The preparation and characterization of Li4Ti5O12carbon nano-tubes - 副本

Electrochimica Acta 53(2008)7756–7759
Contents lists available at ScienceDirect
Electrochimica
Acta
j o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /e l e c t a c t
a
The preparation and characterization of Li 4Ti 5O 12/carbon nano-tubes for lithium ion battery
Junjie Huang,Zhiyu Jiang ?
Department of Chemistry,Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials,Fudan University,Shanghai 200433,China
a r t i c l e i n f o Article history:
Received 3March 2008
Received in revised form 22April 2008Accepted 15May 2008
Available online 21May 2008Keywords:Li 4Ti 5O 12
Carbon nano-tube High rate capability Lithium ion battery Nano-particles
a b s t r a c t
Li 4Ti 5O 12/carbon nano-tubes (CNTs)composite was prepared by sol–gel method while Ti(OC 4H 9)4,LiCH 3COO ·2H 2O and the n -heptane containing CNTs were used as raw materials.The characters of Li 4Ti 5O 12/CNTs composite were determined by XRD,SEM,and TG methods.Its electrochemical prop-erties were measured by charge–discharge cycling and impedance tests.It was found that the prepared Li 4Ti 5O 12/CNTs presented an excellent rate capability and capacity retention.At the charge–discharge rate of 5C and 10C ,its discharge capacities were 145and 135mAh g ?1,respectively.After 500cycles at 5C ,the discharge capacity retained as 142mAh g ?1.It even could be cycled at the rate of 20C .The excellent electro-chemical performance of Li 4Ti 5O 12/CNTs electrode could be attributed to the improvement of electronic conductivity by adding conducting CNTs and the nano-size of Li 4Ti 5O 12particles in the Li 4Ti 5O 12/CNTs composite.
?2008Published by Elsevier Ltd.
1.Introduction
Spine Li 4Ti 5O 12has been viewed as one promising alternative to graphite to be an anode material in lithium ion batteries.It is due to its unique characters,such as:small dimensional change dur-ing charge/discharge process,which may enable a long and stable cycle life,and high insertion potential of 1.55V,which can avoid the reduction of electrolyte on the surface of electrode [1,2].Addi-tionally,Li 4Ti 5O 12also has excellent lithium ion mobility [3].The combination of zero strain insertion properties and high lithium mobility makes it very attractive to be an anode material in lithium ion battery.But right now its rate performance is limited heavily due to the low electronic conductivity of material.
