Hydrothermal synthesis, characterization and photocatalytic properties of Zn2SnO4 nanocrystal

Materials Science and Engineering A432(2006)
221–225
Hydrothermal synthesis,characterization and photocatalytic
properties of Zn2SnO4nanocrystal
Xiangdong Lou a,??,Xiaohua Jia a,Jiaqiang Xu b,?,Shuangzhi Liu a,Qiaohuan Gao b
a College of Chemistry and Environmental Science,Henan Normal University,
Henan Key Laboratory for Environmental Pollution Control,Xinxiang,Henan453007,PR China
b Zhengzhou University of Light Industry,College of Materials and Chemical Engineering,Zhengzhou,Henan450002,PR China
Received26March2006;received in revised form2June2006;accepted8June2006
Abstract
Spinel zinc stannate(Zn2SnO4)samples were prepared one-step by a hydrothermal process at200?C for24h.The crystal structure of the samples was characterized by X-ray diffraction(XRD).The speci?c surface areas of the nanocrystal powders were measured by Brunauer–Emmett–Teller (BET)method with a gas adsorption analyzer.The crystal morphology and particle size were analyzed using a transmission electron microscopy (TEM).The results show that the product is polycrystalline Zn2SnO4,and the average speci?c surface area of nanocrystal powder is about62m2/g, the particles have mono-dispersive cubic shape and the grain size is about20nm.Furthermore,the photocatalytic activity of the samples was appraised by means of the liquid phase photocatalytic degradation of various water-soluble dyes(K-NR,B-RN and B-GFF)under UV and visible light irradiation.The effects of catalyst loading and reacting time on the photocatalytic activity were also investigated.The results show that Zn2SnO4can photocatalytically decompose dyes in water solution after2h irradiation and the degradation can reach about100%.
?2006Elsevier B.V.All rights reserved.
Keywords:Nanometer material;Spinel-type structure;Zn2SnO4;Hydrothermal method
1.Introduction
The elimination of toxic chemicals from wastewater is presently of great concern,because their complete biodegra-dation is usually very slow and requires several days or weeks. These pollutants may originate from industrial applications or from household and personal care areas.Especially toxic organic compounds found in wastewater would bring out serious pollu-tion and must be removed or destroyed to an acceptable level before discharged to receiving waters.Recently,it has been demonstrated that semi-conducting materials mediating photo-catalytic oxidation of organic compounds can be an alternative to conventional methods for the removal of organic pollutants in water and air[1,2].Several studies on photocatalytic degradation of dyes have been reported[3–9].A variety of semiconductor materials(oxides,sul?des,etc.)acted as photocatalysts have already been used,for example,TiO2and ZnO have attracted ?Corresponding author.Tel.:+8637163556078;fax:+8637163556078.??Corresponding author.
E-mail addresses:xujiaqiang@https://www.360docs.net/doc/bb3574991.html,(J.Xu),
xhjia2003@https://www.360docs.net/doc/bb3574991.html,(X.Lou)..extensive attention in the past two decades[10].Titanium(IV) oxide suspended in water has been proven to be one of the most active photocatalyst.However,it has been shown that the photocatalytic activity of TiO2is limited by fast charge–carrier recombination and low interfacial charge-transfer rates of pho-togenerated carriers[11].On the other hand,since its band gap is large(E g=3.2eV),TiO2cannot absorb visible light and only makes use of3–5%of the solar beam that can reach the earth [12].Therefore,the search for novel materials in heterogeneous photocatalysis with high performance has been a matter of inter-est in the last years.
Compound oxide Zn2SnO4is a very important material in advanced technologies,such as gas sensor[13],negative elec-trode material for Li-ion battery[14],and synergistic?ame retardants[15].In addition,it shows high photocatalytic activity to benzene[16],this material is a promising candidate for the degradation of various water-soluble dyestuffs with high photo-catalytic activity.To the best of our knowledge,little attention has been paid to Zn2SnO4.Further studies are necessary to put the new materials into practice.
In this paper,Zn2SnO4nanocrystal was synthesized at the temperature of200?C for24h by one-step hydrothermal method
0921-5093/$–see front matter?2006Elsevier B.V.All rights reserved. doi:10.1016/j.msea.2006.06.010
222X.Lou et al./Materials Science and Engineering A432(2006)221–225
under proper ions concentration and pH value.These sam-ples were used as catalysts for the photodegradation of water-soluble dyes,such as reactive dyes K-NR,B-RN and B-GFF. These results show that Zn2SnO4nanocrystal prepared by the hydrothermal method is a promising material for photodegradat-ing water-soluble dyes due to its high photocatalytic activities.
2.Experimental
2.1.Preparation of spinel Zn2SnO4nanocrystal powders
In this paper,Zn2SnO4nanocrystal was synthesized by using a mild hydrothermal method.The zinc acetate(ZnAc2·2H2O, analytical grade or A.R.)and tin tetrachloride(SnCl4·5H2O, analytical grade)were used as the starting materials without further puri?cation,they were dissolved into distilled water to form two transparent solutions,respectively.Then the zinc acetate solution was added into tin tetrachloride slowly.Sodium hydroxide(NaOH)solution was added dropwise into the mix-ture under magnetic stirring.The?nal mixture charged into the Te?on-lined autoclave with75%degree of?ll,the autoclave was washed without any impurity ions presence before the experi-ment.Then the mixture was reacted at200?C for24h in the autoclave.After the reaction was terminated,the autoclave was cooled naturally to room temperature.The precipitate was?l-tered,washed with distilled water,and dried at100?C for6h.
