各种物质物理化学参数使用手册

STANDARD ITS-90 THERMOCOUPLE TABLES
The Instrument Society of America (ISA) has assigned standard letter designations to a number of thermocouple types having specified emf-temperature relations. These designations and the approximate metal compositions which meet the required relations, as well as the useful temperature ranges, are given below:
Type B(Pt + 30% Rh) vs. (Pt + 6% Rh) 0 to 1820°C
Type E(Ni + 10% Cr) vs. (Cu + 43% Ni)-270 to 1000°C Type J Fe vs. (Cu + 43% Ni)-210 to 1200°C Type K (Ni + 10% Cr) vs. (Ni + 2% Al + 2% Mn + 1% Si)-270 to 1372°C Type N (Ni + 14% Cr + 1.5% Si) vs. (Ni + 4.5% Si + 0. 1% Mg) -270 to 1300°C Type R(Pt + 13% Rh) vs. Pt-50 to 1768°C Type S (Pt + 10% Rh) vs. Pt -50 to 1768°C
Type T Cu vs. (Cu + 43% Ni)-270 to 400°C
The compositions are given in weight percent, and the positive leg is listed first. It should be emphasized that the standard letter designations do not imply a precise composition but rather that the specified emf-temperature relation is satisfied.
The first set of tables below lists, for each thermocouple type, the emf as a function of temperature on the International Temperature Scale of 1990 (ITS-90). The coefficients in the equation used to generate the table are also given. The second set of tables gives the inverse relationships, i.e., the coefficients in the polynomial equation which expresses the temperature as a function of thermocouple emf. The accuracy of these equations is also stated.
Further details and tables at closer intervals may be found in Reference 1.
REFERENCES
1. Burns, G. W., Seroger, M. G., Strouse, G. F., Croarkin, M. C., and Guthrie, W.F.,
Temperature-Electromotive Force Reference Functions and Tables for the
Letter-Designated Thermocouple Types Based on the ITS-90, Nat. Inst. Stand. Tech.
(U.S.) Monogr. 175, 1993.
2. Schooley, J. F., Thermometry, CRC Press, Boca Raton, FL, 1986.
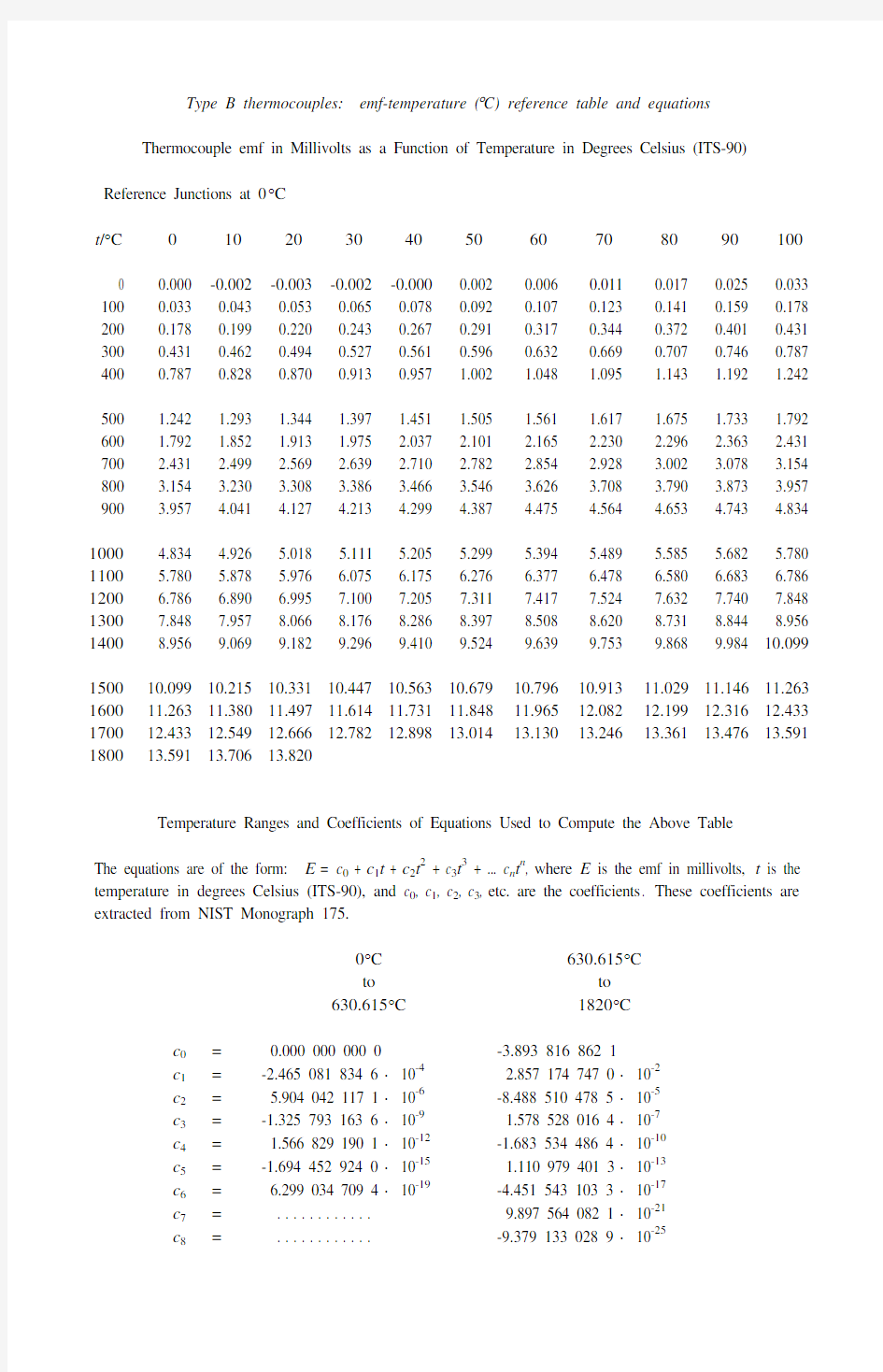
Type B thermocouples: emf-temperature (°C) reference table and equations
Thermocouple emf in Millivolts as a Function of Temperature in Degrees Celsius (ITS-90)
Reference Junctions at 0°C
t/°C0102030405060708090100
0 0.000 -0.002 -0.003 -0.002 -0.000 0.002 0.006 0.011 0.017 0.025 0.033 100 0.033 0.043 0.053 0.065 0.078 0.092 0.107 0.123 0.141 0.159 0.178 200 0.178 0.199 0.220 0.243 0.267 0.291 0.317 0.344 0.372 0.401 0.431 300 0.431 0.462 0.494 0.527 0.561 0.596 0.632 0.669 0.707 0.746 0.787 400 0.787 0.828 0.870 0.913 0.957 1.002 1.048 1.095 1.143 1.192 1.242
500 1.242 1.293 1.344 1.397 1.451 1.505 1.561 1.617 1.675 1.733 1.792 600 1.792 1.852 1.913 1.975 2.037 2.101 2.165 2.230 2.296 2.363 2.431 700 2.431 2.499 2.569 2.639 2.710 2.782 2.854 2.928 3.002 3.078 3.154 800 3.154 3.230 3.308 3.386 3.466 3.546 3.626 3.708 3.790 3.873 3.957 900 3.957 4.041 4.127 4.213 4.299 4.387 4.475 4.564 4.653 4.743 4.834 1000 4.834 4.926 5.018 5.111 5.205 5.299 5.394 5.489 5.585 5.682 5.780 1100 5.780 5.878 5.976 6.075 6.175 6.276 6.377 6.478 6.580 6.683 6.786 1200 6.786 6.890 6.995 7.100 7.205 7.311 7.417 7.524 7.632 7.740 7.848 1300 7.848 7.957 8.066 8.176 8.286 8.397 8.508 8.620 8.731 8.844 8.956 1400 8.956 9.069 9.182 9.296 9.410 9.524 9.639 9.753 9.868 9.984 10.099
1500 10.099 10.215 10.331 10.447 10.563 10.679 10.796 10.913 11.029 11.146 11.263 1600 11.263 11.380 11.497 11.614 11.731 11.848 11.965 12.082 12.199 12.316 12.433 1700 12.433 12.549 12.666 12.782 12.898 13.014 13.130 13.246 13.361 13.476 13.591 1800 13.591 13.706 13.820
Temperature Ranges and Coefficients of Equations Used to Compute the Above Table
The equations are of the form: E= c0 + c1t + c2t2 + c3t3 + ... c n t n, where E is the emf in millivolts, t is the temperature in degrees Celsius (ITS-90), and c0, c1, c2, c3, etc. are the coefficients. These coefficients are extracted from NIST Monograph 175.
0°C630.615°C
to to
630.615°C1820°C
c0= 0.000 000 000 0-3.893 816 862 1
c1=-2.465 081 834 6 × 10-4 2.857 174 747 0 × 10-2
c2= 5.904 042 117 1 × 10-6-8.488 510 478 5 × 10-5
c3=-1.325 793 163 6 × 10-9 1.578 528 016 4 × 10-7
c4= 1.566 829 190 1 × 10-12-1.683 534 486 4 × 10-10
c5=-1.694 452 924 0 × 10-15 1.110 979 401 3 × 10-13
c6= 6.299 034 709 4 × 10-19-4.451 543 103 3 × 10-17
c7= . . . . . . . . . . . . 9.897 564 082 1 × 10-21
c8= . . . . . . . . . . . .-9.379 133 028 9 × 10-25
Type E thermocouples: emf-temperature (°C) reference table and equations
Thermocouple emf in Millivolts as a Function of Temperature in Degrees Celsius (ITS-90)
Reference Junctions at 0°C
t/°C0-10-20-30-40-50-60-70-80-90-100
-200 -8.825 -9.063 -9.274 -9.455 -9.604 -9.718 -9.797 -9.835
-100-5.237 -5.681 -6.107 -6.516 -6.907 -7.279 -7.632 -7.963 -8.273 -8.561 -8.825 00.000 -0.582 -1.152 -1.709 -2.255 -2.787 -3.306 -3.811 -4.302 -4.777 -5.237
t/°C0102030405060708090100
0 0.000 0.591 1.192 1.801 2.420 3.048 3.685 4.330 4.985 5.648 6.319 100 6.319 6.998 7.685 8.379 9.081 9.789 10.503 11.224 11.951 12.684 13.421 200 13.421 14.164 14.912 15.664 16.420 17.181 17.945 18.713 19.484 20.259 21.036 300 21.036 21.817 22.600 23.386 24.174 24.964 25.757 26.552 27.348 28.146 28.946 400 28.946 29.747 30.550 31.354 32.159 32.965 33.772 34.579 35.387 36.196 37.005
500 37.005 37.815 38.624 39.434 40.243 41.053 41.862 42.671 43.479 44.286 45.093 600 45.093 45.900 46.705 47.509 48.313 49.116 49.917 50.718 51.517 52.315 53.112 700 53.112 53.908 54.703 55.497 56.289 57.080 57.870 58.659 59.446 60.232 61.017 800 61.017 61.801 62.583 63.364 64.144 64.922 65.698 66.473 67.246 68.017 68.787 900 68.787 69.554 70.319 71.082 71.844 72.603 73.360 74.115 74.869 75.621 76.373 1000 76.373
Temperature Ranges and Coefficients of Equations Used to Compute the Above Table
The equations are of the form: E = c0 + c1t + c2t2 + c3t3 + ... c n t n, where E is the emf in
millivolts, t is the temperature in degrees Celsius (ITS-90), and c0, c1, c2, c3, etc. are the coefficients. These coefficients are extracted from NIST Monograph 175.
-270°C0°C
to to
0°C1000°C
c0= 0.000 000 000 0 0.000 000 000 0
c1= 5.866 550 870 8 × 10-2 5.866 550 871 0 × 10-2
c2= 4.541 097 712 4 × 10-5 4.503 227 558 2 × 10-5
c3=-7.799 804 868 6 × 10-7 2.890 840 721 2 × 10-8
c4=-2.580 016 084 3 × 10-8-3.305 689 665 2 × 10-10
c5=-5.945 258 305 7 × 10-10 6.502 440 327 0 × 10-13
c6=-9.321 405 866 7 × 10-12-1.919 749 550 4 × 10-16
c7=-1.028 760 553 4 × 10-13-1.253 660 049 7 × 10-18
c8=-8.037 012 362 1 × 10-16 2.148 921 756 9 × 10-21
c9=-4.397 949 739 1 × 10-18-1.438 804 178 2 × 10-24
c10=-1.641 477 635 5 × 10-20 3.596 089 948 1 × 10-28
c11=-3.967 361 951 6 × 10-23. . . . . . . . . . . .
c12=-5.582 732 872 1 × 10-26. . . . . . . . . . . .
c13=-3.465 784 201 3 × 10-29. . . . . . . . . . . .
Type J thermocouples: emf-temperature (°C) reference table and equations
Thermocouple emf in Millivolts as a Function of Temperature in Degrees Celsius (ITS-90)
Reference Junctions at 0°C
t/°C0-10-20-30-40-50-60-70-80-90-100
-200 -7.890 -8.095
-100 -4.633 -5.037 -5.426 -5.801 -6.159 -6.500 -6.821 -7.123 -7.403 -7.659 -7.890 0 0.000 -0.501 -0.995 -1.482 -1.961 -2.431 -2.893 -3.344 -3.786 -4.215 -4.633 t/°C0102030405060708090100
0 0.000 0.507 1.019 1.537 2.059 2.585 3.116 3.650 4.187 4.726 5.269 100 5.269 5.814 6.360 6.909 7.459 8.010 8.562 9.115 9.669 10.224 10.779 200 10.779 11.334 11.889 12.445 13.000 13.555 14.110 14.665 15.219 15.773 16.327 300 16.327 16.881 17.434 17.986 18.538 19.090 19.642 20.194 20.745 21.297 21.848 400 21.848 22.400 22.952 23.504 24.057 24.610 25.164 25.720 26.276 26.834 27.393
500 27.393 27.953 28.516 29.080 29.647 30.216 30.788 31.362 31.939 32.519 33.102 600 33.102 33.689 34.279 34.873 35.470 36.071 36.675 37.284 37.896 38.512 39.132 700 39.132 39.755 40.382 41.012 41.645 42.281 42.919 43.559 44.203 44.848 45.494 800 45.494 46.141 46.786 47.431 48.074 48.715 49.353 49.989 50.622 51.251 51.877 900 51.877 52.500 53.119 53.735 54.347 54.956 55.561 56.164 56.763 57.360 57.953
1000 57.953 58.545 59.134 59.721 60.307 60.890 61.473 62.054 62.634 63.214 63.792 1100 63.792 64.370 64.948 65.525 66.102 66.679 67.255 67.831 68.406 68.980 69.553 1200 69.553
Temperature Ranges and Coefficients of Equations Used to Compute the Above Table
The equations are of the form: E = c0 + c1t + c2t2 + c3t3 + ... c n t n, where E is the emf in millivolts, t is the temperature in degrees Celsius (ITS-90), and c0, c1, c2, c3, etc. are the coefficients. These coefficients are extracted from NIST Monograph 175.
