Enhanced Photocatalytic Activity of Nafion-Coated TiO2

Enhanced Photocatalytic Activity of Nafion-Coated TiO 2
M .S .V O H R A A N D K .T A N A K A *
National Institute of Materials and Chemical Research,1-1Higashi,Tsukuba,Ibaraki 305-8565,Japan
Photocatalytic degradation (PCD)of aqueous paraquat was accelerated by the addition of either phosphate or sulfate salt.Attachment of these anions to the TiO 2surface possibly results in increased adsorption of the cationic paraquat species and in turn its photocatalysis rate.The same effect was obtained more consistently using the Nafion (an anionic polymer)-coated TiO 2.Enhanced PCD of
paraquat and some amine compounds was noted.However the anionic and neutral compounds were not affected significantly.Nafion proved to be stable against photocatalysis.It has been suggested that the degradation rate is larger for the cationic compounds with higher p K a .For a phenol -paraquat -TiO 2system,paraquat degradation did not
begin till near-complete phenol https://www.360docs.net/doc/ef12467144.html,ing the Nafion-coated TiO 2,both phenol and paraquat degradations started simultaneously.Nevertheless,complete paraquat removal still took longer than phenol.
Introduction
It has been recognized that TiO 2is the most efficient photocatalyst for environmental applications as compared with the other semiconductors (1).However,its activity needs to be enhanced especially for water purification.Several methods have been proposed to increase the TiO 2efficiency,e.g.,incorporation of an adsorbent such as activated carbon into TiO 2(2-4).Tanaka and Robledo showed that the adsorption of substrate onto TiO 2significantly affects its photocatalysis rate (5).
The present study discusses an efficient method of increasing the substrate adsorption by modifying the TiO 2surface charge,which in turn improves the photocatalytic activity specifically for the cationic pollutants.It has been shown (6,7)that modifications of TiO 2surface charge by the inorganic anions either reduced the photocatalytic activity (sulfate,phosphate,chloride)or had no influence (nitrate,perchlorate).However,we found that the addition of sulfate and phosphate species enhanced the photocatalytic degra-dation of paraquat (cationic species).These results led us to consider better modifications of the TiO 2surface.
Paraquat,a widely used herbicide,was chosen as a representative cationic pollutant to evaluate the activity of modified TiO 2.Paraquat is very toxic and can cause irrevers-ible lung damage upon ingestion.Also,water and soil contamination at its point of application remains a concern.In the surface soils,paraquat may photodecompose in several weeks (8).However,paraquat in the subsurface environment may remain intact for several years.
Recently,Moctezuma et al.studied the paraquat PCD employing TiO 2(9).The paraquat PCD rate was much higher
as compared with its direct photolysis.Tennakone and Kottegoda also studied aqueous paraquat photocatalysis using TiO 2(10).Use of both UV and sunlight caused paraquat degradation.However,no paraquat removal was observed in the absence of TiO 2.The present study reports that surface modification of TiO 2causes an increase in the photocatalytic degradation of paraquat and other cationic compounds.
Experimental Section
All the chemicals used were of reagent grade.TiO 2(Fujititan TP-2)used in the present work was of anatase form,and its specific surface area is 17.3m 2/g (11).Paraquat (1,1-dimethyl-4,4-bipyridinium dichloride)was purchased from Dr.Eh-renstorfer GmbH,Germany.Asulam (methysulfanilyl car-bamate),ethylamine,trimethylamine,morpholine,and phenol were obtained from Wako Pure Chemical Industries Ltd.Chloride,nitrate,sulfate,and phosphate species were added as sodium salts.Nafion solution [5wt %solution,a product of DuPont,SE -5112,copolymer of perfluorosulfonic acid and poly(tetrafluoroethylene)]was purchased from Aldrich Chemical Co.
