Salt Stress–Induced Disassembly of Arabidopsis Cortical Microtubule

Salt Stress–Induced Disassembly of Arabidopsis Cortical Microtubule Arrays Involves26S Proteasome–Dependent Degradation of SPIRAL1C W
Songhu Wang,a Jasmina Kurepa,a Takashi Hashimoto,b and Jan A.Smalle a,1
a Plant Physiology,Biochemistry,Molecular Biology Program,Department of Plant and Soil Sciences,University of Kentucky, Lexington,Kentucky40546
b Graduate School of Biological Sciences,Nara Institute of Science and Technology,Ikoma,Nara630-0192,Japan
The dynamic instability of cortical microtubules(MTs)(i.e.,their ability to rapidly alternate between phases of growth and shrinkage)plays an essential role in plant growth and development.In addition,recent studies have revealed a pivotal role for dynamic instability in the response to salt stress conditions.The salt stress response includes a rapid depolymerization of MTs followed by the formation of a new MT network that is believed to be better suited for surviving high salinity. Although this initial depolymerization response is essential for the adaptation to salt stress,the underlying molecular mechanism has remained largely unknown.Here,we show that the MT-associated protein SPIRAL1(SPR1)plays a key role in salt stress–induced MT disassembly.SPR1,a microtubule stabilizing protein,is degraded by the26S proteasome,and its degradation rate is accelerated in response to high salinity.We show that accelerated SPR1degradation is required for a fast MT disassembly response to salt stress and for salt stress tolerance.
INTRODUCTION
The ubiquitin/26S proteasome system(UPS)regulates many fundamental cellular processes by controlling the degradation rates of numerous proteins(Hershko and Ciechanover,1998; Vierstra,2009).For the majority of UPS substrates,the concerted action of E1,E2,and E3enzymes leads to the covalent attach-ment of a multiubiquitin chain to the protein destined for degra-dation.The polyubiquitinated target protein is then degraded by the26S proteasome(Glickman,2000),an evolutionarily conserved multicatalytic protease that contains an enclosed proteolytically active core particle and one or two regulatory particles(RPs).The main roles of the RPs are in substrate rec-ognition,which is performed by RP non-ATPase subunits(RPNs), and in the unfolding and translocation of substrates to the core particle by RP triple A ATPase subunits(RPTs)(Smalle and Vierstra,2004;Kurepa and Smalle,2008).
In Arabidopsis thaliana,like in other eukaryotes,proteasome mutants and proteasome activity inhibitors are used to uncover the identity of UPS-regulated pathways and UPS target poteins (Kurepa and Smalle,2008).Loss of function of RP subunits RPN1a and RPN10,for example,revealed that the26S protea-some is essential for cell division and expansion,the modulation of responses to hormones and proteostatic drugs,and game-tophyte development(Smalle et al.,2003;Kurepa et al.,2008, 2009a,2009b,2010;Wang et al.,2009).These studies also revealed that the stress responses of rpn1a and rpn10mutant plants are altered:compared with the wild type,proteasome mutants are more tolerant of oxidative stress and less tolerant of protein misfolding stresses such as heat shock and salt stress (Smalle et al.,2003;Kurepa et al.,2008;Wang et al.,2009). The most frequently used proteasome inhibitor is MG132,a reversible,cell-permeable peptidyl aldehyde that inhibits the proteasome-speci?c chymotrypsin-like protease b5(Lee and Goldberg,1998).Studies using MG132have shown that the UPS is involved in the regulation of plant cell microtubule(MT) networks(Yanagawa et al.,2002;Oka et al.,2004;Sheng et al., 2006;S.Wang et al.,2011).MTs are polymers of a/b-tubulin heterodimers,which are incorporated into MTs directionally so that a-tubulin is exposed at the so-called lagging(2)and b-tubulin at the leading(+)ends.MTs play important roles in numerous cellular processes,including cell division and directional cell expansion(Nogales,2001;Sedbrook and Kaloriti,2008).
The polymerization-depolymerization dynamics of MTs are essential for their functionality in cellular processes,and they include two types of GTP hydrolysis-dependent dynamic behav-iors:dynamic instability and treadmilling.Treadmilling is the net growth of MTs at their(+)ends and shortening at their(2)ends, and dynamic instability is the switching between episodes of growth(polymer assembly)and shortening(polymer disassembly) at the(+)ends.The transition from growing to shortening is a dynamic instability parameter called catastrophe,and transition from shortening to growing is known as rescue.Growth,shrink-age,catastrophe,and rescue rates depend on MT-associated
1Address correspondence to jsmalle@https://www.360docs.net/doc/1e10795561.html,.
The author responsible for distribution of materials integral to the
?ndings presented in this article in accordance with the policy described
in the Instructions for Authors(https://www.360docs.net/doc/1e10795561.html,)is:Jan A.Smalle
(jsmalle@https://www.360docs.net/doc/1e10795561.html,).
C Some?gures in this article are displayed in color online but in black
and white in the print edition.
W Online version contains Web-only data.
The Plant Cell,Vol.23:3412–3427,September2011,https://www.360docs.net/doc/1e10795561.html,?2011American Society of Plant Biologists.All rights reserved.
(+)-end-tracking proteins(+TIPs)(Hirokawa,1994;Mandelkow and Mandelkow,1995;Lloyd and Hussey,2001;Schuyler and Pellman,2001;Sedbrook,2004;Hamada,2007;Sedbrook and Kaloriti,2008;Lyle et al.,2009a,2009b).
A large number of+TIPs have been identi?ed,and some of them are found in widely diverged species,suggesting that they regulate a basic,evolutionarily conserved component of the dynamic instability process(Bisgrove et al.,2004).For example,Arabidop-sis has functional homologs of end binding protein1(EB1)and cytoplasmic linker-associated protein1(CLASP1),which were ?rst identi?ed in human cells(Chan et al.,2003;Mathur et al.,2003; Galjart,2005;Vaughan,2005;Ambrose et al.,2007;Kirik et al., 2007;Bisgrove et al.,2008;Komaki et al.,2010).Other+TIPs,such as SPIRAL1(SPR1),are found only in plants(Nakajima et al.,2004, 2006;Sedbrook et al.,2004).Because plants overexpressing SPR1have increased tolerance to MT-destabilizing drugs,and spr1mutants have morphological defects consistent with altered MT stability,it has been concluded that this protein,similar to EB1 and CLASP1,is important for the regulation of dynamic instability (Nakajima et al.,2004,2006;Sedbrook et al.,2004;Abe and Hashimoto,2005;Ishida et al.,2007).
Similar to other eukaryotes,the structure of the plant MT cy-toskeleton is modulated not only by various developmental cues, but also in response to environmental signals and stress condi-tions(Smertenko et al.,1997;Himmelspach et al.,1999;Mathur and Chua,2000;Wang and Nick,2001;Abdrakhamanova et al., 2003;Van Bruaene et al.,2004;C.Wang et al.,2007,2011; Hamant et al.,2008).For example,short-term salt stress pro-motes the reorientation of MTs in maize(Zea mays)roots from transverse to parallel relative to the longitudinal axis and in tobacco(Nicotiana tabacum)BY-2cells from a structured to a seemingly random organization(Blanca?or and Hasenstein, 1995;Dhonukshe et al.,2003).Long-term salt stress also affects cortical MT organization in Arabidopsis and suppresses the right-handed helical growth of spr1mutants(Shoji et al.,2006). Furthermore,prolonged exposure to salt stress conditions was shown to trigger a biphasic response starting with a massive MT depolymerization phase followed by the formation of new MT networks that are thought to be better suited for surviving high salinity(Wang et al.,2007).Because treatments with a MT-destabilizing drug improved survival and growth under salt stress conditions and treatments with a MT-stabilizing drug caused salt stress hypersensitivity,the initial MT depolymerization response is believed to be essential for maintaining salt stress tolerance (Wang et al.,2007).
The speci?c mechanism that mediates the initial,salt stress–induced MT destabilization is currently not well understood. Because all tested proteasome mutants are hypersensitive to salt stress,we hypothesized that the MT reorganization needed for salt stress tolerance might be impaired in these lines due to an altered dynamic instability of MTs.Since the dynamic instability is regulated by the action of MAPs and in particular+TIPs,we tested if one of these proteins is conditionally degraded by the proteasome to allow MT restructuring required for an optimal salt stress response.Here,we show that in Arabidopsis,salt stress accelerates the26S proteasome–dependent degradation of SPR1and that this facilitates MT disassembly and promotes
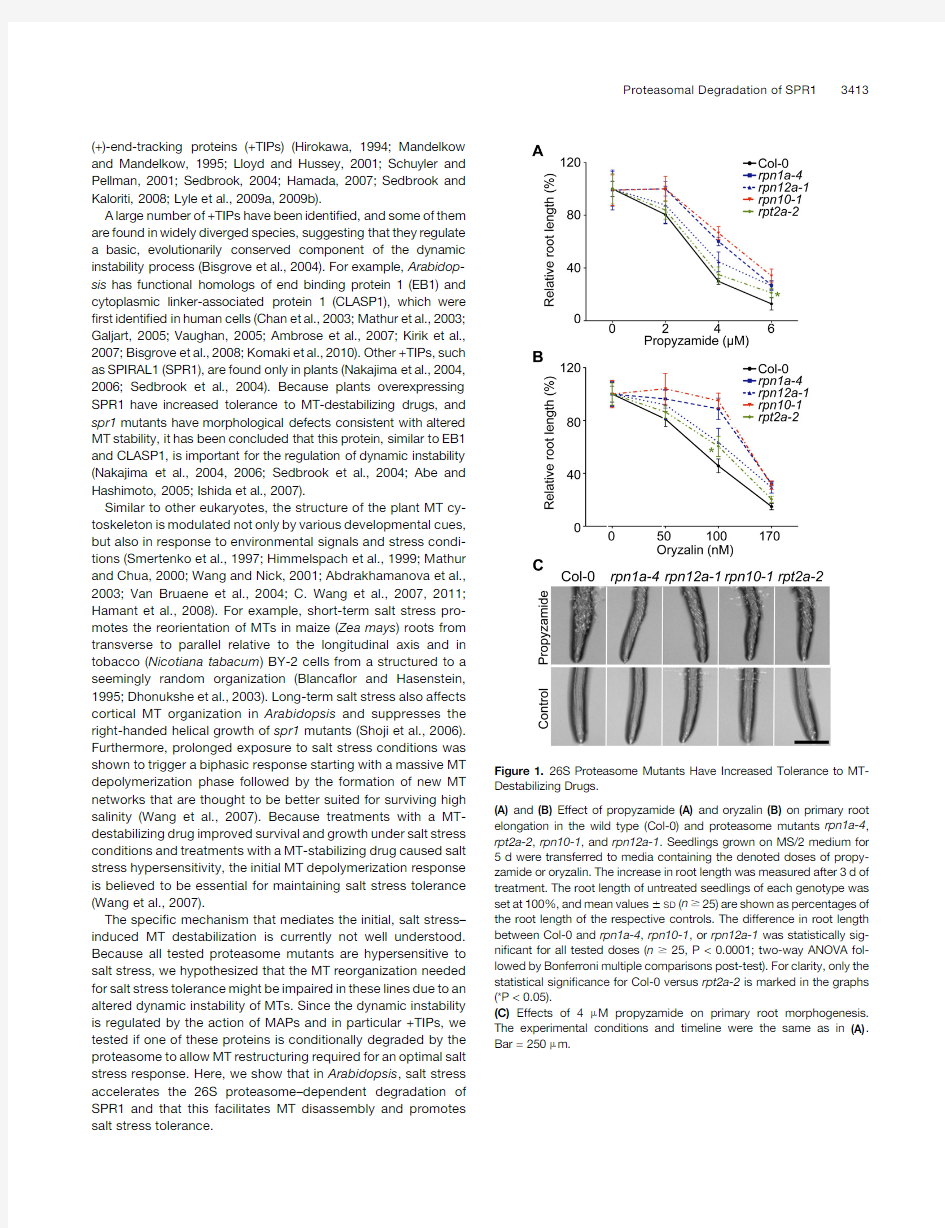
Figure1.26S Proteasome Mutants Have Increased Tolerance to MT-Destabilizing Drugs.
(A)and(B)Effect of propyzamide(A)and oryzalin(B)on primary root elongation in the wild type(Col-0)and proteasome mutants rpn1a-4, rpt2a-2,rpn10-1,and rpn12a-1.Seedlings grown on MS/2medium for 5d were transferred to media containing the denoted doses of propy-zamide or oryzalin.The increase in root length was measured after3d of treatment.The root length of untreated seedlings of each genotype was set at100%,and mean values6SD(n$25)are shown as percentages of the root length of the respective controls.The difference in root length between Col-0and rpn1a-4,rpn10-1,or rpn12a-1was statistically sig-ni?cant for all tested doses(n$25,P<0.0001;two-way ANOVA fol-lowed by Bonferroni multiple comparisons post-test).For clarity,only the statistical signi?cance for Col-0versus rpt2a-2is marked in the graphs (*P<0.05).
(C)Effects of4m M propyzamide on primary root morphogenesis. The experimental conditions and timeline were the same as in(A). Bar=250m m.
Proteasomal Degradation of SPR13413
RESULTS
26S Proteasome Mutants Have Increased Tolerance to
MT-Destabilizing Drugs
To assay the stability of cortical MTs in26S proteasome mutants, we?rst tested the responses of rpn1a-4,rpt2a-2,rpn10-1,and rpn12a-1mutants to the MT-destabilizing drugs oryzalin,which binds to a-tubulin,and propyzamide,which binds to b-tubulin (Nakamura et al.,2004;Lyons-Abbott et al.,2010).We showed previously that the total26S proteasome activity in the four tested proteasome mutants is affected to different levels,with rpt2a-2carrying the weakest and rpn10-1the strongest defect in 26S proteasome function(Kurepa et al.,2008).We determined the effects of MT-destabilizing drugs by measuring their inhibi-tion of root elongation and their promotion of root tip swelling, two plant growth responses that were shown to be caused by MT disassembly(Baskin et al.,1994).
All proteasome mutants were more tolerant to both propyza-mide and oryzalin(Figures1A and1B).The strongest mutant, rpn10-1,was the most tolerant,whereas the weakest mutant, rpt2a-2,showed a statistically signi?cant increase in propyza-mide and oryzalin tolerance only at a single dose(6m M for propyzamide,P<0.05;100nM for oryzalin,P<0.05).In addi-tion,the propyzamide tolerance of the double mutant rpn1a-4 rpn10-1was further increased compared with the respective single mutants(see Supplemental Figure1online).
We also analyzed the morphology of propyzamide-treated roots.Earlier reports have shown that seedlings grown on media supplemented with MT-destabilizing drugs contain swollen pri-mary roots(Furutani et al.,2000).After a3-d-long treatment with 4m M propyzamide,root swelling was obvious in the Columbia-0 (Col-0)plants,whereas the diameter of primary roots of all proteasome mutants did not increase(Figure1C;see Supple-mental Figure1C online).
