Tafenoquine-Succinate-SDS-MedChemExpress
伏硫西汀 结构式

伏硫西汀结构式
伏硫西汀(Fluoxetine)是一种常用的抗抑郁药物,属于选择性5-羟色胺再摄取抑制剂(SSRI)类药物。
它的结构式如下所示:
伏硫西汀的分子式为C17H18F3NO,分子量为309.33 g/mol。
它是一种白色结晶粉末,不溶于水,溶于有机溶剂。
伏硫西汀主要通过抑制5-羟色胺的再摄取来发挥抗抑郁作用。
5-羟色胺是一种神经递质,参与了情绪、睡眠、食欲和认知功能的调节。
抑郁症患者往往存在着5-羟色胺功能的改变,伏硫西汀能够增加5-羟色胺在突触间隙的浓度,从而改善患者的抑郁症状。
伏硫西汀的治疗效果通常需要几周的时间才能显现出来。
在使用伏硫西汀的过程中,患者应严格按照医生的指导进行用药,不可随意停药或更改剂量。
同时,患者在用药期间应定期复诊,医生会根据患者的病情进行调整。
除了抑郁症,伏硫西汀还可用于治疗其他精神障碍,如焦虑症、强迫症和神经性厌食症。
然而,伏硫西汀也有一些副作用,如头痛、失眠、恶心、食欲不振等。
在使用过程中,患者应密切关注自身的症状,如出现严重的副作用,应立即告知医生。
伏硫西汀作为一种选择性5-羟色胺再摄取抑制剂,是一种常用的抗抑郁药物。
它通过增加5-羟色胺的浓度来改善抑郁症状。
患者在使用伏硫西汀时应遵循医生的指导,并密切关注自身的症状。
氟哌噻吨杂质
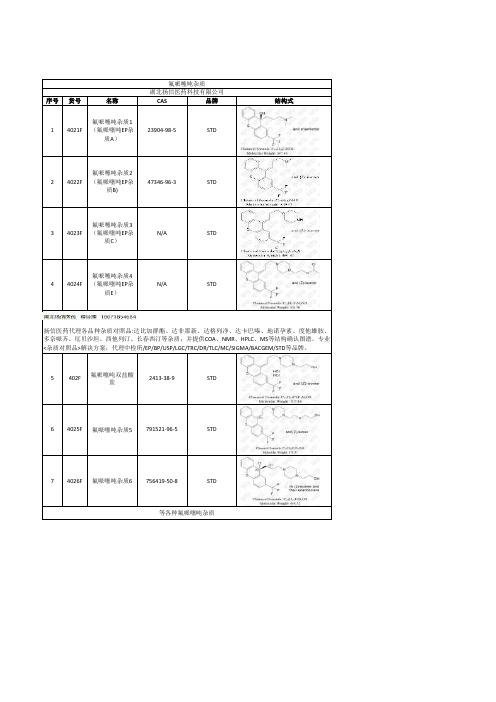
序号货号名称CAS 品牌结构式14021F 氟哌噻吨杂质1
(氟哌噻吨EP 杂
质A )23904-98-5STD
24022F 氟哌噻吨杂质2(氟哌噻吨EP 杂
质B)47346-96-3STD
34023F 氟哌噻吨杂质3(氟哌噻吨EP 杂
质C )N/A STD
44024F 氟哌噻吨杂质4(氟哌噻吨EP 杂
质E )N/A STD
5402F 氟哌噻吨双盐酸
盐2413-38-9STD
64025F 氟哌噻吨杂质5791521-96-5STD
74026F 氟哌噻吨杂质6756419-50-8STD
氟哌噻吨杂质湖北扬信医药科技有限公司扬信医药代理各品种杂质对照品:达比加群酯、达非那新、达格列净、达卡巴嗪、地诺孕素、度他雄胺、多奈哌齐、厄贝沙坦、西他列汀、长春西汀等杂质;并提供COA 、NMR 、HPLC 、MS 等结构确认图谱。
专业<杂质对照品>解决方案,代理中检所/EP/BP/USP/LGC/TRC/DR/TLC/MC/SIGMA/BACGEM/STD 等品牌。
等各种氟哌噻吨杂质。
3_种常用碳青霉烯类抗生素血药浓度UPLC-MS

