PbCaSn Alloys in Lead-Acid Batteries

Journal of Power Sources161(2006)
666–675
Corrosion management of PbCaSn alloys in lead-acid batteries:Effect of composition,metallographic state and voltage conditions
E.Rocca?,G.Bourguignon,J.Steinmetz
Universit′e Henri Poincar′e-Nancy I,Laboratoire de Chimie du Solide Min′e ral UMRCNRS7555,BP239,54506Vandoeuvre-Les-Nancy,France
Received15November2005;received in revised form22March2006;accepted10April2006
Available online7July2006
Abstract
Since several years,lead calcium-based alloys have supplanted lead antimony alloys as structural materials for positive grids of lead-acid batteries in many applications,especially for VRLA batteries.Nevertheless,the positive grid corrosion probably remains one of the causes of rapid and premature failure of lead-acid batteries.The objective of the present study is to present a comprehensive study of the PbCaSn alloy corrosion in function of their composition,metallographic state and voltage conditions(discharge,overcharge,?oating and cycling conditions).For that,four alloys PbCaSn x wt.%(x=0,0.6,1.2,2)were synthesized in two extreme metallurgical conditions and tested by four electrochemical lab-tests. Weight loss measurements and analyses by SEM,EPMA and XRD allowed to monitor the oxidation tests and to characterize the corrosion layers after the oxidation tests.
The results show that the tin level in PbCaSn alloys should be adapted on the calcium concentration and the rate of overageing process,to maintain the bene?cial effect of tin in service during the battery lifetime.According to our results,a Sn/Ca ratio of2.5gives good corrosion resistance in all potential conditions.Nevertheless,when tin level is too high,the corrosion layers can peel off from the metal,which involves a lack of cohesion between the collector and the paste,in cycling conditions.
The anodic potential undergone by the metal is a second main factor determining the corrosion,especially the?oating conditions and the frequency of deep discharge and overcharge.Thus the adjustment of the charge controller parameters of a battery system is a necessity to increase the lifetime of the grids and maintain a good rechargeability.
?2006Elsevier B.V.All rights reserved.
Keywords:Corrosion;Lead-acid batteries;Lead alloys
1.Introduction
Since10years,several ageing processes of lead-acid batter-ies leading to a loss of capacity were identi?ed and reviewed [1–4].Three main kinds of premature capacity loss are now accepted.The?rst one,named PCL-1,results in an increase of the resistance between the positive active mass and the metallic grid,and is general due to the growth of an insulate corrosion layer on the grid.The second capacity loss,named PCL-2,is the result of a swelling and a loss of cohesion of positive active mass,due to the growth of coarse PbSO4crystals during the charge/discharge cycles.More recently,a third mechanism of capacity loss especially in VRLA batteries was mentioned.It is ?Corresponding author.Tel.:+33383684668;fax:+33383684611.
E-mail address:emmanuel.rocca@lcsm.uhp-nancy.fr(E.Rocca).the consequence of the oxygen cycle and is named PCL-3.In fact,the electrochemical reduction of a large quantity of oxygen on the negative plates depolarizes the negative electrode,and may lead to a possible degradation by an irreversible sulfatation process.
The current collector in lead alloys is the strong advantage but also the weak point of lead-acid batteries.Indeed lead alloys assure a good chemical continuity between the lead oxide active mass and the collector responsible for the good adherence of the active mass.Nevertheless lead alloys are subjected to corrosion phenomenon in sulfuric acid.
During the past10years,the development of lead calcium-based alloys have produced an important improvement of lead-acid batteries leading to use new grid manufacturing processes such as continuous casting,rolling,expanding of the alloys,and manufacturing of low-maintenance batteries such as VRLA bat-teries[5,6].
0378-7753/$–see front matter?2006Elsevier B.V.All rights reserved. doi:10.1016/j.jpowsour.2006.04.140
E.Rocca et al./Journal of Power Sources161(2006)666–675667
Nowadays,lead calcium-based alloys have replaced lead anti-mony alloys as structural materials for positive grids of lead-acid batteries in many applications.Nevertheless,the positive grid corrosion probably remains one of the causes of rapid and pre-mature failure of lead-acid battery,especially for the automotive batteries and stand-by applications,as been reported by many studies[2–7].
Two main types of PbCaSn alloy corrosion were generally reported in the literature[8,9]:
?The growth of a PbO layer between the alloy and the active mass in the deep discharge conditions.Because of the poor electric conductivity of PbO,the recharge of the active mass becomes very dif?cult and almost impossible in some cases.?The oxidation of lead into PbO2at high anodic potential.This kind of corrosion provokes the irreversible consummation of metal by forming large pits then can induce the mechanical break of the grids.This phenomenon occurs in overcharge conditions especially during the charge at high current called sometimes“boost charge”.
So,the parameters of the charge controller which regulates the?oating conditions,the depth of discharge and the charge (and“boost charge”)of batteries in service seems to be one of the main factors in?uencing the lifetime of the grid.The com-position of lead–calcium–tin alloys is also the subject of many questions and studies in the literature.The incorporation of tin is now accepted as a solution to reduce the corrosion problems in positive grids.However,according to the Prengaman’s observa-tions,a very corrosion-resistant alloy for the positive grid may be a disadvantage for the battery service[10].
So,for a same set of alloys,the purpose of this work was to obtain an understanding description of the corrosion behavior of PbCaSn alloys at several anodic potential simulating differ-ent batteries conditions:the deep discharge,the overcharge,the ?oating conditions and a typical charge–discharge cycle of a lead-acid battery.This corrosion behavior was studied in func-tion of tin content and metallurgical state of alloys.In fact,the hardness of PbCaSn alloys increases with time after casting, leading?rst to the aged metallurgical state,through the contin-uous precipitation in the lead matrix of very?ne intermetallic phase,(Pb1?x Sn x)3Ca.With time,these metallurgical transfor-mations of ageing are followed by a discontinuous precipitation, which provokes a large decrease of the alloy hardness.This last metallurgical state called overageing results on a lamellar and coarse precipitation of the(Pb1?x Sn x)3Ca phases[11].
For this study,PbCaSn x wt.%(x=0,0.6,1.2,2)alloys were cast in the same conditions and stabilized with different thermal treatments in a given metallurgical state.Electrochemical mea-surements allowed monitoring the oxidation tests.Then optical microscopy(OM),scanning electron microscopy(SEM),elec-tron probe microanalysis(EPMA)and X-ray diffraction(XRD) were used to characterize the corrosion layers.
2.Experimental
2.1.Synthesis of alloys
The Pb–Ca0.08wt.%–Sn x wt.%alloys(with x=0,0.6,1.2, 2)were synthesised from Pb–Ca0.12wt.%master alloy pro-duced by CEAC(Compagnie Europ′e enne des Accumulateurs), pure lead(99.95%GoodFellow)and pure tin(99.99%Prolabo). The raw materials were melted in alumina crucibles for1h at 650?C under nitrogen?ux,and casting in an iced copper mould to form plates with a size of60mm×60mm×3mm.After casting,the composition of each alloy was veri?ed by atomic absorption spectroscopy once it was chemically dissolved.
