改过(35)Preparation and Research of Porous Hydroxyapatite Whiskers and KGM Composite Bone Scaffolds

Preparation and Research of Porous
Hydroxyapatite Whiskers/KGM Composite Bone Scaffolds
Huang Minghua1, a, Chen Qinghua2, b, Lei Li2, c,
Wang Duancheng2, d and Yan Tingting2, e
1Faculty of Mechanical and Electrical Engineering of Kunming University of
Science and Technology, Kunming, 650500, China
2Faculty of Materials Science and Engineering of Kunming University of
Science and Technology, Kunming, 650500, China
a huangminghuajj@https://www.360docs.net/doc/d8568471.html,,
b chenqinghua_yn1@https://www.360docs.net/doc/d8568471.html,,
c kmust2007ll@https://www.360docs.net/doc/d8568471.html,,
d625296056@https://www.360docs.net/doc/d8568471.html,, e ttyan@https://www.360docs.net/doc/d8568471.html,
Keywords: Hydroxyapatite Whisker (HAPw), Konjac Glucomannan (KGM), Crosslinking Agent, Freeze-Drying Method, Porosity
Abstract.Sol-gel method and freeze-drying method were adopted to prepare the porous
HAPw/KGM composite bone scaffolds and ammonia was used as a crosslinking agent. The porosity, average pore diameter, compressive strength and degradation rate in vitro were measured according to the related standard. The curves of each factor and lever affecting comprehensive properties were drew through the orthogonal design L9 (34) experiment. SEM and XRD were applied in characterization. The results show that the optimal preparation program of the composite scaffolds is KGM (2g), HAPw (4.5g), ammonia (0.1 ml) and the freeze temperature (-20 ° C); the prepared scaffolds are porous three-dimensional network structures; the porosity of optimal scaffold is more than 90%; the average pore diameter is between 200-300μm; the compressive strength is about
0.8Mpa and the degradation rate is about 50% within 9 weeks.
Introduction
At present, the bone scaffolds and the products of bone tissue engineering are still in the stage of basic research and there exists a larger gap away from the requirements and standards of clinical applications. Research and development of the ideal bone scaffolds and the products of bone tissue engineering, which can effectively promote formation of new bones and complete regeneration of bone tissue, becomes focus and difficulties. For bone tissue engineering, the ideal scaffolds must have the following basic characteristics [1, 2]: 1) Good biocompatibility and bio-safety. That can be fully compatible with the living body and can participate in living body metabolism. 2) Good osteoinductivity and osteoconductivity that can guide the growth of new bones. 3) High porosity, suitable pore diameter and three-dimensional interconnected-pore structures. That can sever as the extracellular matrix of seed cells and be convenient for selective adhesion of different-function cells and generate relevant specific reaction [3]. At the same time, such structures can provide specific signals of growth and differentiation to regulate cell migration, proliferation, differentiation and growth[4] and can facilitate angiogenesis and delivery of nutrients. 4) The appropriate biodegradation rate that can match with the growth of new bone and can provide place for the regeneration of bone tissue. 5) Good plasticity and suitable mechanical properties[5].
From the perspective of bionic extracellular matrix and on the basis of early studies, the research on the porous HAPw/KGM composite bone scaffolds was put forward. In human natural bone, the type1 collagen fibers-based organic matter accounts for about 34% and the needle-like hydroxyapatite crystals -based inorganic matter accounts for about 65% (60nm× (5-20) nm or 40nm×3nm). The synthetic acicular HAP whiskers and the needle-like hydroxyapatite crystals of human bone have the same chemical composition and crystal structures. The special biochemical reaction can occur in interface between the bioactive HAPw and the host tissue, so both of them can form direct bone integration. The konjac glucomannan is natural neutral polysaccharides and has good biocompatibility as well as plays an important role in cell adhesion, proliferation and differentiation [6, 7, 8, 9]. This experiment adopted the sol-gel method and the freeze-drying method and used ammonia as
a crosslinking agent to successfully prepare the porous HAPw/KGM composite bone scaffolds with the three-dimensional network structure. The porosity, the average pore diameter, the compressive strength and the degradation rate in vitro can meet the requirements of scaffold materials of bone tissue engineering.
