lactic starter cultures和乳酸乳球菌共培养-后酸

81 Journal of Food Protection,Vol.64,No.1,2001,Pages81–86
Copyright q,International Association for Food Protection
The Effects of Cultivating Lactic Starter Cultures with
Bacteriocin-Producing Lactic Acid Bacteria
A.OUMER,S.GARDE,P.GAYA,M.MEDINA,AND M.NUN?EZ*
Departamento de Tecnolog?′a de Alimentos,INIA,Carretera de La Corun?a Km7,Madrid,28040Spain
MS00-108:Received3April2000/Accepted17July2000
ABSTRACT
The effects of bacteriocins produced by six strains of lactic acid bacteria on9mesophilic and11thermophilic commercial starter cultures were investigated in mixed cultures of commercial starters with bacteriocin-produc ing strains in milk.The bacteriocins produced by the test organisms were nisin A,nisin Z,lacticin481,enterocin AS-48,a novel enterocin,and a novel plantaricin.Mesophilic commercial starters were in most cases tolerant of bacteriocins,with only two of the starters being partially inhibited,one by four and the other by two bacteriocins.The aminopeptidase activities of mesophilic starters were generally low,and only one of the combinations of mesophilic starter–bacteriocin producer gave double the aminopep-tidase activity of the starter culture without the bacteriocin producer.Thermophilic commercial starters were more sensitive to bacteriocins than mesophilic starters,with six thermophilic starters being partially inhibited by at least one of the bacteriocins.
Their aminopeptidase activities were generally higher than those of the mesophilic starters.The aminopeptidase activities of seven thermophilic starters were increased in the presence of bacteriocins,by factors of up to9.0as compared with the corresponding starter cultures alone.Bacteriocin-produc ing strains may be used as adjunct cultures to mesophilic starters for the inhibition of pathogens in soft and semihard cheeses,because mesophilic starters are rather tolerant of bacteriocins.
Bacteriocin producers may also be used as adjunct cultures to thermophilic starters of high aminopeptidase activity,more sensitive to lysis by bacteriocins than mesophilic starters,for the acceleration of ripening in semihard and hard cheeses.
Starter cultures commonly used in cheese manufacture are either de ned-strain starters,each consisting of a few phage-unrelated strains,or mixed-strain starters,consisting of unde ned or poorly de ned mixtures of relatively high numbers of strains,frequently derived from artisanal cul-tures(17).Lactic acid bacteria contribute signi cantly to avor formation in cheese by their metabolic activities(3). Peptides derived from caseins by the action of rennet and milk plasmin are hydrolyzed by starter enzymes to small peptides and free amino acids(14,16)that contribute di-rectly,or after further transformation,to the typical avor of cheese(6,8,26).The high population reached by starter lactic acid bacteria during the rst hours of cheese manu-facture,generally over109CFU/g,represents an important catalytic potential for transforming amino acids and other products into aromatic compounds(5,6).However,part of this enzymatic potential is not fully effective until the death and lysis of the cells,when rupture of the membrane allows the liberation of intracellular enzymes into the cheese ma-trix(1,9).Early starter lysis will favor the access of intra-cellular enzymes to their substrates and may accelerate the development of cheese avor(22).
Lactic acid bacteria produce a wide range of bacterio-cins(4,19,28).Nisin,traditionally used in the prevention of gas-blowing defect in cheese,also serves to inhibit path-ogens such as Listeria monocytogenes in Camembert cheese(18).Lacticin3147has been successfully used to *Author for correspondence.Tel:34913476799;Fax:34913572293;inhibit L.monocytogenes in cottage cheese(20)and to con-trol the growth of nonstarter lactic acid bacteria in Cheddar cheese(25).Enterococcal bacteriocins exhibited a strong antilisterial activity in milk and cheese(11,23),and pedi-ocin PA-1reduced the levels of L.monocytogenes on the surface of Munster cheese(7).Inoculation of milk with bacteriocin-producing strains of lactic acid bacteria was shown to be a successful procedure for enhancing the lysis of starter bacteria in cheese(10,22).
The tolerances of commercial starter cultures to bac-teriocins,which will depend on the individual resistance to bacteriocins of the strains included in each starter,are not well known.The use of bacteriocin-producing strains as adjunct cultures,either to inhibit pathogens in cheese or to enhance lysis of starter cells in order to accelerate avor formation,requires previous studies.Therefore,the abilities of mesophilic and thermophilic commercial starters to tol-erate various bacteriocins and the lysis of starter cells by these bacteriocins were investigated.
MATERIALS AND METHODS
Commercial starter cultures and bacteriocin-producing strains.The starter cultures used in the present work,kindly pro-vided by Chr.Hansen(Madrid,Spain)and Rhodia Iberia(Madrid, Spain),were given laboratory designations to hide the identity of the industrial sources.The nine mesophilic starters used were cod-ed MS1to MS9.Starters MS1to MS7each consisted of a few Lactococcus lactis subspp.cremoris and lactis strains,whereas starters MS8and MS9were LD-type,and consisted of https://www.360docs.net/doc/867328084.html,ctis subspp.cremoris and lactis,including biovar diacetylactis,and
J.Food Prot.,Vol.64,No.1
82
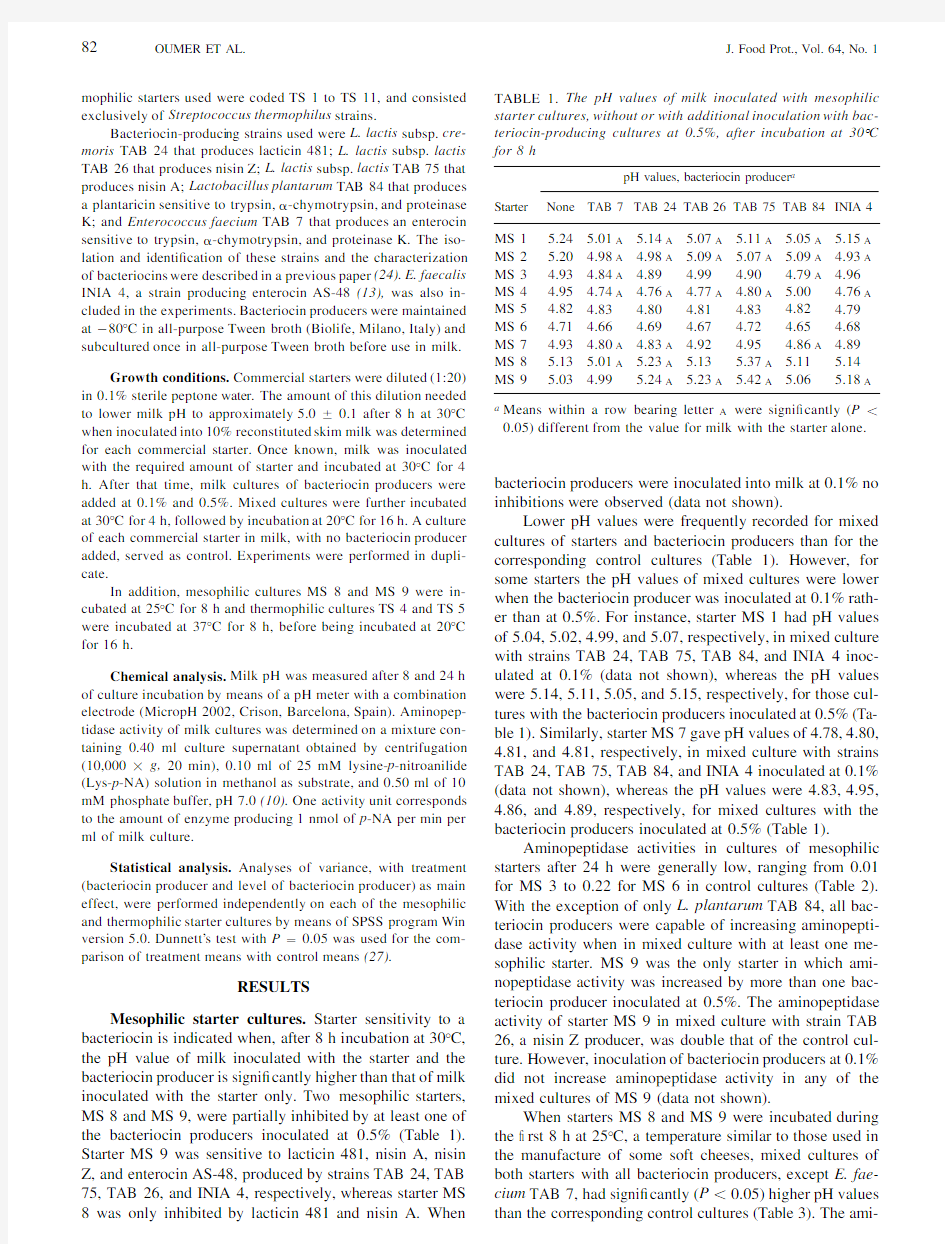
OUMER ET AL.TABLE 1.The pH values of milk inoculated with mesophilic starter cultures,without or with additional inoculation with bac-teriocin-producing cultures at 0.5%,after incubation at 308C for 8h
Starter
pH values,bacteriocin producer a
None
TAB 7TAB 24TAB 26TAB 75TAB 84INIA 4
MS 1MS 2MS 3MS 4MS 5MS 6MS 7MS 8MS
9 5.245.204.934.954.824.714.935.135.03 5.01A 4.98A 4.84A 4.74A 4.834.664.80A 5.01A 4.99 5.14A 4.98A 4.894.76A 4.804.694.83A 5.23A 5.24A 5.07A 5.09A 4.994.77A 4.814.674.925.135.23A 5.11A 5.07A 4.904.80A 4.834.724.955.37A 5.42A 5.05A 5.09A 4.79A 5.004.824.654.86A 5.115.06 5.15A 4.93A 4.964.76A 4.794.684.895.145.18A
a
Means within a row bearing letter A were signi cantly (P ,0.05)different from the value for milk with the starter alone.
