Adsorption of Arsenic on Conditioned Layered Double Hydroxides-Column Experiments and Modeling

Adsorption of Arsenic on Conditioned Layered Double Hydroxides:Column Experiments and Modeling
Megha Dadwhal,Mayur M.Ostwal,Paul K.T.Liu,?Muhammad Sahimi,and Theodore T.Tsotsis*
Mork Family Department of Chemical Engineering and Materials Science,Uni V ersity of Southern California,Uni V ersity Park,Los Angeles,California 90089-1211
The removal of As(V)by conditioned,calcined layered double hydroxide (LDH)adsorbents is investigated in continuous-?ow,packed-bed columns,in order to study the effect of important operating parameters,such as the in?uent As concentration,the pH,the adsorbent particle size,and the ?ow rate.Earlier bed saturation and breakthrough were observed at higher ?ow rates and in?uent concentrations.On the other hand,a decrease in the adsorbent particle size and the in?uent pH resulted in an increase in the number of bed volumes at breakthrough.A column model which accounts for external,liquid-?lm mass transport and for diffusion and adsorption in the adsorbent particles is utilized.Two different adsorption models are employed,which were shown previously to be capable of predicting the As(V)uptake by LDH adsorbents.They are a conventional homogeneous surface diffusion model and a bidisperse pore model,the latter viewing the LDH particles as assemblages of microparticles and taking into account bulk diffusion in the intraparticle pore space,and surface diffusion within the microparticles themselves.Both models are found capable of predicting the ?ow-column experimental results.
1.Introduction
The rapid increase worldwide in industrial activity has,unfortunately,resulted in the inadvertent discharge of a number of heavy metals and in the pollution of drinking wells,streams,and lakes.Of such metals,arsenic,due to its extreme toxicity 1and the serious threat that it poses to human health and the environment,has attracted global concern.It is essential,therefore,that arsenic-impacted waters be cleaned up prior to further use or discharge.A number of cleanup techniques are currently available,including precipitation -?ltration,ion ex-change,membrane separation,and adsorption;the last technique has been found superior to other methods of water reuse applications,in terms of the initial capital cost,simplicity of the design,and ease of operation.So far,a number of conventional adsorbents,such as various clays and other naturally occurring minerals,2,3activated alumina,4-6mesopo-rous alumina,7ferric hydroxide,8and ferrihydrite,9have been https://www.360docs.net/doc/c7207740.html,yered double hydroxides (LDH),which offer a large interlayer surface to host diverse anionic species 10-12with the additional advantage of being potentially easily recyclable,are also attracting current research interest.
In our previous papers,11,12the adsorption of As (but also of Se)on Mg -Al -CO 3-LDH adsorbents was studied in batch experiments in order to investigate the adsorption kinetics and isotherms.The effect of such factors as the As and Se oxidation state,the wastewater’s pH and temperature,and the presence of various competing anions was also investigated.The Mg -Al -CO 3-LDH proved to be an ef?cient adsorbent for the removal of trace levels of As and Se from aqueous solutions,even in the presence of common competing ions,such as CO 32-and HPO 42-.
During the batch adsorption runs with the as-prepared LDH adsorbents,the solution pH was shown to vary signi?cantly,with some dissolution of Al and Mg from the LDH structure
also detected.Though these metals are not currently regulated,they are bound to be a source of concern for the eventual use of these materials in drinking water applications.A conditioning procedure for these adsorbents was subsequently developed,which was shown 12to both reduce the Al and Mg dissolution and to temper the change in solution pH.
The adsorption kinetics and isotherms on a number of conditioned LDH,each with a different average particle size,were studied in batch experimental runs.12The data were ?tted using both a conventional homogeneous surface diffusion model,as well as a bidisperse pore model which views the LDH particles as assemblages of microparticles and takes into account both the bulk diffusion in the interparticle pore space,and the surface diffusion within the microparticles themselves.When the homogeneous surface diffusion model was used to describe the experimental data,the estimated effective particle diffusiv-ities were shown to increase with increasing particle size.On the other hand,the bidisperse pore model predicted that the diffusivities for the microparticles are fairly insensitive to their size.
The ability of LDH to remove As (and Se)has been con?rmed by the batch experiments,but the performance of practical packed-bed sorption column using such LDH materials needs further investigation,as there are currently no data and design methodologies available for the ef?cient optimal design of such systems.This is also generally true for the design of all large-scale adsorption systems used for environmental remediation which,typically,requires copious quantities of preliminary design information,usually gathered in an extensive series of pilot-plant experiments that are time-consuming and expensive.The methodology proposed here aims to alleviate some of the design burden.It involves conducting small-scale batch experi-mental runs aimed at studying the kinetics as well as the equilibrium sorption characteristics of various proposed adsor-bents,including the effect of such parameters as the feed concentration,pH,and temperature.The batch experiments are combined with a mathematical model that properly describes the mass transfer and adsorption processes within the adsorbent in packed-bed sorption columns,in order to predict full-scale
*To whom correspondence should be addressed.E-mail:tsotsis@https://www.360docs.net/doc/c7207740.html,.Phone:2137402069.Fax:2137408053.?
Current address:Media &Process Technology,Inc.,155William Pitt Way,Pittsburgh,PA 15238.
Ind.Eng.Chem.Res.2009,48,2076–2084
207610.1021/ie800878n CCC:$40.75 2009American Chemical Society
Published on Web 01/15/2009
column performance and dynamics,and to study the effect of various design and operating parameters.With the aid of such a design model the optimal conditions for the column operation can be predicted without the need for extensive pilot-plant experiments that are time-consuming and expensive.
The principal objectives of this study are,therefore,(i)to validate the proposed design methodology and(ii)to use the models developed to identify and quantify the impact of important solution and operating parameters,such as the in?uent As concentration,pH,sorbent particle size,and?ow rate during arsenic removal in?xed beds.In what follows,we?rst describe the design models developed;we then describe the experimental data that have been generated and use them to validate the design models.The models are then utilized to describe the column dynamics as a function of the various parameters that character-ize column behavior.
2.Model Development
Any model describing arsenic uptake in?ow columns must take into account the phenomena that occur at the column level, as well as the transport and sorption phenomena within the adsorbent particles themselves.We assume that axial dispersion and channeling effects in the column are negligible,and since the arsenic in?uent concentration is typically low,solution?ow velocity is taken to be constant.Therefore,the mass balance for arsenic in the?owing liquid phase in a packed column con?guration is given by
u ?C
?z
+
?C
?t
+
1-ε
ε
?q c
?t
·F)0(1)
where u is the average axial velocity of the?owing?uid(cm/ min),described by the following equation
u)
Q
πD2ε/4
(2)
where Q0is the volumetric?ow rate fed to the column,D is the inside column diameter,andεis the column void fraction (assumed to be the same with the void fraction in the cross-sectional area).In eq1,C is the solute(As(V))concentration in the?uid phase(μg/L),t is the time(min),z is the axial coordinate(cm),q c is the average concentration of solute in the solid phase(μg/g),and F is the adsorbent particle density(g/ cm3).
The initial(IC)and boundary(BC)conditions,consistent with the laboratory experiments,are
C)0,q c)0,t)0(3)
C)C
,z)0(4) Equations1-4must be coupled with the equation that describes solute transport at the liquid-solid interface.Equating the solute uptake term[F(?q c/?t)in eq1]to the?ux from the bulk phase to the particle surface through the liquid boundary layer surrounding the spherical adsorbent particle yields the desired equation:
F ?q c
?t
)
3k
f
R
M
(C-C
MS
)(5)
where k f is the external mass transfer coef?cient,C MS is the liquid-phase solute concentration at the solid-liquid interface (μg/L),and R M is the particle radius(cm).