To improve the conductivity of spinel Li 4Ti 5O 12,several ef?cient ways have been proposed,including the substitution of a small quantity of Li +or Ti 4+by super-valent metal ions (V 5+,Mn 4+,Fe 3+,Ni 2+,Cr 3+and Mg 2+)[4–7];the deposition of metallic grains or car-bon on Li 4Ti 5O 12particle surface [8,9].The reports showed that these modi?cations on Li 4Ti 5O 12could improve its electrochemical behavior greatly.In this paper,for the ?rst time,carbon nano-tubes (CNTs)were used to modify Li 4Ti 5O 12.The material CNTs have been applied in the many ?elds such as shielding and absorbing electro-magnetic radiation,?eld emission,thermal conducting,hydrogen storing,and catalyzing due to its special properties,including
?Corresponding author.Tel.:+862165643980;fax:+862165641740.E-mail address:zyjiang@https://www.360docs.net/doc/dc11396833.html, (Z.Jiang).high ratio of length/diameter,unique conductivity,strength and ?exibility.Here,the synthetic process and the electrochemical char-acteristics of Li 4Ti 5O 12/CNTs are introduced.The results showed that the prepared Li 4Ti 5O 12/CNTs presented excellent rate capabil-ity and cycling performance.2.Experimental
Li 4Ti 5O 12was prepared through sol–gel method using the raw materials of Ti(OC 4H 9)4,LiCH 3COO ·2H 2O (LiAc ·2H 2O).Firstly,Ti(OC 4H 9)4was mixed with n -heptane in the ratio of 1:10(weight ratio),and LiAc ·2H 2O was dissolved in 90%ethanol in the ratio of 1:20(weight ratio),respectively.Then,the LiAc ethanol solution was dropped slowly into Ti(OC 4H 9)4n -heptane solution (at the molar ratio of Li:Ti =4.4:5)under magnetic stirring.In this drop-ping process the solution turn to white as Ti(OC 4H 9)4hydrolyzing.After 1h stirring,the white sol–gel solution was treated at 65?C to remove solvents gradually,and became a precursor ?nally.Then,the precursor was heat-treated at 450?C in N 2atmosphere for 5h,and further calcinated at 800?C for another 10h.Finally,a white powder of spinel Li 4Ti 5O 12was obtained.The process for preparing Li 4Ti 5O 12/CNTs was similar to that of Li 4Ti 5O 12prepara-tion,except certain amount of CNT (based on the ratio of 10wt.%CNT in Li 4Ti 5O 12/CNTs composite)were mixed in the Ti(OC 4H 9)4n -heptane solution in the ?rst stage.The color of Li 4Ti 5O 12/CNTs was grey.All reagents are from Sinopharm Group Chemical Reagent https://www.360docs.net/doc/dc11396833.html,Ts are multi-wall CNTs produced from Shenzhen Nan-otechnologies Co.Ltd.The diameters of CNTs particles are about
0013-4686/$–see front matter ?2008Published by Elsevier Ltd.doi:10.1016/j.electacta.2008.05.031
J.Huang,Z.Jiang /Electrochimica Acta 53(2008)7756–7759
7757
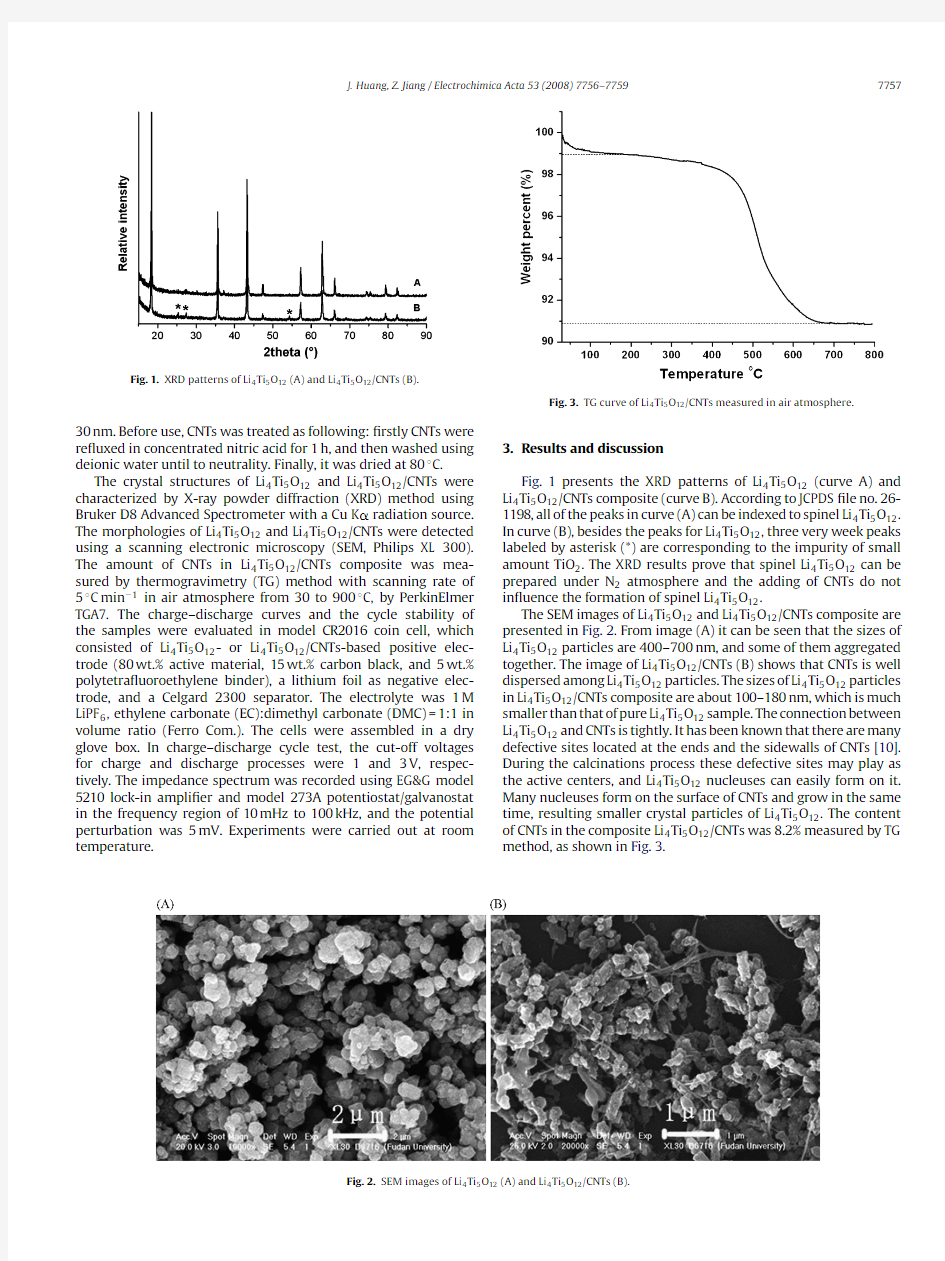
Fig.1.XRD patterns of Li 4Ti 5O 12(A)and Li 4Ti 5O 12/CNTs (B).