mophilic starters used were coded TS 1to TS 11,and consisted exclusively of Streptococcus thermophilus strains.
Bacteriocin-produci ng strains used were https://www.360docs.net/doc/867328084.html,ctis subsp.cre-moris TAB 24that produces lacticin 481;https://www.360docs.net/doc/867328084.html,ctis https://www.360docs.net/doc/867328084.html,ctis TAB 26that produces nisin Z;https://www.360docs.net/doc/867328084.html,ctis https://www.360docs.net/doc/867328084.html,ctis TAB 75that produces nisin A;Lactobacillus plantarum TAB 84that produces a plantaricin sensitive to trypsin,a -chymotrypsin,and proteinase K;and Enterococcus faecium TAB 7that produces an enterocin sensitive to trypsin,a -chymotrypsin,and proteinase K.The iso-lation and identi cation of these strains and the characterization of bacteriocins were described in a previous paper (24).E.faecalis INIA 4,a strain producing enterocin AS-48(13),was also in-cluded in the experiments.Bacteriocin producers were maintained at 2808C in all-purpose Tween broth (Biolife,Milano,Italy)and subcultured once in all-purpose Tween broth before use in milk.Growth https://www.360docs.net/doc/867328084.html,mercial starters were diluted (1:20)in 0.1%sterile peptone water.The amount of this dilution needed to lower milk pH to approximately 5.060.1after 8h at 308C when inoculated into 10%reconstituted skim milk was determined for each commercial starter.Once known,milk was inoculated with the required amount of starter and incubated at 308C for 4h.After that time,milk cultures of bacteriocin producers were added at 0.1%and 0.5%.Mixed cultures were further incubated at 308C for 4h,followed by incubation at 208C for 16h.A culture of each commercial starter in milk,with no bacteriocin producer added,served as control.Experiments were performed in dupli-cate.
In addition,mesophilic cultures MS 8and MS 9were in-cubated at 258C for 8h and thermophilic cultures TS 4and TS 5were incubated at 378C for 8h,before being incubated at 208C for 16h.
Chemical https://www.360docs.net/doc/867328084.html,k pH was measured after 8and 24h of culture incubation by means of a pH meter with a combination electrode (MicropH 2002,Crison,Barcelona,Spain).Aminopep-tidase activity of milk cultures was determined on a mixture con-taining 0.40ml culture supernatant obtained by centrifugation (10,0003g,20min),0.10ml of 25mM lysine-p -nitroanilide (Lys-p -NA)solution in methanol as substrate,and 0.50ml of 10mM phosphate buffer,pH 7.0(10).One activity unit corresponds to the amount of enzyme producing 1nmol of p -NA per min per ml of milk culture.
Statistical analysis.Analyses of variance,with treatment (bacteriocin producer and level of bacteriocin producer)as main effect,were performed independently on each of the mesophilic and thermophilic starter cultures by means of SPSS program Win version 5.0.Dunnett’s test with P 50.05was used for the com-parison of treatment means with control means (27).
RESULTS
Mesophilic starter cultures.Starter sensitivity to a bacteriocin is indicated when,after 8h incubation at 308C,the pH value of milk inoculated with the starter and the bacteriocin producer is signi cantly higher than that of milk inoculated with the starter only.Two mesophilic starters,MS 8and MS 9,were partially inhibited by at least one of the bacteriocin producers inoculated at 0.5%(Table 1).Starter MS 9was sensitive to lacticin 481,nisin A,nisin Z,and enterocin AS-48,produced by strains TAB 24,TAB 75,TAB 26,and INIA 4,respectively,whereas starter MS bacteriocin producers were inoculated into milk at 0.1%no inhibitions were observed (data not shown).
Lower pH values were frequently recorded for mixed cultures of starters and bacteriocin producers than for the corresponding control cultures (Table 1).However,for some starters the pH values of mixed cultures were lower when the bacteriocin producer was inoculated at 0.1%rath-er than at 0.5%.For instance,starter MS 1had pH values of 5.04,5.02,4.99,and 5.07,respectively,in mixed culture with strains TAB 24,TAB 75,TAB 84,and INIA 4inoc-ulated at 0.1%(data not shown),whereas the pH values were 5.14,5.11,5.05,and 5.15,respectively,for those cul-tures with the bacteriocin producers inoculated at 0.5%(Ta-ble 1).Similarly,starter MS 7gave pH values of 4.78,4.80,4.81,and 4.81,respectively,in mixed culture with strains TAB 24,TAB 75,TAB 84,and INIA 4inoculated at 0.1%(data not shown),whereas the pH values were 4.83,4.95,4.86,and 4.89,respectively,for mixed cultures with the bacteriocin producers inoculated at 0.5%(Table 1).
Aminopeptidase activities in cultures of mesophilic starters after 24h were generally low,ranging from 0.01for MS 3to 0.22for MS 6in control cultures (Table 2).With the exception of only L.plantarum TAB 84,all bac-teriocin producers were capable of increasing aminopepti-dase activity when in mixed culture with at least one me-sophilic starter.MS 9was the only starter in which ami-nopeptidase activity was increased by more than one bac-teriocin producer inoculated at 0.5%.The aminopeptidase activity of starter MS 9in mixed culture with strain TAB 26,a nisin Z producer,was double that of the control cul-ture.However,inoculation of bacteriocin producers at 0.1%did not increase aminopeptidase activity in any of the mixed cultures of MS 9(data not shown).
When starters MS 8and MS 9were incubated during the rst 8h at 258C,a temperature similar to those used in the manufacture of some soft cheeses,mixed cultures of both starters with all bacteriocin producers,except E.fae-cium TAB 7,had signi cantly (P ,0.05)higher pH values
J.Food Prot.,Vol.64,No.1STARTER CULTURES AND BACTERIOCIN PRODUCERS83 TABLE2.The aminopeptidase activities in milk inoculated with
mesophilic starter cultures,without or with additional inoculation
with bacteriocin-produ cing cultures at0.5%,after incubation at
308C for8h followed by incubation at208C for16h
Starter
Aminopeptidase activities on Lys-p-NA,
bacteriocin producer a
None TAB7TAB24TAB26TAB75TAB84INIA4
MS1 MS2 MS3 MS4 MS5 MS6 MS7 MS8 MS90.09
0.06
0.01
0.10
0.06
0.22
0.13
0.19
0.09
0.10
0.09
0.07A
0.05A
0.03A
0.15
0.12
0.09A
0.16A
0.10
0.10A
0.01
0.12
0.07
0.12A
0.10A
0.22
0.17A
0.10
0.07
0.02
0.11
0.06
0.11A
0.12
0.18
0.18A
0.04A
0.08
0.02
0.08
0.05
0.13A
0.10A
0.15A
0.16A
0.09
0.09
0.02
0.10
0.02A
0.17
0.10A
0.15A
0.12
0.07
0.06
0.03
0.08
0.03A
0.06A
0.11
0.17
0.14A
a Means within a row bearing letter A were signi cantly(P,
0.05)different from the value for milk with the starter alone.TABLE4.The pH values of milk inoculated with thermophilic starter cultures,without or with additional inoculation with bac-teriocin-producin g cultures at0.5%,after incubation at308C for 8h
Starter
pH values,bacteriocin producer a
None TAB7TAB24TAB26TAB75TAB84INIA4 TS1
TS2
TS3
TS4
TS5
TS6
TS7
TS8
TS9
TS10
TS11
4.88
4.99
4.69
4.96
5.03
4.96
5.00
4.80
4.86
4.89
4.80
4.92
5.12A
4.74
4.99
5.02
5.03
5.09A
4.86
4.91
4.94
4.87
4.91
5.08A
4.74
4.99
5.04
4.98
5.00
4.78
4.84
4.87
4.80
4.91
5.27A
4.74
5.22A
5.13A
5.07A
5.08
4.86
4.93
4.96
4.87
4.91
5.28A
4.73
5.31A
5.16A
5.06A
5.09A
4.90A
4.93
4.93
4.87
4.83
5.05A
4.73
4.95
5.07
5.02
5.05
4.86
4.84
4.96
4.84
4.88
5.13A
4.72
4.95
5.03
5.07A
5.06
4.84
4.90
4.95
4.80
a Means within a row bearing letter A were signi cantly(P,
0.05)different from the value for milk with the starter alone.
