MTT方法说明

For life science research only. Not for use in diagnostic procedures. FOR IN VITRO USE ONLY.
Cell Proliferation Kit I (MTT)
Colorimetric assay (MTT based) for the non-radioactive quantification of cell proliferation and viability
Cat. No. 1 465 007Version 3, October 1999
1 Kit (for 2500 tests)
Store at ?15 to ?25°C
Introduction
The determination of cellular proliferation, viability and activation are key areas in a wide variety of cell biolog-ical approaches. The need for sensitive, quantitative, reliable and automated methods led to the develop-ment of standard assays. Such an example is based on the capability of the cells to incorporate a radioactively labeled substance ([3H])- thymidine), or to release a radioisotope such as [51Cr] after cell lysis. Alternatively, the incorporation of 5-bromo-2’-deoxyuridine (BrdU)* in place of thymidine is monitored as a parameter for DNA synthesis and cellular proliferation in immuno-histo- and cytochemistry, in a cell ELISA and FACS analysis. (kits and reagents for these applications are available from Roche Molecular Biochemicals).
Cell proliferation and viability assays are of particular importance for routine applications. Tetrazolium salts (e.g. MTT, XTT, WST-1) are especially useful for assaying the quantification of viable cells, because they are cleaved to form a formazan dye (fig. 1; for UV absor-bance spectrum, see fig. 2) only by metabolic active cells.
Fig.1: Metabolization of MTT to a formazan salt by viable cells.
Fig. 2: Comparison of UV-spectra of MTT labeling reagent (dotted line) and the formazan salt after solubilization with solubilization solution.
a b o r b a n c e
The non-radioactive, colorimetric assay system using MTT was first described by Mosmann, T. et al. (1) and improved in subsequent years by several other investi-gators (2–6). The assay is designed for the spectro-photometric quantification of cell growth and viability (1, 3, 5–7) without the use of radioactive isotopes. It is used for the measurement of cell proliferation in response to growth factors, cytokines and nutrients (1–3, 6, 8–12) (see fig. 4).