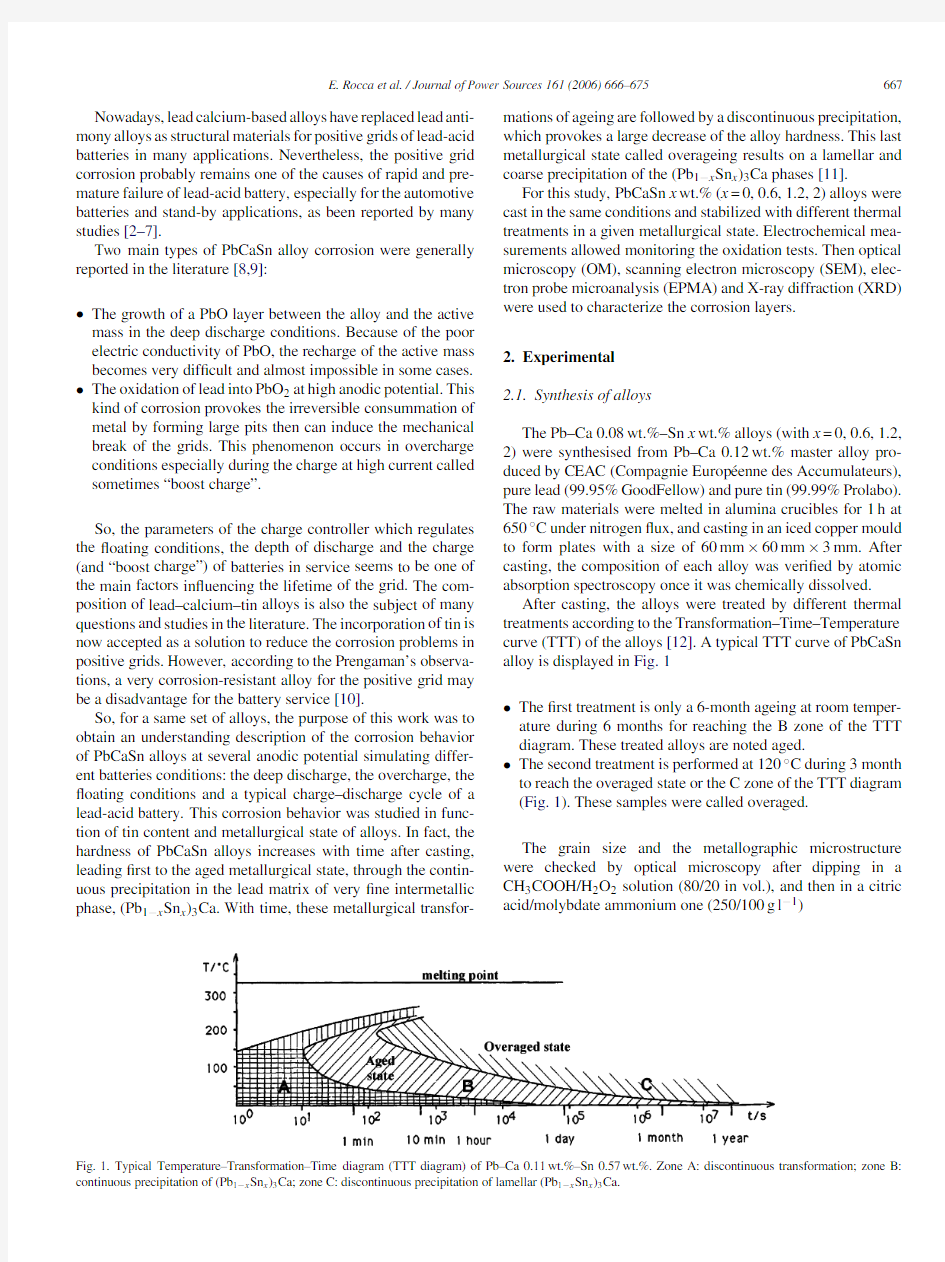
After casting,the alloys were treated by different thermal treatments according to the Transformation–Time–Temperature curve(TTT)of the alloys[12].A typical TTT curve of PbCaSn alloy is displayed in Fig.1
?The?rst treatment is only a6-month ageing at room temper-ature during6months for reaching the B zone of the TTT diagram.These treated alloys are noted aged.
?The second treatment is performed at120?C during3month to reach the overaged state or the C zone of the TTT diagram (Fig.1).These samples were called overaged.
The grain size and the metallographic microstructure were checked by optical microscopy after dipping in a CH3COOH/H2O2solution(80/20in vol.),and then in a citric acid/molybdate ammonium one(250/100g l?1
)
Fig.1.Typical Temperature–Transformation–Time diagram(TTT diagram)of Pb–Ca0.11wt.%–Sn0.57wt.%.Zone A:discontinuous transformation;zone B: continuous precipitation of(Pb1?x Sn x)3Ca;zone C:discontinuous precipitation of lamellar(Pb1?x Sn x)3Ca.
668 E.Rocca et al./Journal of Power Sources161(2006)666–675 Table1
Alloy description(grain size,type of heat treatment and Vickers hardness after
heat treatment)
Pb–Ca0.08wt.%–Sn x wt.%(wt.%)Grain size
(?m)
Heat treatment Hardness
(Hv)
0100±10Aged,overaged13,7
0.6120±10Aged,overaged16,8
1.2140±15Aged,overaged17,8 2120±15Aged,overaged18,11
2.2.Oxidation tests
Electrochemical tests were performed in a three-electrode electrochemical cell connected to an EG&G Princeton273 A potentiostat driven by a computer.The lead alloys used as working electrode(2.8cm2)was a disk of3cm of diameter.It was placed horizontally at the bottom of the cell under a Pt-grid(50cm2)used as auxiliary electrode.The distance between working and auxiliary electrode was2cm.Only one side of the working electrode was in contact with electrolyte.The amount of the electrolyte was70ml.The reference electrode was a K2SO4-saturated mercurous sulfate electrode(E=+0.658V ver-sus SHE),and all working electrode potentials are given versus this reference electrode.
The working electrode was mechanically polished with suc-cessively?ner grades of SiC emery papers up to1200,then with colloidal silica dispersed in water(particle size:0.1?m).Sam-ples were?nally rinsed with distilled water and ethanol then dried.
Four oxidations tests were set up according the battery man-ufacturers and literature reporting potential measurements of positive grid in batteries in service.These tests are described below:
?The discharge tests:The?rst test was in deep discharge conditions at+0.7V for7days in0.5M H2SO4at room tem-perature.The second one is close to the conditions of usual discharge state,at+0.9V for7days in0.5M H2SO4.?The overcharge test:+1.5V for5days in5M H2SO4at50?C.?The?oating test:+1.1V for7days in0.5M H2SO4at room temperature.
?The cycling test:A cycle is composed of2h of overcharge at1.5V,a potential decrease at0.2mV s?1until0.7V,2h of deep discharge time at0.7V,?nally a potential increase
until Fig.2.OM micrograph of PbCaSn0.6wt.%(a and b)and PbCaSn2wt.%(c and d)in metallurgical aged state(a and c)and overaged state(b and d).
E.Rocca et al./Journal of Power Sources 161(2006)666–675669
1.5V .This test goes on for 20cycles,or 5days in 5M H 2SO 4at room temperature.
In these conditions of tests,no strati?cation and density change of H 2SO 4electrolyte was observed.In addition,in over-charge conditions (high voltage),the volume of gas produced by the working and auxiliary electrodes was measured.The oxygen volume was deduced by calculation of the hydrogen volume by assuming that the total current measured between the two elec-trodes was used for the reduction of water in hydrogen gas on the Pt-grid auxiliary electrode.2.3.Corrosion layer analysis
After the oxidation tests,the samples were embedded in epoxy resin and the metallographic cross-sections were polished in the same way as the working electrode.
The XRD and SEM techniques (Hitachi S-2500equipped with a EDS detector)was applied to determine the morphology and the composition of the corrosion layers.EPMA (Cameca SX-50apparatus)analysis was used to precise the compositions in some cases.For the overcharge test,the corrosion of the alloys was characterized through metallic weight loss measurements.The sample weight was measured before oxidation and after dissolution of the corrosion products after test in acetic acid,hydrazine,water solution (1/3,1/3,1/3in vol.).3.Results
3.1.Characterization of alloys
The cross-section of each alloy was observed after metal-lographic chemical attack.The grain sizes,listed in Table 1,are almost similar for the entire alloy,around 120?m (Fig.2).Fig.2(a)and (b)show typical micrographs of aged state and complete overaged state for the PbCaSn 0.6wt.%alloy.On Fig.2(b)(overaged state),the dissolution of the coarse precip-itates during the metallographic etching induces an important roughness,and partially masks the grain boundaries.These two different metallurgical states are also characterized by
different
Fig.3.i =f (t )curves during the oxidation of aged PbCaSn alloys in overcharged conditions (+1.5V ,5M H 2SO 4,50?
C).
Fig.4.(a)Weight loss and (b)oxygen gas evolution of PbCaSn alloys after 5days of oxidation in overcharged conditions (+1.5V ,5M H 2SO 4,50?
C).
Fig.5.Typical metallographic cross section of PbCaSn alloys after 5days of oxidation in overcharged conditions (+1.5V ,5M H 2SO 4,50?C).
670 E.Rocca et al./Journal of Power Sources 161(2006)
666–675
Fig.6.SEM observations of pickled surface:PbCaSn 0wt.%(a)and PbCaSn 2wt.%(b)after the overcharged test of metallic surfaces.
hardness values as mentioned in Table 1.The aged alloys have the highest Vickers value due to the precipitation of a nano-metric (Pb 1?x Sn)3Ca intermetallic phase in the ?-lead matrix through a continuous transformation.In opposite,the hardness of the overaged alloys is close to that of pure lead because of the coalescence of the ?ne precipitates to form coarse and less hardening (Pb 1?x Sn)3Ca precipitates through a discontinuous precipitation.On the micrograph of the PbCaSn 2wt.%overaged alloy,we can observe that the grains are only partially overaged even after several months of treatment at 120?C (Fig.2(d)).This observation explains that the hardness value of overaged PbCaSn 2wt.%remains high.As observed by several authors in the literature,the presence of high content of tin inhibits the overageing process of PbCa alloys [12,13].3.2.Overcharge tests
At +1.5V in 5M H 2SO 4,the current recorded during the overcharge test for the set of the aged alloys is almost constant
with time,as observed in Fig.3.The measurements for the over-aged alloys show the same behavior.In all cases,presence of tin leads to a decrease of the oxidation current,which is correlated to a decrease of the weight loss of alloys due to corrosion and a decrease of the oxygen evolution,as displayed in Fig.4(a)and (b).According to measurements of the oxygen gas evolution,weight loss and current,the calculation of the faradic rate of each process (oxygen release and corrosion)shows that 99.5–99.9%of the oxidation current is due to the water oxidation to oxygen.According to Fig.4,the presence of tin increases the oxygen evolution overvoltage of the alloys.