Experiment Section
Materials. Hydroxyapatite whiskers (Ca10 (PO4)6(OH)2, self-made in my laboratory), konjac glucomannan (Chengdu Cooperation Konjac Food Co. Ltd., the konjac glucomannan ≥ 9 0.0%), 25% ammonia (NH3H2O, analytical purity, Tianjin Chemical Reagent No.3 Factory)
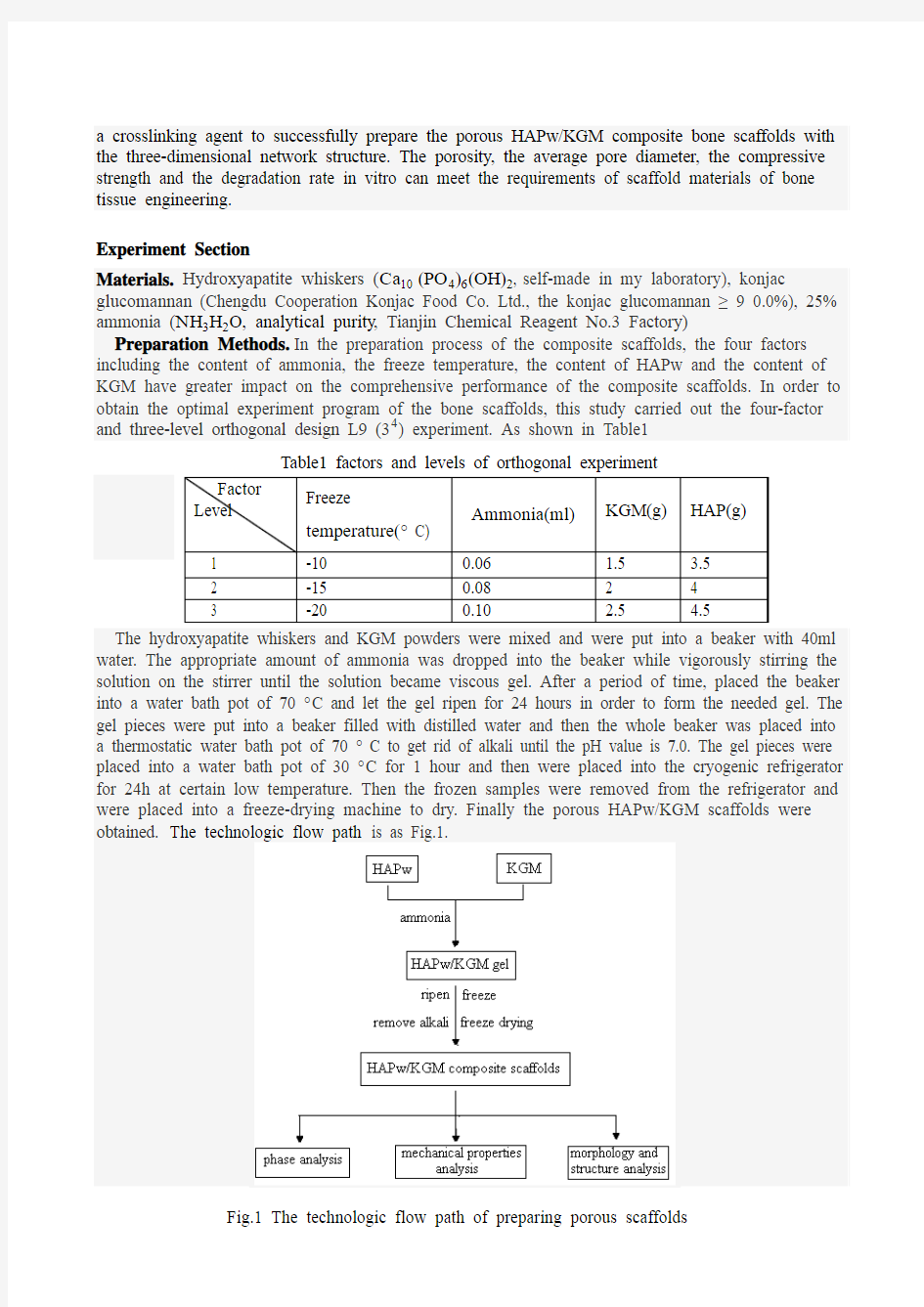
Preparation Methods.In the preparation process of the composite scaffolds, the four factors including the content of ammonia, the freeze temperature, the content of HAPw and the content of KGM have greater impact on the comprehensive performance of the composite scaffolds. In order to obtain the optimal experiment program of the bone scaffolds, this study carried out the four-factor and three-level orthogonal design L9 (34) experiment. As shown in Table1
Table1 factors and levels of orthogonal experiment
The hydroxyapatite whiskers and KGM powders were mixed and were put into a beaker with 40ml water. The appropriate amount of ammonia was dropped into the beaker while vigorously stirring the solution on the stirrer until the solution became viscous gel. After a period of time, placed the beaker into a water bath pot of 70 ° C and let the gel ripen for 24 hours in order to form the needed gel. The gel pieces were put into a beaker filled with distilled water and then the whole beaker was placed into a thermostatic water bath pot of 70 ° C to get rid of alkali until the pH value is 7.0. The gel pieces were placed into a water bath pot of 30 ° C for 1 hour and then were placed into the cryogenic refrigerator for 24h at certain low temperature. Then the frozen samples were removed from the refrigerator and were placed into a freeze-drying machine to dry. Finally the porous HAPw/KGM scaffolds were obtained. The technologic flow path is as Fig.1.
