,使用不少于表2和表3中规定的样品量。参照上文,脂肪状的油膏和油包水的乳液可用豆蔻酸异丙酯稀释至1%浓度,如需要可加热,但不超过40℃。在特殊情况下,加热温度可适当增加但不超过44℃。供试液制备好后尽快过滤,并参照油和油性溶液的要求进行试验。
PREFILLED SYRINGES
预装填的注射器
For prefilled syringes without attached sterile needles, expel the contents of each syringe into one or two separate membrane filter funnels or into separate pooling vessels prior to transfer. If a separate sterile needle is attached, directly expel the syringe contents as indicated above, and proceed as directed for Aqueous Solutions. Test the sterility of the needle, using Direct Inoculation under Method Suitability Test.
对于没有附无菌针头的预装填注射器,在转移之前,将每个注射器的内容物排出一个或两个单独的膜过滤器漏斗,或独立并联的容器。如果附有单独的灭菌针头,直接按照上面的规定将注射器内容物直接排出,并按照关于水性溶液的规定进行试验。参照方法适用性试验项下的直接接种法,检查针头的无菌情况。
SOLIDS FOR INJECTION OTHER THAN ANTIBIOTICS
非抗生素注射用固体
Constitute the test articles as directed on the label, and proceed as directed for Aqueous Solutions or Oils and Oily Solutions, whichever applies. [NOTE—If necessary, excess diluent can be added to aid in the constitution and filtration of the constituted test article. ]
按照其标签上的规定配制供试物品,并按照适用的关于水性溶液或油和油性溶液的规定继续进行。(注:如需要,可以加入额外的稀释剂以帮助对已配制的供试物品进行再配制和过滤)。
ANTIBIOTIC SOLIDS FOR INJECTION
注射用抗生素固体
Pharmacy Bulk Packages, <5 g—From each of 20 containers, aseptically transfer about 300 mg of solids, into a sterile 500-mL conical flask, dissolve in about 200 mL of Fluid A (see Diluting and Rinsing Fluids for Membrane Filtration), and mix; or constitute, as directed in the labeling, each of 20 containers and transfer a quantity of liquid or suspension, equivalent to about 300 mg of solids, into a sterile 500-mL conical flask, dissolve in about 200 mL of Fluid A, and mix. Proceed as directed for Aqueous Solutions or Oils and Oily Solutions, whichever applies.
原料包装,<5g 取20个容器,无菌操作从每个容器中取约300mg固体至500mL
无菌锥形瓶,使用约200mL稀释剂A溶解并混匀(参见用于膜过滤的稀释剂和冲洗液),或取20个容器,每个容器均按照标签上的规定配制,并从每个容器中将相当于300mg固体的液体转移至500mL无菌锥形瓶,使用约200mL稀释剂A溶解并混匀。按照适合的关于水性溶液或油和油性溶液的规定,继续操作。Pharmacy Bulk Packages, ≥5 g—From each of 6 containers, aseptically transfer about 1 g of solids into a sterile 500-mL conical flask, dissolve in about 200 mL of Fluid A, and mix; or constitute, as directed in the labeling, each of 6 containers and transfer a quantity of liquid, equivalent to about 1 g of solids, into a sterile 500-mL conical flask, dissolve in about 200 mL of Fluid A, and mix. Proceed as directed for Aqueous Solutions.
原料包装,≥5g 取6个容器,无菌操作从每个容器中取约1g固体转移至500mL 无菌锥形瓶,使用约200mL稀释剂A溶解并混匀;或取6个容器,每个均按照标签的规定配制,将相当于1g固体的液体转移至500mL无菌锥形瓶,使用约200mL 稀释剂A溶解并混匀。按照水性溶液的规定,继续操作。
ANTIBIOTIC SOLIDS, BULKS, AND BLENDS
抗生素固体、原料及预混物
Aseptically remove a sufficient quantity of solids from the appropriate amount of containers (see Table 2), mix to obtain a composite, equivalent to about 6 g of solids, and transfer to a sterile 500-mL conical flask. Dissolve in about 200 mL of Fluid A, and mix. Proceed as directed for Aqueous Solutions.
无菌操作从适当数量的容器中(见表2)分别取出足够数量的固体,混匀得6g 固体的混合物,至500mL无菌锥形瓶。使用约200mL稀释剂A溶解并混匀。按照水性溶液的规定,继续操作。
STERILE AEROSOL PRODUCTS
无菌气(喷)雾剂产品
For fluid products in pressurized aerosol form, freeze the containers in an alcohol-dry ice mixture at least at –20 for about 1 hour. If feasible, allow the propellant to escape before aseptically opening the container, and transfer the contents to a sterile pooling vessel. Add 100 mL of Fluid D to the pooling vessel, and mix gently. Proceed as directed for Aqueous Solutions or Oils and Oily Solutions, whichever applies.
对于以加压气(喷)雾剂形式存在的液体产品,在大约-20℃的“酒精-干冰”混合物中冷冻约1小时。若可行,在以无菌操作打开容器之前去除抛射剂,并将内容物转移至一个无菌的容器。向容器中加入100mL稀释剂D,并轻轻混匀。并根据情况,按照水性溶液或油和油性溶液的规定继续操作。
DEVICES WITH PATHWAYS LABELED STERILE
标示为无菌的具有管道的医疗器械
Aseptically pass not less than 10 pathway volumes of Fluid D through each device tested. Collect the fluids in an appropriate sterile vessel, and proceed as directed for Aqueous Solutions or Oils and Oily Solutions, whichever applies.
以无菌操作使用稀释剂D冲洗每个器械样本,并且冲洗量不少于器械管道体积的
10倍。在适当的无菌容器中收集冲洗液,并根据情况,按照水性溶液或油和油性溶液的规定继续操作。
In the case of sterile, empty syringes, draw sterile diluent into the barrel through the sterile needle, if attached, or through a sterile needle attached for the purpose of the test, and express the contents into a sterile pooling vessel. Proceed as directed above.
对于无菌的空注射器,如果附有无菌针头,可直接将无菌稀释剂吸取至注射器,或者使用一个专为此试验准备的无菌针头吸取稀释剂,并将内容物压出至合并容器中。按照上述内容继续进行。
Direct Inoculation of the Culture Medium
培养基的直接接种法
Transfer the quantity of the preparation to be examined prescribed in Tables 2 and 3 directly into the culture medium so that the volume of the product is not more than 10% of the volume of the medium, unless otherwise prescribed.
