水的粘度计算表
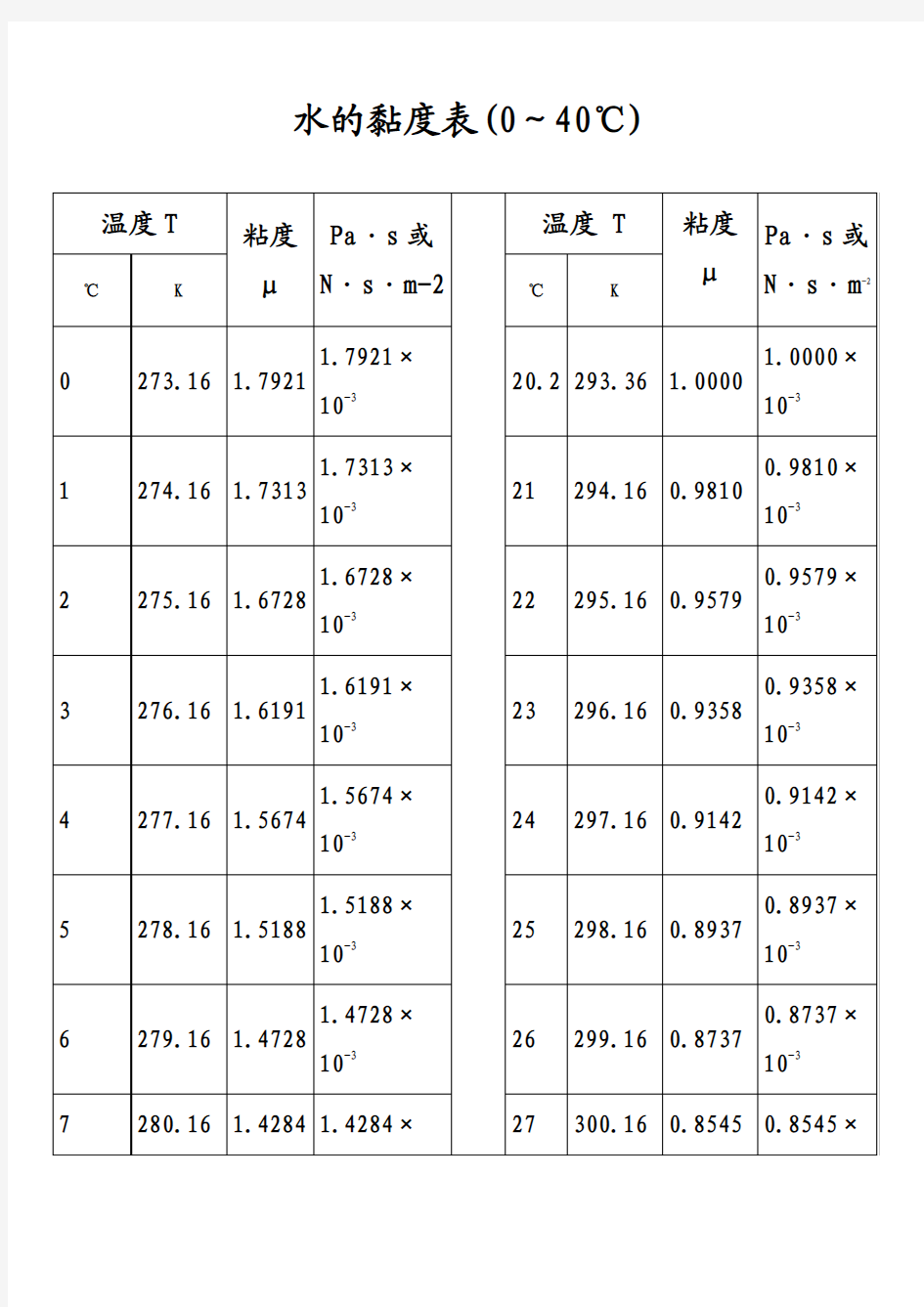

水的黏度表(0~40℃)
水的物理性质
F3 Viscosity decreases with pressure
(at temperatures below 33°C)
Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.
Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces [558] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due
to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.
The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.
Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.