The value of the external mass transfer coef?cient(k f)depends on the?ow conditions in the column.In this study,k f is determined by an empirical correlation obtained by Williamson et al.,13namely,
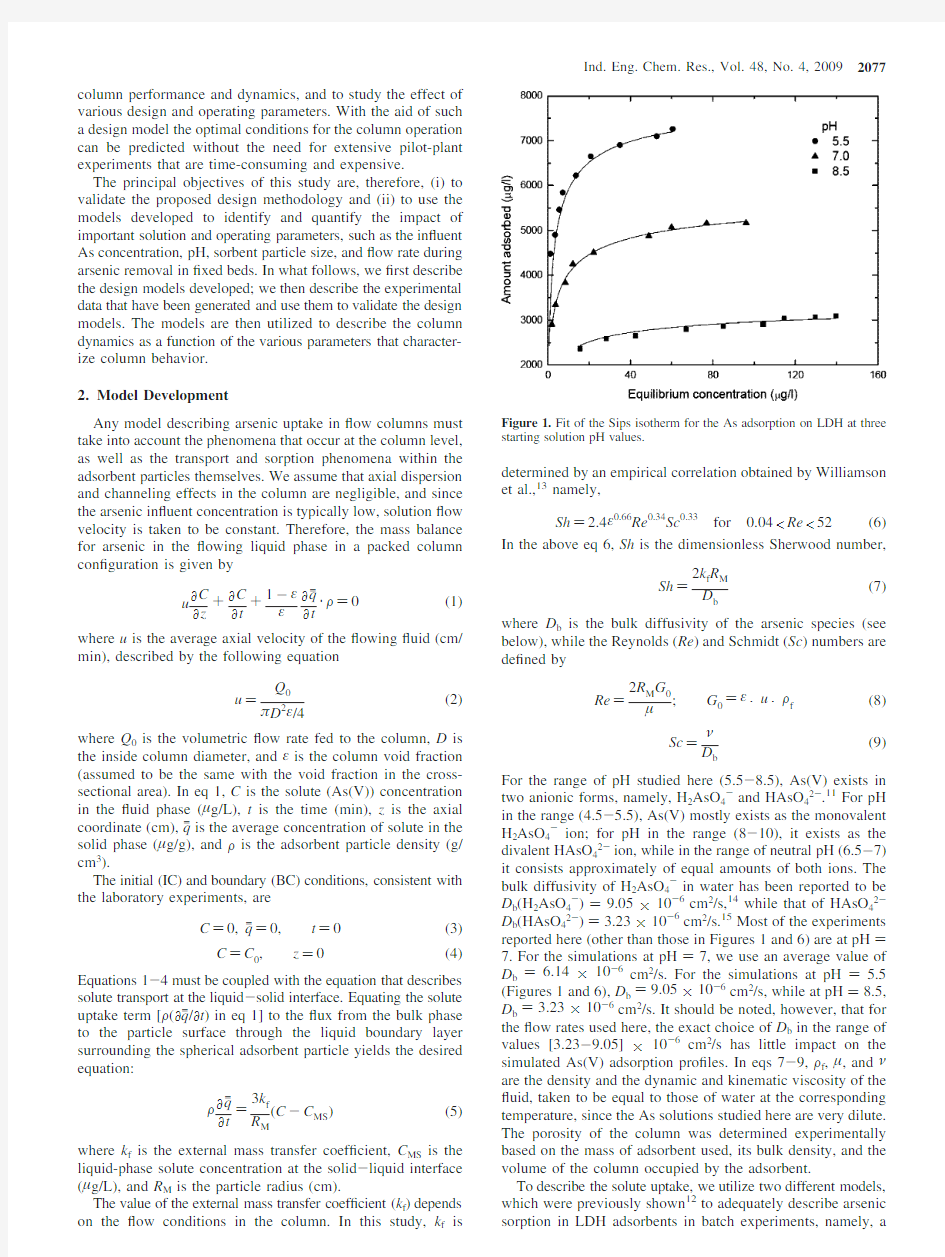
Sh)2.4ε0.66Re0.34Sc0.33for0.04 Sh) 2k f R M D b (7) where D b is the bulk diffusivity of the arsenic species(see below),while the Reynolds(Re)and Schmidt(Sc)numbers are de?ned by Re) 2R M G μ ;G )ε×u×F f (8) Sc) ν D b (9) For the range of pH studied here(5.5-8.5),As(V)exists in two anionic forms,namely,H2AsO4-and HAsO42-.11For pH in the range(4.5-5.5),As(V)mostly exists as the monovalent H2AsO4-ion;for pH in the range(8-10),it exists as the divalent HAsO42-ion,while in the range of neutral pH(6.5-7) it consists approximately of equal amounts of both ions.The bulk diffusivity of H2AsO4-in water has been reported to be D b(H2AsO4-))9.05×10-6cm2/s,14while that of HAsO42-D b(HAsO42-))3.23×10-6cm2/s.15Most of the experiments reported here(other than those in Figures1and6)are at pH) 7.For the simulations at pH)7,we use an average value of D b)6.14×10-6cm2/s.For the simulations at pH)5.5 (Figures1and6),D b)9.05×10-6cm2/s,while at pH)8.5, D b)3.23×10-6cm2/s.It should be noted,however,that for the?ow rates used here,the exact choice of D b in the range of values[3.23-9.05]×10-6cm2/s has little impact on the simulated As(V)adsorption pro?les.In eqs7-9,F f,μ,andνare the density and the dynamic and kinematic viscosity of the ?uid,taken to be equal to those of water at the corresponding temperature,since the As solutions studied here are very dilute. The porosity of the column was determined experimentally based on the mass of adsorbent used,its bulk density,and the volume of the column occupied by the adsorbent. To describe the solute uptake,we utilize two different models, which were previously shown12to adequately describe arsenic sorption in LDH adsorbents in batch experiments,namely, a Figure1.Fit of the Sips isotherm for the As adsorption on LDH at three starting solution pH values. Ind.Eng.Chem.Res.,Vol.48,No.4,20092077 homogeneous surface diffusion model(HDSM)and a bidisperse pore model(BPM).We brie?y describe below the two models (more detailed discussions are given in our original publica-tion12). 2.1.Homogenous Surface Diffusion Model.The HSDM is based on the assumption that the transport of species is determined by external mass transfer in the liquid phase and by intraparticle diffusion resistance in the form of surface diffusion within the adsorbent particle.Assuming homogeneous spherical adsorbent particles,a concentration-independent dif-fusivity(this assumption was relaxed in ref12without offering any particular advantage in model?t),the intraparticle transport and solute uptake is described by the following equation,12 ?q ?t ) D i r M 2 ? ?r M(r M2 ?q ?r M)(10) where q is the solute loading in the sorbent phase(μg/g),D i is the intraparticle diffusion coef?cient(cm2/s),and r M is the radial distance(cm)measured from the center.The boundary and initial conditions(BC and IC)are the following: q)0,t)0(11) ?q ?r M )0,r M )0(12) F D i ?q ?r M |r M)R M)k f(C-C MS),r M)R M(13) At the external surface of the particle(r M)R M),instantaneous equilibrium is assumed between the metal concentrations in the liquid and solid phases,which are coupled using the Sips isotherm,as obtained from the adsorption experiments(see further discussion below). q) Kq s C MS n 1+KC MS n ,r M )R M (14) The average solute concentration in the adsorbent particle is de?ned as: q c) 3 R M 3∫0 R M qr M 2d r M (15) 2.2.Bidisperse Pore Model.The BPM assumes that the adsorbent particle is an agglomerate of a number of equal size microparticles.A porous intercrystalline region forms in between the microparticles.Before the metal anion adsorbs inside the microparticle,it must be transported from the particle surface through the intercrystalline region to the surface of the micro-particles.In the intercrystalline porous region,transport is described by the following equation,12,16 εM ?C M ?t +(1-ε M )F s ?q j μ ?t )ε M D M r M 2 ? ?r M[r M2 ?C M ?r M](16) The BC and IC are the following: C M )0,t)0(17) ?C M ?r M )0,r M )0(18) εM D M ?C M ?r M |r M )R M )k f (C-C MS ),r M )R M (19) where C M is the solute concentration in the intercrystalline porous region(μg/L),q jμis the volume-averaged solute con-centration in the solid phase(microparticles)(μg/g)s see eq25below s R M is the particle external radius(cm).D M is the intercrystalline porous region diffusivity(cm2/g),given by(εM/Γ)D b withεM being the void fraction in the porous region,and Γ,the tortuosity. Transport and adsorption in the microparticles are described by ?q μ ?t ) D μ r μ 2 ? ?r μ(rμ2 ?q μ ?r μ)(20) with the BC and IC being q μ )0,t)0(21) ?q μ ?r μ )0,r μ )0(22) q μ ) Kq S C M n(r M ,t) 1+KC M n(r M ,t) ,r μ )R μ (23) where qμis the solute concentration(μg/g)in the microparticle, Dμis the microparticle diffusivity(cm2/s),and Rμis the microparticle radius(cm).Equation23is the Sips isotherm, which has been shown experimentally to describe arsenic sorption in the LDH adsorbent(see below).The average concentration throughout the adsorbent particle is de?ned as q c) 3 F R M 3∫0 R M r M 2[(1-ε M )F s q j μ +ε M C M ]d r M (24) where qμis given by q j μ ) 3 R μ 3∫0 Rμ q μ r μ 2d r μ (25) Equations1-25above are made dimensionless by de?ning the following dimensionless variables and groups: η) r M R M ) r μ R μ V j) V V C j M ) C M C C j MS ) C MS C C j) C C Q) q q s Q c) q c q s Q μ ) q μ q s Q j μ ) q j μ q s t R ) V Q τ) t t R and setting D j i ) D i t R R M 2 D j M ) D M t R R M 2 D j μ ) D μ t R R μ 2 Λ)KC0n ) R M t R k f ω) F q s C ωj) F s q s C ψ) D i R M k f ) 2 Sh′ ψj) D M R M k f ) 2 Sh′′Sh′) 2R M k f D i Sh′′) 2R M k f D M The column equations above are converted to the following dimensionless form, ?C j ?V j +ε ?C j ?τ +(1-ε)ω ?Q c ?τ )0(26) with the BC and IC being C j)0,Q c)0,τ)0(27) 2078Ind.Eng.Chem.Res.,Vol.48,No.4,2009 C j)1,V j)0(28) ω?Q c ?τ )3(C j-C j MS )(29) The HSDM equations reduce to ?Q ?τ) D j i η2 ? ?η (η2?Q ?η )(30) with the IC and BC being Q)0,τ)0(31) ?Q ?η )0,η)0(32) ωψ?Q ? |η)1)(C j-C j MS),η)1(33) Q) ΛC j MS n 1+ΛC j MS n ,η)1(34) Q c)3 ∫ 1 Qη2dη(35) In the intercrystalline region,the BPM equations are reduced to ?C j M ?τ+ (1-ε M ) εM ωj ?Q j μ ?τ ) D j M η2 ? ?η[η2?C j M ?η](36) with the BC and IC given by C j M )0,Q j μ )0,τ)0(37) ?C j M ?η )0,η)0(38) ψjεM ?C j M ?η|η)1)(C j-C j MS),η)1(39) whereas in the microparticles we have ?Q μ?τ) D j μ 2 ? ? ( 2?Qμ? )(40) with BC and IC being Q μ )0,τ)0(41) ?Q μ ?η )0, )0(42) Q μ) ΛC j M n(η,τ) 1+ΛC j M n(η,τ) , )1(43) Q j μ )3∫01Qμ 2d (44) Q c)3 F∫01 [(1-εM)F s Q jμ+εM C0q s C j M]η2dη(45) To solve the above dimensionless equations,a FORTRAN program was developed,by which the governing equations for the?uid and solid phases were solved by using a?nite-difference technique,which gives rise to tridiagonal matrices. For the HSDM model eqs26-35were solved simultaneously, with Q c,the average dimensionless solute concentration in the adsorbent particle,calculated using eq35.For the BPM model, eqs26-29were simultaneously solved with eqs36-45,with Q c calculated using both Q jμ,the average dimensionless solute concentration in the microparticles,and C j M,the dimensionless solute concentration in the macropores. 