按照表2和表3规定的供试品取样量,将该供试品直接转移到培养基中,除另有规定外,接种量不得超过培养基体积的10%。
If the product to be examined has antimicrobial activity, carry out the test after neutralizing this with a suitable neutralizing substance or by dilution in a sufficient quantity of culture medium. When it is necessary to use a large volume of the product, it may be preferable to use a concentrated culture medium prepared in such a way that it takes into account the subsequent dilution. Where appropriate, the concentrated medium may be added directly to the product in its container.
如果供试品具有抗菌活性,使用适宜的中和剂或采用培养基稀释法消除其抗菌活性后,再进行该试验。当供试品体积较大时,最后配制使用浓缩培养基,以便后续的稀释需要。若条件适宜,浓缩培养基可以直接加入到装有产品的容器中。
OILY LIQUIDS
油性液体
Use media to which have been added a suitable emulsifying agent at a concentration shown to be appropriate in the Method Suitability Test, for example polysorbate 80 at a concentration of 10 g per L.
使用已经加入了适当乳化剂的培养基,乳化剂浓度需经方法适用性试验证明适用于该试验,例如浓度为每升10g的聚山梨酯80。
OINTMENTS AND CREAMS
油膏和乳膏
Prepare by diluting to about 1 in 10 by emulsifying with the chosen emulsifying agent in a suitable sterile diluent such as Fluid A (see Diluting and Rinsing Fluids for Membrane Filtration). Transfer the diluted product to a medium not containing an emulsifying agent.
按照1:10比例将选定的乳化剂混匀在适当的无菌稀释液中,例如稀释剂A(见用于膜过滤的稀释剂和冲洗液),以含有乳化剂的稀释液来制备供试液。将供试
液转移至不含乳化剂的培养基中。
Incubate the inoculated media for not less than 14 days. Observe the cultures several times during the incubation period. Shake cultures containing oily products gently each day. However, when Fluid Thioglycollate Medium is used for the detection of anaerobic microorganisms, keep shaking or mixing to a minimum in order to maintain anaerobic conditions.
接种后的培养基培养不少于14天。在培养中隔日观察。每天轻轻摇动含有油性产品的培养基。但是,当使用硫乙醇酸盐流体培养基用于检测厌氧微生物培养基时,尽量减少摇动,以维持厌氧条件。
CATGUT AND OTHER SURGICAL SUTURES FOR VETERINARIAN USE
兽医用肠线和其他外科缝合线
Use for each medium not less than the quantities of the product prescribed in Tables 2 and 3. Open the sealed package using aseptic precautions, and remove three sections of the strand for each culture medium. Carry out the test on three sections, each 30-cm long, which have been cut off from the beginning, the center, and the end of the strand. Use whole strands from freshly opened cassette packs. Transfer each section of the strand to the selected medium. Use sufficient medium to cover adequately the material to be tested (20 mL to 150 mL).
在每个培养基中,使用不少于表2和表3中所规定的检验品。无菌操作打开封闭的包装,使用从刚刚打开的包装盒取出整股线,从该线的前端、中间、末端分别截取每段30cm的产品进行试验,每种培养基均接种由该股线截取的3部分供试物料。使用充足的培养基,以充分覆盖供试物料(20~150mL)。
SOLIDS
固体
Transfer a quantity of the product in the form of a dry solid (or prepare a suspension of the product by adding sterile diluent to the immediate container), corresponding to not less than the quantity indicated in Tables 2 and 3. Transfer the material so obtained to 200 mL of Fluid Thioglycollate Medium, and mix. Similarly, transfer the same quantity to 200 mL of Soybean–Casein Digest Medium, and mix. Proceed as directed above.
转移干燥固体产品(或通过加入无菌稀释剂在中间容器中配制的该产品的悬浮液),检验量不少于表2和表3中的规定。将供试品接种至200mL硫乙醇酸盐流体培养基中,混匀。同法接种至200mL大豆-酪蛋白消化物培养基中,混匀。按照上述规定继续操作。
PURIFIED COTTON, GAUZE, SURGICAL DRESSINGS, AND RELATED ARTICLES
脱脂棉花、纱布、外科敷料及相关物品
From each package of cotton, rolled gauze bandage, or large surgical dressings being tested, aseptically remove two or more portions of 100- to 500-mg each from the innermost part of the sample. From individually packaged, single-use materials, aseptically remove the entire article. Immerse the portions or article in each medium, and proceed as directed above.
从待检的每个包装中的棉花、卷状纱布绷带、大块外科敷料中,以无菌操作从中心部位取出2个或更多份样品,每份样品100~500mg。对独立包装的一次性使用样品,以无菌操作取出整个样品。将这些取样样品或整个样品浸没在各个培养基中,继续按照上述内容操作。
STERILE DEVICES
无菌器械
Articles can be immersed intact or disassembled. To ensure that device pathways are also in contact with the media, immerse the appropriate number of units per medium in a volume of medium sufficient to immerse the device completely, and proceed as directed above. For extremely large devices, immerse those portions of the device that are to come into contact with the patient in a volume of medium sufficient to achieve complete immersion of those portions.
样品可以完整地拆开后浸没在培养中。为确保器械管道与培养基接触,使用足够体积的培养基浸没整个器械零部件,并按照上述内容继续操作。对于极大的器械,将该器械中将要与患者接触的部分,浸没到足够体积的培养基中。
For catheters where the inside lumen and outside are required to be sterile, either cut them into pieces such that the medium is in contact with the entire lumen or fill the lumen with medium, and then immerse the intact unit.
对于管道内部的腔体和外部均要求无菌的导尿管,将它们切片,这样培养基就可以接触整个腔体,或者用培养基填充腔体,然后浸没整个器械。
OBSERVATION AND INTERPRETATION OF RESULTS
结果的观察和判断
At intervals during the incubation period and at its conclusion, examine the media for macroscopic evidence of microbial growth. If the material being tested renders the medium turbid so that the presence or absence of microbial growth cannot be readily determined by visual examination, 14 days after the beginning of incubation transfer portions (each not less than 1 mL) of the medium to fresh vessels of the same medium, and then incubate the original and transfer vessels for not less than 4 days.