After the overcharge test,SEM analyses of the cross sections of all alloys present a thick corrosion layer mainly constituted by ?-PbO 2and a deep intergranular corrosion (Fig.5).As shown in Fig.6,after removing of the corrosion products,both intergran-ular and intragranular corrosions are important;nevertheless,the intragranular corrosion is markedly reduced in presence of tin,which explains the lower weight loss of PbCaSn 2wt.%in comparison to PbCaSn 0and 0.6
wt.%.
Fig.7.Typical SEM micrographs on PbCaSn 0.6wt.%(a)and PbCaSn 2wt.%after oxidation during 7days in deep discharge conditions (+0.7V ,H 2SO 40.5M,room temperature).
E.Rocca et al./Journal of Power Sources161(2006)666–675
671
Fig.8.Thickness of PbO layer measured on PbCaSn alloys after oxidation for 7days in deep discharge conditions(+0.7V,H2SO40.5M,room temperature).3.3.Discharge and deep discharge tests
In conditions of deep discharge at+0.7V,all the PbCaSn
alloys are covered with a duplex corrosion layer constituted with ?-PbO and lead sulfate PbSO4,except the most tin-concentrated alloy,PbCaSn2wt.%,for which only PbSO4is detected as cor-
rosion product(Fig.7(a)and(b)).
After7days of oxidation,the curves of PbO thickness versus
tin content in PbCaSn alloys exhibit a usual bell-shape reported
also by a previous study for1day of oxidation(Fig.8)[14].This
behavior is similar for the two series of alloys with a higher max-
imum for the overaged one.No PbO is present on the PbCaSn
2wt.%,whatever the metallurgical state after7days of deep
discharge test.
The aged alloys were also oxidized at a higher potential,
+0.9V,which is representative of the normal discharge of the
lead-acid batteries.After7days of oxidation,the SEM and
EPMA analysis of the low tin-concentrated alloys cross-sections
reveal a complex corrosion layer,PbO/PbO x/PbSO4,with a
thick Fig.9.Metallographic cross-sections of aged PbCaSn alloys after oxidation during7days in modi?ed deep discharge conditions(+0.9V,H2SO40.5M,room temperature).(a)PbCaSn0.6wt.%;(b)EPMA analysis of the corrosion layer of PbCaSn0.6wt.%;(c)PbCaSn2wt.%.
672 E.Rocca et al./Journal of Power Sources 161(2006)
666–675
Fig.10.Typical observations of PbCaSn alloys after oxidation in ?oating conditions (+1.1V ,5M H 2SO 4,room temperature):(a)metallographic cross-section and (b)surface.
layer of ?-PbO,as observed in Fig.9(a)and (b).In contrast,the oxidation of a PbCaSn 2wt.%leads to a thinner layer,having only a duplex PbO 2/PbSO 4structure (Fig.9(c)).3.4.Floating conditions
After an oxidation at +1.1V in 5M H 2SO 4at room temper-ature for 5days,all the PbCaSn tested alloys display the same kind of corrosion layer.A typical example is displayed in Fig.10.The measured oxidation current is particularly very low,and the corrosion layer is very thin,below 1–2?m.It is mainly consti-tuted by ?-PbO 2containing a small part of ?-PbO 2according to XRD analysis.These observations are consistent with the work of some authors claiming that the corrosion rate of lead in sulfu-ric acid presents a minimum for oxidation potentials in a range of +1to +1.3V [15,4].3.5.Cycling conditions
Only the aged alloys were tested in cycling conditions.The potential cycle and a typical current transient are displayed in Fig.11.In these conditions of tests,no signi?cant change of charge during the successive cycles was observed in func-tion of time.The current peaks observed during the increase and decrease of potential corresponds to the classical oxida-tion and reduction peaks recorded on a lead alloys voltampero-gram [16,14].Fig.12shows the metallographic cross-sections observed after the cycling test in function of the tin content in the alloys.The overall corrosion layer thickness decreases with the increase of the tin content in alloys.The cross sections,dis-played in Fig.12(a)and (b)for PbCaSn 0and 0.6wt.%,present a corrosion layer with a lamellar structure formed by successive layers of PbO and PbO 2.For the low tin content alloys,the corro-sion layers are thick (around 20–30?m),compact and adhesive
to the metal.The tests are stopped after the deep discharge step which explains presence of a thick PbO layer at the interface metal/corrosion layer.In contrast,on the high tin content alloys (1.2and 2wt.%),the corrosion layers are thinner,contain no visible PbO layer,and seem to be more homogenous;but they are more brittle than that growing on low tin content alloys and easily peeled off from the metal (Fig.12(c)and (d)).4.Discussion
For increasing the speci?c energy of the lead-acid batteries,the reduction of the inactive material in the plate can be reached by the choice of a corrosion-resistant alloy to manufacture the current collector and the mechanical holder for the active mass.However,the control of the corrosion phenomenon is essential to design the grid and to increase its lifetime.
Through the results of this study,the management of the cor-rosion phenomenon of PbCaSn alloys depends on three main factors:the metallurgical state of the alloys,their composition especially the tin level and the electrochemical potential of the
metal.
Fig.11.Potential cycle E =f (t )of the cycling test (a)and typical current transient curve i =f (t )recorded during the cycling test (b).
E.Rocca et al./Journal of Power Sources161(2006)666–675
673
Fig.12.Metallographic cross-sections of aged PbCaSn alloys after the cycling test:(a)0wt.%;(b)0.6wt.%;(c)1.2wt.%;(d)2wt.%.
4.1.Effect of metallurgical state
Lead metal has a low melting point(T f=600K),which means that the diffusion of alloying elements can occur at room tem-perature and provokes many changes in the properties of alloys with time.The change in the mechanical properties of PbCaSn alloys due to the ageing were studied and characterized by many authors[17,11].Figs.4and6demonstrate also that the ageing of PbCaSn alloys can have a strong in?uence on the corrosion rate in overcharge conditions.The weight loss and the oxygen evo-lution of the overaged alloys are almost systematically higher than that of aged alloys.As shown by a previous study[13],the overageing process cause an increase of tin content in precipi-tates and a decrease of tin content in the lead matrix,so the bad corrosion resistance of the overaged alloys is closely related to a low tin content in lead matrix all along the overageing pro-cess.Another factor responsible for the high corrosion rate in overcharge conditions is the O2gas evolution.In lead alloys,the addition of calcium drastically decreases the overpotential of the O2evolution on lead;nevertheless,the addition of tin increases it[18,19].So,the reducing of the tin content in the lead matrix in the overageing process involves a decrease of the O2evolution overpotential and an increase of the mechanical action of the O2 evolution on the corrosion layer.
Thus,the overageing process has a double negative action on the corrosion behavior of PbCaSn alloys in overcharge:decrease of the tin content in matrix weakens its corrosion resistance and increase of the O2evolution induces a higher peeling of the corrosion layer.
In deep discharge conditions,the aged alloys have a lower PbO thickness than that measured on overaged alloys.Previous study has proved that the tin content determines the PbO growth rate.A low content(<1%)accelerates the lead by enhancing the ionic transport through a doping in the oxide lattice(Pb II/Sn IV)
674 E.Rocca et al./Journal of Power Sources161(2006)666–675
[14].Whereas at higher content in the metal(>1.5–2wt.%),tin oxide precipitates in PbO layer,limits the oxygen diffusion and inhibits the PbO growth.So in PbCaSn alloys,our results show that when a large part of tin is trapped in the intermetallic phases in the overaged state,its doping effect on the PbO growth lowers. In the high tin PbCaSn alloys(>1.5wt.%),the tin level in the lead matrix is suf?cient to completely inhibit the PbO growth whatever the metallurgical state.
4.2.Tin effect
In practice,many authors report that addition of tin has a ben-e?cial effect on the corrosion behavior of the grids in batteries. Our results show that tin allows reducing the metal consumma-tion in overcharge conditions by a decrease of the O2evolution, and inhibits the PbO growth responsible of the battery internal resistance increase in discharge conditions.