Changes in rpn10-1MT Dynamics
To analyze the stability of MTs in proteasome mutants further,we compared the dynamics of individual MTs in Col-0and rpn10-1 backgrounds(Figure2).Previous studies have shown that green ?uorescent protein(GFP)fusions with a-(GFP-TUA6)or b-tubulin (GFP-TUB6)are incorporated into MTs and can be used to visualize MT dynamics in vivo(Ueda et al.,1999;Nakamura et al., 2004;Abe and Hashimoto,2005).However,whereas GFP-TUB6–labeled MTs have wild-type properties,the incorporation of GFP-TUA6changes MT dynamics and induces right-handed helical growth(Nakamura et al.,2004;Abe and Hashimoto, 2005).Thus,we chose to introduce the35S:GFP-TUB6trans-gene into the rpn10-1mutant and analyzed MT dynamics using confocal time-lapse imaging.
Kymographs of individual MT(+)-ends and representative life history plots showed that in rpn10-1,the MT growth phase was longer and the shrinkage phase was shorter than in the wild type (Figure2).Indeed,most of the parameters of MT dynamic instability were altered in the rpn10-1background(Table1). The average growth rate of the leading ends and the frequency of while the frequency of rescue events in rpn10-1was increased. Thus,individual MTs in rpn10-1were less dynamic and more prone to polymerization.In contrast with the(+)-end,there was no signi?cant difference in(2)-end dynamics between rpn10-1 and the wild type(Table1),implying that it is the process of dynamic instability and not treadmilling that was affected by the inhibition of proteasome activity.These results combined with the increased tolerance of proteasome mutants to MT-destabilizing drugs indicated that26S proteasome–dependent proteolysis plays an important role in the regulation of MT dy-namic instability.
SPR1Mediates the Tolerance of Proteasome Mutants
to Propyzamide
Previous studies showed that overexpression of the plant-speci?c+TIP SPR1leads to increased propyzamide
tolerance
Figure2.MT Plus-End Dynamics Are Altered in the rpn10-1Mutant.
(A)Kymograph of a160-s-long recording showing(+)-end dynamics of individual MTs.Four-day-old seedlings expressing35S:GFP-TUB6in the Col-0and rpn10-1backgrounds were used for time-lapse confocal imaging.Epidermal cells of upper hypocotyl regions were photographed every4s.Bar=5m m.
(B)The life history plots(length versus time)of three GFP-TUB6–labeled MTs in Col-0and three GFP-TUB6–labeled MTs in the rpn10-1back-ground.MT length was measured from confocal micrographs using Image J.
3414The Plant Cell
(Nakajima et al.,2004).Since proteasome mutants are also more tolerant of MT-destabilizing drugs(Figure1)and have altered MT dynamics,(Figure2),we tested whether SPR1is a26S protea-some target and whether its stabilization in proteasome mutants can explain the observed phenotypes.
To assay SPR1stability,we used cycloheximide(CHX)to inhibit de novo protein synthesis.After a12-h-long CHX treat-ment,the SPR1level was reduced to;30%of the control.This decrease was blocked by the proteasome inhibitor MG132, suggesting that SPR1is an unstable protein that is targeted for 26S proteasome–dependent proteolysis(Figure3A).Indeed,the SPR1level was increased in26S proteasome mutants rpn1a-4 and rpn10-1and was even more abundant in the rpn1a-4 rpn10-1double mutant(Figure3B).The SPR1mRNA abundance remained unchanged in the proteasome mutant backgrounds, con?rming that the increase in SPR1protein level was caused by a posttranscriptional mechanism(see Supplemental Figure2 online).Finally,CHX chase immunoblotting analyses(Yewdell et al.,2011)showed that the SPR1degradation rate was de-creased in proteasome mutant backgrounds(Figures3C and 3D),con?rming a role for the UPS in regulating SPR1abundance. To test if SPR1accumulation is a cause for the increased tolerance of proteasome mutants to MT-destabilizing drugs,we crossed the spr1-3mutation into the rpn1a-4and rpn10-1mutant backgrounds(Figure4;see Supplemental Figure3online). Analyses of the root tip morphology in propyzamide-treated double mutants showed that the reduced root tip swelling in proteasome mutants was suppressed by the spr1-3mutation (Figure4A).To quantify the effect of the spr1-3mutation,we measured the width of the primary root in the elongation zone (Figure4B).As expected,the propyzamide treatment did not promote any substantial root swelling in the proteasome mu-tants.The spr1-3mutation caused a mild increase in root width compared with the wild type(344.565m m and372.967m m for Col-0and spr1-3,respectively;n=25,P<0.001).By contrast, the root width of both rpn1a-4spr1-3and rpn10-1spr1-3double mutants was strongly increased compared with the respective single proteasome mutants(n=25,P<0.001),although the increases in swelling did not reach the wild-type level(P<0.01for Col-0).We concluded that the removal of SPR1suppressed the propyzamide tolerance of proteasome mutants but did not fully revert this phenotype to the wild-type level,suggesting the existence of other MT-stabilizing proteins that are also degraded by the26S proteasome.
The root elongation assay con?rmed that SPR1contributes to the propyzamide tolerance of proteasome mutants(Figure4C). The rpn1a-4spr1-3and rpn10-1spr1-3double mutants dis-played an increase in right-handed helical growth(see Sup-plemental Figure3online).For example,the right-handed root skewing of spr1-3was enhanced in both the rpn1a-4and rpn10-1mutant backgrounds.This was the most striking in rpn1a-4spr1-3seedlings that had upward growing roots(see Supplemental Figure3C online).However,low propyzamide doses fully suppressed the enhanced root skewing phenotype in both double mutants(see Supplemental Figure4online),allow-ing us to compare the propyzamide tolerance levels of all lines by the root elongation assay.Similar to the root tip swelling response,the root elongation assay showed that spr1-3sup-presses the propyzamide tolerance of both the rpn1a-4and rpn10-1mutants(Figure4C).
To analyze directly the effects of spr1-3on MT stability,we attempted to cross the35S:GFP-TUB6transgene into the spr1-3 and rpn10-1spr1-3mutants.We were unable to isolate spr1-3 or rpn10-1spr1-3mutants expressing GFP-TUB6in spite of screening more than1000F2seedlings,and we hypothesized that the35S:GFP-TUB6transgene is located in the proximity of the SPR1locus.However,analysis of the35S:GFP-TUB6locus revealed that the T-DNA is not linked to SPR1(data not shown). The reason GFP-TUB6expression and the spr1-3mutation are incompatible is currently unknown.As an alternative,we intro-duced the35S:GFP-TUA6transgene into the spr1-3and rpn10-1 spr1-3mutants.To assay the MT stability in these lines,we treated4-d-old seedlings with a high dose of propyzamide to ensure a fast and complete MT disruption(20m M propyzamide for1h)and analyzed epidermal cells of the upper hypocotyl regions by confocal microscopy(Figure4D).In rpn10-1,the GFP-TUA6–labeled cortical MTs remained partially intact,while MTs in the wild-type and rpn10-1spr1-3cells were disrupted,and the
Table1.Dynamic Instability Parameters in Col-0and rpn10-1
Dynamic Parameters (+)-Ends(à)-Ends
Col-0rpn10-1Col-0rpn10-1
Growth rate(m m/min) 5.4464.61 4.0462.73* 1.9161.15 1.7261.03
Shrinkage rate(m m/min)10.368.898.9168.44 3.5364.16 3.8264.17 Catastrophe(events/second)0.0410.030*0.2120.196
Rescue(events/second)0.0960.141*0.0470.051
Time spent on growth67.2%76.6%9.3%10.7%
Time spent on pause9.6%11.2%48.4%48.7%
Time spent on shrinkage20.4%12.3%42.4%40.6%
Dynamicity(m m/min) 5.9161.46 4.1962.41* 1.6761.92 1.9961.28
MT dynamic instability parameters were quanti?ed from confocal micrographs.Velocities were calculated from38leading and22lagging ends for 35S:GFP-TUB6in Col-0and45leading and32lagging ends for35S:GFP-TUB6in the rpn10-1background.Total measurements include1111and 1763velocities(4-s intervals)for GFP-TUB6in Col-0and rpn10-1,respectively.Dynamic parameters are expressed as mean6SD.Statistical signi?cance was calculated using Student’s t test comparing the mutant and wild-type values(*P<0.05).
Proteasomal Degradation of SPR13415
Collectively,these experiments suggested that the stabilization of SPR1in proteasome mutants is a cause for both their in-creased tolerance to MT-destabilizing drugs and altered MT dynamicity.
Salt Stress Promotes Proteolysis of SPR1
Based on earlier reports of the salt stress hypersensivitiy of26S proteasome mutants(Smalle et al.,2003;Wang et al.,2009)and the fact that salt stress tolerance requires MT disassembly function increases salt stress sensitivity by stabilizing SPR1, which in turn leads to increased MT stability.To test this,we?rst determined if salt stress leads to a conditional26S proteasome–dependent degradation of SPR1,which could facilitate the re-arrangement of cortical MTs needed for salt tolerance. Prolonged treatment(16h)of4-d-old Col-0seedlings with150 mM NaCl caused a decrease in SPR1level,and this decrease was blocked by MG132(Figure5A).CHX-chase immunoblotting analyses showed that a16-h-long NaCl treatment led to an ;80%reduction of the SPR1level in Col-0,whereas the SPR1 level in rpn1a-4and rpn10-1mutants was reduced by only;30
and;20%,respectively(Figures5B and5C).These results
indicated that the salt stress–induced decrease in SPR1level was a result of proteasome-dependent degradation.
During treatments with a higher concentration of salt(200mM), degradation of SPR1was detectable already after3h of incu-bation(see Supplemental Figures5A to5C online).To test if high salt concentrations promote SPR1degradation via a global increase in26S proteasome–dependent proteolysis,we moni-tored total proteasome activity by analyzing the abundance of polyubiquitinated proteins and by measuring proteasomal che-motryptic activity(see Supplemental Figures5D and5E online). After5h of exposure to200mM NaCl,we observed a signi?cant decrease in the SPR1protein level,no change in the overall levels of ubiquitinated proteins,and a mild but signi?cant decrease in proteasome activity.We concluded that the accelerated proteasome-dependent SPR1degradation during salt stress is not caused by a general increase in proteasome activity but is more likely the result of a speci?c destabilization mechanism that is activated by salt stress.Finally,we con?rmed that the SPR1 depletion in response to salt is indeed the result of a posttrans-criptional mechanism by observing that the SPR1mRNA level did not change in response to salt stress treatments(see Sup-plemental Figure5F online).
Previous studies have shown that cortical MT arrays depoly-merize in response to salt stress(Wang et al.,2007).Since the molecular mechanisms that govern this process were unknown, it remained possible that the salt stress–induced degradation of SPR1re?ects not a direct effect on SPR1,but an indirect effect caused by the MT disassembly.For example,the MT disassem-bly might increase the amount of free SPR1that could be more susceptible to degradation than the tubulin-bound version.To distinguish between these possibilities,we monitored the SPR1 level in Col-0plants treated with propyzamide.In Col-0seedlings treated for3h with10m M propyzamide,the cortical MTs were depolymerized(see Supplemental Figure6online),but the SPR1 level remained the same as in the untreated control(Figure5D). The propyzamide treatment also did not in?uence the salt stress–induced degradation of SPR1(Figure5D).
In addition to ionic stress,high salinity is also known to cause osmotic stress(Zhu,2001).To test if osmotic stress in?uences the stability of SPR1,we treated Col-0seedlings with high doses of the osmolyte mannitol(Figure5E).The mannitol treatments did not affect SPR1stability,suggesting that the signal triggering SPR1destabilization was speci?cally ionic stress(Figure5F).We concluded that salt stress,but not osmotic stress or MT disas-sembly per se,promotes the26S proteasome–dependent deg-
Figure3.SPR1Is a26S Proteasome Target.
(A)Immunoblotting analyses using anti-SPR1and anti-GS antisera. Seven-day-old seedlings were treated with100m M MG132and/or200 m M CHX for16h and used for the extraction of total protein.The anti-GS sera recognizes both the chloroplastic(45kD)and cytosolic(40kD)GS isoforms.
(B)SPR1levels in8-d-old Col-0,rpn1a-4,rpn10-1,and rpn1a-4rpn10-1 seedlings.Anti-RPN1and anti-RPN10sera were used to con?rm the genotype of proteasome mutants.
(C)Representative CHX-chase immunoblots.The stability of SPR1was tested on total protein extract of10-d-old wild-type and mutant seed-lings treated with200m M CHX for the indicated time periods.
(D)Quanti?cation of SPR1stability in CHX-treated Col-0,rpn1a-4,and rpn10-1plants.Immunoblots,representatives of which are shown in(C), were used to quantify signal intensities.The average signal intensity of the zero time point sample for each line was set to100%,and the mean values6SD(n=3)are shown as percentages of the respective control. The asterisks represent the statistical signi?cance of the difference be-tween degradation rates in Col-0and both proteasome mutants(****P< 0.0001;ANOVA followed by Bonferroni multiple comparisons post-test). [See online article for color version of this?gure.]
3416The Plant Cell
SPR1Stabilization Causes Salt Stress Hypersensitivity
To test if SPR1stabilization causes the salt hypersensitivity of26S proteasome mutants(Smalle et al.,2003;Wang et al.,2009),we ?rst analyzed if the increased MT stability is responsible for the changes in salt tolerance(Figure6A),then determined the salt tolerance levels of rpn1a-4spr1-3and rpn10-1spr1-3mutants (Figures6B to6D),and?nally compared the effects of salt stress on the MT arrays of rpn10-1and rpn10-1spr1-3cells(Figure7). To test the relationship between MT stability and salt tolerance, we analyzed the effects of combined salt stress and propyzamide treatments on the root elongation of rpn1a-4and rpn10-1mutants (Figure6A).In the absence of propyzamide,root length of wild-type plants grown on100mM NaCl was;70%of the control,whereas the root length of proteasome mutants was reduced to ;50%.As expected,low doses of propyzamide counteracted the salt-induced inhibition of root elongation in all lines.However, whereas1m M propyzamide led to an8%increase in root length in the wild type(statistically nonsigni?cant,P>0.05,n$15;analysis of variance[ANOVA]with Bonferroni post-test),the increase in root length in the proteasome mutants was;25%(P<0.001,n$15). Thus,since low doses of an MT-destabilizing drug reverted the salt hypersensitivity of proteasome mutants to wild-type levels,we concluded that the increased MT stability in proteasome mutants is indeed responsible for their salt hypersensitivity.
This conclusion,together with our observation that spr1-3 suppressed the increased MT stability in rpn1a-4and rpn10-1 mutants(Figure4),prompted us to test if the spr1-3mutation
also
Figure4.spr1-3Suppresses the Propyzamide Tolerance of Proteasome Mutants.
(A)Effects of4m M propyzamide on primary root morphogenesis in Col-0,rpn1a-4,spr1-3,and the double mutant rpn1a-4spr1-3.Five-day-old seedlings were transferred to fresh MS/2medium or MS/2medium with propyzamide.The seedlings were grown on vertically positioned plates for3d and then photographed.Bar=1mm.
(B)Effects of4m M propyzamide on root width.Seedlings were grown and treated as in(A).The width of primary roots at the elongation zone was measured from micrographs using ImageJ.The signi?cance was analyzed using ANOVA followed by Bonferroni multiple comparisons post-test. Crosses represent the signi?cance of the differences between the untreated Col-0and untreated mutant lines,and asterisks mark the signi?cance between the treated Col-0and treated mutant lines(**P<0.01;???and***P<0.001;and????and****P<0.0001).