2.2.Characterization
The crystal structure of the as-prepared powder was char-acterized by X-ray diffraction(XRD,Germany Bluker D8-Advance)with a Cu K?radiation(wavelengthλ=0.15406nm) operating at20mA and40kV.The mean crystallite sizes,d,were measured from XRD peaks at scan rate of2?/min based on Scher-rer’s equation:D=0.9λ/(βcosθ),whereλis the wavelength of the X-rays,θthe diffraction angle,andβis the full width at half maximum.The speci?c surface area of Zn2SnO4powder was measured by using nitrogen absorption through a gas adsorption analyzer(Model NOV A1000,Quantachrome Co.)according to the Brunauer–Emmett–Teller method.The morphology and particle size of the sample were measured with the help of transmission Electron Microscopy(TEM model JEM-100CXII, JEOL Corporation).The degradation of dyes was studied by a Pekin-Elmer Lambda-17UV–vis spectrometer.
2.3.Photocatalytic activity testing
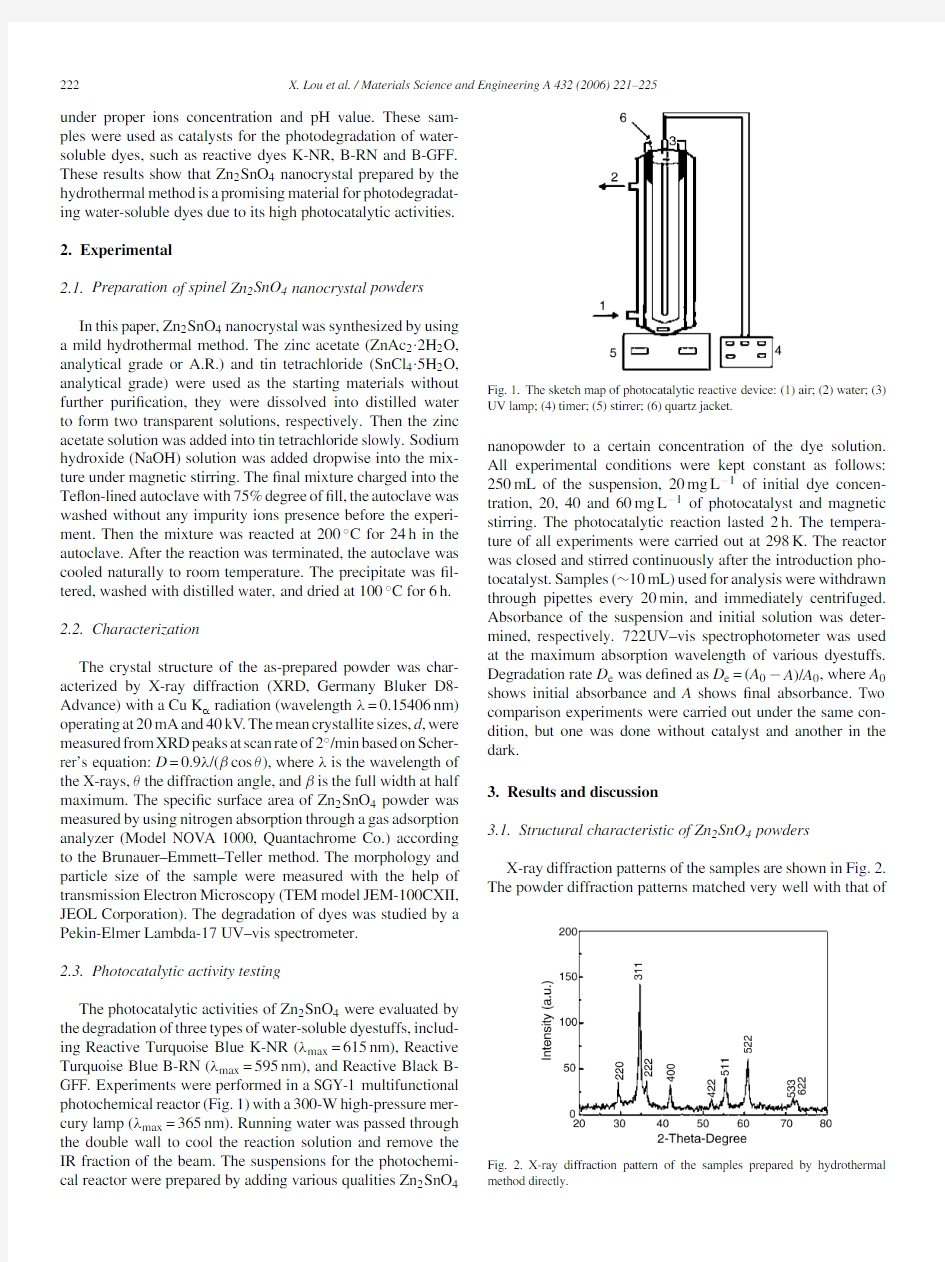
The photocatalytic activities of Zn2SnO4were evaluated by the degradation of three types of water-soluble dyestuffs,includ-ing Reactive Turquoise Blue K-NR(λmax=615nm),Reactive Turquoise Blue B-RN(λmax=595nm),and Reactive Black B-GFF.Experiments were performed in a SGY-1multifunctional photochemical reactor(Fig.1)with a300-W high-pressure mer-cury lamp(λmax=365nm).Running water was passed through the double wall to cool the reaction solution and remove the IR fraction of the beam.The suspensions for the photochemi-cal reactor were prepared by adding various qualities Zn2SnO
4Fig.1.The sketch map of photocatalytic reactive device:(1)air;(2)water;(3) UV lamp;(4)timer;(5)stirrer;(6)quartz jacket.
nanopowder to a certain concentration of the dye solution.