A Pyrex-glass batch type reactor (6cm i.d.and 26cm length)was used for the photocatalysis experiments.Figure 1shows the experimental setup.For each experiment,500mL of 10-4M substrate solution was transferred to the reactor,and 2g of TiO 2was added;a magnetic stirrer was employed for continuous mixing.A 6-W black light lamp was used for illumination.The pH adjustments were made using 1M HClO 4.
Samples were taken at several time intervals and filtered using 0.2-μm pore size Millipore filters.The first three drops of the filtrate were rejected,and the rest of it was collected and analyzed.Paraquat was analyzed employing a Shimadzu LC-10AD HPLC equipped with SCL-10A system controller and SPD-10A UV -Vis detector.The HPLC column was IC -cation -SW TSK gel from the Tosoh.The eluent consisted of 4:1(v:v)of 0.2M NaH 2PO 4?2H 2O (its pH was adjusted to 3using H 3PO 4)and CH 3CN,respectively (12).Phenol was analyzed using the same HPLC unit with an ODS column.Ethylamine,trimethylamine,and morpholine were moni-tored using an ion chromatograph equipped with a JASCO 880PU pump and a Shodex CD-4conductometer.Nitrate and ammonium ions were detected using the same ion chromatograph unit.The columns were Shodex IC-613for
*Corresponding author phone:+81-298-61-4563;fax:+81-298-61-4563;e-mail:ktanaka@home.nimc.go.jp.
FIGURE 1.Schematic illustration of the photocatalytic reactor.1,Pyrex glass tube;2,black light lamp;3,stirrer;4,magnetic stirrer.Environ.Sci.Technol.2001,35,411-
415
10.1021/es001238q CCC:$20.00?2001American Chemical Society
VOL.35,NO.2,2001/ENVIRONMENTAL SCIENCE &TECHNOLOGY
9
411
Published on Web
12/09/2000
nitrate and IC Y-521for the ammonium ions.Acetic and formic acids were determined using a Yokogawa ion chro-matograph (IC 7000series II)equipped with a background suppressor.
The Nafion-coated TiO 2samples were prepared by adding the desired amount of Nafion solution (5wt %)to 2g of TiO 2along with an appropriate amount of methanol for proper mixing and to ensure a homogeneous coating of Nafion onto TiO 2.After being mixed manually,the mixture was dried overnight at room temperature.
To determine the CO 2evolution rate,a 34-mL Pyrex glass vial covered with an air-tight plastic cap was used (Mininert Valve SC-100,GL Sciences Inc.Japan).A total of 75mg of the Nafion-coated TiO 2(2.5-67.5mg of Nafion/g of TiO 2)suspended in 25mL of a 5×10-4M paraquat solution was illuminated by a 500-W super-high-pressure mercury lamp.Headspace CO 2was quantified using a Shimadzu 6AM gas chromatograph.
In the NH 4+desorption experiment,for both TiO 2-only and Nafion-coated TiO 2samples at illumination time >20h,the suspension pH was adjusted to below 3(2and 4mM HNO 3).The difference in the NH 4+concentration (if any)before and after HNO 3addition showed adsorption.