Fig.1 The technologic flow path of preparing porous scaffolds
Sample Measurement and Characterization. The porosity of the samples was measured according to the GB/1966-1996. The distribution of macro-pore and micro-pore diameter was determined by the quantitative metallographic method and statistical method. The compressive strength of the samples was measured based on the GB/1041-92. Quanta200 scanning electron microscopy (SEM) of Philips FEI Company was adopted to analyze scaffold morphology and the pore diameter distribution. The X-ray diffr action instrument (XRD D8 ADVANCE, Germany) was used to analyze the phases of the prepared samples. The degradation rate in vitro of the scaffolds was measured in the compound NaCl physiological saline at the heated incubator of 37 ° C.
Results and Discussion
Comprehensive Property Criteria and Optimal Experiment Program. The comprehensive properties of the scaffolds are judged by the scores of three indicators, the porosity, the average pore diameter and the compressive strength. The share scores of these three indicators are 35, 35 and 30 respectively. G1= (1-(250-x)/250)*35(in which G1is the average pore diameter score of the scaffolds; x is the average pore diameter of the scaffolds and 250μm pore diameter is as full score standard because 250μm pore diameter is beneficial to cell adhesion, migration, proliferation and differentiation in particular). G2=y/100*35(in which G2 is the porosity score of the scaffolds and y is the porosity of the scaffolds). G3 = (z/0.5) * 30(in which G3 is the compressive strength score of the scaffolds; z is the compressive strength of the scaffolds and 0.5MPa compressive strength is as full score standard because 0.5MPa compressive strength can meet the surgical procedure and such scaffolds are not easy to crush[10] and can play a mechanical support in the process of repair). The comprehensive property score of each scaffold is the sum of the scores of three indicators, that is
G=G1+G2+G3.The effect of each factor and level on the comprehensive properties is as Fig. 2.
Fig.2 the curves of each factor and lever affecting comprehensive properties
We can know from Fig.2 when freeze temperature is -20 ° C; ammonia content is 0.1 ml; KGM content is 2g and HAPw content is 4.5g, the comprehensive property scores of the scaffolds reach the maximum. Considering of the 70:30 proportions of the inorganic / organic component in the body’s natural bone, the amount of HAPw is set at 4.5g. Therefore, the optimal preparation program of the porous HAPw/ KGM composite bone scaffolds is as so. The following tested samples are all ones of the optimal preparation program.
XRD Analysis of the Scaffolds. As shown in Fig. 3, KGM powders appear the amorphous
non-crystallization peaks according to (b). (a) is the XRD of the three-dimensional network-structure KGM after crosslink and deacetylation. At 2θ = 11.18 o, 15.42 o, 20.0 o, 26.0 o, 37.2 o appear diffraction peaks. This is because the KGM occurs deacetylation under the action of ammonia alkali and then macromolecular chains close to each other and a large number of hydrogen bonds are formed between molecules, resulting in part of the crystallization peaks. The lattice constant can be calculated as:a=9.21?, b=15.72 ?, c=9.06 ? by MDI Jade (5.0) software. At the same time the diffraction surface can be calculated as: (101), (022), (040), (211), (026) correspondingly at
2θ=11.18o, 15.42o, 20.0o, 26.0o, 37.2o respectively.
