Dissolution+with+EVOH+in+DMSO+and+preliminary+characterization+of+EVOH+membrane

Proceedings of 2011 International Conference
on Advanced Fibers and Polymer Materials,
Aug. 15 – 17, Shanghai, China
Dissolution with EVOH in DMSO and preliminary
characterization of EVOH membrane
WANG Zhewei b, SHEN Xinyuan a, b, LU Xinkun b
(a) State Key Laboratory for Modification of Chemical Fibers and Polymer Materials,
Donghua University, Shanghai 201620, P. R. China.
(b) College of Material Science and Engineering, Donghua University, Shanghai 201620, P. R. China.
Corresponding author: SHEN Xinyuan, shenxy@https://www.360docs.net/doc/d413819803.html,
Abstract:Poly(ethylene-vinyl alcohol) (EVOH) has good biocompatibility, hydrophilicity and other excellent properties. Because of these properties, EVOH is considered to be an ideal material which can be used in the field of biomedical and water treatment. In this article, EVOH was dissolved in DMSO. EVOH membranes were prepared, while PEG 200 was added as pore-causing agent. FT-IR and TG were used to investigate the dissolution of EVOH in DMSO. Rheological behaviour of EVOH/DMSO solution was studied by means of the rheometer. Morphology of EVOH membranes was featured by SEM photographs. The result is prepared for further research on EVOH hollow fiber spinning.
Key words:Poly(ethylene-vinyl alcohol); DMSO; dissolution; membrane
1 Introduction
Poly(ethylene-vinyl alcohol) (EVOH) is usually obtained by saponification of ethylene-vinyl acetate copolymer(EVA). The main properties of EVOH depend on the content of ethylene. Generally speaking, EVOH has high crystallinity and excellent gas barrier property so it has been widely used in food package [1]. In addition, researchers found that EVOH has excellent biocompatibility and hydrophilicity so the application of EVOH in the field of biomedical and water treatment attract more and more interests.
The biocompatibility of usual hemodialysis membranes is not very good, so during the process of hemodialysis, heparins must be used to resist coagulation of blood. But the refine of heparins is very difficult and expensive. Diseases, such as osteoporosis, will happen if patients use heparins frequently [1-3]. Because of its unique properties, especially EVOH membranes can prevent adsorption of protein and coagulation of blood etc, EVOH can be used to make hemodialysis membranes.
In this paper, EVOH was dissolved in DMSO and then EVOH membranes were prepared using the direct immersion precipitation method. FT-IR, TG, rheometer and SEM were used to characteristics the samples.
2 Experimental
2.1 Materials
EVOH containing 38 mol% ethylene was purchased from Nippon Gohsei Co. Dimethyl sulfoxide(DMSO, AR) and glycerol (AR) were purchased from Shanghai Richjoint Chemical Reagent Co. PEG (M n=200, AR) was purchased from Sinopharm Chemical Reagent Co. These reagents were all used without further purification.
2.2 Preparation of the solution
The preparation of the EVOH solution was carried out as follows. An appropriate amount of EVOH was dissolved in DMSO to form a homogeneous solution. PEG 200 was added as pore-casing agent. 15%, 20%, 25% and 30% EVOH/DMSO solution have been prepared for rheological measurements.
2.3 Preparation of the membrane
EVOH was dissolved in DMSO at 40, 50, 70℃. The dissolving time of these three samples is 3, 5 and 7 hours respectively. Membranes were prepared using the direct immersion precipitation method. The solution was cast on a glass plate using a glass stick and then immersed directly in water to effect precipitation. The regenerated EVOH membrane was immersed in water for 6 hours to remove DMSO remained in the membranes and then immersed in 30% glycerol/H2O for 4 hours, followed by dried in the room temperature.
2.4 Fourier transform infrared spectroscopy (FT-IR) analysis
The infrared spectra was recorded using a Fourier transform infrared spectrometer (FT-IR, Nicolet 8700, USA) in a spectral range of 400-4000cm?1 at a resolution of 4cm?1. The scan speed was 0.2 cm/s and 32 scans were taken per sample.