Results and Discussion
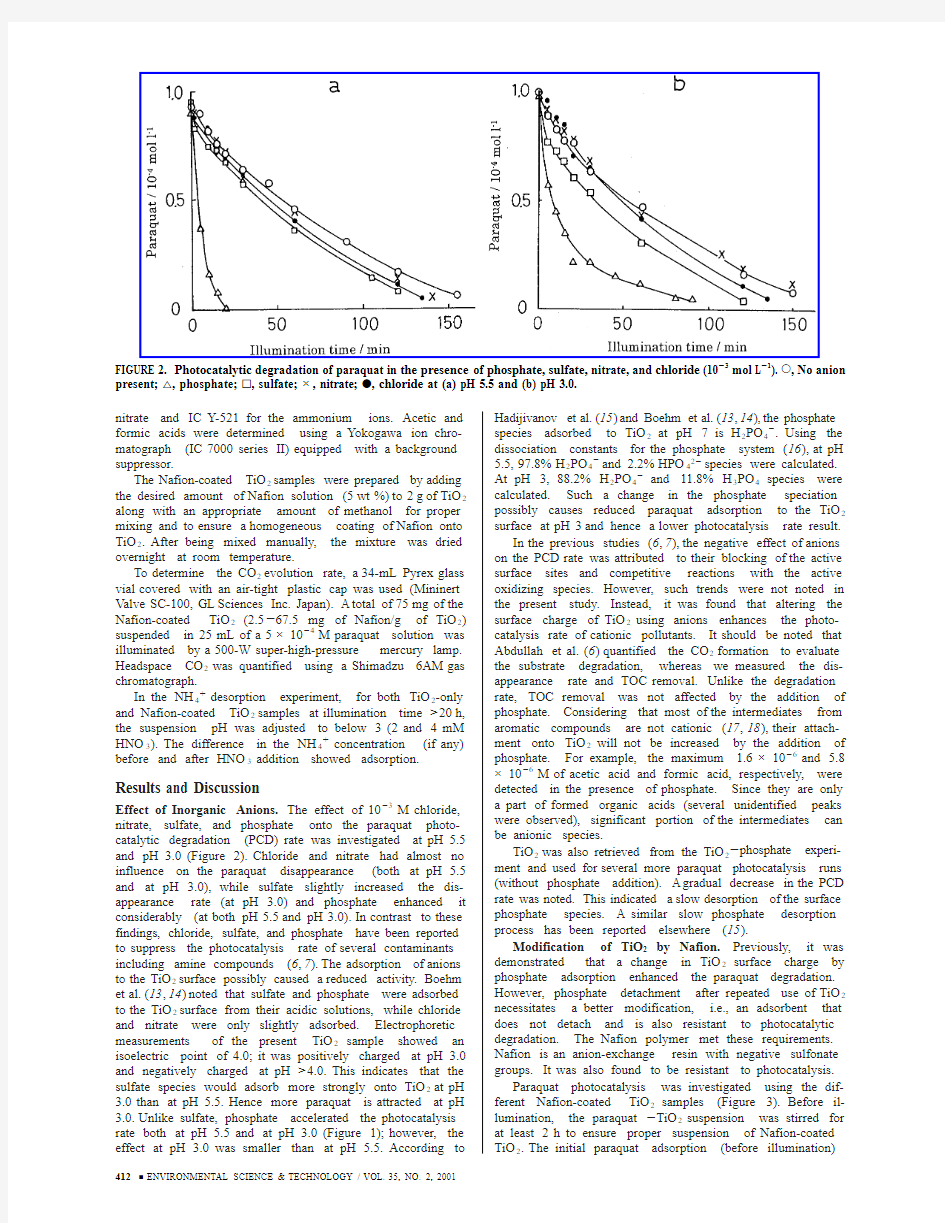
Effect of Inorganic Anions.The effect of 10-3M chloride,nitrate,sulfate,and phosphate onto the paraquat photo-catalytic degradation (PCD)rate was investigated at pH 5.5and pH 3.0(Figure 2).Chloride and nitrate had almost no influence on the paraquat disappearance (both at pH 5.5and at pH 3.0),while sulfate slightly increased the dis-appearance rate (at pH 3.0)and phosphate enhanced it considerably (at both pH 5.5and pH 3.0).In contrast to these findings,chloride,sulfate,and phosphate have been reported to suppress the photocatalysis rate of several contaminants including amine compounds (6,7).The adsorption of anions to the TiO 2surface possibly caused a reduced activity.Boehm et al.(13,14)noted that sulfate and phosphate were adsorbed to the TiO 2surface from their acidic solutions,while chloride and nitrate were only slightly adsorbed.Electrophoretic measurements of the present TiO 2sample showed an isoelectric point of 4.0;it was positively charged at pH 3.0and negatively charged at pH >4.0.This indicates that the sulfate species would adsorb more strongly onto TiO 2at pH 3.0than at pH 5.5.Hence more paraquat is attracted at pH 3.0.Unlike sulfate,phosphate accelerated the photocatalysis rate both at pH 5.5and at pH 3.0(Figure 1);however,the effect at pH 3.0was smaller than at pH 5.5.According to
Hadijivanov et al.(15)and Boehm et al.(13,14),the phosphate species adsorbed to TiO 2at pH 7is H 2PO https://www.360docs.net/doc/ef12467144.html,ing the dissociation constants for the phosphate system (16),at pH 5.5,97.8%H 2PO 4-and 2.2%HPO 42-species were calculated.At pH 3,88.2%H 2PO 4-and 11.8%H 3PO 4species were calculated.Such a change in the phosphate speciation possibly causes reduced paraquat adsorption to the TiO 2surface at pH 3and hence a lower photocatalysis rate result.In the previous studies (6,7),the negative effect of anions on the PCD rate was attributed to their blocking of the active surface sites and competitive reactions with the active oxidizing species.However,such trends were not noted in the present study.Instead,it was found that altering the surface charge of TiO 2using anions enhances the photo-catalysis rate of cationic pollutants.It should be noted that Abdullah et al.(6)quantified the CO 2formation to evaluate the substrate degradation,whereas we measured the dis-appearance rate and TOC removal.Unlike the degradation rate,TOC removal was not affected by the addition of phosphate.Considering that most of the intermediates from aromatic compounds are not cationic (17,18),their attach-ment onto TiO 2will not be increased by the addition of phosphate.For example,the maximum 1.6×10-6and 5.8×10-6M of acetic acid and formic acid,respectively,were detected in the presence of phosphate.Since they are only a part of formed organic acids (several unidentified peaks were observed),significant portion of the intermediates can be anionic species.
