Synthesisandcharacterizationofnovel

Synthesis and characterization of novel thermoresponsive ?uorescence complexes based on copolymers with rare earth
ions
Guihua Cui a ,b ,Shuiying Chen a ,Bao Jiang c ,Yan Zhang a ,Nannan Qiu a ,Toshifumi Satoh d ,Toyoji Kakuchi d ,Qian Duan a ,?
a
Department of Materials Science and Engineering,Changchun University of Science and Technology,Changchun 130022,China b
Department of Chemistry,Jilin Medical College,Jilin 132013,China c
New Technology Research and Development Co.,Ltd.,Dongguan 523087,China d
Division of Biotechnology and Macromolecular Chemistry,Graduate School of Engineering,Hokkaido University,Sapporo 060-8628,Japan
a r t i c l e i n f o Article history:
Received 9April 2013
Received in revised form 4June 2013Accepted 5June 2013
Available online 4July 2013
Keywords:
Poly(N -isopropylacrylamide)Terbium Europium
Lower critical solution temperature (LCST)Atom transfer radical polymerization (ATRP)
a b s t r a c t
The thermo-sensitive and ?uorescent complexes containing Eu(III)or Tb(III)were synthesized and char-acterized,in which cholesterol-g-poly(N -isopropylacrylamide)(PNIPAM)copolymer was used as a poly-mer ligand.The results from the experiments indicated that Eu(III)or Tb(III)was bonded to nitrogen and oxygen atoms in the polymer chain.The ?uorescence lifetimes of the powdered Eu(III)and Tb(III)com-plexes was 11.48ms and 10.71ms,respectively.The maximum emission intensity of the PNIPAM–Eu(III)complex at 613nm and the PNIPAM–Tb(III)complex at 545nm were enhanced about 11.1and 11.3times compared with that of the corresponding rare earth ions,respectively.Additionally,the lower critical solution temperature (LCST)of complexes were slightly higher than those of the copolymers.
ó2013Elsevier B.V.All rights reserved.
1.Introduction
The rare earth ions in a ligand-free cationic state,have a lower absorption/emission ef?ciency in the visible region of the spectrum,but when they bound to organic ligands of high molar absorption coef?cients and form the rare earth complexes,their luminous intensity can be enhanced drastically [1–4].This phenomenon is mainly attributed to the organic ligands which absorb ultraviolet ray energy and transfer to central rare earth ions,so that the charac-teristic ?uorescence will be enhanced.Due to the unique long lumi-nescence lifetimes,sharp emission bands and photostability [5–10],the lanthanide-based complexes have generated much research interest as a new optical components,especially those containing europium (III)or terbium (III),which possess high color purity and produce red-emission (Eu 3+)and green-emission (Tb 3+)are often used as probes and labels in many ?elds such as materials science,biological technology and photoluminescent devices [11–15].
It is an attractive idea to functionalize lanthanide-based com-plexes with stimuli–responsive polymers,and the obtained mate-rials can be used to control the release of guest molecules in immunodiagnostic assays under external stimuli,such as temper-ature,pH,ionic strength [16–18].Perhaps the most extensively studied stimuli-responsive complexes are these modi?ed poly(N-isopropylacrylamide)(PNIPAM).PNIPAM is a well-known thermoresponsive polymer which that can change its appearance from a clear solution to a turbid suspension in water at a relatively lower critical solution temperature (LCST)of 32°C (near that of the human body)[19].Herein we synthesized a new class of composite complexes via conjugating the ?uorescent Eu 3+or Tb 3+with ther-mosensitive and biocompatible cholesterol-g-PNIPAM copolymers.Some polymers such as polymethylmethacrylate [20,21],styrene-co-butylmethacrylate [22],and polyvinylpyrrolidone (PVP)[23,24]were doped with Eu 3+and Tb 3+forming the complexes,in our previous papers we have reported a new composite materi-als from cellulose-g-PNIPAM polymers doped with Eu 3+ions [25],which had large Stokes shifts and long luminescence lifetimes.Once coupled to cholesterol-g-PNIPAM,the complexes should be-come a robust functional probe and is suitable for optical imaging applications.2.Experimental
2.1.Materials and instrumentation
N -isopropylacrylamide (Aldrich,98%)was recrystallized twice from a hexane/benzene mixture (3/2,v/v).Tris(2-(dimethyl-amino)ethyl)amine (Me 6TREN)was synthesized from tris(2-ami-
0925-3467/$-see front matter ó2013Elsevier B.V.All rights reserved.https://www.360docs.net/doc/804523168.html,/10.1016/j.optmat.2013.06.010
Corresponding author.Tel.:+8643185583105;fax:+8643185583105.