水的粘度计算表-水的动力粘度计算公式
水的黏度表(0?40 C)
水的物理性质
F3 Viscosity decreases with p ressure (at temp eratures below 33 Water's p ressure-viscosity behavior [534] can be explained by the in creased p ressure (up to about 150 MPa) caus ing deformatio n, so reduci ng the stre ngth of the hydroge n-bon ded n etwork, which is also p artially res pon sible for the viscosity. This reduct ion in cohesivity more tha n compen sates for the reduced void volume. It is thus a direct con seque nee of the bala nee betwee n hydroge n bonding effects and the van der Waals dis persion forces [558] in water; hydroge n bonding p revaili ng at lower temp eratures and p ressures. At higher p ressures (and den sities), the bala nee betwee n hydroge n bonding effects and the van der Waals dis persi on forces is tipped in favor of the dis persion forces and the rema ining hydroge n bonds are stron ger due Viscous flow occurs by molecules movi ng through the voids that exist betwee n them. As the p ressure in creases, the volume decreases and the volume of these voids reduces, so no rmally in creas ing p ressure in creases the viscosity. |:| k -二 _ r 1 3ire S C 去 * . i i screr - 丁" \ . / . 一 '气:r J J: V .; r "舄 ■ 3 口二 K n PV ■ ■ L T 三 n 曲 ? ■ 5 M r 丐 町寸 -; J 百* " T N ; 【 I bl ■呻口 " 口寸津 a “ d c i 0 290 八 rao 800 i woo Pressure, MPa g 亠 C) Co? 4 — □ ] J %一 M J s 」气1 □ u 古 气 a 15 ?” ”〕 阳 "1 ■ \ ■ ID % ;: s' ¥ 口『 屮 n ◎ 9 r 奇 * =' f f- ::[ 丄 备 IT 记 |B - 3 D ■i 电- 'u O 丰759勺; 】I -一 11 L . P
测定水的粘度系数
水粘度系数的测定 ——车辆工程4班 刘天威 20110402406 1.实验目的 1)掌握用落球法测定水的粘度系数。 2)掌握游标卡尺,停表等实验仪器的使用;了解一种减小实验误差的方法;学习用标准算数误差表示实验结果。 2实验仪器 玻璃圆筒内的待测水,圆筒(有两条标线N1和N2),米尺,停表,游标卡尺,镊子,培养皿,小球(3颗)。 3实验原理 在稳定流动的液体中,因为各层流体的速度不同,因而在相邻的流体层之间会产生切向力,此切向力即为粘性力。实验指出,此粘性力f 正比于两流层间的接触面积S 和该处的速度梯度dv/dx ,即 f =n (dv/dx )S 这就是牛顿粘性定律。式中,比例系数n 称为流体的粘度系数,它只与流体本身的性质和温度有关。 由于液体的粘性,物体在液体中运动时要受到液体的摩擦阻力,当小球在液体中下落时,若下落速度很小,球也很小,且液体在各方向上是无限宽广的,则由斯托克斯公式有 f =6πn r v 式中,v 是小球下落的速度,r 是小球的半径,n 是液体的粘度系数。 小球在液体中下落时,不仅受到流体的阻力,还有自身的重力和水的浮力,三力平衡时,小球等速下落。由三力平衡得 4/3 r v n 6g 3/4g r π03ππρρ+= 式中,0ρ是水的密度,可得 v 9/gr )-(220ρρ=n 因为液体放在容器中总不是无限广阔的,所以小球在无限广阔的液体中下落是不可能的。只考虑管壁的影响。由于小球作匀速运动,则v=L/t ,并以r=d/2,R=D/2,(d 是小球直径,D 是液注直径,L 是小球作匀速运动的距离)得 ) (ρρD /d 71.21L 18/t gd )-(20+=n 4实验装置
水的流量与管径的压力的计算公式
