Hydrothermal Synthesis of Molybdenum Disulfide for Lithium Ion Battery Applications

Chinese Journal of Chemical Engineering, 18(6) 910—913 (2010)
Hydrothermal Synthesis of Molybdenum Disulfide for Lithium Ion Battery Applications*
WANG Shiquan (王石泉)1,2, LI Guohua (李国华)3, DU Guodong (杜国栋)4, JIANG Xueya (江雪娅)2, FENG Chuanqi (冯传启)2, GUO Zaiping (郭再平)4 and KIM Seung- Joo5,**
1 Institue of NT-IT Fusion Technology, Ajou University, Suwon 443-749, Korea
2 Department of Chemistry, Hubei University, Wuhan 430062, China
3 State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology, Zhejiang University of Technol-
ogy, Hangzhou 310032, China
4 Institute for Superconducting and Electronic Materials, University of Wollongong, Northfield Avenue, Wollon-
gong, NSW 2522, Australia
5 Division of Energy Systems Research, Ajou University, Suwon 443-749, Korea
Abstract Molybdenum disulfide nanoflakes were synthesized by a simple hydrothermal process using sodium molybdate and thiourea as reactants at a relatively low temperature. X-ray diffraction (XRD) and transmission elec-tron microscopy (TEM) indicate that the samples have the structure of 2H-MoS2 and the morphology of nanoflakes with the average thickness around 5-10 nm. The results of electrochemical properties indicate that the morphology and size of MoS2 particles have effects on their capacity when they are used as the anode for lithium ion battery.
The as-prepared MoS2 samples have high reversible discharge capacity up to 994.6 mA·h·g?1 for the MoS2-1 elec-trode and 930.1 mA·h·g?1 for the MoS2-2 electrode and show excellent cycling performances. The MoS2-1 electrode has a better cycling stability than the MoS2-2 electrode due to their difference in the uniformity of the samples.
Keywords molybdenum disulfide, chemical synthesis, electrochemical property, electrode material
1 INTRODUCTION
WS2 and MoS2 materials have shown potential applications in the fields of scanning probe micros-copy [1], solid-state lubrication [2], heterogeneous ca-talysis [3], lithium ion battery [4-7] and electrochemi-cal hydrogen storage [8]. Molybdenum disulfide (MoS2) features a layered structure, in which the at-oms are covalently bonded to form two-dimensional layers that are stacked together through weak van der Waals interactions [9]. This characteristic makes them possibly suitable as electrode materials for lithium ion battery [4-6]. For example, Feng et al. [4] reported that MoS2 nanoflakes prepared by a rheological phase reac-tion had a better lithium intercalation/deintercalation behavior. The as-synthesized MoS2 nanoflakes showed good cycling stability over a wide voltage range. The reversible capacity remains 840 mA·h·g?1 after 20 cycles, which is 84% of the initial reversible capacity. Guo et al. [5] prepared MoS2via hydrothermal method and reported that MoS2 electrode delivered a lithium insertion charge capacity of 801 mA·h·g?1, which is much higher than for WS2 powder [6]. Wang et al. [7] prepared WS2 nanotubes and reported that WS2 nano-tubes electrode delivered a lithium insertion capacity of about 915 mA·h·g?1, which is much higher than that of WS2 powder. The electrochemical performance of transition metal disulfides, including MoS2 showed that the particle size and morphology of materials have a great influence on their electrochemical prop-erties. Nanosized materials with the novel morpholo-gies were believed to have somewhat better perform-ance than bulk materials.
In this work, MoS2 nanoflakes were synthesized through a simple hydrothermal process. The as- synthesized MoS2 nanoflakes electrodes can reversi-bly store lithium with discharge capacities up to 994.6 mA·h·g?1 in a voltage range of 0.01-3.0 V vs. Li/Li+ and show a good cycling stability as electrode materials.
2 EXPERIMENTAL
2.1 Sample preparation
2.1.1Synthesis of MoS2-1
1.21 g (0.005 mol) Na2MoO4·2H2O, 1.56 g (0.02 mol) thiourea (NH2CSNH2) and 0.14 g PEG-1000 were dissolved in 30 ml of distilled water. The result-ing solution was transferred into a 50 ml teflon-lined stainless autoclave. The antoclave was heated at 200 °C for 24 h in a furnace and then cooled to room tempera-ture, by shutting off the furnace. The black precipitates were collected by centrifugation, thoroughly washed with distilled water, and then dried in air.
2.1.2Synthesis of MoS2-2
1.21 g Na2MoO4·2H2O and 0.70 g (0.01 mol) NH2OH·HCl were dissolved in 10 ml of distilled wa-ter, respectively. Then the mixture of Na2MoO4 and NH2OH·HCl solution was added into 100 ml round- bottom flask and heated at 90 °C with stirring. Then a
Received 2010-06-21, accepted 2010-11-20.
* Supported partially by the State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology, Zhejiang Univer-sity of Technology and Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0094047).
** To whom correspondence should be addressed. E-mail: sjookim@ajou.ac.kr
Chin. J. Chem. Eng., Vol. 18, No. 6, December 2010911
certain amount of 2 mol·L?1 HCl was added into the above solution to adjust the pH value to about 6. After half an hour, 1.56 g thiourea (NH2CSNH2) was added all at once into the solution with vigorous stirring. The reaction was kept at 90 °C for 10 min. The resulting solution was transferred into a 50 ml teflon-lined stainless autoclave. The autoclave was heated at 180 °C for 12 h in a furnace. Further treatment was similar to that described in MoS2-1.
2.2 Characterization
X-ray diffraction (XRD) patterns were recorded on a Rigaku D/MAX IIA diffractometer using Cu Kαradiation (λ=0.1541 nm). The morphology of the re-sulting compounds was observed using a transmission electron microscope (TEM). Before the characteriza-tion, the samples were dispersed ultrasonically in etha-nol and dropped onto Cu-grid coated with carbon mem-brane. Electrochemical measurements were carried out using a 2032-type coin cell fabricated in an argon-filled glove box (Mbraun, Germany). The working electrode was fabricated in the mass ratio of 70︰20︰10 active material/carbon black/polyvinylidene difluoride, while lithium foil served as counter and reference electrode. The electrolyte was 1 mol·L?1 LiPF6 dissolved in 1︰1 ethylene carbonate (EC) and dimethyl carbonate (DMC). Constant current charge/discharge cycling was con-ducted on a LAND battery tester (CT2001A) in the voltage range between 3.0 and 0.01 V. Cyclic volt-ammetry (CV) was performed on a CHI660C electro-chemical workstation at a scan rate of 0.1 mV·s?1.
3 RESULTS AND DISCUSSION
Although both samples exhibited a broadened fea-ture in XRD patterns due to low crystallinity (Fig. 1), all diffraction lines were indexed to the hexagonal phase of MoS2 (JCPDS file No. 170744).