-210°C760°C
to to
760°C1200°C
c0= 0.000 000 000 0 2.964 562 568 1 × 102
c1= 5.038 118 781 5 × 10-2-1.497 612 778 6
c2= 3.047 583 693 0 × 10-5 3.178 710 392 4 × 10-3
c3=-8.568 106 572 0 × 10-8-3.184 768 670 1 × 10-6
c4= 1.322 819 529 5 × 10-10 1.572 081 900 4 × 10-9
c5=-1.705 295 833 7 × 10-13-3.069 136 905 6 × 10-13
c6= 2.094 809 069 7 × 10-16. . . . . . . . . . . .
c7=-1.253 839 533 6 × 10-19. . . . . . . . . . . .
c8= 1.563 172 569 7 × 10-23. . . . . . . . . . . .
Type K thermocouples: emf-temperature (°C) reference table and equations
Thermocouple emf in Millivolts as a Function of Temperature in Degrees Celsius (ITS-90)
Reference Junctions at 0°C
t/°C0-10-20-30-40-50-60-70-80-90-100
-200 -5.891 -6.035 -6.158 -6.262 -6.344 -6.404 -6.441 -6.458
-100 -3.554 -3.852 -4.138 -4.411 -4.669 -4.913 -5.141 -5.354 -5.550 -5.730 -5.891 0 0.000 -0.392 -0.778 -1.156 -1.527 -1.889 -2.243 -2.587 -2.920 -3.243 -3.554 t/°C0102030405060708090100
0 0.000 0.397 0.798 1.203 1.612 2.023 2.436 2.851 3.267 3.682 4.096 100 4.096 4.509 4.920 5.328 5.735 6.138 6.540 6.941 7.340 7.739 8.138 200 8.138 8.539 8.940 9.343 9.747 10.153 10.561 10.971 11.382 11.795 12.209 300 12.209 12.624 13.040 13.457 13.874 14.293 14.713 15.133 15.554 15.975 16.397 400 16.397 16.820 17.243 17.667 18.091 18.516 18.941 19.366 19.792 20.218 20.644
500 20.644 21.071 21.497 21.924 22.350 22.776 23.203 23.629 24.055 24.480 24.905 600 24.905 25.330 25.755 26.179 26.602 27.025 27.447 27.869 28.289 28.710 29.129 700 29.129 29.548 29.965 30.382 30.798 31.213 31.628 32.041 32.453 32.865 33.275 800 33.275 33.685 34.093 34.501 34.908 35.313 35.718 36.121 36.524 36.925 37.326 900 37.326 37.725 38.124 38.522 38.918 39.314 39.708 40.101 40.494 40.885 41.276
1000 41.276 41.665 42.053 42.440 42.826 43.211 43.595 43.978 44.359 44.740 45.119 1100 45.119 45.497 45.873 46.249 46.623 46.995 47.367 47.737 48.105 48.473 48.838 1200 48.838 49.202 49.565 49.926 50.286 50.644 51.000 51.355 51.708 52.060 52.410 1300 52.410 52.759 53.106 53.451 53.795 54.138 54.479 54.819
Temperature Ranges and Coefficients of Equations Used to Compute the Above Table
The equations are of the form: E = c0 + c1t + c2t2 + c3t3 + ... c n t n, where E is the emf in millivolts, t is the temperature in degrees Celsius (ITS-90), and c0, c1, c2, c3, etc. are the coefficients. In the 0°C to 1372°C range there is also an exponential term that must be evaluated and added to the equation. The exponential term is of the form: c0exp[c1(t-126.9686)2] , where t is the temperature in °C and c0 and c1 are the coefficients. These coefficients are extracted from NIST Monograph 175.
-270°C0°C0°C
to to to
0°C1372°C1372°C
(exponential term)
c0=0.000 000 000 0 -1.760 041 368 6 × 10-2 1.185 976 × 10-1 c1= 3.945 012 802 5 × 10-2 3.892 120 497 5 × 10-2-1.183 432 × 10-4 c2= 2.362 237 359 8 × 10-5 1.855 877 003 2 × 10-5. . . . . . .
c3=-3.285 890 678 4 × 10-7-9.945 759 287 4 ×10-8. . . . . . .
c4=-4.990 482 877 7 × 10-9 3.184 094 571 9 × 10-10. . . . . . .
c5=-6.750 905 917 3 × 10-11-5.607 284 488 9 × 10-13. . . . . . .
c6=-5.741 032 742 8 × 10-13 5.607 505 905 9 × 10-16. . . . . . .
c7=-3.108 887 289 4 × 10-15-3.202 072 000 3 × 10-19. . . . . . .
c8=-1.045 160 936 5 × 10-17 9.715 114 715 2 × 10-23. . . . . . .
c9=-1.988 926 687 8 × 10-20-1.210 472 127 5 × 10-26. . . . . . .
c10=-1.632 269 748 6 × 10-23 . . . . . . . . . . .. . . . . . .
Type N thermocouples: emf-temperature (°C) reference table and equations
Thermocouple emf in Millivolts as a Function of Temperature in Degrees Celsius (ITS-90)
Reference Junctions at 0°C
t/°C0-10-20-30-40-50-60-70-80-90-100
-200 -3.990 -4.083 -4.162 -4.226 -4.277 -4.313 -4.336 -4.345
-100 -2.407 -2.612 -2.808 -2.994 -3.171 -3.336 -3.491 -3.634 -3.766 -3.884 -3.990 0 0.000 -0.260 -0.518 -0.772 -1.023 -1.269 -1.509 -1.744 -1.972 -2.193 -2.407
t/°C0102030405060708090100
0 0.000 0.261 0.525 0.793 1.065 1.340 1.619 1.902 2.189 2.480 2.774 100 2.774 3.072 3.374 3.680 3.989 4.302 4.618 4.937 5.259 5.585 5.913 200 5.913 6.245 6.579 6.916 7.255 7.597 7.941 8.288 8.637 8.988 9.341 300 9.341 9.696 10.054 10.413 10.774 11.136 11.501 11.867 12.234 12.603 12.974 400 12.974 13.346 13.719 14.094 14.469 14.846 15.225 15.604 15.984 16.366 16.748
500 16.748 17.131 17.515 17.900 18.286 18.672 19.059 19.447 19.835 20.224 20.613 600 20.613 21.003 21.393 21.784 22.175 22.566 22.958 23.350 23.742 24.134 24.527 700 24.527 24.919 25.312 25.705 26.098 26.491 26.883 27.276 27.669 28.062 28.455 800 28.455 28.847 29.239 29.632 30.024 30.416 30.807 31.199 31.590 31.981 32.371 900 32.371 32.761 33.151 33.541 33.930 34.319 34.707 35.095 35.482 35.869 36.256 1000 36.256 36.641 37.027 37.411 37.795 38.179 38.562 38.944 39.326 39.706 40.087 1100 40.087 40.466 40.845 41.223 41.600 41.976 42.352 42.727 43.101 43.474 43.846 1200 43.846 44.218 44.588 44.958 45.326 45.694 46.060 46.425 46.789 47.152 47.513 1300 47.513
Temperature Ranges and Coefficients of Equations Used to Compute the Above Table
The equations are of the form: E = c0 + c1t + c2t2+ c3t3 + ... c n t n, where E is the emf in
millivolts, t is the temperature in degrees Celsius (ITS-90), and c0, c1, c2, c3, etc. are the coefficients. These coefficients are extracted from NIST Monograph 175.
-270°C0°C
to to
0°C1300°C
c0= 0.000 000 000 0 0.000 000 000 0
c1= 2.615 910 596 2 × 10-2 2.592 939 460 1 × 10-2
c2= 1.095 748 422 8 × 10-5 1.571 014 188 0 × 10-5
c3=-9.384 111 155 4 × 10-8 4.382 562 723 7 × 10-8
c4=-4.641 203 975 9 × 10-11-2.526 116 979 4 × 10-10
c5=-2.630 335 771 6 × 10-12 6.431 181 933 9 × 10-13
c6=-2.265 343 800 3 × 10-14-1.006 347 151 9 × 10-15
c7=-7.608 930 079 1 × 10-179.974 533 899 2 × 10-19
c8=-9.341 966 783 5 × 10-20-6.086 324 560 7 × 10-22
c9= . . . . . . . . . . 2.084 922 933 9 × 10-25
c10=. . . . . . . . . . -3.068 219 615 1 × 10-29
Type R thermocouples: emf-temperature (°C) reference table and equations
Thermocouple emf in Millivolts as a Function of Temperature in Degrees Celsius (ITS-90) Reference Junctions at 0°C
t/°C0-10-20-30-40-50-60-70-80-90-100
0 0.000 -0.051 -0.100 -0.145 -0.188 -0.226
t/°C0102030405060708090100
0 0.000 0.054 0.111 0.171 0.232 0.296 0.363 0.431 0.501 0.573 0.647
100 0.647 0.723 0.800 0.879 0.959 1.041 1.124 1.208 1.294 1.381 1.469 200 1.469 1.558 1.648 1.739 1.831 1.923 2.017 2.112 2.207 2.304 2.401 300 2.401 2.498 2.597 2.696 2.796 2.896 2.997 3.099 3.201 3.304 3.408 400 3.408 3.512 3.616 3.721 3.827 3.933 4.040 4.147 4.255 4.363 4.471
500 4.471 4.580 4.690 4.800 4.910 5.021 5.133 5.245 5.357 5.470 5.583 600 5.583 5.697 5.812 5.926 6.041 6.157 6.273 6.390 6.507 6.625 6.743 700 6.743 6.861 6.980 7.100 7.220 7.340 7.461 7.583 7.705 7.827 7.950 800 7.950 8.073 8.197 8.321 8.446 8.571 8.697 8.823 8.950 9.077 9.205 900 9.205 9.333 9.461 9.590 9.720 9.850 9.980 10.111 10.242 10.374 10.506
1000 10.506 10.638 10.771 10.905 11.039 11.173 11.307 11.442 11.578 11.714 11.850 1100 11.850 11.986 12.123 12.260 12.397 12.535 12.673 12.812 12.950 13.089 13.228 1200 13.228 13.367 13.507 13.646 13.786 13.926 14.066 14.207 14.347 14.488 14.629 1300 14.629 14.770 14.911 15.052 15.193 15.334 15.475 15.616 15.758 15.899 16.040 1400 16.040 16.181 16.323 16.464 16.605 16.746 16.887 17.028 17.169 17.310 17.451 1500 17.451 17.591 17.732 17.872 18.012 18.152 18.292 18.431 18.571 18.710 18.849 1600 18.849 18.988 19.126 19.264 19.402 19.540 19.677 19.814 19.951 20.087 20.222 1700 20.222 20.356 20.488 20.620 20.749 20.877 21.003
Temperature Ranges and Coefficients of Equations Used to Compute the Above Table
The equations are of the form: E = c0 + c1t + c2t2 + c3t3 + ... c n t n, where E is the emf in millivolts, t is the temperature in degrees Celsius (ITS-90), and c0, c1, c2, c3, etc. are the coefficients. These coefficients are extracted from NIST Monograph 175.
-50°C1064.18°C1664.5°C
to to to
1064.18°C1664.5°C1768.1°C
c0= 0.000 000 000 00. 2.951 579 253 16 1.522 321 182 09 × 102 c1= 5.289 617 297 65 × 10-3-2.520 612 513 32 × 10-3-2.688 198 885 45 × 10-1 c2= 1.391 665 897 82 × 10-5 1.595 645 018 65 × 10-5 1.712 802 804 71 × 10-4 c3=-2.388 556 930 17 × 10-8-7.640 859 475 76 × 10-9-3.458 957 064 53 × 10-8 c4= 3.569 160 010 63 × 10-11 2.053 052 910 24 × 10-12-9.346 339 710 46 × 10-15 c5=-4.623 476 662 98 × 10-14-2.933 596 681 73 × 10-16 . . . . . . . . . . . . .
c6= 5.007 774 410 34 × 10-17. . . . . . . . . . . . .. . . . . . . . . . . . .
c7=-3.731 058 861 91 × 10-20 . . . . . . . . . . . . . . . . . . . . . . . . . .
c8= 1.577 164 823 67 × 10-23 . . . . . . . . . . . . . . . . . . . . . . . . . .
c9=-2.810 386 252 51 × 10-27 . . . . . . . . . . . . . . . . . . . . . . . . . .
Type S thermocouples: emf-temperature (°C) reference table and equations
Thermocouple emf in Millivolts as a Function of Temperature in Degrees Celsius (ITS-90)
Reference Junctions at 0°C
t/°C0-10-20-30-40-50-60-70-80-90-100
0 0.000 -0.053 -0.103 -0.150 -0.194 -0.236
t/°C0102030405060708090100
0 0.000 0.055 0.113 0.173 0.235 0.299 0.365 0.433 0.502 0.573 0.646 100 0.646 0.720 0.795 0.872 0.950 1.029 1.110 1.191 1.273 1.357 1.441 200 1.441 1.526 1.612 1.698 1.786 1.874 1.962 2.052 2.141 2.232 2.323 300 2.323 2.415 2.507 2.599 2.692 2.786 2.880 2.974 3.069 3.164 3.259 400 3.259 3.355 3.451 3.548 3.645 3.742 3.840 3.938 4.036 4.134 4.233
500 4.233 4.332 4.432 4.532 4.632 4.732 4.833 4.934 5.035 5.137 5.239 600 5.239 5.341 5.443 5.546 5.649 5.753 5.857 5.961 6.065 6.170 6.275 700 6.275 6.381 6.486 6.593 6.699 6.806 6.913 7.020 7.128 7.236 7.345 800 7.345 7.454 7.563 7.673 7.783 7.893 8.003 8.114 8.226 8.337 8.449 900 8.449 8.562 8.674 8.787 8.900 9.014 9.128 9.242 9.357 9.472 9.587 1000 9.587 9.703 9.819 9.935 10.051 10.168 10.285 10.403 10.520 10.638 10.757 1100 10.757 10.875 10.994 11.113 11.232 11.351 11.471 11.590 11.710 11.830 11.951 1200 11.951 12.071 12.191 12.312 12.433 12.554 12.675 12.796 12.917 13.038 13.159 1300 13.159 13.280 13.402 13.523 13.644 13.766 13.887 14.009 14.130 14.251 14.373 1400 14.373 14.494 14.615 14.736 14.857 14.978 15.099 15.220 15.341 15.461 15.582
1500 15.582 15.702 15.822 15.942 16.062 16.182 16.301 16.420 16.539 16.658 16.777 1600 16.777 16.895 17.013 17.131 17.249 17.366 17.483 17.600 17.717 17.832 17.947 1700 17.947 18.061 18.174 18.285 18.395 18.503 18.609
Temperature Ranges and Coefficients of Equations Used to Compute the Above Table
The equations are of the form: E = c0 + c1t + c2t2 + c3t3 + ... c n t n, where E is the emf in millivolts, t is the temperature in degrees Celsius (ITS-90), and c0, c1, c2, c3, etc. are the coefficients. These coefficients are extracted from NIST Monograph 175.