All experimental conditions were kept constant as follows:
250mL of the suspension,20mg L?1of initial dye concen-
tration,20,40and60mg L?1of photocatalyst and magnetic
stirring.The photocatalytic reaction lasted2h.The tempera-
ture of all experiments were carried out at298K.The reactor
was closed and stirred continuously after the introduction pho-
tocatalyst.Samples(~10mL)used for analysis were withdrawn
through pipettes every20min,and immediately centrifuged.
Absorbance of the suspension and initial solution was deter-
mined,respectively.722UV–vis spectrophotometer was used
at the maximum absorption wavelength of various dyestuffs.
Degradation rate D e was de?ned as D e=(A0?A)/A0,where A0 shows initial absorbance and A shows?nal absorbance.Two
comparison experiments were carried out under the same con-
dition,but one was done without catalyst and another in the
dark.
3.Results and discussion
3.1.Structural characteristic of Zn2SnO4powders
X-ray diffraction patterns of the samples are shown in Fig.2.
The powder diffraction patterns matched very well with that
of
Fig.2.X-ray diffraction pattern of the samples prepared by hydrothermal method directly.
X.Lou et al./Materials Science and Engineering A432(2006)221–225
223
Fig.3.Typical TEM images of the Zn2SnO4nanoparticles.
the PDF(JCPDS powder diffraction?le)number14-0381,it is shown that the hydrothermal reaction is complete,and the reac-tion is accord with the proper proportion to react.The mean grain size is about18.6nm by Deby–Scherrer https://www.360docs.net/doc/bb3574991.html,pared to the method that mixed ZnO and SnO2ball milled for8h,then sintered them at1350?C for8h to obtained Zn2SnO4[17],the hydrothermal synthetic route has excellent advantages to obtain high crystalline powders with narrow grain size-distribution and high purity without any treatment at high temperature.The surface areas of the samples after measured according to the Brunauer–Emmett–Teller(BET)method are about62m2/g.The lager surface area bene?ts the contact between dyes and cata-lysts and can absorb larger numbers of dyes.So it can increase their photocatalytic activity.
The TEM photograph of the compound oxide Zn2SnO4is given in Fig.3.As seen from the photograph,the compound oxide powder is of an equable distribution except for a little aggregated particulate,and the morphology of the material is homogeneous,and has narrow distribution in grain size.On the other hand,the approximately cubic Zn2SnO4particles were obtained.The average grain size calculated by proportion of the photograph is about20nm,it is uniform with the result calcu-lated from XRD patterns.
3.2.Photocatalytic activity measurement
The photocatalytic degradation of various dyes over different quantities of Zn2SnO4samples were investigated,the results were shown in Figs.4–6.The results showed that the time of the illumination and the quantity of the photocatalyst have obvious in?uence on the degradation of the reactive dyestuff.In general, the effect of the photocatalysis is more obviously at a certain range with the increasing photocatalyst quantity,the results showed in these?gures are accordance with the rule.When the quantity of photocatalyst achieves a certain dosage,it will cause negative in?uence because of the photocatalyst envelop each other,which induce to use the resource of illumination not suf?cient.Because the catalyst loading is large enough, the number of photon tends to a saturation value.The larger catalyst loading probably brings about light blocking which
has Fig.4.The degradation of different reactive dyes as a function of20mg L?1 Zn2SnO4catalyst
loading.
Fig.5.The degradation of different reactive dyes as a function of40mg L?1 Zn2SnO4catalyst loading.
an impact on the photocatalytic ef?ciency and also results in the waste of catalyst.Jiang et al.[18]investigated the in?uence factor during the course of lead titanate doped with zinc and cadmium photocatalysis decomposing reactive turquoise blue. The decolor ratio D will reach a saturation after a rapid increase with the increasing quantity of the photocatalyst.As shown in Figs.4–6,the photocatalytic activity increases with increasing catalyst loading but its increasing tendency is falling down gradually.When the catalyst loading of Zn2SnO4is50mg,the degradation rate is90%.While the loading reaches100mg, the degradation rate is97%,but the degradation rate is100% when the loading is150mg.This phenomenon is similar
to Fig.6.The degradation of different reactive dyes as a function of60mg L?1 Zn2SnO4catalyst loading.
224X.Lou et al./Materials Science and Engineering A432(2006)
221–225
Fig.7.The degradation of Reactive Turquoise Blue K-NR:(a)in the presence of catalyst loading Zn2SnO4,with irradiation;(b)in the presence of Zn2SnO4, without irradiation;(c)in the absence of Zn2SnO4,with irradiation.
that found in the study of other photocatalysts such as TiO2 [19].