E-mail address:duanqian88@https://www.360docs.net/doc/804523168.html, (Q.Duan).
no)ethyl amine(TREN,Aldrich,99%)according to the literature [26].CuCl(Aldrich,99%)was washed successively with acetic acid and ether and then dried and stored under nitrogen.Eu2O3and Tb2O3(Aldrich,99.99%),2-Chloropropionyl chloride(Acros,97%) and Cholesterol(Aldrich,98%)were obtained commercially.
The1H nuclear magnetic resonance(NMR)spectra of monomers and polymers in CDCl3were obtained on a Varian Unity400NMR spectrometer.The molecular weights(M n)and polydispersity(M w/ M n)were measured by a gel permeation chromatograph(GPC) using a Waters510pump and a Model410differential refractom-eter at25°C.THF was used as a mobile phase at a?ow rate of 1.0mL minà1.The LCSTs of the polymer solutions were determined by turbidimetry,using Shimadzu-1240UV–Vis spectrophotometer with a heating rate of0.1°C minà1.FTIR spectra were recorded on a Shimadzu IR-8400S spectrometer.Raman studies have been car-ried out at the wavelength excitation of1064nm using a FT Raman Bruker RFS100spectrophotometer.A Shimadzu RF-5301PC?uo-rescence spectrophotometer was used to obtain?uorescence spec-tra and lifetime measurements.Quantum yields were determined by comparison of the total light emitted from the solutions to the total light emitted from a known standard[Ru(bipy)3]Cl2 [27].The XPS spectra(Mg K a)were recorded with a VG Scienti?c ESCALAB instrument.Elemental analysis(C,H,N)was performed on a(American Perkin–Elmer)2400II CHNS/O elemental analyzer. The lanthanide content was determined by EDTA titration.was placed on one side of an H-shaped ampoule glass and stirred at room temperature.NIPAM and initiator in DMF(3.0mL)were placed on the other side of the ampoule.Nitrogen was bubbled through both mixtures for5min to remove any oxygen.Three freeze–pump–thaw cycles were performed to degas the solution. Both mixtures were placed in an oil bath and thermostated at 80°C for2h.The polymerization was terminated by exposing the mixture to air.The reaction mixture was diluted with DMF and puri?ed using a neutral Al2O3column.Next,the solvent was evaporated,and the remainder was dialyzed in DMF using a cello-phane tube(Spectra/Por6,Membrane).Finally,the solvent was evaporated and a white product was collected by?ltration and dried in a vacuum oven overnight(conversion rate30.2%, M n=4000g molà1,M w/M n=1.08).
2.3.Synthesis of cholesterol-g-PNIPAM/Eu(III)or Tb(III)complexes
A solution of EuCl3or TbCl3and PNIPAMeW
RE3t
:W PNIPAM?0:08:1Tin ethanol was added to a?ask.The mixture was stirred with a magnetic stirring bar for24h.The product was puri?ed and then dried under vacuum at room temperature,yielding the cholesterol-g-PNIPAM/Eu(III)complexes.