-50°C1064.18°C1664.5°C
to to to
1064.18°C1664.5°C1768.1°C
c0= 0.000 000 000 00 1.329 004 440 85 1.466 282 326 36 × 102 c1= 5.403 133 086 31 × 10-3 3.345 093 113 44 × 10-3-2.584 305 167 52 × 10-1 c2= 1.259 342 897 40 × 10-5 6.548 051 928 18 × 10-6 1.636 935 746 41 × 10-4 c3=-2.324 779 686 89 × 10-8-1.648 562 592 09 × 10-9-3.304 390 469 87 × 10-8 c4= 3.220 288 230 36 × 10-11 1.299 896 051 74 × 10-14-9.432 236 906 12 × 10-15 c5=-3.314 651 963 89 × 10-14 . . . . . . . . . . . . . . . . . . . . . . . . . .
c6= 2.557 442 517 86 × 10-17 . . . . . . . . . . . . . . . . . . . . . . . . . .
c7=-1.250 688 713 93 × 10-20 . . . . . . . . . . . . . . . . . . . . . . . . . .
c8= 2.714 431 761 45 × 10-24 . . . . . . . . . . . . . . . . . . . . . . . . . .
Type T thermocouples: emf-temperature (°C) reference table and equations Thermocouple emf in Millivolts as a Function of Temperature in Degrees Celsius (ITS-90) Reference Junctions at 0°C
t/°C0-10-20-30-40-50-60-70-80-90-100 -200 -5.603 -5.753 -5.888 -6.007 -6.105 -6.180 -6.232 -6.258
-100 -3.379 -3.657 -3.923 -4.177 -4.419 -4.648 -4.865 -5.070 -5.261 -5.439 -5.603 0 0.000 -0.383 -0.757 -1.121 -1.475 -1.819 -2.153 -2.476 -2.788 -3.089 -3.379 t/°C0102030405060708090100 0 0.000 0.391 0.790 1.196 1.612 2.036 2.468 2.909 3.358 3.814 4.279 100 4.279 4.750 5.228 5.714 6.206 6.704 7.209 7.720 8.237 8.759 9.288 200 9.288 9.822 10.362 10.907 11.458 12.013 12.574 13.139 13.709 14.283 14.862 300 14.862 15.445 16.032 16.624 17.219 17.819 18.422 19.030 19.641 20.255 20.872 400 20.872
Temperature Ranges and Coefficients of Equations Used to Compute the Above Table
The equations are of the form: E = c0 + c1t + c2t2 + c3t3 + ... c n t n, where E is the emf in millivolts, t is the temperature in degrees Celsius (ITS-90), and c0, c1, c2, c3, etc. are the coefficients. These coefficients are extracted from NIST Monograph 175.
-270°C0°C
to to
0°C400°C
c0=0.000 000 000 0 0.000 000 000 0
c1= 3.874 810 636 4 × 10-2 3.874 810 636 4 × 10-2
c2= 4.419 443 434 7 × 10-5 3.329 222 788 0 × 10-5
c3= 1.184 432 310 5 × 10-7 2.061 824 340 4 × 10-7
c4= 2.003 297 355 4 × 10-8-2.188 225 684 6 × 10-9
c5= 9.013 801 955 9 × 10-10 1.099 688 092 8 × 10-11
c6= 2.265 115 659 3 × 10-11-3.081 575 877 2 × 10-14
c7= 3.607 115 420 5 × 10-13 4.547 913 529 0 × 10-17
c8= 3.849 393 988 3 × 10-15-2.751 290 167 3 × 10-20
c9= 2.821 352 192 5 × 10-17. . . . . . . . . . . .
c10= 1.425 159 477 9 × 10-19. . . . . . . . . . . .
c11= 4.876 866 228 6 × 10-22. . . . . . . . . . . .
c12= 1.079 553 927 0 × 10-24. . . . . . . . . . . .
c13= 1.394 502 706 2 × 10-27. . . . . . . . . . . .
c14=7.979 515 392 7 × 10-31 . . . . . . . . . . . .
Type B thermocouples: coefficients (c i) of polynomials for the computation of temperatures in °C as a function of the thermocouple emf in various temperature and emf ranges
Temperature250°C700°C
Range:to to
700°C1820°C
emf0.291 mV 2.431 mV
Range:to to
2.431 mV1
3.820 mV
c0 =9.842 332 1 × 101 2.131 507 1× 102
c1 = 6.997 150 0 × 102 2.851 050 4 × 102
c2 =-8.476 530 4 × 102-5.274 288 7 × 101
c3 = 1.005 264 4 × 1039.916 080 4
c4 =-8.334 595 2 × 102-1.296 530 3
c5 = 4.550 854 2 × 102 1.119 587 0 × 10-1
c6 =-1.552 303 7 × 102-6.062 519 9 × 10-3
c7 = 2.988 675 0 × 101 1.866 169 6 × 10-4
c8 =-2.474 286 0 -2.487 858 5 × 10-6
Type E thermocouples: coefficients (c i) of polynomials for the computation of temperatures in °C as a function of the thermocouple emf in various temperature and emf ranges
Temperature-200°C0°C
Range:to to
0°C1000°C
emf-8.825 mV0.0 mV
Range:to to
0.0 mV76.373 mV
c0 = 0.000 000 0 0.000 000 0
c1 = 1.697 728 8 × 101 1.705 703 5 × 101
c2 =-4.351 497 0 × 10-1-2.330 175 9 × 10-1
c3 =-1.585 969 7 × 10-1 6.543 558 5 × 10-3
c4 =-9.250 287 1 × 10-2-7.356 274 9 × 10-5
c5 =-2.608 431 4 × 10-2-1.789 600 1 × 10-6
c6 =-4.136 019 9 × 10-38.403 616 5 × 10-8
c7 =-3.403 403 0 × 10-4-1.373 587 9 × 10-9
c8 =-1.156 489 0 × 10-5 1.062 982 3 × 10-11
c9 =. . . . . . .-3.244 708 7 × 10-14
Type J thermocouples: coefficients (c i) of polynomials for the computation of temperatures in °C as a function of the thermocouple emf in various temperature and emf ranges
Temperature-210°C0°C760°C Range:to to to
0°C760°C1200°C
emf-8.095 mV0.0 mV42.919 mV Range:to to to
0.0 mV42.919 mV69.553 mV
c0 = 0.000 000 0 0.000 000 -3.113 581 87 × 103 c1 = 1.952 826 8 ×101 1.978 425 × 101 3.005 436 84 × 102 c2 =-1.228 618 5-2.001 204 × 10-1-9.947 732 30
c3 =-1.075 217 8 1.036 969 × 10-2 1.702 766 30 × 10-1 c4 =-5.908 693 3 × 10-1-2.549 687 × 10-4 -1.430 334 68 × 10-3 c5 =-1.725 671 3 × 10-1 3.585 153 × 10-6 4.738 860 84 × 10-6 c6 =-2.813 151 3 × 10-2-5.344 285 × 10-8. . . . . . .
c7 =-2.396 337 0 × 10-3 5.099 890 × 10-10 . . . . . . .
c8 =-8.382 332 1 × 10-5. . . . . . . . . . . . . .
Type K thermocouples: coefficients (c i) of polynomials for the computation of temperatures in °C as a function of the thermocouple emf in various temperature and emf ranges
Temperature-200°C0°C500°C Range:to to to
0°C500°C1372°C
emf-5.891 mV0.0 mV20.644 mV Range:to to to
0.0 mV20.644 mV54.886 mV
c0 = 0.000 000 0 0.000 000 0-1.318 058 × 102
c1 = 2.517 346 2 × 101 2.508 355 × 101 4.830 222 × 101
c2 =-1.166 287 87.860 106 × 10-2-1.646 031
c3 =-1.083 363 8-2.503 131 × 10-1 5.464 731 × 10-2
c4 =-8.977 354 0 × 10-18.315 270 × 10-2-9.650 715 × 10-4
c5 =-3.734 237 7 × 10-1-1.228 034 × 10-28.802 193 × 10-6
c6 =-8.663 264 3 × 10-29.804 036 × 10-4-3.110 810 × 10-8
c7 =-1.045 059 8 × 10-2-4.413 030 × 10-5 . . . . . . .
c8 =-5.192 057 7 × 10-4 1.057 734 × 10-6 . . . . . . .
c9 =. . . . . . .-1.052 755 × 10-8. . . . . . .
Type N thermocouples: coefficients (c i) of polynomials for the computation of temperatures in °C as a function of the thermocouple emf in various temperature and emf ranges
Temperature-200°C0°C600°C Range:to to to
0°C600°C1300°C
emf-3.990 mV0.0 mV20.613 mV
Range:to to to
0.0 mV20.613 mV47.513 mV
c0 = 0.000 000 0 0.000 00 1.972 485 × 101
c1 = 3.843 684 7 × 101 3.868 96 × 101 3.300 943 × 101
c2 = 1.101 048 5 -1.082 67 -3.915 159 × 10-1
c3 = 5.222 931 2 4.702 05 × 10-2 9.855 391 × 10-3
c4 = 7.206 052 5 -2.121 69 × 10-6-1.274 371 × 10-4
c5 = 5.848 858 6 -1.172 72 × 10-4 7.767 022 × 10-7
c6 = 2.775 491 6 5.392 80 × 10-6. . . . . . . . .
c7 = 7.707 516 6 × 10-1-7.981 56 × 10-8 . . . . . . . . .
c8 = 1.158 266 5 × 10-1. . . . . . . . .. . . . . . . . .
c9 =7.313 886 8 × 10-3. . . . . . . . .. . . . . . . . .
Type R thermocouples: coefficients (c i) of polynomials for the computation of temperatures in °C as a function of the thermocouple emf in various temperature and emf ranges
Temperature-50°C250°C1064°C1664.5°C Range:to to to to
250°C1200°C1664.5°C1768.1°C
emf-0.226 mV 1.923 mV11.361 mV19.739 mV Range:to to to to
1.923 mV13.228 mV19.739 mV21.103 mV
c0 = 0.000 000 0 1.334 584 505 × 101-8.199 599 416 × 101 3.406 177 836 × 104 c1 = 1.889 138 0 × 102 1.472 644 573 × 102 1.553 962 042 × 102-7.023 729 171 × 103 c2 =-9.383 529 0 × 101-1.844 024 844 × 101-8.342 197 663 5.582 903 813 × 102 c3 = 1.306 861 9 × 102 4.031 129 726 4.279 433 549 × 10-1-1.952 394 635 × 101 c4 =-2.270 358 0 × 102-6.249 428 360 × 10-1-1.191 577 910 × 10-2 2.560 740 231 × 10-1 c5 = 3.514 565 9 × 102 6.468 412 046 × 10-2 1.492 290 091 × 10-4. . . . . . . . . .
c6 =-3.895 390 0 × 102-4.458 750 426 × 10-3. . . . . . . . . .. . . . . . . . . .
c7 = 2.823 947 1 × 102 1.994 710 149 × 10-4. . . . . . . . . .. . . . . . . . . .
c8 =-1.260 728 1 × 102-5.313 401 790 × 10-6. . . . . . . . . .. . . . . . . . . .
c9 = 3.135 361 1 × 101 6.481 976 217 × 10-8. . . . . . . . . .. . . . . . . . . .
c10 =-3.318 776 9 . . . . . . . . . .. . . . . . . . . .. . . . . . . . . .
Type S thermocouples: coefficients (c i) of polynomials for the computation of temperatures in °C as a function of the thermocouple emf in various temperature and emf ranges
Temperature-50°C250°C1064°C1664.5°C Range:to to to to 250°C1200°C1664.5°C1768.1°C
emf-0.235 mV 1.874 mV10.332 mV17.536 mV Range:to to to to
1.874 mV11.950 mV17.536 mV18.693 mV
c0 = 0.000 000 00 1.291 507 177 × 101-8.087 801 117 × 101 5.333 875 126 × 104 c1 = 1.849 494 60 × 102 1.466 298 863 × 102 1.621 573 104 × 102-1.235 892 298 × 104 c2 =-8.005 040 62 × 101-1.534 713 402 × 101-8.536 869 453 1.092 657 613 × 103 c3 = 1.022 374 30 × 102 3.145 945 973 4.719 686 976 × 10-1-4.265 693 686 × 101 c4 =-1.522 485 92 × 102-4.163 257 839 × 10-1-1.441 693 666 × 10-2 6.247 205 420 × 10-1 c5 = 1.888 213 43 × 102 3.187 963 771 × 10-2 2.081 618 890 × 10-4. . . . . . . . . .
c6 =-1.590 859 41 × 102-1.291 637 500 × 10-3. . . . . . . . . .. . . . . . . . . .
c7 =8.230 278 80 × 101 2.183 475 087 × 10-5. . . . . . . . . . . . . . . . . . . .
c8 =-2.341 819 44 × 101-1.447 379 511 × 10-7. . . . . . . . . .. . . . . . . . . .
c9 = 2.797 862 60 8.211 272 125 × 10-9. . . . . . . . . .. . . . . . . . . .
Type T thermocouples: coefficients (c i) of polynomials for the computation of temperatures in °C as a function of the thermocouple emf in various temperature and emf ranges
Temperature-200°C0°C
Range:to to
0°C400°C
emf-5.603 mV0.0 mV
Range:to to
0.0 mV20.872 mV
c0 = 0.000 000 0 0.000 000
c1 = 2.594 919 2 × 101 2.592 800 × 101
c2 =-2.131 696 7 × 10-1-7.602 961 × 10-1
c3 =7.901 869 2 × 10-1 4.637 791 × 10-2
c4 = 4.252 777 7 × 10-1-2.165 394 × 10-3
c5 = 1.330 447 3 × 10-1 6.048 144 × 10-5
c6 = 2.024 144 6 × 10-2-7.293 422 × 10-7
c7 = 1.266 817 1 × 10-3. . . . . . . . . .
www.bzfxw.com
15- 16
LABORATORY SOLVENTS AND OTHER LIQUID REAGENTS
This table summarizes the properties of 575 liquids that are commonly used in the laboratory as solvents or chemical reagents. The properties tabulated are:
Data on the temperature dependence of viscosity, dielectric constant, and vapor pressure can be found in the pertinent tables in this Handbook .
REFERENCES
1. Lide, D. R., Handbook of Organic Solvents , CRC Press, Boca Raton, FL, 1994.
2. Lide, D. R., and Kehiaian, H. V ., Handbook of Thermophysical and Thermochemical Data , CRC Press, Boca Raton, FL, 1994.
3. Riddick, J. A., Bunger, W. B., and Sakano, T. K., Organic Solvents, Fourth Edition , John Wiley & Sons, New York, 1986.
4. Fire Protection Guide to Hazardous Materials, 11th Edition , National Fire Protection Association, Quincy, MA, 1994.
5. Urben, P. G., Ed., Bretherick’s Handbook of Reactive Chemical Hazards, 5th Edition , Butterworth-Heinemann, Oxford, 1995
M r :Molecular weight t m :Melting point in ?C
t b :Normal boiling point in ?C
ρ :Density in g/mL at the temperature in ?C indicated by the superscript η :Viscosity in mPa s (1 mPa s = 1 centipoise) ε :Dielectric constant μ:Dipole moment in D
c p :Speci ?c heat capacity of the liqui
d at constant pressur
e at 25?C in J/g K vp:Vapor pressure at 25?C in kPa (1 kPa = 7.50 mmHg)FP:Flash point in ?C
Fl.Lim:Flammable (explosive) limit in air in percent by volume IT Autoignition temperature in ?C
TLV
Threshold limit for allowable airborne concentration in parts per million by volume at 25?C and atmospheric pressure
Name
Mol. Form.