The MTT assay is also useful for the measurement of cytotoxicity. Examples are the quantification of tumor necrosis factor-a or -b effects (13, 14). (see fig. 5) or macrophage induced cell death (15, 16) and the assessment of cytotoxic (17–34) or growth inhibiting agents such as inhibitory antibodies (see fig. 6).
For the replacement of the radioactive [51Cr]-release cytotoxicity assay, protocols using MTT have been developed. The MTT assay is as sensitive as the radio-active method, but shows a significantly lower back-ground especially after long term incubation (34). The MTT assay can also be used to study cell activation (4). Compared to radioactive isotope techniques, the Cell Proliferation Kit I (MTT) is ?safer -no radioactive isotopes are used,?accurate -the absorbance revealed, strongly
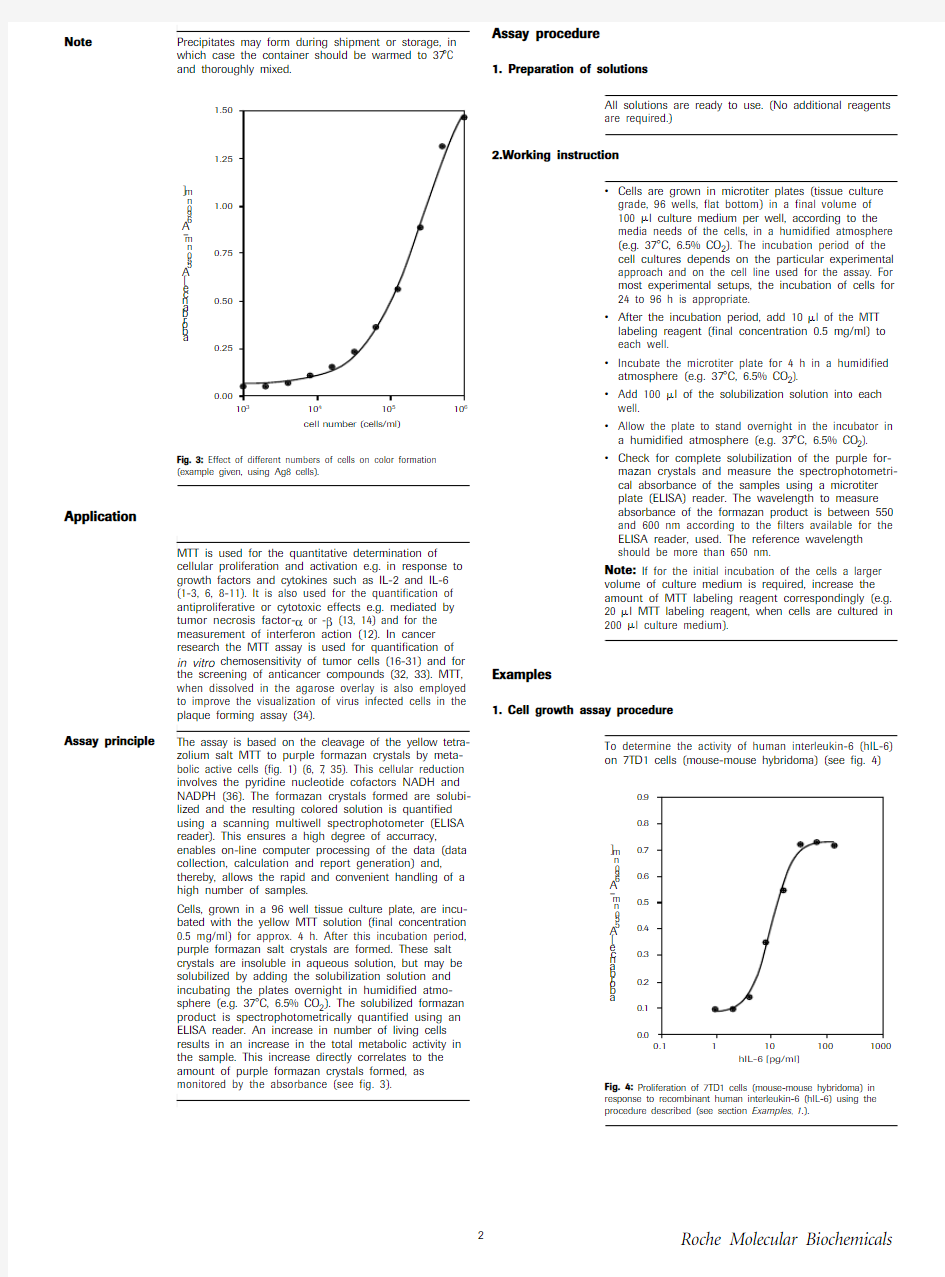
correlates to the cell number, (see fig. 3)
?sensitive -low cell numbers are detected (see fig. 3).?fast -the use of multiwell-ELISA readers
allows for processing a large number of samples,
?easy -no washing steps and no additional
reagents are required,
Product description
The kit contains
1. MTT labeling reagent, (1×, ready-to-use)
5 vials containing 5 ml MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromid) labeling reagent (1x), 5 mg/ml, in phosphate buffered saline (PBS), non-sterile, ready to use.
Stability
Stable at ?15 to ?25°C protected from light. Repeated thaw-freeze cycles do not affect product stability. After thawing, the MTT labeling reagents may be stored pro-tected from light at 2-8°C for up to 4 weeks, in which case a sterile filtration of the reagent is recommended.
2. Solubilization solution, (1×, ready-to-use)
3 bottles solubilization solution (90 ml per bottle), 10% SDS in 0.01 M HCI.
Stability
Stable at 15-25°C.
Note
Precipitates may form during shipment or storage, in which case the container should be warmed to 37°C and thoroughly mixed.