2.5 Thermo gravimetric- differential thermal (TG) analysis
The TG instrument (TG 309F1, USA) was used in this study to test the changes of material mass loss and heat release with temperature. Test conditions were as follows: using an open aluminum sample cell in a nitrogen flow of 20 ml/min from 30℃ to 550℃, and 10℃/min as the rate of increasing temperature. TG–DTA data were collected every second.
2.6 Rheological measurements
The rheological measurements were made on R/S-Plus rheometer (Brookfield Co., USA). The rheological properties of the EVOH/DMSO solution were determined at 20℃, 30℃, 40℃, 50℃, 60℃, 70℃ respectively.
2.7 Microscopic analysis
The prepared membrane was examined for morphological details by JEOL JSM-5600LV scanning electron microscope (SEM) with an acceleration voltage of 10kV.
3 Results and discussion
3.1 Dissolution investigations of EVOH in DMSO
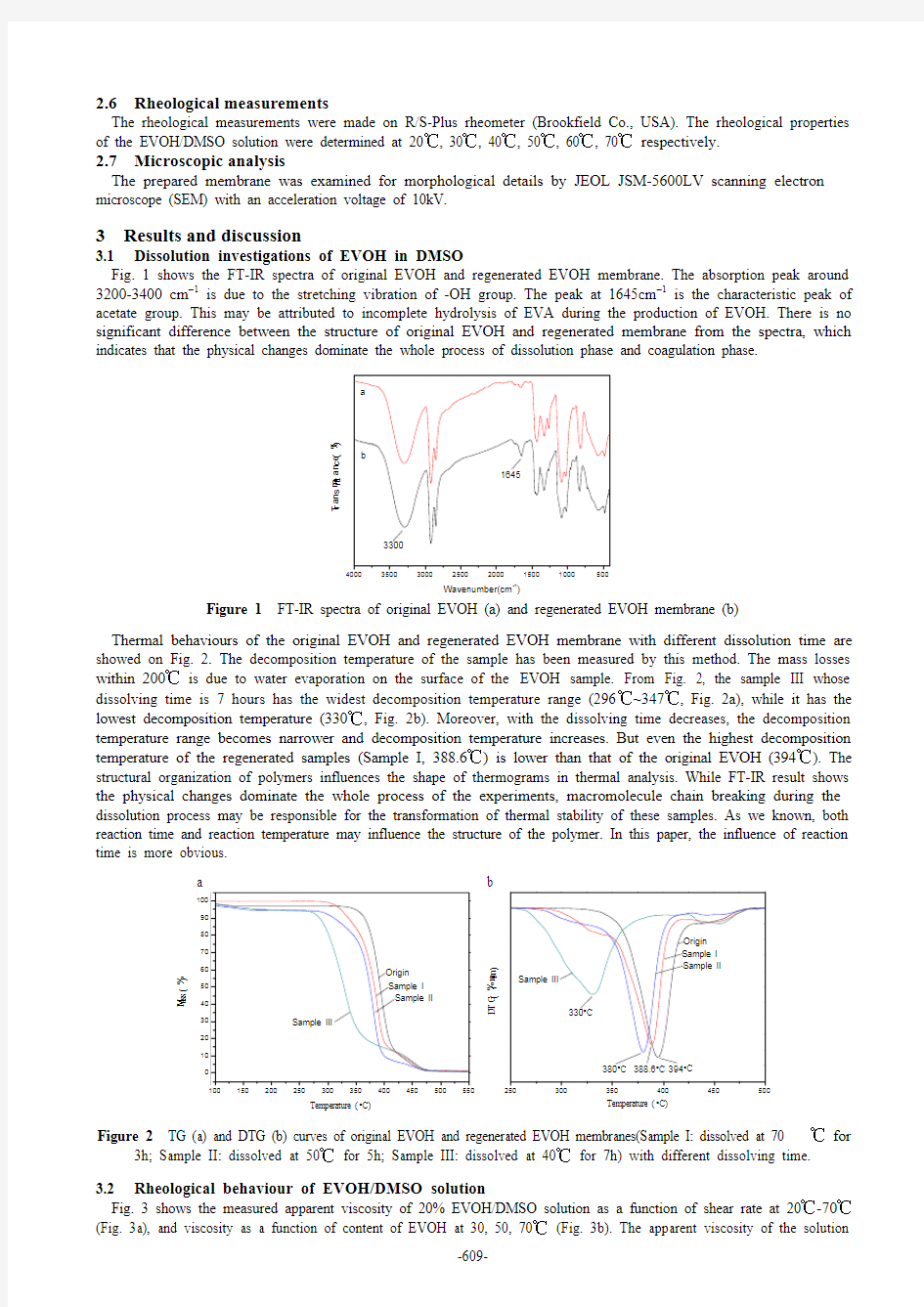
Fig. 1 shows the FT-IR spectra of original EVOH and regenerated EVOH membrane. The absorption peak around 3200-3400 cm ?1 is due to the stretching vibration of -OH group. The peak at 1645cm ?1 is the characteristic peak of acetate group. This may be attributed to incomplete hydrolysis of EVA during the production of EVOH. There is no significant difference between the structure of original EVOH and regenerated membrane from the spectra, which indicates that the physical changes dominate the whole process of dissolution phase and coagulation phase.
4000
3500
3000
2500
2000
1500
1000
500
T r a n s m i t t a n c e (%)
Wavenumber(cm -1
)
3300
1645
a
b
Figure 1 FT-IR spectra of original EVOH (a) and regenerated EVOH membrane (b)
Thermal behaviours of the original EVOH and regenerated EVOH membrane with different dissolution time are showed on Fig. 2. The decomposition temperature of the sample has been measured by this method. The mass losses within 200℃ is due to water evaporation on the surface of the EVOH sample. From Fig. 2, the sample III whose dissolving time is 7 hours has the widest decomposition temperature range (296℃~347℃, Fig. 2a), while it has the lowest decomposition temperature (330℃, Fig. 2b). Moreover, with the dissolving time decreases, the decomposition