3.Experimental Details 3.1.Materials.The sorbent material used in the experiments is a Mg-Al-CO3-LDH with a Mg/Al mole ratio of2.87.It was prepared by the coprecipitation method,proposed by Roelofs et al.17A140mL portion of a solution containing0.7 mol NaOH and0.18mol Na2CO3was added all at once to a second solution containing0.115mol of Mg(NO3)2·6H2O(90 mL)and0.04mol of Al(NO3)3·9H2O(90mL)(corresponding to a Mg/Al ratio of2.87)under vigorous stirring.The thick gel obtained was aged for24h at333K,followed by?ltration and washing with distilled water and was then dried at393K.ICP-MS(inductively coupled plasma mass spectrometry)analysis of the resulting LDH material indicated that its Mg/Al mole ratio is~2.9,which is very close to the Mg/Al ratio of the starting salts. The calcined Mg-Al-LDH was obtained by heating the original LDH in a muf?e furnace at773K for4h in an air atmosphere with heating and cooling rates of2K/min.The calcined LDH was conditioned by shaking it in deionized water for24h(changing the deionized water every6h),and then drying it an air at353K for12h.To obtain different particle size fractions,the conditioned LDH was crushed and sieved using standard testing sieves(VWR).The size fraction collected at50-80mesh(180-300μm)was used for most of the packed-bed adsorption experiments reported in this paper(other than the experiment for the particle size effect).In our prior paper, we investigated sorption kinetics and isotherms for a range of particle sizes.12 As(V)was the metal investigated in the study.The As(V) solutions used for the adsorption experiments were prepared by dilution from a1000ppm ICP standard solution(As(V)in 2%HNO3purchased from Exaxol)using deionized water.For the adsorption experiments,the pH of the working solution was adjusted to the desired initial value using1M NaOH solution. The pH was not adjusted during the adsorption period,but any pH drift was measured and recorded.The As(V)concentration was determined by the ICP-MS. 3.2.Column Experiments.Fixed-bed experiments were conducted in laboratory glass columns with internal diameter, D)0.7cm,and height,H)8.5cm.Two columns with the same diameter and height were employed in the study in order to speed up the experiments(the?ow rate and particle size effect experiments were carried out in one column,while the concentration and pH effect experiments were done in the second column s see discussion to follow).They were packed with conditioned LDH(cotton-wool was placed at the bottom of the column to prevent the LDH particles from eluding from the column during the?ow experiments).They were then rinsed with deionized water for24h to ensure that the LDH particles were densely packed prior to the initiation of the experiments. The feed solution containing arsenic,with a prescribed initial concentration and pH,was then continuously pumped in a down-?ow mode from the reservoir through the columns at various ?ow rates using a peristaltic pump(Alitea model-XV).During the study,the performance of the pump was checked periodically by collecting solution samples at the outlet of the column for predetermined periods of time.Four sample ports(located at heights of2.2, 4.4,6.6,and8.5cm for the?rst column and at heights of1.9,4.0,6.0,and8.5cm for the second column) enabled withdrawing samples from the column for the analysis using3cm3sample syringes.Samples were taken at regular Ind.Eng.Chem.Res.,Vol.48,No.4,20092079 time intervals,until the metal ion concentration in the ef?uent stream at the bottom of the bed became equal to the feed concentration.The metal ion concentrations were determined using ICP-MS.The pH of the in?uent solution was adjusted (in the reservoir)using0.1M NaOH solutions.No pH adjustment was done in the column,but the pH of the ef?uent from the column was measured and recorded with an Accumet Basic AB15pH meter.There was not much change in the pH of the solution in the column,with the ef?uent pH being typically0.1-0.2pH units higher than the pH of the feed.All column experiments were carried out at25°C.Temperature control was carried out by controlling the temperature of the feed solution,and by wrapping the column with a?exible coil through which water at25°C was circulated. 4.Results and Discussion 4.1.Determination of Adsorption Isotherms and Kinetics. We reported in our previous paper12that the adsorption isotherm on conditioned LDH is well expressed by the Sips equation q) K q s C n 1+KC n (46) where q s and K are the maximum sorption capacity and the Sips constant respectively,and n is the parameter characterizing the system heterogeneity.Furthermore,the adsorption parameters are not dependent on the adsorbent particle size.The adsorption isotherms for As(V)on the conditioned LDH for three different initial pH,namely,5.5,7.0,and8.5,are shown in Figure1. The various isotherm parameters(and the“goodness of?t”,as manifested by the R2test)that are used in the simulations below are shown in https://www.360docs.net/doc/c7207740.html,paring the adsorption capacity at these three different pH values shows that lower pH signi?cantly enhances arsenic removal,which may be explained as follows. Since the point of zero charge(pH pzc)for the LDH was reported to be in the range6.8-8.9,18,19the surface of LDH is negatively charged when pH>pH pzc.Therefore,in the higher pH range, the arsenate anionic species will be repelled by the LDH surface. For pH Adsorption kinetics for As(V)were studied in well-stirred batch reactors and presented in our previous publication.12The data are reanalyzed here using both the HSDM and BPM.For the BPM we assumed previously that D M is equal to D b (visualizing the intercrystalline pore region as consisting of straight nonintersecting pores).Here,we relax this assumption and?t the data to estimate D M.Assuming that D M)(εM/Γ)D b, allows one to calculate a pore structure tortuosity.Table2shows the parameters for both models calculated by?tting the experimental data.Note that the BPM provides estimates of Dμ/ Rμ2and D M that depend only weakly on the particle size.As also noted in our previous paper,12that is not the case with the HSDM.Accounting for a concentration-dependent surface diffusivity,or for the particle size distribution of the adsorbent, does not change this conclusion much,with the surface diffusivity still remaining a strong function of the particle size. 4.2.Column Experiments.In practice,the performance of a packed-bed column is evaluated in terms of monitoring the ef?uents concentration(and comparing it with the feed con-centration)as a function of the number of the bed volumes(BV) treated.The number of bed volumes(BV)is de?ned as the volume of metal-laden waste stream treated divided by the volume of the adsorbent bed.20 BV) volume of solution treated volume of packed bed ) Q t V )τ(47) From the above de?nition,BV turns out to be equivalent to the dimensionless timeτ,as de?ned previously.Column“break-through”occurs when the ef?uent concentration from the column is about5%of the in?uent concentration.To utilize the models for?tting the experimental data,we use the parameters in Tables1and2and the external mass transfer coef?cient(k f),calculated by eqs6-9,as described in the previous section. 