30nm.Before use,CNTs was treated as following:?rstly CNTs were re?uxed in concentrated nitric acid for 1h,and then washed using deionic water until to neutrality.Finally,it was dried at 80?C.
The crystal structures of Li 4Ti 5O 12and Li 4Ti 5O 12/CNTs were characterized by X-ray powder diffraction (XRD)method using Bruker D8Advanced Spectrometer with a Cu K ?radiation source.The morphologies of Li 4Ti 5O 12and Li 4Ti 5O 12/CNTs were detected using a scanning electronic microscopy (SEM,Philips XL 300).The amount of CNTs in Li 4Ti 5O 12/CNTs composite was mea-sured by thermogravimetry (TG)method with scanning rate of 5?C min ?1in air atmosphere from 30to 900?C,by PerkinElmer TGA7.The charge–discharge curves and the cycle stability of the samples were evaluated in model CR2016coin cell,which consisted of Li 4Ti 5O 12-or Li 4Ti 5O 12/CNTs-based positive elec-trode (80wt.%active material,15wt.%carbon black,and 5wt.%polytetra?uoroethylene binder),a lithium foil as negative elec-trode,and a Celgard 2300separator.The electrolyte was 1M LiPF 6,ethylene carbonate (EC):dimethyl carbonate (DMC)=1:1in volume ratio (Ferro Com.).The cells were assembled in a dry glove box.In charge–discharge cycle test,the cut-off voltages for charge and discharge processes were 1and 3V,respec-tively.The impedance spectrum was recorded using EG&G model 5210lock-in ampli?er and model 273A potentiostat/galvanostat in the frequency region of 10mHz to 100kHz,and the potential perturbation was 5mV.Experiments were carried out at room
temperature.
Fig.3.TG curve of Li 4Ti 5O 12/CNTs measured in air atmosphere.
3.Results and discussion
Fig.1presents the XRD patterns of Li 4Ti 5O 12(curve A)and Li 4Ti 5O 12/CNTs composite (curve B).According to JCPDS ?le no.26-1198,all of the peaks in curve (A)can be indexed to spinel Li 4Ti 5O 12.In curve (B),besides the peaks for Li 4Ti 5O 12,three very week peaks labeled by asterisk (*)are corresponding to the impurity of small amount TiO 2.The XRD results prove that spinel Li 4Ti 5O 12can be prepared under N 2atmosphere and the adding of CNTs do not in?uence the formation of spinel Li 4Ti 5O 12.
The SEM images of Li 4Ti 5O 12and Li 4Ti 5O 12/CNTs composite are presented in Fig.2.From image (A)it can be seen that the sizes of Li 4Ti 5O 12particles are 400–700nm,and some of them aggregated together.The image of Li 4Ti 5O 12/CNTs (B)shows that CNTs is well dispersed among Li 4Ti 5O 12particles.The sizes of Li 4Ti 5O 12particles in Li 4Ti 5O 12/CNTs composite are about 100–180nm,which is much smaller than that of pure Li 4Ti 5O 12sample.The connection between Li 4Ti 5O 12and CNTs is tightly.It has been known that there are many defective sites located at the ends and the sidewalls of CNTs [10].During the calcinations process these defective sites may play as the active centers,and Li 4Ti 5O 12nucleuses can easily form on it.Many nucleuses form on the surface of CNTs and grow in the same time,resulting smaller crystal particles of Li 4Ti 5O 12.The content of CNTs in the composite Li 4Ti 5O 12/CNTs was 8.2%measured by TG method,as shown in Fig.3
.
