LH and FSH 斑马鱼

Genetic Analysis of Zebrafish Gonadotropin(FSH and LH)Functions by TALEN-Mediated Gene Disruption Zhiwei Zhang,Bo Zhu,and Wei Ge
School of Life Sciences(Z.Z.,B.Z.,W.G.),The Chinese University of Hong Kong,Shatin,New Territories,Hong Kong,China;and Faculty of Health Sciences(Z.Z.,W.G.),University of Macau,Taipa,Macau,China
Vertebrate reproduction is controlled by two gonadotropins(FSH and LH)from the pituitary. Despite numerous studies on FSH and LH in fish species,their functions in reproduction still remain poorly defined.This is partly due to the lack of powerful genetic approaches for functional studies in adult fish.This situation is now changing with the emergence of genome-editing technologies, especially Transcription Activator-Like Effector Nuclease(TALEN)and Clustered Regularly Inter-spaced Short Palindromic Repeats(CRISPR).In this study,we deleted the hormone-specific?-genes of both FSH and LH in the zebrafish using TALEN.This was followed by a phenotype analysis for key reproductive events,including gonadal differentiation,puberty onset,gametogenesis,final maturation,and fertility.FSH-deficient zebrafish(fshb?/?)were surprisingly fertile in both sexes; however,the development of both the ovary and testis was significantly delayed.In contrast,LH-deficient zebrafish(lhb?/?)showed normal gonadal growth,but the females failed to spawn and were therefore https://www.360docs.net/doc/3c2301675.html,ing previtellogenic follicles as the marker,we observed a significant delay of puberty onset in the fshb mutant but not the lhb mutant females.Interestingly,FSH seemed to play a role in maintaining the female status because we repeatedly observed sexual reversal in the fshb mutant.Neither the fshb nor lhb mutation alone seemed to affect gonadal differentiation;however, the double mutation of the two genes led to all males,although the development of the testis was significantly delayed.In summary,our data confirmed some well-known functions of FSH and LH in fish while also providing evidence for novel functions,which would be difficult to reveal using tradi-tional biochemical and physiological approaches.(Molecular Endocrinology29:76–98,2015)
I n vertebrates,reproduction is controlled by two master gonadotrophic hormones from the pituitary gland,FSH and LH.Both gonadotropins belong to the glycoprotein hormone family,consisting of one common?-subunit and one hormone-specific?-subunit(1).In mammals, FSH and LH have been extensively studied,and their functions are clearly defined.FSH stimulates follicular growth and estrogen production by binding to its cognate receptor FSH receptor(FSHR)located on the granulosa cells in the ovary and promotes spermatogenesis in the testis by activating FSHR expressed in the Sertoli cells.On the other hand,LH stimulates androgen production by the theca cells in the ovary,providing a substrate for estrogen production in the granulosa cells,and triggers oocyte maturation and ovulation.
In the testis,LH stimulates androgen production by the Leydig cells(2,3).The clear definition of FSH and LH in mammals has benefited greatly from genetic approaches or gene knockouts in the mouse.In the FSH?-gene knockout(KO)mouse,the mutant females are infertile due to a blockade in folliculogenesis prior to antral follicle formation.However,FSH-deficient males are fertile de-spite having smaller testes and oligospermia(4).In con-trast,the deletion of the LH?-gene causes infertility in both males and females.LH-deficient females have de-fects in folliculogenesis including the degeneration of an-
ISSN Print0888-8809ISSN Online1944-9917
Printed in U.S.A.
Copyright?2015by the Endocrine Society
Received August14,2014.Accepted November11,2014. First Published Online November14,2014Abbreviations:BW,body weight;CRISPR/Cas9,Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated system;dpf,days post fertilization;EV,early vitel-logenic;FG,full grown;FSHR,FSH receptor;gDNA,genomic DNA;GSI,gonadosomatic index;HMA,heteroduplex motility assay;HRMA,high-resolution melt analysis;KO, knockout;PG,primary growth stage;PV,previtellogenic;qPCR,quantitative PCR;RT, reverse transcription;RVD,repeat variable diresidue;SL,standard length;TALEN,Tran-scription Activator-Like Effector Nuclease;WT,wild type.
O R I G I N A L R E S E A R C H
https://www.360docs.net/doc/3c2301675.html, Mol Endocrinol,January2015,29(1):76–98doi:10.1210/me.2014-1256
tral follicles and absence of corpora lutea in the ovary.In males,the lack of LH leads to a decreased testis size, hypoplasia of Leydig cells,blockade of spermatogenesis at the round spermatid stage,and reduced expression of steroidogenic enzymes and secretion of T(5).
In teleosts,the understanding of gonadotropins has taken a long journey,and our knowledge about their functions still remains rudimentary.Although it has been known for many decades in mammals that the pituitary gland produces two gonadotropins since the first report in1931on the follicle-stimulating and luteinizing fractions in the pituitary(6),it was believed for a long time that gonadal development and function in fish were controlled by one single gonadotropin (7–9).The concept of two gonadotropins was proposed later after the mid-1970s based on histochemical and bio-chemical evidence(10–13),but the chemical nature of the molecules was not defined until1988when two chemically distinct gonadotropins,termed GTH-I and GTH-II,were characterized in the chum salmon(Oncorhynchus keta) (14).Ever since then,there have been numerous studies in teleosts demonstrating the duality of gonadotropins,and structural and functional studies have led to the proposal that GTH-I and GTH-II should be renamed to FSH and LH, respectively(15,16).According to the GenBank record in 2014,gonadotropins(FSH and LH)have been sequenced in more than70teleost species.Although the number of fish species whose gonadotropins have been characterized is in-creasing,one major issue that concerns most in the field is the differential functions of the two hormones.
Numerous studies have been reported in a variety of fish species on the physiological roles of FSH and LH(17)and their regulation(18);however,our knowledge of FSH and LH functions still remains fragmentary.It is now generally believed that similar to their mammalian counterparts,fish FSH is mostly involved in promoting early gonadal develop-ment and growth,whereas LH plays an important role in regulating the late stage of gametogenesis,including the final gamete maturation and release(ovulation and spermiation) (19,20).Most studies on gonadotropin functions have been focused on their expression profiles during the reproductive cycles at the transcript and/or protein levels(21–28),effects on gonadal steroidogenesis in males and females(29–34),vitello-genesis in females(35),and final gamete maturation(36–40).
In the zebrafish,our previous studies have shown that different from the gonadotropins in tetrapods,zebrafish FSH and LH show promiscuous recognition of gonadotro-pin receptors(Fshr/fshr and Lhcgr/lhcgr).Recombinant ze-brafish FSH specifically activates its cognitive receptor Fshr expressed in Chinese hamster ovary cells;however,ze-brafish LH can activate both Fshr and Lhcgr although the activation of Fshr requires higher concentrations of ze-brafish LH compared with its activation of Lhcgr(41).A similar phenomenon has also been observed in some other species such as the African catfish(Clarias gariepinus)(28) and Senegalese sole(Solea senegalensis)(31).The physiolog-ical relevance of activating Fshr by LH remains mysterious. Although the expression profiles of zebrafish FSH and LH ?-genes(fshb and lhb)during the reproductive cycle are not fully clear(41),our previous study on their receptors,fshr and lhcgr,has provided strong evidence for their differential roles in controlling folliculogenesis in females(42).Both fshr and lhcgr have a very low expression in the follicles of the primary growth stage(PG).However,the expression of fshr but not lhcgr increases significantly when the follicles are recruited into the secondary growth stage,and its level re-mains high throughout vitellogenic growth before it drops prior to final oocyte maturation.In contrast,the expression of lhcgr starts to increase in late vitellogenic follicles,and its level surges to the peak at the full-grown(FG)stage before oocyte maturation(42,43).
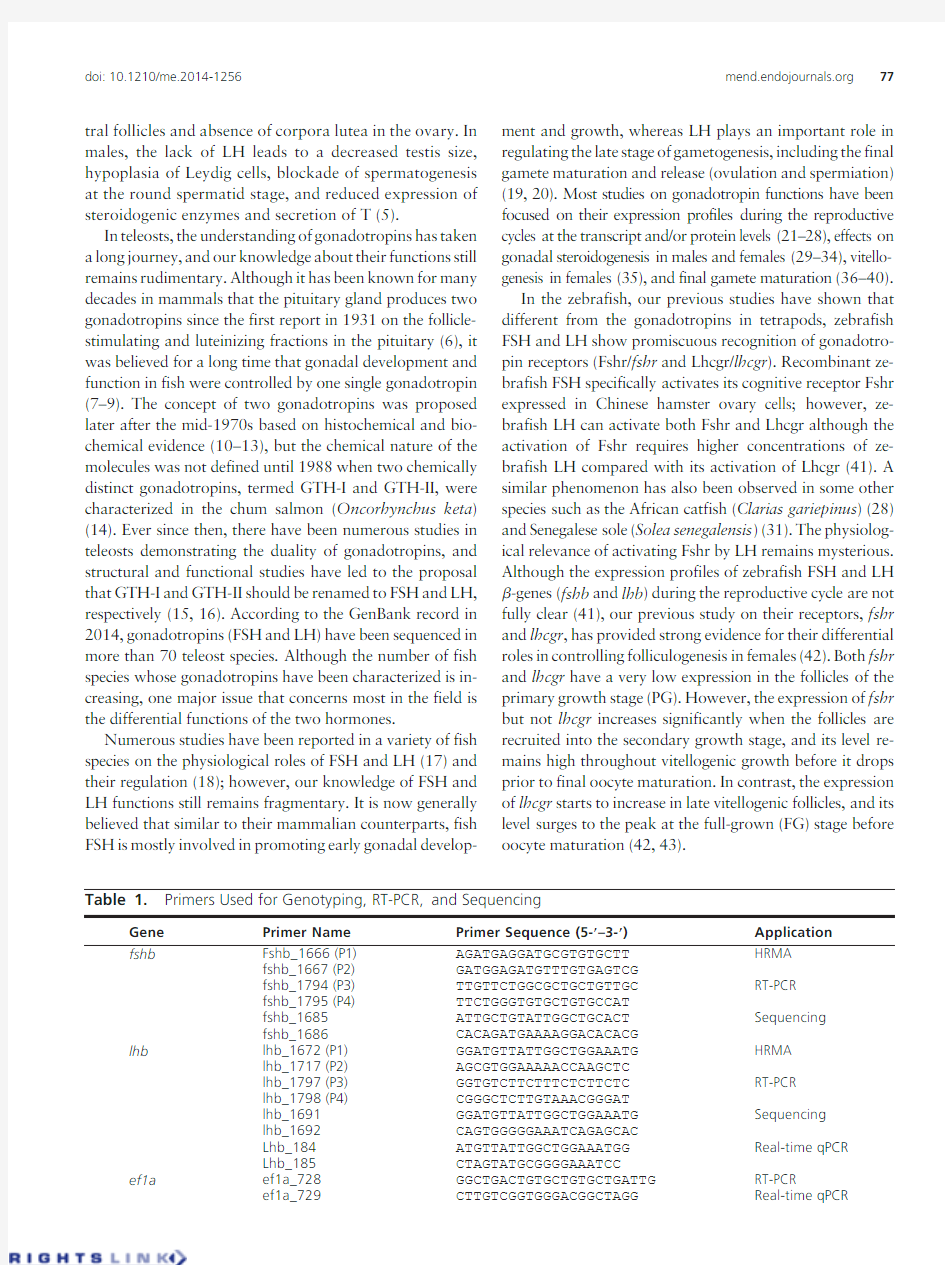
Table1.Primers Used for Genotyping,RT-PCR,and Sequencing
Gene Primer Name Primer Sequence(5-?–3-?)Application
fshb Fshb_1666(P1)AGATGAGGATGCGTGTGCTT HRMA
fshb_1667(P2)GATGGAGATGTTTGTGAGTCG
fshb_1794(P3)TTGTTCTGGCGCTGCTGTTGC RT-PCR
fshb_1795(P4)TTCTGGGTGTGCTGTGCCAT
fshb_1685ATTGCTGTATTGGCTGCACT Sequencing
fshb_1686CACAGATGAAAAGGACACACG
lhb lhb_1672(P1)GGATGTTATTGGCTGGAAATG HRMA
lhb_1717(P2)AGCGTGGAAAAACCAAGCTC
lhb_1797(P3)GGTGTCTTCTTTCTCTTCTC RT-PCR
lhb_1798(P4)CGGGCTCTTGTAAACGGGAT
lhb_1691GGATGTTATTGGCTGGAAATG Sequencing
lhb_1692CAGTGGGGGAAATCAGAGCAC
Lhb_184ATGTTATTGGCTGGAAATGG Real-time qPCR
Lhb_185CTAGTATGCGGGGAAATCC
ef1a ef1a_728GGCTGACTGTGCTGTGCTGATTG RT-PCR
ef1a_729CTTGTCGGTGGGACGGCTAGG Real-time qPCR
doi:10.1210/https://www.360docs.net/doc/3c2301675.html,77
Despite all these studies,our understanding of FSH and LH,especially their relative importance in controlling gonadal development and functions,still remains limited as compared with studies in mammals.This is largely due to the lack of pure forms of homologous hormones with biological activity,the international standard of bioas-says,and more importantly the genetic or knockout ap-proach available in the mouse model.This situation has changed recently with the emergence of powerful ge-nome-editing technologies,in particular the Transcrip-tion Activator-Like Effector Nuclease(TALEN)and Clustered Regularly Interspaced Short Palindromic Re-peats/CRISPR-associated system(CRISPR/Cas9)(13).