Fig. 3: Effect of different numbers of cells on color formation (example given, using Ag8 cells).
Application
MTT is used for the quantitative determination of
cellular proliferation and activation e.g. in response to growth factors and cytokines such as IL-2 and IL-6 (1-3, 6, 8-11). It is also used for the quantification of antiproliferative or cytotoxic effects e.g. mediated by tumor necrosis factor-α or -β (13, 14) and for the measurement of interferon action (12). In cancer research the MTT assay is used for quantification of in vitro chemosensitivity of tumor cells (16-31) and for the screening of anticancer compounds (32, 33). MTT, when dissolved in the agarose overlay is also employed to improve the visualization of virus infected cells in the
plaque forming assay (34).
Assay principle
The assay is based on the cleavage of the yellow tetra-zolium salt MTT to purple formazan crystals by meta-bolic active cells (fig. 1) (6, 7, 35). This cellular reduction involves the pyridine nucleotide cofactors NADH and NADPH (36). The formazan crystals formed are solubi-lized and the resulting colored solution is quantified using a scanning multiwell spectrophotometer (ELISA reader). This ensures a high degree of accurracy,
enables on-line computer processing of the data (data collection, calculation and report generation) and, thereby, allows the rapid and convenient handling of a high number of samples.
Cells, grown in a 96 well tissue culture plate, are incu-bated with the yellow MTT solution (final concentration 0.5 mg/ml) for approx. 4 h. After this incubation period, purple formazan salt crystals are formed. These salt crystals are insoluble in aqueous solution, but may be solubilized by adding the solubilization solution and incubating the plates overnight in humidified atmo-sphere (e.g. 37°C, 6.5% CO 2). The solubilized formazan product is spectrophotometrically quantified using an ELISA reader. An increase in number of living cells results in an increase in the total metabolic activity in the sample. This increase directly correlates to the amount of purple formazan crystals formed, as monitored by the absorbance (see fig. 3).
a b o r b a n c e [A 550 n m –A 690 n m ]
Assay procedure
1. Preparation of solutions
All solutions are ready to use. (No additional reagents are required.)
2.Working instruction
?Cells are grown in microtiter plates (tissue culture grade, 96 wells, flat bottom) in a final volume of 100 ?l culture medium per well, according to the media needs of the cells, in a humidified atmosphere (e.g. 37°C, 6.5% CO 2). The incubation period of the cell cultures depends on the particular experimental approach and on the cell line used for the assay. For most experimental setups, the incubation of cells for 24 to 96 h is appropriate.
?After the incubation period, add 10 ?l of the MTT labeling reagent (final concentration 0.5 mg/ml) to each well.
?Incubate the microtiter plate for 4 h in a humidified atmosphere (e.g. 37°C, 6.5% CO 2).
?Add 100 ?l of the solubilization solution into each well.
?Allow the plate to stand overnight in the incubator in a humidified atmosphere (e.g. 37°C, 6.5% CO 2). ?Check for complete solubilization of the purple for-mazan crystals and measure the spectrophotometri-cal absorbance of the samples using a microtiter plate (ELISA) reader. The wavelength to measure absorbance of the formazan product is between 550 and 600 nm according to the filters available for the ELISA reader, used. The reference wavelength should be more than 650 nm.
Note: If for the initial incubation of the cells a larger volume of culture medium is required, increase the amount of MTT labeling reagent correspondingly (e.g. 20 ?l MTT labeling reagent, when cells are cultured in 200 ?l culture medium).
Examples
1. Cell growth assay procedure
To determine the activity of human interleukin-6 (hIL-6) on 7TD1 cells (mouse-mouse hybridoma) (see fig. 4)
Fig. 4: Proliferation of 7TD1 cells (mouse-mouse hybridoma) in response to recombinant human interleukin-6 (hIL-6) using the procedure described (see section Examples , 1.).
a b o r b a n c e [A 550 n m –A 690 n m ]
Reagents
?Culture medium, e.g. DMEM containing 10% heat inactivated FCS (fetal calf serum), 2 mM glutamine, 0.55 mM L-arginine, 0.24 mM L-asparagine-mono-hydrate, 50 ?M 2-mercaptoethanol, HT-media sup-plement* (1×), containing 0.1 mM hypoxanthine and 16 ?M thymidine. If an antibiotic is to be used, addi-tionally supplement media with penicillin/streptomy-cin* or gentamicin*.