4.2.1.Breakthrough Studies.Figure2shows the dimen-sionless ef?uent concentration C j)C/C0vs dimensionless time τ(BV)at four different dimensionless bed heights(V j)V/V0), of0.22,0.47,0.7,and1.0,and the HSDM and BPM predictions. The time at which the concentration reaches its breakthrough value,C j)0.05,depends on the bed height,of course,in addition to all other column parameters;see the discussion to follow.In Figure2,for example,the breakthrough timeτbr at a dimensionless bed height of0.22is1996,while it is23378at the exit of the column.The adsorption curves in Figure2show the constant pattern behavior,typical of favorable isotherms obtained in our earlier study,11,12with a sharp initial break-through followed by a slow approach to equilibrium.An overshoot is observed in the column experiments reported in Figure2,with the ef?uent concentration at intermediate bed heights being slightly higher than one(as high as1.15),while the exit concentration has yet to reach saturation(C j e1.0). The same behavior is observed with the experiments carried out with the pH of 5.5.The experiments at the higher pH of 8.5did not exhibit any overshoots,on the other hand.The same overshooting behavior was also reported by Chen and Wang,20 while removing Cu and Pb using activated carbon.Chen and Wang theorize that the overshoot indicates that as the adsorption (mass transfer)zone moves down along the column length,some desorption must be occurring in the upper saturated parts of the column.Neither the HSDM nor the BPM,as presented here, account for such desorption phenomena,and are not,therefore, capable of predicting the overshoots in concentration.This de?ciency aside,however,both models do perform well in describing the experimental data at the exit as well as all the internal positions in the column;see also the discussion to follow. 4.2.2.Effect of Changing of the Column Parameters. 4.2.2.1.Effect of Flow Rate.The effect of?ow rate on the column behavior was studied using a200ppb As(V)solution (pH7.0),pumped at various?ow rates,(6-20mL/min)through the column.This range of?ow rates correspond to a range of column residence times,t R,of0.54to0.16min.Figure3shows the concentration at the exit of the column as a function of t (minutes),for four?ow rates(6,8,10,and20mL/min).Shown on the same?gure are the HDSM and BPM predictions.Again, the models perform generally well in predicting the overall column behavior,without any need for adjustable parameters other than the measured values in Tables1and2,and the mass transfer coef?cient calculated by eqs6-9.From the?gure we can calculate the corresponding breakthrough times,t br,for Table1.Sorption Isotherm Parameters for As(V)Uptake on Conditioned LDH Sips isotherm pH q s(μg/g)K(L/g)n R2 5.58045.370.6620.510.94 7.06130.280.6550.450.96 8.53619.900.6490.580.91 2080Ind.Eng.Chem.Res.,Vol.48,No.4,2009 different ?ow rates.As expected,the breakthrough times are a strong function of the column residence times,t R .For a ?ow rate of 6mL/min (t R )0.54min),for example,t br )13205(the HSDM model predicts t br )14505,while the BPM yields t br )15624),while for a ?ow rate of 20mL/min (t R )0.16min),t br )2678(for the HSDM,t br )2206,and the BPM yields t br )3010).On the other hand,the breakthrough time τbr varies from 24233(for the HSDM,τbr )26619,while for the BPM,τbr )28672)for 6mL/min to 16381(for the HSDM,τbr )13494,and for the BPM τbr )18412)for 20mL/min.This decrease in the breakthrough time with increasing ?ow rate is expected,of course,and was also reported by previous investigators.Ko,Porter,and Mckay,21for example,reported a decrease in the uptake with increasing ?ow rate for the sorption of cadmium and copper ions on bone-char in ?xed-beds,and Kundu 22also reported similar results while treating As(V)with iron oxide-coated cement. 4.2.2.2.Effect of the In?uent As Concentration.The in?uent As ion concentration has a signi?cant effect on the column behavior.To investigate this effect,sorption experiments were conducted in a ?xed-bed column (Table 3)with a varying in?uent arsenic concentration (100,200,and 300ppb)at a ?xed pH )7,and a feed ?ow rate of 8mL/min (t R )0.41min).Figure 4shows the concentration at the exit of the column as a function of τfor three in?uent concentrations.Again,the models perform well in predicting the column behavior without the need for any adjustable parameters.For feed concentrations of 300,200,and 100ppb the corresponding experimental τbr values are 15546,23378,and 49056.As expected,the breakthrough times are a strong function of the in?uent concentrations.Similar ?ndings were also reported by other researchers.23,24In terms of the models’ability to predict the breakthrough times,τbr ,the values predicted by the HSDM are 13641,23418,and 47373,while those by the BPM model are 17005,25143,and 47439. 4.2.2.3.Effect of Adsorbent Particle Size.Though most experiments reported here were carried with an adsorbent sample with a particle size distribution in the range 180-300μm,in order to investigate the effect of particle size on the column behavior we also carried out column experiments with a sample with a particle size distribution in the range 90-180μm.Previously,we showed that particle size has a signi?cant effect Table 2.Measured Densities and Porosities and Fitted D μ/R μ2,Γ,and D M Values Using the BPM and Fitted D i Values Using the HSDM for Conditioned LDH with Various Particle Sizes BPM HSDM particle size (μm) solid density (F s )(g/cm 3) particle porosity (εM ) tortuosity factor (Γ) D M (×106cm 2/s) D μ/R μ2(×1061/s) D i (×1011cm 2/s) 53-75 1.9640.38 3.27 1.05 1.35 1.64275-90 1.9830.35 4.190.75 1.14 1.64690-180 1.9750.30 5.000.54 1.00 3.906180-300 1.986 0.31 5.52 0.51 0.86 10.031 Figure 2.Adsorption of 200ppb arsenic solution (pH )7.0),fed at a ?ow rate of 8mL/min by LDH with particle size (180-300μm)at several dimensionless bed depths (V j )in an 8.5cm column.Figure 3.Effect of feed ?ow rate on the breakthrough curves and the corresponding simulation ?ttings at a bed depth of 8.5cm (V j )1.0).Table 3.Properties of the Fixed-Bed Column parameters value height,H (cm)8.5diameter,D (cm)0.7bed porosity,ε 90-180(μm) 0.28180-300(μm) 0.27 Figure 4.Effect of the in?uent arsenic concentration on the breakthrough curves and the corresponding simulation ?ttings at a bed depth of 8.5cm (V j )1.0).Ind.Eng.Chem.Res.,Vol.48,No.4,20092081 on the adsorption kinetics and that the BPM seems to provide a more realistic explanation of this effect (see Table 2).Figure 5compares column behavior (in terms of the concentration at the exit of the column as a function of τ)for the two samples with a different particle size distribution (other conditions for the experiments are in?uent concentration C 0(As))200ppb,pH )7.0,and a ?ow rate of 8mL/min).The results in Figure 5indicate the strong in?uence that the particle size has on the column behavior.The experimental breakthrough time for the adsorbent with the smaller particle size (τbr )36305)is greater than the breakthrough time of that with the larger particle size (τbr )21812).The increased breakthrough time have also been reported by other investigators and were explained by the faster adsorption kinetics exhibited by the smaller particles.25,26 When plotting (using either the HSDM or the BPM),for example,the As(V)concentration pro?les inside the particles (at a large enough τfor the column to have reached saturation)at different column positions,one notes that though for both types of adsorbents the concentration at the particle surface is very close to the equilibrium concentration value (corresponding to the bulk concentration at the position in the column),the same is not true for the interior of the particle.There,for the smaller particles,the As(V)concentration is signi?cantly higher than that for the larger particles.The differences in the concentration pro?les are smaller at the inlet of the column and larger at the end of the column,as expected.When plotting the adsorbed As(V)concentration (per unit volume of column)pro?les along the length of the column,again at saturation one again notes that the column containing the smaller particles has adsorbed more As(V).However,for large size practical columns smaller particles may result in higher ?ow resistances through the column.27 Both the HSDM and the BPM provide a reasonable ?t of the data.They also predict the differences in τbr .The HSDM predicts τbr values of 37786and 23416,while the BPM predicts τbr values of 39072and 25648. 