Both TALEN(44–46)and CRISPR/Cas9(47–49) have been successfully adopted in the zebrafish model to disrupt functional genes.TALEN was first used in the zebrafish to delete the genes golden,ryr3,tbx6,and ryr1a (44),whereas CRISPR/Cas9has been shown to be equally efficient to induce gene disruption in the zebrafish(48). However,all studies reported so far in the zebrafish have aimed at demonstrating the utility of the technologies with phenotypes mostly restricted to the embryonic stages.There has been no report studying functions of genes in adults using these technologies in the zebrafish.
To provide genetic evidence for the function and physio-logical significance of the two gonadotropins in teleosts,we have undertaken the present study by using TALEN ap-proach to disrupt zebrafish FSH and LH?-genes(fshb and lhb).This was followed by a detailed analysis of reproduc-tive phenotypes in both females and males.Our analysis focused on several important events in reproductive devel-opment and function,including gonadal differentiation,pu-berty onset,gametogenesis,final maturation,and fertility. The results from the present study have,for the first time, provided the most comprehensive genetic evidence for go-nadotropin functions in a teleost species.Our data not only confirmed some well-established functions of FSH and LH but also revised some views.More importantly,we have provided evidence for novel functions of these hormones that would be difficult to reveal using traditional biochem-ical and physiological approaches.
Materials and Methods
Zebrafish
The wild type(WT)zebrafish of strain AB was used in the present study to produce mutant lines.Zebrafish were main-tained in flow-through aquaria at28?0.5°C with the photo-period of14hours light and10hours dark.All the experiments performed were licensed by the Government of the Hong Kong Special Administrative Region and were approved by the Ani-mal Experimentation Ethics Committee of the Chinese Univer-sity of Hong Kong.
TALEN design and assembly
The specific TALEN target sites were identified using an online tool(TAL Effector-Nucleotide Targeter:https://tale-nt. https://www.360docs.net/doc/3c2301675.html,/).The selected criteria were followed as de-scribed(44).Gene-specific TALEN constructs were assembled using the TALEN Golden Gate assembly system as described (50).The two TALEN somatic expression backbones, pCS2TAL3DD and pCS2TAL3RR(44),and the plasmids pro-viding repeat variable diresidue(RVD)repeats for Golden Gate cloning(50)were obtained from Addgene.Briefly,two rounds of Golden Gate cloning assembly were performed to generate a TALEN gene with N RVD repeat modules(N?number of RVDs).First,two arrays were generated,corresponding to re-peat modules1–10and11-N
-1
.The resulting vectors that ac-quired correct arrays were confirmed first by the size of repeat array inserts amplified by PCR with the primers flanking the insertion site and then sequencing of the plasmids.Second,the
Figure1.Schematic representation of the genomic structures of
zebrafish fshb(A)and lhb(B)genes and the target sites of TALEN.The
coding and untranslated exon regions are depicted as solid and open
boxes,respectively.The TALEN target sequence for each gene is shown
under each illustration,with the left and right TALEN binding sites
boxed.Arrows indicate the location of primers used for HRMA and the translation initiation codon(ATG)is italicized.
Table2.TALEN-Induced Mutations in F0Embryos and Adults
Gene Postinjection
Embryo Survival
Rate
Postinjection
Abnormal Embryo
Rate
Postinjection
Mutation Rate
at24hpf
Somatic
Mutation Rate
in F0Adults
Germ Line
Transmission
Rate
fshb31/60;52%3/31;10%3/10;30%6/10;60%4/20;20% lhb32/60;53%3/32;10%10/10;100%10/10;100%7/20;35% Abbreviation:hpf,hours post fertilization.Germ line mutation rate is the number of F1fish with mutations among the offspring of one F0fish.
78Zhang et al Genetic Analysis of Zebrafish FSH and LH Functions Mol Endocrinol,January2015,29(1):76–98
two arrays and the sequences encoding the Nth motif were sub-cloned into the backbone vectors (50).The RVD repeat array sequences were cloned into pCS2TAL3DD to generate a left TALEN arm and pCS2TAL3RR to generate a right TALEN arm.The assemblies were confirmed by sequencing.
Injection of TALEN RNA into zebrafish embryos
The 5?-capped mRNA was generated by in vitro transcrip-tion from the customized TALEN plasmid templates using the mMESSAGE and mMACHINE SP6kit (Invitrogen).The cus-tomized TALEN plasmids were linearized with Not I at 37°C for at least 3hours before in vitro transcription.One microgram of linear template was used in the reaction of in vitro transcription,and the yield of the transcribed mRNA was determined by NanoDrop 2000(Thermo Scientific).Fifty picograms of left and right TALEN mRNA each were coinjected into the cytoplasm of one-or two-cell stage zebrafish embryos using the Drummond Nanoject system (Drummon Scientific).
Genomic DNA extraction
The genomic DNA (gDNA)was obtained by the NaOH method (51).Briefly,a single embryo or piece of caudal fin was incubated in 50?L NaOH (50mM).The caudal fin was sam-pled after the fish was anesthetized with MS-222(tricaine meth-anesulphonate,250mg/L;Sigma-Aldrich).The samples were then heated to 95°C until the tissue was noticeably friable,usu-ally 10minutes for the embryo and 20minutes for the fin cut.The solution was then cooled to 4°C,and one tenth volume of 1M Tris-HCL (pH 8.0)was added to neutralize the basic solu-tion.The sample was centrifuged at 13000rpm for 2minutes,and the supernatant was ready for use in PCR.
High-resolution melt analysis (HRMA)
To detect TALEN-induced mutations by HRMA,a 90-to 120-bp amplicon that included the entire genomic target site was generated.Primers flanking the target site were used to amplify the genomic region in a 20?L PCR containing:0.5?l EvaGreen (?20),2?L PCR master mix,2?L embryonic or fin gDNA,and 400nM each forward and reverse primers (Table 1).
The amplification/duplex formation condi-tions were as follows:denaturation at 95°C for 3minutes;40cycles (denatur-ation at 95°C for 15sec;annealing at 62°C for 15sec;extension at 72°C for 20sec);denaturation at 95°C for 15sec;melt curve 70°C-95°C,with a 0.2°C in-crement each step.The amplification was performed on the Bio-Rad CFX96real-time PCR system (Bio-Rad Labo-ratories),and the HRMA analysis was carried out using the Bio-Rad Pre-cision Melt Analysis software (Bio-Rad Laboratories).
The HRMA profile for each gene can be presented as either temperature-shifted melt curve and/or melt peak.The shifted melt curve is sensitive to distin-guish heterozygous (?/?)from ho-mozygous samples (?/?or ?/?),whereas the melt peak is often a better choice to distinguish homozygous mu-tants (?/?)from homozygous wild type (?/?).When the sequence difference is less than 5bp (eg,lhb ),the melt peaks of HRMA profiles often cannot distinguish homozygous mutants (?/?)from the wild type (?/?).To solve this problem,we spiked the unknown samples with wild-type gDNA to create heterodu-plexes,which could be easily detected by either shifted curves or melt peaks.
Heteroduplex motility assay (HMA)
We performed an HMA using PAGE to confirm the mutations detected by HRMA.Briefly,a 90-to 120-bp amplicon that included the entire genomic target site was generated by using the same primers as those for HRMA.A three-step PCR was carried out:95°C for 3minutes,
40
Figure 2.HRMA detection and sequence analysis of TALEN-induced somatic mutations in fshb (A)and lhb (B)genes in F0embryos (1–2dpf).Each curve represents a single embryo.Both normalized,temperature-shifted melt curve (left panel)and melt peak (right panel)are shown.The red curve represents the uninjected control embryo,and the green ones are from the embryos injected with TANEN mRNAs.The arrow indicates the melt peak generated from the injected embryos with
potential somatic mutations.The representative TALEN-induced sequence alterations (indel,insertions and deletions)at targeted locus are shown at the bottom of each panel.Sequences in shadow indicate left and right TALEN binding sites,and red indicate sequence alterations.
doi:10.1210/https://www.360docs.net/doc/3c2301675.html, 79
cycles of95°C for15seconds,60°C for20seconds,and72°C for 20seconds.Five microliters of PCR reaction were electrophoresed on20%polyacrylamide gels at50V for at least10hours,and the gel was stained with ethidium bromide and visualized on Gel Doc XR?System(Bio-Rad Laboratories)(52).
Histological examination
All fish sampled were recorded for their body weight,standard body length,and age before processing for histological examina-tion.Briefly,the fish was anesthetized with MS-222and placed on a petri dish cover to measure body length with a ruler underneath. The body was then gently dried with a piece of tissue for taking body weight on an analytical balance.After the measurements,the fish were either allowed to recover in water or killed by decapita-tion for histological examination.The decapitated fish was imme-diately fixed in Bouin’s solution for at least24hours.The fixed samples were dehydrated and embedded in paraffin and then seri-ally cut into7-?m sections on a Leica microtome.Slides were stained with hematoxylin and eosin and mounted with Canada balsam(Sigma-Aldrich)for microscopic examination.
RNA isolation and RT-PCR
Total RNA was extracted from individual pituitary glands using Tri-Reagent(Molecular Research Center)according to
the Figure3.HRMA(A and B)and HMA(C and D)for mutations in F1and F2fish.The F0founders with mutations detected by HRMA on caudal fin gDNA were crossed with WT partners to generate F1individuals.The gDNA of2-month-old F1progeny was examined by both HRMA and HMA for the presence of mutations.In the HRMA assay(A and B),the smooth red curves are profiles of control WT fish,whereas the heterozygous F1siblings(?/?) display characteristic wavy HRMA profiles shown in different colors.The genotypes were further confirmed by HMA assay on PCR amplicons(C and D), which shows homoduplexes(asterisk)in WT(?/?)and homozygous F2mutants(?/?)and both homoduplexes and heteroduplexes(arrow)in heterozygous F1(?/?).The F1males and females that carried the same mutation were identified and crossed to generate homozygous mutant F2.The region containing the targeted sites was cloned and sequenced after PCR amplification of F1gDNA.The representative mutant sequences are listed at the bottom for each gene,and the sequence in blue was chosen for propagation to establish the mutant lines.
80Zhang et al Genetic Analysis of Zebrafish FSH and LH Functions Mol Endocrinol,January2015,29(1):76–98
manufacturer’s protocol and our previous report(42,53).Re-verse transcription(RT)was performed at37°C for2hours in a total volume of a10-?L reaction solution containing0.5?g oligodeoxythymidine,1?Moloney murine leukemia virus re-verse transcription buffer,0.5mM each deoxynucleotide triphosphate,0.1mM dithiothreitol,and100U Moloney mu-rine leukemia virus reverse transcriptase(Invitrogen).Conven-tional RT-PCR was used to confirm the mutations at mRNA level(15?L containing5?L of1:15diluted RT reaction),and real-time quantitative PCR(qPCR)was performed to
analyze Figure4.Genotype analysis for fshb(A and B)and lhb(C and D)mutants at gDNA(A and C)and mRNA(B and D)levels.Male and female F1 fish with the same mutation were crossed to obtain homozygous F2individuals.The gDNA was isolated from2-month-old F2caudal fins and subjected to HRMA(A and C).The WT(?/?),heterozygous(?/?),and homozygous(?/?)F2fish are labeled in red,green,and blue, respectively.Because the HRMA could not clearly distinguish the homozygous mutant(lhb?/?,blue)from the wild type(lhb?/?,red)well,we spiked the sample with wild-type gDNA to create heteroduplexes(WT?lhb?/?,brown),which could be easily detected by shifted curve and melt peak(arrow).The mutation of fshb and lhb genes at transcription level was further confirmed by RT-PCR(B and D).The primer P3is specific for the deleted sequences,and ef1a is used as the internal control.
doi:10.1210/https://www.360docs.net/doc/3c2301675.html,81
the lhb expression level in individual pituitary glands(20?L containing9.5?L of1:10diluted RT reaction)on the real-time PCR detection system(Bio-Rad Laboratories).The specific primers used are listed in Table1.
Fertility assay
The fertility of different genotypes was assessed by natural mating with fertile partners.Individuals that failed to spawn or produce fertilized embryos after at least10trials were consid-ered infertile.
Data analysis
The expression level of lhb was normalized to that of the internal control ef1a for statistical analysis.All values were expressed as the mean?SEM,and the data were analyzed by a Student’s t test(lhb expression)or a one-way ANOVA followed by a Tukey’s multiple comparison test[body weight(BW),standard length(SL),and gonadosomatic index (GSI)]using Prism6on Macintosh OS X(GraphPad Software).
Results
High efficiency of TALEN-induced mutagenesis of zebrafish fshb and lhb genes
To maximize the chance of inducing loss of function of the targeted gene,the TALEN binding sites were chosen within the second exon immediately down-stream of the translation start site for both fshb and lhb (Figure1).The customized TALEN mRNAs were
effi-Figure5.Effects of fshb(A and B)and lhb(C and D)gene disruption on ovarian growth and spawning in female zebrafish at60dpf.A, Anatomical and histological examination of the ovary in fshb-deficient fish(fshb?/?)and controls(fshb?/?and fshb?/?).B,GSI in controls and fshb-deficient fish(n?3–4,P?.05).C,Anatomical and histological examination of the ovary in lhb-deficient fish(lhb?/?)and controls(lhb?/?and lhb?/?).D,Failed oocyte maturation and ovulation in lhb-deficient fish(lhb?/?)at9:00AM,1hour after lights on.LV,late vitellogenic;MV, midvitellogenic.