Fig.2.SEM images of Li 4Ti 5O 12(A)and Li 4Ti 5O 12/CNTs (B).
7758J.Huang,Z.Jiang /Electrochimica Acta 53(2008)
7756–7759
Fig.4.Charge and discharge curves of Li 4Ti 5O 12electrode at different rates:(A)0.085A g ?1(0.5C ),(B)0.170A g ?1(1C ),(C)0.340A g ?1(2C ),(D)0.850A g ?1(5C ),and (E)1.700A g ?1(10C ).
The charge–discharge curves of Li 4Ti 5O 12electrode at differ-ent current rates from 0.5C to 10C are shown in Fig.4.The curves were recorded after one charge–discharge cycle.Because Li 4Ti 5O 12used in lithium ion batteries is a kind of negative electrode mate-rial,the labels of ‘charge’and ‘discharge’in the ?gure correspond the cathodic and anodic processes,respectively,in order to indi-cate the situation in practical use of lithium ion batteries.As the charge–discharge rate increase,the capacity decreases quickly from 137mAh g ?1(0.5C )to 67mAh g ?1(10C ).Different from that,the CNT–Li 4Ti 5O 12electrode presents a great improvement on the rate capability,as shown in Fig.5.At the rates from 0.5C to 2C ,the vari-ations of discharge capacity and discharge plateau potential are very small.The discharge plateau potentials are near 1.55V,which is the reversible redox potential of spinel Li 4Ti 5O 12,as suggested by Scharner et al.[11].The margin between charge and discharge plateau potentials is only 60mV.It re?ects that the polarization of Li 4Ti 5O 12/CNTs electrode is very low.At the rate of 0.5C ,the discharge capacity is 150mAh for per gram Li 4Ti 5O 12/CNTs com-posite.If corrected by considering the content of Li 4Ti 5O 12in the Li 4Ti 5O 12/CNTs composite (91.8%),the discharge capacity of actual Li 4Ti 5O 12in the composite could be 163mAh g ?1,which is
close
Fig. 5.Charge and discharge curves of Li 4Ti 5O 12/CNTs at different rates:(A)0.085A g ?1(0.5C ),(B)0.170A g ?1(1C ),(C)0.340A g ?1(2C ),(D)0.850A g ?1(5C ),(E)1.700A g ?1(10C ),and (F)3.400A g ?1(20C
).
Fig.6.ac impedance spectra of Li 4Ti 5O 12/Li (A)and Li 4Ti 5O 12/CNTs/Li (B)cells at voltage of 1.56V.
to the theoretical value of 175mAh g ?1.At the current rates of 5C ,and 10C ,the discharge capacities of Li 4Ti 5O 12/CNTs electrodes are 145and 135mAh g ?1,respectively.The discharge capacity at 20C is 106mAh g ?1,which is the 70%of that obtained in the pro-cess discharged at 0.5C .These data show that the electrochemical performance of Li 4Ti 5O 12was greatly improved after modi?ed by https://www.360docs.net/doc/dc11396833.html,pare with Li 4Ti 5O 12,this high rate discharge ability could be attributed to two reasons:The enhancement of electronic conductivity by adding conducting CNTs,which connected with Li 4Ti 5O 12particles tightly;and much smaller size of Li 4Ti 5O 12parti-cles in Li 4Ti 5O 12/CNTs composite than that in Li 4Ti 5O 12sample.The smaller size (100–180nm)makes shorter distance for Li +diffusion in Li 4Ti 5O 12particle,and bene?ts the diffusion process.Moreover,the higher surface area of Li 4Ti 5O 12particles in Li 4Ti 5O 12/CNTs composite results the low current density and low electrochemical reaction resistance during discharge process.