Figure 1 XRD patterns of as-prepared MoS2 samples: (a) MoS2-1 and (b) MoS2-2
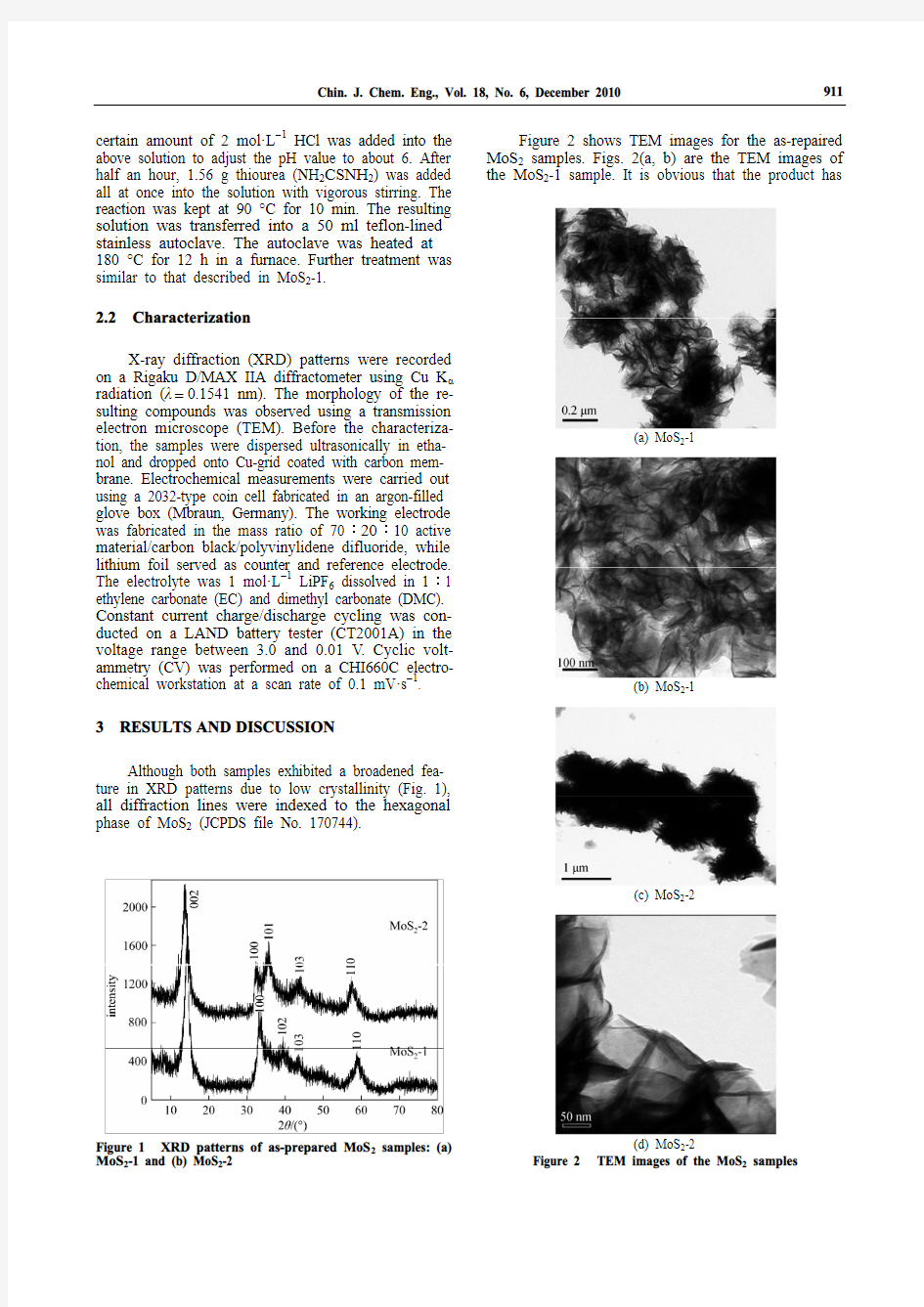
Figure 2 shows TEM images for the as-repaired MoS2 samples. Figs. 2(a, b) are the TEM images of the MoS2
-1 sample. It is obvious that the product has
(a) MoS2
-1
(b) MoS2
-1
(c) MoS2
-2
(d) MoS2-2
Figure 2 TEM images of the MoS2 samples
Chin. J. Chem. Eng., Vol. 18, No. 6, December 2010
912 the shape of nanoflake with the thickness of about 5-10 nm. Figs. 2 (c, d) are the TEM images of the MoS 2-2 sample. The MoS 2-2 sample also shows a flake-like morphology with the thickness of about 10 nm. The nanoflakes in the MoS 2-2 sample were curled at the edges and more aggregated than the MoS 2-1 sample.
Based on the literature [4, 10] and the experimen-tal conditions, the reaction involves a complex process, which contains three steps: (a) the hydrolysis of NH 2CSNH 2 to form H 2S; (b) the reduction of Mo(VI) to produce Mo (IV); (c) the formation of MoS 2 nan-oflakes. The reaction process for the synthesis of MoS 2-1 could be expressed as follows:
222322CS(NH )2H O 2NH CO H S +??→++ (1)
24222224324Na MoO 15CS(NH )6H O 4MoS Na SO 6NaSCN 24NH 9CO ++??→++++ (2) The formation of the MoS 2-2 sample can be expressed as [11]:
222322CS(NH )2H O 2NH CO H S +??→++ (3) 242422
Na MoO 2NH OH HCl 4HCl MoCl 2NaCl 6H O N +?+??→
+++ (4)
422MoCl 2H S MoS 4HCl +??→+ (5)
The electrochemical properties of MoS 2 samples were measured via coin cell testing. Figs. 3 (a) and 3 (b) show the cyclic voltammograms (CVs) of the
MoS 2-1 and MoS 2-2 electrodes, respectively. In the first scanning cycle [Fig. 3 (a)], there are two reduc-tion peaks (at ~0.4 V and ~1.0 V) and the correspond-ing oxidation peaks (at ~1.8 V and ~2.25 V). During the second cycle, the reduction peak at 0.4 V disap-peared, while a new cathodic peak appears at 1.4 V . From the second cycle, the CV curves remain consis-tent and maintain two reduction peaks (at 1.4 V and 1.8 V) and two oxidation peaks (at ~1.7 V and ~2.25 V). For the MoS 2-2 electrode, in the first cycle, there are two reduction peaks (at ~0.4 V and ~1.5 V) and the corresponding oxidation peaks (at ~1.8 V and ~2.25 V). From the second cycle, the CV curves remain consis-tent and maintain two reduction peaks (at 1.5 V and 1.75 V) and two oxidation peaks (at ~1.8 V and ~2.3 V).