3.Results and discussion
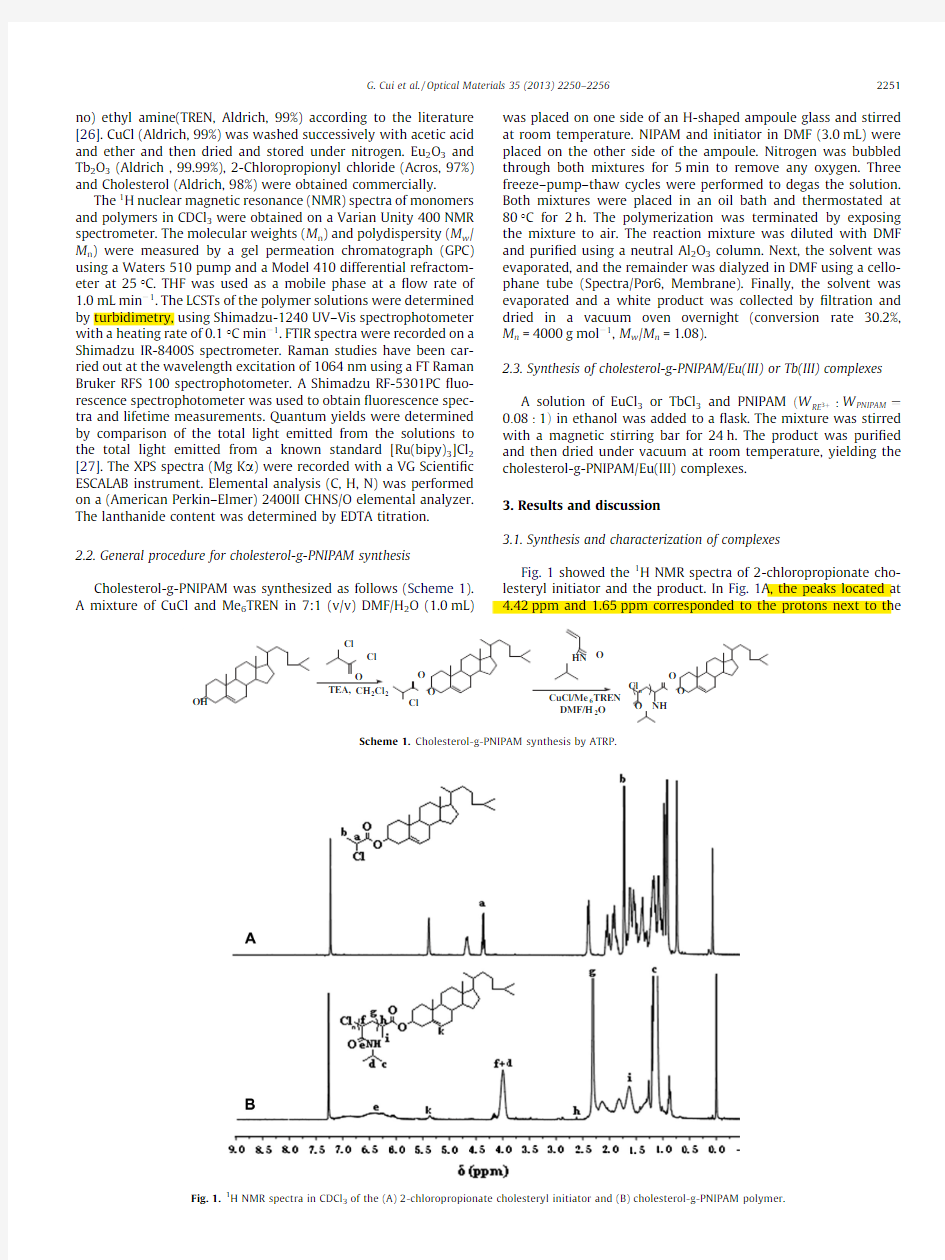
1H NMR spectra in CDCl
3of the(A)2-chloropropionate cholesteryl initiator and(B)cholesterol-g-PNIPAM polymer.