M r
t
m /°C
t b /°C
? /g mL
–1
? /mPa s
?
? /D
c p /J g –1 K –1
vp/kPa
FP/°C
Fl. Lim.
IT/°C
TLV/ppm
Acetaldehyde C 2 H 4 O 44.052-123.3720.10.7834 18 21.0 2.750 2.020120-394-60%175Acetic acid
C 2 H 4 O 2 60.05216.64117.9 1.0446 25 1.056 6.20 1.70 2.053 2.07394-20%46310Acetic anhydride C 4 H 6 O 3 102.089-74.1139.5 1.082
20 0.84322.45 ≈ 2.8 1.6480.68049 2.7-10.3%3165Acetone
C 3 H 6 O 58.079-94.756.050.7845 25 0.30621.01 2.88 2.17530.8-203-13%465500Acetone cyanohydrin C 4 H 7 NO 85.105-19950.932 19 74 2.2-12%688Acetonitrile C 2 H 3 N 41.052-43.8281.650.7857 20 0.36936.64 3.92 2.22911.963-16%
52440Acetophenone C 8 H 8 O 120.14920.5202 1.0281 20 1.68117.44 3.02 1.7030.0497757010
Acetyl bromide C 2 H 3 BrO 122.948-9676 1.6625 16 16.2Acetyl chloride C 2 H 3 ClO 78.497-112.850.7 1.1051 20 0.368
15.8
2.72 1.49138.44390Acrolein C 3 H 4 O 56.063-87.752.60.840 20
3.136.2-26 2.8-31%220Acrylic acid C 3 H 4 O 2 72.06312.5141 1.0511 20 2.02250 2.4-8%4382Acrylonitrile C 3 H 3 N 53.063-83.4877.30.8007 25 33.0 3.87 2.051
4.103-17%4812Allyl alcohol C 3 H 6 O 58.079-12997.00.8540 20 1.218
19.7
1.60
2.392
3.14213-18%3780.5
Allylamine
C 3 H 7 N 57.095-88.253.30.758 20 1.2
33.1
-292-22%
374
2-Amino-2-methyl-1-propanol C 4 H 11 NO 89.13625.5165.50.934 20 673-Amino-1-propanol C 3 H 9 NO 75.10912.4187.50.9824 26 80Aniline C 6 H 7 N 93.127-6.02184.17 1.0217 20 3.857.06 1.13 2.0610.09070 1.3-11%
6152
Anisole
C 7 H 8 O 108.138-37.13153.70.9940 20 1.056
4.30 1.38 1.8400.472
52
475
Antimony(V) chloride Cl 5 Sb 299.0244140 dec 2.34 3.222
Antimony(V) ?uoride F 5 Sb 216.7528.3141 3.10Arsenic(III) chloride AsCl 3 181.280-16130 2.150 1.59Benzaldehyde C 7 H 6 O 106.122-57.1178.8 1.0401 25 17.85 3.0 1.6210.16963192Benzene
C 6 H 6 78.112 5.4980.090.8765 20 0.604
2.28250 1.74112.7
-111-8%
498
0.5
Benzeneacetonitrile C 8 H 7 N 117.149-23.8233.5 1.0205 15 17.87 3.5
113Benzeneethanamine C 8 H 11 N 121.180<01950.9640 25 Benzeneethanol
C 8 H 10 O 122.164-27218.2 1.0202 20 12.31 2.068
96
Benzenemethanethiol C 7 H 8 S 124.204-30194.5 1.058 20 4.705Benzenesulfonyl chloride C 6 H 5 ClO 2 S 176.62114.5251 dec 1.3470 15 28.90Benzenethiol C 6 H 6 S 110.177-14.93169.1 1.0775 20 4.26 1.23 1.5720.5
Benzonitrile C 7 H 5 N 103.122-13.99191.1 1.0093 15 1.267
25.9 4.18 1.602
0.11Benzoyl chloride
C 7 H 5 ClO
140.567
-0.4
197.2
1.2120
20
23.0
0.084
72
15- 17
LABORATORY SOLVENTS AND OTHER LIQUID REAGENTS (continued)
Benzyl acetate C 9 H 10 O 2 150.174-51.3213 1.0550 20 5.34 1.220.9899046010
Benzyl alcohol C 7 H 8 O 108.138-15.4205.31 1.0419 24 5.4711.916 1.71
2.015
0.01593
436
Benzylamine C 7 H 9 N 107.1531850.9813 20 1.624
5.180.0962,2’-Bioxirane
C 4 H 6 O 2 86.090 2.0144 1.113 20 Bis(2-aminoethyl)amine C 4 H 13 N 3 103.166-392070.9569
20
12.62 1.9 2.4620.03
982-7%3581
N,N’ -Bis(2-aminoethyl)-1,2-ethanediamine
C 6 H 18 N 4 146.23412266.510.76Bis(2-chloroethyl) ether C 4 H 8 Cl 2 O 143.012-51.9178.5 1.22 20 21.20 2.6 1.5450.143553%-369
5Bis(chloromethyl) ether C 2 H 4 Cl 2 O 114.958-41.5106 1.323 15 3.510.001
Bis(2-ethylhexyl) phthalate C 24 H 38 O 4 390.557-553840.981 25 5.3 2.84 1.804
218Bis(2-hydroxyethyl) sul ?de C 4 H 10 O 2 S 122.186-10.2282 1.1793 25 28.61
160
298
Boron tribromide BBr 3 250.523-4591 2.60Boron trichloride BCl 3 117.169-10712.6500.911Bromine
Br 2
159.808-7.258.8 3.10280.944 3.148400.4740.1
Bromobenzene C 6 H 5 Br 157.008-30.72156.06 1.4950 20 1.074 5.45 1.700.9830.556515651-Bromobutane
C 4 H 9 Br 137.018-112.6101.6 1.2758 20 0.606
7.315 2.080.798 5.2618 2.6-6.6%
265
2-Bromobutane, (±)-C 4 H 9 Br 137.018-112.6591.3 1.2585 20 8.64
2.239.3221
Bromochloromethane CH 2 BrCl 129.384-87.968.0 1.9344 20 1.70.4119.5200
Bromodichloromethane CHBrCl 2 163.829-5790 1.980 20 Bromoethane C 2 H 5 Br 108.965-118.638.5 1.4604 20 0.374
9.01 2.030.92562.57-8%5115Bromoethene
C 2 H 3 Br 106.949-139.5415.8 1.4933 20 5.63 1.42 1.0071419-15%
530
0.5
2-Bromo-2-methylpropane C 4 H 9 Br 137.018-16.273.3 1.4278 20 10.98 2.17 1.10217.71-Bromopentane C 5 H 11 Br 151.045-88.0129.8 1.2182 20 6.31 2.200.875 1.6832
1-Bromopropane C 3 H 7 Br 122.992-110.371.1 1.3537 20 0.4898.09 2.180.70218.6490
2-Bromopropane C 3 H 7 Br 122.992-89.059.5 1.3140 20 0.4589.46 2.21 1.075
28.93-Bromopropene C 3 H 5 Br 120.976-11970.1 1.398
20 0.471
7.0≈ 1.9
18.6-1 4.4-7.3%
2952-Bromotoluene
C 7 H 7 Br 171.035-27.8181.7 1.4232 20 4.64179Bromotrichloromethane CBrCl 3 198.274-5.65105 2.012 25 2.405 5.35Butanal
C 4 H 8 O 72.106-96.8674.80.8016 20 13.45 2.72
2.27015.7-222-12.5%
2181,3-Butanediol C 4 H 10 O 2 90.121-77207.5 1.0053 20 28.8 2.5210.008
1213951,4-Butanediol C 4 H 10 O 2 90.12120.4235 1.0171 20 31.9
2.58
2.2201212,3-Butanediol C 4 H
10
O 290.1217.6182.5 1.003320 2.363
402
2,3-Butanedione C 4H 6O 286.090-1.2880.980818 4.047.4527Butanenitrile C 4H 7N 69.106-111.9117.60.7936200.55324.83 3.9 2.301 2.5524>1.6%501
1-Butanethiol C 4H 10S 90.187-115.798.50.841620 5.204 1.53 1.898 6.0720.5
2-Butanethiol C 4H 10S 90.187-16585.00.829520 5.64510.8-23Butanoic acid
C 4H 8O 288.106-5.1163.750.952825 1.426 2.98 1.65 2.0270.221722-10%443Butanoic anhydride C 8H 14O 3158.195-752000.96682012.8 1.793540.9-5.8%2791-Butanol C 4H 10O 74.121-88.6117.730.809520 2.5417.84 1.66 2.3910.86371-11%343502-Butanol C 4H 10O 74.121-88.599.510.806320 3.1017.26 1.8 2.656 2.32242-10%4051002-Butanone
C 4H 8O 72.106-86.6479.590.7999250.405
18.56
2.78 2.20112.6-91-11%404200
trans -2-Butenal C 4H 6O 70.090-76102.20.851620 3.67 1.361 4.9213 2.1-15.5%232cis -2-Butenoic acid C 4H 6O 286.09015169 1.0267202-Butoxyethanol C 6H 14O 2118.174-74.8168.40.9015209.30 2.1 2.3780.15694-13%23820Butyl acetate C 6H 12O 2116.158-78126.10.8825200.685 5.07 1.9 1.961 1.66222-8%425150sec -Butyl acetate C 6H 12O 2116.158-98.91120.874820 5.135 1.8731 1.7-9.8%200Butyl acrylate C 7H 12O 2128.169-64.61450.889820 5.25 1.9580.73129 1.7-9.9%2922Butylamine C 4H 11N 73.137-49.177.000.7414200.574
4.71 1.0 2.45012.2-122-10%3125
sec -Butylamine C 4H 11N 73.137<-7262.730.724620 1.28-9tert -Butylamine C 4H 11N 73.137-66.9444.040.69582058.5 1.3 2.62748.4-92-9%380Butylbenzene C 10H 14134.218-87.85183.310.8601200.950
2.359≈ 0 1.8130.150710.8-5.8%410tert -Butylbenzene C 10H 14134.218-57.8169.10.866520 2.359≈0.83
1.7730.280600.7-5.7%
450
Butyl benzoate
C 11H 14O 2178.228-22.4250.3 1.00020 5.52
107tert -Butyl ethyl ether C 6H 14O 102.174-9472.60.73625 2.13
16.55tert -Butyl hydroperoxide C 4H 10O 290.121689 dec 0.896020271-tert -Butyl-4-methylbenzene C 11H 16148.245-521900.861220≈00.09681
Butyl vinyl ether C 6H 12O 100.158-92940.788820 1.25 2.316 6.65-9255?-Butyrolactone C 4H 6O 286.090-43.61204 1.12962039.0
4.27 1.6420.4398Carbon disul ?de CS 276.141-112.146 1.2632200.352
2.6320
1.003
48.2
-30
1-50%90
10
Chloroacetaldehyde C 2H 3ClO 78.497-16.385.5 1.19Chloroacetone
C 3H 5ClO 92.524-44.5119 1.1520Chloroacetyl chloride C 2H 2Cl 2O 112.942-22106 1.420220 2.23 3.330.05
2-Chloroaniline C 6H 6ClN 127.572-1.9208.8 3.3213.40 1.770.0343-Chloroaniline C 6H 6ClN 127.572-10.28230.5 1.21612013.3 1.558705Chlorobenzene
C 6H 5Cl 112.557-45.31131.72 1.1058200.753
5.6895 1.69
1.334
1.6281-10%593
102-Chloro-1,3-butadiene
C 4H 5Cl
88.536
-130
59.4
0.95620
4.914
29.5
-20
4-20%
10
Name
Mol. Form.
M r
t m /°C
t b /°C
?/g mL –1
?/mPa s
?
?/D
c p /J g –1K –1
vp/kPa
FP/°C
Fl. Lim.