When MoS 2 is cycled between 3.0 and 0.01 V as an anode material, two step reactions occur. First, lithium intercalates into the S slab, and the van der Waals S-S bonds must be broken and replaced by Li-S bonds. MoS 2 then decomposes into Mo nanoparticles embedded in a Li 2S matrix, corresponding to two ca-thodic peaks at about 1.0 V and 0.4 V in the first ca-thodic segment in the cyclic voltammograms (CVs), as shown in Figs. 3 (a) and (b). Both the MoS 2-1 and MoS 2-2 samples show these two peaks. In the follow-ing cycles, an extra peak at about 2.0 V appears. This change can be explained by the formation of a gel-like polymeric layer [12].
Figure 4 shows the charge/discharge profiles of the as-prepared MoS 2 electrodes. For the MoS 2-1 electrode delivered a lithium insertion capacity of 1223.1 mA·h·g ?1 for the first cycle when the discharge current density was 50 mA·g ?1, which was much higher than the reported data of the WS 2 nanotubes [7] and MoS 2 nanopowder [6]. The MoS 2-1 electrode re-tained a reversible capacity of 994.6 mA·h·g ?1. The first discharge curve shows two insertion plateaus at ~1.2 V and ~0.8 V . During the first charge, the charge voltage increases gradually at first, then a short plateau at 2.3 V vs . Li/Li + was observed. The MoS 2-2 electrode delivered a lithium insertion capacity of 1138.4 mA·h·g ?1 for the first cycle and it retained a reversible capacity of 930.1 mA·h·g ?1
, as shown in
(a) MoS 2
-1
(b) MoS 2-2
Figure 3 Cyclic voltammograms (CVs) of MoS 2-1 and MoS 2-2 electrodes at a scanning rate of 0.1 mV·s ?1
1st; 2nd; 3rd; 4th; 5th
Figure 4 First and second charge/discharge curves of MoS 2-1 and MoS 2-2 electrodes
MoS 2-1 1st; MoS 2-1 2nd; MoS 2-2 1st; MoS 2-2 2nd
Chin. J. Chem. Eng., Vol. 18, No. 6, December 2010913
Fig. 4. The first discharge curve shows two insertion plateaus at ~1.8 V and ~0.8 V, which is different from the data of MoS2-1 electrode. In the second cycle, a lithium insertion plateau at about 2.2 V and ~1.5 V as well as a slope starting from 1.5 V down to the cutoff voltage of 0.01 V were observed. From the second cycle, only one plateau (2.3 V for the charge curve and 2.0 V for the discharge curve) connected with a slope can be observed for both charge and discharge curves, similar to that of WS2 nanoflakes electrode [13], indi-cating that the intercalation reaction dominates the electrochemical process after the first cycle.
The cyclic performances within 40 cycles of two MoS2 electrodes are shown in Fig. 5. As mentioned above, we can observe the large initial discharge ca-pacities (1223.1 mA·h·g?1 for the MoS2-1 and 1138.4 mA·h·g?1 for the MoS2-2) in the first cycle. The MoS2-1 and MoS2-2 electrodes exhibit reversible spe-cific capacity as high as 994.6 mA·h·g?1 and 930.1 mA·h·g?1, respectively, at a current density of 50 mA·g?1. After the 20th cycles, the discharge capacities of MoS2-1 and MoS2-2 are 814.8 mA·h g?1 and 843.1 mA·h·g?1. Even in the 40th cycles, MoS2-1 and MoS2-2 electrodes still can sustain high capacities above 705.8 mA·h·g?1 and 483.2 mA·h·g?1, respec-tively. The results shown in Fig. 5 are in good agree-ment with the cyclic voltammograms curves (Fig. 4). The reason why the MoS2 nanoflakes electrodes showed such a high capacities, compared to the re-ported MoS2 electrode [5] is probably that lithium ions were not only intercalated into layers of the MoS2 structure to form Li x MoS2 compounds but also in-serted into defect sites of the clusters composed of the MoS2 nanoflakes [4, 7]. The increase in the number of active sites for Li+ insertion has been also observed in other low dimensional electrode materials such as MoO2 nanowires [14, 15]. The MoS2-1 electrode shows a better cycling stability than the MoS2-2 electrode due to the difference in the uniformity of the particle size distribution. Thus, the morphology and size of MoS2 particles have effects to their capacity when they are used as electrodes [4, 7, 16]. MoS2 electrodes, al-though they have relatively high discharge/charge po-tentials, still can be accepted as anode materials. Con-sidering the high reversible capacity and good cycling stability of the as-prepared two MoS2 nanoflakes elec-trodes, MoS2 nanoflakes could be promising alterna-tive anode materials for lithium ion batteries.
4 CONCLUSIONS
In this work, a hydrothermal method was used to synthesize MoS2 nanoflakes. The as-prepared two MoS2 electrodes exhibit high specific capacities and stable cycling stability. The reversible discharge ca-pacities were 994.6 mA·h·g?1 and 930.1 mA·h·g?1, respectively, for the two types of MoS2 nanoflakes prepared. Although the preparation method is simple, MoS2 nanoflakes so obtained could be promising al-ternative anode materials for lithium ion batteries.
REFERENCES
1Homyonfer, M., Alperson, B., Rosenberg, Y., Sapir, L., Cohen, S.R., Hodes, G., Tenne, R., “Intercalation of inorganic fullerene-like
structures yields photosensitive films and new tips for scanning
probe microscopy”, J. Am. Chem. Soc., 119, 2693-2698 (1997).
2Rapport, L., Bilik, Y., Feldman, Y., Homyonfer, M., Cohen, S.R., Tenne, R., “Hollow nanoparticles of WS2 as potential solid-state lu-
bricants”, Nature, 387, 791-793 (1997).
3Mdleni, M.M., Hyeon,T., Suslick, K.S., “Sonochemical synthesis of nanostructured molybdenum sulfide”, J. Am. Chem. Soc., 120,
6189-6190 (1998).
4Feng, C.Q., Ma, J., Li, H., Zeng, R., Guo, Z.P., Liu, H.K., “Synthesis of molybdenum disulfide (MoS2) for lithium ions battery applica-
tions”, Mater. Res. Bull., 44, 1811-1815 (2009).
5Guo, G.H., Hong, J.H., Cong, C.J., Zhou, X.W., “Molybdenum di-sulfide synthesized by hydrothermal method as anode for lithium
rechargeable batteries”, J. Mater. Sci., 40, 2557-2559 (2005).
6Julien, C.M., “Lithium intercalated compounds: Charge transfer and related properties”, Mater. Sci. Eng., 40, 47-102 (2003).
7Wang, G.X., Bewlay, S., Yao, J., Liu, H.K., Dou, S.X., “Tungsten disulfide nanotubes for lithium storage”, Electrochem. Solid-State
Lett., 7, A321-A323 (2004).