G.Cui et al./Optical Materials35(2013)2250–22562251
C was assigned to the stretching vibration (m N A H )of the amino group.The band at 1652cm à1was ascribed to amide [mainly the carbonyl stretching vibration (m C @O )]and the band cm à1was ascribed to amide II [mainly the N A H bending vibration (d N A H )].In Fig.2D,the stretching vibration (m N A H )acylamino group was shifted to 3285cm à1.The bands of amide amide II were shifted to 1640cm à1and 1533cm à1in complex,respectively.The stretching vibration (m N A acylamino group was transferred to 3286cm à1,and the amide I and amide II were transferred to 1641cm àcm à1,respectively in Tb(III)complex in Fig.2E.These changes were more clearly in the low frequency vibrations S2.The Raman of the copolymer and Eu/Tb complexes compared in Fig.S3.The peaks that were present in copolymer
Fig.2.FT-IR spectra for (A)cholesterol,(B)2-chloropropionate cholesteryl,cholesterol-g-PNIPAM and (D)cholesterol-g-PNIPAM/Eu(III)complexes(E)choles-terol-g-PNIPAM/Tb (III)complexes.
Fig.3.(A)XPS spectra of the (a)cholesterol-g-PNIPAM,(b)cholesterol-g-PNIPAM/Eu(III)complexes,(B)XPS spectra of the RECl Temperature dependences of optical transmittance at 500nm obtained aqueous solutions of cholesterol-g-PNIPAM and complex.The LCST was de?ned temperatures corresponding to 10%decrease of transmittance.
2252G.Cui et al./Optical Materials 35(2013)2250–2256
G.Cui et al./Optical Materials35(2013)2250–22562253
(B)photoluminescence emission spectra of the EuCl3,cholesterol-g-PNIPAM/Eu(III)complexes.(C)Excitation and(D)photoluminescence cholesterol-g-PNIPAM/Tb(III)complexes.
The XPS results showed that the average binding energies of O1s and N1s of the complexes were increased comparing with that of cholesterol-g-PNIPAM,thus indicating a decrease in the electron density of O1s and N1s atoms in the complexes.Meanwhile,the average binding energy of Eu4d and Tb4d were decreased,indicating an increase in the electron density of Eu(III)and Tb(III)in the com-plex.The results indicated that the complex were formed by the coordination between Eu(III)or Tb(III)and O and N atoms of acyl-amino group[29].This coordination shifted the electron density from the oxygen and nitrogen of the acylamino groups to the outer orbitals of europium(III)or terbium(III),increasing the outer layer charge density and the shielding effect,which in turn decreased the internal electron binding energy.Additionally,the decrease in the electronic cloud density of the nitrogen atom after coordina-tion was caused by the inductive effect.
By comparing with the XPS results of cholesterol-g-PNIPAM/ Eu(III)complex with the cholesterol-g-PNIPAM/Tb(III)complex, we could observe the binding energy’s increased amplitude of O1s and N1s of Eu(III)complex were higher than which of the Tb(III) complex.This phenomenon was attributed to the reducibility of the europium stronger than the terbium,so the electronic offset degree between the atoms were slightly different of the complex. The IR spectra and the temperature response were similarly be-cause of the electronegativity difference between the europium and the terbium was slightly.
3.2.Thermo-responsive and?uorescent characterization of complexes
The LCSTs of the copolymer and complexes were shown in Fig.4 and Table2,The LCSTs of the complexes were slightly higher than those of cholesterol-g-PNIPAM.For linear PNIPAM homopolymers, Stover and co-workers[30]had recently elucidated that the end-group hydrophobicity and molecular weight could effect on LCST. When cholesterol was grafted to PNIPAM by ATRP,the hydropho-bicity of cholesterol caused the LCST of the copolymer reduced.The LCSTs of the complexes which possessed Eu3+or Tb3+coordinated with PNIPAM were slightly higher than that of copolymer.This phenomenon might be due to Eu3+or Tb3+in complexes coordinat-ing with water molecules in solution,as the coordination bond en-ergy was greater than that of water molecule hydrogen bonds. When the temperature of the solution was near the LCST,more en-ergy was needed to destruct the coordination bonds between Eu3+ or Tb3+and the water molecules,therefore,a higher temperature was required for the phase transition.These?ndings also proved the formation of complexes between Eu3+or Tb3+and PNIPAM.