?Interleukin-6, human (hIL-6) (200 000 U/ml, 2 ?g/ml)*, sterile.
?Cell Proliferation Kit I (MTT).
Procedure
?Seed 7TD1 cells at a concentration of 2 × 103 cells/well in 100 ?l culture medium containing various amounts of IL-6 [final concentration e.g. 0.1–10 U/ml (0.001–0.1 ng/ml)] into microtiter plates (tissue culture grade, 96 wells, flat bottom). Incubate cell cultures for 4 days at 37°C and 6.5% CO 2.
?Add 10 ?l MTT labeling solution and continue as
Reagents
Fig. 5: Determination of the cytotoxic activity of recombinant human TNF-? (h TNF-?) on WEHI-164 cells (mouse fibrosarcoma) using the procedure described (see section Examples, 2.).
Procedure
?Preincubate WEHI-164 cells at a concentration of 1 × 106 cells/ml in culture medium with 1 ?g/ml actinomycin C1 for 3 h at 37°C and 6.5% CO 2.
?Seed cells at a concentration of 5 × 104 cells/well in 100 ?l culture medium containing 1 ?g/ml actino-mycin C 1 and various amounts of hTNF-α (final con-centration e.g. 0.001–0.5 ng/ml) into microtiter plates (tissue culture grade, 96 wells, flat bottom). Incubate cell cultures for 24 h at 37°C and 6.5% CO 2. ?Add 10 ?l MTT labeling solution and continue as described under working instruction (section Assay procedure, 2).
3. Assay procedure for the analysis of neutralizing monoclonal antibodies to growth factors or cytokines
To determine the inhibitory activity of a murine, mono-clonal antibody to human granulocyte-macrophage colony stimulating factor (anti-hGM-CSF) on hGM-CSF activity on TF-1 cells (human erythroleukemic cells) (see fig. 6).
Reagents:
?Culture medium, e.g. RPMI 1640 containing heat inactivated 10% FCS (fetal calf serum), 2 mM L-glutamine. If an antibiotic is to be used, additionally supplement media with penicillin/streptomycin* or gentamicin*.
?hGM-CSF (10 000 U/ml, 1 ?g/ml)*, sterile.
?anti-hGM-CSF (200 ?g/vial)*, lyophilized, sterile. ?Cell Proliferation Kit I (MTT).
Note
Recombinant, human interleukin-3 (hIL-3)*, which also is effective on TF-1 cells, can be used as a negative control (see fig. 6).
Roche Diagnostics GmbH
Roche Molecular Biochemicals Sandhofer Strasse 116D-68305 Mannheim Cell Proliferation Kit II (XTT) flow chart Note: If for the initial incubation of the cells a larger volume of culture medium is required, increase the amount of MTT labeling reagent correspondingly (e.g. 20 ?l MTT labeling reagent, when cells are cultured in 200 ?l culture medium).
References
1Mosmann, T. et al. (1983) J. Immunol. Methods 65, 55–63. 2Tada, H. et al. (1986) J. Immunol. Methods 93, 157–165.
3Denizot, F. & Lang, R. (1986) J. Immunol. Methods 89, 271–277. 4Gerlier, D. & Thomasset, N. (1986) J. Immunol. Methods 94, 57–63.
5Hansen, M. B., Nielsen, S. E. & Berg, K. (1989) J. Immunol. Methods 119, 203–210.6Vistica, D. T. et al. (1991) Cancer Res. 51, 2515–2520.