(C)Root lengths of5-d-old seedlings grown on MS/2media with4m M propyzamide(****P<0.0001;signi?cance for Col-0versus mutants).The statistical analyses and data presentation are as in(B).
(D)In vivo analyses of the MT-destabilizing effect of20m M propyzamide in the Col-0wild type and in the rpn10-1and rpn10-1spr1-3mutants.The35S: GFP-TUA6transgene was crossed into the rpn10-1and rpn10-1spr1-3mutant backgrounds,and the GFP-TUA6–labeled cortical MTs were analyzed by confocal microscopy.Hypocotyl epidermal cells of4-d-old seedlings treated for1h are shown.Bar=20m m.
Proteasomal Degradation of SPR13417
suppresses the salt hypersensitivity of proteasome mutants (Figures 6B and 6D).Salt stress induces a range of morphological alternations in both the aerial parts of the plant and the roots.For example,NaCl treatments lead to reductions in root length and lateral root number,a decrease in leaf and petiole size,and a delay in leaf emergence (Burssens et al.,2000).Furthermore,prolonged or intense salt stress treatments cause leaf chloro-sis,bleaching,and necrosis.All adverse effects of salt stress,including leaf necrosis and the inhibition of root and rosette growth,were apparent at lower doses in rpn10-1compared with the wild type and were suppressed by the spr1-3mutation (Figure 6B).The fresh weight of rpn1a-4spr1-3and rpn10-1spr1-3plants grown for 2weeks on salt-containing media was signi?cantly increased compared with the single rpn1a-4and rpn10-1mutants but did not reach the wild-type levels (Figure 6C).The effects of NaCl on root elongation were measured after 3d of treatment (Figure 6D).On the control media,spr1-3sup-pressed root elongation both in the wild type and in proteasome mutants,probably because it caused the right-hand skewing of roots.On NaCl-containing media,root elongation of rpn1a-4spr1-3and rpn10-1spr1-3plants was increased compared with the respective single 26S proteasome mutants but again did not reach the length of the wild-type roots treated with the same dose of NaCl (Figure 6D).Thus,the spr1-3mutation did indeed suppress the salt hypersensitivity of proteasome mutants,but this suppression was partial.
Next,we performed a time-course analysis of the impact of salt stress on GFP-TUA6–labeled MT arrays in wild-type,spr1-3,rpn10-1,and rpn10-1spr1-3plants (Figure 7).In the wild type,MT disassembly was nearly complete after a 4-h-long exposure to 200mM NaCl (Figure 7A).By contrast,the cortical MT network in rpn10-1cells remained largely intact up until 6h into the treatment.The spr1-3mutation suppressed the delayed MT depolymerization in the rpn10-1mutant,but similarly to the effects of spr1-3on whole-seedling salt tolerance,it did not fully revert the MT disassembly rate back to the wild-type level.Analyses of the number of MTs per unit length con?rmed both the delayed salt response in rpn10-1and the partial suppression of this delay by the spr1-3mutation (Figure 7B).We concluded that the SPR1stabilization in proteasome mutants causes salt hy-persensitivity by slowing down the salt stress–induced depoly-merization of MTs.However,since the proteasome mutant salt stress responses did not completely revert back to the wild-type level when the spr1-3mutation was introduced,we also con-cluded that SPR1is not the only proteasome target that inhibits salt stress–induced MT
depolymerization.
Figure 5.Salt Stress Promotes 26S Proteasome–Dependent Proteolysis of SPR1.
(A)Immunoblotting analyses of SPR1in Col-0seedlings treated with NaCl and MG132.Four-day-old seedlings were treated for 16h with 150mM NaCl alone or 150mM NaCl and 100m M MG132.
(B)CHX-chase immunoblot.Ten-day-old seedlings were transferred to liquid MS/2media containing 200m M CHX and 200mM NaCl.Seedlings were treated for the indicated times.
(C)Quanti?cation of immunoblots represented by those in (B).The signals of the untreated samples were set at 100%.Data are shown as mean values 6SD (n =3)as percentages of the respective controls.The asterisks represent the statistical signi?cance of the difference between the degradation rates in Col-0versus rpn10-1and rpn1a-4(****P <0.0001;ANOVA followed by Bonferroni multiple comparisons post-test).
(D)and (E)Immunoblotting analyses of SPR1in Col-0seedlings treated with the denoted doses of NaCl and (D)propyzamide or mannitol (E).Four-day-old seedlings were transferred to water containing the test compounds,treated for 3h,and used for the isolation of total proteins.3418The Plant Cell
The fast,salt-induced MT depolymerization response in cells of the wild type and rpn10-1spr1-3double mutant was followed by the start of new MT formation(8-h time point;Figure7).By contrast,after8h of treatment,the MT disassembly was just completed and no distinct MTs could be detected in the rpn10-1 seedlings.This con?rms an earlier report stating that fast MT disassembly in response to salt stress is required for the timely formation of new MT networks potentially better suited to toler-ate high salt concentrations(Wang et al.,2007).
Additional proof that increased SPR1abundance is a cause for salt stress hypersensitivity was obtained by analyzing transgenic plants that overexpress SPR1.In theory,plants that overexpress SPR1should respond to salt stress similarly to proteasome mutants in which the SPR1level is increased because of a reduced degradation rate.Previous work already showed that SPR1overexpression indeed leads to increased tolerance to propyzamide in shoot organs(Nakajima et al.,2004).Here,we show that increased tolerance to propyzamide can also be observed in the root-swelling assay and is indeed associated with salt stress hypersensitivity(see Supplemental Figure7 online).Collectively,these results con?rm the importance of SPR1removal for maintaining salt stress tolerance.MG132Promotes MT Stability and Salt
Stress Hypersensitivity
Finally,to independently test the role of SPR1proteolysis in the salt stress–induced restructuring of cortical MT arrays,we ana-lyzed the combined effects of salt,propyzamide,and MG132on Col-0and spr1-3seedlings(Figure8).
Similar to the genetic suppression of proteasome activity, pretreatment of Col-0seedlings with MG132inhibited the propyzamide-induced swelling of root tips(Figure8A).Although MG132also inhibited the swelling of spr1-3roots,this effect was not as strong as in the wild type(Figure8A).MG132also counteracted the inhibitory effect of propyzamide on root elon-gation(Figure8B).Treatment with either MG132or propyzamide inhibited root elongation in Col-0and spr1-3seedlings.However, while MG132reduced the root lengths to;85%of the respec-tive controls,the propyzamide treatment caused a more severe growth inhibition(to;30%of the control values).Roots of Col-0 plants treated with both MG132and propyzamide were;50% longer than when treated with propyzamide alone,indicating that the proteasome inhibitor counteracts the effect of the MT destabilizing drug.Furthermore,this MG132effect was
also
Figure6.spr1-3Suppresses the Salt Hypersensitivity of26S Proteasome Mutants.
(A)Salt hypersensitivity of proteasome mutants was suppressed by propyzamide treatments.Five-day-old seedlings grown on MS/2medium were transferred to fresh MS/2plates or MS/2plates with100mM NaCl and the indicated doses of propyzamide.Plates were positioned vertically,and the root lengths were measured after3d.Data represent relative root length(n$20)with SD.The average root length of untreated Col-0seedlings was set at100%.
(B)Five-day-old seedlings grown on MS/2medium were transferred to fresh MS/2medium or MS/2containing100mM NaCl.Test plates were positioned vertically,and plants were grown for2weeks.The arrows highlight necrotic spots in the rpn10-1mutant grown on100mM NaCl.
(C)and(D)The salt tolerance levels were quanti?ed by comparing the fresh weight after a2-week-long treatment(C)and root lengths after a3-d-long treatment(D).Data are shown as mean values6SD of three independent experiments(n$15for each).The asterisks represent the signi?cances of the differences between the double mutants and the respective single proteasome mutants and were calculated using ANOVA followed by Bonferroni
Proteasomal Degradation of SPR13419
partially attenuated in the spr1-3mutant,implying a role for SPR1 in the MG132-induced MT stabilization(Figure8B).
To test if MG132also increases salt sensitivity,we analyzed the response of GFP-TUB6–labeled MT arrays and the growth of Col-0and spr1-3on medium supplemented with200mM NaCl (Figures8C to8E).Indeed,MG132suppressed the salt-induced depolymerization of cortical MTs(Figure8C)and decreased the ever,compared with the wild type,MG132was less effective in suppressing leaf expansion(Figure8D)and root elongation of salt-treated spr1-3seedlings(Figure8E),con?rming that the salt hypersensitivity caused by proteasome inhibition requires SPR1 function.Thus,we?nd that the increased MT stability and salt stress hypersensitivity of proteasome mutants can be phe-nocopied by treating wild-type plants with the proteasome inhibitor MG132.
DISCUSSION
Posttranscriptional and,in particular,proteasome-dependent regulation of tubulin/MT system dynamics have been described in considerably greater detail in animals than in plants.MT dynamics depend on the availability of tubulin heterodimers and on the activities of MAPs,and recent studies have shown that proteasome-dependent protein degradation determines the sta-bility of both MT dynamics determinants.For example,the role of the proteasome and tubulin-speci?c chaperones in the degra-dation of tubulins released by MT depolymerization has been documented in animals(Bhamidipati et al.,2000;Ren et al., 2003;Bartolini et al.,2005;Voloshin et al.,2010).Depolymeriza-tion of MTs also leads to the proteasome-dependent degrada-tion of tubulins in plants,but the molecular players that prime tubulin for proteolysis and that deliver tubulin heterodimers(or monomer-chaperone complexes)to the proteasome are still unidenti?ed(S.Wang et al.,2011).
In animals,the proteasome has also been shown to regulate MT dynamics by in?uencing the stability of MAPs(David et al., 2002;Petrucelli et al.,2004;Peth et al.,2007;Poruchynsky et al., 2008;Ban et al.,2009).Our study extends this observation to plants and reveals that proteasome-dependent stability control is essential for the restructuring of MT arrays and the?ne-tuning of MT dynamics.We show that genetic and pharmacological inactivation of26S proteasome activity in Arabidopsis leads to an increase in MT stability and,consequently,to an improved tolerance of MT destabilizing drugs.The increased MT stability in proteasome mutants was largely the result of stabilization of the plant-speci?c+TIP SPR1.Thus,the accumulation of SPR1 resulting from either overexpression of the SPR1transgene (Nakajima et al.,2004)or a reduced degradation rate(this study) is suf?cient to promote increased MT stability in plant cells. Although the increased stability of SPR1in26S proteasome mutants implies that this MAP is targeted for degradation in a ubiquitin-dependent manner,we were unable to detect any candidate ubiquitinated SPR1forms with higher molecular weights.Similar results were described for several other known proteasome targets(Dill et al.,2001;Lopez-Molina et al.,2001; Xie et al.,2002;Smalle et al.,2003;Gagne et al.,2004),sug-gesting that the detection of ubiquitin conjugates can be tech-nically challenging.Proteomic studies have shown that only a very small fraction of any given26S proteasome target protein exists in its ubiquitinated form(Kaiser and Tagwerker,2005). Furthermore,it can be envisioned that even upon partial stabi-lization in proteasome mutant backgrounds,the ubiquitinated SPR1forms remain undetectable due to the actions of ubiquitin
Figure7.SPR1Stabilization in rpn10-1Delays the Salt-Induced Disas-sembly of Cortical MT Arrays.
(A)Visualization of GFP-TUA6–labeled cortical MTs in upper hypocotyl epidermal cells from4-d-old Col-0,rpn10-1,and rpn10-1spr1-3seed-lings treated with200mM NaCl for the indicated times.Bar=10m m.
(B)The density of cortical MTs per unit length in upper hypocotyl cells was determined by counting the MTs crossing the longitudinal axis of a cell.For each line and treatment,a minimum of32hypocotyl cells from?ve separate seedlings was photographed and used for measurements.The asterisks represent the signi?cance of the difference between Col-0and the mutants treated for the same time and were calculated using ANOVA followed by Bonferroni multiple comparisons post-test(****P<0.0001). [See online article for color version of this?gure.]
3420The Plant Cell
ubiquitinated proteins back to their unmodi?ed forms (Smalle and Vierstra,2004;Kaiser and Tagwerker,2005).
While our data show that SPR1stabilization plays a pivotal role in the altered MT dynamics of proteasome mutants,they also suggest the involvement of other MAPs.For example,whereas the increased rescue frequencies and decreased frequencies of catastrophe (Figure 2,Table 1)that re?ect an overall increase in MT stability in rpn10-1cells are in agreement with the predicted effects of increased SPR1action,the slower growth of MTs (Table 1)cannot be explained by an increase in SPR1activity and suggests that loss of proteasome function also affects one or more proteins that control the MT polymerization rate.Another example is presented in Figure 4:The spr1-3mutation did not fully suppress the increased propyzamide tolerance of protea-some mutants,suggesting the stabilization of one or more additional proteins that promote MT stability.The involvement of MT-stabilizing factors other then SPR1could also explain the observation that the right skewing of spr1-3roots was enhanced in the rpn10-1spr1-3and rpn1a-4spr1-3double mutants (see Supplemental Figure 3online).The spr1mutants are unusual in that they combine a decrease in MT stability with the right skewing of roots even though this developmental phenotype is typically the result of MT stabilization (Sedbrook and Kaloriti,2008).Because spr1-3partially reversed the increased MT stability of proteasome mutants (Figure 4),we expected that the right skewing of spr1-3roots would also be reversed.However,since the right skewing was enhanced in the double mutants,we propose that this phenotype re?ects the function of other MT-stabilizing MAPs whose activity is enhanced by their stabilization in the proteasome mutant backgrounds.
Our observation that MT stability depends on the SPR1degradation rate suggested that the activity of this protein is controlled posttranslationally and implied the existence of de-velopmental or environmental cues that in?uence MT dynamics by regulating SPR1stability.Indeed,we found that SPR1pro-teolysis is enhanced under salt stress conditions (Figure 5)and that SPR1stabilization leads to salt stress hypersensitivity in 26S proteasome mutants (Figure 6).Furthermore,we showed that the increased salt stress sensitivity of proteasome mutants is caused by a slower rate of MT disassembly,which is a direct result of the SPR1stabilization.These results are in agreement with the results of an earlier study that described that suppression of MT disassembly by the MT-stabilizing drug paclitaxel caused salt stress hypersensitivity (Wang et al.,
2007).
Figure 8.MG132Stabilizes MTs and Increases the Sensitivity to Salt Stress.
(A)and (B)Four-day-old Col-0and spr1-3seedlings were transferred to MS/2medium containing 4m M propyzamide,40m M MG132,or 4m M propyzamide and 40m M MG132.Plants were grown vertically for three days before the root tips were photographed ([A];bar =0.5mm),and the root lengths were measured (B).In (B),data represent an average root length with SD (n =26),and the asterisks represent the signi?cances of the differences between the wild type and spr1-3(****P <0.0001;ANOVA followed by Bonferroni multiple comparisons post-test).
(C)Visualization of GFP-TUB6–labeled cortical MTs in upper hypocotyl epidermal cells.Four-day-old seedlings were pretreated with 100m M MG132or DMSO in liquid MS/2medium for 6h,and then 20m M propyzamide or 200mM NaCl was added.Propyzamide treatments were done for 1h and the NaCl treatments for 3h prior to microscopy.Bars =10m m.