The photoluminescence spectrum of the RECl3and RE(III)com-plexes were shown in Fig.5.In Fig.5A EuCl3(curve A1)showed negligible ultraviolet absorption.Cholesterol-g-PNIPAM/Eu(III)complexes exhibited a wide excitation peak around355nm with the intensity stronger than that of the corresponding peak of EuCl3, which were attributed to the p–p?transition by exciting the car-bonyl and amide group of complexes.The reasonable explanation was that this conjugated structure would increase the electron delocalization and the absorption of the ultraviolet light,so the intensity of excitation peak enhance sharply.In Fig.5B,curve (B1)exhibited very weak emission peaks characteristic of Eu(III). Cholesterol-g-PNIPAM/Eu(III)displayed four strong,narrow emis-sion peaks at579,591,613and650nm,corresponding to the 5D
?7F J,(J=0,1,2,3)electronic transitions,respectively,which occurred from the excited state D to the multiplet F.Owing to the shielding of the4f orbital from the environment by an outer shell of5s and5p orbitals,the f–f absorption bands were very nar-row,so the most pronounced peak was situated at613nm and its half-width was less than10nm.TbCl3and terbium(III)complexes exhibited similarly situation in Fig.5C and D.Cholesterol-g-PNI-PAM/Tb(III)complexes showed a different excitation and emission spectra compared with TbCl3.Terbium(III)complexes had an apparent absorption peak at351nm,and was detected as typical internal transitions4f?4f of the Tb3+ion5D4?7F i(i=6,5,4 and3)four characteristics emission at487,545,583,616nm. The maximum emission intensity of the PNIPAM–Eu(III)complex at613nm and the PNIPAM–Tb(III)complex at545nm were en-hanced about11.1and11.3times compared with that of the cor-responding rare earth ions,respectively.The intensity decay curve were shown in Fig.6,which followed an exponential decay.
A quantum yield and the?uorescent lifetime of the emission could be calculated from the experimental data,which were shown in Table3.
The enhancement of the ef?ciency for photoemission in com-plexes could be explained by the coordination ability of the organic counterpart of the host structure of the polymer,which was strong enough to stabilize the position of the complex neighborhood after the incorporation process,and the absorption coef?cient of the or-ganic ligand was magnitude larger than the inherent absorption coef?cient of rare earth ions.The direct coordination of an organic ligand to Eu(III)or Tb(III)could improve the energy-transfer rate, so the initial strong absorption of the ultraviolet energy that ex-cites the ligand to the excited singlet(S1)state,by an energy migration via intersystem crossing from the S1state to a ligand triplet(T)state,the energy is then nonradiatively transferred from
Table3
The quantum yield(U)and lifetime of the complexes.
Compounds U Lifetime(ms) Cholesterol-g-PNIPAM/Eu(III)0.16711.48
Cholesterol-g-PNIPAM/Tb(III)0.15810.71
2254G.Cui et al./Optical Materials35(2013)2250–2256
the lowest triplet state of the ligand to a resonance state of the coordinated Eu(III)or Tb(III)[31].The energy undergoes a multi-photon relaxation and the subsequent emission in the visible region.