Nevertheless the tin effect is not only determined by its con-centration in the alloys.We have to consider also its availability in the alloys.Indeed,when tin is trapped in large(Pb1?x Sn x)3Ca precipitates during the overageing process,tin is generally less ef?cient.So the limit of tin level for a positive effect on the cor-rosion behavior effect depends on the calcium level in alloy and the rate of the overageing reaction.Consequently,the effect of a given tin content is closely related to the metallurgical state of alloys.
Even in cycling conditions,the corrosion layer thickness is related to the tin content.Higher the tin content,lower the lead alloy corrosion,as we can see on Fig.12.Unfortunately,the interface metal/corrosion layer formed on high tin content alloy is very brittle so the corrosion layer may easily peel off.This lab-observation can be correlated by the remarks of some authors who sometimes observe a loss of cohesion between high tin content grid and positive active mass in the positive plates[10]. This loss of cohesion should be due to loss of adherence of the corrosion layer in some cases on high tin level alloys,because of the absence of the PbO layer acting as a self adhesive compound between lead metal and PbO2.
4.3.Effect of anodic potential
Through this study,we can distinguish four potential zones for the positive grid,with four corrosion behaviors.The?rst is located at low potentials below+0.7to+0.8V and is the typical zone of deep discharge.At these potentials,the corrosion rate is low,but the growth of the insulating corrosion layer quickly increases the battery internal resistance.Previous studies have proved that the PbO growth kinetic is almost linear with time [14,20].
The second potential zone around+0.9,+1V leads to the formation of complex and thick corrosion layer Pb/PbO/PbO x/PbO2where the PbO x sub-layer seems to be a melt of PbO and PbO2.In this potential zone,the corrosion is more important than that in the?rst one and the risk of increase of internal resistance is also important.
The third potential range is limited around+1.3,+1.4V and is representative of charge state or?oating conditions of batteries. The measured oxidation current is very low and the corrosion rate is also very low,which was reported by some authors[15]. In this potential range,only the formation PbO2layer takes place without the water oxidation into O2.The PbO2growth is par-ticularly slow and characterized by a logarithmic kinetic law, which was shown by previous in situ ellipsometric study[21].
The fourth potential zone corresponds to the overcharge conditions behind+1.5V,and is characterized by an impor-tant intergranular corrosion and oxygen release.The oxidation of water to oxygen has a predominant mechanical effect and tends to break and remove the PbO2layer.The high corro-sion rate in this potential zone can be explained by a cycle growth/breaking/peeling/growth of the corrosion layer.
This Fig.13.Schematic mechanism corrosion layer growth on lead alloys in function of potential.
E.Rocca et al./Journal of Power Sources161(2006)666–675675
mechanism provokes a rapid consummation of the metal until
the mechanical breaking of the grid bars in some cases.
In all the potential range,the mechanism of lead oxidation
seems to be governed by the oxygen(O2?)diffusion in the oxide,
as displayed on Fig.13.At low potential(+0.7,+0.8V),the
PbO growth is controlled by the O2?diffusion in the lamel-
lar crystallographic structure of?-PbO,as shown by18O-tracer
experiments[20].At higher potential(+0.9,+1V),the O2?
diffusion increases until the saturation of the lead monoxide
at the interface PbO/electrolyte and leads to the formation of
PbO2/PbO x.At+1.3V,the O2?concentration is so high that ?-PbO2is directly formed on the surface and blocks the O2?diffusion because of its compact rutile crystallographic struc-
ture[22].Then at very high potential,the mechanical effect of
the oxygen release is the main responsible of the peeling of the
PbO2layer and explains the continuous consummation of the
metal by a continuous formation of PbO2.
5.Conclusions
The corrosion management of the positive grids in PbCaSn
alloys should take two main factors into account:the composi-
tion of the alloy and the electric cycles undergone by the lead
batteries.For many applications,a tin level of1.2–1.6wt.%
seems to be a good compromise.This tin concentration allows
the regulation of the O2?diffusion in the corrosion layer espe-
cially by limiting the PbO growth,and reduces the O2release.
Thus,it reduces the mechanical effect of O2bubbles which is the
main cause of the continuous PbO2growth in overcharge con-
ditions.Nevertheless to maintain the bene?cial effect of tin in
service during the battery lifetime,the tin level in alloys should
be adapted to the calcium level and the rate of the overageing
process.In fact the ef?ciency of tin addition is closely related
to the metallurgical state of the alloys.According to our results,
a Sn/Ca ratio of2.5gives the best corrosion resistance in many
cases.Nevertheless,when tin level is too high,the corrosion
layers can peel off the metal,which involves a lack of cohesion
between the collector and the paste in cycling conditions.
The anodic potential is a second main factor determining
the alloy corrosion.In service,a too high?oating current can
maintain the positive alloy grid at a high potential and induces
an irreversible corrosion of the positive grids.The same phe-
nomenon occurs when the“boost charges”are too frequent and
not closely controlled.In discharge conditions,the cycling at
low state-of-charge,for the photovoltaic applications,provokes
the formation of a PbO/PbO x layer by maintaining a low anodic potential of the positive grids.Thus the adjustment of the param-eters of the charge controller of a batteries system is a necessity for increasing the lifetime of the grids and for maintaining a good rechargeability.
The challenge for the development of corrosion-resistant grid alloys is a low corrosion rate and an adherent corrosion layer in all the batteries conditions.If additions of tin are nowadays essential,addition of other elements seems to be necessary to improve the adhesion of the oxide of low corrosion rates alloys. These four lab-tests developed in this article may be good tools to test future alloys compositions.
Acknowledgement
This study was?nancially supported by french governemen-tal organization ADEME(Convention N?9905057). References
[1]A.F.Hollenkamp,J.Power Sources36(1991)567.
[2]R.D.Prengaman,JOM(2001)36.
[3]A.Cooper,P.T.Moseley,J.Power Sources113(2003)200.
[4]P.Ruetschi,J.Power Sources127(2004)33.
[5]R.D.Prengaman,J.Power Sources67(1997)267.
[6]N.Y.Tang,E.M.L.Valeriote,J.Sklarchuk,J.Power Sources59(1996)63.
[7]R.J.Ball,R.Kurian,R.Evans,R.Stevens,J.Power Sources109(2002)
189.
[8]K.R.Bullock,M.A.Butler,J.Electrochem.Soc.133(1986)1085.
[9]R.Miraglio,R.Albert,A.El Ghachcam,J.Steinmetz,J.P.Hilger,J.Power
Sources53(1995)53.
[10]R.D.Prengaman,J.Power Sources95(2001)224.
[11]L.Bouirden,J.P.Hilger,J.Hertz,J.Power Sources33(1991)27.
[12]J.P.Hilger,L.Bouirden,J.Power Sources J.Alloys Compd.236(1996)
224.
[13]A.Maitre,G.Bourguignon,J.M.Fiorani,J.Steinmetz,J.Ghanbaja,P.
Lailler,Mater.Sci.Eng.A A340(2002)103.
[14]E.Rocca,J.Steinmetz,Electrochim.Acta44(1999)4611.
[15]P.Ruetschi,R.T.Angstadt,J.Electrochem.Soc.111(1964)1323.
[16]Y.Yamamoto,K.Fumino,T.Ueda,M.Namlbu,Electrochim.Acta37
(1992)199.
[17]H.Borchers,H.Assmann,Metallurgia(Isernberg,Germany)33(9)(1979)
936.
[18]B.Monahov,D.Pavlov,D.Petrov,Proceedings of the14th Annual Battery
Conference on Applications and Advances,Long Bead,CA,USA,1999, pp.275–279.
[19]https://www.360docs.net/doc/dc16862733.html,m,J.D.Douglas,R.Pillig,D.A.J.Rand,J.Power Sources48(1994)
219.
[20]E.Rocca,J.Steinmetz,S.Weber,J.Electrochem.Soc.146(1999)54.
[21]N.Stein,G.Bourguignon,L.Raboin,L.Broch,L.Johann,E.Rocca,Thin
Solid Films455/456(2004)735–741.
[22]J.Leciejewiec,I.Padlo,Naturwissenschaften49(1962)373.
