Eu stabilized α-sialon ceramics derived from SHS-synthesized powders

Eu stabilized a -sialon ceramics derived from SHS-synthesized powders
Jiu-Xin Jiang a ,Pei-Ling Wang a ,*,Wan-Bao He b ,Wei-Wu Chen a ,Han-Rui Zhuang b ,
Yi-Bing Cheng c ,Dong-Sheng Yan a
a
The State Key Lab of High Performance Ceramics and Superfine Microstructure,Shanghai Institute of Ceramics,Chinese Academy of Sciences,
Shanghai 200050,P .R.China
b
The Center of Structural Ceramics,Shanghai Institute of Ceramics,Chinese Academy of Sciences,Shanghai 200050,P .R.China
c
School of Physics and Materials Engineering,Monash University,Clayton,Victoria 3800,Australia
Received 24June 2004;received in revised form 6August 2004;accepted 28August 2004
Available online 27October 2004
Abstract
The characteristics of Eu-stabilized a -sialon ceramics derived from self-propagating high-temperature synthesis (SHS)Eu a -sialon powders without and with the addition of Y 2O 3are investigated.The results showed that the amount of a -sialon phase formed in sintered Eu a -sialon composition was much less than that in SHS-ed powder when the composition was hot-pressed at 18008C for 1h,while the transformation of a -sialon to h -sialon phase did occur at the same time,which could be attributed to the metastability of SHS-ed powder because of the high heating and cooling rate during the SHS process and the reduction of Eu 3+to Eu 2+under the reduction conditions during hot pressing.By addition of Y 2O 3into SHS-ed Eu a -sialon powder,thus to form (Y ,Eu)a -sialon phase in the sintered sample,the stability of a -sialon phase was improved,as the ratio of a -sialon to a -sialon was increased from 70wt.%in SHS-ed powder to 83wt.%in the sintered product by 50mol%of Y 2O 3added into SHS-ed powder.D 2004Elsevier B.V .All rights reserved.
Keywords:Eu a -sialon;SHS;Phase assemblages;Y 2O 3;Hot pressing;UV–VIS absorption
1.Introduction
a -Sialon can accept some cations into its structure,and in conventional synthesis methods,these cations include Li +,Mg 2+,Ca 2+,Y 3+,and some lanthanide ions with the exception of Ce 3+,Pr 3+,and Eu 3+[1,2].It has been reported that the largest cations to be incorporated into the interstices of a -sialon structure have an ionic radius of c 1.02.It is therefore considered that Ce 3+,with an ionic radius of 1.032,would be too large to be absorbed into the a -sialon structure [3,4].According to the consideration of ionic radius,Eu 3+,with an ionic radius of 0.952,would be expected to stabilize the a -sialon structure.However,previous work has shown that the Eu-doped a -sialon phase was not found (Ref.[5]and references
therein).Biswas et al.[6]verified the spontaneously reduction of Eu 3+ions to Eu 2+at the presence of Al 3+during the densification of the Al-codoped sol–gel silica by the emission spectra,excitation spectra,and UV–VIS absorption spectra.Shen et al.[7,8]have reported that some trivalent rare-earth cations with nearly filled or half-4f electron configurations,e.g.,Yb 3+,Eu 3+,and Sm 3+,are easily reduced to the divalent state during the sintering of Si 3N 4-based ceramics,as the sintering process takes place under reducing conditions.Therefore,even if the ionic radius of Eu 3+is smaller than Nd 3+,it is thought that the Eu 2+ion (1.162)produced by reduction of Eu 3+is too large to be incorporated into the a -sialon structure.The Eu a -sialon material was studied by the SPS technique,and the phase assemblage consisted a -sialon,a -sialon,and P-phase (EuSi 9Al 19ON 31)[9].The same composition pre-pared by hot pressing contains a -sialon,h -sialon,and two new phases,which were respectively assumed as S-phase (Eu 2Al x Si 12àx N 16àx O 2+x )and Eu-containing AlN-based
0167-577X/$-see front matter D 2004Elsevier B.V .All rights reserved.doi:10.1016/j.matlet.2004.08.032
*Corresponding author.Tel.:+862152412324;fax:+862152413122.E-mail address:plwang@https://www.360docs.net/doc/b29222295.html, (P.-L.Wang).