https://www.360docs.net/doc/bb3574991.html,parison experiments
Fig.7shows the results of three comparison experiments carried out in the system of Zn2SnO4suspensions.As shown,a slight loss occurs after2h irradiation with a high-pressure mer-cury lamp in the absence of Zn2SnO4,indicating that the dye is photo-stable basically.In the presence of Zn2SnO4but without irradiation,the dye concentration decreases quickly at starting, and then reaches a saturation value,which is due to the adsorp-tion of dye molecule on the catalyst.In the presence of catalyst and light,the degradation of dye increases with irradiation time. An obvious decrease of the substrate’s absorbance is found after 2h in the presence of Zn2SnO4in the dark,which is attributed to the adsorption of the substrate onto the catalyst.It is well known that Zn2SnO4belongs to the structure of spinel-type. Chen et al.[20]recognize that the compound oxide Zn2SnO4 has n-type electric and oxygen de?ciency Zn2SnO4?X de?cient structure by studying the de?ciency of it.To maintain the charge balance,the forming of oxygen vacancies should result in the increasing of absorbed oxygen.The densities of reactive OH?become higher when absorbed oxygen is increased,which facil-itates the photocatalytic reaction.When the reception energy bigger than band gap energy of material,the photo-degradation processing will happen.Then the particles produce high-energy electron–hole pairs,which produce oxidation–reduction sys-tem.These electron–hole pairs react with water and dissolved oxygen to produce?OH free radicals with high chemical activ-ity,and react with the dyes molecule absorbed on the surface of Zn2SnO4substrate,thus the dyes molecule will be photo-degradated because the oxidation–reduction reaction happens. Comparing curves(b)and(a),it is seen that these two curves are obviously different,implying that adsorption is slight respon-sible to the decrease of dye concentration in both cases.The degradation comes up to97%after120min of irradiation of curve(a)compared with42.4%that of(b),suggesting that pho-tocatalytic reaction leads to the degradation of dyes and that Zn2SnO4has high photocatalytic activity.This also denotes that the presence of semiconductor as well as UV light irradiation
is Fig.8.UV–vis absorption spectra of Reactive Turquoise Blue K-NR:(a)initial solution and(b)in the presence of Zn2SnO4,with irradiation2h.
indispensable for the photocatalytic reaction to take place and the degradation of dyes occurs photocatalytically on the catalyst surface.In order to prove the result of the dyes degradation,the UV–vis was used to characterize the results after reaction.It can be seen that from Fig.8,the UV–vis patterns show that large molecules were decomposed small molecules after illumination 2h,because the main absorption peaks of dyes almost disappear at all.It indicates that the dyes are surely decomposed by pho-tocatalytic action,and the results of the UV–vis are accordance with that of the above experiment.
4.Conclusion
Compound oxide Zn2SnO4nanometer material with the structure of spinel-type was synthesized one-step by the hydrothermal process under mild reaction condition.The obtained particles took on good crystalline and dispersing,its mean grain size is about20nm.Nanocrystal Zn2SnO4prepared by hydrothermal directly exhibited high photocatalytic activity to various reactive dyes because of its great speci?c surface area, such as Reactive Turquoise Blue K-NR,Reactive Turquoise Blue B-RN,and Reactive Black B-GFF.The degradation rate for20mg L?1dyes can reach about100%after under a300-W high-pressure mercury lamp(λmax=365nm)irradiating2h when the content of catalyst is40mg L?1.Thus the Zn2SnO4 nanometer material was synthesized directly by hydrothermal method is promising candidate for the degradation of various water-soluble dyes with high photocatalytic.
Acknowledgements
We appreciate the?nancial supports of Henan Outstanding Youth Science Fund(no.0312*******).We also show great appreciation to the Key Laboratory of special functional mate-rials of Henan University and the Henan Key Laboratory for environmental pollution control of Henan Normal University.
References
[1]K.Nagaveni,G.Sivalingam,M.S.Hegde,G.Madras,Environ.Sci.
Technol.38(2004)1600–1604.
[2]X.Z.Li,H.Liu,L.F.Cheng,H.J.Tong,Environ.Sci.Technol.37(2003)
3989–3994.
[3]N.M.Mahmoodi,M.Arami,N.Y.Limaee,N.S.Tabrizi,J.Colloid Interf.
Sci.295(2006)159–164.
[4]A.Akyol,M.Bayramo?g lu,J.Hazard.Mater.124(2005)241–246.
[5]A.Akyol,H.C.Yatmaz,M.Bayramoglu,Appl.Catal.B:Environ.54
(2004)19–24.
X.Lou et al./Materials Science and Engineering A432(2006)221–225225
[6]S.Chakrabarti,B.K.Dutta,J.Hazard.Mater.112(2004)269–278.
[7]C.Hachem,F.Bocquillion,O.Zahraa,M.Bouchy,Dyes Pigments49
(2001)117–125.
[8]T.Sauer,https://www.360docs.net/doc/bb3574991.html,o,H.J.Jose,R.F.P.M.Moreira,J.Photochem.Photo-
biol.A149(2002)147–154.
[9]M.M.Kosanic,J.S.Trickovic,J.Photochem.Photobiol.A149(2002)
247–251.
[10]M.R.Hoffmann,S.T.Martin,W.Y.Choi,D.W.Bahnemann,Chem.Rev.
95(1995)69–96.
[11]S.T.Martin,H.Herrmann,W.Choi,M.R.Hoffmann,J.Chem.Soc.,
Faraday Trans.90(1994)3315–3322.
[12]X.S.Niu,H.H.Li,G.G.Liu,J.Mol.Catal.A:Chem.232(2005)89–93.
[13]J.H.Yu,G.M.Choi,J.Electroceram.8(2002)249–255.[14]F.Belliard,P.A.Connor,J.T.S.Irvine,Solid State Ionics135(2000)
163–167.
[15]A.Petsom,S.Roengsumran,A.Ariyaphattanakul,P.Sangvanich,Polym.
Degrad.Stabil.80(2003)17–22.
[16]C.Wang,X.M.Wang,J.Mater.Sci.37(2002)2989–2996.
[17]M.N.Niu,F.Q.Bao,Z.Q.Fu,Acta Sci.Nat.Univ.Intramongolicae4
(1992)185–187.
[18]Zh.J.Jiang,J.Dai,Zh.L.Zhang,G.Q.Bian,D.X.Tang,Chinese J.Appl.
Chem.18(2001)552–555.