小学joinin剑桥英语单词汇总
JOIN IN 学生用书1 Word List Starter Unit 1.Good afternoon 下午好 2.Good evening 晚上好 3.Good morning 早上好 4.Good night 晚安 5.Stand 站立 Unit 1 6.count [kaunt] (依次)点数 7.javascript:;eight [eit] 八 8.eleven [i'levn] 十一 9.four [f?:] 四 10.five [faiv] 五 11.flag [fl?g] 旗 12.guess [ges] 猜 13.jump [d??mp] 跳 14.nine [nain] 九 15.number ['n?mb?] 数字 16.one [w?n] 一 17.seven ['sevn] 七 18.six [siks] 六 19.ten [ten] 十 20.three [θri:] 三 21.twelve [twelv] 十二 22.two [tu:] 二 23.your [ju?] 你的 24.zero ['zi?r?u] 零、你们的 Unit 2 25.black [bl?k] 黑色26.blue [blu:] 蓝色 27.car [kɑ:] 小汽车 28.colour ['k?l?] 颜色 29.door [d?:] 门 30.favourite [feiv?rit]javascript:; 特别喜爱的 31.green [gri:n] 绿色 32.jeep [d?i:p] 吉普车 33.orange ['?:rind?] 橙黄色 34.pin k [pi?k] 粉红色 35.please [pli:z] 请 36.purple ['p?:pl] 紫色 37.red [red] 红色 38.white [wait] 白色 39.yellow ['jel?u] 黄色 Unit 3 40.blackboard ['bl?kb?:d] 黑板 41.book [buk] 书 42.chair [t???] 椅子 43.desk [desk] 桌子 44.pen [pen] 钢笔 45.pencil ['pensl] 铅笔 46.pencil case [keis] 笔盒 47.ruler ['ru:l?] 尺、直尺 48.schoolbag [sku:l] 书包 49.tree [tri:] 树 50.window ['wind?u] 窗、窗口 Unit 4 51.brown [braun] 棕色 52.cat [k?t] 猫
joinin剑桥小学英语
Join In剑桥小学英语(改编版)入门阶段Unit 1Hello,hello第1单元嗨,嗨 and mime. 1 听,模仿 Stand up Say 'hello' Slap hands Sit down 站起来说"嗨" 拍手坐下来 Good. Let's do up Say 'hello' Slap hands Sit down 好. 我们来再做一遍.站起来说"嗨"拍手坐下来 the pictures. 2 再听一遍给图画编号. up "hello" hands down 1 站起来 2 说"嗨" 3 拍手 4 坐下来说 3. A ,what's yourname 3 一首歌嗨,你叫什么名字 Hello. , what's yourname Hello. Hello. 嗨. 嗨. 嗨, 你叫什么名字嗨,嗨. Hello, what's yourname I'm Toby. I'm Toby. Hello,hello,hello.嗨, 你叫什么名字我叫托比. 我叫托比 . 嗨,嗨,嗨. I'm Toby. I'm Toby. Hello,hello, let's go! 我是托比. 我是托比. 嗨,嗨, 我们一起! Hello. , what's yourname I'm 'm Toby. 嗨.嗨.嗨, 你叫什么名字我叫托比.我叫托比. Hello,hello,hello. I'm 'm Toby. Hello,hello,let's go! 嗨,嗨,嗨.我是托比. 我是托比. 嗨,嗨,我们一起! 4 Listen and stick 4 听和指 What's your name I'm Bob. 你叫什么名字我叫鲍勃. What's your name I'm Rita. What's your name I'm Nick. 你叫什么名字我叫丽塔. 你叫什么名字我叫尼克. What's your name I'm Lisa. 你叫什么名字我叫利萨. 5. A story-Pit'sskateboard. 5 一个故事-彼德的滑板. Pa:Hello,Pit. Pa:好,彼德. Pi:Hello,:What's this Pi:嗨,帕特.Pa:这是什么 Pi:My new :Look!Pi:Goodbye,Pat! Pi:这是我的新滑板.Pi:看!Pi:再见,帕特! Pa:Bye-bye,Pit!Pi:Help!Help!pi:Bye-bye,skateboard! Pa:再见,彼德!Pi:救命!救命!Pi:再见,滑板! Unit 16. Let's learnand act 第1单元6 我们来边学边表演.
Join In剑桥小学英语.doc
Join In剑桥小学英语(改编版)入门阶段 Unit 1Hello,hello第1单元嗨,嗨 1.Listen and mime. 1 听,模仿 Stand up Say 'hello' Slap hands Sit down 站起来说"嗨" 拍手坐下来 Good. Let's do itagain.Stand up Say 'hello' Slap hands Sit down 好. 我们来再做一遍.站起来说"嗨"拍手坐下来 2.listen again.Number the pictures. 2 再听一遍给图画编号. 1.Stand up 2.Say "hello" 3.Slap hands 4.Sit down 1 站起来 2 说"嗨" 3 拍手 4 坐下来说 3. A song.Hello,what's yourname? 3 一首歌嗨,你叫什么名字? Hello. Hello.Hello, what's yourname? Hello. Hello. 嗨. 嗨. 嗨, 你叫什么名字? 嗨,嗨. Hello, what's yourname? I'm Toby. I'm Toby. Hello,hello,hello. 嗨, 你叫什么名字? 我叫托比. 我叫托比 . 嗨,嗨,嗨. I'm Toby. I'm Toby. Hello,hello, let's go! 我是托比. 我是托比. 嗨,嗨, 我们一起! Hello. Hello.Hello, what's yourname? I'm Toby.I'm Toby. 嗨.嗨.嗨, 你叫什么名字? 我叫托比.我叫托比. Hello,hello,hello. I'm Toby.I'm Toby. Hello,hello,let's go! 嗨,嗨,嗨.我是托比. 我是托比. 嗨,嗨,我们一起! 4 Listen and stick 4 听和指 What's your name? I'm Bob. 你叫什么名字? 我叫鲍勃. What's your name ? I'm Rita. What's your name ? I'm Nick. 你叫什么名字? 我叫丽塔. 你叫什么名字? 我叫尼克. What's your name ? I'm Lisa. 你叫什么名字? 我叫利萨. 5. A story-Pit'sskateboard. 5 一个故事-彼德的滑板. Pa:Hello,Pit. Pa:好,彼德. Pi:Hello,Pat.Pa:What's this? Pi:嗨,帕特.Pa:这是什么? Pi:My new skateboard.Pi:Look!Pi:Goodbye,Pat! Pi:这是我的新滑板.Pi:看!Pi:再见,帕特! Pa:Bye-bye,Pit!Pi:Help!Help!pi:Bye-bye,skateboard! Pa:再见,彼德!Pi:救命!救命!Pi:再见,滑板! Unit 16. Let's learnand act 第1单元6 我们来边学边表演.