TABLE3.The pH values of milk inoculated with two mesophilic starter cultures,without or with additional inoculation with bacte-riocin-producing cultures at0.5%,after incubation at258C for8h and the aminopeptidase activities(AA)after incubation at258C for 8h followed by incubation at208C for16h
Starter
Bacteriocin producer a
None TAB7TAB24TAB26TAB75TAB84INIA4
MS8 MS9pH
AA
pH
AA
5.24
0.16
5.04
0.08
5.28
0.18
5.07
0.10
5.42A
0.07A
5.19A
0.07
5.39A
0.22A
5.20A
0.08
5.38A
0.14
5.18A
0.16A
5.37A
0.12A
5.12A
0.13A
5.44A
0.27A
5.15A
0.06
nopeptidase activities of cultures of both starters after8h
at258C followed by16h at208C were higher in mixed cultures with TAB26or INIA4in the case of MS8,or in mixed cultures with TAB75or TAB84in the case of MS9,than in the corresponding control cultures.The mixed culture of MS9with nisin A-producing https://www.360docs.net/doc/867328084.html,ctis https://www.360docs.net/doc/867328084.html,ctis TAB75showed higher aminopeptidase activ-ity than the corresponding control culture after incubation at258C or308C during the rst8h(Tables2and3).
Thermophilic starter cultures.Thermophilic starters were more sensitive to bacteriocins than mesophilic starters, with6out of11thermophilic starters being partially inhib-ited by at least one of the bacteriocin producers inoculated at0.5%(Table4).There was no signi cant inhibition or stimulation of any of the other thermophilic starters by bac-teriocin https://www.360docs.net/doc/867328084.html,ctis https://www.360docs.net/doc/867328084.html,ctis strains TAB26 and TAB75,respectively,producing nisin Z and nisin A, were the most effective inhibitors of thermophilic starters. When strain TAB26was inoculated at0.1%,pH values of mixed cultures with starters TS2,TS4,and TS5were 5.06,5.04,and5.09,respectively(data not shown),lower than the pH values of corresponding cultures with0.5% inocula of bacteriocin producers but signi cantly higher than those of the corresponding control cultures(Table4), which indicates inhibition of the starters by bacteriocins.Similarly,pH values of mixed cultures of starters TS2and TS4with strain TAB75inoculated at0.1%were5.07and 5.06,lower than the pH values of corresponding cultures with0.5%inocula but signi cantly(P,0.05)higher than those of the corresponding control cultures(Table4).
Aminopeptidase activities of thermophilic starters after 24h were considerably higher than those of mesophilic starters,ranging in control cultures from0.07for TS8to 1.06for TS4(Table5).The aminopeptidase activities of seven thermophilic starters were higher in cultures with bacteriocin producers at0.5%inocula than in the corre-sponding control cultures.Nisin A-producing https://www.360docs.net/doc/867328084.html,ctis https://www.360docs.net/doc/867328084.html,ctis TAB75at0.5%inoculum achieved9.0-,2.0-, and5.3-fold increases in the aminopeptidase activities of starters TS4,TS5,and TS7,respectively(Table5).How-ever,starters TS4,TS5,and TS7in mixed cultures with strain TAB75inoculated at0.1%gave aminopeptidase ac-tivity values of1.21,0.97,and0.22,respectively,that were not signi cantly different from those of the corresponding control cultures.
Thermophilic starters TS4and TS5incubated during the rst8h at378C,a temperature similar to those used in the manufacture of some hard cheese varieties,had lower pH values than after incubation at308C.No signi cant dif-
J.Food Prot.,Vol.64,No.1 84OUMER ET AL.
TABLE5.The aminopeptidase activities in milk inoculated with
thermophilic starter cultures,without or with additional inocu-
lation with bacteriocin-produ cing cultures at0.5%,after incu-
bation at308C for8h followed by incubation at208C for16h
Starter
Aminopeptidase activities on Lys-p-NA,
bacteriocin producer a
None TAB7TAB24TAB26TAB75TAB84INIA4
TS1 TS2 TS3 TS4 TS5 TS6 TS7 TS8 TS9 TS10 TS110.26
0.26
0.07
1.06
0.93
0.34
0.14
0.07
0.08
0.52
0.16
0.17
0.17A
0.12A
1.09
0.80
0.41
0.33A
0.02A
0.11
0.60
0.20
0.19
0.21A
0.20A
1.42A
1.07
0.42
0.40A
0.03
0.10
0.61
0.19
0.16
0.21A
0.07
1.72A
1.05
0.37
0.21A
0.03
0.12
0.58
0.19
0.20
0.38A
0.11A
9.54A
4.91A
0.58A
0.28A
0.07
0.09
0.90A
0.18
0.22
0.24
0.13A
1.12
0.83
0.42
0.36A
0.02A
0.11
0.58
0.20
0.22
0.22A
0.16A
1.16
1.02
0.49A
0.41A
0.07
0.11
0.68
0.17
a Means within a row bearing letter A were signi cantly(P,
0.05)different from the value for milk with the starter alone.
TABLE6.The pH values of milk inoculated with two thermophilic starter cultures,without or with additional inoculation with bac-teriocin-producing cultures at0.5%,after incubation at378C for8h and the aminopeptidase activities(AA)after incubation at378C for8h followed by incubation at208C for16h
Starter
Bacteriocin producer a
None TAB7TAB24TAB26TAB75TAB84INIA4
TS4 TS5pH
AA
pH
AA
4.72
0.33
4.81
0.22
4.71
0.56A
4.81
0.23
4.70
0.37
4.83
0.39A
4.71
0.40
4.88
0.44A
4.78
0.70A
4.88
0.47A
4.68
0.37
4.75
0.23
4.69
0.36
4.74
0.27
ferences between pH values of TS4or TS5cultures with 0.5%inocula of bacteriocin producers and the correspond-ing control cultures were recorded(Table6).The amino-peptidase activity values of starter TS4were signi cantly (P,0.05)higher in mixed culture with enterocin-produc-ing strain TAB7or nisin A-producing strain TAB75than in the corresponding control culture,whereas those of start-er TS5were signi cantly(P,0.05)higher in mixed cul-tures with lacticin481-producing strain TAB24,nisin Z-producing strain TAB26,or nisin A-producing strain TAB 75than in the corresponding control culture(Table6).
DISCUSSION
The inhibition of mesophilic starters by bacteriocin-producing strains can be of industrial signi cance in cheese making,as it may result in fermentations in which acid production is markedly https://www.360docs.net/doc/867328084.html,ctococcin-producing L. lactis https://www.360docs.net/doc/867328084.html,ctis DPC3286added to milk at1.5%inoc-ulum together with a starter consisting of https://www.360docs.net/doc/867328084.html,ctis subsp. cremoris HP caused a delay of2h in the time to reach pH 5.2,which is required for milling Cheddar cheese(22). Acidi cation was also retarded in cheese made with0.1% E.faecalis INIA4as adjunct culture to an LD-type starter that had after24h a pH value0.40units higher than that of control cheese(10).In the present work,starters MS8 and MS9were the only mesophilic starters inhibited by at least one of the bacteriocin producers at0.5%inocula(Ta-ble1).In mixed cultures with0.1%inocula of bacteriocin producers these starters were not inhibited,most probably because of bacteriocin production rates not suf cient to re-tard pH decreases by inhibiting strains included in the start-ers.
