体外消化实验
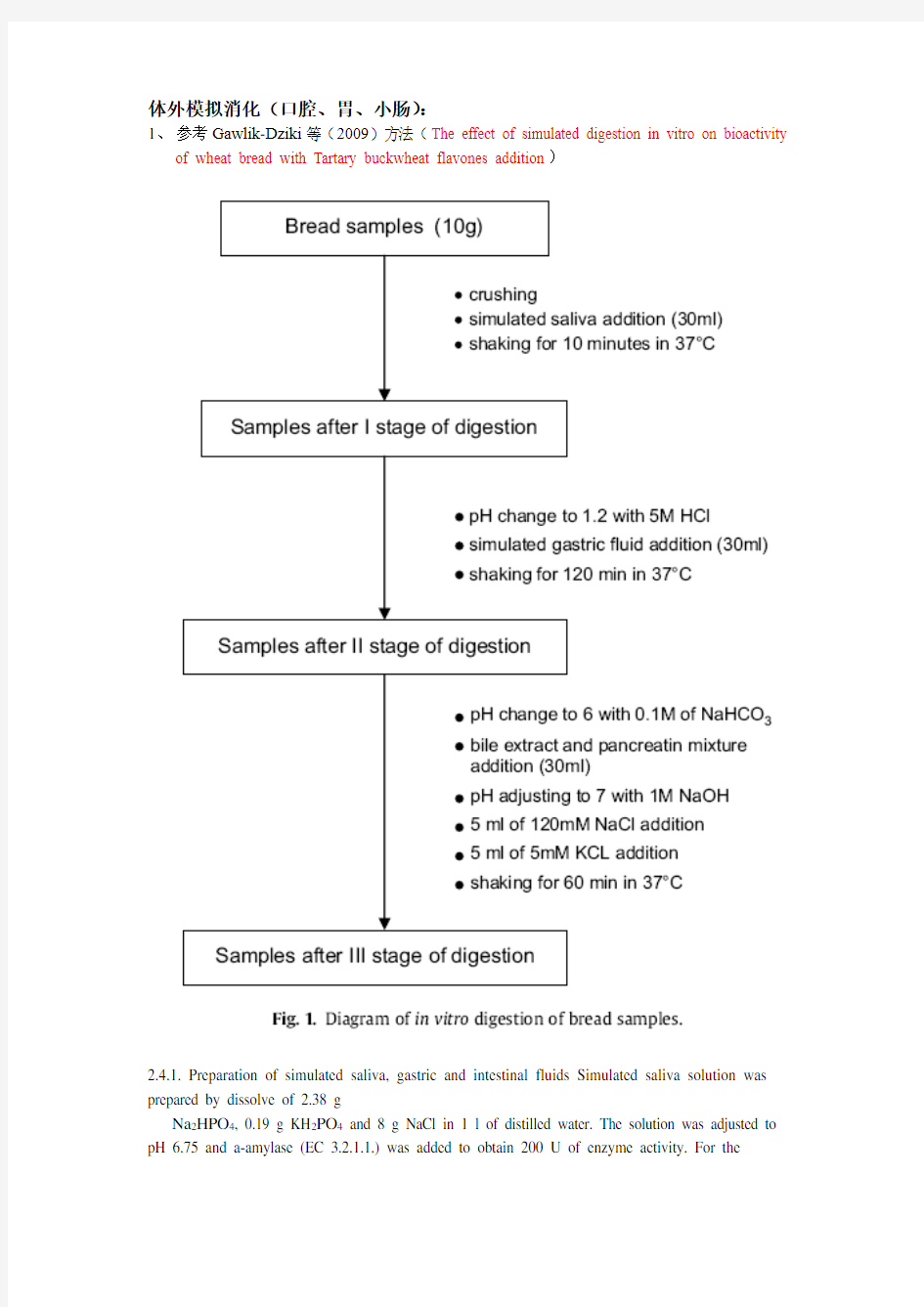
体外模拟消化(口腔、胃、小肠):
1、参考Gawlik-Dziki等(2009)方法(The effect of simulated digestion in vitro on bioactivity
of wheat bread with Tartary buckwheat flavones addition)
2.4.1. Preparation of simulated saliva, gastric and intestinal fluidsSimulated saliva solution was prepared by dissolve of 2.38 g
Na2HPO4, 0.19 g KH2PO4 and 8 g NaCl in 1 l of distilled water. Thesolution was adjusted to pH 6.75 and a-amylase (EC 3.2.1.1.) wasadded to obtain 200 U of enzyme activity. For the gastric
digestion,0.32% pepsin (from porcine stomach mucosa, pepsin A, EC 3.4.23.1)dilution in 0.03 M NaCl, pH 1.2 was prepared. Simulated intestinal.juice was prepared by dilution of 0.05 g of pancreatin and 0.3 g ofbile extract in 35 ml 0.1 M NaHCO3.