[19]M.Muruganandham,M.Swaminathan,Sol.Energy Mater.Sol.C81
(2004)439–457.
[20]Z.Y.Chen,Y.Jia,Z.D.Zhang,Y.T.Qian,J.China Univ.Sci.Technol.
17(1987)343–346.
Synthesis Writing
Synthesis Writing (Sun) Sun1 Sun Chunjuan Professor Guo Shuqing Advanced—Writing (0901011019) 17Nov.2011 Failed Compulsory Education System in American In modern society, most people think it is an important and responsible process for students to receive education from the school. And people hold that education is not only a part of culture [1], but also an important reference for students’ jobs [2]. Thus, it seems that compulsory education plays a key role in the education field. However, the facts always go against the intentions. Compulsory education also brings a lot trouble to American society at the same time. For example, kill the children’s interests, limit their thoughts and put a lot pressure for their job employment. To begin with, it is a failure not to consider the problem of human diversity and individual instructions before the establishment of the compulsory education system. There is no accounting for tastes. Everyone has their own interests. Thus, one teacher in a class is not enough to take care of everyone, and teachers have to cast all the instructions into one uniform pattern (Murray N. Rothbard). As a result, students’ interests are not developed and they are absent-minded in the class. After a long time, they learn little from the class so that more illiteracy and ignorance appear in the America in spite of the attendance law (Samuel L. Blumenfeld). “Y ou can lead a horse to water but you can’t make him drink.” Y ou compel them to have the boring class and they just sit there without thoughts. It’s just a waste of time.
fluid inclusions
The application of fluid inclusions in the mineralization lijin The department of geochemistry,Yangtze university Abstract. Fluid inclusion analysis is an important tool in modern studies of mineral deposits, as reflected by the statistics indicating that about a quarter of the papers published in Economic Geology contain fluid inclusion studies. Fluid inclusions play an important role in the classification of mineral deposits and in the study of the composition, temperature and pressure of mineralizing fluids. Among the principal mechanisms of ore precipitation, flu-id phase separation and fluid mixing derive their key evidence mainly from studies of fluid inclusions. Data on mineralizing fluid composition obtained from fluid inclusion analysis are key to understanding how metals were transported in hydrothermal fluids. Recent progresses in metal transport in vapor have been mainly contributed by fluid inclusion studies. Data on fluid temperature and pressure from fluid inclusion studies provide important constraints on hydrodynamic models of mineralization. Most metal ore deposits are formed in the geological fluid.The formation and characteristics of hydrothermal ore deposits are closely related including temperature, pressure and composition. Although these information can be gained through the study of macro geological characteristics of ore deposit and the geochemical characteristics of the mineral , but the composition of ore-forming fluid, temperature, and pressure from fluid inclusion is the most direct evidence.Fluid inclusion is the only remain in ancient ore-forming fluid. So, the study of fluid inclusion becomes one of the important ways of genesis research naturally. For Economic Geology sampling survey, the proportion of fluid inclusion research papers, from 5% in 1975 to 15% in 1985, 27% in 1995, then remained at about 25%, about 1 in 4 papers of deposits essay involves the study of fluid inclusions. Although the fluid inclusion research has expanded to petroleum geology, magma, and the earth's interior processes, etc.its mian application in the field of ore deposit research . The application of fluid inclusions in ore deposit has a lot of monographs. but these works focus on basic principles , methods of the study and the characteristics of different deposit types.This paper mainly discusses the application of fluid inclusions in the study of ore deposits. Keywords: geochemistry, fluid inclusions, hydrothermal deposits, mineralizing fluids, ore precipitation, metal transportation. 1.Fluid inclusion is one of the basis of the classification of the ore deposit According to the geological characteristics and genesis, ore deposit can be divided into different types, But at present very few scholars classify the ore deposits completely according to the geological characteristics, such as shear zone gold deposits, stratabound lead-zinc deposit or causes such as high temperature
(H2N(C2H4)2NH2)[V4O10]ic951237c