TiO 2was also retrieved from the TiO 2-phosphate experi-ment and used for several more paraquat photocatalysis runs (without phosphate addition).A gradual decrease in the PCD rate was noted.This indicated a slow desorption of the surface phosphate species.A similar slow phosphate desorption process has been reported elsewhere (15).
Modification of TiO 2by Nafion.Previously,it was demonstrated that a change in TiO 2surface charge by phosphate adsorption enhanced the paraquat degradation.However,phosphate detachment after repeated use of TiO 2necessitates a better modification,i.e.,an adsorbent that does not detach and is also resistant to photocatalytic degradation.The Nafion polymer met these requirements.Nafion is an anion-exchange resin with negative sulfonate groups.It was also found to be resistant to photocatalysis.Paraquat photocatalysis was investigated using the dif-ferent Nafion-coated TiO 2samples (Figure 3).Before il-lumination,the paraquat -TiO 2suspension was stirred for at least 2h to ensure proper suspension of Nafion-coated TiO 2.The initial paraquat adsorption (before
illumination)
FIGURE 2.Photocatalytic degradation of paraquat in the presence of phosphate,sulfate,nitrate,and chloride (10-3mol L -1).O ,No anion present;4,phosphate;0,sulfate;×,nitrate;b ,chloride at (a)pH 5.5and (b)pH 3.0.412
9
ENVIRONMENTAL SCIENCE &TECHNOLOGY /VOL.35,NO.2,2001
increased with an increase in the Nafion amount(Figure3). For example,using67.5mg of Nafion/g of TiO2,approximately 87%paraquat adsorption resulted at time zero(Figure3): adsorption of paraquat was confirmed by retrieving TiO2, desorbing paraquat in0.1N HCl,and measuring the UV spectrum.At a higher Nafion volume,it was difficult to ascertain whether the paraquat disappearance results from PCD or adsorption.Therefore,the determination of an optimum Nafion amount was not possible.Considering this, CO2formation was quantified(Figure4).Experiments were conducted using5×10-4M paraquat solution and different Nafion-coated TiO2samples;other details are given in the Experimental Section.The CO2results helped to find the optimum Nafion amount for paraquat mineralization.Figure 4illustrates that initially the CO2evolution rate increases with an increase in the Nafion amount.This is followed by a plateau(or maximum).However,any further increase in the Nafion amount caused a decrease in the CO2formation rate.It seems that too much Nafion retards the degradation either by blocking the active sites(on TiO2)or scattering the incident light.The highest PCD rate was observed at about 2.3-4.5mg of Nafion/g of TiO2.Also at4.5mg of Nafion the initial paraquat adsorption onto TiO2was around10%,which is much smaller as compared with the other Nafion amounts used(Figure3)and may not affect the evaluation of the photocatalytic acitivity much.Therefore4.5mg of Nafion/g of TiO2was used for all further investigations.