Fig.7shows the relationship between the temperature and the emission intensity of the peak at613nm of the cholesterol-g-PNI-PAM/Eu(III)complexes and at547nm of the cholesterol-g-PNI-PAM/Tb(III)complexes,respectively.When the temperature was lower than the LCST of the complexes,the emission intensities change little bit with increasing the temperature for the complex. While the temperature was higher than the LCST of the complexes, the emission intensities increased signi?cantly.This phenomenon was related to the change in the structures of the PNIPAM,the complexes were water-soluble below the LCST,so their conforma-tions were not changed.While the temperature was higher than the LCST,the complexes were changed from hydrophilic to the hydrophobic,the conformations of the water-insoluble complexes changed,so the emission intensity increase.
4.Conclusion
The novel poly(cholesterol-g-NIPAM)-Eu(III)/Tb(III)complexes were formed by the interaction between cholesterol-g-PNIPAM and Eu(III)/Tb(III)ions.The coordination among the oxygen and nitrogen of the acylamino group and Eu3+/Tb3+provided the com-plexes with the intensive characteristic?uorescence of Eu(III)/ Tb(III),the maximum emission intensity of the cholesterol-g-PNI-PAM/Eu(III)complex at613nm and the cholesterol-g-PNIPAM/ Tb(III)complex at545nm were enhanced about11.1and11.3 times compared to that of the corresponding Eu(III)/Tb(III),respec-tively.Additionally,the LCST of complexes were slightly higher than those of the copolymers.The complex might be able to prove the reference for new applications in?uorescence,and biomedical ?eld,which can broaden the application of temperature-sensitive PNIPAM.
Acknowledgements
We are grateful to National Natural Science Foundation of China (50903009),Jilin Science&Technology Department,Science and Technology Development Project(20070556,20100115and 201201120),Science and Technology Bureau of Changchun City Project(2008280)Foundation for Strategical Research for?nancial support.The authors would like to thank all reviewers of this arti-cle for their comments and suggestions.The authors are also grate-ful to Prof.Dr.Xingquan He for help with FT-IR analyses and to Prof.Xiaoyun Mi for running the?uorescence spectrophotometer and Prof.Dr.Xinglin Li for the XPS analyses.
Appendix A.Supplementary material
Supplementary data associated with this article can be found, in the online version,at https://www.360docs.net/doc/804523168.html,/10.1016/j.optmat.2013.
06.010.
References
[1]https://www.360docs.net/doc/804523168.html,w,K.L.Wong,Y.Y.Yang,Q.Y.Yi,G.H.Jia,W.T.Wong,P.A.Tanner,Inorg.
Chem.46(2007)9754–9759.
[2]N.Sabbatini,M.Guardigli,J.M.Lehn,Coord.Chem.Rev.123(1993)201–228.
[3]O.Moudam,B.C.Rowan,M.Alamiry,P.Richardson,B.S.Richards,A.C.Jones,N.
Robertson,https://www.360docs.net/doc/804523168.html,mun.19(2009)6649–6687.
[4]S.J.Butler,D.Parker,Chem.Soc.Rev.42(2013)1652–1666.
[5]J.J.Yu,D.Parker,R.Pal,R.A.Poole,M.J.Cann,J.Am.Chem.Soc.128(2006)
2294–2299.
[6]J.P.Leonard,P.Jensen,T.McCabe,R.D.Peacock,P.E.Kruger,T.Gunnlaugsson,J.
Am.Chem.Soc.129(2007)10986–10987.
[7]A.Cha,G.E.Snyder,P.R.Selvin,F.Bezanilla,Nature402(1999)809–813.
[8]B.Wang,J.Hai,Q.Wang,T.Li,Z.Yang,Angew.Chem.,Int.Ed.50(2011)3063–
3066.
[9]J.Wang,R.Wang,J.Yang,Z.Zheng,M.D.Carducci,T.Cayon,N.Peyghambarian,
G.E.Jabbour,J.Am.Chem.Soc.123(2001)6179–6180.
[10]A.P.Bassett,S.W.Magennis,P.B.Glover,D.J.Lewis,N.Spencer,S.Parsons,R.M.
Williams,L.D.Cola,Z.Pikramenou,J.Am.Chem.Soc.126(2004)9413–9424.