3种常用碳青霉烯类抗生素血药浓度UPLC-MS/MS检测方法的建立Δ秦怡1*,张瑞霞2,吕雅瑶2,翁莉莉1,张弋2 #(1.天津医科大学一中心临床学院,天津 300192;2.天津市第一中心医院药学部,天津 300192)中图分类号 R917;R978.1文献标志码 A 文章编号 1001-0408(2024)03-0343-05DOI 10.6039/j.issn.1001-0408.2024.03.14摘要目的建立3种临床常用碳青霉烯类抗生素——厄他培南(ETP)、亚胺培南(IPM)、美罗培南(MEM)血药浓度检测的超高效液相色谱-质谱联用(UPLC-MS/MS)法。
方法血浆样品经甲醇沉淀蛋白后,以3种抗生素的稳定性同位素(ETP-D4、IPM-D4、MEM-D6)为内标,采用ACQUITY UPLC BEH C18(2.1 mm×50 mm,1.7μm)色谱柱分离;流动相为98%乙腈+2%水+0.1%甲酸和98%水+2%乙腈+0.1%甲酸,梯度洗脱;流速为0.3 mL/min;柱温为40 ℃;采用正离子、多反应监测模式进行扫描分析。
结果该方法专属性良好,在ETP、IPM、MEM 0.2~200、0.1~100、0.1~100μg/mL范围内线性良好(r2≥0.993),批内、批间精密度和准确度良好(RE均≤5.14%,RSD均≤11.15%),基质效应、提取回收率较一致(RSD≤12.99%)。
结论本实验建立了一种可以同时定量ETP、IPM、MEM血药浓度的UPLC-MS/MS法,该方法样品前处理简单、检测时间短、所需样品量少,可满足临床需求。
关键词碳青霉烯类抗生素;超高效液相色谱-质谱联用;血药浓度;厄他培南;亚胺培南;美罗培南Establishment of UPLC-MS/MS method for the determination of plasma concentration of three common carbapenem antibioticsQIN Yi1,ZHANG Ruixia2,LYU Yayao2,WENG Lili1,ZHANG Yi2(1. First Central Clinical College of Tianjin Medical University,Tianjin 300192,China;2. Dept. of Pharmacy,Tianjin First Central Clinical Hospital,Tianjin 300192, China)ABSTRACT OBJECTIVE To establish a UPLC-MS/MS method for the determination of plasma concentration of three carbapenem antibiotics,i.e. ertapenem (ETP),imipenem (IPM)and meropenem (MEM).METHODS After protein precipitation with methanol,the plasma samples were separated by ACQUITY UPLC BEH C18column (2.1mm×50mm,1.7μm)using stable isotopes of three antibiotics (ETP-D4,IPM-D4,MEM-D6)as the internal standard. The mobile phases were 98%acetonitrile +2% water +0.1%formic acid and 98%water +2%acetonitrile +0.1%formic acid,by gradient elution. The flow rate was 0.3mL/min and the column temperature was 40 ℃. Scanning analysis was performed in the positive ion and multiple reaction monitoring mode. RESULTS The method had good specificity,good linearity (r2≥0.993)in the range of 0.2-200,0.1-100and 0.1-100μg/mL of ETP,IPM and MEM,and good intra-batch and inter-batch precision and accuracy (all RE≤5.14%,all RSD≤11.15%),the matrix effect and extraction recovery were consistent (RSD≤12.99%). CONCLUSIONS This study establishes the UPLC-MS/MS method to simultaneously quantify the plasma concentration of ETP,IPM and MEM. The method has the advantages of simple pretreatment, short detection time and small sample quantity to meet clinical requirement.KEYWORDS carbapenem antibiotics; UPLC-MS/MS; plasma concentration; ertapenem; imipenem; meropenem碳青霉烯类抗生素具有抗菌谱广、抗菌活性强、耐药率低的特点,已成为治疗重症感染的主要选择。
特索芬辛