8Chen, J., Kuriyama, N., Yuan, H.T., Takeshita, H.T., Sakai, T., “Electrochemical hydrogen storage in MoS2 nanotubes”, J. Am.
Chem. Soc., 123, 11813-11814 (2001).
9Sibernagel, B.G., “Lithium intercalation complexes of layered transi-tion metal dichalcogenides: An NMR survey of physical properties”,
Solid State Commun., 17, 361-365 (1975).
10Tian, Y., He, Y., Shang, J., Zhu, Y.F., “Hydrothermal synthesis and characterization of lamellar MoS2”, Acta Chimica Sinica, 62,
1807-1810 (2004). (in Chinese)
11Tian, Y.M., Zhao, X., Shen, L.C., Meng, F.Y., Tang, L.Q., Deng, Y.H., Wang, Z.C., “Synthesis of amorphous MoS2 nanospheres by
hydrothermal reaction”, Mater. Lett., 60, 527-529 (2006).
12Wang, Q., Li, J.H., “Facilitated lithium storage in MoS2 overlayers supported on coaxial carbon nanotubes”, J. Phys. Chem. C, 111,
1675-1682 (2007).
13Feng, C.Q., Huang, L.F., Guo, Z.P., Liu, H.K., “Synthesis of tung-sten disulfide (WS2) nanoflakes for lithium ion battery application”,
Electrochem. Commun., 9, 119-122 (2007).
14Yang, L.C., Gao, Q.S., Tang, Y., Wu, Y.P., Holze, R., “MoO2 synthe-sized by reduction of MoO3 with ethanol vapor as an anode material
with good rate capability for the lithium ion battery”, J. Power
Sources, 179, 357-360 (2008).
15Gao, Q., Yang, L., Lu, X., Mao, J., Zhang, Y., Wu, Y., Tang, Y., “Syn-thesis, characterization and lithium-storage performance of MoO2/carbon
hybrid nanowires”, J. Mater. Chem., 20, 2807-2812 (2010).
16Li, H., Li, W., Chen, W.X., Wang, J.M., “Electrochemical lithia-tion/delithiation performances of 3D flowerlike MoS2 powders pre-
pared by ionic liquid assisted hydrothermal route”, J. Alloys Compd.,
471, 442-447 (2009).
Figure 5 Typical cycling performances of two as-prepared MoS2 electrodes
■MoS2-1;●MoS2-2
普通员工辞职申请书范文【三篇】
普通员工辞职申请书范文【三篇】 尊敬的xx人力资源部: 您好! 因为个人职业规划和一些现实因素,经过慎重考虑之后,特此提出离职申请,敬请批准。 在xx工作一年多的时间里,我有幸得到了各位领导及同事们的倾心指导及热情协助,在本职工作和音乐专业技能上,我得到了很大水准的提升,在此感谢xx提供给我这个良好的平台,这个年多的工作经验将是我今后职业生涯中的一笔宝贵财富。 在这里,特别感谢各位领导在过去的工作、生活中给予的大力支持与协助;尤其感谢xx,xx等,一年来对我的信任和关照,感谢所有给予过我协助的同事们。 望批准我的申请,并请协助办理相关离职手续,在正式离开之前我将认真继续做好当前的每一项工作。 祝公司事业蓬勃发展,前景灿烂。 申请人:### 20xx年xx月xx日 【篇二】 尊敬的韩总: 作为一名在酒店工作了大半年的员工,我对酒店有着一种格外亲切的感觉。每一个人在他年轻的时候,都有很多第一次,我当然也不例外。
我的第一份工作是在酒店,我最青春的三年也是在酒店度过的。 在这里,我学会了很多东西,能够跟同事们在一起工作,我觉得很开心,这里的每一位都是我的大哥大姐,我的叔叔阿姨,是他们教给了 我在学校里面学不到的知识,如何为人、如何处事、如何工作……在 酒店里,领导们也对我十分的关心,从刚进入酒店开始,我就感受到 从上至下的温暖。因为我是酒店里年龄还一般,还不算小,也从来没 有在这么大的集体里生活过,自不过然的,心里面就会产生一种被呵 护的感觉。这是一种以前在集体里未曾有过的感觉,很温馨,很自豪,而且它一直陪伴着我,直到我离开…… 但这种感觉不会随着我的离开而走远,我想我永远也不会忘记, 毕竟我以前生活在一个温暖而又温馨的集体里。韩总,还记得第一次 跟您近距离接触和理解是在20xx.3.16号。随着时间的流逝,斗转星移,您多年积累的工作经验与个人才华也得到充分的施展。您是我们 酒店的经理。在我上班之前,制定了一系列的政策与方针,重新定位 了酒店的经营策略,持续地尝试新的机制与奖励、分配办法,力争让 酒店的经济效益持续迈上新高,也让酒店员工的福利待遇如芝麻开花 一般节节高樊。,这才是为员工谋利益的举动,这才是一位被员工在 心里面所认可的经理。 而我,作为这个集体的一份子,更加感觉到您对员工的关心与培养。您肯定想到,酒店要想在竞争激烈的社会中立于不败之地,人才 的培养与发展是不可忽视的环节之一。因为我自身水平的不足,近期 的工作让我觉得力不从心,所以想公司提出了辞呈,忘领导批准。 申请人:### 20xx年xx月xx日 【篇三】 尊敬的公司领导:
范本政府申请报告范文
政府申请报告要怎么写呢?以下是为大家分享的3篇政府申请报告范文,供大家参考借鉴,欢迎浏览! 政府申请报告范文一: 关于向xx人民政府申请xx项目优惠政策的报告 xx人民政府: xx旅游地产暨现代农业建设项目是由(陕西xx集团)下属陕西xx房地产开发有限公司与xx房地产开发有限公司共同投资,根据《汉中市保护利用总体规划》,本着保护性利用自然资源的原则,在兼顾环境效益,合理利用生态资源,统筹城乡发展的基础上,力图打造的一个具有养生体验功能的高科技集旅游地产和现代农业示范区有机整合的地方发展项目。 一、xx旅游地产暨现代农业建设项目简介 xx旅游地产暨现代农业建设项目,将充分运用“文化传承、产品核心竞争力、价值附着”三大策略、“传统农业、现代农业、未来农业”三大板块、“互惠、分享、共赢”三大原则,进行整体规划。项目规划将综合考虑国家及汉中生态保护相关政策,把协调发展“三农问题”作为根本出发点。项目结合项目地良好的自然环境、当前现代农业的发展契机、西北地区休闲市场巨大需求,结合项目单位自身的人才、技术、管理、资金等资源等优势条件,利用农业为主线的链条式发展,以高端科技农业为主打,以规模特色农业为品牌,以休闲体验旅游为提升,将农业和旅游产业有机结合。建设国内外先进农业技术的引进转化示范区、农业专家课题示范区和自驾旅游集散中心。带动旅游观光、生态体验、餐饮住宿等辐射经济效益的升级。体现休闲自然的生活态度和生活方式,成为全国性的农业科技示范教育基地。实现第一产业、第二产业和第三产业的联动、互利和产业链升级发展。 xx项目以农业发展为基础,休闲养生体验为主题,旅游产业为拓展。项目实施后,将积极促进和改善xx区域的生态环境,打造优质生态宜居游乐生活,增进居民幸福度和营造社会和谐度。 项目总投入约亿,项目直接收益较高,可带来超过2亿的税收和6亿的衍生收益,并能解决农村剩余劳动力2000以上,可为这些劳动力带来每年2万元以上的收入,给武乡区域带来极大的综合效益。 二、项目投资单位简介 本项目投资单位:陕西xx房地产开发有限公司;具体实施单位:xx房地产开发有限公司;两公司均为陕西xx集团全资下属单位。 陕西xx集团是经由区政府领导、经贸局和xx房地产开发有限公司共同努力引入汉中,并成功注资落户的外来企业。 陕西xx集团,是以煤炭、电力为主导产业,以资源综合开发利用为宗旨,坚持节能环保发展观的新型能源企业。该公司先后被榆林市委、市人民政府授予“榆林市十佳企业”、“榆林市非公有制纳税十强企业”、“挂牌重点保护
fluid inclusions