(D)and (E)Four-day-old Col-0and spr1-3seedlings were transferred to MS/2medium containing 100mM NaCl,50m M MG132,or both.Seedlings were photographed,and the root lengths were measured after 3d of treatment.In (E),data represent an average root length with SD (n $25),and the asterisks represent the signi?cances of the differences between the wild type and spr1-3(****P <0.0001,***P <0.001,and **P <0.01;ANOVA followed Proteasomal Degradation of SPR13421
Arabidopsis26S proteasome mutants are also characterized by a decrease in growth rate that re?ects a reduced rate of mitosis (Kurepa et al.,2009b).Since MT depolymerization is also known to inhibit mitosis,an alternative explanation for the salt stress hyper-sensitivity of26S proteasome mutants is that the reduced mitotic rates make them more susceptible to the growth inhibitory effects of salt stress.However,this explanation is unlikely because the spr1-3mutation suppressed the salt hypersensitivity but not the reduced growth rate of proteasome mutants(Figure6).Further-more,the SPR1overexpression lines were also salt hypersensitive but did not display any decrease in growth rate(see Supplemental Figure7online),thus con?rming that the increased SPR1abun-dance in26S proteasome mutants is indeed the most likely cause for their salt stress hypersensitivity.
Collectively,our data reveal an important role for proteasome-dependent regulation of SPR1in the survival of plants challenged by high salinity.Figure9outlines a model that summarizes the role of SPR1in the MT disassembly response to salt stress.According to this model,SPR1is a moderately stable protein under normal growth conditions and is localized predominantly at the growing ends of MTs where it inhibits their disassembly. Upon salt stress,26S proteasome–dependent degradation of SPR1is accelerated,and the MT depolymerization needed for the survival of plant cells under salt stress is facilitated.By contrast,salt stress–induced SPR1degradation is attenuated in proteasome mutants,thus slowing down MT disassembly and causing salt stress hypersensitivity.Since the spr1-3mutant was not more tolerant to salt stress than the wild type,we can also conclude that this destabilization mechanism is strong and fast enough to suppress SPR1activity to a level where it does not interfere with the salt-induced MT disassembly process.Ac-cordingly,we propose that SPR1destabilization does not initiate the salt-induced MT disassembly,but its removal is required to allow the timely completion of this process.
The rapid MT depolymerization response to salt stress is thought to facilitate the formation of a new MT network that
allows
Figure9.Model Summarizing the Role of SPR1Proteolysis in the Salt Stress Tolerance of Wild-Type and Proteasome Mutant Cells.
SPR1,a(+)-end MAP,is degraded by the26S proteasome(26SP).In26S proteasome mutants,the degradation rate is reduced and the MTs are more stable due to SPR1accumulation.Upon salt stress perception,a still unknown mechanism leads to the increased proteasome-dependent degradation of SPR1that facilitates MT depolymerization.In proteasome mutants,salt stress–induced degradation of SPR1is reduced,which slows down MT depolymerization and causes salt stress hypersensitivity.This schematic is simpli?ed for the purpose of clarity:no other(+)-end MAPs but SPR1are 3422The Plant Cell
cells to better withstand the damaging impacts of high salt concentrations.Indeed,in plant cells exposed to prolonged salt stress,the initial massive MT depolymerization was followed by the formation of new MT networks(Wang et al.,2007).It has been established that salt stress leads to a reduction and reorientation of cell expansion and that these growth alterations are important for adapting to and surviving high salinity(Munns and Tester, 2008).As major determinants of the direction and rate of cell expansion,MT networks would indeed have to be rapidly reor-ganized to promote and facilitate such changes in growth.The new MT network has a more random MT organization compared with cells of unstressed plants(Wang et al.,2007).This corre-sponds well with the need for a reduced growth rate,as rapid cell elongation tends to require a transverse orientation of MTs to the direction of growth(Chan et al.,2011;Crowell et al.,2011). Because MT depolymerization is known to increase calcium channel activity(Thion et al.,1998),the initial MT disassembly response is also thought to be important for increasing the cytosolic calcium concentration,which is a major requirement for the adaptation to salt stress(Wang et al.,2007;Mahajan et al., 2008).The calcium burst was also shown to be essential for the formation of new MTs after prolonged salt stress exposure, suggesting that the initial MT depolymerization response not only allows the development of new MT networks but also establishes optimal conditions for MT synthesis(Wang et al.,2007).
While our study highlights the importance of proteasome-dependent proteolysis in the regulation of MT dynamics during salt stress,it also raises a number of questions.The?rst question relates to the stress-speci?c effects on MTs.We have shown that osmotic stress does not cause MT disassembly and does not promote SPR1destabilization.Other stresses,such as cold, heat,and treatments with nanoparticles(Smertenko et al.,1997; S.Wang et al.,2011),induce changes in plant MT networks,and future studies need to address whether this is also mediated via the stability control of MAPs.Whereas SPR1is currently the only known26S proteasome target among the plant MAPs,the partial suppression of26S proteasome mutant MT phenotypes by spr1-3suggests the existence of other proteins with SPR1-like activities that are also stabilized when proteasome function is impaired.Some obvious candidates to consider are the family of SPR1-like proteins,which were shown to have functions similar to SPR1(Nakajima et al.,2006),and the evolutionarily conserved, MT-stabilizing protein EB1,which is known to be targeted for proteasome-dependent proteolysis in human cells(Peth et al., 2007)and was reported to interact with SPR1potentially to regulate directional plant cell expansion(Kaloriti et al.,2007). The second question relates to the mechanism by which the perception of the salt stress signal leads to proteasome-dependent degradation of SPR1.Signal-induced site-speci?c phosphory-lation often initiates the ubiquitin-dependent targeting of a pro-tein to the26S proteasome(Chen et al.,1995;Matsuzaki et al., 2003;Smalle and Vierstra,2004).Phosphorylation is also a general regulation mechanism that reduces the binding af?nity of MAPs for MTs and thus leads to MT destabilization(Drewes et al.,1998;Matenia and Mandelkow,2009;Beck et al.,2010). On the other hand,it has been reported that a mitogen-activated protein kinase(MAPK)cascade plays a critical role in the salt salt stress–induced proteasome-dependent control of MT de-polymerization could involve a salt stress–activated MAPK cas-cade that leads to the phosphorylation of SPR1,followed by its interaction with a speci?c ubiquitin ligase and degradation by the 26S proteasome.This sequence of events would require that a salt stress–responsive MAPK localizes close to SPR1or is relocated to SPR1upon stress(i.e.,constitutively or conditionally associated with MTs).Recent studies in Arabidopsis identi?ed a number of MAPKs(MAK18and MAK4)involved in stress signal-ing that are associated with MTs or are involved in the regulation of MT dynamics(Walia et al.,2009;Beck et al.,2010).On the other hand,the SPR1protein contains nine putative phosphor-ylation sites(as predicted by NetPhos2.0),and one of them(Thr-76)is a part of MAPK consensus sequence PXS/TP or S/TP, suggesting that the potential for MAPK-dependent SPR1regu-lation is indeed an interesting topic for future research. METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana plants were grown on plates containing half-strength Murashige and Skoog medium with1%Suc(MS/2)as described previ-ously(Smalle et al.,2003).Plants were grown in a controlled environment chamber at228C with continuous light(140m mol photons m22s21).The proteasome mutants rpn1a-4,rpt2a-2,rpn10-1,and rpn12a-1(all in Col-0 background,and all carrying the kanamycin resistance gene)have been described(Kurepa et al.,2008;Wang et al.,2009).The spr1-3mutant and GFP-TUA6and GFP-TUB6overexpression lines(all in Col-0background) have also been described(Nakajima et al.,2004;Abe and Hashimoto, 2005).For the generation of double mutants,putative homozygous dou-ble mutants were selected based on their phenotypes,and their geno-types were con?rmed by immunoblotting analyses.
Treatments
Oryzalin and propyzamide were purchased from Sigma-Aldrich and MG132from Enzo Life Sciences.All drugs were made as10003stocks. MG132,CHX,and propyzamide were dissolved in DMSO and oryzalin in 100%ethanol.All control experiments included a13dose of the solvent. For all root elongation and root tip assays,plants grown on vertically positioned MS/2plates for5d were transferred to drug-or mock-supplemented media.Test plates were positioned vertically,and the root length was marked daily.After3d of treatments,plants were photo-graphed.Photomicrographs of representative root tips were taken with an Olympus SZX12microscope equipped with a DP12camera.For stress tolerance assays,4-or5-d-old seedlings were transferred to test plates with NaCl or mannitol and were grown vertically.For all morphometric analyses,the relevant parameter was measured from digital images using ImageJ(https://www.360docs.net/doc/1e10795561.html,/ij/).Unless speci?ed otherwise,data are presented as mean values,and the error bars represent standard devi-ation.Statistical signi?cance was determined by ANOVA tests followed by post hoc Bonferroni multiple comparison test.Post hoc statistical signi?cance is indicated in the?gures by asterisks or crosses.For all experiments,descriptive statistics,plotting,and the hypothesis testing were done using Prism5.0d software(GraphPad Software).
Confocal Microscopy Analysis of MTs and Scoring
MT distributions were analyzed in35S:GFP-TUA6and35S:GFP-TUB6
Proteasomal Degradation of SPR13423
Olympus Fluoview FV1000confocal laser scanning microscope equipped with an argon ion laser for the excitation of GFP(excitation488nm and barrier500to550nm)essentially as described(S.Wang et al.,2011).For all experiments,4-d-old seedlings were used,and epidermal cells of upper hypocotyl regions were analyzed.Except for the MT dynamics measurements,seedlings were mounted,incubated,and observed in water or an aqueous solution of the tested drugs.For the measurement of individual MT dynamics,seedlings were mounted in liquid MS/2medium, and time-lapse imaging was performed at1%laser power and4-s in-tervals for3to6min.Images were processed and analyzed using ImageJ as previously described(Buschmann and Lloyd,2008).The dynamics and the frequency of catastrophe and rescue were calculated as described (Dhonukshe and Gadella,2003;Abe and Hashimoto,2005).To quantify MT network density,MTs crossing the middle long axis of upper hypo-cotyl cells were counted.The results were expressed as MT number per unit distance as described previously(Ishida et al.,2007). Immunoblotting Analyses
For all analyses,plants were weighed and transferred to1.7-mL tubes for treatment.After treatment,plants were blotted,frozen in liquid N2,and ground in three volumes of23Laemmli sample buffer.Total proteins were separated by SDS-PAGE(14%acrylamide separating gel for SPR1 analyses,8%for RPN1analyses,and10%for all other analyses). Proteins were transferred to nitrocellulose membranes(Hybond C-Extra; GE)and probed as previously described(S.Wang et al.,2011).Antibodies against RPN1and SPR1were described(Nakajima et al.,2004;Wang et al.,2009).To test if the signal intensity for SPR1in all experiments falls within the linear dynamic range of the assay,immunoblots with a dilution series of total protein extracts were probed with anti-SPR1(dilution 1:1000),developed using chemiluminescent substrate(Thermo Scienti?c Pierce ECL Plus substrate)and analyzed using the linear best?t analyses (see Supplemental Figure8online).The anti-RPN10sera were obtained from Enzo Life Sciences.The anti-glutamine synthase(GS)serum was purchased from Agrisera AB.GS was assayed as a control.This is a stable protein,and its steady state levels are not affected by inhibition of proteasome activity(Kurepa et al.,2010).Horseradish peroxidase–conjugated and alkaline phosphatase–conjugated secondary antibodies were purchased from Santa Cruz Biotechnology.
Transcript Analyses
Total RNA was isolated using TRIzol reagent(Invitrogen).RNA was treated with TURBO DNase(Ambion),and the RNA concentrations were determined spectrophotometricaly(NanoDrop2000;Thermo Scienti?c). One microgram of RNA per sample was used for the synthesis of the?rst-strand cDNA(iScript reverse transcription supermix;Bio-Rad).
For the quanti?cation of SPR1transcripts,real-time RT-PCR was performed using the StepOne real-time PCR system(Applied Biosys-tems)and the DyNAmo Flash SYBR Green qPCR kit(Finnzymes)in total reaction volume of20m L.The SPR1primers used were59-AGCCTGCA-GAGCTTAACAAG-39and59-TGAACTTTGGTCGAAGGACG-39,and the primers for reference genes ACT2,ACT8,EF-1a,and GADPH were as described(Czechowski et al.,2005).Selection of the reference gene(s) best for the normalization in each experiment was calculated using geNorm(Vandesompele et al.,2002).
Analyses of Ubiquitin Conjugates and Proteasome Activity
Immunoblotting analysis of ubiquitin conjugates was done as described (Kurepa et al.,2008).The antipolyubiquitinated protein serum was from Enzo Life Sciences.For proteasome activity assays,samples were ground in1.25volumes of extraction buffer as described(Kurepa et al.,(Bio-Rad),and total proteasome activity was measured using the Suc-LLVY-AMC assay as described(Kurepa et al.,2008).
Generation of Transgenic Plants Overexpressing SPR1
To generate the overexpression construct,SPR1cDNA was ampli?ed using attB PCR primers attB1SPR159-GGGGACAAGTTTGTACAAAAA-AGCAGGCTTAATGGGTCGTGGAAACAGC-39and attB2SPR159-GGG-GACCACTTTGTACAAGAAAGCTGGGTCTTACTTGCCACCAGTGAAGA-39. The cDNA was introduced into pDONR221via BP reaction and then to pEarlyGate100(Earley et al.,2006)by LR recombinase(Invitrogen). Transgenic plants were selected on MS/2plates containing10m M L-phosphinothricin(Gold Biotechology),and homozygous T3plants were used for the analyses.
Accession Numbers
Sequence data from this article can be found in The Arabidopsis Information Resource(https://www.360docs.net/doc/1e10795561.html,/)under the following accession numbers:RPN1a(At2g20580),RPT2a(At4g29040),RPN10 (At4g38630),RPN12a(At1g64520),SPR1(At2g03680),TUA6(At4g14960), and TUB6(At5g12250).Arabidopsis T-DNA insertion mutants and trans-genic lines and their identi?cation numbers are SALK_027970(rpn1a-4), SALK_005596(rpt2a-2),35S:GFP-TUA6(CS6551),and35S:GFP-TUB6 (CS6550).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure1.Visible Phenotypes and Propyzamide Re-sponses of the Double Mutant rpn1a-4rpn10-1.
Supplemental Figure2.The Steady State SPR1Transcript Levels in Proteasome Mutants Are the Same as in the Wild Type.
Supplemental Figure3.Right-Handed Helical Growth Is Enhanced in rpn1a-4spr1-3and rpn10-1spr1-3Mutants.
Supplemental Figure4.Propyzamide Treatment Reverts Ampli?ed Root Skewing in the rpn1a-4spr1-3Mutant.
Supplemental Figure5.Effects of NaCl Treatments on the SPR1 Transcript Level,SPR1Protein Abundance,Accumulation of Ubiq-uitinated Proteins,and Proteasome Activity.
Supplemental Figure6.Treatment with10m M Propyzamide Causes Depolymerization of Cortical MTs.
Supplemental Figure7.Overexpression of SPR1Increases MT Stability and Leads to Salt Stress Hypersensitivity.
Supplemental Figure8.Linear Relationship between SPR1Abun-dance and Signal Intensity on Immunoblots Developed Using Chemi-luminescent Substrate.