1、如何用潜水泵的管径来计算水的流量 Q=4.44F*((p2- p1)/ P 0.5 流量Q,流通面积F,前后压力差p2-p1,密度p, 0.5是表示0.5次方。以上全部为国际单位制。适用介质为液体,如气体需乘以一系数。 由Q = F*v可算出与管径关系。 以上为稳定流动公式。 2、请问流水的流量与管径的压力的计算公式是什么? 管道的内直径205mm,高度120m,管道长度是1800m,请问每小时的流量是多少?管道的压力是多少,管道需要采用多厚无缝钢管? 问题补充: 从高度为120米的地方用一根管道内直径为205mm管道长度是1800米放水下来,请问每个小时能流多少方水?管道的出口压力是多少?在管道出口封闭的情况下管道里装满水,管道底压力有多大 Q=[H/ ( SL )]人(1/2) 式中管道比阻S=10.3* 门人2/9人5.33)=10.3*0.012人2/(0.205人5.33)=6.911 把H=120米,L=1800米及S=6.911代入流量公式得 Q=[120/ ( 6.911*1800 ) ]A(1/2) = 0.0982 立方米/秒= 353.5 立方米/时 在管道出口封闭的情况下管道里装满水,管道出口挡板的压力可按静水压力计算: 管道出口挡板中心的静水压强P=pgH=1000*9.8*180=1764000 帕 管道出口挡板的静水总压力为 F : F=P* (3.14dA2 /4 ) =1764000* (3.14*0.205八2 /4 ) =58193.7 牛顿 3、管径与流量的计算公式 请问2寸管径的水管,在0.2MPA压力的情况下每小时的流量是多少?这个公式是如何计算出来的? 流体在水平圆管中作层流运动时,其体积流量Q与管子两端的压强差Ap管的半径r,长 度L,以及流体的粘滞系数n有以下关系: Q=nX「人4 XA p/(8 n L) 4、面积,流量,速度,压力之间的关系和换算方法、 对于理想流体,管道中速度与压强关系:P + p V2/2 =常数,V2表示速度的平方。 流量二速度X面积,用符号表示Q =VS 5、管径、压力与流量的计算方法 流体在一定时间内通过某一横断面的容积或重量称为流量。用容积表示流量单位是L/s或
水的粘度计算表
水的粘度计算表 Company number:【WTUT-WT88Y-W8BBGB-BWYTT-19998】
水的黏度表(0~40℃)
水的物理性质
360 109 370 264 F3 Viscosity decreases with pressure (at temperatures below 33°C) Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity. Water's pressure-viscosity behavior [] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the between hydrogen bonding effects and the van der Waals dispersion forces [] in water; hydrogen bonding prevailing at lower
水力公式
长距离输水管道水力计算公式的选用 1. 常用的水力计算公式: 供水工程中的管道水力计算一般均按照均匀流计算,目前工程设计中普遍采用的管道水力计算公式有: 达西(DARCY )公式: g d v l h f 22 **=λ (1) 谢才(chezy )公式: i R C v **= (2) 海澄-威廉(HAZEN-WILIAMS )公式: 87 .4852.1852.167.10d C l Q h h f ***= (3) 式中h f ------------沿程损失,m λ―――沿程阻力系数 l ――管段长度,m d-----管道计算内径,m g----重力加速度,m/s 2 C----谢才系数 i----水力坡降; R ―――水力半径,m Q ―――管道流量m/s 2 v----流速 m/s C n ----海澄――威廉系数 其中大西公式,谢才公式对于管道和明渠的水力计算都适用。海澄-威廉公式影响参数较小,作为一个传统公式,在国内外被广泛用于管网系统计算。三种水力计算公式中 ,与管道内壁粗糙程度相关的系数均是影响计算结果的重要参数。 2. 规范中水力计算公式的规定 3. 查阅室外给水设计规范及其他各管道设计规范,针对不同的设计条件,推荐采用的水力 计算公式也有所差异,见表1: 表1 各规范推荐采用的水力计算公式
4. 公式的适用范围: 3.1达西公式 达西公式是基于圆管层流运动推导出来的均匀流沿程损失普遍计算公式,该式适用于任何截面形状的光滑或粗糙管内的层流和紊流。公式中沿程阻力系数λ值的确定是水头损失计 算的关键,一般采用经验公式计算得出。舍维列夫公式,布拉修斯公式及柯列勃洛克(C.F.COLEBROOK )公式均是针对工业管道条件计算λ值的著名经验公式。 舍维列夫公式的导出条件是水温10℃,运动粘度1.3*10-6 m 2/s,适用于旧钢管和旧铸铁管,紊流过渡区及粗糙度区.该公式在国内运用教广. 柯列勃洛可公式 )Re 51 .27.3lg( 21 λ λ +?*-=d (Δ为当量粗糙度,Re 为雷诺数)是根据大量工业管道试验资料提出的工业管道过渡区λ值计算公式,该式实际上是泥古拉兹光滑区公式和粗糙区公式的结合,适用范围为4000 ”=上 P _ 从单位中看出,吕帧nr 含运动要索(号间和长度)'不含动 力要素。所以它更能反映流体的运动特性■运 其流动性越好。 * J 冠度莉示另对动力粘度均有影响,但压力的影响很小?通常只 需等虑温度的影响。温度对液休和气体粘性的影响截然不同遇J 升高时,液体的粘性降低,气体的粘性增加。这是因为液体的粘性 连要晁液斥於手之I'可的内茶万引竈丽?度升高时,内聚力减弱, 故粘性降低『而造成气体粘性的主要原因在于气体分子的热运动, 温度越高?热运动越强烈,所以粘性就越大。 不同温度下?水和空气的粘度可从表1七和1-4中査得。 温度 /V Wf 度 p /kg ? m~3 禎度 7 /N ? n>7 力 xpa 动 "/ 运动曾 y X 10^ /m 2 ? 8 丨 弹性模数 E X 10$ /Pa 0 999.8 9805 1.781 1.785 2.02 5 1000. 0 9807 1.518 1.519 2. 06 10 999.7 9804 1.307 1. 306 2.10 ? 15 999. 1 9798 1. 139 1.139 2.15 20 998.2 9789 1.002 1 1? 003 2. 18 25 997.0 9777 0. 890 0. 893 ? ? 2. 22 30 995.7 9764 0. 