The application of fluid inclusions in the mineralization lijin The department of geochemistry,Yangtze university Abstract. Fluid inclusion analysis is an important tool in modern studies of mineral deposits, as reflected by the statistics indicating that about a quarter of the papers published in Economic Geology contain fluid inclusion studies. Fluid inclusions play an important role in the classification of mineral deposits and in the study of the composition, temperature and pressure of mineralizing fluids. Among the principal mechanisms of ore precipitation, flu-id phase separation and fluid mixing derive their key evidence mainly from studies of fluid inclusions. Data on mineralizing fluid composition obtained from fluid inclusion analysis are key to understanding how metals were transported in hydrothermal fluids. Recent progresses in metal transport in vapor have been mainly contributed by fluid inclusion studies. Data on fluid temperature and pressure from fluid inclusion studies provide important constraints on hydrodynamic models of mineralization. Most metal ore deposits are formed in the geological fluid.The formation and characteristics of hydrothermal ore deposits are closely related including temperature, pressure and composition. Although these information can be gained through the study of macro geological characteristics of ore deposit and the geochemical characteristics of the mineral , but the composition of ore-forming fluid, temperature, and pressure from fluid inclusion is the most direct evidence.Fluid inclusion is the only remain in ancient ore-forming fluid. So, the study of fluid inclusion becomes one of the important ways of genesis research naturally. For Economic Geology sampling survey, the proportion of fluid inclusion research papers, from 5% in 1975 to 15% in 1985, 27% in 1995, then remained at about 25%, about 1 in 4 papers of deposits essay involves the study of fluid inclusions. Although the fluid inclusion research has expanded to petroleum geology, magma, and the earth's interior processes, etc.its mian application in the field of ore deposit research . The application of fluid inclusions in ore deposit has a lot of monographs. but these works focus on basic principles , methods of the study and the characteristics of different deposit types.This paper mainly discusses the application of fluid inclusions in the study of ore deposits. Keywords: geochemistry, fluid inclusions, hydrothermal deposits, mineralizing fluids, ore precipitation, metal transportation. 1.Fluid inclusion is one of the basis of the classification of the ore deposit According to the geological characteristics and genesis, ore deposit can be divided into different types, But at present very few scholars classify the ore deposits completely according to the geological characteristics, such as shear zone gold deposits, stratabound lead-zinc deposit or causes such as high temperature
辞职报告文本辞职报告范文大全
辞职报告文本辞职报告范文大全 辞职报告 (篇一) 尊敬的领导: 我很遗憾自己在这个时候向公司正式提出辞职申请。 来到公司也已经快两年了,在这近两年里,得到了公司各位同事的多方帮助,我非常感谢公司各位同事。正是在这里我有过欢笑,也有过泪水,更有过收获。公司平等的人际关系和开明的工作作风,一度让我有着找到了依靠的感觉,在这里我能开心的工作,开心的学习。或许这真是对的,由此我开始了思索,认真的思考。 但是最近我感觉到自己不适合做这份工作,同时也想换一下环境。我也很清楚这时候向公司辞职于公司于自己都是一个考验,公司正值用人之际,公司新的项目的启动,所有的后续工作在公司上下极力重视下一步步推进。也正是考虑到公司今后在这个项目安排的合理性,本着对公司负责的态度,为了不让公司因我而造成的决策失误,我郑重向公司提出辞职。 我考虑在此辞呈递交之后的2—4周内离开公司,这样您将有时间去寻找适合人选,来填补因我离职而造成的空缺,同时我也能够协助您对新人进行入职培训,使他尽快熟悉工作。 能为公司效力的日子不多了,我一定会把好自己最后一班岗,做好工作的交接工作,尽力让项目做到平衡过渡。离开这个公司,离开
这些曾经同甘共苦的同事,很舍不得,舍不得领导们的尊尊教诲,舍不得同事之间的那片真诚和友善。 在短短的两年时间我们公司已经发生了巨大可喜的变化,我很遗 憾不能为公司辉煌的明天贡献自己的力量。我只有衷心祝愿公司的业绩一路飙升!公司领导及各位同事工作顺利! (篇二) 尊敬的办公室人力资源管理领导: 我向公司正式提出辞职。 我自**日进入公司,到现在已经一年有余了,正是在这里我开始 踏上了社会,完成了自己从一个学生到社会人的转变。在过去的一 年多里,公司给予了我许多学习和锻炼的机会,开阔眼界、增长见识。我对公司给予的照顾表示忠心的感谢!但是,经过近段时间的思考, 我越来越迷惘!我越来越觉得现在的工作、生活离自己想要的越来越远。所以,我必须离开,去过我思想深处另一种有别于目前的生活。我想,生活应该是在选择到适合自己的道路以后,再持之以恒地坚持! 公司目前已经过了一年最忙的时间,是充电、整顿、储备人才的 时刻。相信,我的离开会很快有新生力量补充。因为这不是我想要的工作、生活状态,所以,我现在对工作没有激情、对生活也极其懒散。本着对公司负责的态度,为了不让公司其他同事受到我消极情绪 * ,也为了不让公司因为我出现业务上的纰漏等,我郑重向公司提出辞职,望公司给予批准! 祝公司稳步发展,祝公司的领导和同事们前程似锦、鹏程万里!