[11]P.Huhtinen,M.Kivela,O.Kuronen,V.Hagren,H.Takalo,H.Tenhu,T.Lovgren,
H.Harma,Anal.Chem.77(2005)2643–2648.
[12]Q.Zheng,H.Dai,M.E.Merritt,C.Malloy,C.Y.Pan,W.H.Li,J.Am.Chem.Soc.127
(2005)16178–16188.
[13]Y.Hasegawa,H.Kawai,K.Nakamura,N.Yasuda,Y.Wada,S.Yanagida,J.Alloys
Compd.408(2006)669–674.
[14]K.Kuriki,Y.Koike,Y.Okamoto,Chem.Rev.102(2002)2347–2356.
[15]P.X.Xi,K.Cheng,X.L.Sun,Z.Z.Zeng,S.H.Sun,https://www.360docs.net/doc/804523168.html,mun.48(2012)2952–
2954.
[16]X.S.Feng,D.Taton,R.Borsali,E.L.Chaikof,Y.Gnanou,J.Am.Chem.Soc.128
(2006)11551–11562.
[17]R.I.Mousta?ne,V.L.Bobyleva,A.V.Bukhovets,V.R.Garipova,T.V.Kabanova,
V.A.Kemenova,G.J.Vanden Mooter,Pharm.Sci.100(2011)874–875.
[18]C.C.Rafael,P.Jessica,P.S.Isabel,P.J.Jorge,F.B.Antonio,M.L.M.Luis,Adv.Funct.
Mater.19(2009)3070–3076.
[19]H.G.Schild,Polym.Sci.17(1992)163–249.
[20]M.M.Silva,V.Z.Bermudez,L.D.Carlos,A.P.P.Almedia,M.J.Smith,J.Mater.
Chem.9(1999)1735–1740.
[21]R.F.Sosa,M.H.Flores,R.T.Rodriguez,A.F.Munoz,Rev.Mexicana Fís.49(2003)
519–524.
relationship between the temperature and the emission intensity of the peak at613nm of the(A)cholesterol-g-PNIPAM/Eu(III)complexes and at cholesterol-g-PNIPAM/Tb(III)complexes.
[22]M.S.Iovu,A.M.Andriesh,S.A.Buzurniuc,V.I.Verlan,C.I.Turta,V.E.Zubareva,
M.I.Caraman,J.Non-Cryst.Solids355(2009)1890–1892.
[23]V.I.Verlan,M.S.Iovu,I.Culeac,Y.Nistor,C.I.Turta,V.E.Zubareva,J.Non-Cryst.
Solids357(2011)1004–1007.
[24]V.I.Verlan,M.S.Iovu,I.Culeac,Y.H.Nistor,C.I.Turta,V.E.Zubareva,J.Non-
Cryst.Solids360(2013)21–25.
[25]G.H.Cui,Y.H.Li,T.T.Shi,Z.G.Gao,N.N.Qiu,T.Satoh,T.Kakuchi,Q.Duan,
Carbohydr.Polym.94(2013)77–81.[26]M.Ciampolini,N.Nardi,Inorg.Chem.5(1966)41–44.
[27]Y.Cai,M.Q.Chen,H.N.Ji,X.H.Huang,J.Shen,Acta Polym Sin4(2003)
599–602.
[28]K.Nakamatu,Bull.Chem.Soc.Jpn55(1982)2697–2702.
[29]D.P.Drolet,D.M.Manuta,A.J.Lees,A.Katnani,G.Dand Coyle,J.Inorg.Chim.
Acta146(1988)173–177.
[30]Y.Xia,N.Burke,H.Stover,Macromolecules39(2006)2275–2283.
[31]H.F.Zhao,X.H.Huang,M.Q.Chen,Smart Mater.Struct.16(2007)2600–2604.
2256G.Cui et al./Optical Materials35(2013)2250–2256