82Zhang et al Genetic Analysis of Zebrafish FSH and LH Functions Mol Endocrinol,January2015,29(1):76–98
cient in inducing somatic mutation at the targeted loci. When analyzed by HRMA at24hours after fertiliza-tion,the mutation rates were30%(3of10)and100% (10of10)for fshb and lhb,respectively(Table2).The PCR amplicons were cloned and sequenced to confirm the mutations,and the sequences of some representa-tive mutations are shown in Figure2.The melt curves of mutation-carrying embryos showed significant shift-ing as compared with those of the control.Despite the shifting,the curves were generally smooth,indicating mosaicism of the mutations(Figure2).The remaining F0founder embryos were raised to adulthood and genotyped by HRMA on gDNA isolated from the fin cuts.Most individuals harbored somatic mutations,with60%(6of10)for fshb and100%(10of10)for lhb.To evaluate the efficiency of germ line transmis-sion of the mutations,individual F0fish carrying mu-tations were crossed with wild-type partners to obtain F1offspring.The gDNA was isolated from individual F1embryos from one F0fish and analyzed by HRMA. About20%(4of20)F1embryos for fshb and35%(7 of20)for lhb were heterozygous mutants,demonstrat-ing successful germ line transmission(Table2). Establishment of fshb and lhb KO lines
TALEN proteins existed in the embryos for less than1 day after injection(data not shown).Because there are approximately25–50primordial germ cells in the
ze-Figure6.Catch-up ovarian growth in fshb-deficient mutant(fshb?/?)of different ages(68,70and90pdf).PG,primary growth;PV, previtellogenic;EV,early vitellogenic;MV,midvitellogenic;LV,late vitellogenic;and FG,full-grown.
doi:10.1210/https://www.360docs.net/doc/3c2301675.html,83
brafish at 24days post fertilization (dpf)(54),the germ-lines of F0founders are likely mosaic;however,the level
of mosaicism may not be as high as that in the somatic
cells.In most of the fshb and lhb F0founders screened,as
many as four mutant sequences could be identified in the
F1embryos from individual F0fish.With the high fecundity of the zebrafish,it is possible to obtain sibling male and female F1individuals that carry the same DNA sequence alteration,making it possible to obtain homozygous mutants in F2generation.HRMA profiles
of three different mutated sequences at the targeted loci of fshb and lhb from young adult F1fish (?/?;2mo)are shown in Figure 3,A and B.Different from the smooth HRMA curves from mosaic F0fish,the curves from the heterozygous F1individuals showed typical wavy patterns,indicating the existence of three DNA helixes (wild type,mutant,and hybrid).An HMA also confirmed the genotypes of the heterozygous F1individuals with the presence of both hetero-duplex and homoduplex molecules (Figure 3,C and D).For fshb ,a 10-bp deletion (?10)leading to a frame shifting of the coding se-quence was chosen to establish the
mutant line for phenotype analysis,and a 5-bp deletion for lhb (?5)was selected (Figure 3).
Among sibling F1fish,we successfully identified males
and females that carried the same mutant sequences for
both fshb ?10/?and lhb ?5/?.Homozygous fshb ?/?(?10)
and lhb ?/?
(?5)were obtained in F2,and their genotypes were confirmed by HRMA (Figure 4,A and C)and HMA (Figure 3,C and D).
To further confirm the successful deletion of the fshb and lhb genes,we also performed RT-PCR analysis for fshb and lhb mRNAs in the pituitary to ensure no
expres-
Figure 7.Embryonic offspring from fshb and lhb -deficient zebrafish (fshb ?/?and lhb ?/?).The fertility of homozygous fshb and lhb mutant fish was assessed by natural breeding between mutants or with wild-type fish.Both male and female fshb mutants were fertile with healthy and
viable progeny (A),whereas male lhb mutant was fertile but not females (B).Twenty-two lhb -deficient females were examined,but none succeeded in spawning (Figure
5).
Figure 8.Delayed puberty onset in female fshb ?/?mutants of different ages (45–80dpf).Juvenile females were sampled at different ages (45–80pdf)for histological analysis.The upper panel shows low-magnification view of the cross-sections,highlighting the location and relative size of the ovaries (boxed),whereas the lower panel is the close-up of the ovary showing follicle composition.A,The ovaries from control fish (fshb ?/?and fshb ?/?)with normal growth rate and advanced follicle stages.B,The ovaries of mutant fish (fshb ?/?)with smaller size and PG follicles only.The age,genotype,SL,BW,and stage of follicles in the ovary are shown in the table.
84Zhang et al Genetic Analysis of Zebrafish FSH and LH Functions Mol Endocrinol,January 2015,29(1):76–98
sion of functional transcripts.Specific primers (P3)over the deleted sequences of fshb ?/?(?10)and lhb ?/?(?5)were used for the amplification (P3?P4).The result clearly showed the lack of expression of functional tran-scripts in the mutant pituitary glands.As controls,prim-ers that flank the mutant sites (P1and P4)could amplify the transcripts as expected with the amplicons from the homozygous mutants slightly smaller than the homozy-gous and heterozygous individuals (?/?and ?/?)(Fig-ure 4,B and
D).
Figure 9.Delayed puberty onset in female fshb ?/?mutants of the same age (55dpf).Juvenile females of the same age were sampled for
morphological and histological analysis.A,The ovaries from control fish (fshb ?/?and fshb ?/?)with PV follicles (arrow).B,The ovaries of mutant fish (fshb ?/?)with PG follicles only.The age,genotype,SL,BW,and stage of follicles in the ovary are shown in the table (C).
doi:10.1210/https://www.360docs.net/doc/3c2301675.html, 85
Delayed ovarian growth and follicle development in FSH-deficient but not LH-deficient zebrafish
In our aquarium system,the zebrafish starts spawning around 2months after fertilization,although the fecun-dity is not high at this young stage.We first analyzed the
gross morphology and histology of the ovary in both the controls (fshb ?/?and fshb ?/?;lhb ?/?and lhb ?/?)and homozygous mutants (fshb ?/?and lhb ?/?)at 60dpf.With similar standard length and body weight,the FSH-deficient females (fshb ?/?)showed apparently smaller ab-domens compared with the control individuals (homozygous fshb ?/?and heterozygous fshb ?/?).It was indeed difficult to identify the gen-der of these fish until after dissection and with the help of microscope.The ovaries of the mutant fish were significantly smaller than those of the control individuals (Figure 5A),as demonstrated by the gonadoso-matic index (GSI ?gonadal weight/total body weight;P ?.05)(Figure 5B).Dissection and histological analysis showed that the ovaries from the control fish (?/?and ?/?)contained a full range of developing follicles from the PG (stage I)to the FG (stage III)stage,in agreement with the fact that these fish had started spawning.In contrast,the ovaries from the mutant FSH-defi-cient fish (?/?)contained mostly the PG follicles with a few follicles at the PV (previtellogenic;stage II),suggesting a significant block at the PG-PV transition and delayed devel-opment into the vitellogenic growth (Figure 5A).
In contrast,the lack of LH did not seem to have any effect on ovarian growth.The LH-deficient female fish (lhb ?/?)at 60dpf showed no dif-ference from the control of both ho-mozygous (lhb ?/?)and heterozygous (lhb ?/?)fish at either gross anatomi-cal or histological level.The ovaries in all three genotypes showed full development with all stages of fol-licles present from PG to FG stage (Figure 5C).
Failed spawning in LH-deficient but not FSH-deficient female zebrafish
Although the LH-deficient fe-males showed normal ovarian growth in contrast to that of
FSH-
Figure 10.Normal puberty onset in female lhb ?/?mutants.Both control (lhb ?/?and lhb ?/?)(A)and mutant fish (lhb ?/?)(B)had PV follicles with cortical alveoli (arrow)in their ovaries.The fish had similar age (50–55dpf)and body size.The age,genotype,SL,BW,and stage of follicles in the ovary are shown in the table (C).
86Zhang et al Genetic Analysis of Zebrafish FSH and LH Functions Mol Endocrinol,January 2015,29(1):76–98
deficient fish,these fish were infertile because they failed to spawn to produce fertilizable eggs.We examined all genotypes (lhb ?/?,lhb ?/?,and lhb ?/?)for spawning.All control individuals of homozygous (lhb ?/?)and heterozygous (lhb ?/?)genotypes spawned successfully (10fish each),producing fully vital offspring.In con-trast,none of the homozygous mutant individuals (lhb ?/?)succeeded in spawning (22in total).Further examination of the ovaries at 9:00AM when the light turned on showed that the control ovaries normally contained large number of mature and ovulated eggs with the chorion membrane separated from the egg and expanded;in contrast,only FG but immature follicles (no germinal vesicle breakdown)were present in the mutant ovaries,indicating a failure of final oocyte maturation (Fig-ure 5D).A few individual follicles were sometimes found to have undergone germinal vesicle breakdown in the ovary of the lhb KO mutant fish,but they were never ovulated (data not shown).
Interestingly,although the FSH-deficient females showed significantly delayed ovarian growth as de-scribed above at 60dpf,the development could quickly catch up in the month afterward.As shown in Figure 6,after a significant delay,the ovaries in
FSH-deficient
Figure 11.No effect of fshb and lhb gene disruption on spermatogenesis in the testis at mature stage (80dpf).Phenotypically male individuals were sampled at 80dpf and their gross morphology and histology were observed.All the individuals contained typical white thread-like testes (arrow),and histological examination showed normal cellular composition of testis in all genotypes for both fshb (A)and lhb (B).The fertility of these male fish had been tested by natural spawning (Figure 7).sc,spermatocytes;sg,spermatogonia;sz,spermatozoa.
doi:10.1210/https://www.360docs.net/doc/3c2301675.html, 87
fish(fshb?/?)gradually picked up the growth and be-came fully grown at90dpf(Figure6).In contrast to the LH-deficient females,the FSH-deficient females could successfully spawn eventually with vital offspring after the ovaries completed the growth(Figure7).Delayed puberty onset in FSH-deficient but not
LH-deficient females
Puberty onset is a very important event in reproductive development.The mechanisms underlying puberty onset in vertebrates remain elusive and poorly elucidated,and
they Figure12.Retarded spermatogenesis in FSH-deficient males(fshb?/?)at early developmental stage(64dpf).With the help of HRMA,male individuals of different genotypes were sampled at64dpf for histological examination.A,The testes of control fish(fshb?/?and fshb?/?)with normal size and spermatogenesis.B,The testes of mutant fish(fshb?/?)with smaller size and delayed spermatogenesis.The upper panel shows low-magnification view of the cross-sections highlighting the location and relative size of the testes(boxed),whereas the lower panel is the close-up of the testis showing stage of spermatogenesis.C,The age,genotype,SL,and BW of the fish shown.
88Zhang et al Genetic Analysis of Zebrafish FSH and LH Functions Mol Endocrinol,January2015,29(1):76–98
are entirely unknown in teleosts,especially with regard to the roles of gonadotropins in the process.After analyzing the impacts of FSH and LH deficiency on ovarian growth at the time of maturity as described above,we shifted our attention to the stage when puberty starts,which is marked by the appearance of the first cohort of PV follicles in the ovary.As we reported recently,the puberty onset in female zebrafish depends largely on body growth rather than age,and we have defined100mg body weight and1.80cm standard body length to be critical thresholds for the initiation of puberty(55).These criteria were also used in the present experiment to help with the evaluation of puberty onset and the impacts of FSH and LH deficiency.
We examined six homozygous mutant individuals (fshb?/?)of different ages(45–80dpf)with SL and BW, ranging from1.70to2.65cm and99to320mg,respec-tively,covering the point when puberty onset is expected to occur(1.80cm SL and100mg BW).As shown in Figure8,none of these mutant fish had PV follicles in the ovary,suggesting a significant delay of puberty onset in FSH-deficient females despite the fact that most individ-uals had crossed the thresholds in terms of either BW or SL or both.Female mutants could not break through the PG-PV transition even at70–80dpf with an SL of2.35 and2.65cm,well above the1.80-cm threshold defined previously(55).Furthermore,a histological examina-tion of whole-body cross-sections showed an overall smaller ovarian size in the mutants as compared with those in the controls.In contrast,all control fish of both homozygous and heterozygous genotypes(?/?and?/?) showed a normal initiation of puberty with PV and ad-vanced follicles[early vitellogenic(EV)and late vitello-genic]appearing in the ovary once the SL and BW ap-proached or crossed the defined threshold levels(1.80cm and100mg)(Figure8).
To rule out the influence of age on the result shown above in Figure8,we carried out one additional experiment by using fish of the same age at55dpf,when the puberty onset is expected to have happened according to our recent report (53).The result was consistent with that described in Figure 8.The control fish(?/?and?/?)with SL and BW above the threshold levels all had PV follicles well formed in the ovary(fshb251and fshb257).Even those slightly below the thresholds(fshb244and fshb245)showed signs of early PV follicles.However,none of the fshb KO mutant fish (?/?)showed any sign of PV follicle development,even in those with body sizes much bigger than the controls (Figure9).
In contrast to FSH-deficient fish,no significant pu-berty delay was observed in LH-deficient females.We performed the analysis at50–55dpf,the time when pu-berty onset is normally expected to occur(53,55),on individuals with SL and BW around or above the defined threshold levels(1.80–1.95cm and97–143mg).All in-dividuals had PV follicles well formed in the ovary with no difference among the three genotypes(lhb?/?,lhb?/?and lhb?/?)(Figure10).