798 0. 800 2. 25 40 992. 2 9730 0. 653 0. 658 2. 28 50 988.0 - 9689 0. 547 0. 553 2. 29 60 983. 2 9642 0. 466 0. 474 2. 28 70 977.8 9589 0. 404 0. 413 2. 25 80 971.8 9530 0. 354 0. 364 2. 20 90 955.3 9468 0.315 0. 326 2.14 ioo ] 95g ?4 9399 | 0? 282 | 0.294 [ 2? 07 _ 表1-3 (1-13) 标准大气下水的物理性质 st 04 1A 77 水的黏度表(0~40℃) 温度T Pa ·s 或 粘度μ 温度T 粘度μPa·s 或℃K N·s·m-2 ℃K N·s·m-2 0 273.16 1.7921 1.7921 × 10 -3 20.2 293.36 1.0000 1.0000 × 10 -3 1 274.16 1.7313 1.7313 × 10 -3 21 294.16 0.9810 0.9810 × 10 -3 2 275.16 1.6728 1.6728 × 10 -3 22 295.16 0.9579 0.9579 × 10 -3 3 276.16 1.6191 1.6191 × 10 -3 23 296.16 0.9358 0.9358 × 10 -3 4 277.16 1.5674 1.5674 × 10 -3 24 297.16 0.9142 0.9142 × 10 -3 5 278.1 6 1.5188 1.5188 × 10 -3 25 298.16 0.8937 0.8937 × 10 -3 6 279.16 1.4728 1.4728 × 10 -3 26 299.16 0.8737 0.8737 × 10 -3 7 280.16 1.4284 1.4284 × 10 -3 27 300.16 0.8545 0.8545 × 10 -3 8 281.16 1.3860 1.3860 × 10 -3 28 301.16 0.8360 0.8360 × 10 -3 9 282.16 1.3462 1.3462 × 10 -3 29 302.16 0.8180 0.8180 × 10 -3 10 283.16 1.3077 1.3077 × 10 -3 30 303.16 0.8007 0.8007 × 10 -3 11 284.16 1.2713 1.2713 × 10 -3 31 304.16 0.7840 0.7840 × 10 -3 12 285.16 1.2363 1.2363 × 10 -3 32 305.16 0.7679 0.7679 × 10 -3 13 286.16 1.2028 1.2028 × 10 -3 33 306.16 0.7523 0.7523 × 10 -3 14 287.16 1.1709 1.1709 × 10 -3 34 307.16 0.7371 0.7371 × 10 -3 15 288.16 1.1404 1.1404 × 35 308.16 0.7225 0.7225 ×10 -3 10 -3 16 289.16 1.1111 1.1111 ×36 309.16 0.7085 0.7085 × 0.1 1.0 2.5 5.010.015.020.025.030.035.040.045.050.055.060.065.070.075.080.001750.01750.01750.01750.01750.01740.01740.01740.01740.01730.01730.01730.01720.01720.01720.01720.01710.01710.01710.0101300.01300.01300.01300.01300.01300.01300.01290.01290.01290.01290.01290.01280.01280.01280.01280.01280.01280.01280.0201000.01000.01000.01000.01000.01000.0999.0999.0998.0997.0997.0996.0996.0995.0994.0994.0993.0992.0991.030797.0797.0797.0797.0797.0797.0797.0797.0797.0797.0797.0797.0796.0796.0796.0796.0796.0796.0796.040651.0651.0652.0652.0652.0652.0653.0653.0653.0653.0654.0654.0654.0654.0655.0655.0655.0656.0656.050544.0544.0544.0545.0545.0546.0546.0547.0547.0548.0548.0549.0549.0550.0550.0551.0551.0554.0554.060463.0463.0563.0464.0464.0465.0466.0467.0467.0468.0469.0469.0470.0471.0471.0472.0473.0473.0474.070400.0401.0401.0401.0402.0403.0404.0404.0405.0406.0407.0408.0408.0409.0410.0411.0412.0412.0413.080351.0351.0351.0352.0353.0354.0355.0355.0356.0357.0358.0359.0360.0361.0362.0362.0363.0364.0365.090311.0311.0312.0312.0313.0314.0315.0316.0317.0318.0319.0320.0321.0322.0323.0324.0325.0326.0326.010012.1279.0279.0280.0281.0282.0283