4.2.2.4.Effect of pH.Finally,to examine the effect that the pH has on the column behavior,experiments were carried out in which the feed pH was varied in the range 5.5-8.5.Figure 6compares column behavior for three different pH values (5.5,7.0,and 8.5),for the adsorbent with particle size in the range 180-300μm,and with an initial arsenic concentration of 200ppb.Lower pH generally result in better column behavior,as was expected based on the isotherm studies reported in Figure 1.The experimental τbr are 36521(pH )5.5),23378(pH )7),and 12308(pH )8.0).The corresponding τbr values predicted by the HSDM are 39308,23418,and 14174while the values predicted by the BPM are 42717,26052,and 17004.5.Conclusions The focus of this study was on studying arsenic adsorption in ?ow column experiments.The variables examined in this study included in?uent As concentration,pH,sorbent particle size characteristics,and ?ow rate.Adsorption behavior was characterized in terms of arsenic concentration at the exit of the column (as well as at various lengths along the column)as a function of the number of bed volumes treated by the column.A key column characteristic is the number of bed volumes at breakthrough,de?ned as the time when the ef?uent concentration is equal to 5%of the in?uent concen-tration.The experimental results show that the breakthrough time increases upon decreasing the sorbent particle size,the in?uent stream?ow rate,and column feed concentration.It was also observed that,as the in?uent pH was decreased,the column performance improved signi?cantly and the breakthrough times increased. Two adsorption models (HSDM and BPM),previously developed to ?t the batch experimental data 12were applied to the column experiments.Both models predicted the qualitative trends quite https://www.360docs.net/doc/c7207740.html,ing the root-mean-square deviation (rmsd)(also known as the root-mean-square error (RMSE))method to compare the two models (with respect to their ability to ?t the experimental data)proved inconclusive,as in some cases HSDM provides a better ?t (lower rmsd),while in other cases the BPM was superior.Acknowledgment This research was supported by the California Institute of Energy Ef?ciency and the U.S.Department of Energy.Nomenclature C )bulk liquid-phase concentration (μg/L)C 0)initial liquid-phase concentration (μg/L) C j )dimensionless bulk liquid-phase concentration C M )solute concentration in the intercrystalline pore space (μ g/L) Figure 5.Effect of the adsorbent particle size on the breakthrough curves and the corresponding simulation ?ttings at a bed depth of 8.5cm (V j ) 1.0). Figure 6.Effect of the in?uent stream pH on the breakthrough curves and the corresponding simulation ?ttings at a bed depth of 8.5cm (V j )1.0).2082Ind.Eng.Chem.Res.,Vol.48,No.4,2009 C j M)dimensionless solute concentration in the intercrystalline pore space C MS)liquid-phase concentration at the solid-liquid interface(μg/ L) C j MS)dimensionless liquid-phase concentration at the solid-liquid interface D)diameter of the column(cm) D b)bulk diffusivity(cm2/s) D i)intraparticle diffusion coeffecient(cm2/s) D j i)dimensionless intraparticle diffusion coeffecient D M)mesopore diffusivity(cm2/s) D j M)dimensionless mesopore diffusivity Dμ)micropore diffusivity(cm2/s) D jμ)dimensionless micropore diffusivity G0)super?cial mass velocity of?ow through the bed(g/cm2·min) H)height of the column(cm) K)Sips constant k f)external mass transfer coef?cient(cm/s) n)exponential factor in the Sips-type isotherm q)solute concentration in the adsorbent particle(μg/g) q s)maximum sorption capacity(μg/g) Q)dimensionless solute concentration in the adsorbent particle qμ)solute concentration in the microsphere(μg/g) Qμ)dimensionless solute concentration in the microsphere q jμ)volume-averaged adsorbate concentration in the microsphere (μg/g) Q jμ)dimensionless volume-averaged adsorbate concentration in the microsphere q c)volume-averaged adsorbate concentration in the whole particle (μg/g) Q c)dimensionless volume-averaged adsorbate concentration in the whole particle Q0)volumetric?ow rate fed to the column(mL/min) Re)Reynolds number r M)radial distance in particle(cm) R M)particle radius(cm) rμ)radial distance in microparticle(cm) Rμ)microparticle radius(cm) Sh)Sherwood number Sh′)Sherwood number Sh′′)Sherwood number Sc)Schmidt number t)time(min) t R)residence time in the column(min) u)average axial velocity of the?owing?uid in the interstitial spaces(cm/min) V)column volume coordinate(cm3) V0)total column volume(cm3) V j)dimensionless column volume coordinate z)axial distance coordinate(cm) Greek Symbols ε)void fraction in the bed εM)void fraction of the porous region in the particle Γ)tortuosity factor of the porous region in the particle F)particle density(g/cm3) F f )density of water(g/cm3) F s )solid density(g/cm3) μ)dynamic viscosity of water(g/cm·s)ν)kinematic viscosity of water(cm2/s)η)dimensionless particle radius )dimensionless microparticle radius τ)dimensionless time Λ)dimensionless Sips constant )dimensionless mass transfer coef?cient ω)dimensionless solute concentration in the adsorbent particle ωj)dimensionless solute concentration in the microspheres ψ)dimensionless group ψj)dimensionless group Literature Cited (1)Basu,A.;Mahata,J.;Gupta,S.;Giri,A.K.Genetic Toxicology of a Paradoxal Human Carcinogen,Arsenic:A Review.Mutat.Res.2001,488, 171. (2)Manning,B.A.;Goldberg,S.Adsorption and Stability of Arsenic(III) at the Clay Mineral-Water Interface.En V iron.Sci.Technol.1997,31,2005. (3)Bowell,R.J.Sorption of Arsenic by Natural Iron Oxides and Oxyhydroxides in Soil.Appl.Geochem.1994,9,279. (4)Clifford,D.;Lin,C.C.Ion exchange,Activated Alumina and Membrane Process for Arsenic Removal from Groundwater In Proceedings of the45th Annual Engineering Conference,University of Kansas, Lawrence,KS,Feb,1995. (5)Trotz,M.A.Porous Alumina Packed Bed Reactors:A Treatment Technology for Arsenic Removal.Ph.D.Dissertation,Stanford University, Stanford,CA,2002. (6)Lin,T.F.;Wu,J.K.Adsorption of Arsenite and Arsenate within Activated Alumina Grains:Equilibrium and Kinetics.Water Res.2001,35 (8),2049. (7)Kim,Y.H.;Kim,C.M.;Choi,I.;Rengaraj,S.;Yi,J.H.Arsenic Removal Using Mesoporous Alumina Prepared via a Templating Methodol. En V iron.Sci.Technol.2004,38,924. (8)Hayes,K.F.;Roe,A.L.;Brown,G.E.;Hodgson,K.O.;Leckie, J.O.;Parks,G.A.In Situ X-ray Absorption Study of Surface Complexes: Selenium Oxyanions on a-FeOOH.Science.1987,238,783. (9)Raven,K.P.;Jain, A.;Loepert,R.H.Arsenite and Arsenate Adsorption on Ferrihydrite:Kinetic,Equilibrium and Adsorption