Hydrothermal Syntheses and Structural Characterization of Layered Vanadium Oxides Incorporating Organic Cations:r-, -(H3N(CH2)2NH3)[V4O10]and r-, -(H2N(C2H4)2NH2)[V4O10] Yiping Zhang,?,?Robert C.Haushalter,*,?and Abraham Clearfield*,? NEC Research Institute,4Independence Way,Princeton,New Jersey08540,and Department of Chemistry,Texas A&M University,College Station,Texas77843 Recei V ed September26,1995X Four new layered mixed-valence vanadium oxides,which contain interlamellar organic cations,R-(H3N(CH2)2- NH3)[V4O10](1a), -(H3N(CH2)2NH3)[V4O10](1b),R-(H2N(C2H4)2NH2)[V4O10](2a),and -(H2N(C2H4)2NH2)- [V4O10](2b),have been prepared under hydrothermal conditions and their single-crystal structures determined: 1a,triclinic,space group P1h,a)6.602(2)?,b)7.638(2)?,c)5.984(2)?,R)109.55(3)°, )104.749- (2)°,γ)82.31(3)°,Z)1;1b,triclinic,P1h,a)6.387(1)?,b)7.456(2)?,c)6.244(2)?,R)99.89(2)°, )102.91(2)°,γ)78.74(2)°,Z)1;2a,triclinic,P1h,a)6.3958(5)?,b)8.182(1)?,c)6.3715(7)?,R )105.913(9)°, )104.030(8)°,γ)94.495(8)°,Z)1;2b,monoclinic,space group P21/n,a)9.360(2)?,b )6.425(3)?,c)10.391(2)?, )105.83(1)°,Z)2.All four of the compounds contain mixed-valence V5+/V4+vanadium oxide layers constructed from V5+O4tetrahedra and pairs of edge-sharing V4+O5square pyramids with protonated organic amines occupying the interlayer space. Introduction The contemporary interest in vanadium oxide bronzes reflects not only their interesting electronic and magnetic properties1 but also their complex structural chemistry,associated with the ability of vanadium to adopt a variety of coordination geometries in various oxidation states.In addition to the conventional alkali-metal bronzes A x V2O5,2a class of organic-based vanadium bronzes are also known.While most of the alkali-metal bronzes have been prepared at high temperatures,the organic-based vanadium bronzes are prepared at room temperature or slightly higher via intercalation reactions with vanadium pentoxide xerogels,V2O5?n H2O.The V2O5?n H2O host possesses a porous layered structure and is capable of intercalating a variety of neutral and charged guest species such as alkali-metal ions,3 alkylamines,4alcohols,5pyridine,6benzidine,7etc.The insertion of amines or metal complexes into V2O5hosts has also been reported.8The resulting intercalation compounds usually retain the lamellar structure with the guest species and water molecules occupying the interlayer regions.Partial reduction of V5+to V4+of the oxide layers has been observed to accompany the intercalation reactions with organic amines.In the cases of aniline9and thiophene,10the reduction of the vanadium oxide host,and the simultaneous oxidative polymerization of the guest molecules in the interlayer regions,have been observed.These intercalation compounds with reduced vanadium sites constitute an interesting class of organic-inorganic composite materials that can be viewed as molecular or polymer vanadium bronzes by analogy to alkali-metal bronzes.2However,the structural information about these intercalation compounds is very limited due to their amorphous or semicrystalline nature and lack of high-quality single crystals. Hydrothermal techniques,in combination with organic tem-plates,have been recently demonstrated to be well suited for the synthesis and crystal growth of reduced oxomolybdenum and oxovanadium phosphates and vanadium phosphonates.A series of novel organically templated molybdenum and vana-dium phosphates and vanadium phosphonates with molecular, two-dimensional layered,and three-dimensional open-frame-work structures have been prepared under hydrothermal condi-tions.11In contrast,hydrothermal synthesis of vanadium oxides using organic templates remains relatively unexplored.12While there are many examples of alkali-metal vanadium oxide bronzes with three-dimensional or two-dimensional structures in which the alkali metals occupy the channels or the interlayer regions, analogous organically templated vanadium oxides with3-D open *To whom all correspondence should be addressed. ?Texas A&M University. ?NEC Research Institute. X Abstract published in Ad V ance ACS Abstracts,August1,1996. (1)Murphy,D.W.;Christian,P.A.Science1979,205,651. (2)Hagenmuller,P.In Non-Stoichiometric Compounds,Tungsten Bronzes, Vanadium Bronzes and Related compounds;Bevan,D.J.,Hagen-muller,P.,Eds.;Pergamon Press:Oxford,U.K.,1973;Vol.1. (3)Lemordant,D.;Bouhaouss,A.;Aldebert,P.;Baffier,N.Mater.Res. Bull.1986,21,273. (4)Paul-Boucour,V.;Aldebert,P.Mater.Res.Bull.1983,18,1247. (5)Aldebert,P.;Baffier,N.;Legendre,J.-J.;Livage,J.Re V.Chim.Miner. 1982,19,485.Aldebert,P.;Baffier,N.;Gharbi,N.;Livage,J.Mater. Res.Bull.1981,16,949.Lemordant,D.;Bouhaouss,A.;Aldebert, P.;Baffier,N.J.Chim.Phys.Phys.-Chim.Biol.1986,83,105. (6)Ruiz-Hitzky,E.;Casal,B.J.Chem.Soc.,Faraday Trans.11986,82, 1597. (7)Hasbah,H.;Tinet,D.;Crespin,M.M.;Erre,R.;Setton,R.;Van Damme,H.J.Chem.Soc.,https://www.360docs.net/doc/bb3574991.html,mun.1985,935. (8)Kanatzidis,M.;Marks,T.J.Inorg.Chem.1987,26,783and references therein. (9)Kanatzidis,M.;Wu,C.-G.J.Am.Chem.Soc.1989,111,4139. (10)Kanatzidis,M.;Wu,C.-G.;Marcy,H.O.;DeGroot,D.C.;Kannewurf, C.R.Chem.Mater.1990,2,222. (11)Haushalter,R.C.;Mundi,L.A.Chem.Mater.1992,4,31.Soghomo- nian,V.;Chen,Q.;Haushalter,R.C.;Zubieta,J.;O’Connor,C.J. Science1993,259,1596.Soghomonian,V.;Chen,Q.;Haushalter,R. C.;Zubieta,J.Angew.Chem.,Int.Ed.Engl.1993,32,610.Soghomo- nian,V.;Chen,Q.;Haushalter,R.C.;Zubieta,J.Chem.Mater.1993, 5,1690.Soghomonian,V.;Chen,Q.;Haushalter,R.C.;Zubieta,J., Chem.Mater.1993,5,1595.Soghomonian,V.;Haushalter,R.C.; Chen,Q.;Zubieta,J.Inorg.Chem.1994,33,1700.Zhang,Y.; Clearfield,A.;Haushalter,R.C.J.Solid State Chem.1995,117,157. Zhang,Y.;Clearfield,A.;Haushalter,R.C.Chem.Mater.1995,7, 1221. (12)Huan,G.-H.;Johnson,J.W.;Jacobson,A.J.;Merola,J.S.J.Solid State Chem.1991,91,385.Duan,C.-Y.;Tian,Y.-P.;Lu,Z.-L.;You, X.-Z.;Huang,X.-Y.Inorg.Chem.1995,34,1. 4950Inorg.Chem.1996,35,4950-4956 S0020-1669(95)01237-7CCC:$12.00?1996American Chemical Society
