Chloroxine_DataSheet_MedChemExpress
Study_on_the_pharmacological_activities_and_chemic

ReviewStudy on the pharmacological activities and chemicalstructures of Viburnum dilatatumZhiheng Gao, Yufei Xi, Man Wang, Xiaoxiao Huang*, Shaojiang Song*Key Laboratory of Computational Chemistry-Based Natural Antitumor Drug Research &Development, Liaoning Province, School of Traditional Chinese Materia Medica, ShenyangPharmaceutical University, Shenyang 110016, ChinaAbstractViburnum dilatatum (jiami in Chinese), belonging to the Caprifollaceae family, is widely distributed in Japan and China. Phytochemical investigations of Viburnum dilatatum (V. dilatatum) have resulted in the isolation of triterpenoids, phenolic glycosides essential oil, norisoprenoids, etc. Research results have shown that the chemical constituents of V. dilatatum possess various pharmacological activities, including antihyperglycemic, antioxidant activity and antiulcer effects. This study reviewed the chemical constituents and pharmacological activities of V. dilatatum to provide practical and useful information for further research and development of this plant.Keywords: Viburnum dilatatum; pharmacological activity; chemical structures1 IntroductionViburnum dilatatum (called jiami in Chinese, gamazumi in Japanese and snowball tree in English), beloinging to family Caprifoliaceae, is a deciduous low tree distributed widely in the hills of northern China and Japan [1]. There are many types of chemical constituents in Viburnum dilatatum (V. dilatatum), including triterpenoids, * Author to whom correspondence should be addressed. Address:School of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, 103 Wenhua Rd., Shenyang 110016, China; Tel.: +86-24-43520793 (Xiaoxiao Huang); +86-24-43520707 (ShaojiangSong);E-mail:*******************(XiaoxiaoHuang); ****************(ShaojiangSong).Received: 2021-04-16 Accepted: 2022-08-28phenolic glycosides and norisoprenoids [2-4]. The leaves have been utilized as a traditional Chinese medicine, and phenolic compounds have been reported as the main active chemical component of the leaves. Many researchers have analyzed the functions of these medicinal components and found that these components have good antioxidant antihyperglycemic and antiulcer effects. For example, the gamazumi crude extract obtained from the squeezed juice of the fruit prevented oxidative injury in rats [5]. This review described the chemical structures and pharmacological activities of V. dilatatum, so as to help readers understand comprehensively the research progress of V. dilatatum and provide help for the development of V. dilatatum.2 Chemical constituents and structuresPrevious reports have indicated that the main chemical constituents of V. dilatatum are phenolic glycosides and triterpenoids.2.1 Phenolic glycosidesThirteen phenolic glycosides were isolated and identified from V. dilatatum by extensive spectroscopic methods, namely p -hydroxyphenyl-6-O -trans-caffeoyl-β-D -glucoside (1) [6], p -hydroxyphenyl-6-O -trans-caffeoyl-β-D -alloside (2) [6], 4-allyl-2-methoxyphenyl-6-O -β-D -apiosyl(1→6)-β-D -glucoside (3) [6], 1-(4’-hydroxy-3’-methoxypheny1)-2-[2’’-hydroxy-4’’-(3’’’-hydroxypropyl)]-1,3-propanediol-l-O -β-D -glucopyranoside (erythro isomer) (4-7) [7], neochlorogenic acid methyl ester (8-9) [7], cryptochlorogenic acid methyl ester (10-11) [7], cyanidin-3-sambubioside (Cy-3-sam) (12) [8], cyanidin-3-glucoside (Cy-3-glc) (13) [8], 5-O -caffeoyl-4-methoxyl quinic acid (4-MeO-5-CQA) (14) [8], chlorogenic acid (5-CQA) (15) [8], quercetin (16) [8], 2-(glucopyranosyloxy)-benzyl-3-(glucopyranosyloxy)-benzoate (17) [9] and jiamizioside E (18) [10]. These