Nafion product information shows an ion-exchange capacity of0.9mequiv/g.Therefore,the total ion-exchange capacity of2g of TiO2covered with9mg of Nafion(0.2mL of Nafion/2g of TiO2)is0.81×10-5equiv or0.4×10-5mol (2equiv/mol of paraquat).Paraquat adsorption before illumination was also almost0.5×10-5mol(10%from500 mL of10-4M solution,Figure3).This suggests that most of the sulfonate groups on Nafion-coated TiO2are utilized for paraquat attachment.
Paraquat PCD experiments conducted at pH3and pH6 did not show any marked differences.This indicates that both the sulfonate(group of the Nafion)and the paraquat dissociation are not significantly influenced by such a change in the pH.
The initial TOC removal(up to2h)was quite rapid,both for the Nafion-coated and for the plain TiO2.However after 2h,the TOC removal rate decreased.Also,comparing the Nafion-coated and plain TiO2results(Figure5),the TOC removal rate was slightly larger for the Nafion-coated TiO2 before2h.However,after2h this order was reversed(Figure 5).Major reaction intermediates produced after2h may be anionic species.Consequently,their adsorption and hence PCD on Nafion-coated TiO2may not be favorable.This may cause an overall slower TOC removal for the Nafion-coated TiO2.As mentioned later,a slightly negative effect on the acetic and formic acid degradation was noted.
Results for the plain TiO2study after20h of illumination showed95%original N conversion to NH4+(Figure5). Similarly results for the Nafion-coated TiO2(4.5mg/g of TiO2 after20h illumination)also revealed approximately70%NH4+ production.However,the NH4+concentration(after20h) decreased with an increase in the Nafion amount(Figure6). It can be suggested that part of the NH4+attaches to the surface Nafion species;the percent adsorption increases with an increase in the surface Nafion amount.The adsorption was confirmed by measuring NH4+desorption at lower pH (see Experimental Section).For TiO2coated by67.5mg of Nafion,NH4+adsorption was25%of the total SO3-group; it was assumed that a part of nitrogen formed NO3-(8×10-6 M).
On the other hand,five experiments conducted using the same Nafion-TiO2each time showed an increasing PCD NH4+concentration.Saturation of the TiO2surface sites with repeated use may leave fewer sites for NH4+adsorption.This in turn may cause a gradual increase in the aqueous NH4+ concentration.In summary,CO2and NH4+were the major mineralization products(Figures4and5).Nitrate,if detected, was present in small amounts(<8×10-6M).
It is important that Nafion-coated TiO2should retain its activity even after several uses.This was confirmed by checking the activity of4.5mg of Nafion-coated TiO2.Five
FIGURE3.Photocatalytic degradation of paraquat on Nafion-coated TiO2.Plain TiO2:O.Nafion-TiO2:b,2.25;4,4.5;0,45;and×,67.5 mg/g TiO2.
FIGURE4.Effect of the Nafion amount on CO2evolution rate from the degradation of paraquat.O,initial60min;4,initial180min.FIGURE5.TOC removal and NH4+formation from the degradation of paraquat on Nafion-coated TiO2(4.5mg/g TiO2).TOC:O,on plain TiO2;4,on Nafion-coated TiO2.NH4+:b,on plain TiO2;2,on Nafion-coated TiO2.