Materials Letters 59(2005)205–
209
https://www.360docs.net/doc/b29222295.html,/locate/matlet
polytypoid phase however[8].Eu a-sialon was also investigated by duplex sintering additives Eu2O3and CaO[10],in which the a-sialon could be the single crystalline phase with a trace amount of h-Si3N4or h-sialon in the sintered samples by increasing the amount of Eu in combination with the Ca a-sialon system until the amount of doped Eu reached70at.%.
Self-propagating high-temperature synthesis(SHS)tech-nology has been used to synthesize a-sialon powder from both stoichiometrical compositions and some industrial wastes through proper selection of composition in our previous work[11,12].SHS can also be applied for the synthesis of a-sialons doped by large rare-earth ions[13], like Ce3+,Pr3+,and Eu3+,which could not be accommo-dated into the a-sialon structure by conventional sintering methods.It would be interesting to study the stability of Eu a-sialon materials starting from SHS-ed Eu a-sialon powder.To investigate the effect of the addition of Y2O3 on phase assemblage of sintered material,Y2O3added SHS-ed Eu a-sialon powder was used as the starting material.For comparison,(Eu,Y)a-sialon ceramics were also prepared by hot pressing derived from SHS-synthesized(Eu,Y)a-sialon powder.
2.Experimental
The following starting powders were used in the present work:Si3N4(UBE E10,Japan,2wt.%O),AlN(Wuxi, China,1.3wt.%O),Eu2O3(99.9%),Y2O3(99.9%),Si (99%),and Al(99%).The nominal composition of Eu a-sialon for SHS was designed,lies on the tie line Si3N4–9AlN/RE2O3,corresponding to a-sialon composition of m=1.5and n=0.75,i.e.,Eu0.5Si9.75Al2.25O0.75N15.25.For the SHS purpose of(Eu,Y)a-sialon powder,the composition was(Eu0.25Y0.25)Si9.75Al2.25O0.75N15.25.The hot-pressed samples of SHS-ed Eu and(Eu,Y)a-sialon powders were named as Eu1Y0and Eu1Y1,respectively.The molar ratio of Y2O3added to SHS-ed Eu a-sialon powder(Eu0.5Si9.75 Al2.25O0.75N15.25)was0.25:1.0and0.50:1.0,corresponding to weight ratios of0.0774:1.0and0.1548:1.0,in the samples symbolized as Eu4Y1and Eu4Y2,respectively.
The SHS-ed Eu a-sialon powders,without and with the addition of Y2O3,were hot-pressed in flowing N2at1800 8C for1h under a pressure of20MPa in a graphite furnace. The hot-pressed Eu1Y1sample was prepared under the same condition.
The bulk densities of the samples were measured according to the Archimedes principle.Phase assemblages of the powders and sintered samples were based on X-ray diffraction data from a Guinier–H7gg camera with use of Cu K a1radiation and Si as an internal standard.The photo-graphs obtained were evaluated with a computerized scanner system[14],and the lattice parameters of a-sialon phase were calculated by program PIRUM.The semi-quantitative analysis was carried out on the crystalline content based on the calibration curves.The UV–VIS absorption spectra were carried out on UV-3101PC(SHI-MADZU).The microstructure of the hot-pressed Eu4Y1 and Eu4Y2samples was observed on SEM(Hitachi S-570). The samples were etched in melted NaOH for seconds before SEM observation.
3.Results and discussion
3.1.Characteristics of hot-pressed Eu a-sialon ceramics starting from SHS-ed powder
It was found in our previous work[13]that the crystalline phases in SHS-ed Eu a-sialon powder consisted of a-sialon,h-sialon,AlN-polytypoids,and a small amount of S-phase(Eu2Al x Si12àx N16–x O2+x)that was firstly reported by Shen et al.[15]and Grins et al.[16].In contrast,the a-sialon phase was the major crystalline phase with very trace amount of AlN-polytypoids phase included in the SHS-ed(Eu,Y)a-sialon powder,which was consistent with the previous view that some large RE3+ ions could enter the a-sialon structure when Y2O3is simultaneously added with the RE oxides[17].