常用标点符号用法简表.doc
常用标点符号用法简表 标点符号栏目对每一种汉语标点符号都有详细分析,下表中未完全添加链接,请需要的同学或朋友到该栏目查询。名称符号用法说明举例句号。表示一句话完了之后的停顿。中国共产党是全中国人民的领导核心。逗号,表示一句话中间的停顿。全世界各国人民的正义斗争,都是互相支持的。顿号、表示句中并列的词或词组之间的停顿。能源是发展农业、工业、国防、科学技术和提高人民生活的重要物质基础。分号;表示一句话中并列分句之间的停顿。不批判唯心论,就不能发展唯物论;不批判形而上学,就不能发展唯物辩证法。冒号:用以提示下文。马克思主义哲学告诉我们:正确的认识来源于社会实践。问号?用在问句之后。是谁创造了人类?是我们劳动群众。感情号①!1.表示强烈的感情。2.表示感叹句末尾的停顿。战无不胜的马克思主义、列宁主义、毛泽东思想万岁!引号 ②“ ” ‘ ’ ╗╚ ┐└1.表示引用的部分。毛泽东同志在《论十大关系》一文中说:“我们要调动一切直接的和间接的力量,为把我国建设成为一个强大的社会主义国家而奋斗。”2.表示特定的称谓或需要着重指出的部分。他们当中许多人是身体好、学习好、工作好的“三好”学生。 3.表示讽刺或否定的意思。这伙政治骗子恬不知耻地自封为“理论家”。括号③()表示文中注释的部分。这篇小说环境描写十分出色,它的描写(无论是野外,或是室内)处处与故事的发展扣得很紧。省略号④……表示文中省略的部分。这个县办工厂现在可以生产车床、电机、变压器、水泵、电线……上百种产品。破折号⑤——1.表示底下是解释、说明的部
分,有括号的作用。知识的问题是一个科学问题,来不得半点的虚伪和骄 傲,决定地需要的倒是其反面——诚实和谦逊的态度。2.表示意思的递进。 团结——批评和自我批评——团结3.表示意思的转折。很白很亮的一堆洋 钱!而且是他的——现在不见了!连接号⑥—1.表示时间、地点、数目等 的起止。抗日战争时期(1937-1945年)“北京—上海”直达快车2.表 示相关的人或事物的联系。亚洲—太平洋地区书名号⑦《》〈〉表示 书籍、文件、报刊、文章等的名称。《矛盾论》《中华人民共和国宪法》《人 民日报》《红旗》杂志《学习〈为人民服务〉》间隔号·1.表示月份和日期 之间的分界。一二·九运动2.表示某些民族人名中的音界。诺尔曼·白求 恩着重号.表示文中需要强调的部分。学习马克思列宁主义,要按照毛泽 东同志倡导的方法,理论联系实际。······
Joinin小学五年级英语教案
Join in 小学五年级英语教案 介休市宋古二中上站小学庞汝君 Unit 6 Friends 单元目标: 1、单词:sport music kite cap car book dog cat rat frog spider butterfly 2、句型:Who is your best friend ? (重点) How old is he ? When is his birthday ? What’s his favourite food ? 3、段落:介绍自己的好朋友 My best friend’s name is Toby . He is ten years old . His favourite colour is red . His birthday is in May . He has got a dog . 4、语法:第三人称的人称代词和物主代词(难点) 他he 他的his 她she 她的her 一般现在时第三人称单数动词+s
5、故事:Emma , Jackie and Diana . Are you all right ? What’s the matter ? Don’t be silly . We can play together . The first class 一、教学目标: 两个句型1、Who is your best friend ? 2、How old is he ? 二、教学过程: 1、Talk about your best friend . (1)教师说句子Who is your best friend ? My best friend is Anna. 让学生先领会句子的意思,然后模仿, 小组练习对话并上台表演。 (2)第二个句子How old is he ? He is nine years old . 象上面一样练习,等到学生掌握后把两个问句连起来做 问答操练。
常用标点符号用法含义
一、基本定义 句子,前后都有停顿,并带有一定的句调,表示相对完整的意义。句子前后或中间的停顿,在口头语言中,表现出来就是时间间隔,在书面语言中,就用标点符号来表示。一般来说,汉语中的句子分以下几种: 陈述句: 用来说明事实的句子。 祈使句: 用来要求听话人做某件事情的句子。 疑问句: 用来提出问题的句子。 感叹句: 用来抒发某种强烈感情的句子。 复句、分句: 意思上有密切联系的小句子组织在一起构成一个大句子。这样的大句子叫复句,复句中的每个小句子叫分句。 构成句子的语言单位是词语,即词和短语(词组)。词即最小的能独立运用的语言单位。短语,即由两个或两个以上的词按一定的语法规则组成的表达一定意义的语言单位,也叫词组。 标点符号是书面语言的有机组成部分,是书面语言不可缺少的辅助工具。它帮助人们确切地表达思想感情和理解书面语言。 二、用法简表 名称
句号① 问号符号用法说明。?1.用于陈述句的末尾。 2.用于语气舒缓的祈使句末尾。 1.用于疑问句的末尾。 2.用于反问句的末尾。 1.用于感叹句的末尾。 叹号! 2.用于语气强烈的祈使句末尾。 3.用于语气强烈的反问句末尾。举例 xx是xx的首都。 请您稍等一下。 他叫什么名字? 难道你不了解我吗?为祖国的繁荣昌盛而奋斗!停止射击! 我哪里比得上他呀! 1.句子内部主语与谓语之间如需停顿,用逗号。我们看得见的星星,绝大多数是恒星。 2.句子内部动词与宾语之间如需停顿,用逗号。应该看到,科学需要一个人贡献出毕生的精力。 3.句子内部状语后边如需停顿,用逗号。对于这个城市,他并不陌生。 4.复句内各分句之间的停顿,除了有时要用分号据说苏州园林有一百多处,我到过的不外,都要用逗号。过十多处。 顿号、用于句子内部并列词语之间的停顿。
五年级下英语月考试卷全能练考Joinin剑桥英语(无答案)
五年级下英语月考试卷-全能练考-Join in剑桥英语 姓名:日期:总分:100 一、根据意思,写出单词。(10′) Fanny:Jackie, ________[猜] my animal,my ________最喜欢的 animal.OK? Jackie:Ok!Mm…Has it got a nose? Fanny:Yes,it’s got a big long nose,…and two long ________[牙齿]. Jackie:Does it live in ________[美国]? Fanny:No,no.It lives ________[在] Africa and Asia. Jackie:Can it ________[飞]? Fanny:I’m sorry it can’t. Jackie:Is it big? Fanny:Yes,it’s very big and ________[重的]. Jackie:What ________[颜色] is it?Is it white or ________[黑色]? Fanny:It’s white. Jackie:Oh,I see.It’s ________[大象]. 二、找出不同类的单词。(10′) ()1 A.sofa B.table C.check ()2 A.noodle https://www.360docs.net/doc/dc16862733.html,k C.way ()3 A.fish B.wolf C.lion ()4 A.bike B.car C.write ()5 A.tree B.farm C.grass ()6 A.big B.small C.other ( )7 A.eat B.listen C.brown
剑桥小学英语Join_In
《剑桥小学英语Join In ——Book 3 下学期》教材教法分析2012-03-12 18:50:43| 分类:JOIN IN 教案| 标签:|字号大中小订阅. 一、学情分析: 作为毕业班的学生,六年级的孩子在英语学习上具有非常显著的特点: 1、因为教材的编排体系和课时不足,某些知识学生已遗忘,知识回生的现象很突出。 2、有的学生因受学习习惯及学习能力的制约,有些知识掌握较差,学生学习个体的差异性,学习情况参差不齐、两级分化的情况明显,对英语感兴趣的孩子很喜欢英语,不喜欢英语的孩子很难学进去了。 3、六年级的孩子已经进入青春前期,他们跟三、四、五年级的孩子相比已经有了很大的不同。他们自尊心强,好胜心强,集体荣誉感也强,有自己的评判标准和思想,对知识的学习趋于理性化,更有自主学习的欲望和探索的要求。 六年级学生在英语学习上的两极分化已经给教师的教学带来很大的挑战,在教学中教师要注意引导学生调整学习方式: 1、注重培养学生自主学习的态度 如何抓住学习课堂上的学习注意力,吸引他们的视线,保持他们高涨的学习激情,注重过程的趣味化和学习内容的简易化。 2、给予学生独立思考的空间。 3、鼓励学生坚持课前预习、课后复习的好习惯。 六年级教材中的单词、句子量比较多,难点也比较多,学生课前回家预习,不懂的地方查英汉词典或者其它资料,上课可以达到事半功倍的效果,课后复习也可以很好的消化课堂上的内容。 4、注意培养学生合作学习的习惯。 5、重在培养学生探究的能力:学习内容以问题的方式呈现、留给学生更多的发展空间。 二、教材分析: (一).教材特点: 1.以学生为主体,全面提高学生的素质。
(完整版)JOININ小学三年级英语(上册)重点知识归纳
小学三年级英语(上册)重要知识点归纳 一、单词 Unit 1学习文具:pen (钢笔) pencil (铅笔) pencil-case ( 铅笔盒) ruler(尺子) eraser(橡皮) crayon (蜡笔) book (书) bag (书包) sharpener (卷笔刀) school (学校) Unit 2身体部位:head (头) face( 脸) nose (鼻子) mouth (嘴) eye (眼睛)leg (腿) ear (耳朵) arm (胳膊)finger (手指) leg (腿) foot (脚)body (身体) Unit 3颜色:red (红色的) yellow (黄色的)green (绿色的)blue (蓝色的) purple (紫色的) white (白色的) black (黑色的) orange (橙色的) pink (粉色的)brown (棕色的) Unit 4动物:cat (猫)dog (狗)monkey (猴子)panda (熊猫)rabbit( 兔子) duck (鸭子)pig (猪)bird (鸟) bear (熊)elephant (大象)mouse (老鼠) squirrel (松鼠) Unit 5食物:cake (蛋糕)bread (面包) hot dog (热狗) hamburger (汉堡包) chicken (鸡肉)French fries (炸薯条)coke (可乐)juice (果汁)milk (牛奶) water (水)tea (茶) coffee (咖啡) Unit 6数字:one (一) two (二) three (三)four (四) five (五)six( 六)seven (七) eight (八) nine( 九) ten( 十)doll (玩具娃娃) boat (小船)ball (球) kite (风筝) balloon (气球) car (小汽车)plane (飞机) 二、对话 1、向别人问好应该说――A: Hello! (你好!) B: Hi! (你好!) 2、问别人的名字应该说-――A:What's your name? 你的名字是什么? B:My name's Chen Jie. 我的名字是陈洁。 3、跟别人分手应该说――A: Bye.\ Good bye!(再见) B: See you.(再见) \ Goodbye.(再见) 4、A: I have a pencil\ bag\ruler 我有一只铅笔\书包\尺子。 B: Me too . 我也有。 5、早上相见应该说-――A: Good morning. 早上好!