7Maehara, Y. et al. (1986) Eur. J. Cancer Clin. Oncol . 23, 273–276. 8Heeg, K. et al. (1985) J. Immunol. Methods 77, 237–246.
9Hooton, J. W. L., Gibbs, C. & Paetkan, U. (1985) J. Immunol. 135, 2464–2473. 10Mosmann, T. R. & Fong, T. A. T. (1989) J. Immunol. Methods 116, 151–158. 11Ohno, M. & Abe, T. (1991) J. Immunol. Methods 145, 199–203.
12Berg, K., Hansen, M. B. & Nielsen, S. E. (1988) J. Interferon Res . 8, (suppl. 1), S. 67. 13Green, L. M., Reade, J. L. & Ware, C. F. (1984) J. Immunol. Methods 70, 257–268.14Espevik, T. & Nissen-Meyer, J. (1986) J . Immunol. Methods 95, 99–105.
15Ferrari, M., Fornasiero, M. C. & Isetta, A. M. (1990) J. Immunol. Methods 131, 165–172. 16Van de Loosdrecht, A. A. et al. (1991) J. Immunol. Methods 141, 15–22. 17Cole, S. P. C. (1986) Cancer Chemother. Pharmacol 17, 259–263. 18Carmichael, J. et al. (1987) Cancer Res . 47, 936–942.
19Twentyman, P. R. & Luscombe, M. (1987) Br. J. Cancer 56, 279–285. 20Park, J.-G. et al. (1987) Cancer Res. 47, 5875–5879.
21Horiuchi, N. et al. (1988) Cancer Chemother. Pharmacol. 22, 246–250. 22Pieters, R. et al. (1988) Cancer Letters 41, 323–332.
23McHale, A. P. & McHale, L. (1988) Cancer Letters 41, 315–321.
24Edmondson, J. M., Armstrong, L. S. & Martinez, A. O. (1988) J. Tissue Culture Methods 11, 15–17.
25Faircloth, G. T., Stewart, D. & Clement, J. J. (1988) J. Tissue Culture Methods 11, 201–205. 26Campling, B. G. et al. (1988) Leukemia Res. 12, 823–831. 27Pieters, P. et al. (1989) Br. J. Cancer 59, 217–220.
28Twentyman, P. R., Fox, N. E. & Rees, K. H. (1989) Br. J. Haematol. 71, 19–24. 29Plumb, J. A., Milroy, R. & Kaye, S. B. (1989) Cancer Res. 49, 4435–4440. 30Alley, M. C. et al. (1988) Cancer Res . 48, 589–601. 31Heo, D. S. et al. (1990) Cancer Res. 50, 3681–3690.
32Ruben, R. L. & Neubauer, R. H. (1987) Cancer Treat. Rep. 71, 1141–1149. 33Rubinstein, L. V. et al. (1990) J. Natl. Canc. Inst. 82, 1113–1118. 34Shanafelt, A. B. (1991) BioTechniques 3, 330.
35Slater, T. F., Sawyer, B. & Str ?uli, U. (1963) Biochim.
36
Berridge, M. V. & Tan, A. S. (1993) Arch. Biochem. Biophys. 303, 474.