【优质】向政府写申请书范文-优秀word范文 (3页)
本文部分内容来自网络整理所得,本司不为其真实性负责,如有异议或侵权请及时联系,本司将立即予以删除! == 本文为word格式,下载后可方便编辑修改文字! == 向政府写申请书范文 向政府写申请书范文:企业向政府申请书范文 ******建设局: 为了更好的贯彻落实国家对资源综合利用的指导思想,促进合理的节约资源,提高资源利用率,保护环境实现经济社会的可持续发展的战略方针。 ************有限责任公司顺应形势发展,准备在******投资建设一条具有轻质、阻燃、保温、抗震性强并具有可持续发展的轻集料小型空心砌块建筑材 料生产线。 此项目的投资建设能达到节约能源、保护土地、变废为宝及综合治理环境 污染的目的。 投资建设此项目我公司具有以下优势: 一、轻集料小型空心砌块是以矿渣、炉渣、粉煤灰加有石硝为骨料,以水泥为胶结料,被广泛使用于工业与民用建筑的非承重砌块、承重砌块、保温块。 是一种节能、节土、利废的可持续发展的建筑材料。 该产品生产工艺无二次污染产生,市场前景广阔变废为宝,造福后代并具 有极高的社会效益和环境效益。 而我公司经营煤矿及煤炭销售多年,常年与矿渣、粉煤灰、水泥等物接触。 二、我公司为******热力公司供运供热用煤已有多年,合作非常融洽。
随着******城市建设规模的不断扩大,******热力公司现在每年冬季供热用煤需要4万多吨,为了保证冬季的正常供暖,秋季储存煤就非常关键,但是热力公司的场地有限,无法大量储存煤,加之供热产生的炉渣占地面积也很大。 为此双方约定由我公司申请30亩土地,其中一半无偿作为热力公司储煤场地,一半用于我公司建设轻集料小型空心砌块生产线使用,同时供热产生的炉渣及时运送到本厂作为生产原料。 充分体现了双方互利互惠的原则。 综上所述,建设轻集料小型空心砌块生产线,即符合国家产业政策,同时为确保******冬季正常供暖,热力公司秋季储煤的问题也得到了解决。 因此,恳请******建设局审批30亩土地作为************有限责任公司建设轻集料小型空心砌块生产线和******热力公司储煤场地为盼。 ************有限责任公司 20**年月日 向政府写申请书范文:向政府申请资金请示范文 县政府: XX镇政府办公楼建于X年X月,迄今XX年,由于该楼建筑时间长,加之建筑质量不好等原因,部分房间的墙体出现裂缝,虽小有修缮但仍存在屋顶掉块、墙围脱落等现象,该楼已存在安全隐患,不适宜继续办公,必须进行修缮。 经多方论证,修缮费用预算为XX万元,因镇政府资金短缺,特向县政府申请修缮办公楼经费,我们一定加强对招投标和工程质量的管理,指派专人负责办公楼修缮事宜,做到专款专用,严格质量、严格纪律,请予以支持为盼。 当否,请批示。 附:XX镇政府办公楼修缮预算开支一览表。 X县X镇人民政府
(H2N(C2H4)2NH2)[V4O10]ic951237c
Hydrothermal Syntheses and Structural Characterization of Layered Vanadium Oxides Incorporating Organic Cations:r-, -(H3N(CH2)2NH3)[V4O10]and r-, -(H2N(C2H4)2NH2)[V4O10] Yiping Zhang,?,?Robert C.Haushalter,*,?and Abraham Clearfield*,? NEC Research Institute,4Independence Way,Princeton,New Jersey08540,and Department of Chemistry,Texas A&M University,College Station,Texas77843 Recei V ed September26,1995X Four new layered mixed-valence vanadium oxides,which contain interlamellar organic cations,R-(H3N(CH2)2- NH3)[V4O10](1a), -(H3N(CH2)2NH3)[V4O10](1b),R-(H2N(C2H4)2NH2)[V4O10](2a),and -(H2N(C2H4)2NH2)- [V4O10](2b),have been prepared under hydrothermal conditions and their single-crystal structures determined: 1a,triclinic,space group P1h,a)6.602(2)?,b)7.638(2)?,c)5.984(2)?,R)109.55(3)°, )104.749- (2)°,γ)82.31(3)°,Z)1;1b,triclinic,P1h,a)6.387(1)?,b)7.456(2)?,c)6.244(2)?,R)99.89(2)°, )102.91(2)°,γ)78.74(2)°,Z)1;2a,triclinic,P1h,a)6.3958(5)?,b)8.182(1)?,c)6.3715(7)?,R )105.913(9)°, )104.030(8)°,γ)94.495(8)°,Z)1;2b,monoclinic,space group P21/n,a)9.360(2)?,b )6.425(3)?,c)10.391(2)?, )105.83(1)°,Z)2.All four of the compounds contain mixed-valence V5+/V4+vanadium oxide layers constructed from V5+O4tetrahedra and pairs of edge-sharing V4+O5square pyramids with protonated organic amines occupying the interlayer space. Introduction The contemporary interest in vanadium oxide bronzes reflects not only their interesting electronic and magnetic properties1 but also their complex structural chemistry,associated with the ability of vanadium to adopt a variety of coordination geometries in various oxidation states.In addition to the conventional alkali-metal bronzes A x V2O5,2a class of organic-based vanadium bronzes are also known.While most of the alkali-metal bronzes have been prepared at high temperatures,the organic-based vanadium bronzes are prepared at room temperature or slightly higher via intercalation reactions with vanadium pentoxide xerogels,V2O5?n H2O.The V2O5?n H2O host possesses a porous layered structure and is capable of intercalating a variety of neutral and charged guest species such as alkali-metal ions,3 alkylamines,4alcohols,5pyridine,6benzidine,7etc.The insertion of amines or metal complexes into V2O5hosts has also been reported.8The resulting intercalation compounds usually retain the lamellar structure with the guest species and water molecules occupying the interlayer regions.Partial reduction of V5+to V4+of the oxide layers has been observed to accompany the intercalation reactions with organic amines.In the cases of aniline9and thiophene,10the reduction of the vanadium oxide host,and the simultaneous oxidative polymerization of the guest molecules in the interlayer regions,have been observed.These intercalation compounds with reduced vanadium sites constitute an interesting class of organic-inorganic composite materials that can be viewed as molecular or polymer vanadium