ACKNOWLEDGMENTS
This work was supported in part by the Kentucky Science and Engi-neering Foundation(Grant148-502-06-189)and the Kentucky Tobacco Research and Development Center.We thank the ABRC for providing seeds of the GFP-TUA6and GFP-TUB6transgenic lines.
AUTHOR CONTRIBUTIONS
The research was designed by S.W.under guidance from J.A.S.The
3424The Plant Cell
New analytical tools were contributed by T.H.The data were analyzed and the article was written by S.W.,J.K.,and J.A.S.
Received August1,2011;revised August30,2011;accepted September 12,2011;published September27,2011.
REFERENCES
Abdrakhamanova, A.,Wang,Q.Y.,Khokhlova,L.,and Nick,P. (2003).Is microtubule disassembly a trigger for cold acclimation? Plant Cell Physiol.44:676–686.
Abe,T.,and Hashimoto,T.(2005).Altered microtubule dynamics by expression of modi?ed a-tubulin protein causes right-handed helical growth in transgenic Arabidopsis plants.Plant J.43:191–204. Ambrose,J.C.,Shoji,T.,Kotzer,A.M.,Pighin,J.A.,and Wasteneys, G.O.(2007).The Arabidopsis CLASP gene encodes a microtubule-associated protein involved in cell expansion and division.Plant Cell 19:2763–2775.
Ban,R.,Matsuzaki,H.,Akashi,T.,Sakashita,G.,Taniguchi,H.,Park, S.Y.,Tanaka,H.,Furukawa,K.,and Urano,T.(2009).Mitotic regulation of the stability of microtubule plus-end tracking protein EB3by ubiquitin ligase SIAH-1and Aurora mitotic kinases.J.Biol. Chem.284:28367–28381.
Bartolini, F.,Tian,G.,Piehl,M.,Cassimeris,L.,Lewis,S.A.,and Cowan,N.J.(2005).Identi?cation of a novel tubulin-destabilizing pro-tein related to the chaperone cofactor E.J.Cell Sci.118:1197–1207. Baskin,T.I.,Wilson,J.E.,Cork, A.,and Williamson,R.E.(1994). Morphology and microtubule organization in Arabidopsis roots ex-posed to oryzalin or taxol.Plant Cell Physiol.35:935–942.
Beck,M.,Komis,G.,Mu¨ller,J.,Menzel,D.,and Samaj,J.(2010). Arabidopsis homologs of nucleus-and phragmoplast-localized kinase 2and3and mitogen-activated protein kinase4are essential for microtubule organization.Plant Cell22:755–771.
Bhamidipati,A.,Lewis,S.A.,and Cowan,N.J.(2000).ADP ribosyla-tion factor-like protein2(Arl2)regulates the interaction of tubulin-folding cofactor D with native tubulin.J.Cell Biol.149:1087–1096. Bisgrove,S.R.,Hable,W.E.,and Kropf, D.L.(2004).+TIPs and microtubule regulation.The beginning of the plus end in plants.Plant Physiol.136:3855–3863.
Bisgrove,S.R.,Lee,Y.R.,Liu,B.,Peters,N.T.,and Kropf,D.L.(2008). The microtubule plus-end binding protein EB1functions in root responses to touch and gravity signals in Arabidopsis.Plant Cell20: 396–410.
Blanca?or,E.B.,and Hasenstein,K.H.(1995).Growth and microtubule orientation of Zea mays roots subjected to osmotic stress.Int.J.Plant Sci.156:774–783.
Burssens,S.,Himanen,K.,van de Cotte,B.,Beeckman,T.,Van Montagu,M.,Inze′,D.,and Verbruggen,N.(2000).Expression of cell cycle regulatory genes and morphological alterations in response to salt stress in Arabidopsis thaliana.Planta211:632–640. Buschmann,H.,and Lloyd,C.W.(2008).Arabidopsis mutants and the network of microtubule-associated functions.Mol.Plant1:888–898. Chan,J.,Calder,G.M.,Doonan,J.H.,and Lloyd,C.W.(2003).EB1 reveals mobile microtubule nucleation sites in Arabidopsis.Nat.Cell Biol.5:967–971.
Chan,J.,Eder,M.,Crowell,E.F.,Hampson,J.,Calder,G.,and Lloyd, C.(2011).Microtubules and CESA tracks at the inner epidermal wall align independently of those on the outer wall of light-grown Arabi-dopsis hypocotyls.J.Cell Sci.124:1088–1094.
Chen,Z.,Hagler,J.,Palombella,V.J.,Melandri, F.,Scherer, D.,
phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway.Genes Dev.9:1586–1597.
Crowell, E.F.,Timpano,H.,Desprez,T.,Franssen-Verheijen,T., Emons,A.M.,Ho¨fte,H.,and Vernhettes,S.(2011).Differential regu-lation of cellulose orientation at the inner and outer face of epidermal cells in the Arabidopsis hypocotyl.Plant Cell23:2592–2605. Czechowski,T.,Stitt,M.,Altmann,T.,Udvardi,M.K.,and Scheible, W.R.(2005).Genome-wide identi?cation and testing of superior reference genes for transcript normalization in Arabidopsis.Plant Physiol.139:5–17.
David,D.C.,Lay?eld,R.,Serpell,L.,Narain,Y.,Goedert,M.,and Spillantini,M.G.(2002).Proteasomal degradation of tau protein.J. Neurochem.83:176–185.
Dhonukshe,P.,and Gadella,T.W.,Jr.(2003).Alteration of microtubule dynamic instability during preprophase band formation revealed by yellow?uorescent protein-CLIP170microtubule plus-end labeling. Plant Cell15:597–611.
Dhonukshe,P.,Laxalt, A.M.,Goedhart,J.,Gadella,T.W.,and Munnik,T.(2003).Phospholipase d activation correlates with micro-tubule reorganization in living plant cells.Plant Cell15:2666–2679. Dill,A.,Jung,H.S.,and Sun,T.P.(2001).The DELLA motif is essential for gibberellin-induced degradation of RGA.Proc.Natl.Acad.Sci. USA98:14162–14167.
Drewes,G.,Ebneth,A.,and Mandelkow,E.M.(1998).MAPs,MARKs and microtubule dynamics.Trends Biochem.Sci.23:307–311. Earley,K.W.,Haag,J.R.,Pontes,O.,Opper,K.,Juehne,T.,Song,K., and Pikaard, C.S.(2006).Gateway-compatible vectors for plant functional genomics and proteomics.Plant J.45:616–629. Furutani,I.,Watanabe,Y.,Prieto,R.,Masukawa,M.,Suzuki,K., Naoi,K.,Thitamadee,S.,Shikanai,T.,and Hashimoto,T.(2000). The SPIRAL genes are required for directional control of cell elonga-tion in Arabidopsis thaliana.Development127:4443–4453. Gagne,J.M.,Smalle,J.,Gingerich,D.J.,Walker,J.M.,Yoo,S.D., Yanagisawa,S.,and Vierstra,R.D.(2004).Arabidopsis EIN3-binding F-box1and2form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3degradation.Proc.Natl. https://www.360docs.net/doc/1e10795561.html,A101:6803–6808.
Galjart,N.(2005).CLIPs and CLASPs and cellular dynamics.Nat.Rev. Mol.Cell Biol.6:487–498.
Glickman,M.H.(2000).Getting in and out of the proteasome.Semin. Cell Dev.Biol.11:149–158.
Hamada,T.(2007).Microtubule-associated proteins in higher plants. J.Plant Res.120:79–98.
Hamant,O.,Heisler,M.G.,Jo¨nsson,H.,Krupinski,P.,Uyttewaal,M., Bokov,P.,Corson,F.,Sahlin,P.,Boudaoud,A.,Meyerowitz,E.M., Couder,Y.,and Traas,J.(2008).Developmental patterning by mechanical signals in Arabidopsis.Science322:1650–1655. Hershko,A.,and Ciechanover,A.(1998).The ubiquitin system.Annu. Rev.Biochem.67:425–479.
Himmelspach,R.,Wymer,C.L.,Lloyd,C.W.,and Nick,P.(1999). Gravity-induced reorientation of cortical microtubules observed in vivo.Plant J.18:449–453.
Hirokawa,N.(1994).Microtubule organization and dynamics dependent on microtubule-associated proteins.Curr.Opin.Cell Biol.6:74–81. Ishida,T.,Kaneko,Y.,Iwano,M.,and Hashimoto,T.(2007).Helical microtubule arrays in a collection of twisting tubulin mutants of Arabidopsis https://www.360docs.net/doc/1e10795561.html,A104:8544–8549. Kaloriti,K.,Galva, C.,Parupalli, C.,Khalifa,N.,Galvin,M.,and Sedbrook,J.C.(2007).Microtubule associated proteins in plants and the processes They manage.J.Integr.Plant Biol.48:1164–1173. Kaiser,P.,and Tagwerker,C.(2005).Is this protein ubiquitinated? Methods Enzymol.399:243–248.
Proteasomal Degradation of SPR13425
and Hu¨lskamp,M.(2007).CLASP localizes in two discrete patterns on cortical microtubules and is required for cell morphogenesis and cell division in Arabidopsis.J.Cell Sci.120:4416–4425.
Komaki,S.,Abe,T.,Coutuer,S.,Inze′, D.,Russinova, E.,and Hashimoto,T.(2010).Nuclear-localized subtype of end-binding 1protein regulates spindle organization in Arabidopsis.J.Cell Sci. 123:451–459.
Kurepa,J.,Karangwa, C.,Duke,L.S.,and Smalle,J.A.(2010). Arabidopsis sensitivity to protein synthesis inhibitors depends on 26S proteasome activity.Plant Cell Rep.29:249–259.
Kurepa,J.,and Smalle,J.A.(2008).Structure,function and regulation of plant proteasomes.Biochimie90:324–335.
Kurepa,J.,Toh-E, A.,and Smalle,J.A.(2008).26S proteasome regulatory particle mutants have increased oxidative stress tolerance. Plant J.53:102–114.
Kurepa,J.,Wang,S.,Li,Y.,and Smalle,J.(2009a).Proteasome regulation,plant growth and stress tolerance.Plant Signal.Behav.4: 924–927.
Kurepa,J.,Wang,S.,Li,Y.,Zaitlin,D.,Pierce,A.J.,and Smalle,J.A. (2009b).Loss of26S proteasome function leads to increased cell size and decreased cell number in Arabidopsis shoot organs.Plant Physiol.150:178–189.
Lee,D.H.,and Goldberg,A.L.(1998).Proteasome inhibitors:Valuable new tools for cell biologists.Trends Cell Biol.8:397–403.
Lloyd,C.,and Hussey,P.(2001).Microtubule-associated proteins in plants—Why we need a MAP.Nat.Rev.Mol.Cell Biol.2:40–47. Lopez-Molina,L.,Mongrand,S.,and Chua,N.H.(2001).A postgermi-nation developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5transcription factor in Arabidopsis.Proc.Natl. https://www.360docs.net/doc/1e10795561.html,A98:4782–4787.
Lyle,K.,Kumar,P.,and Wittmann,T.(2009a).SnapShot:Microtubule regulators I.Cell136:380.
Lyle,K.,Kumar,P.,and Wittmann,T.(2009b).SnapShot:Microtubule regulators II.Cell136:566.
Lyons-Abbott,S.,Sackett,D.L.,Wloga,D.,Gaertig,J.,Morgan,R.E., Werbovetz,K.A.,and Morrissette,N.S.(2010).a-Tubulin mutations alter oryzalin af?nity and microtubule assembly properties to confer dinitroaniline resistance.Eukaryot.Cell9:1825–1834.
Mahajan,S.,Pandey,G.K.,and Tuteja,N.(2008).Calcium-and salt-stress signaling in plants:shedding light on SOS pathway.Arch. Biochem.Biophys.471:146–158.
Mandelkow, E.,and Mandelkow, E.M.(1995).Microtubules and microtubule-associated proteins.Curr.Opin.Cell Biol.7:72–81. Matenia, D.,and Mandelkow, E.M.(2009).The tau of MARK:A polarized view of the cytoskeleton.Trends Biochem.Sci.34:332–342. Mathur,J.,and Chua,N.H.(2000).Microtubule stabilization leads to growth reorientation in Arabidopsis trichomes.Plant Cell12:465–477. Mathur,J.,Mathur,N.,Kernebeck,B.,Srinivas,B.P.,and Hu¨lskamp, M.(2003).A novel localization pattern for an EB1-like protein links microtubule dynamics to endomembrane organization.Curr.Biol.13: 1991–1997.
Matsuzaki,H.,Daitoku,H.,Hatta,M.,Tanaka,K.,and Fukamizu,A. (2003).Insulin-induced phosphorylation of FKHR(Foxo1)targets to proteasomal https://www.360docs.net/doc/1e10795561.html,A100:11285–11290. Munns,R.,and Tester,M.(2008).Mechanisms of salinity tolerance. Annu.Rev.Plant Biol.59:651–681.
Nakajima,K.,Furutani,I.,Tachimoto,H.,Matsubara,H.,and Hashimoto,T.(2004).SPIRAL1encodes a plant-speci?c microtubule-localized protein required for directional control of rapidly expanding Arabidopsis cells.Plant Cell16:1178–1190.
Nakajima,K.,Kawamura,T.,and Hashimoto,T.(2006).Role of the SPIRAL1gene family in anisotropic growth of Arabidopsis thaliana.Nakamura,M.,Naoi,K.,Shoji,T.,and Hashimoto,T.(2004).Low concentrations of propyzamide and oryzalin alter microtubule dynam-ics in Arabidopsis epidermal cells.Plant Cell Physiol.45:1330–1334. Nogales,E.(2001).Structural insight into microtubule function.Annu. Rev.Biophys.Biomol.Struct.30:397–420.
Oka,M.,Yanagawa,Y.,Asada,T.,Yoneda,A.,Hasezawa,S.,Sato, T.,and Nakagawa,H.(2004).Inhibition of proteasome by MG-132 treatment causes extra phragmoplast formation and cortical micro-tubule disorganization during M/G1transition in synchronized to-bacco cells.Plant Cell Physiol.45:1623–1632.
Peth,A.,Boettcher,J.P.,and Dubiel,W.(2007).Ubiquitin-dependent proteolysis of the microtubule end-binding protein1,EB1,is con-trolled by the COP9signalosome:Possible consequences for micro-tubule?lament stability.J.Mol.Biol.368:550–563.
Petrucelli,L.,et al.(2004).CHIP and Hsp70regulate tau ubiquitination, degradation and aggregation.Hum.Mol.Genet.13:703–714. Poruchynsky,M.S.,Sackett,D.L.,Robey,R.W.,Ward,Y.,Annunziata, C.,and Fojo,T.(2008).Proteasome inhibitors increase tubulin poly-merization and stabilization in tissue culture cells:A possible mech-anism contributing to peripheral neuropathy and cellular toxicity following proteasome inhibition.Cell Cycle7:940–949.
Ren,Y.,Zhao,J.,and Feng,J.(2003).Parkin binds to a/b tubulin and increases their ubiquitination and degradation.J.Neurosci.23:3316–3324.