IT/°C
TLV/ppm
15-18
1-Chlorobutane C 4H 9Cl 92.567-123.178.40.8857200.422
7.276 2.05 1.891
13.7-122-10%
240
2-Chlorobutane C 4H 9Cl 92.567-131.368.20.8732208.564 2.0421.0
-10Chlorocyclohexane C 6H 11Cl 118.604-43.81142 1.000207.9505 2.132Chlorodibromomethane CHBr 2Cl 208.280-20120 2.45120Chloroethane C 2H 5Cl 64.514-138.412.30.923909.45 2.05 1.617160
-504-15%519100
2-Chloroethanol
C 2H 5ClO 80.513-67.5128.6 1.20192025.80 1.78605-16%4252-Chloroethyl vinyl ether C 4H 7ClO 106.551-70108 1.04952027(Chloromethyl)benzene C 7H 7Cl 126.584-45179 1.100420 6.854 1.8 1.440.164671%-585
1
Chloromethyl methyl ether C 2H 5ClO 80.513-103.559.5 1.0631024.91-Chloro-2-methylpropane C 4H 9Cl 92.567-130.368.50.8773207.027 2.00 1.71319.9-62-8.7%
2-Chloro-2-methylpropane C 4H 9Cl 92.567-25.6050.90.8420209.663 2.13 1.86742.7
01-Chloronaphthalene C 10H 7Cl 162.616-2.5259 1.188025 5.04 1.57 1.307121>558
1-Chlorooctane C 8H 17Cl 148.674-57.8183.50.873420 5.05 2.00 1.335701-Chloropentane C 5H 11Cl 106.594-99.0108.40.882020 6.654 2.16 4.3613 1.6-8.6%2602-Chlorophenol C 6H 5ClO 128.5569.4174.9 1.263420 3.597.40 1.4680.308641-Chloropropane
C 3H 7Cl 78.541-122.946.50.8899200.3348.588 2.05 1.683
45.8<-18 2.6-11%5202-Chloropropane
C 3H 7Cl 78.541-117.1835.70.8617200.303
2.17
68.9
-32 2.8-11%
593
3-Chloro-1,2-propanediol C 3H 7ClO 2110.540213 dec 1.3251831.03-Chloropropanenitrile C 3H 4ClN 89.524-51175.5 1.157320762-Chloropropene C 3H 5Cl 76.525-137.422.60.9017208.92 1.647110-37 4.5-16%3-Chloropropene C 3H 5Cl 76.525-134.545.10.9376200.3148.2 1.94 1.63548.9-32
2.9-11%
4851Chlorosulfonic acid ClHO 3S 116.525-80152 1.752-Chlorotoluene C 7H 7Cl 126.584-35.8159.0 1.0825200.964 4.721 1.56 1.318
0.482
504-Chlorotoluene C 7H 7Cl 126.5847.5162.4 1.0697200.837
6.25 2.21
Chromyl chloride Cl 2CrO 2154.900-96.5117 1.910.025
trans -Cinnamaldehyde C 9H 8O 132.159-7.5246 1.04972017.72o -Cresol C 7H 8O 108.13831.03191.04 1.032735 6.76 1.45 2.1600.04181>1.4%5995m -Cresol C 7H 8O 108.13812.24202.27 1.03392012.91
12.44 1.48 2.0800.01986>1.1%5585p -Cresol
C 7H 8O 108.13834.77201.98 1.01854013.05
1.48
2.0440.01786>1.1%5585
Cyanogen chloride CClN 61.471-6.513 1.18620 2.8331Cyclobutane C 4H 856.107-90.712.60.703800157<10>1.8%Cyclohexane C 6H 1284.159 6.5980.730.7739250.894 2.0243≈0 1.84113.0-201-8%245300Cyclohexanol C 6H 12O 100.15825.93160.840.96242057.516.40 2.0790.10681-9%30050Cyclohexanone C 6H 10O 98.142-27.9155.430.947820 2.0216.1 2.87 1.8560.53441-9%42025Cyclohexene C 6H 1082.143-103.582.980.8110200.625 2.21760.33 1.805
11.8-12>1.2%310300Cyclohexylamine C 6H 13N 99.174-17.81340.819120 1.944 4.547 1.3 1.20311-9%293101,3-Cyclopentadiene C 5H 666.102-85410.8021200.41958.575Cyclopentane C 5H 1070.133-93.449.30.7457200.413
1.9687≈0 1.8374
2.3-252%-
361
600
Cyclopentanol C 5H 10O 86.132-17.5140.420.94882018.5 2.1190.29451Cyclopentanone
C 5H 8O 84.117-51.90130.570.94872013.58 3.3 1.84 1.5526cis -Decahydronaphthalene C 10H 18138.250-42.9195.80.896520 3.04 2.219≈0 1.6780.10trans -Decahydronaphthalene C 10H 18
138.250-30.4187.30.865925 1.948
2.184≈0
1.653
0.164
54
1-5%255
Decamethylcyclopentasiloxane C 10H 30O 5Si 5370.770-382100.959320 2.50Decanal C 10H 20O 156.265-4.0208.50.83015Decane
C 10H 22142.282-29.6174.150.7266250.838 1.9853≈ 0
2.2100.170510.8-5.4%210Decanoic acid C 10H 20O 2172.26531.4268.70.885840 2.7611-Decanol C 10H 22O 158.281 6.9231.10.82972010.917.93 2.3410.009822881-Decene
C 10H 20140.266-66.3170.50.7408200.756 2.136≈ 0 2.1440.210<55235Diacetone alcohol C 6H 12O 2116.158-44167.90.938720 2.80
18.2 3.2 1.905
0.224582-7%643
50Dibenzyl ether
C 14H 14O 198.260 1.8298 1.042820 3.821135
Dibromodi ?uoromethane CBr 2F 2209.816-110.122.760.66110100
1,2-Dibromoethane C 2H 4Br 2187.8619.84131.6 2.168325
1.595 4.9612 1.20.724 1.55Dibromomethane
CH 2Br 2173.835-52.597 2.4969200.9807.77 1.430.61 6.121,2-Dibromotetra ?uoroethane C 2Br 2F 4259.823-110.3247.35 2.14925 2.340.6943.4Dibutylamine C 8H 19N 129.244-62159.60.7670200.918 2.765 1.0 2.2660.34471-6%Dibutyl ether
C 8H 18O 130.228-95.2140.280.7684200.637 3.0830 1.17 2.1360.89825 1.5-7.6%194Di-tert -butyl peroxide C 8H 18O 2146.228-401110.70420
3.43
18Dibutyl phthalate
C 16H 22O 4278.344-35340 1.04652016.63
6.58 2.82 1.789157>0.5%402Dibutyl sebacate C 18H 34O 4314.461-10344.50.940515 4.54 2.48 1.968178>0.4%
365
Dibutyl sul ?de C 8H 18S 146.294-79.71850.838620 4.29 1.61 1.94376
Dichloroacetic acid C 2H 2Cl 2O 2128.94210194 1.5634208.33o -Dichlorobenzene C 6H 4Cl 2147.002-17.0180 1.305920 1.32410.12 2.50 1.1050.18662-9%64825m -Dichlorobenzene
C 6H 4Cl 2147.002-24.8173 1.288420 1.044 5.02
1.72
1.163
0.25272trans -1,4-Dichloro-2-butene C 4H 6Cl 2124.997 1.0155.4 1.183250.005
Dichlorodimethylsilane C 2H 6Cl 2Si 129.061-1670.3 1.0642518.9<21 3.4-9.5%1,1-Dichloroethane
C 2H 4Cl 2
98.959
-96.9
57.3
1.175720
0.46410.10 2.06 1.276
30.5
-17
5-11%
458100
Name
Mol. Form.
M r
t m /°C
t b /°C
?/g mL –1
?/mPa s
?
?/D
c p /J g –1K –1
vp/kPa
FP/°C
Fl. Lim.
IT/°C
TLV/ppm
15-19
1,2-Dichloroethane C 2H 4Cl 298.959-35.783.5 1.2454250.77910.42 1.8 1.29810.6136-16%413101,1-Dichloroethene C 2H 2Cl 296.943-122.5631.6 1.21320 4.60 1.34 1.14880.0-287-16%5705cis -1,2-Dichloroethene C 2H 2Cl 296.943-80.060.1 1.2837200.4459.20 1.90 1.20126.863-15%460200trans -1,2-Dichloroethene C 2H 2Cl 296.943-49.848.7 1.256520
0.317 2.140 1.20544.22
6-13%460200Dichloromethane
CH 2Cl 284.933-97.240 1.3266200.413
8.93 1.60 1.192
58.213-23%
556
50
(Dichloromethyl)benzene C 7H 6Cl 2161.029-17205 1.2625 6.9 2.10.061,1-Dichloropropane
C 3H 6Cl 2112.98688.1 1.1321209.091,2-Dichloropropane, (±)-C 3H 6Cl 2112.986-100.5396.4 1.1560208.37 1.8 1.320 6.62
213-15%55775
1,3-Dichloropropane C 3H 6Cl 2112.986-99.5120.9 1.17852510.27 2.082,3-Dichloropropene C 3H 4Cl 2110.9701094 1.2112015
2.6-7.8%
2,4-Dichlorotoluene C 7H 6Cl 2161.029-13.5201 1.247620 5.68 1.700.055
Dicyclohexylamine C 12H 23N 181.318-0.1256 dec 0.912320>99Diethanolamine C 4H 11NO 2105.13628268.8 1.09662025.75 2.8 2.22<0.011722-13%6620.46
1,1-Diethoxyethane C 6H 14O 2118.174-100102.250.825420 3.80 1.4 2.01 3.68-212-10%2301,2-Diethoxyethane C 6H 14O 2118.174-74.0121.20.835125
3.90
2.195 4.3327205Diethylamine
C 4H 11N 73.137-49.855.50.7056200.319
3.6800.92
2.313
30.1
-232-10%
3125
N,N -Diethylaniline C 10H 15N 149.233-38.8216.30.930720 5.1585630o -Diethylbenzene C 10H 14134.218-31.21840.880020 2.59457395m -Diethylbenzene C 10H 14134.218-83.9181.10.860220 2.36956450p -Diethylbenzene C 10H 14134.218-42.83183.70.862020 2.259550.7-6%430Diethyl carbonate C 5H 10O 3118.131-431260.969225 2.820 1.10 1.80 1.6325Diethylene glycol
C 4H 10O 3106.120-10.4245.8 1.11971530.231.82 2.3 2.3070.0011242-17%
224
Diethylene glycol diethyl ether C 8H 18O 3162.227-451880.906320 5.70 2.10482Diethylene glycol dimethyl ether C 6H 14O 3134.173-681620.9434200.989
7.23
2.0 2.0430.31567Diethylene glycol monobutyl ether C 8H 18O 3162.227-682310.955320 2.188Diethylene glycol monoethyl ether C 6H 14O 3134.1731960.988520 1.6 2.243
0.01796Diethylene glycol monoethyl ether acetate
C 8H 16O 4176.211-25
218.5 1.009620 1.80.029110425Diethylene glycol monomethyl ether
C 5H 12O 3120.147193
1.03520 1.6
2.2560.024961-23%240Diethyl ether C 4H 10O 74.121-116.234.50.7138200.224
4.2666 1.15 2.36971.7
-452-36%180400
Diethyl maleate C 8H 12O 4172.179-8.8223 1.0662207.560121350
Diethyl malonate C 7H 12O 4160.168-50200 1.0551207.550 2.54 1.77993Diethyl oxalate C 6H 10O 4146.141-40.6185.7 1.0785208.266 2.49
1.7840.030
76Diethyl phthalate C 12H 14O 4222.237-40.5295 1.232147.86 1.647
161>0.7%457Diethyl succinate C 8H 14O 4174.195-21217.7 1.040220 6.09890Diethyl sulfate C 4H 10O 4S 154.185-24208 1.1722529.2104
436
Diethyl sul ?de C 4H 10S 90.187-103.9192.10.8362200.422
5.723 1.54 1.9007.78Diiodomethane CH 2I 2267.836
6.1182 3.321120
5.32
1.080.5000.172Diiodosilane H 2I 2Si 283.911-1150Diisobutylamine C 8H 19N 129.244-73.5139.60.723
0.97229Diisopentyl ether C 10H 22O 158.281172.50.777720 2.817
1.23
2.3940.210Diisopropylamine C 6H 15N 101.190-618
3.90.7153200.393 1.1510.7-1 1.1-7.1%3165Diisopropyl ether C 6H 14O 102.174-85.468.40.7192250.379 3.805 1.13 2.12219.9-281-8%4432501,2-Dimethoxyethane C 4H 10O 290.121-69.208
4.50.8637250.455
7.30 2.1459.93-2202Dimethoxymethane C 3H 8O 276.095-105.1420.859320 2.6440.7 2.12953.1-322-14%2371000Dimethylacetal
C 4H 10O 290.121-113.264.50.85012022.9N,N -Dimethylacetamide C 4H 9NO 87.120-18.591650.9372250.927
38.85
3.7 2.0160.075
702-12%490
102,3-Dimethylaniline C 8H 11N 121.180<-15221.50.99312097>1%
0.52,6-Dimethylaniline C 8H 11N 121.18011.22150.984220 1.63 1.971960.5N,N -Dimethylaniline C 8H 11N 121.180 2.42194.150.955720 1.300 4.90 1.68 1.7716337152,2-Dimethylbutane C 6H 1486.175-98.849.730.6444250.351 1.869≈ 0 2.22742.5-48 1.2-7%4055002,3-Dimethylbutane
C 6H 1486.175-128.1057.930.6616200.361
1.889≈ 0
2.201
31.3-29
1.2-7%
405
500
3,3-Dimethyl-2-butanone C 6H 12O 100.158-52.5106.10.72292512.73 4.27Dimethylcarbamic chloride C 3H 6ClNO 107.539-33167 1.16825Dimethyl disul ?de
C 2H 6S 294.199-84.67109.74 1.0625209.6
1.8 1.551 3.8224N ,N -Dimethylethanolamine C 4H 11NO 89.136-591340.886620N,N -Dimethylformamide C 3H 7NO 73.094-60.481530.9445250.794
38.25 3.82 2.0600.439582-15%445102,6-Dimethyl-4-heptanone C 9H 18O 142.238-41.5169.40.8062209.91 2.7
2.0900.23
491-7%396251,1-Dimethylhydrazine C 2H 8N 260.098-57.2063.90.79122 2.731-152-95%2490.01
Dimethyl phthalate C 10H 10O 4194.184 5.5283.7 1.19052014.36
8.66 1.561146>0.9%
4902,6-Dimethylpyridine C 7H 9N 107.153-6.1144.010.9226207.33 1.7 1.7280.746
Dimethyl sulfate C 2H 6O 4S 126.132-31.7188 dec 1.33222055.0831880.1
Dimethyl sul ?de C 2H 6S 62.134-98.2437.330.8483200.284 6.70 1.554 1.90164.4-37 2.2-20%206Dimethyl sulfoxide C 2H 6OS 78.13317.89189 1.101025 1.98747.24 3.96 1.9580.084953-42%2151,4-Dioxane
C 4H 8O 2
88.106
11.85
101.5
1.033720
1.177
2.2189
1.726
4.95
12
2-22%
180
20
Name
Mol. Form.
M r
t m /°C
t b /°C
?/g mL –1
?/mPa s
?
?/D
c p /J g –1K –1
vp/kPa
FP/°C
Fl. Lim.