Normal development of testis in FSH and
LH-deficient males at maturation stage
We also analyzed the phenotypes of male fshb and lhb KO zebrafish at80dpf.Surprisingly,neither fshb nor lhb KO mutants(?/?)showed significant signs of abnormal-ity in their testis development and spermatogenesis at gross anatomical and histological levels as compared with the con-trols.All stages of sperm cells including spermatogonia(sg), spermatocytes(sc),and spermatozoa(sz)were present in all individuals of the three genotypes(Figure11).Furthermore, all mutant fshb?/?and lhb?/?males were fertile as demon-strated by their successful spawning with females to produce normal offspring(Figure7).The lhb?/?males were tested with WT females as the lhb?/?females were unable to spawn and therefore infertile as described above.
Despite the normal fertility of fshb?/?and lhb?/?males at the mature stage,there was evidence for a role of gonadotropins in promoting early testis development,es-pecially for FSH.As shown in Figure12,when examined at64dpf when all control males(fshb?/?and fshb?/?) Figure13.Expression of lhb in the pituitary glands of control
(fshb?/?)and FSH-deficient(fshb?/?)female(A)and male(B)fish(130 dpf).Total RNA from each pituitary gland was reverse transcribed for real-time qPCR quantification of lhb expression,which was normalized to ef1a(mean?SEM,n?6).*,P?.05vs control.
doi:10.1210/https://www.360docs.net/doc/3c2301675.html,89
had their testes well developed with all stages of sper-matogenesis present,there was an obvious delay of sper-matogenesis in the fshb KO mutant fish (?/?),although all mutant fish examined had larger body sizes than those of the controls.The testes of the mu-tant fish contained germ cells mostly at the early stages of spermatogene-sis including spermatogonia and spermatocytes,and the germ cells of advanced stages were seldom pres-ent in the mutant testes.Further-more,the overall sizes of the testes were also much smaller than those in the controls (Figure 12).Increased expression of lhb in the pituitary glands of FSH-deficient fish
The results described above (Fig-ures 6and 12)showed that although there was a significant retardation of gonadal growth in FSH-deficient fe-males and males,the growth of ovary and testis could both catch up after a period of delay,leading to normal fertility in both sexes.One mechanism for the catch-up growth was likely due to the cross-activa-tion of FSH receptor Fshr by LH as we reported previously (41).To pro-vide supportive evidence for this hy-pothesis,we examined the expres-sion of lhb in the pituitary glands of FSH-deficient females and males (130dpf).Interestingly,the expres-sion level of lhb increased signifi-cantly in the pituitaries of female fshb ?/?fish.The level increased also in males,but it was not statistically significant due to high individual variation (Figure 13).We were un-able to measure plasma FSH and LH levels in the zebrafish because of the lack of specific RIAs in this species.
Evidence for a role of FSH in maintaining female status in mature adults
During our large-scale screening by histology,we discovered two fshb ?/?mutant fish with ovotestis
(fshb65and fshb140).This was never found in any other genotypes including lhb
?/?
mutants.Fshb65(68dpf)had a testis on one side but ovotestis on the other side with ovarian tissue being dominant,whereas in fshb140(72dpf),the
testis
Figure 14.Sexual reversal in FSH-deficient adult zebrafish.Two fish at 68and 72dpf were
identified with ovotestis.Fshb65(?/?)was fixed and sectioned as a whole with gonadal location
clearly shown on both sides,whereas for fshb140(?/?),the gonads were isolated for fixation and
sectioning.sc,spermatocytes;sg,spermatogonia;sz,spermatozoa.90Zhang et al Genetic Analysis of Zebrafish FSH and LH Functions Mol Endocrinol,January 2015,29(1):76–98
issue was dominant.The ovarian and testis tissues were well separated without much mingling in both fish,and the ovarian tissues contained well-developed vitellogenic follicles (PV and EV)with some undergoing degeneration
or apoptosis (Figure 14).The pres-ence of vitellogenic follicles indi-cated that the fish were likely geno-typically female,and they might possibly be undergoing sex reversal.No evidence for the
involvement of FSH and LH
alone in gonadal differentiation The discovery of hermaphroditic fshb ?/?fish in adult stage prompted us to speculate a possible role for gonadotropins in influencing go-nadal differentiation.To address this issue,we did an experiment to analyze the development of gonads at 35–40dpf (35dpf for fshb and 40dpf for lhb ).This time window was chosen because our previous study has shown that the zebrafish ovary forms from the undifferentiated so-called ovary-like state around 30dpf,whereas the formation of testis lagged behind by approximately 2weeks,ie,approximately 45dpf (55).Our result showed that all four genotypes analyzed (fshb ?/?and fshb ?/?;lhb ?/?and lhb ?/?)had fe-males with well-formed ovaries con-taining early perinucleolar oocytes (fshb302and fshb309,lhb76and lhb80)and undifferentiated gonads,which might likely develop into tes-tis as shown by early signs of sper-matogenesis (presence of spermato-gonia and spermatocytes)(fshb300and fshb298,lhb94and lhb90)(Fig-ure 15).We did not observe any dif-ference between heterozygous fish (fshb ?/?and lhb ?/?)and homozy-gous mutants (fshb ?/?and lhb ?/?).
Production of all-male offspring with double mutations of fshb and lhb
The significant phenotypes of de-layed development of ovary and tes-tis but normal fertility in the end in fshb ?/?fish led us to hypothesize
that LH could play a compensatory role due to its cross-activation of Fshr.This idea was supported by the evi-dence that the expression of lhb increased in fshb ?/?
fish
Figure 15.No effect of fshb and lhb gene disruption on gonadal differentiation.To investigate whether gonadotropins play any roles in gonadal differentiation,samples were taken at 35dpf for fshb and 40dpf for lhb .At 35dpf,both control (fshb ?/?)and mutant (fshb ?/?)had already completed gonad differentiation,with no significant difference between the two https://www.360docs.net/doc/3c2301675.html,pared with the ovary,the formation of testis was slower.The same was observed for control and mutant lhb at 40dpf.
doi:10.1210/https://www.360docs.net/doc/3c2301675.html, 91
(Figure 13).To provide further evidence for this hypoth-esis,we created a mutant zebrafish with double mutations of fshb and lhb.Interestingly,lack of FSH and LH led to all-male offspring.At 55dpf,the control fish expressing either fshb and lhb alone or both (fshb ?/?/lhb ?/?,fshb ?/
?/lhb ?/?
,and fshb ?/?/lhb ?/?)all had normal ovarian de-velopment;in contrast,the gonads of double-mutant fish (fshb ?/?/lhb ?/?)all stayed at very early stage without obvious signs of sexual differentiation (Figure 16).Fur-ther analysis of elder fish (100–120dpf)showed that all double-mutant fish developed into males (Figure 17A)(n ?31),whereas the control fish (fshb ?/?/lhb ?/?,n ?14;fshb ?/?/lhb ?/?,n ?32)exhibited normal gonadal differentiation and development (Figure 17B)with an ex-pected sex ratio (?50%females)(Figure 17C).The all-male phenotype did not seem to be due to sex reversal because no oocytes were observed in any individuals at any stage.The level of spermatogenesis in double-mutant
fish varied significantly among indi-viduals.Some individuals showed no development beyond spermato-gonial stage at 110dpf (Fish 1),whereas others exhibited develop-ment of spermatocytes and even ma-ture spermatozoa (Fish 2–5,100–120dpf).In general,most mutant fish had small testis size compared with those in control fish of similar or even younger age (Figure 17,A and B).Despite the significant de-lay in spermatogenesis,the double-mutant fish were fertile and they could breed with WT females to produce normal offspring (Figure 17D).
Discussion
Using TALEN technology,the pres-ent study analyzed the functions of FSH and LH hormones in zebrafish reproduction,which not only con-firmed some aspects of FSH and LH functions proposed before but also provided evidence for novel func-tions of these hormones that would be otherwise difficult to demon-strate with other approaches.
Roles of FSH and LH in folliculogenesis
In the zebrafish model,our previ-ous study on FSH and LH receptors (Fshr and Lhcgr)has provided important clues to the functions of FSH and LH in females,which to some extent agrees with the generally accepted concept that FSH plays a major role in stimulat-ing follicle growth via its cognate receptor Fshr,whereas LH is involved in final maturation and ovulation.How-ever,there has been no direct evidence to verify this con-cept.By deleting the fshb and lhb genes from the genome,we provide the most comprehensive and conclusive ge-netic evidence so far in any teleost species for roles of these two important reproductive hormones.
Loss of the fshb gene and therefore the FSH hormone caused a significant retardation of follicle growth in fe-male zebrafish,especially at the PG-PV transition.This is similar to the phenotype observed in the FSH-deficient mice whose follicle development is blocked at the antral stage,resulting in female infertility (4).Interestingly,
de-
Figure 16.Gonadal development in fshb and lhb double mutants at 55dpf.A,Control fish expressing both fshb and lhb (fshb ?/?/lhb ?/?)or fshb and lhb alone (fshb ?/?/lhb ?/?,fshb ?/?/lhb ?/?)had normal ovarian formation.Arrows indicate PV follicles.B,Double mutations of fshb and lhb (fshb ?/?/lhb ?/?)led to the retarded development of the gonads (testis?)(circled and labeled by arrow).
92Zhang et al Genetic Analysis of Zebrafish FSH and LH Functions Mol Endocrinol,January 2015,29(1):76–98
spite a significant blockade at the PG-PV transition in the absence of FSH,PV follicles could still emerge after a delay in the zebrafish ovary,which differs from the situ-ation in the mouse (4).Once entering the PV stage,the follicles in fshb KO fish could quickly pick up the growth to complete the process of vitellogenesis in about one month,although the growth rate seemed slower than nor-mal,which takes about 2weeks according to our previous studies (42,56).This phenomenon raises an interesting question.After a delayed activation,what eventually trig-gers some follicles to break the blockade at PG-PV tran-sition to enter the vitellogenic growth?The answer re-mains unknown.We speculate that LH likely plays a compensatory role.
Our previous study has shown that LH can cross-acti-vate Fshr in the zebrafish at high concentrations (41),and this promiscuous activation of Fshr would allow a com-pensatory role for LH to drive folliculogenesis in the ab-sence of FSH.When the PG follicles grow to their full size prior to entering the PV stage,the expression of Fshr increases (42,43),therefore increasing the responsiveness of the follicles to FSH and probably,to a lesser extent,LH as well.Without FSH,LH may play a role in initiating the PG-PV transition albeit at slower rate due to its lower potency in stimulating Fshr (Figure 18).This idea is fur-ther supported by our evidence that lhb expression in the pituitaries of FSH-deficient fish increased in both females and males.It is conceivable that such an increase in LH biosynthesis may contribute to the compensatory role of LH in the absence of FSH.The mechanism for the in-creased lhb expression is unknown,and it will be an in-teresting issue to address in future studies.
The promiscuous activation of Fshr by LH has been reported in several species including the zebrafish (41).However,the functional significance of this partial acti-vation of Fshr by LH has remained entirely unknown and mysterious.The genetic approach adopted in this study by deleting the fshb and lhb genes seems to have provided a conceivable answer to the question.Despite this,we still need to be cautious in that the compensatory role of LH was revealed in the absence of FSH,which is a nonphysi-ological condition.Further studies on the fshr mutant will provide insight into this
issue.
Figure 17.All-male development in fshb and lhb double mutants at 100–120dpf.A,Double mutations of fshb and lhb (fshb ?/?/lhb ?/?)
produced all-male offspring with delayed development of the testis (circled).B,Control fish (fshb ?/?/lhb ?/?,fshb ?/?/lhb ?/?,fshb ?/?/lhb ?/?)had normal testis growth (circled)and spermatogenesis.C,Sex ratio in fshb and lhb double mutants.D,Normal fertility of fshb and lhb double mutants.sc,spermatocytes;sg,spermatogonia;sz,spermatozoa.
doi:10.1210/https://www.360docs.net/doc/3c2301675.html, 93
In contrast to FSH,the lack of LH had no observable effect on follicle growth in the zebrafish but caused infer-tility due to failed oocyte maturation and ovulation in vivo.This is in contrast to the infertility in LH-deficient female mice whose ovaries are small with abnormal antral follicles (5).This result also suggests that FSH plays no compensatory role for LH in controlling final oocyte mat-uration and ovulation in the zebrafish.
Roles of FSH and LH in female puberty onset
In fish,the appearance of the first wave of PV follicles in the ovary is considered a marker for puberty onset in females (55,57,58).Little is known about the mecha-nisms that trigger and control puberty onset in fish spe-cies.We previously proposed that FSH could be a poten-tial factor that triggers puberty onset in females because of the significant increase of fshr expression in PV follicles (42).However,our subsequent study on ontogeny of fshb and lhb expression during development led us to a differ-ent view that instead of FSH,it was likely LH that played a role in the event because the expression of lhb in the pituitary was very low before puberty but increased dra-matically when the first wave of PV follicles appeared in the ovary (53).This view,however,is not supported by the evidence from the present study.In contrast to the loss of fshb ,which caused a significant delay in PV follicle development,the disruption of the lhb gene had no effect at all on the timing of the PG-PV transition.Based on this,
we can conclude that the previously observed surge in lhb expression at puberty onset is the consequence rather than the cause of puberty onset.