The lower pH values frequently recorded for mixed cultures of mesophilic starters with bacteriocin producers as compared with those of the corresponding control cul-tures(Table1)may be explained by the combined lactic acid production of the starter and the bacteriocin producer in each mixed culture.The particular phenomenon of start-ers MS1and MS7that gave lower pH values when cul-tivated in mixed cultures with0.1%inocula of some bac-teriocin producers than in mixed cultures with0.5%inocula or in the corresponding control cultures(Table1),could be due to the lysis of sensitive strains of those starters by the bacteriocins at concentrations low enough to allow growth of the less sensitive strains that might be stimulated by in-tracellular compounds from lysed cells.Certainly,differ-ences in the sensitivities to enterocin AS-48of Lactococcus and Leuconostoc strains included in mesophilic starter CH-N01have been reported(10).
Mesophilic starters MS2,MS3,and MS9had higher aminopeptidase activities in at least one of the mixed cul-tures with bacteriocin producers than in the corresponding control cultures(Table2),most probably because of the lysis of sensitive strains by bacteriocins followed by the leakage of intracellular enzymes from dead cells.Higher postproline dipeptidyl aminopeptidase activity was record-ed in Cheddar cheese made with a lactococcin-producing adjunct culture than in control cheese(22).Aminopeptidase activities on Lys-p-NA and Leu-p-NA were3.9-and1.7-fold higher,respectively,in cheese made from milk inoc-ulated with an enterocin-producing adjunct culture than in control cheese(10).
Thermophilic starters have been reported to be more sensitive to antibiotics than mesophilic starters(2).They also have higher sensitivities to bacteriocins,as inferred from the differences between pH values of mixed cultures with bacteriocin producers and pH values of the corre-sponding control cultures(Table4).The lytic effect of bac-teriocins on thermophilic starters was shown by the consid-erable increases in aminopeptidase activities when ther-
J.Food Prot.,Vol.64,No.1STARTER CULTURES AND BACTERIOCIN PRODUCERS85
mophilic starters TS4,TS5,and TS7were cultivated together with nisin A-producing TAB75inoculated at0.5% as compared with the corresponding control cultures(Table 5).
When thermophilic cultures TS4and TS5were in-cubated at378C during the rst8h(Table6)with no bac-teriocin producers added,aminopeptidase activities were lower than when they were incubated at308C during the rst8h,in spite of the lower pH values reached that in-dicated enhanced growth of thermophilic starters at a tem-perature close to their optimum.The lower pH values at 378C may have partially inhibited and decreased amino-peptidase activities of thermophilic streptococci,as previ-ously reported for lactococci and lactobacilli aminopepti-dases(15,21).In mixed cultures,378C was a less favorable temperature than308C for growth of most of the bacteriocin producers tested,which probably produced a lower amount of bacteriocin,resulting in a less marked increase in ami-nopeptidase activity(Tables5and6).
From the results obtained in the present work,the use of bacteriocin-producing strains as adjunct cultures to me-sophilic starters for pathogen inhibition seems a generally feasible procedure,because de ned-strain mesophilic start-ers were in most cases tolerant to bacteriocins.Each par-ticular combination of starter with adjunct culture would require preliminary trials to determine inoculation rates such that both starter and adjunct culture grew and that the bacteriocin was produced at a convenient concentration. Contrarily,the use of bacteriocin producers as adjunct cul-tures to de ned-strain mesophilic starters in order to en-hance the lysis of starter cells and accelerate cheese rip-ening will be impeded frequently by the tolerance of these starters to bacteriocins.More complex mixtures such as those in LD-type mixed-strain starters containing both tol-erant strains that would grow producing lactic acid and sen-sitive strains that would be lysed by bacteriocins delivering their contents to the cheese matrix as a source of avor compounds might serve for the purpose of accelerated rip-ening(10).
A different conclusion was drawn for thermophilic starters.These starters are commonly used in hard cheeses that,due to their lower pH and moisture content values, have a lesser risk of contamination by pathogens and in which the use of bacteriocins is not therefore required. Some thermophilic strains are a valuable source of intra-cellular peptidases(12),a characteristic that could be ex-ploited to accelerate ripening of some hard and semihard cheeses by lysing sensitive strains with in situ produced bacteriocins,provided that a correct curd acidi cation was assured by the inclusion of bacteriocin-tolerant strains in the starter culture.
ACKNOWLEDGMENTS
Financial support from projects SC96-051(INIA)and ALI96-2511, and grants of A.Oumer,Comisio′n Interministerial de Ciencia y Tecno-log′a,and S.Garde,Comunidad Auto′noma de Madrid,are acknowledged.
REFERENCES
1.Chapot-Chartier,M.-P.,C.Deniel,M.Rousseau,L.Vassal,and J.-
C.Gripon.1994.Autolysis of two strains of Lactococcus lactis dur-
2.Cogan,T.M.1972.Susceptibility of cheese and yoghurt starter bac-
teria to antibiotics.Appl.Microbiol.23:960–965.
3.Cogan,T.M.1995.Flavour production by dairy starter cultures.J.
Appl.Bacteriol.79:49S–64S.
4.Coventry,M.J.,J.B.Gordon,A.Wilcock,K.Harmark,B.E.Da-
vidson,M.W.Hickey,A.J.Hillier,and J.Wan.1997.Detection of bacteriocins of lactic acid bacteria isolated from foods and compar-ison with pediocin and nisin.J.Appl.Microbiol.83:248–258.
5.Crow,V.L.,T.Coolbear,P.K.Gopal,F.G.Martley,L.L.McKay,
and H.Riepe.1995.The role of autolysis of lactic acid bacteria in the ripening of cheese.Int.Dairy J.5:855–875.
6.Engels,W.J.M.,and S.Visser.1996.Development of cheese avour
from peptides and amino acids by cell-free extracts of Lactococcus lactis subsp.cremoris B78in a model https://www.360docs.net/doc/867328084.html,k Dairy J.
50:3–17.
7.Ennahar,S.,O.Assobhei,and C.Hasselmann.1998.Inhibition of
Listeria monocytogenes in a smear-surface soft cheese by Lactoba-cillus plantarum WHE92,a pediocin AcH producer.J.Food Prot.
61:186–191.
8.Fox,P.F.,and J.M.Wallace.1997.Formation of avor compounds
in cheese.Adv.Appl.Microbiol.45:17–85.
9.Fox,P.F.,J.M.Wallace,S.Morgan,C.M.Lynch,E.J.Niland,and
J.Tobin.1996.Acceleration of cheese ripening.Antonie van Leeu-wenhoek70:271–297.
10.Garde,S.,P.Gaya,M.Medina,and M.Nun?ez.1997Acceleration
of avour formation in cheese by a bacteriocin-producing adjunct lactic culture.Biotechnol.Lett.19:1011–1014.
11.Giraffa,G.,D.Carminati,and G.Torri Tarelli.1995.Inhibition of
Listeria innocua in milk by bacteriocin-producing Enterococcus fae-cium7C5.J.Food Prot.58:621–623.
12.Gomez,M.J.,P.Gaya,M.Nun?ez,and M.Medina.1998.Strepto-
coccus thermophilus as adjunct culture for a semi-hard cows’milk https://www.360docs.net/doc/867328084.html,it78:501–511.
13.Joosten,H.M.L.J.,M.Nun?ez,B.Devreese,J.van Beeumen,and
J.D.Marugg.1996.Puri cation and characterization of enterocin4,
a bacteriocin produced by Enterococcus faecalis INIA4.Appl.En-
viron.Microbiol.62:4220–4223.
14.Kunji,E.R.S.,I.Mierau,A.Hagting,B.Poolman,and W.N.Kon-
ings.1996.The proteolytic systems of lactic acid bacteria.Antonie van Leeuwenhoek70:187–221.
https://www.360docs.net/doc/867328084.html,an,H.,S.E.Tan,P.Bruinenberg,G.Limsowtin,and M.Broome.
1998.Aminopeptidase activities of starter and non-starter lactic acid bacteria under simulated Cheddar cheese ripening conditions.Int.
Dairy J.8:267–274.
https://www.360docs.net/doc/867328084.html,ne,C.N.,and P.F.Fox.1997.Role of starter enzymes during
ripening of Cheddar cheese made from pasteurized milk under con-trolled microbiological conditions.Int.Dairy J.7:55–63.
17.Limsowtin,G.K.Y.,I.B.Powell,and E.Parente.1996.Types of
starters,p.101–129.In T.M.Cogan and J.-P.Accolas(ed.),Dairy starter cultures.VCH Publishers,Inc.,New York.
18.Maisnier-Patin,S.,N.Deschamps,S.R.Tatini,and J.Richard.1992.
Inhibition of Listeria monocytogenes in Camembert cheese made with a nisin-producing https://www.360docs.net/doc/867328084.html,it72:249–263.
19.Mart′nez,B.,J.E.Sua′rez,and A.Rodr′guez.1995.Antimicrobials
produced by wild lactococcal strains isolated from homemade chees-es.J.Food Prot.58:1118–1123.
20.McAuliffe,O.,C.Hill,and R.P.Ross.1999.Inhibition of Listeria
monocytogenes in cottage cheese manufactured with a lacticin3147-producing starter culture.J.Appl.Microbiol.86:251–256.
21.McDonnell,M.,P.Bouchier,R.J.Fitzgerald,and G.O’Cuinn.1999.
Puri cation and characterization of a lysine-p-nitroanilide hydrolase,
a broad speci city aminopeptidase,from the cytoplasm of Lacto-
coccus lactis subsp.cremoris AM2.J.Dairy Res.66:257–270. 22.Morgan,S.,R.P.Ross,and C.Hill.1997.Increasing starter cell lysis
in Cheddar cheese using a bacteriocin-producing adjunct.J.Dairy Sci.80:1–10.
23.Nun?ez,M.,J.L.Rodr′guez,E.Garc′a,P.Gaya,and M.Medina.
1997.Inhibition of Listeria monocytogenes by enterocin4during the manufacture and ripening of Manchego cheese.J.Appl.Microbiol.
J.Food Prot.,Vol.64,No.1 86OUMER ET AL.
24.Rodr′guez,E.,B.Gonza′lez,P.Gaya,M.Nun?ez,and M.Medina.
2000.Diversity of bacteriocins produced by lactic acid bacteria iso-lated from raw milk.Int.Dairy J.10:15–25.
25.Ryan,M.P.,M.C.Rea,C.Hill,and R.P.Ross.1996.An application
in Cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin,lacticin3147.Appl.
Env.Microbiol.62:612–619.
26.Salles,C.,C.Septier,F.Roudot-Algaron,A.Guillot,and P.X.Etie′-
vant.1995.Sensory and chemical analysis of fractions obtained by
gel permeation of water-soluble Comte′cheese extracts.J.Agric.
Food Chem.43:1659–1668.
27.Steel,R.G.D.,and J.H.Torrie.1980.Multiple comparisons,p.172–
194.In C.Napier and J.W.Maisel(ed.),Principles and procedures of statistics,a biometrical approach.McGraw-Hill,Singapore.
28.Vaughan,E.E.,E.Caplice,R.Looney,N.O’Rourke,H.Coveney,
C.Daly,and G.Fitzgerald.1994.Isolation from food sources,of
lactic acid bacteria that produced antimicrobials.J.Appl.Bacteriol.
76:118–123.
构建食品级的重组乳酸乳球菌表达纳豆激酶
构建食品级表达纳豆激酶的乳酸乳球 菌重组菌株 冯浩余凤云单凤娟闫达中* 武汉工业学院生物与制药工程学院湖北武汉 430023 摘 要:纳豆激酶(Nattokinase,NK)是从日本传统食品纳豆中分离到的、具有高效溶栓活性的蛋 白激酶,它能够很好地弥补传统溶血栓药物的缺陷,极有可能开发为新一代口服制剂用于血栓性 疾病的预防和治疗。以HisH基因作为同源重组的靶位点,构建整合型表达载体pFY008,通过同 源重组的方法将含有纳豆激酶原基因的表达盒PnisA-aprN整合到乳酸菌的基因组上,实现纳豆 激酶在乳酸乳球菌中食品级的表达。构建的食品级基因工程菌安全性高、稳定性好,并赋予了其 溶栓的功能。本研究为生产新型溶栓保健乳酸饮料提供理论和技术支持。 关键词: 纳豆激酶;同源重组;乳酸乳球菌 中图分类号:Q786 文献标识码:A文章编号: 脚注收稿日期: 基金项目:湖北省教育厅中青年项目(Q200718003) 武汉工业学院研究生教育创新计划资助(09cx018) 作者简介:冯浩(1988-),男,硕士研究生,研究方向为微生物学。E-mail:sqfenghao@https://www.360docs.net/doc/867328084.html, *通讯作者:闫达中(1968-),男,副教授,研究方向为微生物学。E-mail:yandz6808@https://www.360docs.net/doc/867328084.html, Construction of a Recombinant Lactococcus lactis to Realize Food-grade Expression of Nattokinase FENG Hao YU Feng-yun SHAN Feng-juan YAN Da-zhong* College of biological and pharmaceutical engineering, Wuhan Polytechnic University, Wuhan 430023, China Abstract: Nattokinase (NK) is a protein kinase with strong fibrinolytic property, seperated from Japanese traditional food natto. Because it could make up for the shortcomings of traditional thrombolytic drug well, it may be used as a new kind of oral agents for thrombotic diseases. An integrative expression vector pFY008 was constructed with hisH gene as the homologous recombination target site. In order to realize nattokinase gene (aprN) food-grade expression in Lactococcus lactis, the expression cassette PnisA-apr N gene was integrated into the genome of Lactococcus lactis by homologous recombination. A food-grade recombinant Lactococcus lactis was constructed to produce a thrombolytics protein. The recombinant Lactococcus lactis was endowed with fibrinolytic property. Meanwhile, it had fine stability and high safety. The work mentioned above has provided a theory and technical support for the development of a new lactic acid drink with thrombolytic activity. Keywords: Nattokinase; Homologous recombination; Lactococcus lactis 1992年NADAMURA等人[1]采用鸟枪法首次从分泌纳豆激酶的枯草芽孢杆菌中克隆到包括调控序列在内的纳豆激酶全长基因aprN,并对1473 bp的aprN编码序列进行分析。纳豆激酶是分子量较小的单链蛋白,易被人体吸收;并可以直接降解交联纤维蛋白,且对纤维蛋白原不敏感,而且能促进内源性t-PA(Tissue-type plasminogen activator)增生[2],药效时间长,价格低廉;有一定的抗胰酶水解能力,可由肠吸收入血,口服效果好,具备一定的抗凝血功能,安全性高。纳豆激酶很好地弥补了传统溶血栓药物的缺陷,极有可能作为新一代口服制剂用于血栓性疾病的预防和治疗。 近年,纳豆激酶作为功能性食品、食品添加剂和普通食品发展的十分迅速。一些制药公司已转向生产功能性食品,这也是保健食品发展的趋势。就世界范围而言,所有的制药企业也都在努力占领保健品市场。仅美国和欧洲的营养品和功能性食品的市场销售额就达5000亿美元,并以17-20%的增长率,逐年增加,一些制药企业开始生产功能性食品。
PCR技术在乳酸菌分类鉴定中的应用
PCR技术在乳酸菌分类鉴定中的应用 王庭柱,高学军,杨振宇 东北农业大学教育部乳品科学重点实验室(150030) E-mail:wangtingzhu1980@https://www.360docs.net/doc/867328084.html, 摘要:近年来,随着分子生物学和生物信息学的迅速发展,特别是作为生物技术里程碑的PCR技术以及核酸测序和电泳技术的不断改进与完善,产生了许多新的分类学方法,如RAPD、PCR-RFLP、T-RFLP、ARDRA、PCR-SSCP、PCR-DGGE、PCR-TGGE、AFLP、REP-PCR、S-PCR、LCR、LH-PCR、SBCS以及小卫星序列多态性和序列同源性分析等。本文即论述了这些技术在乳酸菌分类鉴定中的应用及其优势和局限性。 关键词:乳酸菌,PCR,分类,鉴定,分子生物学 1. 引言 乳酸菌(lactic acid bacteria, LAB)是一大类缺乏细胞色素、糖代谢主要以乳酸为终产物的革兰氏阳性非芽孢细菌,其过氧化氢酶反应为阴性、耐氧耐酸、营养要求复杂并且严格发酵。LAB这个名称就细菌分类学而言实属一个非正式、非规范的名称。目前从自然界中已发现的这类细菌在分类学上至少可划分为23个属,涉及到的有关菌种则更多,其代表性的菌属有乳杆菌属、乳球菌属、链球菌属、双歧杆菌属、肠球菌属、明串珠菌属、气球菌属、肉杆菌属、酒球菌属、足球菌属、四体球菌属和漫游球菌属等[1,2]。 传统的LAB鉴定方法主要依赖于表型分析,包括形态学观察、生长需要及特性、发酵图谱、细胞壁蛋白分析、血清学以及脂肪酸甲基酯分析等,其中有些技术已被证明适用于某些LAB的鉴定,但是也普遍意识到表型分析的一些缺点,如重现率及辨识能力低、相似的表型特性并不等同于相似的或者关系密切的基因型[3]。表型试验可能的固有问题是,不是每一给定种内的所有菌株都有一个共同的性状,而且即使是同一菌株也可能呈现出一定的生化变异性。此外,实验操作的一点改变也可能产生错误的结果。因此基于表型试验的常规技术并不能对菌株做出明确的鉴定[4]。一般来讲,准确地鉴定一株乳酸菌至菌种的水平,至少需要17种表型实验[5],但是微生态系统的分离株需要更为简单快速的鉴定方法。因此,需要进行表型特性的分子水平研究和遗传特性的研究。 只有分子生物学技术才能解决LAB鉴定的复杂性。近年来发展起来的核酸技术如DNA 杂交和特定靶序列的扩增技术,对LAB的鉴定是一种改进和补充。在LAB分类学方面,代表性的遗传学方法是限制酶分析(restriction enzyme analysis, REA)的多态性(如PFGE、RFLP 和核糖分型)和PCR技术[3]。REA是进行基因组DNA的限制性内切酶消化。脉冲场凝胶电泳(pulsed-field gel electrophoresis, PFGE)是经酶切后,分离出大量的基因组片段;限制性片段长度多态性(restriction fragment length polymorphism, RFLP)指的是酶切后含同源序列的酶切片段在长度大小或数目上的差异;而核糖分型(Ribotyping)是以rDNA和/或其间隔区域作为探针,与基因组限制片段进行杂交。PFGE是菌株分类中最有识别力的方法,但是基因组DNA的所有限制片段的完全分离是个困难;迄今,最佳辨识力的遗传探针是种特异性的[6];而PCR技术以及以PCR为基础的遗传指纹技术和群落分析技术甚至可以进行高度菌株特异性的LAB鉴定。 PCR技术的创立打开了LAB快速鉴定的大门,其不仅操作简便、快速可靠并且灵敏高效,而且还克服了对培养的依赖性(自然界中有85~99.9%的微生物至今还不能进行纯培
乳酸菌抗菌机理
乳酸菌抗菌机理 乳酸菌的抗菌机理涉及其产生的各种代谢产物,包括酸性物质、乳酸菌素、二氧化碳和过氧化氢等。其中酸性物质可以消耗大量细胞能量并影响细胞膜的稳定性;乳酸菌素可作用于细胞膜,造成膜内物质和能量的泄漏。 乳酸菌是一类可发酵碳水化合物产生大量乳酸的细菌的通称,在自然界和食物中广泛存在。乳酸菌是最早被人类用于食品储藏加工的微生物之一,早在公元前6000年,人们就懂得利用乳酸菌发酵食物。他们发现食物经过一定的处理和储存就可改善风味、延长储存期和增加食物的安全性。迄今人们已明确了许多乳酸菌在生产安全优质食品中所起重要作用的生物学机理[1~2]:乳酸菌可以发酵食物中碳水化合物,分泌乳酸菌素,产生有机酸、酒精和二氧化碳等,来抑制一些腐败菌或致病菌的生长及改善食品的品质和风味,同时经过发酵,乳酸菌可以增加食品的可消化性并产生一些维生素、抗氧化剂。近几年,乳酸菌抑制食品中一些腐败菌和致病菌的作用引起人们的极大关注。虽然现代生物技术和安全体系(如HACCP)已被普遍的引入食品加工行业,但食品的安全问题仍然威胁着人类,每年都有许多关于食物中毒和食源性疾病散发或爆发的报道,同时,人们正力图追求不含化学防腐剂及各种添加剂的天然的安全食品。解决这问题需要发展新的食品保鲜技术来控制食品中腐败菌和致病菌的生长。国内外学者对之开展了大量的研究并建立了许多方法,其中最引人注目的就是利用乳酸菌来加强食品安全性和延长储存期。
1乳酸菌产生的酸性物质及其抑菌作用 1.1乳酸菌产生的酸性物质乳酸菌可产生对食品中微生物具有抑制作用的酸性物质,主要是乳酸菌的代谢终产物及中间产物,包括乳酸、乙酸、乙醇等。 1.2酸性物质对食品微生物的抑制作用一般细菌生长的最适pH 值为6~7,若低于该值,细菌的生长速率将大大降低或不生长甚至死亡,这在腐败性微生物上尤为可见。乳酸菌产生的酸性物质对食品中微生物的抑制作用已在许多实验中得到证实,这种抑菌作用取决于3个相互影响的因素:1.介质的pH值; 2.酸的离解程度; 3.酸的种类。 从20世纪70~80年代,国内外学者就开始建立pH值对食品中各种腐败菌和致病菌抑制作用的预测模型。但在这些模型中都是用无机酸如盐酸、磷酸来降低pH值,而乳酸菌产生的多是一些含羧基的弱有机酸。只有未离解的弱有机酸进入细菌细胞才能有效的发挥抑菌作用。这些有机酸的离解度取决于其pKa和pH值,可以用Henderson-Hasselbach公式计算:pH=pKa+log([A-] / [HA])。从中不难看出介质的pH值影响酸的离解,若在pH值固定条件下酸的pKa决定了其离解度。因此乳酸菌产生的弱酸的抗菌能力取决于介质的pH值及酸的种类(pKa)。由于胞质的pH值相对较高,当非离解的酸通过细胞膜进入胞质,就发生离解使细胞质酸化并释放酸性阴离子。这就给微生物带来两种后果:首先,若微生物要维持其胞内的pH值,就得动用ATP酶来清除质子,这将消耗大量细胞能量,加重细胞的代谢负担;其次,细胞内阴性酸离子的积聚可影响细胞膜的稳定性并抑制其传递
乳酸乳球菌N3816发酵培养基的优化研究
龙源期刊网 https://www.360docs.net/doc/867328084.html, 乳酸乳球菌N3816发酵培养基的优化研究 作者:申秋华 来源:《安徽农学通报》2013年第19期 摘要:以乳酸乳球菌株(Lactococcus lactis subsp. lactis N3816)为出发菌株,通过单因子试验和正交设计方法对该菌株产Nisin发酵培养基进行优化。结果表明:该菌株产Nisin的最 适碳源、复合氮源和生长因子分别为:蔗糖0.8%、蛋白胨2.0%、酵母膏1.0%、Tween80 1.5%。 关键词:乳链球菌肽;培养基;效价 中图分类号 TS207 文献标识码 A 文章编号 1007-7731(2013)19-34-03 乳链球菌肽(Nisin)是乳酸乳球菌的某些菌株在代谢过程中合成的一种具有较强抑菌作 用的多肽类细菌素。成熟的乳链球菌肽分子含有34个氨基酸残基,分子量约为3 510 Da,分子式为C143H228N42O37S7。乳链球菌肽前分子含有57个氨基酸,前体分子依次经过将部分丝氨酸和苏氨酸酶促转化为脱水氨基酸、链内形成5个硫醚环、细胞质膜移位和断裂去除N端23肽而形成[1-3]。 Nisin对引起食品腐败的绝大部分革兰氏阳性细菌,特别是对产芽孢的细菌如芽孢杆菌、梭状芽孢杆菌有很强的抑制作用。另外,Nisin与某些表面活性剂、螯合剂和络合剂联合使用时还能抑制部分革兰氏阴性菌。因此,与其它乳酸菌产生的细菌素相比,Nisin具有较宽的抑菌谱。Nisin属于天然食品防腐剂的一种,自1951年首次应用于食品保藏以来,Nisin已先后 被数10个国家和地区广泛使用。目前,Nisin应用于乳制品、肉制品、罐装食品和蛋白食品等的防腐保鲜中,成为越来越受人们重视的一种安全、高效、无毒、天然的食品防腐剂[4]。 随着Nisin在食品行业中应用的普及,获得产量较高且生产成本较低的Nisin成为工业化 应用的前提。为了提高其产量,人们开始研究一些发酵工艺因素对Nisin发酵生产的影响。本文主要是在实验室条件下对乳酸乳球菌N 3816进行研究,通过改变碳源、氮源和添加吐温的方式获得发酵培养基的最优配比。 1 材料与方法 1.1 试验材料 Nisin标准品购于sigma公司。 1.2 菌种乳链菌肽生产菌:乳酸乳球菌(Lactococcus lactis subsp. lactis N 3816),由实验室保藏。效价检测指示菌:藤黄微球菌(Micrococcus luteus),购自中国科学院微生物研究所菌种保藏中心。
乳球菌属
乳球菌属 (一)乳球菌属的特征 乳球菌属的特征:球或卵圆形细胞,单生、成对或成链状,有时因细胞伸长似杆状、致使某些乳球菌错误地分到乳杆菌属内,乳杆菌属原先的这两个种现已重新分类,分别作为乳酸球菌的两个亚种。 乳球菌是革兰氏阳性,兼性厌氧菌,不运动。通常不溶血,仅有某些乳酸乳球菌的菌株显示微弱的a-溶血反应。所有的乳球菌通常能在4%NaCl生长,仅乳酸乳球菌乳脂亚种。只耐2%NaCl。乳球菌能在10℃下生长,但不能在45℃下生长,此生长温度特征可用于区分它们与链球菌及肠球菌。大多数的乳球菌能与N 型抗血清起反应,但并非乳球菌属的所有菌株都能与之反应,从鸡粪和河水中分离的与N型抗血清起反应的某些运动菌株在遗传上与乳球菌、肠球菌或链球菌无密切关系。 (二)乳球菌属内种和亚种的特征 从核酸杂交和比较血清学研究结果说明乳球属的成员关系密切,但又不同与肠球菌和链球菌。乳球菌属内乳酸乳球菌又形成单一的DNA同源群。但按其表型特征又足以将它们分为三个亚种。 对于乳球菌属内的格氏乳球菌和鱼病肠球菌的关系问题,近年来又有些研究报道。Teixeira等从患轻度乳腺炎的水牛体分离到一些非典型的格氏乳球菌的菌株,他们将这些菌株的全细胞蛋白图像与格氏乳球菌和鱼病肠球菌的模式菌株的图像进行对比,并用肠球菌的基因探针试验以及使用羟磷灰石法进行DNA相关性的研究,结果证明这些分离的格氏乳球菌菌株与鱼病肠球菌极相似,认为它们同属一个种。 (三)乳球菌的应用 乳球菌中乳酸乳球菌3个亚种的一些菌株在乳制品的生产中占有较重要的地位。它们的单一或混合培养物可产生不同类型的乳酪和发酵奶,培养黄油和生产酪蛋白。 乳酸乳球菌的一些菌株可产生细菌素,如Nisin ,Lactococcin和Bacteriocin 等。Nisin这种乳球菌肽的抗菌物质作为一种无毒的天然食品防腐剂,已被50多个国家和地区广泛应用与乳制品、罐头食品、鱼类制品和乙醇饮料等的防腐和保鲜。在食品中加入十万分之几到万分之几的这种物质,就足以抑制引起食品腐败的许多革兰氏阳性菌的生长和繁殖。
乳酸菌1
乳酸菌的耐酸机制 摘要:对乳酸菌耐酸机理进行了初步介绍, 主要从以下几个方面进行 了阐述, 包括质子泵机制、蛋白质及RNA修复、细胞膜及代谢方式的改变和碱生成等, 以期为人们了解乳酸菌耐酸的生理生化机制提供借鉴, 为研究者对乳酸菌耐酸性研究提供理论指导。 关键词:乳酸菌; 耐酸性; 机理 Review on the Mechanism of Acid Tolerance of Lactic Acid Bacteria Abstract:This review provided the possible acid tolerance mechanism of Lactic acid bacteria, including proton pump, repair of protein and RNA, cell membrane and metabolic ways change, production of alkali and so on. The purpose of this article was to make comprehensive understandings of the mechanism for acid tolerance of Lactic acid bacteria and provide a theoretical basis for the research work related to Lactic acid bacteria. Key words:Lactic acid bacteria; acid tolerance; mechanism 引言 乳酸菌是一类能利用可发酵糖产生大量乳酸的细菌的通称。它们在自然界分布广泛,可栖居于人和动物的肠道及其他器官中。在土壤、植物根际和许多的人类食品、动物饲料,还有自然界的湖泊和污泥以及一些临床样品中都发现有乳酸菌的存在。很久以前人们就利用乳酸菌来发酵动物(乳、肉、鱼等)和植物制品(蔬菜、葡萄酒、橄榄等)生产各种各样的产品。随着食品发酵工业的不断发展壮大,乳酸菌的经济效益不断在增长,因为虽然它们在发酵食品中的含量非常少,但是对食品的感官品质和质量却有决定作用。因此,发酵剂菌株的质量功能特性和生长特性对于产品的成功发酵是非常必要的。 乳酸菌不但包括在食品发酵中使用的一般认为安全的微生物,而且还包括胃肠道中普遍存在的共生体和具有潜在益生作用的益生菌。对这些微生物来说,食品和胃肠道中的酸性环境对它们的生存是一个很大的挑战。例如,益生菌的最佳
乳酸菌基础知识培训
乳酸菌基础知识 1.乳酸菌及其分类 1.1 什么是乳酸菌? 乳酸菌指发酵糖类主要产物为乳酸的一类无芽孢,革兰氏染色阳性细菌的总称。凡是能从葡萄糖或乳糖的发酵过程中产生乳酸的细菌统称为乳酸菌。乳酸菌发酵原理是在酶的催化作用下将葡萄糖转化为乳酸,同时放出能量提供给其自身生命活动。这是一群相当庞杂的细菌,目前至少可分为43个属,共有373个种和亚种。 1.2 乳酸菌的分类位置 界:细菌界Bacteria; 门:厚壁菌门Firmicutes; 纲:芽孢杆菌纲Bacilli; 目:乳杆菌目Lactobacillales; 科:乳杆菌科Lactobacillaceae; 属:乳杆菌属Lactobacillus Beijerinck. 1.3 乳酸菌的分类 乳酸菌大体上可分为两大类。一类是动物源乳酸菌,一类是植物源乳酸菌。因为动物源取自动物,因菌种常处于相对不稳定状态,其生物功效也较不稳定,且在大量食用时,很容易导致人体动物蛋白过敏,即排斥反应。而植物源乳酸菌,因为取自植物易被人体认可,不论摄取多大的量,人体不会产生异体蛋白排斥反应,且植物源乳酸菌比动物源者更具有活力,能比动物源多8倍的数量到达人体小肠内定植,从而发挥其强大而稳定的生物功效。 1.4 乳酸菌的分布 乳酸菌广泛分布于人体和动物的消化系统、呼吸系统、泌尿系统、口腔系统、皮肤系统和粪便,乳汁和乳制品,植物的果实、枝叶和根茎,腐烂的植物体,发酵动植物食品和饮料,堆肥、土壤和淤泥、污水,以及若干种临床样品等。它们是生物界中重要一员,对人类、动物、植物的生存起着不可替代的重要作用。乳酸菌在人体的食道中很少,在胃中乳酸菌数量为103个/ml左右,肠道中的乳酸菌数量为105个/g左右。 1.5 常用的乳酸菌 ①①用于乳酸发酵工业和乳酸钙生产的德氏乳杆菌(L.delbrueckii); ②肠膜状明串珠菌(Leuconostocmesenteroides)是制药工业上生产右旋糖酐的重要菌种,但也是制糖工业的一种害菌,常使糖汁发粘稠而无法加工。 ③用于乳制品发酵加工的嗜热链球菌和保加利亚乳杆菌; ④用于蔬菜、水果和青贮饲料乳酸发酵的植物乳酸杆菌; ⑤用于益生菌胶囊生产的长双歧杆菌、青春双歧杆菌、动物双歧杆菌、干酪乳杆菌、嗜酸乳杆菌、鼠李糖乳杆菌(LGG )等。 ⑥用于乳酸菌素生产的嗜酸乳酸杆菌为主的复合乳酸菌菌种。 2.乳酸菌的发展 2.1 乳酸菌的起源 早在4~5000年前人类就已经在使用乳酸菌。在佛教教典、《圣经·创世纪》、
第四讲 乳酸菌类型及生物活性次生代谢产物
第四讲乳酸菌及益生菌种类的功能及具有生物活性的次生代谢产物 一、乳酸菌及益生菌 1、定义及分类 (1)乳酸菌 (2)益生菌 2.生物活性 3.生长特性及应用、产品开发 二、生物活性的次生代谢产物 1、细菌素 (1)定义及种类 (2)特性 Nisin是正常菌群中某些种类的乳酸乳球菌合成和分泌的一种对大多数G+菌有强大杀灭作用的细菌素。Nisin对营养细胞的作用主要是在细胞膜上,它可以抑制细菌细胞壁中肽聚糖的生物合成,使细胞质膜和磷脂化合物的合成受阻,从而导致细胞内物质外泄,甚至引起细胞裂解。作为一种天然的生物性食品防腐剂和抗菌添加剂,Nisin已广泛应用于乳制品、罐头食品、高蛋白食品及饲料工业中。添加400IU/g的产品储存11天,其含菌数比对照组低4个对数期,产品的感官可接受性较高。Nisin对乳酸菌株的影响也较大,如控制适宜的添加量(一般在5-100mg/kg),能对某些乳酸菌株有明显的抑制作用,造成菌株的活力下降、产酸受抑、生长不良,但乳酸菌总数变化不大。 nisin的研究现状 乳链菌肽是由乳酸乳球菌产生的由基因编码的多肽抗生素,含有34个氨基酸残基,分子中含有5种稀有氨基酸,即氨基丁酸(ABA)、脱氢丙氨酸(DHA)、β-甲基脱氢丙氨酸(DHB)、羊毛硫氨基酸( ALA-S-ALA)和β-甲基羊毛硫氨基酸(ALA-ABA),它们通过硫醚键形成五个内环。 乳链菌肽的抑菌谱包括营养体和芽孢,对葡萄球菌、梭菌、芽孢杆菌和利斯特菌有强烈的抑制作用,对酵母和霉菌没有作用。迄今为止,乳链菌肽已在全世界约60多个国家和地区被用作食品防腐剂]。我国对乳链菌肽的研究始于1989年,中国科学院微生物研究所在国家自然科学基金项目和中国科学院“八五”重点科研基金项目资助下,完成了对乳链菌肽的基础研究,研究成果现已批量生产,投放市场。1992年3月29日,我国卫生部食品监督部门签发了乳链菌肽在国内的使用合格证明,同时将乳链菌肽列入1992年10月1日实施的国标GB2760-86中的增补品种,可用于罐藏食品、植物蛋白食品、乳制品和肉制品的保藏中。乳链菌肽用于食品中的优点是安全无毒,它的LD50约为7000Kg,允许使用的最大剂量是33000IU/Kg,乳链菌肽是一种短肽,可以被体内的α-胰凝乳蛋白酶降解;不影响食品的色香味;在食品加工中可以降低杀菌温度、减少热处理时间,改变食品的营养价值、风味和结构等性状。国内外的研究者在对乳链菌肽的分子结构、分子形成过程、物理化学性质、抗菌机理和毒性等进行了广泛的研究 产nisin菌株在发酵食品中的应用 在干酪中的应用 早在1951Hirsch等研究发现nisin产生菌乳酸乳球菌乳酸亚种在Swiss型干酪中抑制厌
乳酸菌模式株中英文对照表
乳酸菌常见种属中英文对照表 1. Lactobacillus(共151个种及亚种) 序号英文名称中文名称 1 Lactobacillus acetotolerans 耐酸乳杆菌 2 Lactobacillus acidifarinae 酸面乳杆菌 3 Lactobacillus acidipiscis 酸鱼乳杆菌 4 Lactobacillus acidophilus 嗜酸乳杆菌 5 Lactobacillus agilis 敏捷乳杆菌 6 Lactobacillus algidus 低温乳杆菌 7 Lactobacillus alimentarius 消化乳杆菌 8 Lactobacillus amylolyticus 解淀粉乳杆菌 9 Lactobacillus amylophilus 嗜淀粉乳杆菌 10 Lactobacillus amylotrophicus 发酵淀粉乳杆菌 11 Lactobacillus amylovorus 食淀粉乳杆菌 12 Lactobacillus animalis 动物乳杆菌 13 Lactobacillus antri 胃窦乳杆菌 14 Lactobacillus apodemi 田鼠乳杆菌 15 Lactobacillus aquaticus 16 Lactobacillus aviarius subsp. araffinosus 鸟乳杆菌不解棉籽糖亚种 17 Lactobacillus aviarius subsp. aviarius 鸟乳杆菌鸟亚种 18 Lactobacillus bifermentans 双发酵乳杆菌 19 Lactobacillus bobalius 巴博乳杆菌 20 Lactobacillus brevis 短乳杆菌 21 Lactobacillus buchneri 布氏乳杆菌 22 Lactobacillus cacaonum 23 Lactobacillus camelliae 茶叶乳杆菌 24 Lactobacillus capillatus 多毛乳杆菌 25 Lactobacillus casei 干酪乳杆菌 26 Lactobacillus ceti 鲸乳杆菌 27 Lactobacillus coleohominis 人阴道乳杆菌 28 Lactobacillus collinoides 丘状菌落乳杆菌 29 Lactobacillus composti 堆肥乳杆菌 30 Lactobacillus concavus 曲面乳杆菌 31 Lactobacillus coryniformis subsp. coryniformis 棒状乳杆菌棒状亚种 32 Lactobacillus coryniformis subsp. torquens 棒状乳杆菌曲屈亚种 33 Lactobacillus crispatus 卷曲乳杆菌 34 Lactobacillus crustorum 面包乳杆菌 35 Lactobacillus curvatus 弯曲乳杆菌 36 Lactobacillus delbrueckii subsp. delbrueckii 德氏乳杆菌德氏亚种 37 Lactobacillus delbrueckii subsp. indicus 德氏乳杆菌印度亚种 38 Lactobacillus delbrueckii subsp. lactis 德氏乳杆菌乳亚种
乳酸菌得分类
乳酸菌的分類 1.乳酸菌的分類: (1)是一類可發酵利用碳水化合物而產生大量乳酸的細菌。 (2)大多數細菌都能產生或多或少的乳酸 →不能認為凡是產乳酸的細菌都是乳酸菌。 2.乳酸菌的分類体系→(1)生化狀態分類法(2)化學分類法。 3.按照Bersy細菌學手冊中的生化分類法: 乳桿菌屬(Lactobacillus) 鏈球菌屬(Streptococeus) 乳酸菌有5個屬明串珠菌屬(Leuconostoc) 雙歧桿菌屬(Bifidobacterium) 片球菌屬(Pediococcus) 每個屬中又有很多菌種,一些菌種還包括數個亞種。 4.乳桿菌屬: (1)一般呈細長的桿狀,大小為0.5~1 μm x 2~10 μm,常呈 鏈狀排列。G(+),無芽孢菌,微需氧。 (2)目前已實際應用的,主要有: 德氏乳桿菌(L. delbrueckii)、保加利亞乳桿茵(L. bulgaricus)瑞士乳桿菌(L. helviticus)、嗜酸乳桿菌(L. acido phlus)和乾 酪乳桿菌(L. casei)及其亞种等;短乳桿茵(L. brevis)和發酵 乳桿菌(L. fementi)。 5.鏈球菌屬乳酸菌: (1)一般呈短鏈或長鏈狀排列,為無芽孢G(+),兼性厭氧。
(2)有些屬於人或動物的病原菌: 如能引起牛乳房炎的無乳鏈球菌(S. agalactiae)和人咽喉炎的溶血鏈球菌(S.haemolytlcus)。 (3)有些能引起食物變質而常用于特種乾酪(羅馬諾乾酪)製造上 ,如糞鏈球菌(S. faecalis)和液化鏈球菌(S. liquefaciens)。 (4)有些則是發酵乳製品生產上常用的菌種,如乳酸鏈球菌(S. lactis)、丁二酮乳酸鏈球菌(S. diacetilactis)、乳酪鏈球菌 (S.creamoris)和嗜熱乳鏈球菌(S. thermophilus)等。 6.明串珠菌屬: (1)呈圓形或卵圓形,菌体排列成鏈狀。經常存在于水果、蔬菜 中,能在高濃度的含糖食品中生長。 (2)該屬乳酸菌可發酵利用葡萄糖產生CO2、乙酸和乳酸。依据 此特性可將明串珠菌從鏈球菌中區別開(鏈球菌不產生CO2,而且生成L-乳酸)。 (3)常見的菌种有腸膜明串珠菌(Leuc. Mesenteroides)及其乳脂亞 种(Leuc. Cremoris)和葡聚糖亞種(Leuc. Dextranicun)、蝕橙明串珠菌(Lcuc. Citrovorum)、乳酸明串珠茵(Leuc. Lactis)和酒明串珠菌(Leuc. Oenos)等。它們均可用在發酵乳製品生產上。 (4)腸膜明串珠菌乳脂亞种(又稱乳脂明串珠菌)最為常見,它 能發酵檸檬酸產生特殊風味物質,故又稱風味菌、香氣菌或產香菌。 7.雙歧桿菌: (1)因菌体尖端呈分支枝狀(如Y或V字形)而得名,是無芽孢