IT/°C
TLV/ppm
15-20
1,3-Dioxolane C 3H 6O 274.079-97.2278 1.06020 1.19 1.59314.62Dipentyl ether C 10H 22O 158.281-691900.783320 2.798 1.20 1.57957170Dipropylamine
C 6H 15N 101.190-63109.30.7400200.517
2.923
1.03
2.500
3.21
17
299
Dipropylene glycol monomethyl ether C 7H 16O 3148.200-80188.30.95100
Dipropyl ether C 6H 14O 102.174-114.890.080.7466200.396 3.38 1.21 2.1698.3521 1.3-7%188Dodecane
C 12H 26170.334-9.57216.320.749520 1.383 2.0120≈ 0 2.2060.01674>0.6%
2031-Dodecanol C 12H 26O 186.33323.92600.830924 5.82 2.3511272751-Dodecene C 12H 24168.319-35.2213.80.758420 1.20 2.152≈ 0 2.1430.01979Epichlorohydrin C 3H 5ClO 92.524-26118 1.181220 1.073
22.6
1.8 1.422
2.2314-21%4110.51,2-Epoxybutane C 4H 8O 72.106-1506
3.40.829720 1.891
2.039
31.7
-22
1.7-19%
439
1,2-Epoxy-4-(epoxyethyl)cyclohexane C 8H 12O 2140.180<-55227 1.0966200.1
1,2-Ethanediamine C 2H 8N 260.09811.141170.89792013.82 1.99 2.872 1.62403-12%385101,2-Ethanediol
C 2H 6O 262.068-12.69197.3 1.11352016.06
41.4 2.28 2.3940.011113-22%39835
1,2-Ethanediol, diacetate C 6H 10O 4146.141-31190 1.1043207.7 2.34 2.121
0.03088
1.6-8.4%
482
1,2-Ethanediol, dinitrate C 2H 4N 2O 6152.062-22.3198.5 1.49182028.260.0090.05
1,2-Ethanedithiol C 2H 6S 294.199-41.2146.1 1.234207.26 2.03Ethanethiol C 2H 6S 62.134-147.8835.00.8315250.287 6.667 1.60 1.89870.3-17 2.8-18%3000.5Ethanol
C 2H 6O 46.068-114.1478.290.789320 1.07425.3 1.69 2.4387.87133-19%3631000Ethanolamine C 2H 7NO 61.08310.5171 1.01802021.131.94 2.3 3.2010.05863-24%
410
34-Ethoxyaniline C 8H 11NO 137.179 4.6254 1.0652167.43116Ethoxybenzene C 8H 10O 122.164-29.43169.810.965120 1.197
4.216 1.45 1.8700.204632-Ethoxyethanol
C 4H 10O 290.121-701350.92532513.38 2.1 2.3390.71433-18%23552-Ethoxyethyl acetate C 6H 12O 3132.157-61.7156.40.9740207.567 2.2 2.8450.24562-8%3795Ethyl acetate
C 4H 8O 288.106-83.877.110.9003200.423
6.0814 1.78 1.93712.6-42-12%426400Ethyl acetoacetate C 6H 10O 3130.141-45180.8 1.03681014.0 1.9060.095571-10%295Ethyl acrylate C 5H 8O 2100.117-71.299.40.923420 6.05 1.96 5.1410 1.4-14%3725Ethylamine C 2H 7N 45.084-80.516.50.689158.7 1.22 2.884141-164-14%3855N -Ethylaniline C 8H 11N 121.180-63.5203.00.962520 2.05 5.8785Ethylbenzene C 8H 10106.165-94.96136.190.8626250.631 2.44630.59 1.726 1.28211-7%
432100
Ethyl benzoate C 9H 10O 2150.174-34212 1.041525 6.20 2.00 1.63888490Ethyl butanoate C 6H 12O 2116.158-98121.30.8735250.639
5.18 1.74
1.963
2.0124463
2-Ethyl-1-butanol C 6H 14O 102.174<-151470.832620 6.190.20657Ethyl chloroacetate C 4H 7ClO 2122.551-21144.3 1.1585200.640
64Ethyl chloroformate C 3H 5ClO 2108.524-80.695 1.1352209.73616500
Ethyl cyanoacetate C 5H 7NO 2113.116-22.5205 1.06542031.62 2.17 1.947110Ethyleneimine C 2H 5N 43.068-77.9560.8322518.3 1.9028.9-11 3.3-55%3200.5Ethyl formate C 3H 6O 274.079-79.654.40.9208200.380
8.57 1.9
2.015
32.3
-203-16%455100
2-Ethylhexanal
C 8H 16O 128.212<-1001630.854020440.9-7.2%1902-Ethyl-1,3-hexanediol C 8H 18O 2146.228-402440.93252218.731273602-Ethyl-1-hexanol C 8H 18O 130.228-70184.60.831925 6.27
7.58 1.74 2.4380.019
730.8-9.7%2312-Ethylhexyl acetate C 10H 20O 2172.265-801990.871820 1.8711-8%268Ethyl lactate
C 5H 10O 3118.131-26154.5 1.03282015.4 2.4
2.150
46>1.5%
400
Ethyl 3-methylbutanoate C 7H 14O 2130.185-99.3135.00.865620 4.71
1.07Ethyl 2-methylpropanoate C 6H 12O 2116.158-88.2110.10.86820 3.25
13Ethyl nitrite
C 2H 5NO 275.067180.89915-354-50%90Ethyl propanoate C 5H 10O 2102.132-73.999.10.8843250.501
5.76 1.74
1.920 4.9712 1.9-11%440
Ethyl silicate C 8H 20O 4Si 208.329-82.5168.80.932020 2.50 1.749 1.175210
Eucalyptol C 10H 18O 154.2490.8176.40.926720 4.570.26048Fluorobenzene C 6H 5F 96.102-42.1884.73 1.0225200.550 5.465 1.60 1.52310.4
-15Fluorosulfonic acid FHO 3S 100.070-89163 1.726Formamide CH 3NO 45.041 2.49220 1.133420 3.34111.0 3.73 2.38915410Formic acid CH 2O 246.0268.3101 1.22020 1.60751.1 1.425 2.151 5.755018-57%4345Furan C 4H 4O 68.074-85.6131.50.9514200.361 2.940.66 1.68680.0-362-14%Furfural
C 5H 4O 296.085-38.1161.7 1.159420 1.587
42.1 3.5 1.6980.29602-19%3162Furfuryl alcohol
C 5H 6O 298.101-14.6171 1.12962016.85 1.9 2.0790.097752-16%49110
Germanium(IV) chloride Cl 4Ge 214.42-51.5086.55 1.880Glycerol
C 3H 8O 392.09418.1290 1.261320934
46.53 2.6
2.377<0.011993-19%370Glycerol triacetate C 9H 14O 6218.203-78259 1.1583207.11 1.763<0.01
138
1%-
433
Glycerol trioleate C 57H 104O 6885.432-40.91515 3.109Heptanal C 7H 14O 114.185-43.4152.80.8132259.07 2.015Heptane
C 7H 16100.202-90.5598.40.6795250.387 1.9209≈0 2.242 6.09-41-7%
204400
Heptanoic acid C 7H 14O 2130.185-7.17222.20.912425 3.84 3.04 2.0392751-Heptanol C 7H 16O 116.201-33.2176.450.821920 5.8111.75 2.3422-Heptanone
C 7H 14O
114.185
-35
151.05
0.811120
0.714
11.95
2.6 2.037
0.49391-8%393
50
Name
Mol. Form.
M r
t m /°C
t b /°C
?/g mL –1
?/mPa s
?
?/D
c p /J g –1K –1
vp/kPa
FP/°C
Fl. Lim.
IT/°C
TLV/ppm
物质性质查询网站汇总
物质性质查询网站汇总.txt每天早上起床都要看一遍“福布斯”富翁排行榜,如果上面没有我的名字,我就去上班。谈钱不伤感情,谈感情最他妈伤钱。我诅咒你一辈子买方便面没有调料包。物质性质查询网站汇总 1 化学工程师资源主页 该站点由西弗吉尼亚大学校友Christopher M.A.Haslego维护。该主页有非常丰富的化学工程方面的内容,其中包括一些查找物性数据比较好的站点: https://www.360docs.net/doc/249935889.html,/physinternetzz.shtml 1.1 物性数据https://www.360docs.net/doc/249935889.html,/data.xls 该数据库是浏览型数据库,含有470多种纯组分的物性数据,如分子量、冰点、沸点、临界温度、临界压力、临界体积、临界压缩、无中心参数、液体密度、偶极矩、气相热容、液相热容、液体粘度、反应标准热、蒸气压、蒸发热等。 1.2聚合物和大分子的物理性质数据库https://www.360docs.net/doc/249935889.html,/~athas/databank/intro.html 该数据库是浏览型数据库。含有200多种线性大分子的物性数据,如熔融温度、玻璃转换温度、热容等。该站点不仅提供物理性质,还提供一些供估计物质物理性质的软件,如PhysProps from G&P Engineering、Prode's thermoPhysical Properties Generator(PPP)等。 1.3 https://www.360docs.net/doc/249935889.html,/~jrm/thermot.html 该站点可查294种组分的热力学性质,还可以根据Peng Robinson状态方程计算纯组分或混合物的性质:包括气液相图、液体与气体密度、焓、热容、临界值、分子量等数据。 1.4 https://www.360docs.net/doc/249935889.html, G&P Engineering是一个软件,提供物质的28种物理性质并估算其它18种物理性质。 2 由美国国家标准技术研究院开发的数据库 2.1 标准参考数据库化学网上工具书https://www.360docs.net/doc/249935889.html,/chemistry/ 该数据库是一种检索型数据库,检索方法非常简单,可通过化学物质名称、分子式、部分分子式、CAS登记号、结构或部分结构、离子能性质、振动与电子能、分子量和作用进行检索,可检索到的数据包括分子式、分子量、化学结构、别名、CAS登记号、气相热化学数据、凝聚相热化学数据、液态常压热容、固态常压热容、相变数据、汽化焓、升华焓、燃烧焓、燃烧熵、各种反应的热化学数据、溶解数据、气相离子能数据、气相红外光谱、质谱、紫外/可见光谱、振动/电子能及其参考文献。
天然气物理化学性质
海底天然气物理化学性质 第一节海底天然气组成表示法 一、海底天然气组成 海底天然气是由多种可燃和不可燃的气体组成的混合气体。以低分子饱和烃类气体为主,并含有少量非烃类气体。在烃类气体中,甲烷(CH 4 )占绝大部分, 乙烷(C 2H 6 )、丙烷(C 3 H 8 )、丁烷(C 4 H 10 )和戊烷(C 5 H 12 )含量不多,庚烷以上 (C 5+)烷烃含量极少。另外,所含的少量非烃类气体一般有氮气(N 2 )、二氧化 碳(CO 2)、氢气(H 2 )、硫化氢(H 2 S)和水汽(H 2 O)以及微量的惰性气体。 由于海底天然气是多种气态组分不同比例的混合物,所以也像石油那样,其物理性质变化很大,它的主要物理性质见下表。 海底天然气中主要成分的物理化学性质 名称分 子 式 相 对 分 子 质 量 密度 /Kg ·m-3 临界 温度 /℃ 临 界 压 力 /MP a 粘度 /KP a ·S 自 燃 点 / ℃ 可燃性 限 /% 热值 /KJ·m-3 (15.6℃, 常压) 气体 常数 / Kg· m· (Kg ·K)-1 低 限 高 限 全 热 值 净 热 值 甲烷CH 4 16. 043 0.71 6 -82. 5 4.6 4 0.01( 气) 6 4 5 5. 15. 372 62 334 94 52.8 4 乙烷C 2 H 6 30. 070 1.34 2 32.2 7 4.8 8 0.009( 气) 5 3 3. 2 12. 45 661 51 602 89 28.2 丙烷C 3 H 8 44. 097 1.96 7 96.8 1 4.2 6 0.125( 10℃) 5 1 2. 37 9.5 937 84 862 48 19.2 3 正丁烷n-C 4 H 10 58. 12 2.59 3 152. 01 3.8 0.174 4 9 1. 86 8.4 1 121 417 108 438 14.5 9 异丁烷i-C 4 H 10 58. 12 2.59 3 134. 98 3.6 5 0.194 1. 8 8.4 4 121 417 108 438 14.5 9 氨He 4.0 03 0.19 7 -267 .9 0.2 3 0.0184 211. 79 氮N 228. 02 1.25 -147 .13 3.3 9 0.017 30.2 6
空气物性参数表
空气物性参数表 工程热力学研究的对象是热能转化成机械能的规律和方法,以及提高转化效率的途径。热力学第一定律说明了能量在传递和转化时的数量关系,即某一物体失去的热量必然等于另一物体所得到的热量。热力学第二定律是研究能量传递和转移过程进行的方向、条件和深度等规律问题,其中最根本的是关于方向的问题。热不可能自发地、不付代价地、从低温物体传至高温物体。 1. 导热:也称热传导,是指物体各部分之间不发生相对位移时,依靠分子、原子及自由电子等微观粒子的热运动而产生的热量传递现象。例如,物体内部热量从温度较高的部分传递到温度较低的部分,以及温度较高的物体把热量传递给与之接触的温度较低的另一物体都是导热现象。 2. 热对流:简称对流,是指流体内部各部分之间发生相对位移,冷热流体相互掺混而引起的热量传递现象。热对流现象仅能发生在流体内部,而且必然伴随有导热现象。 3. 热辐射:物体通过电磁波来传递能量的方式称为辐射。物体会因各种原因发出辐射能,其中因热的原因而发出辐射能的现象称为热辐射。(由物体表面直接向外界发射可见和不可见射线,在空间传递能量的现象称为热辐射。它是一种非接触传递能量的方式。)
4. 温度:是指物体冷热的程度。是指物质微观粒子(分子、电子等)热运动激烈程度的衡量。 5. 导热系数λ(导热率):它表示物质导热能力的大小。由实验取得。单位:W/m.℃ 6. 换热系数α(放热系数、给热系数):表示当流体与壁面间的温差为1℃时,在单位时间内,通过单位面积的热量。放热系数的大小反映出对流换热过程的强烈程度。单位:W/m2.℃ 7. 传热系数k:传热温差为1℃时,在单位时间内,通过单位面积的热量。它反映传热过程的强烈程度。单位:W/m2.℃ 8. 导温系数α(热扩散率):表示物体中热扩散的快慢程度。是材料传播温度变化能力大小的指标。α=λ/ρc 由实验取得。单位:m2/s 9. 热阻Rt:热转移过程中的阻力称为热阻。Rt=△t/Q 10. 比热c:物体温度升高1度所需的热量叫热容,单位物量的物体温度升高1度所需的热量叫比热容,简称比热。根据计量物量的单位不同,有质量比热、容积比热、摩尔比热之分。质量比热单位:kJ/kg.℃;
各种物质物理化学参数使用手册
STANDARD ITS-90 THERMOCOUPLE TABLES The Instrument Society of America (ISA) has assigned standard letter designations to a number of thermocouple types having specified emf-temperature relations. These designations and the approximate metal compositions which meet the required relations, as well as the useful temperature ranges, are given below: Type B(Pt + 30% Rh) vs. (Pt + 6% Rh) 0 to 1820°C Type E(Ni + 10% Cr) vs. (Cu + 43% Ni)-270 to 1000°C Type J Fe vs. (Cu + 43% Ni)-210 to 1200°C Type K (Ni + 10% Cr) vs. (Ni + 2% Al + 2% Mn + 1% Si)-270 to 1372°C Type N (Ni + 14% Cr + 1.5% Si) vs. (Ni + 4.5% Si + 0. 1% Mg) -270 to 1300°C Type R(Pt + 13% Rh) vs. Pt-50 to 1768°C Type S (Pt + 10% Rh) vs. Pt -50 to 1768°C Type T Cu vs. (Cu + 43% Ni)-270 to 400°C The compositions are given in weight percent, and the positive leg is listed first. It should be emphasized that the standard letter designations do not imply a precise composition but rather that the specified emf-temperature relation is satisfied. The first set of tables below lists, for each thermocouple type, the emf as a function of temperature on the International Temperature Scale of 1990 (ITS-90). The coefficients in the equation used to generate the table are also given. The second set of tables gives the inverse relationships, i.e., the coefficients in the polynomial equation which expresses the temperature as a function of thermocouple emf. The accuracy of these equations is also stated. Further details and tables at closer intervals may be found in Reference 1. REFERENCES 1. Burns, G. W., Seroger, M. G., Strouse, G. F., Croarkin, M. C., and Guthrie, W.F., Temperature-Electromotive Force Reference Functions and Tables for the Letter-Designated Thermocouple Types Based on the ITS-90, Nat. Inst. Stand. Tech. (U.S.) Monogr. 175, 1993. 2. Schooley, J. F., Thermometry, CRC Press, Boca Raton, FL, 1986.