Roles of FSH and LH in spermatogenesis
One of the most striking discoveries in the present study was the role of FSH and LH in testis development and spermatogenesis in males.In the absence of FSH and LH alone or both,the males were all fertile with a normal ability to spawn and produce vital offspring,although fshb ?/?mutant and fshb ?/?/lhb ?/?double-mutant fish had slower testis growth.This result suggests that differ-ent from our traditional view on the importance of neu-roendocrine regulation of gonadal development and func-tion (59),the classical reproductive axis involving FSH and LH may play a less-than-expected role in the repro-duction of male zebrafish.It is likely that after the initial promotion by FSH to trigger male puberty in juveniles,the process of spermatogenesis becomes independent of pituitary gonadotrophic hormones.This agrees with a view in mammals that FSH plays a role in initiating sper-matogenesis but not in maintaining the process in adults (4,60,61),although this concept still remains controver-sial (62).In FSH-deficient mice,the males are fertile but oligospermic with smaller testes (4),which agrees well with our finding in the zebrafish.As for the role of LH,our discovery is in sharp contrast to that in mammals.The LH-deficient zebrafish males seemed normal with full
ca-
Figure 18.Hypothetical model for roles of FSH and LH in controlling follicle development and maturation in the zebrafish ovary.FSH rather than LH plays a key role in setting the timing of puberty onset in females,and FSH is a dominant hormone in controlling follicle growth afterward.In the absence of FSH,LH will kick in to play a role in initiating follicle activation (PG-PV transition)and promoting its growth via its cross-reaction with the FSH receptor (Fshr).At the end of folliculogenesis,it is LH but not FSH that plays a critical role in triggering final oocyte maturation and ovulation in females.In addition to promoting follicle growth,FSH may also play a role in maintaining female status in adult,probably through stimulating and maintaining aromatase expression in the ovary.
94Zhang et al Genetic Analysis of Zebrafish FSH and LH Functions Mol Endocrinol,January 2015,29(1):76–98
pacity to reproduce.In LH-deficient mice,however,both males and females are infertile(5).The major regulators of spermatogenesis in adult male zebrafish may come from other endocrine axes,in particular the soma-totrophic axis.This view is supported by in vitro evi-dence that the spermatogenesis in cultured zebrafish testis could be promoted by IGF-1alone,which stimu-lated the development of spermatogonia to spermato-cytes and spermatids(63).
Roles of FSH and LH in gonadal differentiation One interesting discovery in the present study was the hermaphroditic fish in fshb KO mutants at the adult stage.Although we should be cautious in interpreting the result due to the small sample size,it raises a possibility for a role of FSH in maintaining female status after sexual differentiation.It is likely that despite the compensatory role of LH in supporting folliculogenesis,the lack of FSH may lead to a lower expression level of aromatase in the ovary,making the fish susceptible to both internal and external influences that favor a conversion of ovary into testis through sex reversal.This idea is supported by re-cent studies in the zebrafish that demonstrated sex rever-sal in mature adult females after treatment with aroma-tase inhibitor(64,65).
To test this hypothesis,we went on to generate a mu-tant zebrafish that carries double mutations of fshb and lhb.Unexpectedly but interestingly,all double mutant fish turned out to be males with normal but retarded testis growth and spermatogenesis.The production of all-male offspring raises an interesting question on the role of go-nadotropins in sexual differentiation.Our previous study has demonstrated that during zebrafish life cycle,the ex-pression of lhb appeared first at the time of gonadal dif-ferentiation,and its signal could be detected only in the pituitaries of individuals whose gonads contained well-developed oocytes;in contrast,there was no detectable lhb expression in those with either undifferentiated go-nads or testis.This has led us to hypothesize that gonad-otropins may likely play a role in gonadal differentiation (53).The all-male phenotype of fshb and lhb double mu-tation provides a strong support to this hypothesis.The effect of LH on ovarian differentiation may be subtle in the presence of FSH;however,it becomes significant in the absence of FSH.
Table3.Phenotype Comparison of FSHB/Fshb/fshb and LHB/Lhb/lhb Mutants in Humans,Mice,and Zebrafish
Gene Gender Species Phenotype Reference fshb Female Human 1.Hypogonadism(66–68)
2.Delayed puberty
3.Amenorrhea
4.Infertile
Mouse 1.Arrested folliculogenesis at antral formation(4)
2.Infertile
Zebrafish 1.Delayed puberty Present study
2.Slower folliculogenesis
3.Fertile
Male Human 1.Hypogonadism(69)
2.Azoospermia
Mouse 1.Smaller testis(4)
2.Fertile
Zebrafish 1.Slower progression of initial spermatogenesis Present study
2.Fertile
lhb Female Mouse 1.Small ovary(5)
2.Abnormal folliculogenesis
3.Degenerating antral follicles
4.Absence of corpora lutea
5.Infertile
Zebrafish 1.Normal folliculogenesis Present study
2.Failed oocyte maturation and ovulation
3.Infertile
Male Human 1.Delayed puberty(70)
2.Infertile
Mouse 1.Smaller testis(5)
2.Reduced testosterone level
3.Blocked spermatogenesis at round spermatid stage
4.Infertile
Zebrafish 1.Normal spermatogenesis Present study
2.Fertile
doi:10.1210/https://www.360docs.net/doc/3c2301675.html,95
斑马鱼作为研究营养与生长的模式生物:向水产鱼类的营养基因组学的研究提供参考
斑马鱼作为研究营养与发育的模式生物:为水产鱼类的营养基因组学的研究提供参考摘要 斑马鱼是最普遍的用来研究毒理学、发育生物学、神经生物学和分子遗传学的模式生物。人们提出把它当做一个可能的研究鱼类营养与发育的模型。斑马鱼用于这一领域研究的好处是它们尺寸小、生殖周期短(12-14周)可以产出大量的卵。后来有了大量的分子工具,同时可以通过基因分析获得相关信息,但是斑马鱼仍然在鱼类的营养基因组学的研究中当做模式生物使用。作为模式生物,对其每一个特点的研究都是细微的,这是因为这些特点是用来推理几个生物过程是如何在相关生物身上发生的,同时为扩增我们在鱼类营养和发育机制方面的知识做出重大的贡献。这篇综述的目的是展示斑马鱼在营养和发育方面的相关研究,从而说明斑马鱼作为研究鱼类营养基因组学的模式生物的价值。我们特别强调斑马鱼中由营养因素导致的基因表达和遗传变异可以用来阐明水产养殖鱼类中的类似过程。 关键字:斑马鱼,发育,营养,营养基因组学,比较基因组学 前言