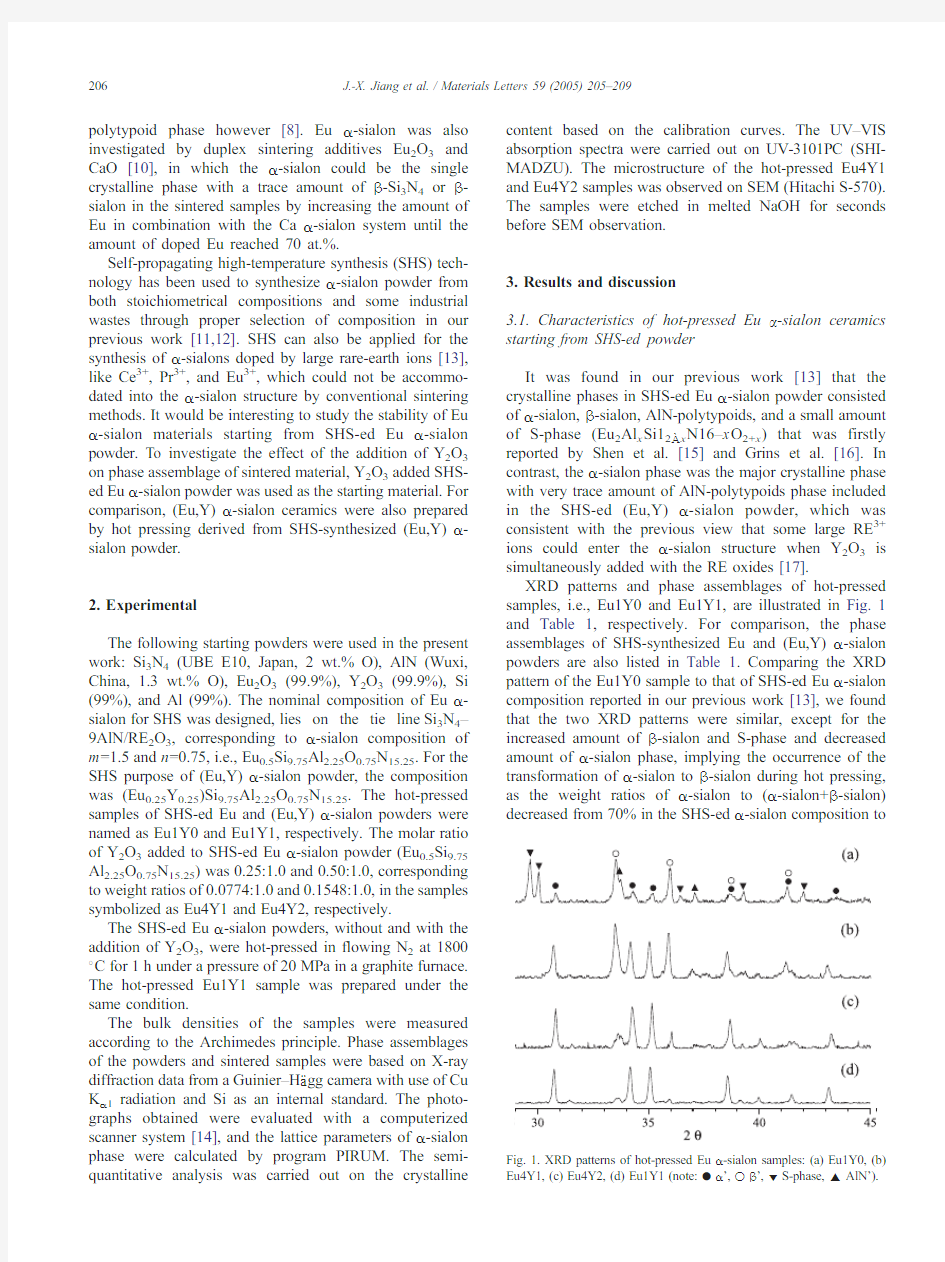
XRD patterns and phase assemblages of hot-pressed samples,i.e.,Eu1Y0and Eu1Y1,are illustrated in Fig.1 and Table1,respectively.For comparison,the phase assemblages of SHS-synthesized Eu and(Eu,Y)a-sialon powders are also listed in https://www.360docs.net/doc/b29222295.html,paring the XRD pattern of the Eu1Y0sample to that of SHS-ed Eu a-sialon composition reported in our previous work[13],we found that the two XRD patterns were similar,except for the increased amount of h-sialon and S-phase and decreased amount of a-sialon phase,implying the occurrence of the transformation of a-sialon to h-sialon during hot pressing, as the weight ratios of a-sialon to(a-sialon+h-sialon) decreased from70%in the SHS-ed a-sialon composition
to Fig.1.XRD patterns of hot-pressed Eu a-sialon samples:(a)Eu1Y0,(b) Eu4Y1,(c)Eu4Y2,(d)Eu1Y1(note:.a’,o h’,z S-phase,E AlN’).