Note: A reference list for microtiter tetrazolium assays (e.g. MTT, XTT, WST-1)
is available on request.* available from Roche Molecular Biochemicals
Step Procedure
Vol./well
Time T emp. (°C)Perform tissue culture using 96 well microtiter plates
(tissue culture grade, flat-bottom)100?l
24-96 h
37°C
1
Add MTT labeling reagent and incubate in a humidified atmosphere
10 ?l 4 h 37°C
2Add solubilization solution and incubate in a humidified atmosphere
100 ?l overnight 37°C
3Evaluate microtiter plate with the use of an ELISA reader at 550–600 nm with a reference wavelength of ? 650 nm
___
Related Products
Argentina 541 954 5555; Australia (02) 9899 7999; Austria (01) 277 87; Belgium (02) 247 4930;
Brazil +55 (11) 3666 3565; Bulgaria +35929625408; Cameroon 237-370269; Canada (450) 0867050; (800) 361 2070; Chile 00 56 (2) 22 33 737 (central) 00 56 (2) 22 32 099 (Exec); China 8621 6427 5586; Colombia 0057-1-3412797; Cyprus +357-2-311362; Czech Republic (0324) 4554, 58 71-2; Denmark +45 363 999 58; Egypt 20-2-3619047; Estonia 372-7-447600; Ethiopia 251-1-552799; Finland +358 9 525 333 66; France 04 76 76 30 87; Germany (0621) 759 8568;Greece 3 (01) 67 40 238; Hong Kong (852) 2485 7596; India +91-22-8379906; Indonesia 62(021) 252 3820 ext. 755; Iran +98-21-8072374 / +98-21-8797027; Israel 972-6- 6380569; Italy 039 247 4109-4181; Japan 03 3432 3155; Kenya +254-2-750112; Korea 82-2-3471-6500;Kuwait +965-4837859; Latvia 371-787828309; Lebanon Fax: 00961-1-399667; Lithuania 370-2-729715; Luxembourg +352-496098; Malta Fax: +356-341087; Morocco Fax: +212-2-944040; Malaysia 60 (03) 755 5039; Mexico (5) 227 8967; Netherlands (036) 539 4911; New Zealand (09) 276 4157; Nigeria +234-1-521767; Norway (47) 23 373300; Philippines (632) 8107246; Poland +48 (22) 22 66 84 305; Portugal (01) 4171717; Republic of Ireland 1 800 40 9041; Romania +40-1-2123763; Russia (49) 621 759 8636 Fax: (49) 621 759 8611; Saudia Arabia +966-1-4010364; Singapore 0065 272 9200; Slovenia +386 61 1363528; South Africa (011)886 2400; South Korea 02 569 6902; Spain (93) 201 4411; Sweden (08) 404 8800; Switzerland +41 (41) 799 6161; T aiwan (02) 736 7125; Thailand 66 (2) 274 07 08 (12 line); Turkey 0090 212216 32 80; United Arab Emirates +971-4-694351; United Kingdom (0800) 521578; USA (800)428 5433. Venezuela Fax: +0058-4810697; Yugoslavia +381 11 137163.
Parameter Detection by Products
Cat. No.BrdU labeling of proliferating cells
?In situ assay ?BrdU Labeling and Detection Kit I ?BrdU Labeling and Detection Kit II ?BrdU Labeling and Detection Kit III ?In Situ Cell Proliferation Kit, FLUOS
1 296 7361 299 9641 444 6111 810 740?ELISA ?Cell Proliferation ELISA, BrdU
(colorimetric)
?Cell Proliferation ELISA, BrdU (chemiluminescent)
1 647 2291 669 915?Single reagents for in situ assays and ELISA applications ?Anti-BrdU* formalin grade
?Anti-BrdU -Fluorescein, formalin grade ?Anti-BrdU -Peroxidase, Fab fragments, formalin grade
?FixDenat
1 170 3761 20
2 6931 585 8601 758 764Measurement of metabolic activity ?Quantification in microtiterplate ?Cell Proliferation Kit I (MTT)?Cell Proliferation Kit II (XTT)?Cell Proliferation Reagent WST-1
1 465 0071 465 0151 644 807Measurement of growth
related
parameters
?In situ assay