斑马鱼已经成为研究在个体发育、神经生物学分子遗传学研究的十分普遍的模式生物(Driever et al. 1994; Roush 1996; Bergeronet al. 2008)。近来,人们提出在鱼类营养和发育的研究方面斑马鱼可以作为一种模式生物(Alestro m etal.2006; Dahm andGeisler 2006; De-Santis and Jerry 2007; Wright et al.2006; Johnston et al. 2008)。 人们一个主要的研究兴趣就是生长发育的特点。因为这与水产养殖行业中,鱼类的生产量和可获取的利益密切相关(De-Santis and Jerry 2007)。这些特点中,表型性状是基因控制的,但也取决于环境因素,这些因素中,又直接由营养条件影响(Moriyama et al.2000)。基因研究工具的发展,使我们有机会去弄清楚数量性状相关的基因的变化,随着那些影响养殖生物生长特征的QTL基因座图谱的建立,在生长发育中鉴别候选基因变得非常有效的(Davis and Hetzel2000; Fjalestad et al. 2003; Reid et al. 2005; Aranedaet al. 2008; Lo Pestri et al. 2009; Dumas et al. 2010)。在这一方面,斑马鱼有比其它养殖动物更加多样的分子工具和可获得的基因分析的数据。 人们建议把斑马鱼作为研究鱼类营养基因组学研究的模式生物,同时期望从这一途径获得的结果可以为水产养殖鱼类提供合适的比较基因组学信息(Metscher and Ahlberg 1999; Drew et al. 2008;Robison et al. 2008; Crollius and Weissenbach 2008)。营养基因组学是一门合营养学和遗传学的学科。是一门通过“营养基因组学”(研究日常食物是如何影响某些基因表达的)和“营养遗传学”(研究基因突变对个体“营养应答”的影响)两种手段研究营养-基因相互作用的一门科
最新生物学常见模式生物资料
模式生物 生物学家通过对选定的生物物种进行科学研究,用于揭示某种具有普遍规律的生命现象。此时,这种被选定的生物物种就是模式生物。比如:孟德尔在揭示生物界遗传规律时选用豌豆作为实验材料,而摩尔根选用果蝇作为实验材料,在他们的研究中,豌豆和果蝇就是研究生物体遗传规律的模式生物。由于进化的原因,许多生命活动的基本方式在地球上的各种生物物种中是保守的,这是模式生物研究策略能够成功的基本基础。选择什么样的生物作为模式生物首先依赖于研究者要解决什么科学问题,然后寻找能最有利于解决这个问题的物种。19世纪末20世纪初,人们就发现,如果把关注的焦点集中在相对简单的生物上则发育现象的难题可以得到部分解答。因为这些生物更容易被观察和实验操作,因此,除了在遗传学研究外,模式生物研究策略在发育生物学中获得了非常广泛的应用,一些物种被大家公认为优良的模式生物,如线虫、果蝇、非洲爪蟾、蝾螈、小鼠等。 随着人类基因组计划的完成和后基因组研究时代的到来,模式生物研究策略得到了更加的重视;基因的结构和功能可以在其它合适的生物中去研究,同样人类的生理和病理过程也可以选择合适的生物来模拟。 目前在人口与健康领域应用最广的模式生物包括,噬菌体、大肠杆菌、酿酒酵母、秀丽隐杆线虫、海胆、果蝇、斑马鱼、爪蟾和小鼠。在植物学研究中比较常用的有,拟南芥、水稻等。随着生命科学研究的发展,还会有新的物种被人们用来作为模式生物。但它们会有一些基本共同点: 1)有利于回答研究者关注的问题,能够代表生物界的某一大类群; 2)对人体和环境无害,容易获得并易于在实验室内饲养和繁殖; 3)世代短、子代多、遗传背景清楚; 4)容易进行实验操作,特别是具有遗传操作的手段和表型分析的方法。 背景 早在20世纪最初的20年中,甚至更早到19世纪,人们就发现,如果把关注的焦点集中在相对简单的生物上则发育的现象难题可以得到部分解答。因为这些生物的细胞数量更少,分布相对单一,变化也较好观察。由于进化的原因,细胞生命在发育的基本模式方面具有相当大的同一性,所以利用位于生物复杂性阶梯较低级位置上的物种来研究发育共通规律是可能的。尤其是当在有不同发育特点的生物中发现共同形态形成和变化特征时,发育的普
硫酸铜对斑马鱼胚胎发育的影响
中国海洋大学实验报告 年月日姓名:专业年级:同组者: 课程:发育生物学实验题目:硫酸铜对斑马鱼胚胎发育的影响 一.【实验目的】 1、锻炼独立开展科学实验、分析和解决实际问题的能力; 2、加深环境对动物受精及早期发育影响的理解 二.【实验原理】 重金属污染是近年渔业环境污染的公害之一。随着工农业的发展,浓度严重超标的一些重金属离子被排入水体而造成污染,对鱼类有毒害作用。目前水体中的重金属主要有Cd、Cu、Pb、Zn等。其中Cu离子就具有较强的毒性,可以和蛋白质中游离的羧基形成不溶性的盐,使蛋白质变性,因此常被用作杀菌剂。 斑马鱼(Danio rerio)是常见的暖水性(21~32℃)观赏鱼,鲤科,个体小(4~5㎝),常年产卵,鱼卵易收集,性成熟周期短且胚胎透明,便于观察药物对其体内器官的影响。雌性斑马鱼可产卵200枚,胚胎在24小时内就可发育成形,繁殖水温24℃时,受精卵经2~3天孵出仔鱼;水温28℃时,受精卵经36小时孵出仔鱼,这使得生物学家可以在同一代鱼身上进行不同的实验,进而研究病理演化过程并找到病因。由于斑马鱼基因与人类基因的相似度达到87%,这意味着在其身上做药物实验所得到的结果在多数情况下也适用于人体,因此它受到生物学家的重视。 在斑马鱼的整个生活周期中,胚胎期和仔稚幼鱼早期发育阶段对重金属污染最为敏感。据报道,波罗的海春天产卵的鲱鱼,在10 ppb 的Cu2+,水的盐度为5.7条件下即对它的受精和发育有影响。30 ppb的Cu2+引起大西洋鲱鱼(Clupea harengus)卵的死亡,而35 ppb 的Cu2+也使太平洋鲱鱼(C.pallasi)的胚胎发生大量死亡[1]。吴玉霖等1990年依据不同金属对褐牙鲆胚胎的滞育、致畸、成活率及孵化率的综合影响指际,得出5种金属对褐牙鲆胚胎的毒性大小顺序为:Cu2+>Zn2+>Cd2+>Pb2+>Cr3+,对仔鱼的毒性大小顺序为Cu2+>Cd2+>Zn2+>Pb2+>Cr3+ [2]。 三.【实验步骤】 1、实验材料 斑马鱼囊胚、CuSO4为分析纯,充分曝气的去离子水、24孔细胞培养板、恒温培养箱、用充分曝气的蒸馏水配置成浓度为5 mg/L 的CuSO4母液,用时稀释。. 2、实验方法 1)硫酸铜浓度梯度的设定 经查阅文献,确定斑马鱼胚胎发育从受精到孵化出幼鱼过程中的CuSO4半致死浓度和安全浓度范围,设定5个浓度梯度和和1个对照组。 研究表明,Cu2+对大银鱼受精卵的安全浓度为0.00112 mg/L[3],对鮸状黄姑鱼仔鱼的安全浓度为0.006mg/L[4],所以设定对照组CuSO4的浓度梯度分别为0(空白对照),0.001 mg/L,0.01 mg/L,0.1 mg/L,1.0 mg/L,2.0 mg/L。每个浓度组设定4个平行组。使用暴晒后的
斑马鱼动物模型的应用介绍
斑马鱼动物模型的应用 斑马鱼(Danio rerio)属于辐鳍亚纲(Actinopterygii)鲤科(Cyprinidae)短担尼鱼属(Danio)的一种硬骨鱼,原产于南亚,是一种常见的热带观赏鱼,因其体侧具有斑马一样暗蓝与银色相间的纹条而得名。 斑马鱼个体小,易于饲养,成体长4-5cm,雄鱼体修长,雌鱼体肥大。可在有限空间里养殖相当大的群体,可满足样本需求量大的研究。斑马鱼发育迅速,在28.5℃培养条件下受精后约40min完成第一次有丝分裂,之后大约每隔15min分裂一次,24h后主要器官原基形成,相当于28d的人类胚胎,幼鱼孵出后约3个月达到性成熟。雌雄鱼通过调控光周期控制14:10(光照:黑暗)产卵时间,成熟鱼每周可产卵一次,一尾雌鱼每次可产卵100-300枚。胚胎体外受精,体外发育,胚体透明,易于观察。受精卵直径约1mm,易于进行显微注射和细胞移植等操作。 一、斑马鱼的品系 经过30多年的研究应用和系统发展,已有约20个斑马鱼品系,斑马鱼基因数据库-ZFIN (http://zfin/org)里有相关的资料可供查询和下载。目前研究中常用的斑马鱼野生型品系主要为AB 品系、Tuebingen(Tu)品系、WIK 品系,斑马鱼基因组计划所用品系是Tu。AB 品系是实验室常用的斑马鱼品系,由单倍体细胞经早期加压法获得。Tu品系斑马鱼具有胚胎致死突变基因,用于基因组测序前敲除该致死突变基因。WIK品系较Tu品系具有更多的形态多样性。此外,还保存有3000多个突变品系和100多个转基因品系。这些品系资源对于利用斑马鱼开展各种科学研究起着很大的推动作用。 二、斑马鱼突变品系的筛选 斑马鱼突变的方法主要有三种:已基亚硝脲(ENU)化学诱导、γ或χ射线照射和插入诱变。ENU是一种DNA烃基化试剂,在生殖细胞减数分裂前诱导碱基对的替换,诱导产生的突变率为0.1%-0.2%,涉及单个基因的突变。射线照射导致染色体大片段的缺失或染色体重排,产生突变率达1%。插入诱变是以逆转录病毒为载体,用显微注射法将目的基因片段导入斑马鱼受精卵,整合到基因组中,干扰正常基因表达。射线照射产生的突变率是ENU 化学诱导的10倍,但由于突变涉及多个基因,突变的表型是受若干基因调控的结果,不利于基因功能的分析,因此,ENU化学诱导法是斑马鱼突变的主要方法。研究含有纯合致死
斑马鱼的胚胎发育与影响因素
鲁东大学生命科学学院学院20 10 -20 11 学年第二学期学院______________ 专业_____________ 年级________ 班________ 学号 _____________姓名______________ 密封线 学生须将文字写在此线以下 《发育生物学》课程论文 课程号:2522080
.关键词:斑马鱼;发育;葡萄糖;溶液浓度;温度;TCDD 一、斑马鱼简介 斑马鱼(zebra fish),又名蓝条鱼、花条鱼、斑马担尼鱼。 斑马鱼是一种常见的热带鱼。斑马鱼体型纤细,成体长3-4cm,对水质要求不高。孵出后约3个月达到性成熟,成熟鱼每隔几天可产卵一次。卵子体外受精,体外发育,胚胎发育同步且速度快,胚体透明。发育温度要求在25-31℃之间。斑马鱼由于个体小,养殖花费少,能大规模繁育,且具许多优点,吸引了众多研究者的注意。经过30多年的研究应用和系统发展,已有约20个斑马鱼品系,斑马鱼基因数据库里有相关 斑马鱼的资料可供查询和下载,方便了研究。斑马鱼的细胞标记技术、组织移植技术、突变技术、单倍体育种技术、转基因技术、基因活性抑制技术等已经成熟,且有数以千计的斑马鱼胚胎突变体,是研究胚胎发育分子机制的优良资源,有的还可做为人类疾病模型。斑马鱼已经成为最受重视的脊椎动物发育生物学模式之一,在其它学科上的利用也显示很大的潜力. 二、斑马鱼的发育过程 1〕卵子的发生 斑马鱼卵子发生过程中乱母细胞的发育是不同步的。在卵子发生早期,核内许多小核仁开始富集,其数目在接下来的时期中可以达到1500个,他们分布在和的外围和靠近内部核膜。 斑马鱼卵子发生过程一般分为5个时期,即StageⅠ-Ⅴ;有时也将StageⅡ和Ⅲ作为一个时期将卵子发生分为4个时期。各个时期的基本特征是: StageⅠ是原始滤泡生长阶段,乱母细胞没有卵黄,是一个有细胞质包围着升值滤泡的圆形球体。其染色体去浓缩并出现灯刷装 状表型,此时DNA高度延伸,形成一个具有典型形态学上的包含RNA和蛋白质的恻环。染色体的这一构型被认为有利于母源性基因的转录激活,其出现与斑马鱼卵母细胞中RNA合成的高速率是一致的。在这时期,卵母细胞和滤泡细胞的微小突起(microvilli)开始伸展并延伸到彼此的区域。一旦形成,这些连接包含粘附连接、细胞桥粒和间隙连接,它们可能与滤泡细胞和卵母细胞间的交流和卵母细胞附近小分子的吸收有关。同时,卵黄膜组分开始在滤泡细胞和卵母细胞之间富集;其他结构,如线粒体、高尔基体和内质网等富集,反映了产生大量产物的需要。 StageⅡ卵母细胞的特点是富集了大量蛋白质和油脂,对于卵子激
斑马鱼性腺促熟及早期发育模式
年月日姓名:专业年级:同组者 科目:发育生物学实验题目:斑马鱼性腺促熟及早期发育模式 一【目的要求】 1、通过实验操作掌握斑马鱼性腺促熟和产卵调控技术 2、通过斑马鱼早期发育的观察,巩固对硬骨鱼胚胎发生的认识 二【实验材料】 (一)器材 培养缸、控温棒、解剖镜、显微镜 (二)试剂 经太阳晒过至少一天的自来水 (三)动物 斑马鱼(Danio sp.) 三【实验内容】 (一)亲鱼培育和性腺促熟 挑选体长大于4厘米的斑马鱼放养于鱼缸中,水温保持在28℃左右,放养数量根据鱼缸中水体体积而定,密度5尾\L左右。饲喂亲鱼用的饲料有活性饲料和配合饲料两种,直接购自于观赏鱼市场,要求每天投喂4次,及时清除残饵,隔天换水,快到繁殖季节时将雌雄分养,加强管理。 (三)繁殖 繁殖前一天中午,将雌雄合养于繁殖缸里,要求雌雄比例为2:1。由于斑马鱼有食卵的习性,为防止亲鱼吞噬鱼卵,可用网孔为2-3毫米的网将亲鱼限制在繁殖缸的上半部活动,以防止亲鱼吞吃鱼卵。一般次日凌晨到中午可以产卵和受精,受精卵便沉降于缸底,将繁殖后的亲鱼及时转移至别的培养缸中,吸取缸底受精卵,剔除异物以及眼观有白色小斑点、畸形异常卵。 (三)斑马鱼胚胎发育模式 孵化期间,培养用水温度控制在25-28℃,每天要及时清除败育卵,并换水1-2次。按时观察记录斑马鱼胚胎的早期发育过程,绘制斑马鱼的胚胎发育模式图。 1、受精卵:斑马鱼的卵呈圆球形,橙黄色、微透明,直径0.8-0.9 mm。在水中,受精卵卵膜(壳膜)迅速膨胀,出现透明的卵周隙,在壳膜上可以看到呈漏斗状的卵膜孔。 2、卵裂:卵子受精后,细胞质迅速向动物极流动,并集中形成帽状的胚盘。卵裂即在胚盘范围内进行,卵裂属于不全裂,盘状卵裂。第一次分裂为经裂,分裂沟自上而下,但不到达底部,结果分为两个相等的不完整分裂球。第二次分裂仍为经裂,分裂面与第一次垂直,仍是不完全分裂,于是分成大小相似的四个分裂球。第三次分裂亦为经裂,两个分裂面在第一次分裂面两侧,并与第一次分裂面平行,形成两排,每排四个,共八个分裂球。第四次分裂仍为经裂,两个分裂面在第二次分裂面的两侧,并与第二次分裂面平行,分裂为四排每排有四个分裂球,共形成十六个分裂球。第五次分裂,有经裂也有纬裂,分裂面己不整齐,分裂球大小也不一致。以后几次分裂,分裂球愈分愈小。 3、囊胚期:由于细胞不断分裂,数目增多,细胞体积逐渐变小,分裂球层次增加,同时在胚盘与卵黄之间产生一空腔,即为胚盘下腔,此时称囊胚期,又分为囊胚早期、中期和晚期,也称高囊胚、中囊胚和低囊胚。 4、原肠胚期:囊胚晚期后,细胞逐渐向植物半球下包,胚盘变扁,开始进入原肠期。当胚盘下包到卵黄的一半时,胚环最大,背唇呈新月状,此即胚盾开始,即原肠早期。胚盘继续下包到胚胎的三分之二时,由于细胞不断集中于胚环的一处,致使该处呈一盾状隆起即
斑马鱼胚胎发育过程中Mef2c的表达
万方数据