剑桥小学英语join in五年级测试卷
五 年 级 英 语 测 试 卷 学校 班级 姓名 听力部分(共20分) 一、Listen and colour . 听数字,涂颜色。(5分) 二、 Listen and tick . 听录音,在相应的格子里打“√”。 (6分) 三、Listen and number.听录音,标序号。(9分) pig fox lion cow snake duck
sheep 笔试部分(共80分) 一、Write the questions.将完整的句子写在下面的横线上。(10分) got it Has eyes on a farm it live sheep a it other animals eat it it Is 二、Look and choose.看看它们是谁,将字母填入括号内。(8分) A. B. C. D.
E. F. G. H. ( ) pig ( ) fox ( ) sheep ( ) cat ( ) snake ( ) lion ( ) mouse ( ) elephant 三、Look at the pictures and write the questions.看图片,根据答语写出相应的问题。(10分) No,it doesn’t. Yes,it is.
Yes,it does. Yes,it has. Yes,it does. 四、Choose the right answer.选择正确的答案。(18分) 1、it live on a farm? 2. it fly?
3. it a cow? 4. it eat chicken? 5. you swim? 6. you all right? 五、Fill in the numbers.对话排序。(6分) Goodbye. Two apples , please. 45P , please. Thank you.
JOININ英语三年级下册课本重点
JOIN IN英语三年级下册课本重点Start unit 1 Words morning afternoon evening night 2 Sentences Good morning !早上好 Good afternoon !下午好 Good evening!晚上好 Good night!晚安 3 Phrases clap your hands 拍拍手 jump up high 往高跳 shake your arms and your legs 晃动你的胳膊和腿 bend your knees 弯曲你的膝盖 touch your toes 摸摸你的脚指
stand nose to nose 鼻子对鼻子站着 Unit 1 Pets 1 Words cat猫dog狗bird 鸟mouse老鼠fish鱼rabbit 兔子frog青蛙hamster仓鼠 budgie鹦鹉tiger老虎monkey 猴子panda熊猫giraffe 长颈鹿elephant 大象bear 熊run跑sit坐fly飞swim游泳roar吼叫eat吃 2 Grammar ★名词的复数:一般在词尾直接加s,不规则变化要牢记: fish-----fish mouse------mice 3 Sentences 1.Have you got a pet ? 你有宠物吗?Yes ,I have. 是的,我有。/No, I haven’t. 不,我没有。 2.2. What have you got ? 你有什么宠物吗?I’ve got a dog . / A dog. 我有一只狗。 3.What colour is the cat ? 你的猫是什么颜色的?It’s black. 它是黑色的。 What iswizard’s pet? 巫师的宠物是什么? 4.What is it ? 它是什么? It’s a rabbit .它是只兔子。
(完整版)剑桥小学英语Joinin六年级复习题(二)2-3单元.doc
2011-2012 学年度上学期六年级复习题(Unit2-Unit3 ) 一、听力部分 1、听录音排序 ( ) () ()() () 2、听录音,找出与你所听到的单词属同一类的单词 () 1. A. spaceman B. pond C . tiger () 2. A.mascots B. potato C . jeans () 3. A. door B. behind C . golden () 4. A. sometimes B. shop C . prince () 5. A. chair B. who C . sell 3、听录音,将下面人物与他的梦连线 Sarah Tim Juliet Jenny Peter 4、听短文,请在属于Mr. Brown的物品下面打√ ( ) ( ) ( ) ( ) ( ) ( ) ( ) 5、听问句选答句 () 1. A. Yes, I am B. Yes, I have C . Yes, you do () 2. A.Pink B. A friendship band C . Yes. () 3. A. OK B. Bye-bye. C . Thanks, too. () 4. A. Monday. B. Some juice. C . Kitty. () 5. A. I ’ve got a shookbag. B. I ’m a student. C . It has got a round face. 6、听短文,选择正确答案 () 1. Where is Xiaoqing from? She is from . A.Hebei B. Hubei C . Hunan () 2. She goes to school at . A.7:00 B.7:30 C . 7:15 () 3. How many classes in the afternoon? classes. A. four B. three C . two () 4. Where is Xiaoqing at twelve o ’clock? She is . A. at home B. at school C .in the park () 5. What does she do from seven to half past eight? She . A.watches TV B. reads the book C. does homework
三年级下学期英语(Joinin剑桥英语)全册单元知识点归纳整理-
Starter Unit Good to see you again知识总结 一. 短语 1. dance with me 和我一起跳舞 2. sing with me 和我一起唱歌 3. clap your hands 拍拍你的手 4. jump up high 高高跳起 5.shake your arms and your legs晃晃你的胳膊和腿 6. bend your knees 弯曲你的膝盖 7. touch your toes 触摸你的脚趾8. stand nose to nose鼻子贴鼻子站 二. 句子 1. ---Good morning. 早上好。 ---Good morning, Mr Li. 早上好,李老师。 2. ---Good afternoon. 下午好。 ---Good afternoon, Mr Brown. 下午好,布朗先生。 3. ---Good evening,Lisa. 晚上好,丽莎。 ---Good evening, Bob. 晚上好,鲍勃。 4. ---Good night. 晚安。 ----Good night. 晚安。 5. ---What’s your name? 你叫什么名字? ---I’m Bob./ My name is Bob. 我叫鲍勃。 6. ---Open the window, please. 请打开窗户。 ---Yes ,Miss. 好的,老师。 7. ---What colour is it? 它是什么颜色? 它是蓝红白混合的。 ---It’s blue, red and white. 皮特的桌子上是什么? 8. ---What’s on Pit’s table? ---A schoolbag, an eraser and two books. 一个书包,一个橡皮和两本书。 9. ---What time is it? 几点钟? 两点钟。 ---It’s two. 10.---What’s this? 这是什么? ---My guitar. 我的吉他。
JOININ英语三年级下册知识点
JOIN IN英语三年级下册 Start unit 1 Words morning afternoon evening night 2 Sentences Good morning !早上好Good afternoon !下午好Good evening!晚上好 Good night!晚安 3 Phrases clap your hands 拍拍手 jump up high 往高跳 shake your arms and your legs 晃动你的胳膊和腿 bend your knees 弯曲你的膝盖 touch your toes 摸摸你的脚指 stand nose to nose 鼻子对鼻子站着 Unit 1 Pets 1 Words cat猫dog狗bird 鸟mouse老鼠fish鱼rabbit 兔子frog青蛙hamster仓鼠 budgie鹦鹉tiger老虎monkey 猴子panda熊猫giraffe 长颈鹿elephant 大象bear 熊run跑sit坐fly飞swim游泳roar吼叫eat吃 2 Grammar
★名词的复数:一般在词尾直接加s,不规则变化要牢记: fish-----fish mouse------mice 3 Sentences 1.Have you got a pet ? 你有宠物吗? Yes ,I have. 是的,我有。/No, I haven’t. 不,我没有。 2.What have you got ? 你有什么宠物吗?I’ve got a dog . / A dog. 我有一只狗。 3.What colour is the cat ? 你的猫是什么颜色的?It’s black. 它是黑色的。 What is wizard’s pet? 巫师的宠物是什么? 4.What is it ? 它是什么? It’s a rabbit .它是只兔子。 5.How many budgies /mice are there? 这里有多少只鹦鹉/老鼠? There are + 数字budgies/mice. 这里有------只鹦鹉/老鼠。 6.Fly like a budgie. 像鹦鹉一样飞。Run like a rabbit. 像兔子一样跑。 Swim like a fish. 像鱼一样游泳。Eat like a hamster. 像仓鼠一样吃东西。 Sit like a dog. 像狗一样坐。Roar like a tiger. 像老虎一样吼叫。 7.What are in the pictures. 图片里面是什么?Animals. 动物。 8. What animals? 什么动物? 9.How many pandas (elephants /bears/ giraffes/ monkeys/ budgies) are there?有多少.? How many + 可数名词的复数形式 Unit 2 The days of the week
外研社剑桥小学英语Join_in四年级上册整体课时教案
外研社剑桥小学英语Join_in四年级上册整体课时教案Starter Unit Let's begin 第一学时: The pupils learn to understand: How are you today? I’m fine/OK. Goodbye. The pupils learn to use: What’s your name? I’m (Alice). How are you? I’m fine. I’m OK. Activities and skills: Decoding meaning from teacher input. Using phrases for interaction in class. Greeting each other. I Introduce oneself. Asking someone’s mane. Teaching process: Step 1: Warm—up. Sing a song. Hello, what’s your name? Step 2: Presentation. 1. What’s your name? I’m…. (1) The teacher introduces herself, saying: Hello, /Good morning, I’m Miss Sun. (2) Asks a volunteer’s name:
What’s your name? The teacher prompt the pupil by whispering, I’m (Alice). (3) Asks all the pupils the same question and help those who need it by whispering I’m…. 2. How are you today? I’m fine. I’m OK. (1) The teacher explains the meaning of How are you today? (2) Tell the class to ask the question all together and introduce two answers, I’m (not very) fine/I’m Ok. (3) Asks all the pupils the same question and make sure that all the students can reply. Step3: Speak English in class. 1. Tells the pupils to open their books at page3, look at the four photographs, and listen to the tape. 2. Asks the pupils to dramatise the situations depicted in the four photographs. A volunteer will play the part of the parts of the other pupils, answering as a group. 第二学时: The pupils learn to use these new words: Sandwich, hamburger, hot dog, puller, cowboy, jeans, cinema, walkman, snack bar, Taxi, clown, superstar.