无菌检查在培养观察期间和下结论时,都要检查该培养基是否有肉眼可见的微生物生长。如果供试物料导致培养基混浊,但无法通过肉眼观察来确定是否存在微生物生长时,培养14天之后,将该培养基的若干部分(每个部分不少于1mL)转接种至含相同新鲜培养基的容器中,培养不少于4天。
If no evidence of microbial growth is found, the product to be examined complies with the test for sterility. If evidence of microbial growth is found, the product to be examined does not comply with the test for sterility, unless it can be clearly demonstrated that the test was invalid for causes unrelated to the product to be examined. The test may be considered invalid only if one or more of the following conditions are fulfilled:
如果未发现微生物生长,则认为该供试产品无菌检查结果合格。若发现微生物生长,除非有充分证据证实此次试验无效或微生物污染与该供试产品无关,否则认
为该供试产品无菌检查不合格。当满足下一个或多个条件时,认为检验结果阳性无效。
a. The data of the microbiological monitoring of the sterility testing facility show
a fault.
b. A review of the testing procedure used during the test in question reveals a fault.
c. Microbial growth is found in the negative controls.
d. After determination of the identity of the microorganisms isolated from the test, the growth of this species (or these species) may be ascribed unequivocally to faults with respect to the material and or the technique used in conducting the sterility test procedur
e.
①该无菌试验设施的微生物监控数据显示实验保障有缺陷。
②通过实验回顾发现实验过程中有疑问提示有实验过程缺陷。
③阳性对照中发现微生物生长。
④阳性结果中的微生物经过菌种鉴别后,确定该(或这些)微生物的生长与实验材料无关和/或与无菌检查方法有关。
If the test is declared to be invalid, it is repeated with the same number of units as in the original test. If no evidence of microbial growth is found in the repeat test, the product examined complies with the test for sterility. If microbial growth is found in the repeat test, the product examined does not comply with the test for sterility.
如果该试验证明无效,应取用与原试验相同量的供试品进行重验。若在重新试验中未发现微生物生长,则认为该产品符合无菌要求。若在重验中发现微生物生长,则该产品不合格。
APPLICATION OF THE TEST TO PARENTERAL PREPARATIONS,
OPHTHALMIC, AND OTHER NONINJECTABLE PREPARATIONS REQUIRED TO COMPLY WITH THE TEST FOR STERILITY 无菌检查在注射药品、眼科和其他必须符合无菌要求的非注射药品中的应用When using the technique of membrane filtration, use, whenever possible, the whole contents of the container, but not less than the quantities indicated in Table 2, diluting where necessary to about 100 mL with a suitable sterile solution, such as Fluid A (see Diluting and Rinsing Fluids for Membrane Filtration).
当使用膜过滤法时,只要可能,就要使用包装容器的全部内容物,但不少于表2中规定的检验量,必要时以适当的无菌溶液将供试品稀释至约100mL,例如液体A(参见用于膜过滤的稀释剂和冲洗液)。
When using the technique of direct inoculation of media, use the quantities shown in Table 2, unless otherwise justified and authorized. The tests for bacterial and fungal sterility are carried out on the same sample of the product to be examined. When the volume or the quantity in a single container is insufficient to carry out the tests, the contents of two or more containers are used to inoculate the different media.
当使用直接接种法时,除非另有证据或授权,使用表2中规定的检验量。可用同
Hamlet经典独白to be, or not to be英汉对照及解析讲解学习
精品文档 精品文档 Hamlet (Act 3, Scene 1, lines 55-86) To be, or not to be: that is the question, Whether'tis nobler in the mind to suffer The slings and arrows of outrageous fortune, Or to take arms against a sea of troubles, And by opposing end them. To die, to sleep; No more; and by a sleep to say we end The heartache, and the thousand natural shocks That flesh is heir to, 'tis a consummation Devoutly to be wished. To die, to sleep. To sleep, perchance to dream: ay, there's the rub; For in that sleep of death what dreams may come When we have shuffled off this mortal coil, Must give us pause. There's the respect That makes calamity of so long life; For who would bear the whips and scorns of time, Th' oppressor's wrong, the proud man's contumely, The pangs of despised love, the law's delay, The insolence of office, and the spurns That patient merit of th'unworthy takes, When he himself might his quietus make With a bare bodkin? Who would fardels bear, To grunt and sweat under a weary life, But that the dread of something after death, The undiscovered country from whose bourn No traveller returns, puzzles the will, And makes us rather bear those ills we have Than fly to others that we know not of? Thus conscience does make cowards of us all, And thus the native hue of resolution Is sicklied o'er with the pale cast of thought, And enterprises of great pith and moment With this regard their currents turn awry And lose the name of action. 生存还是毁灭,这是一个值得考虑的问题;默然忍受命运暴虐的毒箭,或是挺身反抗人世无涯的苦难,这两种行为,哪一种更加高尚?死了;睡着了;什么都完了;要是在这一种睡眠之中,我们心头的创痛,以及其他无数血肉之躯所不能避免的打击,都可以从此消失,那正是我们求之不得的结局。死了;睡着了;睡着了也许还会做梦;嗯,阻碍就在这儿:因为当我们摆脱了这一具朽腐的皮囊以后,在那死的睡眠里,究竟将要做些什么梦,那不能不使我们踌躇顾虑。人们甘心久困于患难之中,也就是为了这个缘故;谁愿意忍受人世的鞭挞和讥嘲、压迫者的凌辱、傲慢者的冷眼、被轻蔑的爱情的惨痛、法律的迁延、官吏的横暴和费尽辛勤所换来的小人的鄙视,要是他只要用一柄小小的刀子,就可以清算他自己的一生?谁愿意负着这样的重担,在烦劳的生命的压迫下呻吟流汗,倘不是因为惧怕不可知的死后,惧怕那从来不曾有一个旅人回来过的神秘之国,是它迷惑了我们的意志,使我们宁愿忍受目前的磨折,不敢向我们所不知道的痛 苦飞去?这样,重重的顾虑使我们全变成了懦夫,决心的赤热的光彩,被审慎的思维盖上了一层灰色,伟大的事业在这一种考虑之下,也会逆流而退,失去了行动的意义。 ——《哈姆雷特》(英·William Shakespeare )中的经典独白soliloquy [s ?'l ?l ?kw ?] This is an internal philosophical debate on the advantages and disadvantages of existence, and whether it is one ’s right to end his/her own life.
哈姆雷特经典台词对白爱情电影英文独白赏析
哈姆雷特经典台词对白爱情电影英文独白赏析经典台词一 To be, or not to be:that is the question: Whether 'tis nobler in the mind to suffer The slings and arrows of outrageous fortune, Or to take arms against a sea of troubles, And by opposing end them? To die: to sleep; No more; and by a sleep to say we end The heart-ache and thousand natural shocks The flesh is heir to, 'tis a consummation Devoutly to be wish'd. To die, to sleep; To sleep; perchance to dream: ay, there's the rub; For in that sleep of death what dreams may come When we have shuffled off this mortal coil, Must guve pause: there's the respect That makes calamity of so long life; For who would bear the whips and scorns of time, The oppressor's wrong, the proud man's delay, The insolence of office and the spurns That patient merit of the unworthy takes, 经典台词二
无菌检查法-中国药典2010第三部-附录XIIA
附录XII A 无菌检查法 无菌检查法系用于检查药典要求无菌的生物制品、医疗器具、原料、辅料及其他品种是否无菌的一种方法。若供试品符合无菌检查法的规定,仅表明了供试品在该检验条件下未发现微生物污染。 无菌检查应在洁净度万级下的局部洁净度百级的单向流空气区域内或隔离系统中进行,其全过程应严格遵守无菌操作,防止微生物污染,防止污染的措施不得影响供试品中微生物的检出。单向流空气区、工作台面及环境应定期按《医药工业洁净室(区)悬浮粒子、浮游菌和沉降菌的测试方法》的现行国家标准进行洁净度验证。隔离系统应按相关的要求进行验证,其内部环境的洁净度须符合无菌检查的要求。日常检验还需对试验环境进行监控。 无菌检查人员必须具备微生物专业知识,并经过无菌技术的培训。 培养基 培养基的制备:培养基按以下处方制备,亦可用按该处方生产的符合规定的脱水培养基。配制后应采用验证合格的灭菌程序灭菌。制备好的培养基应保存在2~25℃避光的环境,若保存于非密闭容器中,一般可在3周内使用;若保存于密闭容器中,一般可在1年内使用。 1、硫乙醇酸盐流体培养基 酪胨(胰酶水解)15.0g 酵母浸出粉 5.0g 葡萄糖 5.0g 氯化钠 2.5g L-胱氨酸0.5g 新配制的0.1%刃天青溶液 1.0mL 硫乙醇酸钠0.5g (或硫乙醇酸0.3mL) 琼脂0.75g 水1000mL 除葡萄糖和刃天青溶液外,取上述成分混合,微温溶解,调pH值为弱碱性,煮沸,滤清,加入水葡萄糖和刃天青溶液,摇匀,调pH值使灭菌后为7.1±0.2。分装至适宜的容器中,其装量与容器高度的比例应符合培养结束后培养基氧化层(粉红色)不超过培养基深度的1/2,灭菌。在供试品接种前,培养基氧化层的高度不得超过培养基深度的1/5,否则,须经1000℃水浴加热至粉红色消失(不超过20分钟)后,迅速冷却,只限加热一次,并防止被污染。 2、改良马丁培养基 胨 5.0g 磷酸氢二钾 1.0g 酵母浸出粉 2.0g 硫酸镁0.5g 葡萄糖20.0g 水1000mL 除葡萄糖外,取上述成分混合,微温溶解,调pH值约为6.8,煮沸;加入putaotang 溶解后,摇匀,滤清,调pH值使灭菌后为6.4±0.2,分装,灭菌。 3、选择性培养基 按上述硫乙醇盐流体培养基或改良马丁培养基的处方及制法,在培养基灭菌或使用前加入适宜的中和剂、灭活剂或表面活性剂,其用量同方法验证试验。 4、营养肉汤培养基 胨10.0g 氯化钠 5.0g 牛肉浸出粉 3.0g 水1000mL 取上述成分混合,微温溶解,调pH值为弱碱性,煮沸,滤清,调pH值使灭菌后为7.2±0.2,分装,灭菌。 5、营养琼脂培养基 按上述营养肉汤培养基的处方及制法,加入14.0g琼脂,调pH值使灭菌后为7.2±0.2,分装,灭菌。
无菌检查方法学验证
无菌检查方法学验证 1. 概述 将规定量的供试品按无菌检查法中的薄膜过滤法进行操作,并接种小于100cfu 的试验菌,同时设置阳性对照,按规定温度培养3~5 天,与各相应的阳性对照管比较: 如含供试品各管中的试验菌均生长良好,则供试品的该检验量在该检验条件下无抑菌作用,可按此法进行供试品的无菌检查;如含供试品的任一管中的试验菌生长微弱、缓慢或不生长,则说明供试品的该检验量在该检验条件下有抑菌作用,应采用增加冲洗量或使用中和剂等方法消除供试品的抑菌作用,并再重新进行验证试验。 2. 验证前提条件 供试品的选择原则:相同配方,不同规格的制品,选择其中装量最大的制品进行验证。种类相同,含量不同的制品,选择含量最高的制品进行验证。照此原则,多规格制 品按下表选择供试品进行验证。 3. 验证方法 3.1菌液制备 3.1.1细菌菌液制备程序 ①取金黄色葡萄球菌、枯草芽抱杆菌、铜绿假单胞菌的冻干菌种管各一支,分别在无菌操作下打开,以毛细吸管加入适量营养肉汤,轻柔吹吸数次,使菌种融化分散后,吸出滴入营养琼脂斜面上,使均匀分布,置30~35C培养18h~24h。用接种环取适量第1代培养菌苔,划线接种于营养琼脂培养基斜面上,于30~35C培养18h~24h o 同法操作,培养得第3代培养物。 ②取生抱梭菌的冻干菌种管一支,在无菌操作下打开,以毛细吸管加入适量营养 肉汤,轻柔吹吸数次,使菌种融化分散后,吸出滴入胃酶肉肝疱斜面上,使均匀分布,置30~35C培养18h~24h o用接种环取适量第1代培养菌苔,划线接种于胃酶肉肝疱斜
面上,于30~ 35°C培养18h~24h。取生抱梭菌的第二代新鲜培养物至硫乙醇酸盐流 体培养基中,30~35C培养18h~24h,得第3代培养物。 ③分别取金黄色葡萄球菌、枯草芽抱杆菌、铜绿假单胞菌的第3代营养琼脂斜面新鲜培养物,用5.0ml吸管吸取3.0ml~5.0ml稀释液加入斜面试管内,反复吹吸,洗下菌苔。随后用5.0ml吸管将洗液移至另一无菌试管中,用电动混合器混合20s或在手掌上振敲80次,以使细菌悬浮均匀,得初步菌悬液。 ④取初步制成的上述菌悬液和生抱梭菌第3代新鲜培养物,用0.9%无菌氯化钠溶液稀释至不高于标准比浊管的浓度,即得每1ml含菌数小于100cfu验证细菌菌液。 3.1.2抱子悬液制备程序 ①取白色念珠菌和黑曲霉的冻干菌种管各一支,分别在无菌操作下打开,以毛细 吸管加入适量营养肉汤,轻柔吹吸数次,使菌种融化分散后,吸出滴入改良马丁琼脂斜面上,使均匀分布,置23~28C培养5~7天。用接种环取适量第1代培养菌苔,划线接种于改良马丁琼脂斜面上,同上述条件培养得第2代培养物。取第2代培养物,同法操作,得第3代培养物。 ②用5.0ml吸管吸取3.0ml~5.0ml0.9%无菌氯化钠溶液加入上述第三代新鲜斜 面试管内,反复吹吸,洗脱抱子,用管口带有薄的无菌棉花或纱布的毛细吸管吸出抱子悬液至无菌试管内,用0.9%无菌氯化钠溶液稀释至不高于标准比浊管的浓度,即得每1ml含菌数小于100cfu验证抱子悬液。 3.2 薄膜过滤 3.2.1 供试品试验组 取规定量供试品,按《无菌检查标准操作细则》薄膜过滤后,如为不含防腐剂供试品,则往其中一只滤筒内接入100ml改良马丁培养基,往另一只滤筒内各接入100ml 的硫乙醇酸盐流体培养基,用5ml灭菌注射器抽取1ml验证菌液,从集菌培养器胶管处注入培养基中;如为含防腐剂供试品,则待供试品过滤完后,连续 3 次用
项目管理基本概念题1
应掌握的基本概念:(以下内容将包含在选择、填空和问答题中) 1、项目的定义 一般认为:项目是一个组织为实现自己既定的目标,在一定的时间、人员和资源约束条件下,所开展的一种具有一定独特性的一次性工作。 2、项目管理的定义 1.项目管理是使用各种管理方法、技术和知识为实现项目目标而对项目各项活动所开展的管理工作。 2.项目管理涉及到对于项目或项目阶段的起始、计划、组织、控制和结束这样五个具体的管理过程(或内容)。 3、一个项目可以划分为四个主要工作阶段: 1.项目的定义与决策阶段 2.项目的计划和设计阶段 3.项目的实施与控制阶段 4.项目的完工与交付阶段 4、现代项目管理知识体系的构成 按照PMI的体系可以划分为如下九个主要的方面。 1.项目集成管理 确保各种项目工作和项目的成功要素能够很好的协调与配合,以及相应的管理理论、方法、工具。 2.项目范围管理 计划和界定一个项目或项目阶段需要完成的工作和必须要完成的工作的管理工作的理论、方法、工具。 3.项目时间管理 又叫项目工期进度管理,是有关如何按时完成项目工作的理论、方法、工具。 4.项目成本管理 又叫项目选价管理,是如何在不超出项目预算的情况下完成整个项目工作,所需的管理理论、方法、工具。 5.项目质量管理 如何确保项目质量,以及保证项目质量所需的管理理论、方法、工具。 6.项目人力资源管理 如何更有效地利用项目所涉及的人力资源,以及在项目人力资源管理方面所需的管理理论、方法、工具。 7.项目沟通管理 如何有效、及时地生成、收集、储存、处理和最有效的使用项目信息,以及在项目信息和沟通管理方面所需的管理理论、方法、工具。 8.项目风险管理 如何识别项目风险、分析项目风险和应对项目风险,以及项目风险管理所需的管理理论方法、工具。 9.项目采购管理 也叫做项目获得管理,是有关从项目组织外部寻求和获得各种商品与劳务的管理,以及这一管理所需的理论、方法、工具。 5、项目管理过程 一个项目的全过程或项目阶段都需要有一个相对应的项目管理过程。这种项目管理过程一般由五个不同的管理具体工作过程构成。 1.起始过程 它包含有:定义一个项目阶段的工作与活动、决策一个项目或项目阶段的起始与否,以及决定是否
无菌检查法-2015版中国药典
无菌检查法-2015版中国药典 无菌检查法系用于检查药典要求无菌的药品、生物制品、医疗器具、原料、辅料、及其他品种是否无菌的一种方法。若供试品符合无菌检查法的规定,仅表明了供试品在该检验条件下未发现微生物污染。 无菌检查应在环境洁净度B 级背景下的局部A 级洁净度的单向流空气区域内或隔离系统中进行,其全过程应严格遵守无菌操作,防止微生物污染,防止污染的措施不得影响供试品中微生物的检出。单向流空气区、工作台面及环境应定期按《医药工业洁净室(区)悬浮粒子、浮游菌和沉降菌的测试方法》的现行国家标准进行洁净度确认。隔离系统应定期按相关的要求进行验证,其内部环境的洁净度须符合无菌检查的要求。日常检验还需对试验环境进行监控。 无菌检查人员必须具备微生物专业知识,并经过无菌技术的培训。 培养基 硫乙醇酸盐流体培养基主要用于厌氧菌的培养,也可用于需气菌培养;胰酪大豆胨液体培养基适用于真菌和需气菌的培养。培养基的制备及培养条件培养基可按以下处方制备,亦可使用按该处方生产的符合规定的脱水培养基。配制后应采用验证合格的灭菌程序灭菌。制备好的培养基应保存在2~25℃、避光的环境,若保存于非密闭容器中,一般在3周内使用;若保存于密闭容器中,一般可在一年内使用。 1. 硫乙醇酸盐流体培养基 酪胨(胰酶水解) 15.0g 酵母浸出粉 5.0g 葡萄糖 5.0g 氯化钠 2.5g L-胱氨酸0.5g 新配制的0.1% 刃天青溶液1.0ml 硫乙醇酸钠0.5g 琼脂0.75g (或硫乙醇酸) (0.3 ml) 纯化水1000ml 除葡萄糖和刃天青溶液外,取上述成分混合,微温溶解,调节pH 为弱碱性,煮沸,滤清,加入葡萄糖和刃天青溶液,摇匀,调节pH 值使灭菌后为7.1±0.2。分装至适宜的容器中,其装量与容器高度的比例应符合培养结束后培2养基氧化层(粉红色)不超过培养基深度的1/2。灭菌。在供试品接种前,培养基氧化层的高度不得超过培养基深度的1/5,否则,须经100℃水浴加热至粉红色消失(不超过20 分钟),迅速冷却,只限加热一次,并防止被污染。除另有规定外,硫乙醇酸盐流体培养基置30~35℃培养。 2. 胰酪大豆胨液体培养基 胰酪胨17.0g 氯化钠 5.0g 大豆木瓜蛋白酶消化物 3.0g 磷酸氢二钾2.5g 葡萄糖(一水合/无水)2.5g (2.3g)纯化水1000mL 除葡萄糖外,取上述成分,混合,微温溶解,滤过,调节pH 值使灭菌后在25℃的pH 值为7.3±0.2,加入葡萄
无菌检验验证方案样本
类别:编号: 部门:页码: 无菌检验方法验证 版次:□新订口替代: ________________ 制定人: ___________________ ____________ H H 日审批会签: _____________________________________________
1.1 确认所用的检查方法适用于一次性活检钳的无菌检查。 1.2 确认检查结果的准确性、可靠性、重复性和可操作性及检查方法的完整 性。 2. 验证范围 适用于我公司生产的一次性活检钳无菌检验。 3. 检验依据 《中国药典》附录无菌检查法 ISO11737-2: 医疗器械的灭菌微生物学方法第一部分: 确认灭菌过程的无菌试验 GB/T14233.2- 医用输液、输血、注射器具检验方法第2部分: 生物学试验方法 4. 验证器材 4.1 检验环境: 无菌检查应在环境洁净度10000 级下的局部100 级的单向流空气 区域内进行操作。 4.2 设备: 医用净化工作台、生物安全柜、无菌检查膜过滤器、电动吸引器、 电热干燥箱、霉菌培养箱、数显生化培养箱、压力蒸汽灭菌器、电子天平、pH 计、冰箱、恒温水浴锅、显微镜等。 4.3 培养基及稀释液: 硫乙醇酸盐流体培养基、改良马丁培养基、pH7.0 氯化钠- 蛋白胨缓冲液、0.1% 蛋白胨水溶液、营养琼脂。 4.4其它实验材料:微孔滤膜(孔径w 0.45um,直径约50mm)、无菌过滤杯、无 菌剪刀、无菌镊子、无菌钳子、无菌手套、吸管(1ml和10ml)、酒精灯、三角烧瓶、接种环、培养皿。
5.1 原理: 活检钳的形状为不溶物, 为微生物转移更彻底, 特选用无菌检查法中的 薄膜过滤法。 5.2 设备器材: 验证中所用器材、设备已经过国家计量单位鉴定合格并有鉴定证书。 5.3 培养基: 验证中所用培养基以经过培养基灵敏度实验检测合格。 5.4 操作步骤: 5.4.1 供试品的取样按照GB/T 2828.1- 相关规定进行逐批取样。 5.4.2 将所需物品, 供试品表面消毒, 经过传递窗, 紫外线照射半小时. 无菌室紫 外线消毒半小时。 5.4.3 操作人员用洗手液、自来水清洗双手, 关闭紫外灯, 换拖鞋, 脱外衣放入衣 柜, 穿洁净白大衣, 进入缓冲间, 再用洗手液、纯化水清洗双手, 用75% 乙醇对手进行消毒, 穿戴无菌衣、帽、口罩、手套等。 5.4.4 进入菌检室, 打开医用洁净工作台风机, 运行至少15分钟以上。 5.4.5 将所需物品由传递窗取出, 两只消毒液桶分别放置于菌检洁净工作台一侧, 其余物品放置于传递窗一侧的方形桌上, 剥去牛皮纸外包装。 5.4.6 医用洁净工作台内点燃酒精灯。打开3个养琼脂平板, 置于医用洁净工作 台的不同位置。 5.4.7 取供试品, 在医用洁净工作台内, 打开包装袋, 小心将供试品取出, 将其剪成 约10cm的小段,浸入500ml 0.1%无菌蛋白胨水溶液中,充分振摇作为供 试液。 5.4.8 将供试液平均转入三个薄膜过滤器的滤杯中, 开启电动吸引器开关进 行过滤,用pH7.0蛋白胨-氯化钠缓冲液每次100ml冲洗滤膜三次,用平口镊子夹取滤膜(过程保持滤膜的完整性) , 一张转移到100ml 改
项目投资的基本概念
项目投资的基本概念 黄大方 一、项目投资的相关概念 1、投资主体 投资人或从债权人也可以作为项目的投资主体(间接投资主体)。这三种人都要从各自的立场分析评价投资项目。 企业项目投资的直接投资主体就是企业本身。 2、项目计算期 项目计算期是指投资项目从投资建设(建设起点)开始到最终清理(终结点)结束整个 过程的全部时间,包括建设期和生产经营期。 n =s+p 从上述数轴中应该明白六点:建设期起点(项目计算期起点);建设期终点(经营期起点);经营期终点(项目计算期终点)。 NCF1 :第1年现金净流量( 假定其全部发生于第1年末现金净流量) NCF2:第2年现金净流量(假定其全部发生于第2年末现金净流量) 注意NCF 与N 、S 、P 之间的换算关系如某项目建设期为3年,生产经营期7年,则: NCF9=NCF (3+6)表示项目计算期第9年,也是生产经营期第6年的净现金流量。 如某项目建设期为3年,生产经营期7年,则:项目计算期第7年即为生产经营期第4年(7-3);生产经营期第2年即为项目计算期第5年(3+2)。 3、投资项目的有关价值指标 1)原始总投资等于企业为使项目完全达到设计生产能力、开展正常经营而垫支的全部现实资金,包括建设投资(固定资产投资、无形资产投资、开办费投资)与流动资金投资。原始总投资可以一次投入,也可以分次投入。 2)投资总额等于原始总投资与建设期资本化利息之和,其中固定资产投资与其资本化利息之和称为固定资产原值。
投资决策中的现金流量,通常由以下几个方面构成: 1、初始现金流量 初始现金流量是指项目开始投资量发生的现金流量。包括: (1)固定资产投资。 (2)其他长期资产投资。 (3)流动资金投资。 (4)原有固定资产的变价收入。 2、营业现金流量 营业现金流量是指项目完成后,就整个寿命周期内由于下沉生产营业所带来的现金流量。此类现金流量可按年计算。其值等于营业现金收入减去营业现金支出和 税金支出后的差额。 应该注意的是,定期损益计算的净收益和营业上实际发生的现金流量是有所不同的。因为根据权责发生制进行定期的损益计算,费用中包括了非现金支出的部分(主要是折旧费、摊销费和利息费)。因此,以定期操作益计算的净收益为基础,可按下式调整计算现金流量: 营业现金流量=定期操作益计算的净收益+非现金支出的成本费用 3、终结点现金流量 终结点现金流量是指项目经济寿命终结时发生的现金流量。主要包括 a)固定资产的变价收入或残值收入 b)原垫支的流量资金回收额。
《哈姆雷特》第三幕第一场精彩对白,to be or not to be
出自《哈姆雷特》第三幕第一场 此段的全文如下: To be, or not to be- that is the question: whether it's nobler in the mind to suffer the slings and arrows of outrageous fortune, or to take arms against a sea of troubles, and by opposing end them? To die:to sleep;no more; andby a sleep to say we end the heartache and the thousand natural shocks that flesh is heir to. 'tis a consummation devoutly to be wish'd. To die: to sleep; To sleep: perchance to dream: ay, there's the rub: for in that sleep of death what dreams may come when we have shuffled off this mortal coil, must give us pause: there's the respect that makes calamity of so long life;For who would bear the whips and scorns of time, the oppressor's wrong, the proud man's contumely, the pangs of despis'd love, the law's delay, the insolence of office, and the spurns that patient merit of the unworthy takes, when he himself might his quietus make with a bare bodkin? Who would fardels bear, to grunt and sweat under a weary life, but that the dread of something after death, the undiscover'd country, from whose bourn no traveller returns, puzzles the will and makes us rather bear those ills we have than fly to others that we know not of? Thus conscience does make cowards of us all; And thus the native hue of resolution is sicklied o'er with the pale cast of thought, and enterprises of great pith and moment with this regard their currents turn awry, and lose the name of action. 编辑本段 译文 生存还是毁灭, 这是个值得考虑的问题: 默然忍受命运暴虐的毒箭, 或是挺身反抗人世无涯的苦难, 通过斗争把他们扫清,这两种行为,哪一种更高贵? 死了,睡着了,什么都完了。倘若在这一种睡眠之中, 我们心头的创痛,以及其他无数血肉之躯所不能避免的打击,都可以从此消失,这正是我们求之不得的结局。死了,睡着了,睡着了也许还会做梦。嗯,阻碍就在这: 因为当我们摆脱了这一具腐朽的皮囊以后, 在那死的睡眠里,究竟将要做些什么梦,那不能不使我们踌躇顾虑。人们甘心久困于患难之中,也就是为了这个缘故。谁愿意忍受人世的鞭挞和讥嘲、压迫者的凌辱、傲慢者的冷眼、被轻蔑的爱情的惨痛、法律的迁延、官吏的横暴和费尽辛勤所换来的小人的鄙视,要是他只用一柄小小的刀子,就可以清算他自己的一生?谁愿意负着这样的重担,在烦劳的生命的压迫下呻吟流汗,倘若
哈姆雷特剧本
Part1: The Ghost 旁白:在一个月黑风高夜,两个守卫的士兵在城堡外看见了一个鬼魂。 Guard 1:哇!那真像老国王汪子熙啊!【睁大眼睛】 Guard 2:但不可能啊!老国王已经去西天了! Guard 1:我们得告诉Hamlet王子的好基友Horatio。也许那鬼魂有事相告呢!【鬼魂继续飘着~】【两士兵下】 旁白:第二个月黑风高的夜晚,Horatio去看那位传说中的鬼魂。鬼魂又出现了。它跟汪子熙穿的一模一样。 Horatio:你到底要干甚?说话呀!!! 【鬼魂盯着他】 Guard 2:他拒绝说话。【凑近Horatio】 Horatio:我得告诉王子了,也许鬼魂会跟他说话呢!【坐沉思状】 Guard 2:此乃不祥之兆啊!也许丹麦即将发生一件坏事!【对Guard1说】 【三人下】 旁白:Hamlet王子最近很桑心。老国王,也就是他的亲生父亲,突然卒了。Gertrude,也就是她的额娘,改嫁给了Hamlet的叔叔,Claudius。所以呢,Claudius 就变成了新国王。但是Hamlet并不喜欢这个叔叔。 Claudius:是时候去思考未来了,Hamlet。我可以变成你的父亲然后你就可以变成下一任主公。【托腮ing】 Gertrude:跟我们呆在一起吧!别去上大学了,你知道,我们是爱你的~【自作多情状~】 Hamlet:我应该遵从您的,夫人。【单膝跪下,稍有轻蔑】 Hamlet独白:我父皇比这个死叔叔好多了!母后他难道智商瞬间降低,与尹玉祺等齐了?! 【三人下】 旁白:Horatio来了,他告诉Hamlet关于那个鬼魂的事情,Horatio与Hamlet在同一所大学里上学很久了,他是Hamlet最好的基友,Hamlet完全信任他。【Hamlet和Horatio聊天ing…】 Hamlet:我恨死这个地方了,我妈和那该死的叔叔在一起的多欢脱!【他们两个人进去,就差没有三个人出来了!】它们是怎么这么快就忘掉我可怜的爸爸的?!Horatio:Hamlet!我昨晚看见你爸了! Hamlet:What do you mean?【大惊】 Horatio:午夜时分去城墙上看看吧!Then, you’ll understand.【故作淡定】 【两人下】【夜晚】 旁白:Hamlet看见了那鬼魂。 Ghost:我是你爸爸的灵魂…如果你还是他儿子…为这场谋杀复仇!!!【声音飘忽】Hamlet:谋杀?!【大惊】 Ghost:我的妻子Gertrude对我不忠…我的哥哥谋杀了我…当我在花园里睡觉【继续飘忽】的时候,哥哥把毒药滴在了我的耳朵里…哦!太阳即将普照大地…我得走了…Hamlet,remember me【渐弱】… 旁白:Hamlet不知道应该怎么做。也许鬼魂在说谎… Hamlet独白:【冷静的】我只能装疯了!没有人会注意到一个可怜的疯子。我会想办法弄清真相的。如果鬼魂的话是真的…我会对叔叔用闪电判定。
哈姆雷特英文评析配汉语翻译对照
○1The character of Hamlet is the most outstanding of his irresolute and.hesitant. Facing the father was poisoned, mother to be possessed, Wang Quan is stolen, countries were coveted enemy of hate, Hamlet has strong revenge wish: " my destiny in the cry, make my whole body each fine blood vessels become like an angry lion. Bones and muscles as hard." "rest, rest, suffering soul! Well, friends, I with great passion, trust you two ; if in the hamlet of weak ability, can have to express to you his friendship, God, I'll quit you. Let us go ; please remember no matter what time should be kept. It is an upside-down era, oh, shit I have to shoulder the responsibility of restructuring the course! Come, let's go together." the oath, Hamlet in this resolutely determined expression of their revenge of the firm belief. But on the other hand, Hamlet to the killing of this seemingly simple actions are shown for ordinary people to understand the concerns of complex, he had several times to kill enemies the chance, but in this complex work, revenge againWork not completed. When he meets by chance to the enemy for their sin, revenge is flashed, and then he was transferred to the opportunity value thinking : " now I just started, he was praying. I do it right now, he is a life died, I will revenge. It need to calculate a. A villain who killed my father, my son, the evil Chinese to go to heaven but then Hamlet first became an ideological struggle. ○2One side is murdered his father 's tooth pain, father dead sound collection, the other side is the life value of serious thought, born with a melancholy temperament. Hamlet lies between the two hard wander. "But is afraid of death went no one back that had never found the land, if there will also do not know how, so will shake, so they prefer to endure the punishment, rather than to some other unknown suffering like this, concerns that we have become a coward, also like this, decisions are colors on white layer of thinking ill ; this can be with vigour and vitality of large for, as a result of this wonder out awkward and lose the name of action." ○3On the interpretation of a revenge plan became a bitter ideological breakthrough. In this breakthrough in the process of Hamlet constantly see the darkness of the society, was trying to put myself into this dark world, with the enemy the same means to deal with the enemy; but the humanity and the faith of justice is making the instinctive resistance. In this kind of give tit for tat the ideological conflict, the evolution of Hamlet for a difficult choice. "People are what a piece of work! Reason is very valuable! How power is infinite! Appearance and manner how end is whole, how wonderful! In action how like an angel! On understanding, how like a god! The universe of China! The intelligent part of the universe! But, for me, this clay extracted. What's" here "Hamlet in a sarcastic tone of speaking the words," this is a hamlet on the status of doubt, also the Hamlet a time order reflect the idea of him with suspicion. View all around the world : human hypocrisy, Yan Liang, heaven 's injustice, finally, finally reached this suspicion state vertex : " to be or not to be" a pressing matter of
无菌检查法
无菌检查法
目录 一、培养基 (2) <一>培养基的制备 (2) <二>培养基的适用性检查 (3) 二、稀释液、冲洗液及其制备方法 (4) 三、方法验证或试验 (4) <一>菌种及菌液制备 (4) <二>验证方法 (4) <三>结果判断 (4) 四、供试品的无菌检查 (4) 五、供试品处理及接种培养基 (5) <一>薄膜过滤法 (5) <二>直接接种法 (6) 六、培养及观察 (7) 七、结果判断 (7)
无菌检查法 无菌检查法系用于检查药典要求无菌的药品、医疗器具、原料、辅料及其他品种是否无菌的一种方法。若供试品符合无菌检查法的规定,仅表明了供试品在该检验条件下未发现微生物污染。 无菌检查应在环境洁净度10000级下的局部洁净度100级的单向流空气区域内或隔离系统中进行,其全过程中必须严格遵守无菌操作,防止微生物污染。单向流空气区、工作台面及环境应定期按《医药工业洁净室(区)悬浮粒子、浮游菌和沉降菌的测试方法》的现行国家标准进行洁净度验证。隔离系统按相关的要求进行验证,其内部环境的洁净度须符合无菌检查的要求。 一、培养基 <一>培养基的制备 培养基可按以下处方制备,也可使用按该处方生产的符合规定的脱水培养基。配制后应采用验证合格的灭菌程序灭菌。制备好的培养基应保存在2~25℃、避光的环境。培养基若保存于非密闭容器中,一般在三周内使用;若保存于密闭容器中,一般可在一年内使用。 1、硫乙醇酸盐流体培养基(用于培养好氧菌、厌气菌) 酪胨(胰酶水解)15g;氯化钠2.5g;葡萄糖5g;新配制的0.1%刃天青溶液1.0ml;L-胱氨酸0.5g(或新配制的0.2%亚甲蓝溶液0.5ml);硫乙醇酸钠0.5g;琼脂0.75g(或硫乙醇酸0.3ml);水1000ml;酵母浸出粉5g 除葡萄糖和刃天青溶液外,取上述成分混合,微温溶解后,调节pH为弱碱性,煮沸,滤清,加入葡萄糖和刃天青溶液,摇匀,调节pH值使灭菌后为7.1±0.2,分装至适宜的容器中,其装量与容器高度的比例应符合培养结束后培养基氧化层(粉红色)不超过培养基深度的1/2。灭菌。在供试品接种前,培养基氧化层的高度不得超过培养基深度的1/5,否则,须经100℃水浴加热至粉红色消失(不超过20分钟)迅速冷却,只限加热一次,并应防止被污染。 硫乙醇酸盐流体培养基置30~35℃培养。 2、改良马丁培养基(用于培养真菌) 胨5g;磷酸氢二钾1g;酵母浸出粉2g;硫酸镁0.5g;葡萄糖20g;水1000ml 除葡萄糖外,取上述成分混合,微温溶解,调节pH值约为6.8,煮沸,加入葡萄糖溶解后,摇匀,滤清,调节pH值使灭菌后为6.4±0.2,分装,灭菌。 改良马丁培养基置23~28℃培养。 3、选择性培养基 按上述硫乙醇酸盐流体培养基或改良马丁培养基的处方及制法,在培养基灭菌或使用前加入适量的中和剂或表面活性剂,如对氨基苯甲酸(用于磺胺类供试品)、聚山梨酯80(用于非水溶性供试品)或β-内酰胺酶(用于β-内酰胺类供试品)等。中和剂或表面活性剂的用量应通过验证。 4、营养肉汤培养基 胨10g;牛肉浸出粉3.0g;氯化钠5g;葡萄糖5.0g;水1000ml。 取上述成分混合,微温溶解,调节pH为弱碱性,煮沸,滤清,调节pH值使 灭菌后为7.2±0.2,分装,灭菌。 5、营养琼脂培养基 按上述营养肉汤培养基的处方及制法,加入14.0g琼脂,调节pH值使灭菌后为7.2
高中语文 1.3《哈姆莱特》《哈姆莱特》中英文经典台词素材 新人教版必修4
《哈姆莱特》中英文经典台词 (中英文) Hamlet SCENE I. Elsinore. The Castle [Enter Hamlet.] Hamlet:To be, or not to be- that is the question: Whether 'tis nobler in the mind to suffer The slings and arrows of outrageous fortune Or to take arms against a sea of troubles And by opposing end them. To die- to sleep- No more; and by a sleep to say we end The heartache and the thousand natural shocks That flesh is heir to. 'Tis a consummation Devoutly to be wish'd. To die- to sleep. To sleep- perchance to dream: ay, there's the rub! For in that sleep of death what dreams may come When we have shuffled off this mortal coil, Must give us pause. There's the respect That makes calamity of so long life. For who would bear the whips and scorns of time, Th' oppressor's wrong, the proud man's contumely, The pangs of despis'd love, the law's delay, The insolence of office, and the spurns That patient merit of th' unworthy takes, When he himself might his quietus make With a bare bodkin? Who would these fardels bear, To grunt and sweat under a weary life, But that the dread of something after death- The undiscover'd country, from whose bourn No traveller returns- puzzles the will, And makes us rather bear those ills we have Than fly to others that we know not of? Thus conscience does make cowards of us all, And thus the native hue of resolution Is sicklied o'er with the pale cast of thought, And enterprises of great pith and moment With this regard their currents turn awry And lose the name of action. 生存或毁灭, 这是个必答之问题: 是否应默默的忍受坎苛命运之无情打击, 还是应与深如大海之无涯苦难奋然为敌, 并将其克服。