空气物性参数表
物性参数: 物性参数主要是材料在制工方面能否达到要求的数据。不同材料有不同的物性参数。比如尼龙,就有很多数据要求,有冲击强度,拉伸强度,融溶指数等等。 传热学中的参数: 工程热力学研究的对象是热能转化成机械能的规律和方法,以及提高转化效率的途径。热力学第一定律说明了能量在传递和转化时的数量关系,即某一物体失去的热量必然等于另一物体所得到的热量。热力学第二定律是研究能量传递和转移过程进行的方向、条件和深度等规律问题,其中最根本的是关于方向的问题。热不可能自发地、不付代价地、从低温物体传至高温物体。 1. 导热:也称热传导,是指物体各部分之间不发生相对位移时,依靠分子、原子及自由电子等微观粒子的热运动而产生的热量传递现象。例如,物体内部热量从温度较高的部分传递到温度较低的部分,以及温度较高的物体把热量传递给与之接触的温度较低的另一物体都是导热现象。 2. 热对流:简称对流,是指流体内部各部分之间发生相对位移,冷热流体相互掺混而引起的热量传递现象。热对流现象仅能发生在流体内部,而且必然伴随有导热现象。 3. 热辐射:物体通过电磁波来传递能量的方式称为辐射。物体会因各种原因发出辐射能,其中因热的原因而发出辐射能的现象称为
热辐射。(由物体表面直接向外界发射可见和不可见射线,在空间传递能量的现象称为热辐射。它是一种非接触传递能量的方式。) 4. 温度:是指物体冷热的程度。是指物质微观粒子(分子、电子等)热运动激烈程度的衡量。 5. 导热系数λ(导热率):它表示物质导热能力的大小。由实验取得。单位:W/m.℃ 6. 换热系数α(放热系数、给热系数):表示当流体与壁面间的温差为1℃时,在单位时间内,通过单位面积的热量。放热系数的大小反映出对流换热过程的强烈程度。单位:W/m2.℃,但是与导热系数不同,它不是物性参数。 7. 传热系数k:传热温差为1℃时,在单位时间内,通过单位面积的热量。它反映传热过程的强烈程度。单位:W/m2.℃ 8. 导温系数α(热扩散率):表示物体中热扩散的快慢程度。是材料传播温度变化能力大小的指标。α=λ/ρc 由实验取得。单位:m2/s 9. 热阻Rt:热转移过程中的阻力称为热阻。Rt=△t/Q 10. 比热c:物体温度升高1度所需的热量叫热容,单位物量的物体温度升高1度所需的热量叫比热容,简称比热。根据计量物量的单位不同,有质量比热、容积比热、摩尔比热之分。质量比热单位:kJ/kg.℃;容积比热单位:kJ/m3.℃;摩尔比热单位:kJ/mol.℃。定压比热用cp表示;定容比热用cv表示。
基本物理量与物化参数的测定
6.基本物理量与物化参数的测定?????????????????????????????? 实验88 化学反应焓变的测定 实验概述 化学反应通常是在等压条件下进行的,此时化学反应的热效应叫做等压热效应Q p。在化学热力学中,则是用反应体系焓H的变化量△H来表示的,简称为焓变。为了有一个比较的统一标准,通常规定100kPa为标准态压力,记为p 。把体系中各固体、液体物质处于p 下的纯物质,气体则在p 下表现出理想气体性质的纯气体状态称为热力学标准态。在标准状态下化学反应的焓变称为化学反应的标准焓变,用△r H 表示,下标“r”表示一般的化学反应,上标“ ”表示标准状态。在实际工作中,许多重要的数据都是在298.15 K下测定的,通常用298.15 K下的化学反应的焓变,记为△r H (298.15K)。 本实验是测定固体物质锌粉和硫酸铜溶液中的铜离子发生置换反应的化学反应焓变: Zn(s) + CuSO4(aq)═ZnSO4(aq)+ Cu(s) △r H m (298.15K)=- 217 kJ?mol-1 这个热化学方程式是表示:在标准状态、298.15 K时,发生了一个单位的反应,即1 mol的Zn与1 mol的CuSO4发生置换反应生成1 mol的ZnSO4和1 mol的Cu,此时的化学反应的焓变△r H m (298.15K)称为298.15 K时的标准摩尔焓变。其单位为kJ?mol-1。 测定化学反应热效应的仪器称为量热计。对于一般溶液反应的摩尔焓变。可用图8.1.1所示的“保温杯式”量热计来测定。 图8.8.1 简易量热计示意图 在实验中,若忽略量热计的热容,则可根据已知溶液的比热容、溶液的密度、浓度、实验中所取溶液的体积和反应过程中(反应前和反应后)溶液的温度变化,求得上述化学反应的摩尔
常见物性参数表word版本
常见物性参数表
常用溶剂 一、乙醇(ethyl alcohol,ethanol)CAS No.:64-17-5 (1)分子式 C2H6O (2)相对分子质量 46.07 (3)结构式 CH3CH2OH, (4)外观与性状:无色液体,有酒香。 (5)熔点(℃):-114.1 (6)沸点(℃):78.3 溶解性:与水混溶,可混溶于醚、氯仿、甘油等多数有机溶剂; 密度:相对密度(水=1)0.79;相对密度(空气=1)1.59; 稳定性:稳定;危险标记 7(易燃液体); 主要用途:用于制酒工业、有机合成、消毒以用作溶剂 不同压力下乙醇物性参数变化 表压液态密 度比热容气体密 度 蒸发 热 分子 量 粘度沸 点 MPa Kg/m3KJ/Kg*K Kg/m3KJ/Kg g/mol MPa*s ℃ 0.06 750.49 2.811 2.4693 830.21 46.07 0.58 90.6 5 0.04 752.35 2.790 2.1825 837.84 46.07 0.59 87 0.02 754.38 2.767 1.8917 845.99 46.07 0.61 83 常压756.65 2.742 1.5966 854.89 46.07 0.63 78.3 5 -0.02 759.50 2.711 1.2984 865.7 6 46.0 7 0.66 72. 8 -0.04 762.93 2.674 0.9936 878.32 46.07 0.6 9 65.9 -0.06 767.38 2.627 0.6806 893.85 46.07 0.74 56.8 2 -0.08 774.37 2.556 0.3559 916.51 46.07 0.83 42.4
物理化学第一章讲义
第一章气体的pVT 关系 §1.1 理想气体状态方程 §1.2 理想气体混合物 §1.3 真实气体的液化及临界参数 §1.4 真实气体状态方程 §1.5 对应状态原理及普遍化压缩因子图 教学重点及难点 教学重点1.理解理想气体模型、摩尔气体常数,掌握理想气体状态方程。 2.理解混合物的组成、理想气体状态方程对理想气体混合物的应用,掌握理想气体的分压定律和分体积定律。 3.了解气体的临界状态和气体的液化,理解液体的饱和蒸汽压。 4.了解真实气体的pV m - p图、范德华方程以及压缩因子和对应状态原理。 教学难点:1.理想气体的分压定律和分体积定律。 前言 宏观的物质可分成三种不同的聚集状态: 气态:气体则最为简单,最易用分子模型进行研究。 液态:液体的结构最复杂,对其认识还很不充分。 固态:结构较复杂,但粒子排布的规律性较强,对其研究已有了较大的进展。 当物质的量n确定后,其pVT 性质不可能同时独立取值,即三者之间存在着下式所示的函数关系:f(p,V, T)= 0也可表示为包含n在内的四变量函数式,即f(p,V,T,n)= 0这种函数关系称作状态方程。 §1-1 理想气体的状态方程 1.理想气体状态方程 (1)气体的基本实验定律: 波义尔定律:PV = 常数(n,T 恒定) 盖·吕萨克定律:V/T = 常数(n,p恒定) 阿伏加德罗定律:V/n=常数(T,p恒定) ( 2 ) 理想气体状态方程 上述三经验定律相结合,可整理得理想气体状态方程:pV=nRT (p: Pa(帕斯卡)V: m3(米3) T:K(开尔文) R(摩尔气体常数): J·mol-1·K-1(焦·摩尔-1·开-1)) 因为摩尔体积V m = V/n,气体的物质的量n=m /M 理想气体状态方程又常采用下列两种形式:p V m=RT、pV=(m/M)RT 2.理想气体模型 (1)分子间力:分为相互吸引和相互排斥,按照兰纳德一琼斯的理 论:E=E吸引+E排斥=-A r6+B r12 由图可知:
纯物质(乙醇)物性参数查询输出结果
纯物质(乙醇)物性参数查询输出结果 (2013/11/17) (1) 常规性质 中文名: 乙醇 英文名: ETHANOL CAS号: 64-17-5 化学式: C2H6O 结构简式: 所属族: 醇 分子量: 46.069 g/mol 熔点: -114.1 C 沸点: 78.29 C 临界压力: 6147.9957 kPa 临界温度: 240.77 C 临界体积: 1.67E-04 m3/mol 偏心因子: 0.645245 临界压缩因子: 0.24 偶极距: 1.69083 debye 标准焓: -234.9500096 kJ/mol 标准自由焓: -167.8499464 kJ/mol 绝对熵: 2.806401E+05 J/kmol/K 熔化焓: 未知 kJ/mol 溶解参数: 10.853 (cal/cm3)1/2 折光率: 1.35941 等张比容: 128.324 (2) 饱和蒸气压 系数(Y单位:Pa) 使用温度范围:159.05 - 513.92K A= 74.475 B= -7164.3 C= -7.327
D= 3.134E-6 E= 2 (3) 液体比热容 系数(Y单位:J/kmol/K) 使用温度范围:159.05 - 390K A= 1.02640E+5 B= -139.63 C= -0.030341 D= 0.0020386 E= 0 (4) 理想气体比热容 系数(Y单位:J/mol/K) 使用温度范围:200 - 1500K A= 49200 B= 1.45770E+5 C= 1662.8 D= 93900 E= 744.7 (5) 液体粘度 系数(Y单位:Pa·s) 使用温度范围:200 - 440K
物理化学化学专业课程目的与要求
物理化学(化学专业) (physical chemistry) 课程目的与要求 一、物理化学课程的作用和地位 物理化学是化学学科的一个重要分支,是化学专业本科生的一门主干基础课。通过本课程的学习,不仅使学生能系统地掌握物理化学的基本知识和理论基础,而且使他们受到严格的科学训练,具备应用物理化学基本原理和方法去分析和解决问题的能力,培养学生的辩证唯物主义世界观、爱国主义精神以及理论联系实际、艰苦奋斗、勇于创新的科学素质。 二、任务和要求 本课程的任务是介绍化学热力学、统计热力学、化学动力学、电化学和界面及胶体化学的基本原理、方法及应用。通过课堂讲授、自习、讨论课、演算习题、计算机辅助教学、考试等教学环节达到本课程的目的,其基本要求如下:(1)、化学热力学:掌握热力学四大定律、重要热力学公式及其物理意义和应用条件,各热力学量的计算中,掌握标准的选择和非理想体系处理的一般方法,掌握热力学函数表的应用。均相系热力学量之间的关系及转化,据以判断化学变化的方向和限度,掌握相平衡和化学平衡的基本原理及其在实际问题中的应用。了解非平衡态热力学的基本概念。 (2)、统计热力学:掌握玻尔兹曼统计的基本原理,能从微观层次理解体系的一些热力学性质,掌握从分子配分函数及自由能函数表计算简单气相反应平衡常数及理想气体与晶体的热力学函数。 (3)、化学动力学:掌握化学动力学的基本概念及化学动力学的唯象基本规律、反应速率常数、活化能的测定和计算方法,掌握反应级数的求算和反应历程推测的基本方法,初步掌握基元反应速率理论的基本内容、均相和多相催化原理、现代光化学的基本原理及了解分子反应动力学的现代进展。 (4)、电化学:掌握电解质溶液的基本概念和理论、电导及其应用,可逆电池热力学及其应用,了解电极过程动力学的基本内容及其应用,了解电化学基础研究的活跃领域。 (5)、界面及胶体化学:掌握表面热力学以及胶体体系的性质及基本规律、表面活性剂的作用及应用等。 基本内容及学时分配 绪言(1学时)
各液体燃料物理化学参数
参数物质甲醇 (methanol)乙醇(ethanol) 二甲醚 (dimethyl ether) 正丁醇 (butyl alcohol) 异丁醇 (isobutyl alcohol) 叔丁醇(tert- butyl alcohol) 乙醚(ether)正庚烷(heptane) 异庚烷 (isoheptane) 正辛烷(octane)异辛烷(isooctane) 化学式/结构式CH4O CH30H C2H6O CH3CH2OH C2H6O CH3OCH3 C4H10O CH3(CH2)3OH C4H10O (CH3)2 CH CH2OH C4H10O (CH3)3 C-OH C4H10O CH3CH2OCH2CH3 C7H16 CH3(CH2)5CH3 C7H16 CH3(CH2)3CH(CH3)2 C8H18 CH3(CH2)6CH3 C8H18 (CH3)2CHCH2C(CH3)3 分子量32.04 46.07 46.07 74.12 74.12 74.12 74.12 100.21 100.21 114.22 114.22 性质无色有酒精气 味易挥发、易 燃的液体 易燃、易挥发 的无色透明液 体 在零下23℃以 上为无色气体, 以下为无色透明 液体 易燃、易挥发 的无色透明液 体,刺激性气 味 易燃、刺激性的无 色透明液体 易燃、无色结 晶,有少量水存 在时为液体,有 樟脑气味 极易燃、易挥发的 无色透明液体 易燃、易挥发、 无色透明液体 无色、易挥发液体无色透明液体 易燃、易挥发有刺激 性气味的无色透明液 体 毒性有剧毒微毒性毒性低于乙醚低毒低毒微毒性低毒低毒低毒低毒低毒沸点(℃)64.7 78.4 -23 117.5 107 82.4 34.6 98.4 90 125.8 99.3 密度(20℃)(g/cm3)0.7912 0.78945 0.0104(气) 0.6616(液) 0.8087 0.802 0.784 0.7135 0.684 0.68 0.7024 0.6919 溶解性溶于水、多种 无机盐以及 醇、醚等多数 有机溶剂 能与水和多种 有机溶剂混溶 溶于水、醇、乙 醚 微溶于水,溶 于乙醇、醚等 多数有机溶剂 易溶于水、乙醇和 乙醚 溶于水、乙醇、 乙醚 溶于低碳醇、苯、 氯仿和油类,微溶 于水 不溶于水,溶于 乙醇、乙醚等 不溶于水,溶于乙 醇、乙醚。 不溶于水,溶于 乙醇、乙醚、 苯、丙酮等多数 有机溶剂 不溶于水,溶于 醚,易溶于醇、丙 酮、氯仿等 C P(20℃)(J/(kg·K)) 2500 2430 2240 2330 2390 3040(27℃) 1862.13 2233 2210.53 2090 C V(20℃)(J/(kg·K)) 2119 1724.0 1760.8 1790.26 热导率 (30℃)( W/(m·K)) 0.2077 0.1621 0.1331 0.1516 0.1338 0.1107 0.12980.13258 0.1225 0.12605 0.10048 粘度 (20℃)(mPa·s) 0.597 1.20 0.0855(气态) 2.948 4.11 3.1 2.95 0.409 0.384 0.542 0.53 参数物质 甲醇 (methanol) 乙醇(ethanol) 二甲醚 (dimethyl ether) 正丁醇 (butyl alcohol) 异丁醇 (isobutyl alcohol) 叔丁醇(tert- butyl alcohol) 乙醚(ether)正庚烷(heptane) 异庚烷 (isoheptane) 正辛烷(octane)异辛烷(isooctane) 活化能(KJ/mol)172.9 176.7 202.6 热值(KJ/mol)727.0 1365.5 1453 2673.2 2667.7 2630.5 2752.9 4806.6 4802.4 5518 5468.98 引燃温度(℃)450 363 350 365 426 470 160 233 280 206 417 最小着火能(mJ)21 45 0.19 最大火焰传播速度 (cm/s) 37 39 50 63(200℃) 58(200℃) 55 125(4bar,120℃) 67(150℃) 60.9(150℃) 火焰火焰呈蓝色完全燃烧发出 淡蓝色火焰; 不完全燃烧有 黄色火焰 火焰略带亮光火焰呈现蓝白色火焰呈现蓝紫色 价格(元/500ml)8~15 5~10 20~30 10~20 18~30 18~25 25~35 13~25 20~30 30~45 40~50
互联网上的物性参数查询