structures are shown in Fig. 1.Fig. 1 Phenolic glycosides isolated from V . dilatatumContinued fig. 12.2 TriterpenoidsThere were about seventeen triterpenoids isolated and characterized from V. dilatatum , such as viburnols A (19) [11], viburnols B (20) [11], viburnols C (21) [11], viburnols D (22) [11], viburnols E (23) [11], viburnols F (24) [12], viburnols G (25) [12], viburnols H (26) [12], viburnols I (27) [12], viburnols J (28) [12],viburnols K (29) [12], viburnudienone B 2methyl ester (30) [13], viburnenone H 2 (31) [13],v i b u r n e n o n e B 2 m e t h y l e s t e r (32) [13], viburnudienone B 1 methyl ester (33) [13], viburnenone H 1 (34) [13], and viburnenone B 2 methyl ester (35) [13]. The structures are shown in Fig. 2.Continued fig. 23 Pharmacological activities3.1 Antioxidant activityOxidative stress caused by free radicals and their derivatives leads to disturbances in redox homeostasis. Reactive oxygen species (ROS) are not only endogenously produced during intracellular metabolic processes but also generated by exogenous stimuli such as UV radiation, pollutants, smoke and drugs. The cell triggers its defense systems or undergoes apoptosis when intracellular oxidative status increases. It influences numerous cellular processes including core signaling pathways, which are associated with development of systematic and chronic disorders, such as aging and cancer. Therefore, it is critical to remove cellular oxidants and restore redox balance.solution of V. dilatatum (GSS) had strong antioxidant activity in vivo and prevent stress-induced oxidative damage by the XYZ-dish method and the澳electron spin resonance (ESR) method [14]. The experimental result showed that the concentrations of lipid peroxide in plasma, liver and stomach in the GSS group were reduced. Furthermore, the activities of plasma lactic dehydrogenase, amylase and creatine phosphokinase are ordinarily increased by stress. However, these activities in the GSS group decreased to that in the control group. It was concluded that gastric ulcer formation, increase of lipid peroxidation in plasma and tissues and elevation of plasma enzymatic activities were confirmed in rats with water immersion restraint stress. It was also found that intake of GSS could protect the stomach and other tissues from oxidative damage.Kim et al. identified and isolated two major anthocyanins by NMR and LC-ESI-MS/MS, namely, cyanidin 3-sambubioside (I) and kuromanin (II) [15]. By the electron spin resonance method, the superoxide anion radical scavenging activities of I and II were evaluated with the IC 50 values of 17.3 and 69.6 µM, and their activities on hydroxyl radicals were evaluated with the IC 50 values of 4.3 and 53.2 mM. As the positive control, the IC 50 values of ascorbic acid were 74.2 µM on superoxide anion radicals and 3.0 mM on hydroxyl radicals, respectively. The above results suggested that these anthocyanins with radical scavenging properties might be the key compounds contributing to the antioxidant activity and physiological effects of V . dilatatum fruits.Woo et al. determined the free radical scavenging capacity of VD (the leaves of V. dilatatum ) [16]. Anti-oxidant activity of the extracts was assessed by the ability to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) or 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radicals. Butylated hydroxytoluene (BHT), a synthetic antioxidant, or α-tocopherol, was used as the positive control in these assays. The experimental result showed that VD inducedincrease in radical scavenging activity. In addition, lipid peroxidation inhibitory activity was determined via measurement of MDA (Malondialdehyde) levels