temperature range becomes narrower and decomposition temperature increases. But even the highest decomposition temperature of the regenerated samples (Sample I, 388.6℃) is lower than that of the original EVOH (394℃). The structural organization of polymers influences the shape of thermograms in thermal analysis. While FT-IR result shows the physical changes dominate the whole process of the experiments, macromolecule chain breaking during the dissolution process may be responsible for the transformation of thermal stability of these samples. As we known, both reaction time and reaction temperature may influence the structure of the polymer. In this paper, the influence of reaction time is more obvious.
Figure 2 TG (a) and DTG (b) curves of original EVOH and regenerated EVOH membranes(Sample I: dissolved at 70℃ for
3h; Sample II: dissolved at 50℃ for 5h; Sample III: dissolved at 40℃ for 7h) with different dissolving time.
3.2 Rheological behaviour of EVOH/DMSO solution
Fig. 3 shows the measured apparent viscosity of 20% EVOH/DMSO solution as a function of shear rate at 20℃-70℃ (Fig. 3a), and viscosity as a function of content of EVOH at 30, 50, 70℃ (Fig. 3b). The apparent viscosity of the solution
250
300
350
400
450
500
Sample III
Sample I Sample II
394°C
388.6°C 380°C
D T G (%/m i n )
Temperature (°C)
330°C
Origin 100
150
200
250
300
350
400450500550
010203040506070
8090100Sample III
Sample II
Temperature (°C)
M a s s (%)
Origin Sample I a
b
Figure 3 (a) Rheological behaviour of 20% EVOH/DMSO solution at different temperatures; (b) Relation between
content of EVOH and zero shear viscosity of EVOH/DMSO solution. decreases while the shear rate increases, which shows the shear thinning behavior. As the temperature decreases the apparent viscosity increases and the shear tinning behaviour becomes more obvious (Fig. 3a). Further it is noticed that the apparent viscosity at 20℃ is approximately six times as that at 70℃ (Fig. 3a), indicating a strong temperature effect on the viscosity of the EVOH/DMSO solution. This can be concluded from Fig. 3b as well. Also in Fig. 3b, it shows the zero shear viscosity has a obvious tendency to increase as the concentration of EVOH increases.