互联网上的物性参数查询 1 化学工程师资源主页 该站点由西弗吉尼亚大学校友Christopher M.A.Haslego维护。该主页有非常丰富的化学工程方面的内容,其中包括一些查找物性数据比较好的站点:(https://www.360docs.net/doc/249935889.html,/physinternetzz.shtml) 1.1 物性数据((https://www.360docs.net/doc/249935889.html,/data.xls) 该数据库是浏览型数据库,含有470多种纯组分的物性数据,如分子量、冰点、沸点、临界温度、临界压力、临界体积、临界压缩、无中心参数、液体密度、偶极矩、气相热容、液相热容、液体粘度、反应标准热、蒸气压、蒸发热等。 1.2 聚合物和大分子的物理性质数据库(https://www.360docs.net/doc/249935889.html,/~athas/databank/intro.html) 该数据库是浏览型数据库。含有200多种线性大分子的物性数据,如熔融温度、玻璃转换温度、热容等。该站点不仅提供物理性质,还提供一些供估计物质物理性质的软件,如PhysProps from G&P Engineering、Prode's thermoPhysical Properties Generator(PPP)等。 1.3 https://www.360docs.net/doc/249935889.html,/~jrm/thermot.html 该站点可查294种组分的热力学性质,还可以根据Peng Robinson状态方程计算纯组分或混合物的性质:包括气液相图、液体与气体密度、焓、热容、临界值、分子量等数据。 1.4 https://www.360docs.net/doc/249935889.html,/ G&P Engineering是一个软件,提供物质的28种物理性质并估算其它18种物理性质。 2 由美国国家标准技术研究院开发的数据库 2.1 标准参考数据库化学网上工具书(https://www.360docs.net/doc/249935889.html,/chemistry/) 该数据库是一种检索型数据库,检索方法非常简单,可通过化学物质名称、分子式、部分分子式、CAS登记号、结构或部分结构、离子能性质、振动与电子能、分子量和作用进行检索,可检索到的数据包括分子式、分子量、化学结构、别名、CAS登记号、气相热化学数据、凝聚相热化学数据、液态常压热容、固态常压热容、相变数据、汽化焓、升华焓、燃烧焓、燃烧熵、各种反应的热化学数据、溶解数据、气相离子能数据、气相红外光谱、质谱、紫外/可见光谱、振动/电子能及其参考文献。 2.2 美国标准技术研究所物理网上工具书(https://www.360docs.net/doc/249935889.html,/) 该站点包括物性常数、原子光谱数据、分子光谱数据、离子化数据、χ-射线、γ-射线数据、放射性计量数据、核物理数据及其它数据库。 3 化学搜索器
大学物理化学公式总结(傅献彩_南京大学第五版)
热力学第一定律 功:δW =δW e +δW f (1)膨胀功 δW e =p 外dV 膨胀功为正,压缩功为负。 (2)非膨胀功δW f =xdy 非膨胀功为广义力乘以广义位移。如δW (机械功)=fdL ,δW (电功)=EdQ ,δW (表面功)=rdA 。 热 Q :体系吸热为正,放热为负。 热力学第一定律: △U =Q —W 焓 H =U +pV 理想气体的内能和焓只是温度的单值函数。 热容 C =δQ/dT (1)等压热容:C p =δQ p /dT = (?H/?T )p (2)等容热容:C v =δQ v /dT = (?U/?T )v 常温下单原子分子:C v ,m =C v ,m t =3R/2 常温下双原子分子:C v ,m =C v ,m t +C v ,m r =5R/2 等压热容与等容热容之差: (1)任意体系 C p —C v =[p +(?U/?V )T ](?V/?T )p (2)理想气体 C p —C v =nR 理想气体绝热可逆过程方程: pV γ=常数 TV γ-1=常数 p 1-γT γ=常数 γ=C p / C v 理想气体绝热功:W =C v (T 1—T 2)=1 1 -γ(p 1V 1—p 2V 2) 理想气体多方可逆过程:W =1 nR -δ(T 1—T 2) 热机效率:η= 2 1 2T T T - 冷冻系数:β=-Q 1/W 可逆制冷机冷冻系数:β= 1 21 T T T - 焦汤系数: μJ -T =H p T ???? ????=-()p T C p H ?? 实际气体的ΔH 和ΔU : ΔU =dT T U V ??? ????+dV V U T ??? ???? ΔH =dT T H P ??? ????+dp p H T ???? ???? 化学反应的等压热效应与等容热效应的关系:Q p =Q V +ΔnRT 当反应进度 ξ=1mol 时, Δr H m =Δr U m +∑B B γRT 化学反应热效应与温度的关系:()()()dT B C T H T H 2 1 T T m p B 1m r 2m r ? ∑??,+=γ 热力学第二定律
化工主要物性参数查询网站
资源]化工主要物性参数查询网站 1 化学工程师资源主页该站点由西弗吉尼亚大学校友Christopher M.A.Haslego维护。该主页有非常丰富的化学工程方面的内容,其中包括一些查找物性数据比较好的站点:(https://www.360docs.net/doc/249935889.html,/physinternetzz.shtml)1.1物性数据((https://www.360docs.net/doc/249935889.html,/data.xls) 该数据库是浏览型数据库,含有470多种纯组分的物性数据,如分子量、冰点、沸点、临界温度、临界压力、临界体积、临界压缩、无中心参数、液体密度、偶极矩、气相热容、液相热容、液体粘度、反应标准热、蒸气压、蒸发热等。1. 2 聚合物和大分子的物理性质数据库(https://www.360docs.net/doc/249935889.html,/~athas/databank/intro.html) 该数据库是浏览型数据库。含有200多种线性大分子的物性数据,如熔融温度、玻璃转换温度、热容等。该站点不仅提供物理性质,还提供一些供估计物质物理性质的软件,如PhysProps from G&P Engineering、Prode's thermoPhysical Properties Generator(PPP)等。1. 3 https://www.360docs.net/doc/249935889.html,/~jrm/thermot.html 该站点可查294种组分的热力学性质,还可以根据Peng Robinson状态方程计算纯组分或混合物的性质:包括气液相图、液体与气体密度、焓、热容、临界值、分子量等数据。1. 4 https://www.360docs.net/doc/249935889.html,/ G&P Engineering是一个软件,提供物质的28种物理性质并估算其它18种物理性质。2 由美国国家标准技术研究院开发的数据库2.1 标准参考数据库化学网上工具书(https://www.360docs.net/doc/249935889.html,/chemistry/) 该数据库是一种检索型数据库,检索方法非常简单,可通过化学物质名称、分子式、部分分子式、CAS登记号、结构或部分结构、离子能性质、振动与电子能、分子量和作用进行检索,可检索到的数据包括分子式、分子量、化学结构、别名、CAS登记号、气相热化学数据、凝聚相热化学数据、液态常压热容、固态常压热容、相变数据、汽化焓、升华焓、燃烧焓、燃烧熵、各种反应的热化学数据、溶解数据、气相离子能数据、气相红外光谱、质谱、紫外/可见光谱、振动/电子能及其参考文献。2.2美国标准技术研究所物理网上工具书(https://www.360docs.net/doc/249935889.html,/) 该站点包括物性常数、原子光谱数据、分子光谱数据、离子化数据、χ-射线、γ-射线数据、放射性计量数据、核物理数据及其它数据库。3 化学搜索器(https://www.360docs.net/doc/249935889.html,/' target=_blank>https://www.360docs.net/doc/249935889.html,/) Chemfinder 化学搜索器是免费注册使用的数据库,是目前网上化合物性质数据最全面的资源。可通过分子式、化学物质名称、分子量或化合物的结构片段来检索,检索结果包括化合物的同义词、结构图形及物理性质,如熔点、沸点、蒸发速率、闪点、折射率、CAS登记号、比重、蒸汽密度、水溶性质及特征等。该数据库目前含有7 5 000种化合物的数据,其中包括几千种最常见化合物的详细资料。使用起来方便、简单。4sigma-aldrich手册(https://www.360docs.net/doc/249935889.html,/saws.nsf/Pages/Custom+Bulk ?EditDocument) 该数据库是一种可检索数据库,可通过产品名称、全文、分子式、CAS登记号等进行检索,检索的结果包括产品名称、登记号、分子式、分子量、贮存温度、纯度、安全数据等。5 热化学性质估计(http:/https://www.360docs.net/doc/249935889.html,/chem/TCPEE/TCPE.htm) 有机化合物热化学性质预测,通过化学物质的结构来预测,可预测到沸点、蒸汽压、临界性质、密度、液相密度、溶解参数、粘度等数据。 6 化学同义词数据库(http://129.79.137.107/cfdocs/libchem/searchu.html) 通过化学物质缩写来检索化合物全称,所检索的缩写部分自动进行左右截词。如检索PVC,则系统检索到CPVC(critical pigment volume concentration、Chlorinated Polyvinyl Chloride)、PVC(pigment volume concentration、polyvinyl chloride)、UPVC(unplasticized poly(vinyl chloride))。7加拿大环境技术中心网(https://www.360docs.net/doc/249935889.html,/cgi-win/oil-prop-cgi.exe?Pat h=\Website\river\) 该数据库是检索型数据库,包含412种原油及油品的性质,包括油来源、含水量、比重、Reid 蒸汽压、非金属含量等。8 https://www.360docs.net/doc/249935889.html,/conversn/constant.htm 该
物性参数表
常用溶剂 一、乙醇(ethyl alcohol,ethanol)CAS No.:64-17-5 (1)分子式 C2H6O (2)相对分子质量 46.07 (3)结构式 CH3CH2OH, (4)外观与性状:无色液体,有酒香。 (5)熔点(℃):-114.1 (6)沸点(℃):78.3 溶解性:与水混溶,可混溶于醚、氯仿、甘油等多数有机溶剂; 密度:相对密度(水=1)0.79;相对密度(空气=1)1.59; 稳定性:稳定;危险标记7(易燃液体); 主要用途:用于制酒工业、有机合成、消毒以用作溶剂 不同压力下乙醇物性参数变化 表压液态密 度比热容气体密 度 蒸发 热 分子 量 粘度沸 点 MPa Kg/m3KJ/Kg*K Kg/m3KJ/Kg g/mol MPa*s ℃0.06 750.49 2.811 2.4693 830.21 46.07 0.58 90.65 0.04 752.35 2.790 2.1825 837.84 46.07 0.59 87 0.02 754.38 2.767 1.8917 845.99 46.07 0.61 83 常压756.65 2.742 1.5966 854.89 46.07 0.63 78.35 -0.02 759.50 2.711 1.2984 865.76 46.07 0.66 72.8 -0.04 762.93 2.674 0.9936 878.32 46.07 0.69 65.9 -0.06 767.38 2.627 0.6806 893.85 46.07 0.74 56.82 -0.08 774.37 2.556 0.3559 916.51 46.07 0.83 42.4
纯物质(乙酸甲酯)物性参数查询输出结果
纯物质(乙酸甲酯)物性参数查询输出结果 (2011-9-16) (1) 常规性质 中文名: 乙酸甲酯 英文名: METHYL ACETATE CAS号: 79-20-9 化学式: C3H6O2 结构简式: 所属族: 乙酯 分子量: 74.0794 g/mol 熔点: 175.15 K 沸点: 330.09 K 临界压力: 4750.0045425 kPa 临界温度: 506.55 K 临界体积: 2.28E-04 m3/mol 偏心因子: 0.331255 临界压缩因子: 0.257 偶极距: 1.67884 debye 标准焓: -98.4465 kcal/mol 标准自由焓: -77.4857 kcal/mol 绝对熵: 0.3197999 kJ/mol/K 熔化焓: 未知 kcal/mol 溶解参数: 9.014 (cal/cm3)1/2 折光率: 1.3589 等张比容: 179.032 (2) 饱和蒸气压 系数(Y单位:Pa) 使用温度范围:175.15 - 506.55K A= 61.267 B= -5618.6 C= -5.6473 D= 2.108E-17 E= 6
(3) 液体热容 系数(Y单位:J/kmol/K) 使用温度范围:253.4 - 373.4K A= 61260 B= 270.9 C= 0 D= 0 E= 0 (4) 理想气体比热容 系数(Y单位:J/mol/K) 使用温度范围:298 - 1500K A= 55500 B= 1.78200E+5 C= 1260 D= 85300 E= 562 (5) 液体粘度 系数(Y单位:Pa·s) 使用温度范围:250 - 425K A= 13.557 B= -187.3 C= -3.6592 D= 0 E= 0
物理化学公式大全
物理化学公式集 热力学第一定律 功:δW=δW e+δW f (1)膨胀功δW e=p外dV 膨胀功为正,压缩功为负。 (2)非膨胀功δW f=xdy 非膨胀功为广义力乘以广义位移。如δW(机械功)=fdL,δW(电功)=EdQ,δW(表面功)=rdA。热Q:体系吸热为正,放热为负。 热力学第一定律:△U=Q—W 焓H=U+pV 理想气体的内能和焓只是温度的单值函数。 热容C=δQ/dT (1)等压热容:C p=δQ p/dT=(?H/?T)p (2)等容热容:C v=δQ v/dT=(?U/?T)v 常温下单原子分子:C v,m=C v,m t=3R/2 常温下双原子分子:C v,m=C v,m t+C v,m r=5R/2 等压热容与等容热容之差: (1)任意体系C p—C v=[p+(?U/?V)T](?V/?T)p (2)理想气体C p—C v=nR 理想气体绝热可逆过程方程: pVγ=常数TVγ-1=常数p1-γTγ=常数γ=C p/ C v 理想气体绝热功:W=C v(T1—T2)=(p1V1—p2V2) 理想气体多方可逆过程:W=(T1—T2) 热机效率:η=冷冻系数:β=-Q1/W 可逆制冷机冷冻系数:β=
焦汤系数:μJ-T==- 实际气体的ΔH和ΔU: ΔU=+ΔH=+ 化学反应的等压热效应与等容热效应的关系:Q p=Q V+ΔnRT 当反应进度ξ=1mol时,Δr H m=Δr U m+RT 化学反应热效应与温度的关系: 热力学第二定律 Clausius不等式: 熵函数的定义:dS=δQ R/T Boltzman熵定理:S=klnΩ Helmbolz自由能定义:F=U—TS Gibbs自由能定义:G=H-TS 热力学基本公式: (1)组成恒定、不作非膨胀功的封闭体系的热力学基本方程: dU=TdS-pdV dH=TdS+Vdp dF=-SdT-pdV dG=-SdT+Vdp (2)Maxwell关系: ==- (3)热容与T、S、p、V的关系: C V=T C p=T Gibbs自由能与温度的关系:Gibbs-Helmholtz公式=- 单组分体系的两相平衡: (1)Clapeyron方程式:=式中x代表vap,fus,sub。 (2)Clausius-Clapeyron方程式(两相平衡中一相为气相):= (3)外压对蒸汽压的影响:p g是在惰性气体存在总压为p e时的饱和蒸汽压。