using mouse liver tissue homogenate treated with various concentrations of the extracts. The concentration-dependent decrease in MDA levels observed was consistent with radical scavenging activities of the extracts. To examine whether VD extracts could protect mam-malian cells from oxidative stress, cultures of a human mammary gland-derived epithelial cell line MCF-7 were treated with each extract prior to challenging them with tBHP. The intracellular ROS (Reactive oxygen species) production was determined with the relative intensity of dichlorofluorescein fluorescence. While intracellular ROS formation was significantly promoted by tBHP treatment, the augmented ROS level was significantly reduced after the treatment with VD extracts.3.2 Antihyperglycemic effectIwai et al. used an oral glucose tolerance test on the diabetic rats [17]. They found that the elevation of plasma glucose level after oral administration of 2 g/kg glucose was suppressed by the repeated administration of the freeze-dried powder of V. dilatatum fruit juice (CEV). The α-glucosidase inhibitory activities of isolated compounds from CEV were also measured. Cyanidin 3-sambubioside and 5-caffeoyl quinic acid A showed inhibitory activity. These results suggested that V. dilatatum fruit had the antihyperglycemic effects.4 ConclusionV. dilatatum is distributed widely in the hills of northern China and Japan. Currently, the studies on V. dilatatum have been conducted at home and abroad, but few studies focus on its chemical components and pharmacological activities. Previousphytochemical investigations showed that the constituents of V. dilatatum included triterpenoids, phenolic glycosides, norisoprenoids and other compounds. This study describes thirteen phenolic glycosides and seventeen triterpenoids and their different degrees of antihyperglycemic, antioxidant activity and antiulcer effects, aiming to provide a reference for further studies on V. dilatatum and pharmaceutical development.References[1] Jeffrey B, Harborne A. Colour atlas of medicinal plantsof Japan. Phytochemistry, 1981, 20: 1467.[2] Miyazawa M, Hashidume S, Takahashi T, et al. Aromaevaluation of gamazumi (Viburnum dilatatum) by aroma extract dilution analysis and odour activity value.Phytochem Anal, 2012, 23: 208-213.[3] Kurihara T, Kikuchi M. Studies on the constituentsof flowers. IV. On the components of the flower of Viburnum dilatatum Thunb. J Health Sci, 1975, 95: 1098-1102.[4] Machida K, Kikuchi M. Norisoprenoids from Viburnumdilatatum. Phytochemistry, 1996, 41: 1333-1336. [5] Iwai K, Onodera A, Matsue H. Mechanism of preventiveaction of Viburnum dilatatum Thunb (gamazumi) crude extract on oxidative damage in rats subjected to stress. J Sci Food Agric, 2010, 83: 1593-1599.[6] Machida K, Nakano Y, Kikuchi M. Phenolic glycosidesfrom Viburnum dilatatum. Phytochemistry, 1991, 30: 2013-2014.[7] Machida K, Kikuchi M. Phenolic compounds fromViburnum dilatatum. Phytochemistry, 1992, 31: 3654-3656.[8] Kim MY, Iwai K, Matsue H. Phenolic compositions ofViburnum dilatatum Thunb. fruits and their antiradical properties. J Food Compos Anal, 2005, 18: 789-802. [9] Lu D, Yao S. Phenolic glycoside from the roots ofViburnum dilatatum. Nat Prod Commun, 2009, 4: 945-946.[10] Wu B, Zeng X, Zhang Y. New metabolite fromViburnum dilatatum. Nat Prod Commun, 2010, 5: 1097-1098.[11] Machida K, Kikuchi M. Viburnols: Novel triterpenoidswith a rearranged dammarane skeleton from Viburnum dilatatum. Tetrahedron Lett, 1996, 37: 4157-4160. [12] Machida K, Kikuchi M. Viburnols: Six noveltriterpenoids from Viburnum dilatatum. Tetrahedron Lett, 1997, 38: 571-574.