Envelopes. En V iron.Sci.Technol.1998,32,344. (10)Cavani,F.;Tri?ro,F.;Vaccari,A.Hydrotalcite-Type Anionic Clays: Preparation,Properties and Applications.Catal Today.1991,11,173. (11)Yang,L.;Shahrivari,Z.;Liu,P.K.T.;Sahimi,M.;Tsotsis,T.T. Removal of Trace Levels of Arsenic and Selenium from Aqueous Solutions by Calcined and Uncalcined Layered Double Hydroxides(LDH).Ind.Eng. Chem.Eng.2005,44(17),6804–6815. (12)Yang,L.;Dadwhal,M.;Shahrivari,Z.;Ostwal,M.;Liu,P.K.T.; Sahimi,M.;Tsotsis,T.T.Adsorption of Arsenic on Layered Double Hydroxides:Effect of the Particle Size.Ind.Eng.Chem.Eng.2006,45 (13),4742–4751. (13)Williamson,J.E.;Bazaire,K.E.;Geankoplis,C.J.Liquid-Phase Mass Transfer at Low Reynolds Numbers.Ind.Eng.Chem.Fund.1963,2 (2),126–129. (14)Lide,D.R.Hanbook of Chemistry and Physics,77th ed.;CRC Press:Boca Raton,FL,1996-1997;pp5-99. (15)Vrijenhoek,E.M.;Waypa,J.J.Arsenic Removal from Drinking Water by a“loose”nano?ltration membrane.Desalination.2000,130(3), 265–277. (16)Cen,P.L.;Yang,R.T.Analytical Solution for Adsorber Break-through Curves with Bidisperse Sorbents(Zeolites).AICHE.1986,32(10), 1635–1641. (17)Roelofs,J.C.A.A.;Van Bokhoven,J.A.;Van Dillen,A.J.;Geus, J.W.;De Jong,K.P.The Thermal Decomposition of Mg-Al Hydrotalcites: Effects of Interlayer Anions and Characteristics of the Final Structure. Chem.s Eur.J.2002,8(24),5571–5579. (18)Das,D.P.;Das,J.;Parida,K.Physicochemical Characterization and Adsorption Behavior of Calcined Zn/Al Hydrotalcite-like Compound (HTLs)Towards Removal of Fluoride from Aqueous Solution.J.Colloid Interface Sci.2003,261(2),213–220. (19)Manju,G.N.;Gigi,M.C.;Anirudhan,T.S.Hydrotalcite as Adsorbent for the Removal of Chromium(VI)from Aqueous Media: Equilibrium Studies.Ind.J.Chem.Technol.1999,6(3),134–141. (20)Chen,J.P.;Wang,X.Removing Copper,Zinc,and Lead Ion by Granular Activated Carbon in Pretreated Fixed Bed Columns.Sep.Purif. Technol.2000,19,157–167. (21)Ko,D.C.K.;Porter,J.F.;Mckay,G.Film-pore Diffusion Model for the Fixed-Bed Sorption of Copper and Cadmium Ions onto Bone Char. Wat.Res.2001,35(16),3876–3886. (22)Kundu,S.;Gupta,A.K.Analysis and Modeling of Fixed Bed Column Operations on As(V)Removal by Adsorption onto Iron Oxide-Coated Cement(IOCC).J.Colloid Interface Sci.2005,290(1),52–60. (23)Chen,J.P.;Yoon,J.T.;Yiacoumi,S.Effects of Chemical and Physical Properties of In?uent on Copper Sorption onto Activated Carbon Fixed Bed Columns.Carbon.2003,41,1635–1644. Ind.Eng.Chem.Res.,Vol.48,No.4,20092083 (24)Han,R.;Zhang,J.;Zou,W.;Xiao,H.;Shi,J.;Liu,H.Biosorption of Copper(II)and Lead(II)from Aqueous Solution by Chaff in a Fixed-Bed Column.J.Hazard.Mater.2006,113(1-3),262–268. (25)Vaishya,R.C.;Gupta,S.K.Arsenic(V)Removal by Sulphate Modi?edIron Oxide-Coated Sand(SMIOCS)in a Fixed Bed Column.Water Qual.Res.J.Can.2006,41(2),157–163. (26)Malkoc,E.;Nuhoglu,Y.Removal of Ni(II)ions from aqueous solutions using waste of tea factory:Adsorption on a?xed-bed column. J of Hazard.Mat.2006,B135,328–336. (27)Inglezakis,V.J.;Grigoropoulou,H.Effects of Operating Conditions on the Removal of Heavy Metals by Zeolite in Fixed Bed Reactors.J. Hazard.Mater.B.2004,112,37–43. Recei V ed for re V iew June3,2008 Re V ised manuscript recei V ed October21,2008 Accepted December3,2008 IE800878N 2084Ind.Eng.Chem.Res.,Vol.48,No.4,2009 On the contrary Onthecontrary, I have not yet begun. 正好相反,我还没有开始。 https://www.360docs.net/doc/c7207740.html, Onthecontrary, the instructions have been damaged. 反之,则说明已经损坏。 https://www.360docs.net/doc/c7207740.html, Onthecontrary, I understand all too well. 恰恰相反,我很清楚 https://www.360docs.net/doc/c7207740.html, Onthecontrary, I think this is good. ⑴我反而觉得这是好事。 https://www.360docs.net/doc/c7207740.html, Onthecontrary, I have tons of things to do 正相反,我有一大堆事要做 Provided by jukuu Is likely onthecontrary I in works for you 反倒像是我在为你们工作 https://www.360docs.net/doc/c7207740.html, Onthecontrary, or to buy the first good. 反之还是先买的好。 https://www.360docs.net/doc/c7207740.html, Onthecontrary, it is typically american. 相反,这正是典型的美国风格。 222.35.143.196 Onthecontrary, very exciting. 恰恰相反,非常刺激。 https://www.360docs.net/doc/c7207740.html, But onthecontrary, lazy. 却恰恰相反,懒洋洋的。 https://www.360docs.net/doc/c7207740.html, Onthecontrary, I hate it! 恰恰相反,我不喜欢! https://www.360docs.net/doc/c7207740.html, Onthecontrary, the club gathers every month. 相反,俱乐部每个月都聚会。 https://www.360docs.net/doc/c7207740.html, Onthecontrary, I'm going to work harder. 我反而将更努力工作。 https://www.360docs.net/doc/c7207740.html, Onthecontrary, his demeanor is easy and nonchalant. 相反,他的举止轻松而无动于衷。 https://www.360docs.net/doc/c7207740.html, Too much nutrition onthecontrary can not be absorbed through skin. 太过营养了反而皮肤吸收不了. https://www.360docs.net/doc/c7207740.html, Onthecontrary, I would wish for it no other way. 正相反,我正希望这样 Provided by jukuu Onthecontrary most likely pathological. 反之很有可能是病理性的。 https://www.360docs.net/doc/c7207740.html, Onthecontrary, it will appear clumsy. 反之,就会显得粗笨。 https://www.360docs.net/doc/c7207740.html, 期刊影响因子的“含金量”是多少 这是一个以标准衡量的世界。既然吃饭都有米其林餐厅评级作为参考,更何况严谨的学术科研成果。 期刊影响因子长久以来被学术界视为一个重要的科研水平参考指标。在一本影响因子高的期刊发表论文,科研人员的科研能力和成果也更容易获得认同。然而,部分科学家已对这一指标能否真正反映单篇论文乃至作者学术水平提出质疑,加上每年发布这一指标的汤森路透公司在本月早些时候宣布把相关业务转售给两家投资公司,影响因子未来能否继续维持其「影响力」令人存疑。 广泛影响 根据汤森路透发布的信息,该公司已同意将旗下知识产权与科学业务作价35.5亿美元出售给私募股权公司Onex和霸菱亚洲投资。这一业务包括了世界知名的科技文献检索系统「科学引文索引」(简称SCI)以及定期发布的《期刊引证报告》,其中的期刊影响因子是一本学术期刊影响力的重要参考。 新华社记者就此事咨询了汤森路透,该公司一位发言人说,这一交易预计今年晚些时候完成,在此之前该公司还会继续拥有并运营这项业务,「我们将在不影响这项业务开展和质量的前提下完成交易」。 帝国理工学院教授史蒂芬·柯里接受记者采访时说,他对汤森路透用来计算期刊影响因子所使用的数据是否可靠本来就有一定顾虑,「我不确定汤森路透的这次交易是否产生影响,但这项业务的接盘方如果未来能够保证这方面的透明度也是一件好事」。 影响因子的计算方法通常是以某一刊物在前两年发表的论文在当年被引用的总次数,除以该刊物前两年发表论文的总数,得出该刊物当年的影响因子数值。理论上,一种刊物的影响因子越高,影响力越大,所发表论文传播范围也更广。鉴于全球每个科研领域中都有大量专业期刊,如果有一个可靠的指标能告诉研究人员哪个期刊影响力更大,他们就能更高效地选择在一个高质量平台上发表科研成果。 但这又引申出一个现象,即许多科研机构、高校甚至学术同行越来越依赖影响因子来评判一篇论文甚至作者本身的科研水平,进而影响他们的职称评定和获取科研项目资助等机会。 业内争议 这种过度依赖影响因子的做法引起不少业内争议。来自帝国理工学院、皇家学会等科研机构学者以及《自然》《科学》等期刊出版方的高级编辑,合作撰写了一份报告分析其中弊端,并提出相关改进方案。这篇报告已在近期被分享到一个公开的预印本服务器上供同行审阅。 报告分析了包括《自然》《科学》在内11份学术期刊在2013年至2014年间所刊发论文被引用次数的分布情况,这些数据也您身边的论文好秘书:您的原始资料与构思,我按您的意思整理成优秀论文论著,并安排出版发表,企鹅1550116010自信我会是您人生路上不可或缺的论文好秘书被用来计算2015年相关刊物的影响因子。 报告作者发现,多数论文被引用次数都达不到发表它们的期刊的影响因子数值水平,比如《自然》在这期间所刊发论文中的74.8% 在2015年获得的引用次数就低于这本期刊当年影响因子所显示的水平,《科学》的情况也类似。报告说,这主要是因为这些期刊中有一小部分论文被引用次数非常高,导致影响因子在均值计算过程中出现偏差。 报告详细描述了如何更准确地计算出期刊所刊发论文被引用次数的分布状况,并呼吁各家期刊将这些基础数据公布出来,减少学术界对影响因子的过度依赖。 一般过去式 时间状语:yesterday just now (刚刚) the day before three days ag0 a week ago in 1880 last month last year 1. I was in the classroom yesterday. I was not in the classroom yesterday. Were you in the classroom yesterday. 2. They went to see the film the day before. Did they go to see the film the day before. They did go to see the film the day before. 3. The man beat his wife yesterday. The man didn’t beat his wife yesterday. 4. I was a high student three years ago. 5. She became a teacher in 2009. 6. They began to study english a week ago 7. My mother brought a book from Canada last year. 8.My parents build a house to me four years ago . 9.He was husband ago. She was a cooker last mouth. My father was in the Xinjiang half a year ago. 10.My grandfather was a famer six years ago. 11.He burned in 1991 提高学术期刊影响因子的途径 作者:李勤来源:《今传媒》 美国科技信息研究所所长尤金?加菲尔德首先用论文的被引证频次来测度期刊的影响力,1963年美国科技信息研究所正式提出和使用影响因子这一术语。期刊在某年的影响因子是指该刊前两年发表论文在统计当年被引用的总次数除以该刊前两年发表论文的总数。由影响因子的定义可知,期刊的影响因子反映在一定时期内期刊论文的平均被引率。影响因子的三个基本要素是论文量、时间和被引次数,也就是说,期刊所刊发论文的被引情况决定了该期刊的影响因子。总的说来,一篇论文的被引次数越多,说明它的学术影响力越大,同样也表明它的学术质量较高、创新性较强。因为影响因子高的期刊具有较广泛的读者群和比较高的引用率。