VOL.35,NO.2,2001/ENVIRONMENTAL SCIENCE&TECHNOLOGY9
413
degradation experiments were completed employing fresh paraquat solutions but the same TiO 2sample (each time).The initial TiO 2activity was retained even after five uses (Figure 7).
It is also important that the Nafion polymer should not degrade during the photocatalysis process.The stability of Nafion against photocatalysis was confirmed by illuminating 500mL of deionized water containing 2g of Nafion-coated TiO 2(135mg of Nafion/g of TiO 2);samples were collected at several time intervals,and sulfate salt and TOC analysis were conducted.No appreciable increase either in sulfate salt or in TOC was observed.Similarly,a 4.5-mg Nafion -TiO 2sample was suspended in 500mL of deionized water and then illuminated for 10days.After this time,the TiO 2sample was retrieved and used for 10-4M paraquat PCD.No significant change in the photocatalytic activity of TiO 2was noted.Hence,continuous illumination for 10days did not degrade the Nafion coating.
The effect of Nafion coating on the degradation of other ionic and nonionic compounds was also investigated.Degradation of ethylamine was accelerated by Nafion coating at pH 5.9.However,this effect was smaller at pH 3.0.A similar trend was also observed for trimethylamine,although the effect of Nafion was comparatively smaller.No appreciable Nafion effect on morpholine,asulam,and sodium acetate degradation was noted (both at pH 5.9and at pH 3.0).A slightly negative effect was noted for sodium acetate and phenol (1.4and 1.5times longer illumination time was required for 50%degradation,respectively).
These results suggest that Nafion at 4.5mg/g of TiO 2covers only a part of the TiO 2surface;therefore,the sulfonate groups
(of Nafion)only slightly affect the adsorption of neutral and negatively charged compounds.The effect of Nafion coating was correlated to the dissociation constant of nitrogen-containing compounds (Figure 8).To our knowledge,the p K a value for paraquat is not available.However,for some N -alkyl-substituted pyridine compounds it is reported to be larger than 11(19), e.g.,11.4for 1,4,5,6-tetrahydro-1,2-dimethylpyridine (20).In Figure 8,the latter value (11.4)was taken as an approximate value for paraquat.Also,the p K a of asulam was assumed to be the same as that of aniline (21).Figure 8indicates that Nafion coating is more effective for cationic pollutants with a larger p K a value.Adsorption of pollutants onto Nafion-coated TiO 2may be affected by several factors beside the electrostatic interactions.A study on steric hindrance due to the structure of pollutants is in progress.The selectivity of both plain and Nafion-coated TiO 2to a mixture of phenol and paraquat was also studied.The control experiment with plain TiO 2demonstrated that the degradation of phenol is not affected by the presence of paraquat but that of the paraquat is suppressed markedly,which started only after complete phenol degradation.However for Nafion-coated TiO 2,the degradation of paraquat was less affected by the phenol,and the two compounds started to degrade simultaneously (Figure 9).It can be suggested that phenol occupies the active and/or adsorption sites of plain TiO 2and thus prevents paraquat from attaching to the TiO 2surface.However,as the Nafion-coated TiO 2has strong binding sites for paraquat attachment,both paraquat and phenol degradation start
simultaneously.
FIGURE 6.Concentration of free NH 4
+from
the
degradation of paraquat on TiO 2coated with different amounts of Nafion(after 20h of photocatalysis,1×10-4M paraquat).
FIGURE 7.Stability of photocatalytic activity of Nafion-coated TiO 2(4.5mg/g)against repeated uses.O ,first use;0,second use;],third use;×,fourth use;4,fifth use.
FIGURE 8.Effect of Nafion coating vs the dissociation constant.t 1/2and t naf.1/2are the times for 50%degradation using the plain and Nafion-coated TiO 2,respectively.