.0284.0285.0286.0287.0288.0289.0290.0291.0292.0293.0294.0295.011012.5252.0253.0254.0255.0256.0257.0258.0259.0260.0261.0262.0263.0264.0265.0266.0267.0268.0269.012012.9230.0230.0231.0232.0233.0234.0235.0236.0237.0238.0239.0241.0242.0243.0244.0245.0246.0247.013013.3211.0212.0212.0213.0214.0215.0216.0218.0219.0220.0221.0222.0223.0224.0225.0226.0227.0228.014013.7195.0195.0196.0197.0198.0199.0200.0201.0203.0204.0205.0206.0207.0208.0209.0210.0211.0213.015014.2181.0182.0182.0183.0184.0185.0187.0188.0189.0190.0191.0192.0193.0194.0196.0197.0198.0199.016014.6169.0169.0170.0171.0172.0173.0175.0176.0177.0178.0179.0180.0181.0183.0184.0185.0186.0187.017015.0159.0159.0160.0161.0162.0163.0164.0165.0166.0168.0169.0170.0171.0172.0173.0174.0176.0177.018015.415.0150.0150.0151.0153.0154.0155.0156.0157.0158.0159.0161.0162.0163.0164.0165.0166.0168.019015.815.4141.0142.0143.0144.0145.0147.0148.0149.0156.0151.0153.0154.0155.0156.0157.0158.0160.020016.215.9134.0135.0136.0137.0138.0139.0141.0142.0143.0144.0145.0146.0148.0149.0150.0151.0152.021016.616.3127.0128.0129.0130.0132.0133.0134.0135.0136.0138.0139.0140.0141.0142.0143.0145.0146.022017.016.7122.0122.0123.0124.0126.0127.0128.0129.0130.0132.0133.0134.0135.0136.0138.0139.0140.023017.417.216.8117.0118.0119.0120.0122.0123.0124.0125.0126.0128.0129.0130.0131.0132.0133.0134.024017.817.617.3112.0113.0114.0115.0117.0118.0119.0120.0121.0123.0124.0125.0126.0127.0128.0129.025018.218.117.8107.0109.0110.0111.0112.0113.0115.0116.0117.0118.0119.0121.0122.0123.0124.0126.026018.618.518.3103.0104.0106.0107.0108.0109.0111.0112.0113.0114.0115.0117.0118.0119.0120.0122.027019.018.918.718.4101.0102.0103.0104.0105.0107.0108.0109.0110.0112.0113.0114.0115.0117.0118.028019.419.419.219.097.098.299.4101.0102.0103.0104.0106.0107.0108.0109.0111.0112.0113.0114.029019.819.819.719.593.694.996.197.498.699.9101.0102.0104.0105.0106.0107.0109.0110.0111.030020.320.220.220.190.591.793.094.395.596.898.199.3101.0102.0103.0104.0106.0107.0108.031020.720.720.620.686.688.389.491.192.493.894.996.197.598.499.7101.0102.0103.0103.032021.121.121.121.121.684.585.987.789.290.692.092.994.395.596.697.899.0100.0102.033021.421.521.621.722.480.482.184.185.887.588.890.091.192.493.594.896.097.298.334021.921.922.022.223.076.078.280.282.184.085.586.988.089.290.591.893.194.395.535022.322.322.422.723.625.473.075.978.580.282.183.684.886.287.588.990.291.492.636022.722.822.923.224.125.766.870.673.776.378.380.381.583.284.786.287.488.790.037023.123.223.423.724.626.029.664.368.572.074.276.778.380.281.983.584.986.287.538023.523.623.824.225.026.328.853.763.267.570.673.075.177.379.180.982.383.784.939024.924.024.224.625.426.628.634.956.163.067.069.972.374.376.378.279.781.282.640024.324.424.625.025.826.928.632.145.757.362.866.569.371.773.775.577.379.080.3410 