影响因子的高低客观地反映了期刊和编辑吸引高质量稿件的能力。所以,我们在评价期刊时,影响因子为重要的评价指标之一。许多作者在投稿时,也将影响因子高的期刊作为投稿首选。图书馆或研究院、资料室在选择订阅期刊或优化馆藏期刊时,也把期刊的影响因子作为重要的参考标准之一。而且影响因子也是筛选中文核心期刊的一项重要指标。因此,作为期刊工作者,努力提高期刊的影响因子十分必要。分析学术期刊的计量指标情况,决定影响因子高低的因素通常有这样几点: 一、影响因子的影响因素 一是论文发表时滞。论文发表时滞(DPA)是指期刊论文的出版日期与编辑部收到该文章的日期之时间差,以月为单位。它是衡量期刊时效性的重要指标,与期刊的影响因子和被引频次有密切关系。因为在计算影响因子时,期刊被引频次中两年的时间限制可导致不同刊物中论文的被引证次数有较大的差异。出版周期短的刊物更容易获得较高的影响因子。因而在同一学科领域的研究论文,特别是研究热点领域内的论文,首先被公开发表的论文更有可能引起较大的影响或者被别人引证。 二是论文学术水平。论文的学术质量直接制约着期刊影响因子的提高。学术质量较高的论文,容易被同行认可,引用率自然就高,影响因子也高。相反,学术质量较差的论文,不会被同行认可,得不到同行研究者的重视,引用率自然就低,影响因子也较低。在各类文章中,具有原创性的学术论文常常被研究人员参考和引用。同时有争议的学术讨论更容易获得同行的广泛关注,而普通的介绍性论文则不太被人们关注。 三是参考文献的数量和质量。由于影响因子是根据期刊的引文计算出来的,通常参考文献的内容越新颖,信息质量越高,影响因子就越高。准确的参考文献有助于作者在有限的篇幅中阐述论文的研究背景及其相关的观点和论据。同时可以方便读者追溯有关的参考资料进一步研究问题。统计分析表明,期刊的影响因子主要取决于论文的平均引文数、引证半衰期及论文的被引证率。所以,参考文献数量较多的论文它的平均引文数量就比较大,而且参考文献越准确,读者查阅参考文献就更方便,读者能分享文献信息资源就越多。 根据我们的分析研究,提高学术期刊影响因子,应该在以下几个方面用功夫: ⒈鼓励高质量论文在我国首先发表 ●I wonder if it’s because I have been at school for so long that I’ve grown so crazy about going home. ●It is because she wasn’t well that she fell far behind her classmates this semester. ●I can well remember that there was a time when I took it for granted that friends should do everything for me. ●In order to make a difference to society, they spent almost all of their spare time in raising money for the charity. ●It’s no pleasure eating at school any longer because the food is not so tasty as that at home. ●He happened to be hit by a new idea when he was walking along the riverbank. ●I wonder if I can cope with stressful situations in life independently. ●It is because I take things for granted that I make so many mistakes. ●The treasure is so rare that a growing number of people are looking for it. ●He picks on the weak mn in order that we may pay attention to him. ●It’s no pleasure being disturbed whena I settle down to my work. ●I can well remember that when I was a child, I always made mistakes on purpose for fun. ●It’s no pleasure accompany her hanging out on the street on such a rainy day. ●I can well remember that there was a time when I threw my whole self into study in order to live up to my parents’ expectation and enter my dream university. ●I can well remember that she stuck with me all the time and helped me regain my confidence during my tough time five years ago. ●It is because he makes it a priority to study that he always gets good grades. ●I wonder if we should abandon this idea because there is no point in doing so. ●I wonder if it was because I ate ice-cream that I had an upset student this morning. ●It is because she refused to die that she became incredibly successful. ●She is so considerate that many of us turn to her for comfort. ●I can well remember that once I underestimated the power of words and hurt my friend. ●He works extremely hard in order to live up to his expectations. ●I happened to see a butterfly settle on the beautiful flower. ●It’s no pleasure making fun of others. ●It was the first time in the new semester that I had burned the midnight oil to study. ●It’s no pleasure taking everything into account when you long to have the relaxing life. ●I wonder if it was because he abandoned himself to despair that he was killed in a car accident when he was driving. ●Jack is always picking on younger children in order to show off his power. ●It is because he always burns the midnight oil that he oversleeps sometimes. ●I happened to find some pictures to do with my grandfather when I was going through the drawer. ●It was because I didn’t dare look at the failure face to face that I failed again. ●I tell my friend that failure is not scary in order that she can rebound from failure. ●I throw my whole self to study in order to pass the final exam. ●It was the first time that I had made a speech in public and enjoyed the thunder of applause. ●Alice happened to be on the street when a UFO landed right in front of her. ●It was the first time that I had kept myself open and talked sincerely with my parents. ●It was a beautiful sunny day. The weather was so comfortable that I settled myself into the SCI收录期刊的学术影响力「范本」 SCI收录期刊的学术影响力本文简介:SCI是目前国际上三大检索系统中最著名的一种,它的引文索引表现出独特的科学参考价值[5],虽然目前对于SCI的作用存在不同观点,但是SCI数据库中的JCR仍是被国际普遍认可的了解世界优秀期刊的重要渠道之一[6]。笔者运用WebofScience数据库的权威数据,分析主要国家。感领域SCI收录期刊的主 SCI收录期刊的学术影响力本文内容: SCI是目前国际上三大检索系统中最著名的一种,它的引文索引表现出独特的科学参考价值[5],虽然目前对于SCI的作用存在不同观点,但是SCI数据库中的JCR仍是被国际普遍认可的了解世界优秀期刊的重要渠道之一[6]。笔者运用WebofScience数据库的权威数据,分析主要国家。感领域SCI收录期刊的主要引证指标,并对比了主要国家的发展差异,分析差异出现的原因,以掌握当前世界。感学类SCI期刊的总体发展状况及主要国家在。感领域所处的国际地位[7],为中国。感学及。感学国际期刊的发展提供一定的参考尧为中国从事。感领域相关工作的科研人员投稿及合作提供借鉴尧为编辑办刊提供经验指导。 1研究对象 叶科学引文索引曳渊ScienceCitationIndex,简称SCI冤是世界公认的自然科学领域最权威的研究成果与期刊检索和评价工具[8]。通过WebofScience渊WOS 冤数据库的叶期刊引证报告曳渊JournalCitationReports,简称JCR[9]冤,检索2020年要2020年被SCI收录的主题属于。感渊RemoteSensing冤的国际期刊,共31种。为保证数据的连续性和可对比性,同时兼顾尽量保留最多的期刊数量,使更多的国家参与分析的原则,从2020年起向前追溯,至少拥有近3年连续JCR 收录数据的。感学期刊为对象进行分析,共有期刊25种。其中,自2020年起有JCR数据的期刊有2种,JournaloftheIndianSocietyofRemoteSensing和RemoteSensingLetters曰自2020年起的有3种,EuropeanJournalofRemoteSensing尧GeocartoInternational和RemoteSensing,其余20种为自2020年起拥有连续5年收录数据的期刊渊表1冤。对25种期刊的语言尧创刊年份尧影响因子及分区等信息逐项查找核实,WOS 高中英语~词性~句子成分~语法构成 第一章节:英语句子中的词性 1.名词:n. 名词是指事物的名称,在句子中主要作主语.宾语.表语.同位语。 2.形容词;adj. 形容词是指对名词进行修饰~限定~描述~的成份,主要作定语.表语.。形容词在汉语中是(的).其标志是: ous. Al .ful .ive。. 3.动词:vt. 动词是指主语发出的一个动作,一般用来作谓语。 4.副词:adv. 副词是指表示动作发生的地点. 时间. 条件. 方式. 原因. 目的. 结果.伴随让步. 一般用来修饰动词. 形容词。副词在汉语中是(地).其标志是:ly。 5.代词:pron. 代词是指用来代替名词的词,名词所能担任的作用,代词也同样.代词主要用来作主语. 宾语. 表语. 同位语。 6.介词:prep.介词是指表示动词和名次关系的词,例如:in on at of about with for to。其特征: 介词后的动词要用—ing形式。介词加代词时,代词要用宾格。例如:give up her(him)这种形式是正确的,而give up she(he)这种形式是错误的。 7.冠词:冠词是指修饰名词,表名词泛指或特指。冠词有a an the 。 8.叹词:叹词表示一种语气。例如:OH. Ya 等 9.连词:连词是指连接两个并列的成分,这两个并列的成分可以是两个词也可以是两个句子。例如:and but or so 。 10.数词:数词是指表示数量关系词,一般分为基数词和序数词 第二章节:英语句子成分 主语:动作的发出者,一般放在动词前或句首。由名词. 代词. 数词. 不定时. 动名词. 或从句充当。 谓语:指主语发出来的动作,只能由动词充当,一般紧跟在主语后面。 宾语:指动作的承受着,一般由代词. 名词. 数词. 不定时. 动名词. 或从句充当. 介词后面的成分也叫介词宾语。 定语:只对名词起限定修饰的成分,一般由形容 M A: Has the case been closed yet? B: No, the magistrate still needs to decide the outcome. magistrate n.地方行政官,地方法官,治安官 A: I am unable to read the small print in the book. B: It seems you need to magnify it. magnify vt.1.放大,扩大;2.夸大,夸张 A: That was a terrible storm. B: Indeed, but it is too early to determine the magnitude of the damage. magnitude n.1.重要性,重大;2.巨大,广大 A: A young fair maiden like you shouldn’t be single. B: That is because I am a young fair independent maiden. maiden n.少女,年轻姑娘,未婚女子 a.首次的,初次的 A: You look majestic sitting on that high chair. B: Yes, I am pretending to be the king! majestic a.雄伟的,壮丽的,庄严的,高贵的 A: Please cook me dinner now. B: Yes, your majesty, I’m at your service. majesty n.1.[M-]陛下(对帝王,王后的尊称);2.雄伟,壮丽,庄严 A: Doctor, I traveled to Africa and I think I caught malaria. B: Did you take any medicine as a precaution? malaria n.疟疾 A: I hate you! B: Why are you so full of malice? malice n.恶意,怨恨 A: I’m afraid that the test results have come back and your lump is malignant. B: That means it’s serious, doesn’t it, doctor? malignant a.1.恶性的,致命的;2.恶意的,恶毒的 A: I’m going shopping in the mall this afternoon, want to join me? B: No, thanks, I have plans already. mall n.(由许多商店组成的)购物中心 A: That child looks very unhealthy. B: Yes, he does not have enough to eat. He is suffering from malnutrition. 意见应以事实为根据. 3 来自辞典例句 192. The bombers swooped ( down ) onthe air base. 轰炸机 突袭 空军基地. 来自辞典例句 193. He mounted their engines on a rubber base. 他把他们的发动机装在一个橡胶垫座上. 14 来自辞典例句 194. The column stands on a narrow base. 