[13] Machida K, Kikuchi M. Studies on the Constituents ofViburnum Species. XIX. Six New Triterpenoids from Viburnum dilatatum Thunb. Chem Pharm Bull, 1999, 47: 692-694.[14] Iwai K, Onodera A, Matsue H, et al. Antioxidant activityand inhibitory effect of Gamazumi (Viburnum dilatatum THUNB.) on oxidative damage induced by water immersion restraint stress in rats. Int J. Food Sci Nutr, 2001, 52: 443-451.[15] Kim MY, Iwai K, Onodera A, et al. Identification andAntiradical Properties of Anthocyanins in Fruits of Viburnum dilatatum Thunb. J Agric Food Chem, 2003, 51: 6173-6177.[16] Woo YJ, Lee HJ, Jeong YS, et al. Antioxidant Potentialof Selected Korean Edible Plant Extracts. Bio Med Res Int, 2017, 2017: 1-9.[17] Iwai K, Kim MY, Akio O, et al. Alpha-glucosidaseinhibitory and antihyperglycemic effects of polyphenols in the fruit of Viburnum dilatatum Thunb. J Agric Food Chem, 2006, 54: 4588-4592.。
洋甘菊提取液MSDS英文版

1. IDENTIFICATION OF THE SUBSTANCE/TREPARATION AND THE COMPANY/UNDERTAKING3.HAZARDS IDENTIFICATION4. FIRST AID MEASURESMATERIAL SAFETY DATA SHEETProduct name:Supplier:Tel:EMERGENCY OVERVIEW: May cause skin irritation and/or dermatitisPrinciple routes of exposure: Inhalation: Ingestion: Skin contact: Eye contact:SkinMay cause irritation of respiratory tract May be harmful if swallowed May cause allergic skin reaction Avoid contact with eyesStatements of hazard MAY CAUSE ALLERGIC SKIN REACTION.Statements of Spill of Leak Label Eliminate all ignition sources. Absorb and/or contain spill with inert materials (e.g., sand, vermiculite). Then place in appropriate container. For large spills, use water spray to disperse vapors, flush spill area. Prevent runoff from entering waterways or sewers.General advice:POSITION/INFORMATION ON INGREDIENTSInhalation:Skin contact:Ingestion:Eye contact:Protection of first – aiders:Medical conditions aggravated by exposure: In the case of accident or if you fell unwell, seek medical advice immediately (show the label where possible).Move to fresh air, call a physician immediately.Rinse immediately with plenty of water and seek medical adviceDo not induce vomiting without medical advice.In the case of contact with eyes, rinse immediately with plenty of water and seek medical advice.No information availableNone knownSuitable extinguishing media:Specific hazards:Special protective equipment for firefighters:Flash point:Autoignition temperature:NFPA rating Use dry chemical, CO2, water spray or “alcohol” foam Burning produces irritant fumes.As in any fire, wear self-contained breathing apparatus pressure-demand, MSHA/NIOSH (approved or equivalent) and full protective gearNot determinedNot determinedNFPA Health: 1 NFPA Flammability: 1 NFPA Reactivity: 0Personal precautions: Environmental precautions: Methods for cleaning up: Use personal protective equipment.Prevent product from entering drains.Sweep up and shovel into suitable containers for disposalStorage:7. HANDLING AND STORAGE5.FIRE-FIGHTING MEASURES6. ACCIDENTAL RELEASE MEASURESRoom temperature Handling:Safe handling advice: Incompatible products:Use only in area provided with appropriate exhaust ventilation.Wear personal protective equipment.Oxidising and spontaneously flammable productsEngineering measures: Respiratory protection: Skin and body protection:Eye protection: Hand protection: Hygiene measures:Ensure adequate ventilation.Breathing apparatus only if aerosol or dust is formed. Usual safety precautions while handling the product will provide adequate protection against this potential effect. Safety glasses with side-shieldsPVC or other plastic material glovesHandle in accordance with good industrial hygiene and safety practice.Melting point/range: Boiling point/range: Density: Vapor