bronzes by analogy to alkali-metal bronzes.2However,the structural information about these intercalation compounds is very limited due to their amorphous or semicrystalline nature and lack of high-quality single crystals. Hydrothermal techniques,in combination with organic tem-plates,have been recently demonstrated to be well suited for the synthesis and crystal growth of reduced oxomolybdenum and oxovanadium phosphates and vanadium phosphonates.A series of novel organically templated molybdenum and vana-dium phosphates and vanadium phosphonates with molecular, two-dimensional layered,and three-dimensional open-frame-work structures have been prepared under hydrothermal condi-tions.11In contrast,hydrothermal synthesis of vanadium oxides using organic templates remains relatively unexplored.12While there are many examples of alkali-metal vanadium oxide bronzes with three-dimensional or two-dimensional structures in which the alkali metals occupy the channels or the interlayer regions, analogous organically templated vanadium oxides with3-D open *To whom all correspondence should be addressed. ?Texas A&M University. ?NEC Research Institute. X Abstract published in Ad V ance ACS Abstracts,August1,1996. (1)Murphy,D.W.;Christian,P.A.Science1979,205,651. (2)Hagenmuller,P.In Non-Stoichiometric Compounds,Tungsten Bronzes, Vanadium Bronzes and Related compounds;Bevan,D.J.,Hagen-muller,P.,Eds.;Pergamon Press:Oxford,U.K.,1973;Vol.1. (3)Lemordant,D.;Bouhaouss,A.;Aldebert,P.;Baffier,N.Mater.Res. Bull.1986,21,273. (4)Paul-Boucour,V.;Aldebert,P.Mater.Res.Bull.1983,18,1247. (5)Aldebert,P.;Baffier,N.;Legendre,J.-J.;Livage,J.Re V.Chim.Miner. 1982,19,485.Aldebert,P.;Baffier,N.;Gharbi,N.;Livage,J.Mater. Res.Bull.1981,16,949.Lemordant,D.;Bouhaouss,A.;Aldebert, P.;Baffier,N.J.Chim.Phys.Phys.-Chim.Biol.1986,83,105. (6)Ruiz-Hitzky,E.;Casal,B.J.Chem.Soc.,Faraday Trans.11986,82, 1597. (7)Hasbah,H.;Tinet,D.;Crespin,M.M.;Erre,R.;Setton,R.;Van Damme,H.J.Chem.Soc.,https://www.360docs.net/doc/3612311541.html,mun.1985,935. (8)Kanatzidis,M.;Marks,T.J.Inorg.Chem.1987,26,783and references therein. (9)Kanatzidis,M.;Wu,C.-G.J.Am.Chem.Soc.1989,111,4139. (10)Kanatzidis,M.;Wu,C.-G.;Marcy,H.O.;DeGroot,D.C.;Kannewurf, C.R.Chem.Mater.1990,2,222. (11)Haushalter,R.C.;Mundi,L.A.Chem.Mater.1992,4,31.Soghomo- nian,V.;Chen,Q.;Haushalter,R.C.;Zubieta,J.;O’Connor,C.J. Science1993,259,1596.Soghomonian,V.;Chen,Q.;Haushalter,R. C.;Zubieta,J.Angew.Chem.,Int.Ed.Engl.1993,32,610.Soghomo- nian,V.;Chen,Q.;Haushalter,R.C.;Zubieta,J.Chem.Mater.1993, 5,1690.Soghomonian,V.;Chen,Q.;Haushalter,R.C.;Zubieta,J., Chem.Mater.1993,5,1595.Soghomonian,V.;Haushalter,R.C.; Chen,Q.;Zubieta,J.Inorg.Chem.1994,33,1700.Zhang,Y.; Clearfield,A.;Haushalter,R.C.J.Solid State Chem.1995,117,157. Zhang,Y.;Clearfield,A.;Haushalter,R.C.Chem.Mater.1995,7, 1221. (12)Huan,G.-H.;Johnson,J.W.;Jacobson,A.J.;Merola,J.S.J.Solid State Chem.1991,91,385.Duan,C.-Y.;Tian,Y.-P.;Lu,Z.-L.;You, X.-Z.;Huang,X.-Y.Inorg.Chem.1995,34,1. 4950Inorg.Chem.1996,35,4950-4956 S0020-1669(95)01237-7CCC:$12.00?1996American Chemical Society
辞职申请书范文大全500字
辞职申请书范文大全500字 辞职申请书500字 辞职一般是提前30天向上级或公司递交辞职,无需公司批准,30天之后您就能顺利辞职了,以下是为大家搜集的范文,欢迎阅读! 尊敬的公司领导: 由于工作调动,现正式向公司提出调离原工作岗位。 舍不得,舍不得这里的人,舍不得自己曾经的付出。每一次出差、每一次报价、每一次谈判、每一次争吵,在飞机上、在吉普车上、在会议室里、在工地上,所有这一切,都充斥着我的记忆,那么清晰,就像是在昨天。但时间的指针总是忠诚地一步一步往前走,昨天终究会结束。 在公司四年半的时间里,我收获了很多,除了朋友和知识,更 重要的是,我到了成长的快乐。感谢命运,让我在最青春的年华里遇到了装备公司;感谢公司领导,你们的关注和欣赏让我一直充满自信,你们的指点和教诲让我在成长的路上少走了很多弯路;感谢公司的同事,和你们的沟通,轻松愉悦;感谢我自己,能够一直保持着一份纯净,真诚地付出,真诚地享受每一次收获。
鉴于目前的身体及生活状态,自认为不能够为公司创造更大的价值,现向公司提出辞职。 虽然我不能在这里继续“战斗”下去,但真心的希望,xx公司能够梦想成真,在世界的舞台上舞出属于自己的精彩。 此致 敬礼! 辞职人: 20xx年xx月xx日 尊敬的x总: 您好! 转眼间,我到公司已有X年了,这X年的工作时间里,虽然我的工作并不是尽善尽美,但在公司同事们的帮助,尤其是您的信任与教导下,我也努力的去完成每一项您布置给我的工作,都用了自己的
热情努力去对待。凭心而论,我开始对基础工程毫无了解,但在您这里我基本了解了基础工程,使我学到了很多东西,特别是一些做人的道理和对生活的理解。在这里,我真诚的对袁总说一声:谢谢您了! 但犹豫再三,经过了长时间的考虑,我还是写了这封辞职申请书。 加入公司以来,您对我的信任、教导与严格要求,令我非常感动,也成为激励我努力工作的动力。在您及同事们的热心指导与悉心帮助下,我在工程技术和管理能力方面都有了一定的提高。我常想,自己应该用一颗感恩的心,去回报您及公司对我的栽培,真的想用自己的努力去做好您交给的每一份工作任务,但自己的能力真的很有限,有很多地方没有做得能让您满意,所以对过去工作中失误与不足的地方,我真诚的对您说声抱歉,请您原谅! 经过这段时间的思考,我觉得我可能技术能力方面有所不足, 也缺少工作的积极性和脚踏实地的工作精神,没能很好的适应这个工作,所以一直没有把工作做到令您满意的程度。这是我在以后的人生中需要注意的地方,也是袁总经常教导我的地方,我一定会铭记于心! 再一次真诚地感谢您及公司全体同事对我的关爱与帮助!