3.3 Morphology of EVOH membrane
The cross section of the regenerated EVOH membrane is evaluated by scanning electron microscopy. As can be seen, the membrane presented an asymmetric structure consisting of many thin and long pores orderly arranged on the top and macro voids spread near the bottom of the membrane (Fig. 4a). Fig. 4b shows the magnified photograph of a macro void near the bottom. The wall of the macro void which consisted of EVOH particles is skinless. The asymmetric structure is due to the difference of the diffusion rate between the top and the bottom during the coagulation phase. As the bottom of the membrane touched the glass, the diffusion rate at the bottom is much slower than that near the top [4, 5].
Figure 4 SEM photographs of cross section of the regenerated EVOH membrane
with PEG200 as pore-causing agent.
4 Conclusions
In this work, EVOH was dissolved in DMSO and PEG 200 was added as pore-causing agent. Membranes were prepared using the direct immersion precipitation method. From the analysis of FT-IR and TG, we find that the physical changes dominate the whole process of dissolution phase and coagulation phase. The influence of dissolving time to the thermal stability of regenerated EVOH is more obvious than that of the dissolving temperature. Both the temperature factor and concentration factor have been considered during the rheological behaviour analysis of EVOH/DMSO solutions. The apparent viscosity of EVOH/DMSO solution increases as the temperature increases or the concentration of EVOH decreases. SEM study presented an asymmetric structure consisting of many thin and long pores orderly arranged on the top and macro voids spread near the bottom of the membrane. The asymmetric structure may be due to the difference of the diffusion rate between the top and the bottom during the coagulation phase. Further investigations based on the results can be made on the spinning of EVOH hollow fiber for dialysis membranes in the field of biomedical science.
Acknowledgement
This research was financially supported by State Key Laboratory for Modification of Chemical Fibers and Polymer
(b)
V i s c o s i t y (P a ?s )
Shear rate (s -1
)
a
V i s c o s i t y (P a ?s )
C(%)
b
(a)
Top
Materials (No. LZ0902).
References
[1] J. Lima, M. Felisberti.J. MEMBRANE SCI., 2009, 344, 237-243.
[2] D. Cava, L. Cabedo, E. Gimenez, et al. Polymer Testing, 2006, 25, 254-261.
[3] H. Matsuyama, T. Iwatani, Y. Kitamura, et al. J. Appl. Polymer Sci., 2001, 79, 2449-2455.
[4] T. H. Young, C. W. Lin, L. P. Cheng, et al. Biomaterials, 2001, 22, 1771-1777.
[5] T. H. Young, L. P. Cheng, H. Y. Lin. Polymer, 2000, 41, 377-383.
Dissolution with EVOH in DMSO and preliminary characterization
of EVOH membrane
作者:WANG Zhewei, SHEN Xinyuan, LU Xinkun
作者单位:WANG Zhewei,LU Xinkun(College of Material Science and Engineering, Donghua University, Shanghai 201620, P.R.China), SHEN Xinyuan(State Key Laboratory for Modification of Chemical Fibers and
Polymer Materials,Donghua University, Shanghai 201620, P.R.China College of Material Science
and Engineering, Donghua University, Shanghai 201620, P.R.China)
本文链接:https://www.360docs.net/doc/d413819803.html,/Conference_WFHYXW471017.aspx