pressure: Evaporation rate: Vapor density: Solubility (in water): Flash point:Autoignition temperature:No Data available at this time. No Data available at this time. No data available No data available No data available No data available No data available Not determined Not determinedStability: Stable under recommended storage conditions. Polymerization: None under normal processing.Hazardous decomposition products: Thermal decomposition can lead to release of irritating gases and vapours such as carbon oxides.Materials to avoid: Strong oxidising agents.10. STABILITY AND REACTIVITY9. PHYSICAL AND CHEMICAL PROPERTIES8. EXPOSURE CONTROLS/PERSONAL PROTECTION11. TOXICOLOGICAL INFORMATIONConditions to avoid: Exposure to air or moisture over prolonged periods.Product information Acute toxicityChronic toxicity:Local effects: Chronic exposure may cause nausea and vomiting, higher exposure causes unconsciousness.Symptoms of overexposure may be headache, dizziness, tiredness, nausea and vomiting.Specific effects:May include moderate to severe erythema (redness) and moderate edema (raised skin), nausea, vomiting,headache.Primary irritation: Carcingenic effects: Mutagenic effects: Reproductive toxicity:No data is available on the product itself. No data is available on the product itself. No data is available on the product itself. No data is available on the product itself.Mobility:Bioaccumulation: Ecotoxicity effects: Aquatic toxicity:No data available No data available No data availableMay cause long-term adverse effects in the aquatic environment.12. ECOLOGICAL INFORMATION13. DISPOSAL CONSIDERATIONSWaste from residues/unused products:Contaminated packaging:Waste disposal must be in accordance with appropriate Federal, State and local regulations. This product, if unaltered by use, may be disposed of treatment at a permitted facility or as advised by your local hazardous waste regulatory authority. Residue from fires extinguished with this material may be hazardous.Do not re-use empty containers.UN/Id No:Not regulated14. TRANSPORT INFFORMATIONDOTProper shipping name: Not regulatedTGD(Canada)WHMIS hazard class: Non - controlledIMDG/IMOIMDG – Hazard Classifications Not ApplicableIMO – labels:15. REGULATORY INFOTMATION International Inventories16. OTHER INFORMATIONPrepared by: Health & SafetyDisclaimer: The information and recommendations contained herein are based upon tests believed to be reliable.However, XABC does not guarantee the accuracy or completeness NOR SHALL ANY OF THIS INFORMATION CONSTITUTE A WARRANTY, WHETHER EXPRESSED OR IMPLIED, AS TO THE SAFETY OF THE GOOD, THE MERCHANTABILITY OF THE GOODS, OR THE FITNESS OF THE FITNESS OF THE GOODS FOR A PARTICULAR PURPOSE. Adjustment to conform to actual conditions of usage maybe required. XABC assumes no responsibility for results obtained or for incidental or consequential damages, including lost profits arising from the use of these data. No warranty against infringement of any patent, copyright or trademark is made or implied.End of safety data sheet。
LOXO-101_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:LOXO–101 is a potent, ATP competitive TRK inhibitor with IC50s in low nanomolar range for inhibition of all TRK family members in binding and cellular assays, with 100x selectivity over other kinases, with 2 to 20 nM cellular potency against the TRKA, TRKB, and TRKC kinases.In vivo: A single dose (30 mg/kg) of LOXO–101 reduced tyrosine phosphorylation of TRKA and downstream signal transduction (pERK)in the tumor >80%. LOXO–101 was well tolerated up to 200 mg/kg/day for 14 d in this model.In vitro: LOXO–101 was evaluated for off–target kinase enzyme inhibition against a panel of 226 non–TRK kinases at a compound concentration of 1,000 nM and ATP concentrations near the Km for each enzyme. LOXO–101 demonstrated greater than 50%inhibition for only one non–TRK kinase (TNK2 IC50 = 576 nM).References:[1]. Nagasubramanian R et al. Infantile Fibrosarcoma With NTRK3–ETV6 Fusion Successfully Treated With the Tropomyosin–Related Kinase Inhibitor LOXO–101. Pediatr Blood Cancer. 