As can be seen from (c) and (d) in Fig. 3, the diffraction peaks of the composite scaffolds are basically consistent with the diffraction peaks of hydroxyapatite whiskers. This explains that
inorganic phases in the composite scaffolds are mainly HAPw. The XRD of the c omposite scaffolds does not appear the KGM crystallization peaks because the polymer of KGM has relatively lower crystallinity compared with the ceramic phase of HAPw and the KGM content in the composite scaffolds is smaller.
Scanning Electron Microscopy (SEM) Morphological Analysis of the Scaffolds. From the Fig.4 we can learn that the majority of pore diameters of the samples is distributed between
200-300μm . This is very suitable for the transfer of nutrients and cell proliferation and differentiation. The various pores in the scaffolds are formed in the freeze-drying process since firstly the absorbed water in the crosslinked network-structure gel becomes ice crystals and then ice crystals sublimate. The hydroxyapatite whiskers exist in the pore walls of the KGM alone and do not form any bond with KGM so the hydroxyapatite whiskers improve mechanical properties of the composite scaffolds unobviously.
(a) XRD of KGM after crosslink (b) XRD of KGM powders
(c) XRD of HAPw (d) XRD of the composite scaffolds
Fig.3 XRD patterns of samples
Fig.4 SEM morphology of the porous HAPw/KGM composite bone scaffolds
The Analysis of Degradation Behavior in Vitro. As can be seen from Fig.5, the degradation rate of each sample shows the significant increase trend with time before 9 weeks but the rate increase slows after 9 weeks. This is because the degraded matter before 9 weeks is mainly the HAPw and the konjac glucomannan degradation is relatively slow due to the three-dimensional network-structure by crosslink. The degradation rate of samples is about 50% within 9 weeks.
Fig.5 degradation rate curves of the scaffold samples
Conclusion
In this study, the Sol-gel method and the freeze-drying method are adopted to prepare the porous HAPw/KGM composite bone scaffolds successfully. T he optimal preparation program of the scaffolds is determined as: KGM (2g), HAPw (4.5g), ammonia (0.1 ml) and the freeze temperature (-20 ° C) based on the orthogonal design L9 (34) experiment and the curves of each factor and lever affecting comprehensive properties.
The obtained optimal HAPw/KGM composite bone scaffold is the porous three-dimensional network structure which porosity is more than 90%; the average pore diameter is mainly distributed between 200-300μm; the compressive strength is about 0.8Mpa; the degradation rate in vitro is about 50% within 9 weeks and the degradation rate is larger before 9 weeks but the degradation rate is smaller after 9 weeks.
These porous three-dimensional network structures of the scaffolds can be formed due to icy crystals sublimation in the freeze-drying process and KGM crosslink after deacetylation under the action of ammonia alkali. The hydroxyapatite whiskers exist in the pore walls of the KGM alone and do not form any bond with KGM.
Acknowledgment
This work is financially supported by Science and Technology Project of Yunnan Province (2011lA008).The authors would like to thank them.
References
[1]Deepak Vashishth: International Journal of Fatigue Vol. 29 (2007), p. 1024-1033
[2]Robert P. Lanza, Robert Langer and Joseph Vacanti: Tissue Engineering Principles, translated
by Z.M. Zhang (Chemical Industry Press, China 2006, In Chinese)
[3]Marc Andre Meyers, Po-Yu Chen, Albert Yu-Min Lin et al: Progress in Materials Science Vol.
53(2008), p. 1-206
[4] F. Sun, X. Pang, I. Zhitomirsky: Mater. Process Tech. (2008)
[5]W.H. Dietmar: Biomaterials Vol. 21(2000), p. 2529- 2543
[6]J. Pang and Q. Lin: Structural Chemistry V ol. 11(6)(2003), p. 633-642, In Chinese
[7]Y.Q. Zhang, B.J. Xie, X. Gan: Carbohydrate Polymers Vol. 60(2005), p. 27-31
[8]J. Pang, P.S. Zhang and B.B. Kang: Polymer Materials Science and Engineering Vol. 19(6)
(2003), p. 216-219, In Chinese
[9]S. Jing, F. Zhang, Q.H. Chen et al: Chinese Tissue Engineering and Clinical Rehabilitation V ol.
35(2007), p. 7053-7056, In Chinese
[10]S ang-Hoon Rhee, Y asushi Suetsugu, Junzo Tanaka: Biomaterials V ol. 22(2001), p. 2843