Schuyler,S.C.,and Pellman,D.(2001).Microtubule“plus-end-tracking proteins”:The end is just the beginning.Cell105:421–424. Sedbrook,J.C.(2004).MAPs in plant cells:Delineating microtubule growth dynamics and organization.Curr.Opin.Plant Biol.7:632–640. Sedbrook,J.C.,Ehrhardt,D.W.,Fisher,S.E.,Scheible,W.R.,and Somerville,C.R.(2004).The Arabidopsis sku6/spiral1gene encodes a plus end-localized microtubule-interacting protein involved in direc-tional cell expansion.Plant Cell16:1506–1520.
Sedbrook,J.C.,and Kaloriti,D.(2008).Microtubules,MAPs and plant directional cell expansion.Trends Plant Sci.13:303–310.
Sheng,X.,Hu,Z.,Lu¨,H.,Wang,X.,Baluska,F.,Samaj,J.,and Lin,J. (2006).Roles of the ubiquitin/proteasome pathway in pollen tube growth with emphasis on MG132-induced alterations in ultrastructure, cytoskeleton,and cell wall components.Plant Physiol.141:1578–1590.
Shoji,T.,Suzuki,K.,Abe,T.,Kaneko,Y.,Shi,H.,Zhu,J.K.,Rus,A., Hasegawa,P.M.,and Hashimoto,T.(2006).Salt stress affects cortical microtubule organization and helical growth in Arabidopsis. Plant Cell Physiol.47:1158–1168.
Smalle,J.,Kurepa,J.,Yang,P.,Emborg,T.J.,Babiychuk, E., Kushnir,S.,and Vierstra,R.D.(2003).The pleiotropic role of the 26S proteasome subunit RPN10in Arabidopsis growth and develop-ment supports a substrate-speci?c function in abscisic acid signaling. Plant Cell15:965–980.
Smalle,J.,and Vierstra,R.D.(2004).The ubiquitin26S proteasome proteolytic pathway.Annu.Rev.Plant Biol.55:555–590. Smertenko,A.,Dra′ber,P.,Viklicky′,V.,and Opatrny′,Z.(1997).Heat stress affects the organization of microtubules and cell division in Nicotiana tabacum cells.Plant Cell Environ.20:1534–1542.
Teige,M.,Scheikl,E.,Eulgem,T.,Do′czi,R.,Ichimura,K.,Shinozaki, K.,Dangl,J.L.,and Hirt,H.(2004).The MKK2pathway mediates cold and salt stress signaling in Arabidopsis.Mol.Cell15:141–152. Thion,L.,Mazars,C.,Nacry,P.,Bouchez,D.,Moreau,M.,Ranjeva,R., and Thuleau,P.(1998).Plasma membrane depolarization-activated calcium channels,stimulated by microtubule-depolymerizing drugs in wild-type Arabidopsis thaliana protoplasts,display constitutively large activities and a longer half-life in ton2mutant cells affected in the organization of cortical microtubules.Plant J.13:603–610.
3426The Plant Cell
microtubules in living cells of transgenic Arabidopsis thaliana.Proto-plasma206:201–206.
Van Bruaene,N.,Joss,G.,and Van Oostveldt,P.(2004).Reorgani-zation and in vivo dynamics of microtubules during Arabidopsis root hair development.Plant Physiol.136:3905–3919. Vandesompele,J.,De Preter,K.,Pattyn,F.,Poppe,B.,Van Roy,N., De Paepe,A.,and Speleman,F.(2002).Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multi-ple internal control genes.Genome Biol.3:RESEARCH0034. Vaughan,K.T.(2005).TIP maker and TIP marker;EB1as a master controller of microtubule plus ends.J.Cell Biol.171:197–200. Vierstra,R.D.(2009).The ubiquitin-26S proteasome system at the nexus of plant biology.Nat.Rev.Mol.Cell Biol.10:385–397. Voloshin,O.,Gocheva,Y.,Gutnick,M.,Movshovich,N.,Bakhrat,A., Baranes-Bachar,K.,Bar-Zvi, D.,Parvari,R.,Gheber,L.,and Raveh, D.(2010).Tubulin chaperone E binds microtubules and proteasomes and protects against misfolded protein stress.Cell. Mol.Life Sci.67:2025–2038.
Walia,A.,Lee,J.S.,Wasteneys,G.,and Ellis,B.(2009).Arabidopsis mitogen-activated protein kinase MPK18mediates cortical microtu-bule functions in plant cells.Plant J.59:565–575.
Wang,C.,Li,J.,and Yuan,M.(2007).Salt tolerance requires cortical
microtubule reorganization in Arabidopsis.Plant Cell Physiol.48: 1534–1547.
Wang,C.,Zhang,L.-J.,and Huang,R.-D.(2011).Cytoskeleton and plant salt stress tolerance.Plant Signal.Behav.6:29–31.
Wang,Q.Y.,and Nick,P.(2001).Cold acclimation can induce micro-tubular cold stability in a manner distinct from abscisic acid.Plant Cell Physiol.42:999–1005.
Wang,S.,Kurepa,J.,and Smalle,J.A.(2009).The Arabidopsis26S proteasome subunit RPN1a is required for optimal plant growth and stress responses.Plant Cell Physiol.50:1721–1725.
Wang,S.,Kurepa,J.,and Smalle,J.A.(2011).Ultra-small TiO(2) nanoparticles disrupt microtubular networks in Arabidopsis thaliana. Plant Cell Environ.34:811–820.
Xie,Q.,Guo,H.S.,Dallman,G.,Fang,S.,Weissman,A.M.,and Chua, N.H.(2002).SINAT5promotes ubiquitin-related degradation of NAC1 to attenuate auxin signals.Nature419:167–170.
Yanagawa,Y.,et al.(2002).Cell-cycle dependent dynamic change of 26S proteasome distribution in tobacco BY-2cells.Plant Cell Physiol. 43:604–613.
Yewdell,J.W.,Lacsina,J.R.,Rechsteiner,M.C.,and Nicchitta,C.V. (2011).Out with the old,in with the new?Comparing methods for measuring protein degradation.Cell Biol.Int.35:457–462.
Zhu,J.K.(2001).Plant salt tolerance.Trends Plant Sci.6:66–71.
Proteasomal Degradation of SPR13427
Supplemental Figure 1. Visible Phenotypes and Propyzamide Responses of the Double Mutant rpn1a-4 rpn10-1.
(A) Rosette phenotypes of in vitro-grown three-week-old Col-0, rpn1a-4, rpn10-1 and rpn1a-4 rpn10-1 plants. Rosettes of the single proteasome mutants are smaller than those of the the wild type, and this phenotype is further enhanced in the double mutant. Arrowheads point to the first leaves in the double mutant that accumulate more anthocyanins than single mutants or the wild type. The immunoblot showing the absence of both RPN1a and RPN10 subunits in the double mutant is presented in Figure 3B.
(B) The effect of propyzamide on primary root elongation was measured after three days of treatment. Root elongation is shown in relative values due to the large differences in root lengths of the untreated controls. For all lines, the average root length of the untreated control was assigned the value of 100%, and mean values ± SD (n≥25) are shown as percentages of the respective controls. The double mutant had an increased propyzamide tolerance compared to single mutants (p < 0.0001, Bonferroni multiple comparisons post-test), and its root length was significantly increased compared to the untreated double mutant plants (p < 0.001). Statistical significances of the root length of the mutants vs. Col-0 are shown on the graph (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001).
(C) Effect of propyzamide (propyz.) on the diameter of primary roots. Roots were photographed after three days of treatment. Bar = 250 μm.
S P R 1 l e v e l s
Col-0 rpn1a-4
rpn10-1 1.2
1.0 0.8 0.6 0.4 0.2 0
Supplemental Figure 2. The Steady-State SPR1 Transcript Levels in Proteasome Mutants are the Same as in the Wild Type.
Real-time RT-PCR analyses of total RNA isolated from 8-day-old seedlings. Relative SPR1 mRNA levels are expressed as a ratio of SPR1
expression normalized against expression of ACT2
(At3g18780). Test was done in triplicate.
Supplemental Figure 3. Right-Handed Helical Growth is Enhanced in rpn1a-4 spr1-3 and rpn10-1 spr1-3 Mutants.
(A) To confirm that spr1-3 rpn1a-4 and spr1-3 rpn10-1 are homozygous for both mutations, total protein extracts from 10-day-old seedlings were used for immunoblotting analyses with anti-RPN1, anti-RPN10 and anti-SPR1 sera. Immunoblot showing GS isoforms is added as a loading control.
(B) Increased epidermal cell file skewing and increased diameter of etiolated hypocotyls in double mutants. All lines were grown on MS/2 plates in the dark for five days. Bar = 1 mm.
(C) Root phenotypes of ten-day-old Col-0, rpn10-1, spr1-3, rpn10-1 spr1-3 and rpn1a-4 spr1-3 seedlings. Seedlings were grown on vertically positioned plates.
(D) The epidermal cell files in the root elongation zone of plants shown in (C).
(E) Right-handed skewing of true leaves in plants grown on soil for three weeks.
Supplemental Figure 4.Propyzamide Treatment Reverts Amplified Root Skewing in the rpn1a-4 spr1-3 Mutant.
Seedlings were grown for five days on MS/2 plates, and than transferred to fresh MS/2 media or media containing 2 μM propyzamide. The root length was then marked. The photographs were taken after three days of treatment.
让多媒体技术为小学英语教学插上翅膀
让多媒体技术为小学英语教学插上翅膀 摘要:随着科学技术的发展,多媒体技术应用的领域越来越广,在小学英语课堂中的应用给课堂带来了新的面貌,它以生动形象的画面、鲜明多姿的色彩、直观清楚的演示在教育领域逐渐取代传统教学方式,与传统教学方式相比较,多媒体教学有着无法比拟的优势。 关键词:多媒体技术小学英语课堂 正文: 科技日新月异,伴随着科技的进步,多媒体技术也日趋成熟,逐渐地应用到了教育领域,多媒体技术的使用,使得教育领域发生了巨大的变化。多媒体技术贯穿了现代教育中的各个方面,各个学科,新教学手段(媒介)取代旧的就学手段这是教育发展的必然要求,多媒体运用到课堂,使得教育更具时代色彩,这也是事物发展的客观规律。 多媒体技术运用到小学英语教学中,对此我有切身感受,它颠覆了我们传统的教学方式,使得过去单一的教学模式因注入科技成果的运用而发生质的飞跃,如今的英语课堂用“高效优质”、“多姿多彩”、“有声有色”、“精彩纷呈”等这样的词语来形容并不为过。 一、利用多媒体充分调动学生学习积极性 多媒体技术就是利用电脑把文字、图形、影象、动画、声音及视频等媒体信息都数位化,并将其整合在一定的交互式界面上,使电脑具有交互展示不同媒体形态的能力,多媒体技术具有声形俱全与图文并茂的特点,能使一些枯燥抽象的概念、复杂的变化过程、现象各异的运动形式直观而形象地显现在学生面前。。传统的教学手段只有简单的板书和口述,新课标要求教师的角色转换,传统教学中教师是主导,而新课标要求学生的主体地位,教师应该是课堂的组织者。而多媒体技术在英语课堂中的应用就是组织者教师与主体学生联系的一个纽带。而这个纽带不像传统教学的填鸭式教育手段,而是多姿多彩,有声有色的多媒体教学,这样的教学方式,能引起了学生们极大的学习兴趣,而兴趣是最好的老师,有了兴趣他们就愿意用心学,花时间去学,甚至自觉地学,这样久儿久之,就能将
小学英语自我介绍简单七篇-最新范文
小学英语自我介绍简单七篇 小学的时候我们就开始学习英语了,用英语进行自我介绍是我们值得骄傲的哦,以下是小编为大家带来的小学英语自我介绍简单,欢迎大家参考。 小学英语自我介绍1 Goodafternoon, teachers! Today, I`m very happy to make a speech here. Now, let me introduce myself. My name is ZhuRuijie. My English name is Molly. I`m 12. I come from Class1 Grade 6 of TongPu No.2 Primary School. I`m an active girl. I like playing basketball. Because I think it`s very interesting. I`d like to eat potatoes. They`re tasty. My favourite colour is green. And I like math best. It`s fun. On weekend, I like reading books in my room. I have a happy family. My father is tall and strong.My mother is hard-working and tall, too. My brother is smart. And I`m a good student. My dream is
to be a teacher, because I want to help the poor children in the future. Thank you for listening! Please remember me! 小学英语自我介绍2 Hello,everyone,Iamagirl,mynameisAliceGreenIaminClasstwoGrad efive,Iamveryoutgoing,Icandomanyhousework,suchascooking,swe epingthefloor,andIlikesinginganddancing,IamgoodatpiayingSoc cer,IhopeIcanmakegoodfriendswithyou.Thankyou! 小学英语自我介绍3 Hi, boys and girls, good morning, it is my plearsure to take this change to introduce myself, my name is ---, i am 10 years old. About my family, there are 4 people in my family, father, mother, brother and me, my father and mother are all very kind, welcome you to visit my family if you have time! I am a very outgoing girl(boy), I like reading、playing games and listening to music, of course, I also like to make friends with all the
小学生英语自我介绍演讲稿五篇
小学生英语自我介绍演讲稿五篇 小学生英语自我介绍演讲稿(1) Good afternoon, teachers! My name is YangXiaodan. I`m 11 now. I`m from Class2 Grade 5 of TongPu No.2 Primary School. My English teacher is Miss Sun. She`s quiet and kind. She`s short and young. My good friend is ZhangBingbing. She`s 12. She`s tall and pretty. We`re in the same class. We both like English very much. I like painting , listening to music, playing computer games and reading books. My favourite food is chicken. It`s tasty and yummy. I often do my homework and read books on Saturdays. This is me. Please remember YangXiaodan. Thank you very much! 小学生英语自我介绍演讲稿(2) Hi everybody, my name is XX ,what’s your name? I’m 11 years old, how old are you? My favourite sport is swimming, what’s your favourite sport? My favourite food is hamburgers,what food do you like?