J.-X.Jiang et al./Materials Letters59(2005)205–209 206
27%in the hot-pressed sample Eu1Y0.It is noted however that the cell dimensions of the a-sialon phase is kept almost constant before and after hot-pressing.It could be under-stood that the transformation of a-sialon to h-sialon encouraged the formation of a bigger amount of S-phase that was an Eu-containing phase(see Table1).As stated above,the hot-pressed Eu a-sialon composition(Ref.[8]) contains a-sialon,h-sialon,S-phase,and Eu-containing AlN-polytypoids.It is reasonable to think that the AlN-polytypoids phase formed in the Eu1Y0sample may also contain Eu.
Previous studies[18,19]on a-sialon doped by rare-earth ions showed that a-sialon to h-sialon phase transformation could occur during heat treatment between1200and1500 8C,especially in a-sialon systems stabilized by light rare earth elements.These observations suggest that some a-sialon phases formed at elevated temperatures(~18008C) could be thermodynamically unstable at temperatures lower than the initial forming temperature and would transform to the more stable h-sialon phase.In contrast,Ca-a-sialon is found very stable[20]and does not transform to h-sialon even after heat treatment at14508C for200h[21]. However,in our previous study on the phase transformation of(Ca,Mg)a-sialon fabricated from SHS-ed(Ca,Mg)a-sialon powder,the transformation of a-sialon to h-sialon has also been found,which was contributed to the metastable SHS-ed powder formed during very rapid heating and cooling process of SHS[12].The same phenomenon was found in the hot-pressed Eu a-sialon sample starting from SHS-ed powder and could be attributed to the same reason. SHS is a very rapid heating and cooling process,and the reaction temperature could rapidly reach above20008C and reaction could cease within seconds.Synthesis of a-sialon by SHS under a high nitrogen pressure is a complex process as the reactants involved are in liquid or gaseous state inside the combustion chamber,and they could react very rapidly. When the metastable powders were reheated in the sintering temperature,a thermodynamic equilibrium would be achieved in the whole system,and the transformation of a-sialon to h-sialon could occur,resulting in decrease in amount of the a-sialon phase.In contrast,the difference in the amount of a-sialon phase between the SHS-ed(Eu,Y)a-sialon powder and the hot-pressed sample has been small because it changed from100%in the SHS-ed powder to 96%in the sintered sample,implying the limited trans-formation of a-sialon to h-sialon and the contribution of Y3+to stabilize a-sialon structure in both cases.No obvious difference in cell dimension of the a-sialon phase between that of SHS-ed(Eu,Y)a-sialon powder and the correspond-ing sintered sample was found,which also reflects the effect of Y3+on the stability of the a-salon phase.
In addition to the metastable properties of the powders resulted from the SHS process,the spontaneous reduction of Eu3+to Eu2+during the sintering also has a contribution to the transformation of a-sialon to h-sialon that has already been established[8].To clarify the valency of Eu,UV–VIS absorption spectra of Eu2O3powder,SHS-ed Eu a-sialon powder,and Eu1Y0sample were therefore investigated. From the absorption spectra of Eu2O3powder,as shown in Fig.2,it is found that Eu3+has a violent absorption peak in the ultraviolet region(about250nm).In addition,there are three small absorption peaks located in the near ultraviolet region at400,470,and530nm,respectively.However,for the SHS-ed Eu a-sialon powder,two absorption peaks, similar to that of Eu2O3powder and attributed to
the Fig.2.UV–VIS absorption spectra of(a)Eu2O3powder,(b)SHS-ed Eu a-sialon powder,and(c)hot-pressed Eu a-sialon sample.