The resistance and the electrochemical reaction properties of electrodes were further examined by ac impedance method.Fig.6presents the ac impedance spectra of Li 4Ti 5O 12(A)and Li 4Ti 5O 12/CNTs (B)electrodes at the stable voltage of 1.56V,respec-tively.Each curve consists of a depressed semicircle in high–middle frequency region and an oblique straight line in low frequency region.It is well known that the cross-section value of impedance spectra on the real Z axis at highest frequency is the resistance R s
,
Fig.7.Discharge capacity and charge–discharge ef?ciency versus cycle number for Li 4Ti 5O 12/CNTs electrode at the rates of 0.5–20C :(A)0.085A g ?1(0.5C ),(B)0.170A g ?1(1C ),(C)0.340A g ?1(2C ),(D)0.850A g ?1(5C ),(E)1.700A g ?1(10C ),and (F)3.400A g ?1(20C ).
J.Huang,Z.Jiang/Electrochimica Acta53(2008)7756–7759
7759
Fig.8.Cycling performance of Li4Ti5O12/CNTs at5C.
which corresponds the resistance of electrolyte mainly.For both systems they are very similar.The semicircle re?ects the electro-chemical reaction resistance and the double layer capacity of the electrode.The oblique line is corresponding to the Li+diffusion process.
For evaluating the cycling stability of Li4Ti5O12/CNTs,the elec-trode was progressively charged and discharged in a series stage with the rates from0.5C,1C,2C,5C,10C to20C as shown in Fig.7. For each stage the charge–discharge process was taken for10cycles. It was measured,that in the charge–discharged stages with0.5–10C the discharge capacity kept stable.It can even be cycled at20C.After such60charge–discharge cycles,the Li4Ti5O12/CNTs electrode was further charge–discharged at current rate of5C for another500 cycles.The discharge capacity was very stable as shown in Fig.8. In?rst cycle the discharge capacity was145mAh g?1,and after 500charge–discharge cycles the capacity remained as142mAh g?1. Fig.6also shows that the charge and discharge ef?ciency kept almost near100%in this long cycle period.These results show that Li4Ti5O12/CNTs electrode has high rate capability and capacity retention,which can be attributed to the improvement of conduc-tivity and the smaller size of Li4Ti5O12in Li4Ti5O12/CNTs composite by adding CNTs.
4.Conclusions
The electrochemical performance of Li4Ti5O12could be greatly improved by modi?cation of CNTs.The prepared Li4Ti5O12/CNTs composite presented an excellent rate capability and capacity retention.It can be charge–discharged even at discharge rate of 20C.After500cycles at5C the discharge capacity maintained as 142mAh g?1,which was97.9%of initial discharge capacity.This result shows that Li4Ti5O12/CNTs composite was a very promising material and might be used in high rate lithium ion batteries.
Acknowledgement
This work was supported by National Nature Science Foundation of China.
References
[1]K.M.Colbow,J.R.Dahn,R.R.Haering,J.Power Sources26(1989)397.
[2]K.Zaghib,M.Armand,M.Gauthier,J.Electrochem.Soc.145(1998)3135.
[3]S.W.Woo,K.D.K.Kanamura,Electrochim.Acta53(2007)79.
[4]P.Kubiak,A.Garcia,M.Womes,et al.,J.Power Sources119–121(2003)626.
[5]C.H.Chen,J.T.Vaughey,A.N.Jansen,et al.,J.Electrochem.Soc.148(1)(2001)
A102.
[6]A.D.Robertson,L.Trevino,H.Tukamoto,J.T.S.Irvine,J.Power Sources81–82
(1999)352.
[7]K.Mukai,K.Ariyoshi,T.Ohzuku,J.Power Sources146(2005)213.
[8]S.Huang,Z.Wen,J.Zhang,X.Yang,Electrochim.Acta52(2007)3704.
[9]G.J.Wang,J.Gao,L.J.Fua,N.H.Zhao,Y.P.Wua,T.Takamura,J.Power Sources174
(2007)1109.
[10]C.E.Banks,R.R.Moore,T.J.Davis,https://www.360docs.net/doc/dc11396833.html,potom,https://www.360docs.net/doc/dc11396833.html,mun.16(2004)
1804.
[11]S.Scharner,W.Weppner,P.Schmid-Beurmann,J.Electrochem.Soc.146(1999)
857.