FIGURE 9.Photocatalytic degradation of paraquat and phenol in their mixture.Plain TiO 2:b ,paraquat;2,phenol.Nafion-coated TiO 2(4.5mg/g TiO 2):O ,paraquat;4,phenol.
414
9
ENVIRONMENTAL SCIENCE &TECHNOLOGY /VOL.35,NO.2,2001
The present study shows that coating of TiO2by Nafion improves the photocatalytic degradation of cationic pollut-ants.However,no significant change in the degradation rate of neutral and anionic compounds was observed. Literature Cited
(1)Hoffmann,M.R.;Martin,S.T.;Choi,W.;Bahnemann,D.W.
Chem.Rev.1995,95,69-96.
(2)Uchida,H.;Itoh,S.;Yoneyama,H.Chem.Lett.1993,1995-
1998.
(3)Tkeda,N.;Torimoto,T.;Sampath,S.;Kuwabata,S.;Yoneyama,
H.J.Phys.Chem.1995,99,9986-9991.
(4)Torimoto,T.;Ito,S.;Kuwabata,S.;Yoneyama,H.Environ.Sci.
Technol.1996,30,1275-1281.
(5)Tanaka,K.;Robledo,S.M.J.Mol.Catal.1999,144,425-430.
(6)Abdullah,M.;Low,G.K.-C.;Mathews,R.W.J.Phys.Chem.
1990,94,6820-6825.
(7)Kormann,C.;Bahemann,D.W.;Hoffmann,M.R.Environ.Sci.
Technol.1991,25,494-500.
(8)Eisler,R.Paraquat Hazards to Fish,Wildlife,and Invertebrates.
A Synoptic Review;PB91-114231/XAD;National Technical
Information Services(NTIS):Springfield,VA,1990.
(9)Moctezuma,E.;Leyva,E.;Monreal,E.;Villegas,N.;Infante,D.
Chemosphere1999,39,511-517.
(10)Tennakone,K.;Kottegoda,I.R.N.J.Photochem.Photobiol.A
1996,93,79-81.(11)Tanaka,K.;Capule,M.F.;Hisanaga,T.Chem.Phys.Lett.1991,
187,73-76.
(12)Nakagiri,I.;Suzuki,K.;Shiraku,Y.;Kuroda,Y.;Takasu,N.;
Kohama,A.J.Chromatogr.1989,481,434-438.
(13)Flaig-Baummann,R.;Hermann,M.;Boehm,H.P.Z.Anorg.
Allg.Chem.1970,372,296-307.
(14)Boehm,H.P.Discuss.Faraday Soc.1971,264-275.
(15)Hadjiivanov,K.I.;Klissurski,D.G.;Davydov,A.A.J.Catal.1985,
116,498-505.
(16)Handbook of Chemistry and Physics,78th ed.;Lide,D.R.,Ed.;
CRC Press:Boca Raton,FL,1997-1998;pp8-44.
(17)Tanaka K.;Luesaiwong,W.;Hisanaga,T.J.Mol.Catal.A1997,
122,67-74.
(18)Pichat,P.;Guillard,C.;Maillard,C.;Amalric,L.;D’Oliveira,J.-C.
Photocatalytic Purification and Treatment of Water and Air;
Ollis,D.F.,Al-Ekabi,H.,Eds.;Elsevier Science Publishers: Amsterdam,1993;pp207-223.
(19)Perrin,D.D.Dissociation Constants of Organic Bases in Aqueous
Solution;Butterworths:London,1965;p177.
(20)Perrin,D.D.Dissociation Constants of Organic Bases in Aqueous
Solution;Butterworths:London,1965;p175.
(21)Handbook of Chemistry and Physics,78th ed.;Lide,D.R.,Ed.;
CRC Press:Boca Raton,FL,1997-1998;pp8-49. Received for review May9,2000.Revised manuscript re-ceived September19,2000.Accepted October25,2000.
ES001238Q
VOL.35,NO.2,2001/ENVIRONMENTAL SCIENCE&TECHNOLOGY9415