Table1
Phase assemblages of hot-pressed samples without and with the addition of Y2O3and cell dimensions of a-sialon phase
Sample Phase assemblage(wt.%)Cell dimensions of a’Density/g cmà3 Phase assemblage**a’/h’a(2)c(2)V(23)
Eu a’*a’/s,h’/m,AlN’/w S/w70/307.796(1) 5.672(1)298.59–
(Eu,Y)a’*a’/s,AlN’/vw100/07.813(1) 5.688(1)300.68–
Eu1Y0h’/s,S/m,a’/m,AlN’/w27/737.793(3) 5.671(3)298.29 2.38
Eu1Y1a’/s,h’/vw96/47.808(1) 5.687(1)300.26 2.98
Eu4Y1a’/s,h’/s52/487.796(1) 5.673(1)298.65 3.47
Eu4Y2a’/s,h’/mw83/177.806(1) 5.685(2)299.97 3.51 *Eu a’—SHS-ed Eu a-sialon powder;(Eu,Y)a’—SHS-ed(Eu,Y)a-sialon powder,whose phase assemblages referring to Ref.[13].
**a’—a-sialon,h’—h-sialon,AlN’—AlN-polytypoids,S—(Eu2Al x Si12àx N16àx O2+x),s—strong,m—medium,mw—medium weak,w—weak,vw—very weak.
J.-X.Jiang et al./Materials Letters59(2005)205–209207
contribution of Eu3+,are found in the ultraviolet and near ultraviolet regions,with the exception of much lower and higher intensities in the formal and later peak,respectively, than that of the Eu2O3powder.In addition,one absorption peak at320nm appears.It has been reported by Freiser et al.
[22]and Shen and Nygren[7]that Eu2+has strong absorption peaks near the UV regions.However,no absorption spectrum detail dealing with Eu2+has been provided yet.We therefore suggest that the peak at320nm and the higher intensity at400nm are resulted from the contribution of Eu2+.The absorption peak positions of the hot-pressed Eu1Y0sample are similar to that of SHS-ed Eu a-sialon powder,and the only difference between them is the higher intensities of absorption peaks at320and400nm than that of SHS-ed powder,implying the increase in amount of Eu2+in the hot-pressed Eu1Y0sample.This result could be easily understood because the hot-pressing process was carried out under a reducing atmosphere for a longer time than that in the SHS process,facilitating the reduction of Eu3+to Eu2+,which provided additional evidence for the influence of the reduction of Eu3+on the formation of Eu-stabilized a-sialon.
3.2.Effect of the addition of Y2O3on the phase assemblage of Eu a-sialon ceramics
XRD patterns and the phase assemblages of hot-pressed samples,Eu4Y1and Eu4Y2,derived from SHS-ed Eu a-sialon powder with the addition of different amounts of Y2O3,are shown in Fig.1and Table1,respectively.It is found that the amount of a-sialon phase in sintered samples increases with the increase in addition of Y2O3,accom-panying with the decreasing of the amount of the h-sialon
phase and the disappearance of the S-phase and AlN-polytypoids.Although the lattice parameters of a-sialon phase in Eu4Y1are close to that in Eu1Y0,as seen from Table1,almost a doubled amount of the a-sialon phase in the formal sample reveals the contribution of Y3+to stabilize the a-sialon structure.The cell dimensions of a-sialon phase become larger when the molar ratio of Y2O3to SHS-ed Eu a-sialon powder reaches0.50:1.0,as seen from Table1,implying more Y3+absorbed into the a-sialon structure.On the other hand,the addition of Y2O3could lower the comelting temperature in the system and increase the amount of liquid phase during sintering,which can improve the densification of the samples.The higher densities in sintered samples Eu4Y2than Eu4Y1,as listed in Table1,are caused by the increased Y2O3content in the composition.In contrast,the densities of both Eu1Y0and Eu1Y1samples are quite low,and the formal one is even lower,implying that less liquid phase was formed during hot pressing in the two samples,because the densification of nitride ceramics is through the transient liquid formed during sintering.
SEM micrographs of Eu4Y1and Eu4Y2samples are shown in Fig.3(a)and(b),respectively.It is found that the grains mainly have elongated morphology in both samples,but the aspect ratio of grains is higher in the Eu4Y1sample than that in Eu4Y2.According to the XRD analysis,the grains with higher aspect ratio shown in Fig.3(a)would be h-sialon,and the grains with lower aspect ratio in Fig.3(b)belong to a-sialon.