复旦学报(医学版)2006年1月,33(1) 脏的发生与发育过程中的作用提供依据。 材料和方法 斑马鱼胚胎固定收集不同发育时期的AB野 生型斑马鱼(购自俄勒冈大学,斑马鱼养殖系统从美国AquaticHabitats公司引进)胚胎:6hpf(hourspost—fertilization,受精后6h)、7、8、9、10、11、12、13、15、17、20、24、36、48hpf,用4%多聚甲醛溶液固定过夜(至少固定12h),保存于甲醇溶液中,置一20℃备用。 引物设计与合成首先根据Genbank数据库查得斑马鱼Mef2ccDNA序列(Genbank:30575),以包含密码子1319~2385位的基因序列为RNA探针序列,探针序列总长1066bp。RNA探针的引物序列(由上海赛百盛生物有限公司合成)为:For—ward:5'-CTCAAATACGGAAAAGCTAC一3 7Reverse:5'-CGCCCGTGGGACTGATGA GAG一3 7。 PCR扩增以斑马鱼基因组总DNA为模板,用以上两条引物特异扩增RNA探针序列。扩增条件为:95℃预变性2min,95℃变性45S,57℃复性45S,72℃延伸2min,重复30个循环,最后72℃延伸7min。1%琼脂糖凝胶电泳检测扩增产物。 合成反义RNA探针将纯化的扩增产物连接到pGEM—T载体(Promega)中,然后转化到DH5a感受态菌株(博大泰克)中进行克隆。根据Harland的方法[43抽提转染后的连接载体质粒(测序验证质粒中插入序列是否正确),NotI内切酶(NEB)酶切完全,以其为模板转录合成反义RNA探针。 整体原位杂交取不同发育时期的胚胎,用1×PBST溶液洗去多余的甲醇溶液,将胚胎置于65℃水浴进行预杂交3h,然后加入所合成的反义RNA探针65℃水浴杂交过夜。多余的探针用0.2×SSC溶液洗去,加入anti—Dig—AP(Roche)与反义RNA探针结合过夜。未结合的抗体用1XPBST溶液洗去,再加入BCIP/NBT/NTMT溶液显色30min,迅速用1XPBST溶液洗去多余的显色液,在显微镜下观察并记录结果。 结果 探针合成效果琼脂糖凝胶电泳检测结果表明,本实验中设计的引物能够特异性地扩增目标基因片段。图1A为特异扩增的RNA探针序列电泳检测结果,扩增的RNA探针序列大小约1066bp。图1B为酶切后电泳迁移率的改变,2、3泳道是未被酶切的质粒序列,4、5泳道是酶切后的质粒序列。酶切后质粒电泳速率要比未酶切质粒的速率要快。同时,我们将该序列测序后进一步验证(由上海赛百盛生物有限公司测序),连接至pGEM—T载体中的RNA探针的序列是正确的。 图l凝胶分析PCR结果(A)和质粒酶切结果(B)Fig1GelanalysisPCR(A)andplasmid(B) A:1:marker;2-5;RNAprobe.B:1:marker;2-3:Mef2cRNA—pGEMT; 4-5:Mef2cRNA-pGEMTcutbyNot-1 斑马鱼整体原位杂交在斑马鱼胚胎发育早期,Mef2c在胚体中没有自身的转录产物,仅少许从母体自带的Mef2cmRNA存在,胚体染色呈现为弥散均染状态(图2:a,b见封二)。当胚胎逐步发育到13hpf,Mef2c在体节中开始表达,此时约已形成8个体节,表现为在背侧出现两条条带状的深染部位;同时,在心脏中的表达也开始出现,在靠近头侧部出现有深染的片状区域,代表早期的生心区的细胞中有Mef2c表达(图2:cl,c2见封二)。当胚胎发育至15hpf时,体节以及心脏中Mef2c的表达更加明显,Mef2c在体节的表达表现为各个体节之间能清晰区分,并表现为典型的V型结构;同时,心脏中的表达表现为生心区细胞开始集中,逐步靠近体轴开始形成心管结构,体现出染色更为集中,由片状染色转变成为线状(图2:dl,d2见封二)。随着胚胎进一步发育,体节逐步完善,心管逐步形成,Mef2c仍然保持较高的转录水平,体节中表现为随着体节的增多,V型染色的体节也随之增多;在心脏的表达呈现出明显的线管状结构(图2:e,f见封 --)。斑马鱼的胚胎发育随着时间的进展而逐步完 万方数据
斑马鱼简介
它是发育生物学家的得力助手,是医学研究的后起之秀,是环境监测的哨兵,是指示兵,是药物研发的新宠,它就是脊椎类模式动物明星斑马鱼(zebrafish,Danio rario)。 一、斑马鱼基本特点: 原产地:热带淡水鱼,原产于喜马拉雅山南麓的印度、巴基斯坦、孟加拉和尼泊尔等南亚国家。成鱼体长3-100px,略呈纺锤形,头小而稍尖,吻较短,身躯玲珑而纤细,因其体侧具有像斑马一样纵向的暗蓝色与银色相间的条纹而得名。 http://www.mun.ca/biology/desmid/brian/BIOL3530/DEVO_03/ch03f09.jpg 斑马鱼是体外受精发育,胚体透明。胚胎发育快,受精后3天左右孵化出膜,天左右开口进食,约3个月达到性成熟,寿命可达2年以上。斑马鱼可常年产卵,繁殖周期3-4天,一对成年斑马鱼每次可产卵200-300枚,受精率通常在70%以上。养殖温度一般在23-31℃,15℃左右仍可存活,最佳养殖温度在25-28摄氏度之间,pH值在6.8~7.8,硬度在2~6之间。 二、斑马鱼作为模式生物的起源和发展 1938年,美国布朗大学Roosen-Rung教授首次报道斑马鱼发育形态学研究成果。1950年代,美国罗格斯大学K. Kenneth Hisaoka教授首次报道斑马鱼毒理学研究成果。1972年,美国俄勒冈大学George Streisinge教授开始斑马鱼发育生物学研究和模式动物建立工作。1989年,美国俄勒冈大学Monte Westerfield教授出版斑马鱼研究圣经The Zebrafish Book第一版。1998年,首个斑马鱼模式生物数据库ZFIN成立(https://www.360docs.net/doc/3c2301675.html,)。1998年,全球第一家斑马鱼药物研发
斑马鱼性腺促熟及早期发育模式
斑马鱼性腺促熟和早期发育模式 XXX,YYY,ZZZ 一、目的与要求: 1、掌握斑马鱼性腺促熟和产卵调控技术。 2、加深硬骨鱼早期形态发育模式的理解。 二、实验内容: (一)斑马鱼性腺促熟和产卵调控。 1、斑马鱼特性: 斑马鱼一般4月龄性成熟,5月龄鱼繁殖较好;繁殖周期短,一般7天左右。 雌雄分辨:雌性(偏银灰色,体形丰满,腹部膨大、松软,仰腹可见有明显的卵巢轮廓,手摸富有弹性);雄性(偏柠檬色,腹部扁平,身材显得修长)。【如图一、图二】 图一:雄鱼图二:雌鱼 精、卵体外受精,体外发育,且速度快。发育速度与温度密切相关:在25℃的培养条件下,从受精卵到孵化约需36h;在28℃的培养条件下,从受精卵到孵化约需24h,即胚胎发育成熟。 2、斑马鱼繁殖准备: 将亲鱼雌、雄分开饲喂2~3天(要在饲养箱中加一玻璃隔板,将雌、雄分开,但同时相互之间又要能够看到),繁殖时将雌、雄按1:1或2∶1比例放入产卵池中进行产卵受精。在此过程中一般采用10h光照,14h黑暗的光周期。斑马鱼一般在混合的次日凌晨产卵,为防止亲鱼吞噬鱼卵,可用网孔2~3mm的网将亲鱼限制在产卵池的上半部活动,以防止亲鱼吞吃鱼卵。每条雌鱼可产卵300~1000粒。 (二)斑马鱼早期发育观察: 斑马鱼早期胚胎发育主要有以下七个时期(附有相应的时间): (1)合子期Zygote Period(0-0.75h) (2)卵裂期Cleavage Period(0.75-2.2h) (3)囊胚期Blastula Period(2.25-5.25h) (4)原肠胚期Gastrula Period(5.3-10h) (5)体节期Segmentation Period(10-24h) (6)咽期Pharyngula Period(24-48h) (7)孵化期Hatching Period(48-72h)
基于斑马鱼的模式生物的关于人类疾病的最新研究进展
基于斑马鱼的模式生物的关于人类疾病的最新研究进展 摘要:作为一种理想的生物实验模型,斑马鱼在生物学和人类疾病发面有着广泛的科研价值。参考以斑马鱼物生物模型所得的实验研究对于人类疾病的防治有重要的借鉴意义。 关键词:斑马鱼,模式生物,人类疾病 正文: 随着现代生物科学的不断发展,传统的以单一生物模型为研究模板的方式已经不能 满足日益丰富的研究内容,越来越多的生物学研究开始转向以模式生物为研究对象。由于 模式生物的基因在进化的保守性以及遗传密码的通用性,模式生物为其他的实验生物提供 了良好的实验模板,因此,选择合适的模式生物可以使实验祈祷事半功倍的效果。[1] 就目前所用于科学实验的模式生物而言,主要有果蝇,大肠杆菌,斑马鱼,小鼠, 酵母菌和拟南芥等。其中,斑马鱼以其容易捕获,易于饲养,生长周期短,繁殖能力强, 基因组与人类有高度保守性,使得斑马鱼及其胚胎在模式生物研究领域起着不可替代的重 要作用[2]。早在1981年,在Oregon带血的著名遗传学家Streisinger等就在Nature杂志 上发表了第一篇关于斑马鱼的科研论文。自此之后,斑马鱼就开始广泛的运用于发育与遗 传毒理学、生物学、医学、环境毒理学和药物研发等多个领域。[3]在这里主要介绍斑马鱼 及其胚胎在人类疾病模型构建中的研究应用。 一、关于造血疾病的模型研究 斑马鱼血小板和人类的有所差异,主要为带有稀疏细胞质巨大细胞核的有核细胞。但在电镜下表面较为光滑,染色质细密,较易于观察。不过最主要的是两者在生理功能上 具有某些相似性,包括血小板的黏附、激活聚集和释放反应等。所以斑马鱼作为模式生物 研究血小板具有较强的可行性。Gregory等[4]通过将斑马鱼幼鱼暴露在FeCl3中,利用激光损伤的方法损伤血管壁,构建血管闭塞模型。发展了激光介导血栓形成的方法,并通过这 个方法来了解斑马鱼血小板功能的变化。Langenau等[5]将源于小鼠的c-myc基因与斑马鱼 胚胎的Rag2基因融合,再在这个基因的尾部连接上GFP基因,之后植入到斑体细胞,从而影响了造血细胞的的基因表达,建立了斑马鱼白血病模型。 二、关于神经系统疾病的模型研究 在斑马鱼关于人类神经系统的疾病研究主要指关于阿尔茨海默病(AD),帕金森综合 症(PD),亨廷顿舞蹈症(HD)以及肌肉萎缩性脊髓侧索硬化症(ALS)的疾病研究。
ptges3a基因在斑马鱼早期胚胎发育过程中的表达
文章编号:1000 5404(2011)04 0389 03 论著 ptges3a 基因在斑马鱼早期胚胎发育过程中的表达 韩勇军,吴新荣 (510010广州,广州军区广州总医院药剂科) [摘要] 目的 克隆斑马鱼前列腺素E 合酶3a(pro stag land i n E synthase 3a ,pt ges3a)基因,研究其在斑马鱼胚胎早期 发育中的表达情况。方法 提取斑马鱼胚胎的总RNA,制备D I G 标记的ptges3a RNA 反义探针,整胚原位杂交研究ptges3a 在斑马鱼胚胎早期发育过程的表达。结果 ptges3a 在sh i e l d 期前普遍性表达,10体节期在后脑神经龙骨处有特异性表达,18体节期在后脑有特异性表达,在26hpf 时期头部表达较多,并且在肾小管有特异性表达,36hpf ptges3a 在头部较高表达。结论 ptges3a 可能参与了脑部发育和肾小管形成。 [关键词] 斑马鱼;前列腺素E 合成酶;胚胎发育 [中图法分类号] R 321;R345;R 394.2[文献标志码] A [通信作者] 吴新荣,E m a i :l gzwxrong @y a hoo .co m Expression of ptges3a duri ng early develop m ent in zebrafi sh H an Yong j u n,W u X i n rong (D epart m ent of Phar m acy ,G uang zhou G eneral H o spita l o f G uang zhou M ilita ry Co mm and ,G uangzhou ,G uangdong P rov i nce ,510010,Ch i na) [Abstract ] Obj e cti v e To clone prostag land i n E synthase 3a (ptges3a)gene and investigate its te m pora l and spati a l expression pattern i n zebrafish duri n g earl y deve l o pm en.t Methods The to ta l RNA of different phases of zebrafish e m br yos w as ex tracted .D igox i n labe led RNA probe o f ptges3a w as prepared .W ho le e mbryo m ount in situ hybridization w as e mp loyed to investi g ate the expressi o n patter n of ptges3a during zebrafish e mbryogenesis .Results ptges3a transcri p t w as d istri b uted ub i q u itously before sh ield stage .A t 10 so m ite there w ere detectab le expression o f ptges3a gene in the hindbra i n neural kee,l and at 18 so m ite it w as expressed in the h i n dbra i n .Fro m 26to 36h after post fertilizati o n (hp f),h i g h expressi o n o f ptges3a w as found i n the brai n .ptges3a w as a lso detectab le i n the rena l tubule at 26hp.f C onclusi o n The expression patter n of ptges3a during zebrafish e m bryogenesis suggests that it m ay be critical for the developm ent of bra i n and rena l t u bule .Further i n vestigation on the functi o n of ptges3a is needed . [Key words ] zebrafish;prostaglandin E synthase 3a ;e m bryonic developm ent C orrespond i ng au t hor :W u Xi n rong ,E m ai:l gz w xrong @yahoo .co m 前列腺素(prostag land i n ,PG )E 2是一种重要的前列腺素类物质,参与机体多种生理及病理过程,前列腺素E 合成酶(prostaglandin E synthase 3,ptges3)是PGH 2转化为PGE 2过程中的重要终端限速酶。