2.4.1. Preparation of simulated saliva, gastric and intestinal fluids
Simulated saliva solution was prepared by dissolve of 2.38 g Na2HPO4, 0.19 g KH2PO4 and 8 g NaCl in 1 l of distilled water. The solution was adjusted to pH 6.75 and α-amylase (EC 3.2.1.1.) was added to obtain 200 U of enzyme activity. For the gastric digestion, 0.32% pepsin (from porcine stomach mucosa, pepsin A, EC 3.4.23.1) dilution in 0.03 M NaCl, pH 1.2 was prepared. Simulated intestinal juice was prepared by dilution of 0.05 g of pancreatin and 0.3 g of bile extract in 35 ml 0.1 M NaHCO3.
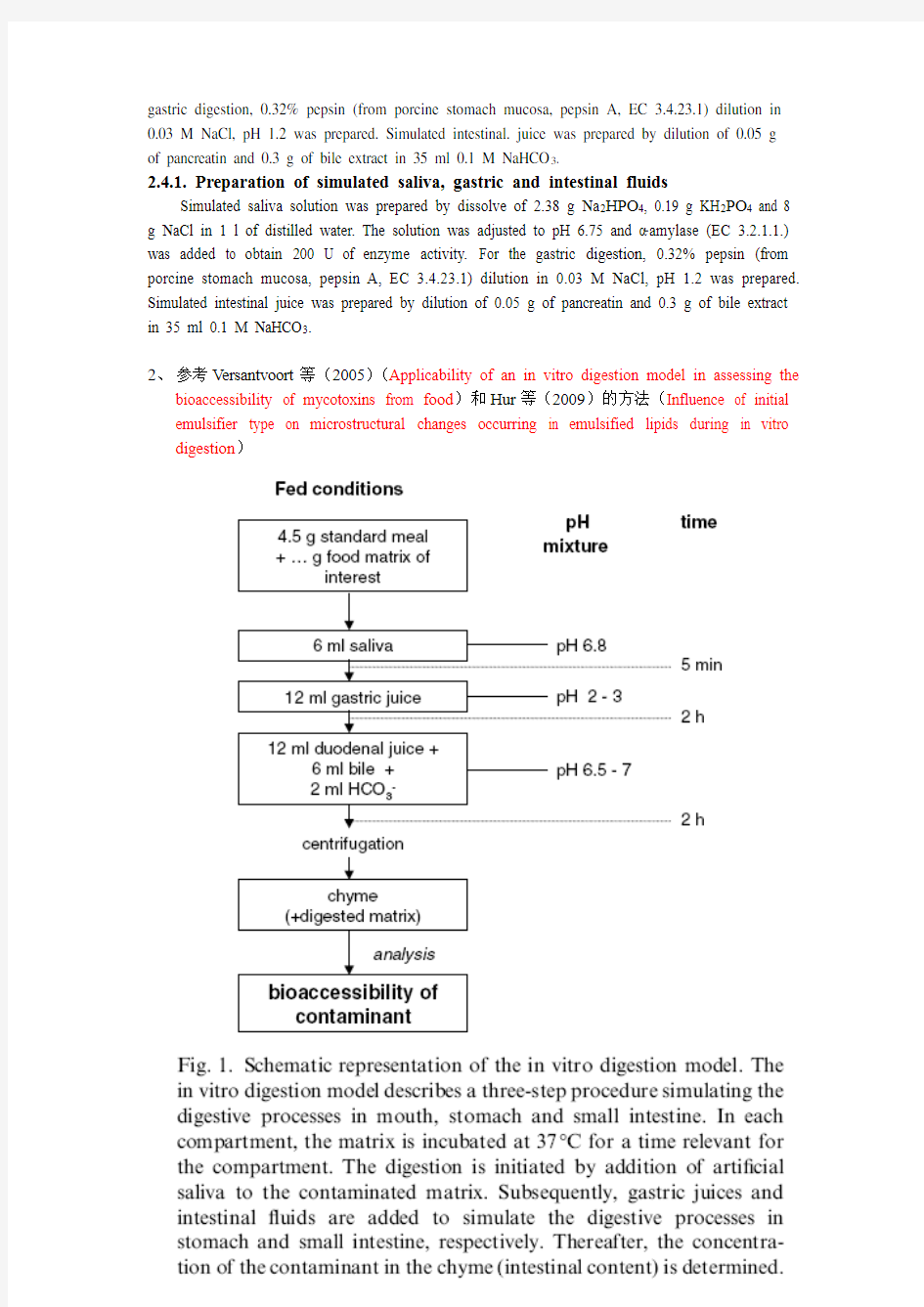
2、参考Versantvoort等(2005)(Applicability of an in vitro digestion model in assessing the
bioaccessibility of mycotoxins from food)和Hur等(2009)的方法(Influence of initial emulsifier type on microstructural changes occurring in emulsified lipids during in vitro digestion)
Table 1
Constituents and concentrations of the various synthetic juices of the in vitro digestion model representing fed conditions
Saliva 唾液Gastric juice 胃液Duodenal juice
十二指肠液
Bile juice 胆汁
Inorganic solution 10ml KCl 89.6
g/l
15.7 ml NaCl 175.3g/l
40 ml NaCl
175.3g/l
30 ml NaCl
175.3g/l
10 ml KSCN
20g/l
3.0 ml NaH2PO4 88.8
g/l
40 ml NaHCO3
84.7 g/l
68.3 ml NaHCO3
84.7 g/l
10 ml NaH2PO4
88.8 g/l
9.2 ml KCl 89.6 g/l
10 ml KH2PO4
8g/l
4.2 ml KCl 89.6 g/l 10 ml NaSO4
57g/l
18 ml CaCl2· 2H2O
22.2 g/l
6.3 ml KCl
89.6 g/l
150 μlHCl 37% g/g 1.7 ml NaCl
175.3 g/l
10 ml NH4Cl 30.6 g/l
10 ml MgCl2 5
g/l
20 ml NaHCO3
84.7 g/l
6.5 ml HCl 37%g/g
180 μlHCl
37%g/g
Organic solution 8ml urea 25g/l 10ml glucose 65g/l 4ml urea 25g/l 10ml urea 25g/l
10 ml glucuronic acid
(葡萄糖醛酸) 2g/l
3.4 ml urea 25g/l
10 ml glucosamine
hydrochloride (盐酸
氨基葡萄糖) 33g/l
Add to mixture
organic
+ inorganic solution
290mg
α-amylase
1g BSA
9ml
CaCl2· 2H2O
22.2 g/l
10ml CaCl2· 2H2O
22.2 g/l
15 mg uric acid 2.5 g pepsin 1g BSA 1.8 g BSA
25 mg mucin 3 g mucin 9 g pancreatin 30 g bile
1.5 g lipase
pH 6.8 ± 0.2 1.30 ± 0.02 8.1 ± 0.2 8.2 ± 0.2 The inorganic and organic solutions are augmented to 500ml with distilled water. After mixing of the inorganic and organic solutions, some further constituents are added and dissolved. If necessary, the pH of the juices is adjusted to the appropriate interval.
另外,
抗性淀粉含量测定:
1、参考Li等(2008)方法(Characterization of maize amylose-extender (ae) mutant starches. Part