柱子竖立在狭窄的地基上. 14 来自辞典例句 195. When one stretched it, it looked like grey flakes on the carvas base. 你要是把它摊直, 看上去就象好一些灰色的粉片落在帆布底子上. 18 来自辞典例句 196. Economic growth and human well - being depend on the natural resource base that supports all living systems. 经济增长和人类的福利依赖于支持所有生命系统的自然资源. 12 1 来自辞典例句 197. The base was just a smudge onthe untouched hundred - mile coast of Manila Bay. 那基地只是马尼拉湾一百英里长安然无恙的海岸线上一个硝烟滚滚的污点. 6 来自辞典例句 198. You can't base an operation on the presumption that miracles are going to happen. 你不能把行动计划建筑在可能出现奇迹的假想基础上. 2019中国最具国际影响力学术期刊排名 日前,中国学术期刊光盘版电子杂志社与清华大学图书馆联合开展研究,以国际化视野,检索了6400多种中国学术期刊被1.4万多种国际学术期刊的引证情况,并且通过计 量分析和同行专家评议,研制了2019年中国学术期刊国际影响力引证报告。这是2019年以来第三次发布该引证报告。本次刊登的人文社会科学类期刊排名基于被国际引证过的人文社科类学术期刊总数的前5%。统计表明,这些期刊已经具备相当国际影响,迈进了国际期刊的门槛。 “2019中国最具国际影响力学术期刊”(人文社会科学) 序号期刊名称国际影响力指数CI “国际他引总被引频次” “国际他引影响因子” 语种主办单位 1 China & World Economy* 896.77 2 250 0.658 英文中国社会科学院世界经济与政治研究所等 2 经济研究 555.766 517 0.094 中文中国社会科学院经济研究所 3 心理学报 512.577 32 4 0.119 中文中国心理学会等 4 文物 450.581 356 0.059 中文文物出版社 5 中国软科学 443.721 279 0.097 中文中国软科学研究会 6 考古 415.946 302 0.062 中文中国社会科学院考古研究所 7 管理世界 409.017 330 0.042 中文中华人民共和国国务院发展研究中心 8 中国语文 399.799 329 0.036 中文中国社会科学院语言研究所 9 中国管理科学 399.443 248 0.086 中文中国优选法统筹法与经济教学研究会等 10 会计研究360.122 197 0.098 中文中国会计学会 11 世界经济与政治 358.621 209 0.086 中文中国社会科学院世界经济与政治研究 所 12 经济地理 341.38 241 0.047 中文中国地理学会等 13 中国社会科学 336.698 182 0.093 中文中国社会科学杂志社 14 社会学研究 321.739 136 0.129 中文中国社会科学院社会学研究所 15 中国工业经济 302.006 194 0.054 中文中国社会科学院工业经济研究所 英语造句大全English sentence 在句子中,更好的记忆单词! 1、(1)、able adj. 能 句子:We are able to live under the sea in the future. (2)、ability n. 能力 句子:Most school care for children of different abilities. (3)、enable v. 使。。。能句子:This pass enables me to travel half-price on trains. 2、(1)、accurate adj. 精确的句子:We must have the accurate calculation. (2)、accurately adv. 精确地 句子:His calculation is accurately. 3、(1)、act v. 扮演 句子:He act the interesting character. (2)、actor n. 演员 句子:He was a famous actor. (3)、actress n. 女演员 句子:She was a famous actress. (4)、active adj. 积极的 句子:He is an active boy. 4、add v. 加 句子:He adds a little sugar in the milk. 5、advantage n. 优势 句子:His advantage is fight. 6、age 年龄n. 句子:His age is 15. 7、amusing 娱人的adj. 句子:This story is amusing. 8、angry 生气的adj. 句子:He is angry. 9、America 美国n. 主语+谓语 1. 理解主谓结构 1) The students arrived. The students arrived at the park. 2) They are listening. They are listening to the music. 3) The disaster happened. 2.体会状语的位置 1) Tom always works hard. 2) Sometimes I go to the park at weekends.. 3) The girl cries very often. 4) We seldom come here. The disaster happened to the poor family. 3. 多个状语的排列次序 1) He works. 2) He works hard. 3) He always works hard. 4) He always works hard in the company. 5) He always works hard in the company recently. 6) He always works hard in the company recently because he wants to get promoted. 4. 写作常用不及物动词 1. ache My head aches. I’m aching all over. 2. agree agree with sb. about sth. agree to do sth. 3. apologize to sb. for sth. 4. appear (at the meeting, on the screen) 5. arrive at / in 6. belong to 7. chat with sb. about sth. 8. come (to …) 9. cry 10. dance 11. depend on /upon 12. die 13. fall 14. go to … 15. graduate from 16. … happen 17. laugh 18. listen to... 19. live 20. rise 21. sit 22. smile 23. swim 24. stay (at home / in a hotel) 25. work 26. wait for 汉译英: 1.昨天我去了电影院。 2.我能用英语跟外国人自由交谈。 3.晚上7点我们到达了机场。 4.暑假就要到了。 5.现在很多老人独自居住。 6.老师同意了。 7.刚才发生了一场车祸。 8.课上我们应该认真听讲。9. 我们的态度很重要。 10. 能否成功取决于你的态度。 11. 能取得多大进步取决于你付出多少努力。 12. 这个木桶能盛多少水取决于最短的一块板子的长度。 综述论文与学术期刊影响力 作者:黄青, 冯有为, HUANG Qing, FENG YonWei 作者单位:中国科学院金属研究所学报信息部,110016,沈阳市沈河区文化路72号 刊名: 中国科技期刊研究 英文刊名:CHINESE JOURNAL OF SCIENTIFIC AND TECHNICAL PERIODICALS 年,卷(期):2009,20(6) 被引用次数:2次 参考文献(8条) 1.冯腊枝综述论文的遴选及审编[期刊论文]-编辑学报 2001(04) 2.查看详情 2009 3.张玉华;潘云涛科技论文影响力相关因素研究[期刊论文]-编辑学报 2007(02) 4.柳晓丽提高科技期刊影响因子的途径探讨[期刊论文]-编辑学报 2006(04) 5.曾建勋如何提高期刊影响因子 2005(02) 6.查看详情 2009 7.2008 Journal Citation Reports' Science Edition (Thomson Reuters,2009) 8.陈浩元科技期刊标准化18讲 1998 本文读者也读过(2条) 1.冯腊枝综述论文的遴选及审编[期刊论文]-编辑学报2001,13(4) 2.王兰英.王连柱.王家勤.张瑞君.雍文明.郑会绍.WANG Lan-ying.WANG Lian-zhu.WANG Jia-qin.ZHANG Rui-jun. YONG Wen-ming.ZHENG Hui-shao基于语料库的综述论文结构式英文摘要文体分析[期刊论文]-实用儿科临床杂志2008,23(23) 引证文献(4条) 1.孙宪民关于提高科技期刊影响力的探讨[期刊论文]-中国科技信息 2011(9) 2.刘晓燕.张成娥.徐晓芹.金继运期刊高被引论文学术特征分析及其启示——以《植物营养与肥料学报》为例[期刊论文]-农业图书情报学刊 2011(10) 3.姚志昌.邢燕萍高校学报学术影响力主要影响因素分析——以《中国矿业大学学报》为例[期刊论文]-中国科技期刊研究 2010(6) 4.武建虎.李小萍.张萍.尤伟杰.郭青.梁秋野.岳建华综合评价《武警医学》杂志的栏目设置[期刊论文]-中国科技期刊研究 2010(6) 本文链接:https://www.360docs.net/doc/c7207740.html,/Periodical_zgkjqkyj200906042.aspx 【it's time to和it's time for】 ——————这其实是一个句型,只不过后面要跟不同的东西. ——————It's time to跟的是不定式(to do).也就是说,要跟一个动词,意思是“到做某事的时候了”.如: It's time to go home. It's time to tell him the truth. ——————It's time for 跟的是名词.也就是说,不能跟动词.如: It's time for lunch.(没必要说It's time to have lunch) It's time for class.(没必要说It's time to begin the class.) They can't wait to see you Please ask liming to study tonight. Please ask liming not to play computer games tonight. Don’t make/let me to smoke I can hear/see you dance at the stage You had better go to bed early. You had better not watch tv It’s better to go to bed early It’s best to run in the morning I am enjoy running with music. With 表伴随听音乐 I already finish studying You should keep working. You should keep on studying English Keep calm and carry on 保持冷静继续前行二战开始前英国皇家政府制造的海报名字 I have to go on studying I feel like I am flying I have to stop playing computer games and stop to go home now I forget/remember to finish my homework. I forget/remember cleaning the classroom We keep/percent/stop him from eating more chips I prefer orange to apple I prefer to walk rather than run I used to sing when I was young What’s wrong with you There have nothing to do with you I am so busy studying You are too young to na?ve I am so tired that I have to go to bed early SCI收录期刊的学术影响力 各位读友大家好,此文档由网络收集而来,欢迎您下载,谢谢 SCI收录期刊的学术影响力本文关键词:影响力,收录,期刊,学术,SCI SCI收录期刊的学术影响力本文简介:SCI是目前国际上三大检索系统中最著名的一种,它的引文索引表现出独特的科学参考价值[5],虽然目前对于SCI的作用存在不同观点,但是SCI数据库中的JCR仍是被国际普遍认可的了解世界优秀期刊的重要渠道之一[6]。笔者运用WebofScience数据库的权威数据,分析主要国家。感领域SCI收录期刊的主 SCI收录期刊的学术影响力本文内容: SCI是目前国际上三大检索系统中最著名的一种,它的引文索引表现出独特的科学参考价值[5],虽然目前对于 SCI的作用存在不同观点,但是SCI数据库中的JCR仍是被国际普遍认可的了解世界优秀期刊的重要渠道之一[6]。笔者运用WebofScience数据库的权威数据,分析主要国家。感领域SCI收录期刊的主要引证指标,并对比了主要国家的发展差异,分析差异出现的原因,以掌握当前世界。感学类SCI期刊的总体发展状况及主要国家在。感领域所处的国际地位[7],为中国。感学及。感学国际期刊的发展提供一定的参考尧为中国从事。感领域相关工作的科研人员投稿及合作提供借鉴尧为编辑办刊提供经验指导。 1研究对象 叶科学引文索引曳渊ScienceCitationIndex,简称SCI冤是世界公认的自然科学领域最权威的研究成果与期刊检索和评价工具[8]。通过WebofScience渊WOS冤数据库的叶期刊引证报告曳渊JournalCitationReports,简称JCR[9]冤,检索2010年要2014年 《The Kite Runner》追风筝的人--------------------------------美句摘抄 1.I can still see Hassan up on that tree, sunlight flickering through the leaves on his almost perfectly round face, a face like a Chinese doll chiseled from hardwood: his flat, broad nose and slanting, narrow eyes like bamboo leaves, eyes that looked, depending on the light, gold, green even sapphire 翻译:我依然能记得哈桑坐在树上的样子,阳光穿过叶子,照着他那浑圆的脸庞。他的脸很像木头刻成的中国娃娃,鼻子大而扁平,双眼眯斜如同竹叶,在不同光线下会显现出金色、绿色,甚至是宝石蓝。 E.g.: A shadow of disquiet flickering over his face. 2.Never told that the mirror, like shooting walnuts at the neighbor's dog, was always my idea. 翻译:从来不提镜子、用胡桃射狗其实都是我的鬼主意。E.g.:His secret died with him, for he never told anyone. 3.We would sit across from each other on a pair of highon the contrary的解析
期刊影响因子的“含金量”是多少
英语造句
提高学术期刊影响因子的途径
学生造句--Unit 1
SCI收录期刊的学术影响力「范本」
英语句子结构和造句
六级单词解析造句记忆MNO
base on的例句
2019中国最具国际影响力学术期刊排名
英语造句大全
(完整版)主谓造句
综述论文与学术期刊影响力
初中英语造句
SCI收录期刊的学术影响力
The Kite Runner-美句摘抄及造句