多项研究[1-3] 结果显示,ptges3在结肠癌、肺癌、头颈部肿瘤、乳腺癌和胃癌中过表达,且与肿瘤的发生、发展密切相关。缺失ptges3的小鼠可以消除炎症,类似于非甾体抗炎药(non steroida l anti i n fla mm atory drug ,NSA I D )作 用的效果[4] ,动脉粥样硬化患者的巨噬细胞中ptges3 有高表达,可诱导血小板破裂[5] 。ptges3可能成为用于治疗疾病和筛选药物的潜在靶点。斑马鱼是近几十年出现的新型模式生物,它有许多优点:个体小、喂养费用便宜;早期胚胎透明,易于观察和操作;产卵多,繁殖周期短。通过序列比对和进化树分析发现斑马鱼ptges3a 与小鼠、人的ptges3具有高度同源性。整胚原 位杂交技术是研究斑马鱼发育相关基因的功能和表达 模式的一种重要手段。本研究通过整胚原位杂交方法分析了ptges3a mRNA 在斑马鱼早期胚胎发育过程中的表达情况,为深入研究这一基因的功能提供基础依据。1 材料与方法1.1 材料 斑马鱼Tubingen 野生型,总RNA 提取试剂盒RNA queous 4PCR K it(Amb i on 公司),PCR 引物由上海生工生物工程有限公司合成,琼脂糖凝胶DNA 回收试剂盒、大肠杆菌DH 5 感受态细胞、普通质粒小提取试剂盒(T i angen 公司),T 4DNA 连接酶、p GM T easy 载体、D IG RNA L abe li ng M i x (R oche 公司),SP 6RNA Po l ym erase(P ro m ega 公司),限制性内切酶Ap a 、Ex T aq 聚合酶(T a K aRA 公司),A nti D i gox i g en i n A P 、染色剂:NBT 和BC I P (R oche 公司)。 1.2 方法 1.2.1 人、小鼠和斑马鱼的ptges3基因进化树分析 从生物技术信息网页(http ://www .ncb.i nl m .n i h .gov /guide /)获取 389 第33卷第4期 2011年2月28日 第 三 军 医 大 学 学 报ACTA ACADEM I AE M EDIC I NAE M ILI TARIS TERTI AE V o.l 33,No .4 Feb .28 2011
发育生物学模式动物
发育生物学近年来研究进展 ———模式动物 摘要: 随着科学技术水平的不断发展,在生命科学、人类医药和健康研究领域,由于一些原因,人们必须寻找一类用作研究的实验动物,通过相互参照,可以用一种动物的生命活动过程成为另一种动物或者人类的参照物。对一些难以在人身上进行的工作, 及一些数量很少的珍稀动物, 或一些因体型庞大、不易实施操作的动物种类, 采用取材容易、操作简便的另一种动物来代替人类或原来的目标动物进行实验研究, 这就是动物实验。为了保证这些动物实验更科学、准确和重复性好, 可以用各种方法把一些需要研究的生理或病理活动相对稳定地显现在标准化的实验动物身上,供实验研究之用。这些标准化的实验动物就称之为模式动物。 关键词:斑马鱼;猪;基因;表达; 通过发育生物学及相关学科的学习,我们了解到了模式动物在生命科学的发展历程中起到了可以说是举足轻重的作用,比如说通过海胆等低等动物模型的构建催生出现代受精生物学和发育生物学;又比如像果蝇模型的建立大大推进了遗传学和发育生物学的进展;酵母和大肠杆菌作为生物模型为现代分子生物学和基因工程技术提供了施展的舞台,线虫模型对基础和应用生物学产生了巨大的推动作用, 并直接导致了细胞凋亡现象的发现, 并开创了一个当代生物医学的全新领域。这些研究成果已经充分证明了模式动物在生命科学研究中的作用。通过查阅近几年来人们关于模式动物的研究进展,我总结了以下几点近几年来关于模式动物上的具有典型性的突破,仅供大家参考。 一、关于斑马鱼的研究 1.Pitx2基因在斑马鱼牙齿发育过程中早期表达的研究 人类Pitx2基因与常染色体显性疾病里格尔综合征的发生有关联, 可导致牙齿和 眼睛的缺陷。斑马鱼的牙齿和人类的牙齿有很多相似之处, 其牙齿位于腮弓之上, 牙齿发育可明确分为蕾状期、帽状期、钟状期和分泌期等各个阶段, 这些特点使得利用斑马鱼作为模式动物研究牙发育和牙再生具有较大优势。 经调查发现,小鼠的牙成型之前, Pitx2基因在整个牙齿发育过程中在成牙上皮中持续表达, 在牙发育的过程中伴有至关重要的角色。而斑马鱼被公认是一种理想的研究器官发育分子生物学机制的模式动物。科学家在对小鼠的胚胎发育研究中发现, Pitx2基因活性的缺失会导致Fgf 8在牙上皮中的向下调节, 也就是说P itx2和Fgf8之间存在正向的反馈回路, 同时Pitx2还是Bmp4信号通路的一个受体。Pitx2敲除的小鼠牙齿发育中断于蕾状期。还有研究发现牙齿的发育与P itx2的量有密切的关系。 实验结果显示, P itx2基因在人发育中的牙胚的表达模式与在小鼠中类似。不论是切牙还是前磨牙, Pitx2基因的表达都只能在牙上皮的蕾状期后期、帽状期和钟状期检测到。在分化良好的牙,Pitx2基因的表达受到成釉细胞的限制。这些结果显示, P itx2基因在人类牙齿牙上皮的发育过程和釉质分化过程中起一定作用[1]。 另一方面,为研究Pitx2基因在斑马鱼牙齿早期发育阶段的表达,本实验利用RT - PCR技术直接克隆P itx2特异性基因片段, 成功制作出针对P itx2的基因探针, 并选取斑马鱼发育早期多个时段的胚胎进行整胚原位杂交,获得P itx2基因在斑马鱼早期发育阶段的表达情况。这对于人们对Pitx2基因的研究更进一步。
斑马鱼
斑马鱼 斑马鱼(zebra fish),又名蓝条鱼、花条鱼、斑马担尼鱼(Brachydaniorerio),原产于印度、孟加拉国。斑马鱼(B. rerio),是淡水水族箱观赏鱼,原产于亚洲,体长约4公分(1.5吋),具暗蓝与银色纵条纹,蓑鮋属鱼类是海水水族箱观赏鱼,鳍棘有剧毒,体具色彩丰富的垂直条纹。有些种类称为蓑鮋(lion-fish)或称狮子鱼、火鸡鱼。由于其基因与人类87%相似,因此广泛应用与生命科学的研究,2009年研究表明,它可能为盲人和耳聋带来福音。 中文学名:斑马鱼别称:蓝条鱼、花条鱼、蓝斑马鱼、印度鱼、印度斑马鱼 二名法:Daniorerio界:动物界 门:脊索动物门Chordata纲:辐鳍鱼纲Actinopterygii 目:鲤形目Cypriniformes科:鲤科Cyprinidae 属:(鱼丹)属Danio种:斑马鱼 D. rerio 简介 斑马鱼(zebra fish),又名蓝条鱼、花条鱼、斑马担尼鱼(Brachydaniorerio),原产于印度、孟加拉国。(里面常见的蓝斑马讲解)是两个非近缘鱼类类群,即鲤形目(Cypriniformes)鲤科(Cyprinidae)短鱼丹属(Brachydanio)淡水鱼类和鮋形目(Scorpaeniformes)鮋科(Scorpaenidae)蓑鮋属(Pterois)海水鱼类的统称。斑马鱼(B. rerio),是淡水水族箱观赏鱼,原产於亚洲,体长约4公分(1.5吋),具暗蓝与银色纵条纹,蓑鮋属鱼类是海水水族箱观赏鱼,鳍棘有剧毒,体具色彩丰富的垂直条纹。有些种类称为蓑鮋(lion-fish)或称狮子鱼、火鸡鱼。斑马鱼是一种常见的热带鱼。斑马鱼体型纤细,成体长3-4cm,对水质要求不高。孵出后约3个月达到性成熟,成熟鱼每隔几天可产卵一次。卵子体外受精,体外发育,胚胎发育同步且速度快,胚体透明。发育温度要求在25-31℃之间。斑马鱼由于个体小,养殖花费少,能大规模繁育,且具许多优点,吸引了众多研究者的注意。经过30多年的研究应用和系统发展,已有约20个斑马鱼品系,斑马鱼基因数据库里有相关斑马鱼的资料可供查询和下载,方便了研究。斑马鱼的细胞标记技术、组织移植技术、突变技术、单倍体育种技术、转基因技术、基因活性抑制技术等已经成熟,且有数以千计的斑马鱼胚胎突变体,是研究胚胎发育分子机制的优良资源,有的还可做为人类疾病模型。斑马鱼已经成为最受重视的脊椎动物发育生物学模式之一,在其它学科上的利用也显示很大的潜力. 特征 体长4~6厘米。体呈纺锤形。背部橄榄色,体侧从鳃盖后直伸到尾未有数条银蓝色纵纹,臀鳍部也有与体色相似的纵纹,尾鳍长而呈叉形。雄鱼柠檬色纵纹;雌的斑马鱼蓝色纵纹加银灰色纵纹。 繁殖方法 喜在水族箱底部产卵,斑马鱼最喜欢自食其卵,一般可选6月龄的亲鱼,在25厘米X25厘米X25厘米的方形缸底铺一层尼龙网板,或铺些鹅卵石,繁殖时产出后即落入网板下面或散落在小卵石的空隙中。选取2~3对亲鱼,同时放入繁殖缸中,一般在黎明到第二天上午10时左右产卵结束,将亲鱼捞出。其卵无粘性,直接落入缸底,到晚上10时左右,没有受精的鱼卵发白,可用吸管吸出。繁殖水温24℃时,受精卵经2~3天孵出仔鱼;水温28℃时,受精卵经36小时孵出仔鱼。雌鱼每次产卵300余枚,最多可达上千枚。水温25℃时,7~8天的仔鱼开食,此时投喂蛋黄灰水,以后再投喂小鱼虫。斑马鱼的繁殖周期约7天左右,一年可连续繁
斑马鱼养殖
斑马鱼作为一种观赏性的模式生物,广泛存在于家庭、酒店和科研实验室,是日常生活中比较常见的一种鱼类,相信很多人也有冲动养殖斑马鱼。但和其他生物的养殖一样,在养殖斑马鱼的过程中需要注意到很多问题。 一、养殖设备的要求。日常室内和科研实验室养殖时需要选择合适且质量合格的水族箱,这样能够保证斑马鱼的正常生长,不会影响日常观赏和科研活动。首先,因为斑马鱼的体型比较娇小,一般是3-4厘米,一般选择比较小的水族箱。但是也是要注意到养殖量,当养殖量很大时,需要选择较大点的水族箱。目前市面上在售的水族箱规格齐全,能够满足不同科研和酒店、家庭养殖斑马鱼的需要。 二、养殖水质的要求。斑马鱼养殖起来很简单,对水温水质的要求要并不高。一般而言,它的养殖水温要保持在27度左右即可,但若水温低于这一范围,斑马鱼也能够调整自身积极适应,不会影响斑马鱼的正常生存。在水质方面,斑马鱼养殖的水质维持在中性或偏软性水都是可以的,均是斑马鱼能够接受的水质区间。当然,在斑马鱼亲鱼繁殖期间,其生存水质需要用软水,因为这样的水质能够提升卵子受精率和孵化率。不少水族箱和养殖缸具有完整且独立的循环系统,及时进行水体流动和循环,以保持良好的水质,达到淡海水养殖均可使用的标准,配有恒温设备,可以根据不同研究和养殖需要调节水温,不会影响其生存环境。 三、养殖饲料的要求。斑马鱼食性相当单一,喂养起来也是十分
便捷,天然饵料和人工配合饲料都可以满足它的生长需要。对于饵料,市面上不少生产厂家能够特别针对斑马鱼配置了相应的饵料,能够满足不同数量及成长阶段的斑马鱼饲养要求。 上海海圣生物实验设备有限公司成立于1997年,是一家从事水生物养殖设备制造的专业生产型企业,专为各高等院校、研究院所度身设计、制造水生物实验养殖系统,如中国水产科学研究生院东海水产研究所、黄海水产研究所、北京大学等。近年来,海圣在斑马鱼养殖设备研发上取得新进展,开发出二代新品,既提升了性能又降低了运行成本。海圣拥有完善的售后服务体系,保修期内免费上门维修,48小时内到达现场。定期回访及维护设备,积极听取和处理反馈意见,提供终身技术支持及配件。它不断吸收国内外先进技术,积极完善生产工艺,获得多项专利,已通过了ISO9001:2015质量管理体系认证、ISO14001:2015环境管理体系认证和ISO 45001:2018职业健康安全管理体系认证,养殖设备达FDA检测标准,无毒无害,质量有保证!
环境对斑马鱼胚胎发育的影响
环境对斑马鱼胚胎发育的干扰 XXX,YYY,ZZZ 一、实验目的及原理: 1、通过斑马鱼早期发育过程的观察,巩固对硬骨鱼胚胎发生的认识。 2、通过设置环境因子(锌离子)来研究其对斑马鱼胚胎发育的影响程度。 3、通过对比对照组和实验组,进一步巩固对硬骨鱼胚胎发育模式的认识。 4、锻炼独立开展科学实验、分析和解决实际问题的能力。 5、加深环境对动物受精及早期发育影响的理解。 二、实验材料与方法: 1、实验材料: 所需胚胎:发育良好的、健康的斑马鱼胚胎(原肠胚早期)36枚。 试剂:硫酸锌(分析纯), 器材:培养容器12孔板,恒温培养箱,显微镜,解剖镜,载玻片,塑料滴管,手表,移液枪100uL、1000uL,量筒100mL*2。
2、实验方法: (1)在本实验中,使用硫酸锌作为实验用环境因子,共设三个梯度,分别为:0,0.1mg/L,1mg/L。每个梯度设4个平行组,每个平行组3个胚胎,共需36个胚胎。 (2)实验处理起始时间:原肠胚早期: 实验处理终止时间:孵化期。 (3)实验操作: A.挑选胚胎: 由于斑马鱼卵受精及发育的不均匀性,要对斑马鱼卵进行挑选。挑选时要挑透明、无白色斑点、卵膜完整的。之后的实验中还要不断将败育卵挑出。 用滴管(塑料滴管口部要剪短且一定要圆滑,并且直径要大于卵径)将选择好的卵依次吸出,然后放入12孔板中进行孵化。 【注意剔除异物眼观有白色小斑点、畸形异常卵。】
B.实验体系: 12孔板培养胚胎,每孔放入3个胚胎,共使用12个孔,36个胚胎。12个孔分别标上A1~A4,B1~B4,C1~C4。A1~A4组作为对照组的三个平行组,B1~B4作为0.1mg/L组的三个平行组,C1~C4作为1mg/L组的三个平行组。 C.实验期间的培养管理: 1.孵化用水: 孵化水温一般要25~28℃,在这个温度范围内,温度越低,孵化所需时间相应越长。水温25℃时,受精卵经48~72h孵出仔鱼,水温28℃时,经36h孵出仔鱼。水温太高或太低,会造成受精卵死亡。[2] 【实验中设定的培养温度为25℃,由于恒温培养箱的示数与实际温度有较大差异,而且在实验后期(80h)提高培养箱温度以加速孵化,所以本次实验实际中的温度并不恒定,暂以25℃为准。】 2.孵卵卫生: (1)定期观察,每12h换一次水,水温要相近,而且每次换水量在原水量的1/3-1/2,以尽量使胚胎缩短适应期,使发育流畅。定期将死卵挑出,以确保不影响其他卵的正常发育。 3、观察记录: (1)前3h实时观察、拍照并记录,之后每天早上(7:00-9:00)、中午(12:30-14:30)、晚上(18:00-21:00)三段时间对斑马鱼胚胎发育不同时期以及畸变进行观察、拍照并记录。 (2)实验指标:实验前,根据文献查询[3],Zn2+可能导致的畸变有:
