AZD1152_722543-31-9_DataSheet_MedChemExpress
Product Name:
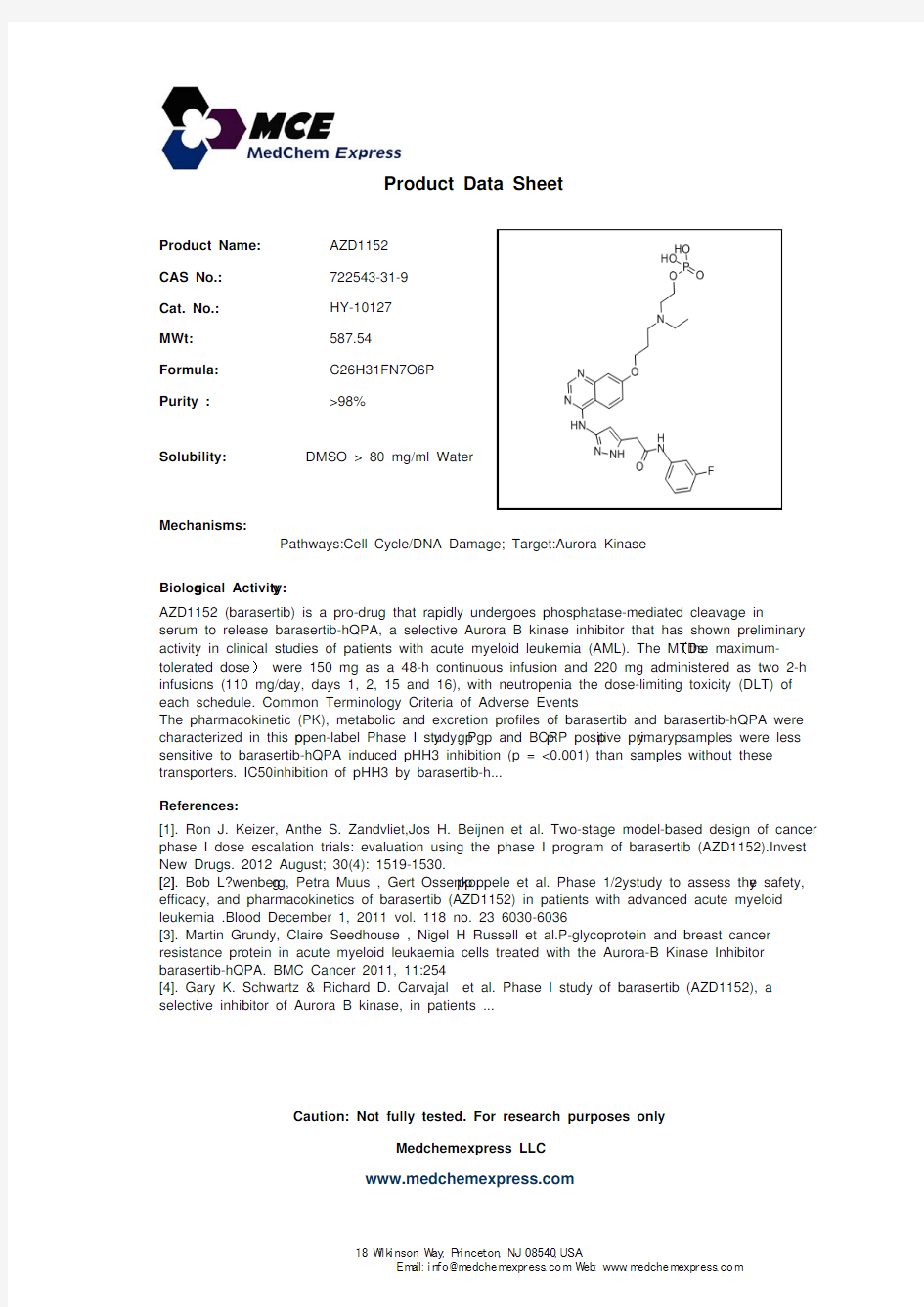
AZD1152CAS No.:
722543-31-9
Product Data Sheet
Cat. No.:
HY-10127MWt:
587.54Formula:
C26H31FN7O6P Purity :
>98%
Solubility:Mechanisms:
Biological Activity:
Pathways:Cell Cycle/DNA Damage; Target:Aurora Kinase
DMSO > 80 mg/ml Water
g y AZD1152 (barasertib) is a pro-drug that rapidly undergoes phosphatase-mediated cleavage in
serum to release barasertib-hQPA, a selective Aurora B kinase inhibitor that has shown preliminary activity in clinical studies of patients with acute myeloid leukemia (AML). The MTDs (the maximum-tolerated dose ) were 150 mg as a 48-h continuous infusion and 220 mg administered as two 2-h infusions (110 mg/day, days 1, 2, 15 and 16), with neutropenia the dose-limiting toxicity (DLT) of
each schedule. Common Terminology Criteria of Adverse Events The pharmacokinetic (PK), metabolic and excretion profiles of barasertib and barasertib-hQPA were characterized in this open-label Phase I study. Pgp and BCRP positive primary samples were less References:
[1]. Ron J. Keizer, Anthe S. Zandvliet,Jos H. Beijnen et al. Two-stage model-based design of cancer phase I dose escalation trials: evaluation using the phase I program of barasertib (AZD1152).Invest
New Drugs. 2012 August; 30(4): 1519-1530.[2]. Bob L?wenberg, Petra Muus , Gert Ossenkoppele et al. Phase 1/2 study to assess the safety,p y gp p p y p
sensitive to barasertib-hQPA induced pHH3 inhibition (p = <0.001) than samples without these transporters. IC50inhibition of pHH3 by barasertib-h...
[]g pp y y efficacy, and pharmacokinetics of barasertib (AZD1152) in patients with advanced acute myeloid
leukemia .Blood December 1, 2011 vol. 118 no. 23 6030-6036[3]. Martin Grundy, Claire Seedhouse , Nigel H Russell et al.P-glycoprotein and breast cancer resistance protein in acute myeloid leukaemia cells treated with the Aurora-B Kinase Inhibitor
barasertib-hQPA. BMC Cancer 2011, 11:254[4]. Gary K. Schwartz & Richard D. Carvajal et al. Phase I study of barasertib (AZD1152), a selective inhibitor of Aurora B kinase, in patients ...
Caution: Not fully tested. For research purposes only
Medchemexpress LLC
https://www.360docs.net/doc/9716001682.html,
18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m