4.Conclusions
After hot pressing of SHS-ed Eu a-sialon powder,part of the a-sialon phase transforms to h-sialon,and the amount of S-phase is also increased in the sintered sample,which could be attributed to the metastability of SHS-ed powder because of the high heating and cooling rate during SHS process and the reduction of Eu3+to Eu2+under the reduction conditions during hot pressing.In contrast,the sintering of SHS-ed(Eu,Y)a-sialon powder does not cause the phase transformation as a-sialon content in the sample keeps almost constant.By addition of Y2O3into the SHS-ed Eu a-sialon powder and formation of(Y,Eu)a-sialon phase in the sintered sample,the ratio of a-sialon to h-sialon was increased from70wt.%in SHS-ed powder to83wt.%in
the Fig.3.Microstructure of hot-pressed samples:(a)Eu4Y1,(b)Eu4Y2.
J.-X.Jiang et al./Materials Letters59(2005)205–209 208
sintered product by50mol%of Y2O3added into SHS-ed powder,revealing that the addition of Y2O3can restrain the transformation of a-sialon to h-sialon. Acknowledgement
The financial support for the research from the National Natural Science Foundation of China and the Energy Saving Investment of China(No.50272073)and the Outstanding Overseas Chinese Scholars Fund of Chinese Academy of Sciences was highly appreciated.
References
[1]S.Hampshire,H.K.Park,D.P.Thompson,K.H.Jack,Nature(Lond.)
274(1978)121.
[2]Z.K.Huang,T.Y.Tien,T.S.Yen,J.Am.Ceram.Soc.69(8)(1986)
C-241.
[3]T.Ekstr f m,K.Jansson,P.O.Olsson,J.Persson,J.Eur.Ceram.Soc.8
(1991)3.
[4]H.Mandal,D.P.Thompson,J.Mater.Sci.Lett.15(1996)1435.
[5]D.P.Thompson,Mater.Sci.Forum47(1989)21.
[6]A.Biswas,C.S.Friend,P.N.Prasad,J.Mater.Lett.39(1999)227.
[7]Z.J.Shen,M.Nygren,Key Eng.Mater.132–136(1997)755.
[8]Z.J.Shen,M.Nygren,P.L.Wang,J.W.Feng,J.Mater.Sci.Lett.17
(1998)1703.
[9]Z.Shen,M.Nygren,J.Eur.Ceram.Soc.21(2001)611.
[10]R.J.Xie,M.Mitomo,K.Uheda,F.F.Xu,Y.Akimune,J.Am.Ceram.
Soc.85(5)(2002)1229.
[11]W.W.Chen,P.L.Wang,D.Y.Chen,B.L.Zhang,J.X.Jiang,Y.B.
Cheng,D.S.Yan,J.Mater.Chem.212(2002)1199.
[12]J.X.Jiang,P.L.Wang,W.B.He,W.W.Chen,H.R.Zhuang,Y.B.
Cheng,D.S.Yan,J.Eur.Ceram.Soc.23(2003)2343.
[13]J.X.Jiang,P.L.Wang,W.B.He,W.W.Chen,H.R.Zhuang,Y.B.
Cheng,D.S.Yan,J.Am.Ceram.Soc.87(2004)703.
[14]K.E.Johansson,T.Palm,P.E.Werner,J.Phys.E:Sci.Instrum.13
(1980)1289.
[15]Z.J.Shen,J.Grins,S.Esmaeilzadeh,H.Ehrenberg,J.Mater.Chem.9
(1999)1019.
[16]J.Grins,S.Esmaeilzadeh,G.Svensson,Z.J.Shen,J.Eur.Ceram.Soc.
19(1999)2723.
[17]T.Ekstr f m,K.Jansson,P.O.Olsson,J.Person,J.Eur.Ceram.Soc.8
(1991)3.
[18]Z.Shen,M.Nygren,Mater.Sci.Forum325–326(2000)191.
[19]H.Mandal,D.P.Thompson,T.Ekstr f m,J.Eur.Ceram.Soc.12(1993)
421.
[20]H.Mandal,D.P.Thompson,J.Eur.Ceram.Soc.19(1999)543.
[21]C.L.Hewett,Y.B.Cheng,B.C.Muddle,M.B.Trigg,J.Eur.Ceram.
Soc.18(1998)417.
[22]M.J.Freiser,S.Methfessel,F.Holtzberg,J.Appl.Phys.39(1968)
900.
J.-X.Jiang et al./Materials Letters59(2005)205–209209