Geological and isotopic evidence for magmatic-hydrothermal
ARTICLE Geological and isotopic evidence for magmatic-hydrothermal origin of the Ag –Pb –Zn deposits in the Lengshuikeng District,east-central China Changming Wang &Da Zhang &Ganguo Wu & M.Santosh &Jing Zhang &Yigan Xu &Yaoyao Zhang Received:7August 2012/Accepted:27March 2014/Published online:8April 2014#Springer-Verlag Berlin Heidelberg 2014 Abstract The Lengshuikeng ore district in east-central China has an ore reserve of ~43Mt with an average grade of 204.53g/t Ag and 4.63%Pb+Zn.Based on contrasting geological characteristics,the mineralization in the Lengshuikeng ore district can be divided into porphyry-hosted and stratabound types.The porphyry-hosted minerali-zation is distributed in and around the Lengshuikeng granite porphyry and shows a distinct alteration zoning including minor chloritization and sericitization in the proximal zone;sericitization,silicification,and carbonatization in the periph-eral zone;and sericitization and carbonatization in the distal zone.The stratabound mineralization occurs in volcano-sedimentary rocks at ~100–400m depth without obvious zoning of alterations and ore minerals.Porphyry-hosted and stratabound mineralization are both characterized by early-stage pyrite –chalcopyrite –sphalerite,middle-stage acanthite –native silver –galena –sphalerite,and late-stage pyrite –quartz –calcite.The δ34S values of pyrite,sphalerite,and galena in the ores range from ?3.8to +6.9‰with an average of +2.0‰.The C –O isotope values of siderite,calcite,and dolomite range from ?7.2to ?1.5‰with an average of ?4.4‰(V-PDB)and from +10.9to +19.5‰with an average of +14.8‰ (V-SMOW),respectively.Hydrogen,oxygen,and carbon iso-topes indicate that the hydrothermal fluids were derived main-ly from meteoric water,with addition of minor amounts of magmatic water.Geochronology employing LA –ICP –MS analyses of zircons from a quartz syenite porphyry yielded a weighted mean 206Pb/238U age of 136.3±0.8Ma considered as the emplacement age of the porphyry.Rb –Sr dating of sphalerite from the main ore stage yielded an age of 126.9±7.1Ma,marking the time of mineralization.The Lengshuikeng mineralization classifies as an epithermal Ag –Pb –Zn deposit. Keywords Stable isotope .Geochemistry .Porphyry .Stratabound .Ag –Pb –Zn .Lengshuikeng Introduction The Lengshuikeng ore district,located in the Jiangxi Province of east-central China (Fig.1a ),contains more than 50ore bodies belonging to seven deposits hosted in granite porphyry,pyroclastic,and carbonate rocks.The ore reserves in Lengshuikeng have been estimated at ~43Mt with average grades of 2.11%Pb,2.61%Zn,204.53g/t Ag,0.08g/t Au,and 0.01%Cd.The ores can be grouped into two types:(1)porphyry-hosted (Yinluling,Baojia,and Yinzhushan)and (2)stratabound (Xiabao,Yinkeng,Yinglin,and Xiaoyuan).The porphyry-hosted mineralization is distributed within and around the Lengshuikeng granite porphyry,whereas the stratabound mineralization occurs in volcano-sedimentary rocks at ~100–400m depth.The spatial distribution of the porphyry-hosted and stratabound ore bodies,their mineral constituents,and the zoning of alteration assemblages are markedly different from those of typical porphyry deposits. Editorial handling:T.Bissig and G.Beaudoin C.Wang (*): D.Zhang :G.Wu :M.Santosh :J.Zhang :Y .Xu :Y .Zhang State Key Laboratory of Geological Processes and Mineral Resources,China University of Geosciences,No.29,Xueyuan Road,Beijing 100083,People ’s Republic of China e-mail:wcm233@https://www.360docs.net/doc/bb3574991.html, Y .Xu No.912Geological Surveying Team,Bureau of Geology and Mineral Exploration and Development,Yingtan 334000,China Miner Deposita (2014)49:733–749DOI 10.1007/s00126-014-0521-8
逻辑综合synthesis(测试版)
综合复习资料(综合测试版) 一、名词解释 1、Synthesis:synthesis is the transformation of an idea into a manufacturable device to carry out an intended function. 2、SOLD(Synopsys On-Line Documentation): It is a website to provide answers. 3、STA(Static Timing Analysis): A method for determining of a circuit meets timing constraints without having to simulate clock cycles. 4、Clock skew:To account for varying delays between the clock network branches. 5、Jitter:Because some uncertain factors,which leads to the clock happen drift. 6、RTL(Register Transfer Level):It is a coding style means describing the register architecture, the circuit topology, and the functionality between registers. 7、TCL(Tool Command Language): It is an “open”, industry-standard language, developed at UCA Berkeley. 8、PVT: STA scales each cell and net delay based on Process, Voltage, and Temperature variations. 9、CTS(Clock Tree Synthesis):Buffer clock timing device in the right place, and avoid the CLOCK to SKEW. 10、BDD(Binary Decision Diagram):The binary decision diagram is used to represent the data structure of the Boolean functions. 二、填空 1、Design objects: Design、Cell、Reference、Port、Pin、Net、Clock 2、The advantages of synthesis: reusability、verifiable、portability、prestige、productivity、abstraction、design tricks 3、Synthesis is Constraint-Driven, is Path-Based. 4、Synthesis=translation + optimization + mapping 5、GTECH has nothing to do with technology. 三、简答 1、Cell-BaBehavioral Level 答:1.Behavioral level 2.RTL Level 3.Logic Synthesis 4.Logic Level Design 5.Circuit Level Design https://www.360docs.net/doc/bb3574991.html,yout Level Design 7.Post Verificationsed-Flow 2、Logic Synthesis Overview 答:1.RTL Design 2.HDLCompiler3.DesignCompiler4.OptimizedGate-level Netlist 3、What .synopsys_dc.setup defined 答:link_library target_library symbol_library