特索芬辛特索芬辛,402856-42-2简介 中文名称 化学名称CAS NO. 结构式 质量规格 特索芬辛 (1R,2R,3S)-3-(3,4-dichlorophenyl)-2-(ethoxymethyl)-8-methyl-8-azabicyclo[3.2.1]octane [402856-42-2] 企标,99%特索芬辛,402856-42-2用途特索芬辛(tesofensine )是一种5-羟色胺-去甲肾上腺素-多巴胺再摄取抑制剂,原先开发用于治疗帕金森病和阿尔茨海默病。
据研究人员在第16届欧洲肥胖病会议上的一份报告,特索芬辛有助于肥胖病人减轻体重,而且减去他们身体合适部位和合适成分(脂肪)的重量。
丹麦科研人员研究了特索芬辛治疗对于身体组成状况,包括体重、脂肪重量、腰围、矢状径(sagittal diameter ,上腹部尺寸)的影响。
研究属于一项随机、双盲、安慰剂对照的Ⅱb 临床试验,有203名体重指数在30~40的肥胖(除此无其它疾病的)男性和女性参与。
经过一个为期2周的饮食控制和体育锻炼导入期,他们被分组进行药物治疗24周,每日分别接受0.25 mg ,0.5 mg ,1.0 mg 剂量特索芬辛或相应的安慰剂。
结果显示特索芬辛对中央型肥胖十分有效。
另一项研究显示低剂量特索芬辛治疗肥胖安全有效。
特索芬辛,402856-42-2厂家武汉东康源厂家拥有独家特索芬辛生产研发基地和独家的特索芬辛生产工艺并引进了先进的生产设备。
与此同时,武汉东康源不断着力于完善特索芬辛的供应体系,积累了大量特索芬辛生产销售经验。
目前东康源的特索芬辛年产量超过20吨,产品远销至欧美,东南亚和非洲等国家,深受高校机关、科研单位、药厂商、质检部门、环保企业、化工生产、农业生产企业的信赖。
“诚信为本,服务至上;康源出品,必属精品",武汉东康源厂家坚持诚信经营,让采购商满意是我们一贯的宗旨:追求品质,为广大采购商供应优质特索芬辛是我们义不容辞的责任。
Gelucire-14-44-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Nov.-23-2018Print Date:Nov.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Gelucire 14/44Catalog No. :HY-Y1892CAS No. :121548-04-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:N/AMolecular Weight:N/ACAS No. :121548-04-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Pure form-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Oil)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
恩美曲妥单抗化学式

恩美曲妥单抗化学式
恩美曲妥单抗化学式:C43H58N10O13
恩美曲妥单抗(Trastuzumab)的化学式为C43H58N10O13,它是一种人源化的IgG1κ单克隆抗体,用于治疗HER2阳性的乳腺癌和胃癌等恶性肿瘤。
恩美曲妥单抗是一种单克隆抗体药物,由人类IgG1κ Fc 区域和人源化的单克隆抗体结合片段组成。
它的化学式为C43H58N10O13,分子量为约1100 kDa。
恩美曲妥单抗的结构中包含了两个重链和两个轻链,每个链都由多个氨基酸组成。
恩美曲妥单抗是一种人源化的单克隆抗体药物,它可以与HER2受体结合,抑制HER2受体的信号转导,从而减少癌细胞的增殖和转移。
恩美曲妥单抗主要用于治疗HER2阳性的乳腺癌和胃癌等恶性肿瘤。
Cisplatin_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jul.-12-2017Print Date:Jul.-12-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :CisplatinCatalog No. :HY-17394CAS No. :15663-27-11.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 2), H300Serious eye damage (Category 1), H318Carcinogenicity (Category 1B), H3502.2 GHS Label elements, including precautionary statementsPictogramSignal word DangerHazard statement(s)H300 Fatal if swallowed.H318 Causes serious eye damage.H350 May cause cancer.Precautionary statement(s)P201 Obtain special instructions before use.P202 Do not handle until all safety precautions have been read and understood.P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P280 Wear protective gloves/ eye protection/ face protection.P301 + P310 IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician.P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contactlenses, if present and easy to do. Continue rinsing.P310 Immediately call a POISON CENTER or doctor/ physician.H300 Fatal if swallowed.H318 Causes serious eye damage.H350 May cause cancer. P321 Specific treatment (see supplemental first aid instructions on this label).P330 Rinse mouth.P405 Store locked up.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:CDDP; cis–DiaminodichloroplatinumFormula:Cl2H6N2PtMolecular Weight:300.05CAS No. :15663-27-14. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data available Flash point No data available Evaporation rate No data available Flammability (solid, gas)No data available Upper/lower flammability or explosive limits No data available Vapor pressure No data available Vapor density No data available Relative density No data available Water Solubility No data available Partition coefficient No data available Auto-ignition temperature No data available Decomposition temperature No data available Viscosity No data available Explosive properties No data available Oxidizing properties No data available 9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 2Additional informationRTECS No.: TP2450000May cause liver irregularities.This information is based on our current knowledge. However the chemical, physical, and toxicological properties have not been completely investigated.12. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONAcute Health Hazard, Chronic Health Hazard.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
GC测定盐酸普拉克索中三乙胺残留量

- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Feb.-01-2019Print Date:Feb.-01-20191. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Tafenoquine (Succinate)Catalog No. :HY-111529ACAS No. :106635-81-81.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:WR 238605 (Succinate)Formula:C28H34F3N3O7Molecular Weight:581.58CAS No. :106635-81-84. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2019 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