24.7 24.8 25.0 25.4 26.1 27.2 28.7 31.3 38.1 50.4 58.1 62.8 66.2 68.9 71.1 73.1 74.9 76.4 77.9 温度t/℃水和水蒸气的动力粘度μ×10E6/(Pa ·s ) 压力 p/MPa 水的黏度表(0~40℃) 水的物理性质 F3 Viscosity decreases with pressure (at temperatures below 33°C) Viscous flow occurs by molecules moving through thevoids that exist betweenthem。 Asthe pressure increa ses,the volumedecreases and the volume of these voids re duces, sonormally increasingpressure increases the v iscosity. Water's pressure—viscosity behavior [534] can beexplained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength ofthe hydrog en-bonded network, which is also partially responsible for the viscosity. This reductionin cohesivity mo re than compensates for the reduced voidvolume. It is thus a directconsequence ofthe balance between hydro gen bonding effects and the van der Waals dispersion forces[558] in water; hydrogen bonding prevailing at lower temperatures and pressures。At higher pressures (and densi ties), the balance between hydrogen bonding effects and the va nder Waals dispersion forces is tipped in favor ofthe dispersion forces and the remaininghydrogen bonds are stronger due to thecloser proximity of thecontributingoxygen atoms [655]. Viscosity, then, increases with pressu re。 The dashed line(opposite)indicates theviscosity minima. 水处理常用计算公式汇总 碳源计算公式 1、碳源选择 通常反硝化可利用的碳源分为快速碳源(如甲醇、乙酸、乙酸钠等)、慢速碳源(如淀粉、蛋白质、葡萄糖等)和细胞物质。不同的外加碳源对系统的反硝化影响不同,即使外加碳投加量相同,反硝化效果也不同。 与慢速碳源和细胞物质相比,甲醇、乙醇、乙酸、乙酸钠等快速碳源的反硝化速率最快,因此应用较多。表1 对比了四种快速碳源的性能。 2、碳源投加量计算 1)氮平衡 进水总氮和出水总氮均包括各种形态的氮。进水总氮主要是氨氮和有机氮,出水总氮主要是硝态氮和有机氮。 进水总氮进入到生物反应池,一部分通过反硝化作用排入大气,一部分通过同化作用进入活性污泥中,剩余的出水总氮需满足相关水质排放要求。 2)碳源投加量计算 同化作用进入污泥中的氮按BOD5 去除量的5%计,即0.05(Si-Se),其中Si、Se 分别为进水和出水的BOD5 浓度。 反硝化作用去除的氮与反硝化工艺缺氧池容大小和进水BOD5 浓度有关。 反硝化设计参数的概念,是将其定义为反硝化的硝态氮浓度与进水BOD5 浓度之比, 表示为Kde(kgNO3--N/kgBOD5)。 由此可算出反硝化去除的硝态氮 [NO3--N]=KdeSi。 从理论上讲,反硝化1kg 硝态氮消耗2.86kgBOD5,即: Kde=1/2.86(kg NO3--N/kgBOD5) =0.35(kg NO3--N/kgBOD5) 污水处理厂需消耗外加碳源对应氮量的计算公式为: N=Ne 计-NsNe 计=Ni - KdeSi - 0.05(Si-Se) 式中: N—需消耗外加碳源对应氮量,mg/L; Ne 计—根据设计的污水水质和设计的工艺参数计算出能达到的出水总氮,mg/L;Ns— 二沉池出水总氮排放标准, mg/L; Kde—0.35,kg NO3--N/kgBOD5; Si—进水BOD5 浓度,mg/L; Se—出水BOD5 浓度,mg/L; Ne 计需通过建立氮平衡方程计算,生化反应系统的氮平衡见图1。 通过计算出的氮量,折算成需消耗的碳量。 除磷计算公式 1、除磷药剂投加量的计算 国内较常用的是铁盐或铝盐,它们与磷的化学反应如式(1)?(2)? Al3++PO3-4→AlPO4↓(1) Fe3++PO3-4→FePO4↓(2)非常用用的流体力学计算常用查表(水、空气中度、不同温度动力粘度、粘度)
水的粘度计算表
水和水蒸气的动力粘度表
水的粘度计算表水的动力粘度计算公式
水处理常用计算公式汇总