常用标点符号主要用法
常用标点符号主要用法 问号 1、用在特指问句后。如:(7)你今年多大了? 2、用在反问句后。如:(8)为什么我们不能刻苦一点呢? ?提示:反问句若语气缓和,末尾可用句号;若语气重可用感叹号。如:(9)国家 主席可以活活被整死;堂堂大元帅受辱骂;……这哪里还有什么尊重可言! 3、用在设问句后。如:(10)我们能让你计划实现吗?不会的。 4、用在选择问句中。如:(11)我们是革命呢,还是要现大洋? ( 12)你到底是去,还是不去? 5、用在表疑问的独词句后。如:(13)我?不可能吧。 ?提示:若疑问句为倒装句,问号应放在句末。如:(14)到底出了什么问题,你的 车?(若说成:“到底出了什么问题?你的车。”则错误。) ?特别提示: 句号、问号均表示句末停顿。句号用于陈述句末尾,问号用于疑问句末尾。有些句 中虽有疑问词,但全句并不是疑问句,句末只能用句号,不能用问号。 例如:(17)……最后应求出铜块的体积是多少? (18)面对千姿百态、纷繁芜杂的期刊世界,有哪位期刊编辑不想通过期刊版面设 计为刊物分朱布白、添花增色呢? (19)关于什么是智力?国内外争论多年也没有定论。 (17) (18) ( 19)三句都是非疑问句,(17) (18)句中问号均应改为句号,(19)句中的问号应改为逗号。 感叹号 ?特别提示: 1、在表感叹或祈使语气的主谓倒装句中,感叹号要放在句末。 如:(20)多么雄伟壮观啊,万里长城! 2、句前有叹词,后是感叹句,叹号放在句末。 如:(21)啊,这儿多么美丽! 下面介绍句中点号的用法。句中点号包括逗号、分号、顿号、和冒号四种。 逗号 提示:复句内各分句之间的停顿,除了有时用分号外,都要用逗号。 顿号 用于句中并列的词、词组之间较小的停顿。 如:(22)邓颖超的品德、人格、风范为中华民族树立了一座精神丰碑。 (23)从1918年起,鲁迅陆续发表了《狂人日记》、《药》、《祝福》等短篇小说。 ?特别提示:以下九种情况不用顿号。 1、不定数的两个数字间不用顿号。 如:(24)你的年龄大概是十六七岁。(不能写成“十六、七岁”) ?【注意】相邻的两个数字而非约数之间要用顿号。
剑桥英语JOININ三年级上册知识点(供参考)
Join in三年级上册知识点 1.见面打招呼 Hello! = Hi! 你好 Good morning! 早上好! Good afternoon! 下午好! Good evening! 晚上好! (注意:Good night的意思是“晚安”) 2.询问身体健康状况 How are you? 你好吗? I’m fine. = I’m OK.我很好。 3.临走分别 Goodbye. = Bye bye. 再见。 See you tomorrow. 明天见。 4.Miss小姐Mr先生 5.询问名字 -What’s your name? 你叫什么名字? -I’m Toby. = My name is Toby. = Toby.我叫托比。 6.Let’s+动词原形 如Let’s go! 我们走吧! Let’s begin! 我们开始吧! 7. Are you ready? 你准备好了吗? I’m ready. 我准备好了。 8.十二个动词 look看listen听mime比划着表达speak讲read读write写draw画colour涂sing唱think想guess猜play玩9.询问物体 -What’s this? 这是什么? -It’s my dog. / It’s a dog. / My dog. 这是我的狗。 10.my/your+名词 如my apple我的苹果 your apple你的苹果 11.数字0-10 zero零one一two二three三four四five五six六seven七eight八nine九ten十12.询问电话号码 -What’s your phone number? 你的电话号码是多少? -It’s . / . 13.guess sth 猜东西 如Guess the number. 猜数字 14. What’s in the box? 盒子里有什么? 15.询问数量 -How many? 多少? -Nine. 九个。 16. Here is / are sth. 这是……
公文写作中标点符号的使用规范
公文写作中标点符号的使用规范 《中华人民共和国国家标准》(GB|T15834—1995)中有《标点符号用法》,其中对标点符号的使用作了明确的规定: 标点符号是辅助文字记录语言的符号,是书面语的有机组成部分,用来表示停顿、语气以及词语的性质和作用。 常用的标点符号有16种,分点号和标号两大类。 点号的作用在于点断,主要表示说话时的停顿和语气。点号又分为句末点号和句内点号——句末点号用在句末,有句号、问号、叹号3种,表示句末的停顿,同时表示句子的语气;句内点号用在句内,有逗号、顿号、分号、冒号4种,表示句内的各种不同性质的停顿。 标号的作用在于标明,主要标明语句的性质和作用。常用的标号有9种,即:引号、括号、破折号、省略号、着重号、连接号、间隔号、书名号和专名号。 一、常用标点符号用法简表 名称符号用法说明举例 句号。表示一句话完了之后的停 顿。 中国共产党是全中国人民的领导核心。 逗号,表示一句话中间的停顿。全世界各国人民的正义斗争,都是互相支持的。 顿号、表示句中并列的词或词组 之间的停顿。 能源是发展农业、工业、国防、科学技术和提高人 民生活的重要物质基础。 分号;表示一句话中并列分句之 间的停顿。 不批判唯心论,就不能发展唯物论;不批判形而上 学,就不能发展唯物辩证法。 冒号:用以提示下文。马克思主义哲学告诉我们:正确的认识来源于社会实践。 问号?用在问句之后。是谁创造了人类?是我们劳动群众。 感情号!1.表示强烈的感情。 2.表示感叹句末尾的停 顿。 战无不胜的马克思主义、列宁主义、毛泽东思想万 岁! 引号“”1.表示引用的部分。毛泽东同志在《论十大关系》一文中说:“我们要
【精品】joinin英语剑桥版五年级(上)单词表
beautiful美丽的,美好的 clean(把。。。)弄干净 hard费劲地;费力地 learn学习;学会 New South Wales新南威尔士州 soon不久,很快 summer holiday暑假 take拍摄 take photos拍照 time一段时间;某段日子 the UK英国 umbrella雨伞 whose谁的 work工作,劳动 both两者,两个都 change转变;转换 come in 进来 come on快点 cook煮,烧;厨师 cousin堂(表)兄弟;表姐妹doctor医师,大夫 driver司机、驾驶员 family家庭 fantastic太好了;极好的 famer农民 grandfather祖父;外祖父 grandmother祖母;外祖母 grandparent祖父;外祖父;祖母;外祖母granny奶奶;外婆 introduce介绍,引见 Little Red Riding Hood小红帽 member成员 nurse护士 phone电话 police警察 problem问题,难题 role角色 run away逃跑 scene一场 taxi出租车 these这些 walk off离开 woods小树林,林地 word字、单词 worker工人 always总是,每次都 band圈,箍,带 because因为
bell铃 bring带来 cool冷静的,沉着的darling亲爱的,宝贝儿dollar美元 easy不担心的,不紧张的everywhere处处 friendship友谊 hurt弄伤; luck好运;幸运 mascot吉祥物 necklace项链 Olympic奥运会的 popular受欢迎的 Puma Ranch美洲狮大牧场 ring戒指 shell贝壳 silver银制的;银色的soft toy毛绒玩具 test测验 watch out小心 answer答案 athlete运动员 collect收集采集 cyclist骑自行车者 end结束;停止 go开始(做某事)kilometre千米,公里 opera house歌剧院 quarter一刻钟,十五分钟say说 special特殊的,特别的spot圆点,斑点 stamp邮票 tell告诉 time次,回 top最佳的,最好的train火车,列车 Yours您真挚的autograph亲笔签名 comic连环画 email电子邮件 for为了要;为了得到gone走了;不见了 hat帽子 postcard明信片 sticker贴纸 take接受