Geological and isotopic evidence for magmatic-hydrothermal
ARTICLE Geological and isotopic evidence for magmatic-hydrothermal origin of the Ag –Pb –Zn deposits in the Lengshuikeng District,east-central China Changming Wang &Da Zhang &Ganguo Wu & M.Santosh &Jing Zhang &Yigan Xu &Yaoyao Zhang Received:7August 2012/Accepted:27March 2014/Published online:8April 2014#Springer-Verlag Berlin Heidelberg 2014 Abstract The Lengshuikeng ore district in east-central China has an ore reserve of ~43Mt with an average grade of 204.53g/t Ag and 4.63%Pb+Zn.Based on contrasting geological characteristics,the mineralization in the Lengshuikeng ore district can be divided into porphyry-hosted and stratabound types.The porphyry-hosted minerali-zation is distributed in and around the Lengshuikeng granite porphyry and shows a distinct alteration zoning including minor chloritization and sericitization in the proximal zone;sericitization,silicification,and carbonatization in the periph-eral zone;and sericitization and carbonatization in the distal zone.The stratabound mineralization occurs in volcano-sedimentary rocks at ~100–400m depth without obvious zoning of alterations and ore minerals.Porphyry-hosted and stratabound mineralization are both characterized by early-stage pyrite –chalcopyrite –sphalerite,middle-stage acanthite –native silver –galena –sphalerite,and late-stage pyrite –quartz –calcite.The δ34S values of pyrite,sphalerite,and galena in the ores range from ?3.8to +6.9‰with an average of +2.0‰.The C –O isotope values of siderite,calcite,and dolomite range from ?7.2to ?1.5‰with an average of ?4.4‰(V-PDB)and from +10.9to +19.5‰with an average of +14.8‰ (V-SMOW),respectively.Hydrogen,oxygen,and carbon iso-topes indicate that the hydrothermal fluids were derived main-ly from meteoric water,with addition of minor amounts of magmatic water.Geochronology employing LA –ICP –MS analyses of zircons from a quartz syenite porphyry yielded a weighted mean 206Pb/238U age of 136.3±0.8Ma considered as the emplacement age of the porphyry.Rb –Sr dating of sphalerite from the main ore stage yielded an age of 126.9±7.1Ma,marking the time of mineralization.The Lengshuikeng mineralization classifies as an epithermal Ag –Pb –Zn deposit. Keywords Stable isotope .Geochemistry .Porphyry .Stratabound .Ag –Pb –Zn .Lengshuikeng Introduction The Lengshuikeng ore district,located in the Jiangxi Province of east-central China (Fig.1a ),contains more than 50ore bodies belonging to seven deposits hosted in granite porphyry,pyroclastic,and carbonate rocks.The ore reserves in Lengshuikeng have been estimated at ~43Mt with average grades of 2.11%Pb,2.61%Zn,204.53g/t Ag,0.08g/t Au,and 0.01%Cd.The ores can be grouped into two types:(1)porphyry-hosted (Yinluling,Baojia,and Yinzhushan)and (2)stratabound (Xiabao,Yinkeng,Yinglin,and Xiaoyuan).The porphyry-hosted mineralization is distributed within and around the Lengshuikeng granite porphyry,whereas the stratabound mineralization occurs in volcano-sedimentary rocks at ~100–400m depth.The spatial distribution of the porphyry-hosted and stratabound ore bodies,their mineral constituents,and the zoning of alteration assemblages are markedly different from those of typical porphyry deposits. Editorial handling:T.Bissig and G.Beaudoin C.Wang (*): D.Zhang :G.Wu :M.Santosh :J.Zhang :Y .Xu :Y .Zhang State Key Laboratory of Geological Processes and Mineral Resources,China University of Geosciences,No.29,Xueyuan Road,Beijing 100083,People ’s Republic of China e-mail:wcm233@https://www.360docs.net/doc/3612311541.html, Y .Xu No.912Geological Surveying Team,Bureau of Geology and Mineral Exploration and Development,Yingtan 334000,China Miner Deposita (2014)49:733–749DOI 10.1007/s00126-014-0521-8
简短辞职申请书范文大全
简短辞职申请书范文大全 想必每一位在职场混迹多年的职场人士都应曾经写过辞职信之类的。在现在这个发展速度如此之快的社会,跳槽也就成了常见现象。而离职前的辞职信是必写的。下面就是小编给大家带来的简短辞职申请书范文大全,希望大家喜欢! 尊敬的xx: 我自xx年来到公司,工作中得到公司和您的培养,个人得到了很大的成长,公司的文化和环境也令我工作得非常开心。 现由于个人原因,我不得不提出辞职,希望能于x年x月x日正式离职,请公司批准我的这份辞职书。并请公司在x月x日前安排好人员接替我的工作,我将尽心交接。 再次对您x年来的培养和指导表示衷心的感谢。 最后祝您及公司的所有同事一切顺利! 此致 敬礼 辞职人:xxx 20xx年x月x日 尊敬的X经理: 您好! 感谢公司在我入职以来的培养关心和照顾,从X年X月份来到[公司]至今,我学到了很多东西,今后无论走向哪里,从事什么,这段经历都是一笔宝贵的财富,我为在彩卡的这段工作经历而自豪。 而今,由于个人原因提出辞职,望领导批准。 辞职人: 20xx年x月x日
公司人事部: 我因为要去美国留学,故需辞去现在的工作,请上级领导批准。 公司的企业文化感化了我,我对公司是深有感情的。我留学归来之后,仍愿意回公司就职。 感谢公司领导和同事在工作中对我的关心和支持,并祝公司兴隆。 辞职人:xxx 20xx年x月x日 尊敬的公司领导: 在递交这份辞呈时,我的心情十分沉重。现在由于我的一些个人原因的影响,无法为公司做出相应的贡献。因此请求允许离开。 当前公司正处于快速发展的阶段,同事都是斗志昂扬,壮志满怀,而我在这时候却因个人原因无法为公司分忧,实在是深感歉意。 我希望公司领导在百忙之中抽出时间受理我的离职事项。 感谢诸位在我在公司期间给予我的信任和支持,并祝所有同事和朋友们在工作和活动中取得更大的成绩。 辞职人: 20xx年x月x日 尊敬的xx: 自xx年入职以来,我一直很喜欢这份工作,但因某些个人原因,我要重新确定自己未来的方向,最终选择了开始新的工作。 希望公司能早日找到合适人手开接替我的工作并希望能于今年5月底前正式辞职。如能给予我支配更多的时间来找工作我将感激不尽,希望公司理解!在我提交这份辞呈时,在未离开岗位之前,我一定会尽自己的职责,做好应该做的事。 最后,衷心的说:“对不起”与“谢谢”! 祝愿公司开创更美好的未来!