I: Relationship between resistant starch contents and molecular structures)
2.3. Resistant starch (RS) content
RS contents of the ae-mutant starch samples were determined using the AOAC Method 991.43 for total dietary fiber (AOAC, 2003) and Englyst’s method (1992) for comparison.
For the AOAC 991.43 method, starch (1.0 g, dry-starch basis, dsb) was suspended in a Mes-tris buffer solution (0.05 M, 40 ml) and hydrolyzed with 500 u of a-amylase from Bacillus
licheniformis (Sigma Chemical, Cat. No. A3403) in a boiling water-bath for 30 min with stir. The sample was then digested with protease from Bacillus licheniformis(5 mg, Sigma Chemical, Cat. No. P3910) at 60 oC in a shaker waterbath for 30 min. The sample dispersion was adjusted to pH 4.4–4.6 by adding HCl and then hydrolyzed with amyloglucosidase (300 U, Sigma chemical, Cat. No. A9913) at 60 oC in a shaker water-bath for 30 min. The digested sample was filtered through a celite layer in a crucible and washed twice with 15 ml of 78% ethanol, twice with 15 ml of 100% ethanol and rinsed with 15 ml of acetone. The remaining sample was dried in an oven at 100 oC overnight. The resistant starch content was calculated using the equation:
(%)RS content = Remaining sample weight (g, dsb)/initial sample weight (g, dsb) ×100%
For RS determined using Englyst’s method (1992), Starch(1.000 g, db) in 20 mL of sodium acetate buffer (0.1 M, pH 5.2)was cooked in a boiling water-bath for 30 min. The starch dispersionwas cooled down to 37 oC, mixed with an enzyme solution(5 mL) consisting of pancreatin extract and amyloglucosidase,and incubated in a water-bath at 37 oC. The pancreatin extractwas prepared as follows; 3.0 g of pancreatin (Sigma, Cat. No.P7545) was suspended in 20 mL deionized water, stirred for10 min at room temperature, and centrifuged at 1500g for10 min. The enzyme solution was prepared by mixing 13.5 mlsupernatant of pancreatin extract, 210 U amyloglucosidase (Sigma,Cat. No. A7095), and 1.0 mL deionized water. The rapid digestiblestarch (RDS) was defined as the total starch digested within thefirst 20 min, and the slowly digestible starch (SDS) was the starchdigested between 20 and 120 min (Englyst et al., 1992). The resistantstarch content was calculated as follows:
(%)RS content = 100%×(total starch – RDS - SDS) (g, dsb)/total starch (g, dsb)