Hydrothermal synthesis of single-crystal CeCO3OH and their thermal conversion to CeO2
Original article Hydrothermal synthesis of single-crystal CeCO3OH and their thermal conversion to CeO2 Kun Gao a,Yi-Yang Zhu a,Da-Qing Tong a,Li Tian a,Zhao-Hui Wang a,b,Xiao-Zu Wang a,* a College of Chemistry and Chemical Engineering,Nanjing University of Technology,Nanjing210009,China b State Key Laboratory of Materials-Oriented Chemical Engineering,Nanjing University of Technology,Nanjing210009,China 1.Introduction In recent years,cerium compounds have been widely used in catalysis[1–4],fuel cells[5]and chemical materials[6–8]due to their speci?c4f energy levels of the Ce-element[9,10].Among all the cerium compounds,cerium carbonate hydroxide,as an important functional material,has been attracted much attention because of its novel electronic properties,optical properties and chemical characteristics arising from their4f electrons[9–12]. Recently,cerium carbonate hydroxide with different morphol- ogies was synthesized by different methods,such as self-assembly, sonochemical[13,14],hydrothermal[15–18],and microwave- assisted hydrothermal route[19].Among all the preparation methods,the hydrothermal process is considered to be an effective and economical route due to its merits of low synthesis temperature,high powder reactivity and versatile shape control [20–23].In a hydrothermal system,CeCO3OH with different structures corresponding to distinct morphologies have been synthesized[18,24,25].There have been suf?cient studies report- ing on the synthesis of different morphologies,for example,Guo et al.reported the synthesis of triangular micro-plate,bundle-like, shuttle-like and?ower-like structures of CeCO3OH by hydrother- mal method[15,16,18].Li and Zhao synthesized single-crystalline CeCO3OH with dendrite-like structures through a facile hydro- thermal method and obtained CeO2by heating CeCO3OH at5008C for6h[26].Zhang et al.synthesized CeCO3OH rhombic micro- plates by the precipitation method in the presence of3- aminopropyltriethoxysilane[28].However,most of these reports on the synthesis of CeCO3OH micro/nanoparticles were prepared using CO(NH2)2or HMT as the alkaline and carbon resource[13– 18]and added surfactant or template to adjust the nucleation and crystal growth of CeCO3OH particles[13,15–18,28],which makes the process complex and raw materials more costly.So it is important to explore a facile method to synthesize morphology- controlled CeCO3OH micro/nanomaterials. In this paper,we report a simple method to synthesize dendrite- like CeCO3OH crystallites using CeCl3á7H2O as the cerium source, triethylenetetramine as both an alkaline and carbon source.The polycrystalline CeO2was obtained by calcination of the precursor at 5008C for4h,partly maintaining the dendrite-like morphology.The optical absorption properties of CeO2were also investigated. 2.Experimental All chemical reagents were of analytical grade without further puri?cation.In a typical synthesis,0.001mol of CeCl3á7H2O was dissolved in60mL deionized water to form a clear solution,and then0.30mL triethylenetetramine was added to the transparent solution in order to completely react with Ce3+at258C for about 0.5h with continued stirring.The resulting homogenous solution Chinese Chemical Letters25(2014)383–386 A R T I C L E I N F O Article history: Received10August2013 Received in revised form8September2013 Accepted26September2013 Available online1December2013 Keywords: CeCO3OH Hydrothermal Cerium carbonate hydroxide Nanostructures A B S T R A C T Hexagonal single-crystalline cerium carbonate hydroxide(CeCO3OH)precursors with dendrite morphologies have been synthesized by a facile hydrothermal method at1808C using CeCl3á7H2O as the cerium source,triethylenetetramine as both an alkaline and carbon source,with triethylenete- tramine also playing an important role in the formation of the dendrite structure.Polycrystalline ceria (CeO2)have been obtained by calcining the precursor at5008C for4h.The morphology of the precursor was partly maintained during the heating process.The optical absorption spectra indicate the CeO2 nano/microstructures have a direct band gap of2.92eV,which is lower than values of the bulk powder due to the quantum size effect.The high absorption in the UV region for CeO2nano/microstructure indicated that this material was expected to be used as UV-blocking materials. ?2013Xiao-Zu Wang.Published by Elsevier B.V.on behalf of Chinese Chemical Society.All rights reserved. *Corresponding author. E-mail address:wangxiaozu@https://www.360docs.net/doc/bb3574991.html,(X.-Z.Wang). Contents lists available at ScienceDirect Chinese Chemical Letters j o u rn a l h om e p a g e:w w w.e l s e v i e r.c o m/l o c a t e/c c l e t 1001-8417/$–see front matter?2013Xiao-Zu Wang.Published by Elsevier B.V.on behalf of Chinese Chemical Society.All rights reserved. https://www.360docs.net/doc/bb3574991.html,/10.1016/https://www.360docs.net/doc/bb3574991.html,let.2013.11.047