小学英语课堂教学活动设计的有效性
小学英语课堂教学活动设计的有效性 在小学英语教学中,活动是实施课堂教学的主要形式,而课堂教学又是在教学活动中得以体现的。因此,作为一名小学英语教师就应在课前精心设计有针对性、实效性的课堂教学活动。教师在课堂教学中应努力引导学生通过活动去体验、感悟、发现和探究,创设贴近学生实际的活动,组织并开展活动教学,提高学生运用英语的能力。因此如何加强小学英语教学有效性,不断提高教学质量,是每一位小学英语教师急需解决的问题。听了这次晋江市举行的研讨课及结合自己的教学实践,我就课堂教学活动设计的有效性谈几点体会。 一、教学目标是教学活动的出发点和回归点 一堂好课必须有一个恰当、实际的教学目标。新课程改革背景下,教师在表述教学目标时,不仅要做到意思明确,还要符合课改的精神和要求;不仅要涉及知识目标,还要涉及能力、情感和价值观。科学、合理的教学目标的设计才能使课堂教学活动更具有方向性、针对性和有效性。因此,教师对课堂教学设计和安排的各个环节的教学活动都必须有明确的目的,每个活动都应以达成教学目标为导向。教师要思考和明确设计活动的目的和意图是什么,通过活动是否能达成教学目标,活动与教学主题和教学目标是否相关,活动是否必要,活动是否体现了教育价值等。在活动实施过程中生成的新目标也应以预设的教学目标为基础。因此,活动从设计到实施都应在教学目标的调控下进行。 二、培养兴趣是有效教学活动的出发点 小学生在刚接触英语时,有新颖感出于好奇,兴趣盎然。但随着学习时间的增加,年级的升高,内容难度的加大,学习的兴趣就会逐渐降低,有的甚至会产生畏惧感。因而,教师在设计小学英语课堂教学活动时应注意以下几点:1、活动要符合小学生的年龄特征小学生具有好动、对新鲜事物易感兴趣。因此,设计教学活动时应形式多样,如对话、唱歌、叙述、演示、游戏、小制作、全身反应法、chant等,让学生的眼、耳、口和四肢都参与到活动中来。2、新课程标准明确指出,要重视从学生的日常生活出发,培养学生实际运用语言的能力。因此活动设计要贴近学生的生活。实践表明,学生对源于自己生活的活动特别感兴趣,并有强烈的参与欲望。教师要关注来自学生生活的各种信息,在设计活动时要以学生的生活为基础,选择符合学生生活经验和认知水平的活动,力图真实地反映学生的生活。让学生融入到学习活动中去,同时让学生享受在用中学英语和在学中用英语的喜悦。如在教学《A new house》一课后,我让学生设计了自己的理想之家,画室内房间图,然后用英语标出图中物品的名称,并用英语作简单的介绍,课后写成小短文。由于活动内容和形式十分贴近学生的生活经历。又富有挑战性,学生兴趣盎然,积极参与,他们发挥了丰富的想像力,设计出的房子五花
让多媒体走进小学英语课堂
让多媒体走进小学英语课堂 多媒体技术整合于英语学科教学,主动适应课程的发展,并与其和谐自然融为一体。作为课程改革不可或缺的条件,多媒体技术在教学中的飞速发展,必将使教师更新教学观念,营造学习环境,完善教学模式,变革评价方法,促进科学与人文统一,让课堂更精彩。那么,多媒体技术在小学英语教学过程中到底有什么作用呢?笔者认为主要表现在以下几个方面: 一、运用多媒体,激发学习兴趣 托尔斯泰说:“成功的教学所需要的不是强制而是激发学生兴趣。”培养学生学习英语的兴趣无疑是小学英语教学的关键所在。学生只有对自己感兴趣的东西才会开动脑筋、积极思考,外国学者在研究可靠学习发生的条件时指出:学生只能学到那些他们有兴趣的东西,在缺乏适当的环境﹑背景和铺垫的时候,学习不能发生。因此,在教学中要重视培养和引导学生学习英语的兴趣爱好。现代信息技术具有形象性﹑直观性﹑色彩鲜艳﹑图像逼真等特点,能将抽象的教学内容变成具体的视听形象,激发情趣,调动学生多种感官同时参与,从而激发学生的学习兴趣,使学生从多角度轻松舒畅地接受知识,并使其记忆长久。 二、运用多媒体,突破教学难点 小学生的思维正处在由具体形象向抽象思维过渡的时期,而有些教学内容之所以成为学生学习的难点就是因为缺乏语境而显得不具体。如果仅凭教师的描述和讲解,往往是教师花了很大精力,教学效果却事倍功半。而运用多媒体演示就能够突破时空限制,化静为动,使抽象的概念具体化、形象化,不仅突破了教材的难点,还可以加深学生对教学内容的理解和掌握,从而提高教学效率,达到事半功倍的效果。 例如:在教学“in、on、behind、under、near”这几个表示空间关系的方位介词时,我利用多媒体设计了这样的动画:通过点按鼠标,一只小猫一会儿跳到书桌上,一会儿藏在桌子里,一会儿蹦到书桌下,一会儿躲到桌子后,一会儿又窜到桌子旁。随着小猫的位置移动,电脑会问:Where is the cat?这样连续演示几次,学生通过观察、思考,就能快速地回答:It’s on the desk\in the desk\under the desk……,动画刺激了学生的感官,诱发了思维,难点不仅易于突出,更易于突破。 三、运用多媒体,提高创新能力 创新能力是未来人才的重要素质,是一个民族兴旺发达的灵魂。因此发挥每个学生潜在的创造因素,培养学生的创新能力,是当前教育工作者研究的重要课题。要想培养创新能力,就要力求使学生处于动眼、动手、动口的主体激活状态。多媒体技术对文本、图形、声音、动画和视频等信息具有集成处理的能力,使教学手段趋于全方位、多层次,它能加速学生感知过程,促进认识深化、加深理解、
小学生英文自我介绍及故事(精选多篇)
小学生英文自我介绍及故事(精选多篇) 第一篇:小学生英文自我介绍 good afternoon everyone! my name is max. my chinese name is dong li zhen. i am six years old. i am in the primary school of jinling high school hexi branch.my family have four people. one,myfather, his name is dongning, he is a worker; two,my mother, her name is liqing,she is a housewife; three,my pet yoyo ,he is a dog; there is one more. now let me see….oh,yes,that is me! i love my family. i have many toys,a car ,a helicopter, two balls, three jumpropes. i like jumpropes best of all,because i jumprope very good. i have a pencilcase ,it’s bule. i have an brown eraser. i have five pencils,they are many colors, and one ruler,it’s white. i like english,i like speak english thank you! 第二篇:小学生英文自我介绍 自我介绍 各位老师好,我叫邵子豪,今年15岁,现在是红星海学校初中二年级学生。 我是一个善良、自信、幽默、乐于助人的阳光男孩,平时我喜欢和同学一起打篮球,还喜欢和爸爸谈古论今,我自引以为豪的是我有一个善解人意的妈妈和博学多才的爸爸,我为我生活在这样一个充满爱和民主的家庭中感到幸福! hello , teachers: mynameisshaoziha o. i’mfifteenyearsold. istudy in hongxinghaimiddeleschool..iamingradetwonow. iamakind,confident,humourousandhelpfulboy..ilikeplaying basketballwithmyfriends. andialsoliketotalkaboutthehsitoryandthecurrent newswithmyfather. i amveryproudofmyfatherandmymotherbecauseoftheirknowledge.
(完整)如何提高小学英语课堂教学的有效性
如何提高小学英语课堂教学的有效性 -----浅析小学英语趣味教学爱因斯坦说过:“兴趣是最好的老师。”为了激发学生的学习兴趣,一个成功的英语教师会创造和谐融洽的师生关系和轻松愉快的学习环境,采用灵活多变的教学方法,让学生在做中学,学中用,学得主动,提高效率。在教学中有意识的培养学生对英语的持久兴趣,激励学生不断处于较佳的学习状态之中,使他们对英语乐学、善学、会学,学而忘我,乐此不疲;还要通过多种手段激发学生实践的热情,加强学生对英语的兴趣,完成新课程标准要求的任务。 一、融洽的师生关系,营造良好的学习氛围 作为一名21世纪的英语教师,要懂得尊重和热爱自己的学生,同他们建立起一种新型、平等、合作的师生关系。教育是充满情感和爱的事业,教师应多于学生进行情感方面的交流,做学生的知心朋友,让学生觉得老师是最值得信任的人,跟老师无话不说、无事不谈,达到师生关系的最佳状态。这就要求教师要深入学生,了解学生的兴趣、爱好、喜怒哀乐情绪的变化,要时时处处关心学生,爱护学生,尊重学生,有的放矢的帮助学生。老师在学生眼中不仅是可敬的师长,更是他们可亲可近的亲密朋友,用爱来激发学生学习英语的兴趣。 二、创设英语教学环境,调动多种感官参与活动 好的开始是成功的一半。在课堂教学中,教师首先要特别注意利用上课前的十分钟创造出一个良好的课堂气氛(Warming up)。比如唱一些英语歌曲,开展如“Let me guess.”或“Follow me.”等有趣的复习知识型游戏,活跃课堂气氛,增加英语课堂的趣味性,引发学生的求知欲。
其次,创造一个轻松愉快的学习环境,教师要以蛮强的热情,全心地投入课堂教学,仪表要洒脱;精神要饱满;表情要轻松愉快;目光要亲切;态度要和蔼;举止要大方、文雅;言吐简洁;语言要纯正、地道、流利;书法要规范、漂亮;版面设计要合理、醒目等。 再次,调动学生多种感官参与学习,学生单靠视觉回忆能再现原内容的70%,而视觉并用能再现原内容的86.5%,所以应该让学生尽可能多的处于英语环境中,并调动多种感官参与活动,让学生从多个角度接受信息。多种感官的运用有益于学生对知识的理解,记忆的加深。它能激发学习兴趣,使学生全面发展。教学中让学生动嘴说、用眼看、用耳听、用笔画、手脑并用,同时可以加强学生对所学语言国家文化的了解。 三、不断更新教学方法,以此获趣 教学不仅是一门技巧,更是一门艺术。教师必须在教学中尽量让学生感到有趣、新奇,这就要求教师顺应时代的发展,与时俱进,充分运用现代教育技术手段,通过模型、图片、幻灯、录像、投影、多媒体课件等,激发学生的想象能力,变抽象为具体,使教学活动变得生动,使学生学得轻松、练得扎实。在教学中教师要善于用各种方法。如“表演法”、“竞赛法”、“游戏法”等。 1.当“小老师”、“模仿秀” 比如“talk about it”这一模块中,我们就可以鼓励学生大胆地模仿,带表情说句子。还可以鼓励学生上讲台当“小老师”,领读句子。让他们模仿教师平时上课时的表情、动作,鼓励他们学者使用课堂用语。 2.把身体语言带入课堂 在教单词或句子时,我们可以带领学生做动作,甚至做夸张的动作或
多媒体对小学英语教学的作用.docx
一、多媒体的设计要符合学生认知规律多媒体的设计应依据学生学习的需求来分析,并采用解决问题的最佳方案,使教学效果达到最优化。 多媒体教学的手段能化被动为主动,化生硬为生动,化抽象为直观,化理论为实践。 根据小学生的认知规律,他们缺乏坚实的理论基础,但直观的操作演示可以弥补这项弱点,可以帮助他们更好地理解和掌握。 对此,学者们已渐有共识这种直观生动的教学形式极大地激发了学生的学习兴趣,也引发了学生丰富的想象力和创造力。 在教学设计过程中,一方面,要运用多媒体的功能优势,将课本中抽象原理形象化,从而激发学生学习的兴趣,提高学习效果;另一方面必须让教学过程符合学生的认知规律,要多为学生创设动手操作的机会。 例如,在教学?一课时,我利用多媒体设计了一次动物聚会,森林里有很多小动物,有些通过图片来呈现,有些通过动画来呈现。 我带领学生边欣赏多媒体演示边复习了小动物的单词,然后引导学生猜每种小动物都来了几个,通过猜测的方式,强调复数的用法。 这里我使用了链接的方式,让学生像在欣赏魔术表演一样,使其出于好奇而变得主动。 通过设计符合学生知识基础和认知能力的多媒体教学内容,不仅能够激发学生学习的积极性和主动性,而且能够促进学生对知识的自我建构和内化。 此外,考虑到学生的年龄特点,在每节课的教学过程中我还格外重视评价的作用,而多媒体技术在这方面也表现出独特的优势。
不论是学习单词、句型,还是与电脑进行互动交流,每个知识点 都有图案背景,并提供过程性评价,随之出现的评价语言和动画无不让学生兴奋异常。 这种符合学生认知规律和个性化特征的内容设计与反馈,不仅使学生们感受到获得成功的喜悦,更达到了快乐交互学习的目的。 二、多媒体的应用要适时、适度和适量信息技术整合于课程不是 简单地将信息技术应用于教学,而是高层次地融合与主动适应。 必须改变传统的单一辅助教学的观点,创造数字化的学习环境,创设主动学习情景,创设条件让学生最大限度地接触信息技术,让信息技术成为学生强大的认知工具,最终达到改善学习的目的。 为保证多媒体与英语教学有效的融合,充分发挥多媒体技术应用的效果,在利用多媒体进行教学设计过程中,要遵循适时、适度和适量的原则,即在恰当的时间选择合适的媒体,适量地呈现教学内容。 在设计多媒体的过程中,要把握教学时机,不同的教学内容和教学环节,决定了多媒体运用的最佳时机也不同,精心策划才能妙笔生花,不能为使用多媒体而使用,不要一味追求手段的先进性、豪华性,而造成课堂教学过程中的画蛇添足。 这就要求教师熟悉多媒体的功能和常用多媒体的种类、特点,以便在教学设计和应用过程中将多媒体与课程学习进行有效地融合,最终提高课堂教学效果。 例如,在学习?这一知识点时,传统的英语课常常是借助钟表或模型来解释某一时刻,讲解整点、几点几分、差几分几点。
小学简单英语自我介绍范文(精选3篇)
小学简单英语自我介绍范文(精选3篇) 小学简单英语自我介绍范文 当换了一个新环境后,我们难以避免地要作出自我介绍,自我介绍可以满足我们渴望得到尊重的心理。但是自我介绍有什么要求呢?下面是收集整理的小学简单英语自我介绍范文,仅供参考,希望能够帮助到大家。 小学简单英语自我介绍1 Good afternoon, teachers! I`m very happy to introduce myself here. I`m Scott. My Chinese name is SunZijing. I`m 13. I`m from Class1 Grade 6 of TongPu No.2 Primary School. I have many hobbies, such as playing ping-pong, playing badminton, listening to music, reading magazines and so on. My favourite food is chicken. It`s tasty and yummy. So I`m very strong. I`m a naughty boy. I always play tricks. And I just like staying in my room and reading books. But I have a dream, I want to be a car-designer. Because I`m a car fan.This is me. Please remember SunZijing! Thank you very much! 小学简单英语自我介绍2 Goodafternoon, teachers! Today, I`m very happy to make a speech here. Now, let me introduce myself. My name is ZhuRuijie.
小学英语教学的有效性
小学英语教学的有效性 发表时间:2011-05-10T09:13:26.643Z 来源:《少年智力开发报》(小学英语)2011年第27期供稿作者:宋晓云[导读] 在小学英语教学中,创设情境让学生感知语言,在真实情境中运用语言会收到意想不到的效果。 河北平泉县榆树林子学区宋晓云 英语教师作为实施新课程标准的主体,应学会如何巧妙自然地寓英语学习于生活体验之中,把新理念推向课堂班级层面,变理想的课程为现实的课程,从而改变学生的学习和思维方式,释放学生的创造潜能。为此在教学中我进行了一些尝试,做到了以下几点,也从中感受到了新课改的实效。 一、采用任务型教学模式 对于一年级的小学英语课堂来讲,“任务型”的课堂教学就是在英语学科的教学目标确定之后,教师需要分析和设计具体任务,完成任务所需要的其他前提知识以及需要采用的教学方法和技巧等,旨在将每一个教学目标拓展为教学活动中可用的具体内容。由于在我的课堂上采用了任务型的教学模式,学生在课堂上都有事可做,并且在做事的过程中体验到了英语的乐趣,英语课也变得丰富多彩,学生从中也体验到了成功的喜悦。 二、创设英语的教学情境 在小学英语教学中,创设情境让学生感知语言,在真实情境中运用语言会收到意想不到的效果。在课堂中我经常利用多媒体设备来突破教学重点和难点,把文字、声音、图像等融为一体,创设学生主动参与语言交际活动的情境,让学生走入情境、理解情境、表演情境以此突破语言关。学生在真实的情境中更容易进入角色,课堂气氛也异常活跃。 三、运用现代教育技术 在小学英语课堂教学上,教师恰当运用现代教育技术,利用多媒体的优势,可以最大限度地激发学生学习的积极性,帮助他们提高学习英语的兴趣。在小学英语课堂中运用现代教育技术,创设教学情景,可以优化课堂教学,有效地培养学生初步运用英语进行交际的能力,激活儿童的思维,促进学生综合能力的提高。 四、使用合理的评价手段 评价也是一门艺术,好的评价手段不仅可以满足学生的成功感,而且可以激励学生产生不断向上的精神。学习评价可采用多种形式:形式性评价与终结性评价相结合,个人评与他人、小组评组合、等级与评语相结合,在教学中要灵活地使用。这样,对学生的评价才是全面的、合理的。 总之,通过教学中的这些尝试,我的英语课堂气氛异常活跃,学生学习积极主动,听说能力大大提高,合作意识和交往能力也大大增强,学生学习英语的兴趣空前高涨。作为教师,我由衷地感到高兴,也切身体会到了新课改所带来的欣慰和喜悦。但这只是一个开端,我们要做的还有很多,还需要不断学习新课改的有关理论,并运用到教学实践中去,不断充实我们的小学英语课堂!