Anti-Casein Kinase 2?Anti-Ki-67 (Ki-S5), formalin grade Anti-PCNA/Cyclin-Fluorescein*Anti-PCNA/Cyclin, formalin grade Anti-Topoisomerase IIa (Ki-S1), formalin grade
Anti-Transferrin Receptor, human
1 499 6021 74
2 3451 205 8111 486 7721 742 3531 118 048
E-mail Address
Country
E-mail Address
Country
argentina.biochem@https://www.360docs.net/doc/bf10083330.html, Argentina Raitis@invitros.lv Latvia
biochem.au@https://www.360docs.net/doc/bf10083330.html, Australia Sakkijha@https://www.360docs.net/doc/bf10083330.html, Lebanon
G erhard.Muehlbauer@https://www.360docs.net/doc/bf10083330.html, Austria G intaras@eksma.lt Lithuania
biochem.be@https://www.360docs.net/doc/bf10083330.html, Belgium diagnostics@prophac.lu Luxembourg
Valent@mbox.cit.bg Bulgaria Vccl@https://www.360docs.net/doc/bf10083330.html,.mt Malta africhem@camnet.cm Cameroon Aiouche.echo@https://www.360docs.net/doc/bf10083330.html,.ma Morocco biochem.ca@https://www.360docs.net/doc/bf10083330.html, Canada biocheminfo.nl@https://www.360docs.net/doc/bf10083330.html, Netherlands https://www.360docs.net/doc/bf10083330.html,@https://www.360docs.net/doc/bf10083330.html, China https://www.360docs.net/doc/bf10083330.html,@https://www.360docs.net/doc/bf10083330.html, New Zealand
Info@https://www.360docs.net/doc/bf10083330.html,.cy Cyprus bofungwu@https://www.360docs.net/doc/bf10083330.html,.ng Nigeria Bm-comp@bm-comp.cz Czech Republic biochem.se@https://www.360docs.net/doc/bf10083330.html, Norway dk.biochem@https://www.360docs.net/doc/bf10083330.html, Denmark biochem.pt@https://www.360docs.net/doc/bf10083330.html, Portugal
ou.melestrum@neti.ee Estonia Topdiag@fx.ro Romania
pharsc.et@https://www.360docs.net/doc/bf10083330.html,.et Ethiopia biochem.sg@https://www.360docs.net/doc/bf10083330.html, Singapore
helsinki.biochem_diagnostics@https://www.360docs.net/doc/bf10083330.html, Finland roche.diagnostics@https://www.360docs.net/doc/bf10083330.html, Slovenia
biochem.fr@https://www.360docs.net/doc/bf10083330.html, France south_africa.bioboffin@https://www.360docs.net/doc/bf10083330.html, South Africa
mannheim.biocheminfo@https://www.360docs.net/doc/bf10083330.html, G ermany biochem.es@https://www.360docs.net/doc/bf10083330.html, Spain
Bm_roche@https://www.360docs.net/doc/bf10083330.html, India biochem.se@https://www.360docs.net/doc/bf10083330.html, Sweden h.hajian@https://www.360docs.net/doc/bf10083330.html, Iran BiochemInfo.CH@https://www.360docs.net/doc/bf10083330.html, Switzerland tubanegin@https://www.360docs.net/doc/bf10083330.html, Iran Jean-Marie.kindbeiter@https://www.360docs.net/doc/bf10083330.html, Tunisia
Dyn@https://www.360docs.net/doc/bf10083330.html,.il Israel bmuae@https://www.360docs.net/doc/bf10083330.html,.ae United Arab Emirates it.biochem@https://www.360docs.net/doc/bf10083330.html, Italy uk.biochem@https://www.360docs.net/doc/bf10083330.html, United Kingdom bmkkbio@cet.co.jp Japan https://www.360docs.net/doc/bf10083330.html,@https://www.360docs.net/doc/bf10083330.html, USA pharmakp@https://www.360docs.net/doc/bf10083330.html, Kenya Mvalentiner@https://www.360docs.net/doc/bf10083330.html,.ve Venezuela Bmskorea@https://www.360docs.net/doc/bf10083330.html, Korea dusica@eunet.yu Y ugoslavia
react@ncc.moc.kw Kuwait biochemts.row@https://www.360docs.net/doc/bf10083330.html, All other countries
https://www.360docs.net/doc/bf10083330.html,/pack-insert/1465007a.pdf