小学生英语自我介绍演讲稿带翻译
小学生英语自我介绍演讲稿带翻译 篇一:小学英语自我介绍演讲稿 Good morning everyone! Today I am very happy to make a speech(演讲) here. Now, let me introduce myself. My name is Chen Qiuxiang. I am from Dingcheng center Primary School. I live in our school from Monday to Friday. At school, I study Chinese, Math, English, Music, Computer, and so on. I like all of them. I am a monitor, so I often help my teacher take care of my class. I think I am a good help. And I like Monday best because we have English, music, computer and on Tuesdays. There are four seasons in a year , but my favourite season is summer. Because I can wear my beautiful dress . When I am at home, I often help my mother do some housework. For example, wash clothes , clean the bedroom, do the dishes and so on . and my mother says I am a good help, too. In my spare time, I have many hobbies. I like reading books , going hiking, flying kites .But I like singing best, and my favourite English song is “The more we get
小学英语运用多媒体教学案例
小学英语运用多媒体教学案例 PEP 三年级上册 Unit 5 Where is my ruler? 案例片段描述:片段一: 在呈现本课重点句型“Where is …?”及其回答“It’s in / on / under …”时,我利用多媒体课件,将此句型形象生动地展现给学: T: Boys and girls, look at the screen,what is it? S: It’s a pencil. T: Wh ere is my pencil? Help to answer: It’s in the desk. 课件变化铅笔的位置,教师继续呈现本课重点: T:Look, where is my pencil now? Help to answer: It’s on the desk. It’s under the desk. 片段二: 在操练和巩固环节,我设计了一个“捉迷藏”的游戏。课件播放动画,本册书中的小松鼠Zip 是本段对话的中心人物。它不断地变换在森林里的位置,然后同学们和它一起捉迷藏,猜猜它在哪里。 ( 课件 ) Zip: Hello, boys and girls, I’m Zip. Can you guess where I am? 之后,画面变化,Zip 藏了起来。这时学生们开始猜测Zip的位置: S1: It’s under the tree. S2: It’s in the house. S3: It’s on the chair. 学生们争先恐后地猜着Zip 的位置,而且都能运用英语的方位词来表达。学生的感受:课后,我对学生在这堂课上的感受进行了一下调查,学生对本节课的方位词都掌握得很不错,还有的同学到下课了还用英语跟同学在玩刚刚zip捉迷藏的游戏。虽然已经下课了,但学生依然沉浸在刚刚课堂上轻松快乐的学习之中。可见多媒体调动学生开口说英语的积极性。
小学生英语自我介绍12篇
小学生英语自我介绍12篇 Hellow,everyone,my name is XX,I e fromXX,now I will tell you something about myself。 I study at XX school my school is very large ,there are at least 1000students in it。I like all my subjects ,such as Chinese,Maths,English and so on。I like Chinese best between these subjects,I think it is very interesting and amazing。this is me,do you know me now? if you want to know me more,please let me know as soon as possible。 Introducing yourself is very important when you meet new people。You always want to make a good impression when telling others about yourself。Allow me to introduce myself。 My name is XX and I'm from XX 。Right now,I'm a student。I study very hard every day。I like going to school because I'm eager to learn。I enjoy learning English。It's my favorite class。I like to make friends and I get along with everyone。This is the introduction I give whenever I meet new people。It tells people a little bit about me and about what I like to do。 小学生英语自我介绍(二十二): Hello,my name is_______。I am_______ years old。Now I am studying in_______PrimarySchool。I am in Grade _______,Class_______。 I live in_______。There are _______ members in my family—
小学英语有效性教学
小学英语有效性教学 小学英语课堂教学应体现有趣,有用,有效。有趣、有用、有效,构成了一个三角形,使得老师展开课堂教学能更有意义。“有效”是指教师的教学方式能促使学生较好地达到三维目标,在整个过程中达到事半功倍之效。“有趣”是指学生具有有意义学习的心向。它需要教师在课堂教学中能够激发学生的学习兴趣,在平时注意培养学生的学习动机。“有用”是指学习的材料使学生感到有价值。它需要教师在课堂教学中,使学生感受到所学知识能够协助他们解决生活中的实际问题。 关键词:课堂教学有效性有趣有用有效教学目标新教材给我们教师带来了新的教学理念,也给我们教师的教与学生的学带来了新的挑战。在新课程理念的指导下,现在的小学英语课堂中教师的教学方式在改变,学生的学习方法在改变,课堂上学生学习英语的兴趣提升了,积极性增强了。小学英语教学的主要途径是课堂教学,而课堂教学又是在教学活动中得以体现的。 所以,注重学生学习,如何使小学英语教学科学有效地发展,怎样使教学成为有意义的教学已成为广大教育工作者共同思考的问题。 教师在日常教学中应该牢固树立以下有效教学的理念:有趣、有用、有效,构成了一个三角形,使得老师展开课堂教学能更有意
义。“有效”是指教师的教学方式能促使学生较好地达到三维目标,在整个过程中起到事半功倍的作用。有效教学注重学生的进步和发展,教师必须确立学生的主体地位,树立“一切为了学生的发展”的思想。“有趣”是指如何使用游戏教学、故事教学、TPR的教学等新的教学方法,来调动学生参与课堂活动的积极性?“有用”是指学习的材料,使学生感到有价值。它需要教师在课堂教学中使学生感受到所学知识能够协助他们解决生活中的实际问题。有效教学需要教师具备一种反思的意识,经常实行行动研究。要求每一位教师持续地反思自己的日常教学行为,持续地改进自己的教学。 有效教学强调教学效果,注重学生通过一段时间在教师引导下努力学习所获得的进步与发展。有效教学应该包括有效果和有效率两个层面。我们知道,小学英语教学的主要途径是课堂教学,而课堂教学又是在教学活动中得以体现的。所以,有效的课堂教学活动是顺利达到教学目标的可靠保障。作者认为提升小学英语课堂教学的有效性,教师应从以下几方面考虑: 一、课堂教学活动要目标明确 新课程理念下,基础教育阶段英语课程的总体目标是:培养学生的综合语言使用水平,学生能够用英语做事情是课堂教学目标达成的主要体现。在小学英语课堂教学中,教师广泛采用贴近学生生活的,易于学生参与体验的任务型教学活动。例如,在教学 PEP 四年级上册 Unit 5“What would you like?”Part A的教学内容时,
小学生英语自我介绍范文(9篇)
小学生英语自我介绍范文(9篇) 如今英语交流越来越普遍了,英语版的自我介绍也很普遍,那 么你知道如何写好英语自我介绍吗?下面是收集的小学生英语自我 介绍范文,欢迎阅读借鉴。更多资讯自我介绍栏目。 Good afternoon, teachers! My name is YangXiaodan. I`m 11 now. I`m from Class2 Grade 5 of TongPu No.2 Primary School. My English teacher is Miss Sun. She`s quiet and kind. She`s short and young. My good friend is ZhangBingbing. She`s 12. She`s tall and pretty. We`re in the same class. We both like English very much. I like painting , listening to music, playing puter games and reading books. My favourite food is chicken. It`s tasty and yummy. I often do my homework and read books on Saturdays. This is me. Please remember YangXiaodan. Thank you very much! Gooda fternoon, teachers! My name is XuLulu. I`m 11. I e from Class1 Grade 5 of TongPu No.2 Primary School. There are 4 people in my family. My father is tall. My mother is pretty.My sister has a long hair. And I`m a good student. However, my home is near the school, I often get up early. Because I must work hard. after school, I like playing puter games and reading books. I`d like to eat apples. They are sweet and healthy. And I like
小学英语教学有效性的策略研究
小学英语教学有效性的策 略研究 Revised by Hanlin on 10 January 2021
《小学英语教学有效性的策略研究》 研究课题鉴定成果概况 课题名称:小学英语教学有效性的策略研究 课题类别:甘肃省教育科学“十二五”规划重点课题 课题批准号:GS(2010)Z045 课题鉴定证书号:GSGB【2013】J005 课题负责人:张旭东 所在单位:金昌市金川区第一小学 课题组参与人员:姚兴瑞刘婷娟杨兆光王文君李其霞张莉杨永涛武 功颜张海生顾秀文 鉴定等级:优秀 为了适应教育发展的趋势,让教师真正走进新课程,构建新课堂,我们结合本市区小学实际,根据自身条件,在充分酝酿的基础上,经多方论证,精心选题,确定《新课改理念下的小学英语有效学习策略研究》上报为省级课题,2010年10月被省教育科研组批复立项为甘肃省教育科学“十一五”重点课题。 三年多来,经过课题组成员不断的实践、反思、总结,我们对新课改理念下的小学英语有效学习的策略、教师的教学手段、有效学习的组织形式、课堂教学的模式、合作学习的有效策略等,进行了具体的研究,下面将我校的课题研究工作总结如下: 一、课题提出的背景 1.新一轮课程改革的需要 《英语课程标准》“倡导学生主动参与合作,交流与探究等多种学习活动,改进学习方式,促进学生相互帮助,体验集体荣誉感和成就感,发展合作精神,使学生真正成为学习的主人。”它要求把学生的交往活动即合作学习纳入语言学习活动。但是随着社会经济的不断发展,很多农民工进城打工创业,他们也渴望自
己的孩子拥有城里孩子所能得到的优质教育,这就造成了城市学校班额太大,再加上学生层次不齐,给我们教育教学提出了严峻的挑战,为了很快适应和扭转这种局面,不断提高教育教学质量,我们提出了在《新课改理念下的小学英语有效学习策略研究》。小组合作学习是一种教学策略,既能充分调动学生学习英语的兴趣和积极性,又能提高学习效果,在大班额环境下实施小组合作学习策略尤为重要。 2.我市教育现状的需要 截至目前,全市原有的162所完全小学已调整为142所;20所独立初中调整为16所,撤并了中小学24所。虽然通过集中力量办较大规模的寄宿制农村中小学,农村中小学教育资源进行有效整合后,改善了办学条件,使农村中小学生接受了良好的学校教育,对提高办学效益和教学质量,缩小城乡教育水平差距起到了推动作用,但是教育资源有效整合的力度还是不大,跟不上时代发展的需要,跟不上广大农村家长对高质量学校的需求。望子成龙、望女成凤的愿望使他们不惜一切代价将孩子送往城市学校读书。目前我市农村学校生源越来越少,班额越来越小,老师过剩。相反,城市学校生源越来越多,班额越来越大。如何解决大班额环境下的教学质量问题,就显得尤为重要。 3.我校实际情况的需要 我校在金昌市范围内是一所比较特殊的城市小学,说特殊是因为我校属城市小学,但是区属职工的子女少,而来自金川区宁远和双湾两镇和外来进城打工的子女多,虽学校各年级都增加了班级,还是满足不了源源不断增加的学生,每个班级学生都在60人左右,这样学校班额大、学生多,班级人数远远超过了正常班级人数的要求。一个专职的小学英语教师要负责的有近三百五十个学生,甚至更多。在英语课堂上,一方面教师感到力不从心;另一方面如果还依然采用“一刀切”的办法进行教学,在长期“齐步走”“一言堂”的课堂教学环境下,小组合作学习也只是简单的分组进行个别的合作学习而已,教师在有限的课堂40分钟内难以真正做到面向全体学生,让每个学生真正参与到活动中,鉴于此我们提出了“大班额环境下小学英语教学小组合作学习有效性”的课题,结合本校的实际,力争以其作为深化并打造我校英语特色学校的着力点,借他人肩膀,加
小学英语多媒体课件的设计思路
小学英语多媒体课件的设计思路 一、多媒体辅助教学的优势和特点 多媒体辅计算机助教学具有生动形象、信息储存量大、交互性强、适合个别化学习等特点。在多媒体环境中,学生可以根据自己和学习水平与需要选择学习内容,高度体现了学生的主体作用。多媒体辅助教学的应用非常灵活,可以进行课堂计算机辅助教学,也可以进行网络教学。它不同于传统教学,其教学质量与教学效率远高于传统教学。特别是用多媒体计算机辅助英语教学,可以提供形象逼真的情景,正确标准的发音,生动有趣的练习游戏,从而大大地激发了小学生学习英语的兴趣,提高教学效率。然而,在多媒体辅助教学条件下,学校育人环境的改善、教学过程的优化、教学质量的提高,都离不开教学课件的质量支持和保证。这不仅取决于计算机硬件的装备和决策水平,而且取决于教学课件的设计水平。因此,制作高质量的多媒体辅助教学课件,首先要设计好课件的脚本。 二、教学课件设计的指导思想和遵循的原则 课件设计的总指导思想是现代教育理论。“现代教育”认为在科学技术飞速发展的今天,人们开始极为重视人的智能开发。“传统教育”这中再现型教学,不利于发挥学生的独创性和知识的综合利用;过分地不恰当地强调教师的主导作用,也会约束以至压抑学生主体作用的发挥。因此,在教
学中必须以学生为中心,尊重学生的需要,培养有个性的学生,强调独立自主地学习,从而形成学生多方面的能力,特别是学生主动的学习能力和学习态度。现代教育的教育教学观,为开发多媒体辅助教学课件的主旨是不谋而合的。因此,课件的设计思路是以现代教育理论为指导思想,同时必须遵循以下几个原则: 1、课件设计的科学性。因为科学设计教学课件是多媒体辅助教学的核心和关键,它决定整个教学系统的成败。优秀的多媒体辅助教学课件应围绕教学大纲,将教学目标、教学内容和教学方法巧妙地贯穿在整个课件的设计制作中,并通过与计算机硬件相结合充分发挥计算机高密度、大容量、迅速准确、灵活高效的优点,调动学习者各种感官,激活其思维,消除其心理障碍,使其生动地去活学活用。具体表现在:紧扣教学大纲,量化教学目标;运用外语教学原则,开发配套课件;具备优良的教学方法。 2、要适应小学生的个性特点。小学阶段的学生年龄一般为6—12周岁。根据皮亚杰的儿童认识发展理论,该年龄阶段的儿童跨越了前运算阶段、具体运算阶段,向形式运算阶段过渡,因此在设计课件时必须使之符合小学生的年龄特征。英语课件中可能受学生年龄特征影响的组合因素有:课件界面、人机交互控制的难易程度、教学信息内容、文本、图形图像、声音、动画、视频、色彩搭配等。因此,课件界