2016 Aug;63(8):1468–70.[2]. LOXO–101, a pan TRK inhibitor, For The Treatment Of TRK–driven Cancers. Lori Kunkel et al.[3]. Robert C. Doebele et al. An oncogenic NTRK fusion in a soft tissue sarcoma patient with response to the tropomyosin–related kinase (TRK) inhibitor LOXO–101. Cancer Discov. 2015 Oct; 5(10): 1049–1057.Product Name:LOXO–101Cat. No.:HY-12866CAS No.:1223403-58-4Molecular Formula:C 21H 22F 2N 6O 2Molecular Weight:428.44Target:Trk Receptor Pathway:Protein Tyrosine Kinase/RTK Solubility:DMSO: ≥ 4.6 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
分子球棍模型 演示文稿1

(63) 乙酸酐 ) (67)乙酸乙酯 ) (71)对苯醌 ) (75)吡咯 ) (79)恶唑 ) (83)苯并噻吩 ) (87) 2-吡喃酮 ) 吡喃酮 (91)吡嗪 ) (95)苯并 吡喃酮 )苯并-4-吡喃酮 (99) D-(-)-赤藓糖 ) 赤藓糖 (103)D-(-)-来苏糖 ) 来苏糖 (107)D-(+)- 葡萄糖 ) (111)D -(-)-古罗糖 ) 古罗糖 (115)异亮氨酸 ) (119)赖氨酸 ) (123)雌酮激素 )
(39) 环己酮 cyclohexanone
(40) 环己酮肟 cyclohexanone-oxime
(41)溴化乙基镁 溴化乙基镁 ethylmagnesium bromide
(42) 三甲基氯硅烷 trimethylsilane chloride
(43)甲苯 甲苯tolune 甲苯
(44) 二甲苯 二甲苯1,4-dimethylbenzene
(103) D-(-)-来苏糖 来苏糖 D-(-)-lyxose
(104) D-(-)-木糖 木糖 D-(-)-xylose
(105) D-(+)-阿洛糖 阿洛糖 D-(-)-allose
(106) D-(+)-阿卓糖 阿卓糖 D-(-)-altrose
(107) D-(+)- 葡萄糖 D-(+)-glucose
(13)乙炔 (ethyne) )
(14)2-丁炔 (2-butyne) ) 丁炔
(15)氯甲烷 ) (chloromethane)
(16)烯丙基氯 ) (3-chloro-1-propene)
(17)碘甲烷 ) (iodomethane)
(18)碘仿 ) (iodoform)
顶空毛细管柱气相色谱法测定饮用水中三氯甲烷和四氯化碳的方法改进

分析检测顶空毛细管柱气相色谱法测定饮用水中三氯甲烷和四氯化碳的方法改进刘 燕(平度市疾病预防控制中心,山东平度 266700)摘 要:目的:改进饮用水中三氯甲烷和四氯化碳的顶空毛细管柱气相色谱检测方法。
方法:用微量注射器量取、配制三氯甲烷和四氯化碳混合标准溶液,用顶空瓶封闭保存,用顶空毛细管柱气相色谱法检验。
结果:三氯甲烷在6.01~60.10 μg·L-1下、四氯化碳在0.202~2.020 μg·L-1下,相关系数分别为0.999 1~0.999 9、0.999 1~0.999 8,回收率分别为91.3%~103.7%、92.9%~106.0%,检出限分别为7.0×10-3μg·L-1、7.0×10-4μg·L-1,精密度分别为2.3%、3.1%。
结论:该方法适用于饮用水中三氯甲烷和四氯化碳的测定,且具有良好的线性关系,准确度和精密度高。
关键词:顶空毛细管柱气相色谱;三氯甲烷;四氯化碳Improvement of Headspace Capillary Column GasChromatography for Determination of Chloroform and Carbon Tetrachloride in Drinking WaterLIU Yan(Pingdu Center for Disease Control and Prevention, Pingdu 266700, China) Abstract: Objective: Improving the headspace capillary column gas chromatography method for the detection of chloroform and carbon tetrachloride in drinking water. Method: Weigh and prepare a mixed standard solution of chloroform and carbon tetrachloride using a micro syringe, seal and store it in a headspace bottle, conduct headspace capillary column gas chromatography testing. Result: Trichloromethane (test result range: 6.01~60.10 μg·L-1), carbon tetrachloride (test result range: 0.202~2.020 μg·L-1), with correlation coefficients ranging from 0.999 1 to 0.999 9 and 0.999 1 to 0.999 8. The recovery rates were 91.3% to 103.7% and 92.9% to 106.0%. The detection limit was 7.0×10-3 μg·L-1 and 7.0×10-4 μg·L-1, with a precision of 2.3% and 3.1%. Conclusion: This method is suitable for the determination of chloroform and carbon tetrachloride in drinking water, and has good linear relationship, accuracy, and precision.Keywords: headspace capillary column gas chromatography; chloroform; carbon tetrachloride氯消毒是当前应用率最高的一种生活饮用水消毒方法,消毒产物主要为有机卤代物,通常包括三氯甲烷、四氯化碳、一氯甲烷、二氯一溴甲烷、一氯二溴甲烷和溴仿等成分。
常用蛋白酶切割位点
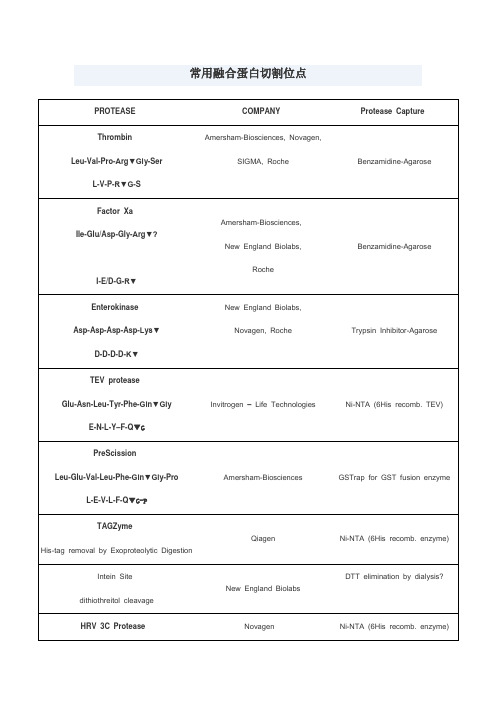
4.溴化氰处理,专一性的切割甲硫氨酸羧基端的肽键。
SIGMA, Roche
Benzamidine-Agarose
Factor Xa
Ile-Glu/Asp-Gly-Arg▼?
I-E/D-G-R▼
Amersham-Biosciences,
New England Biolabs,
Roche
Benzamidine-Agarose
Enterokinase
Asp-Asp-Asp-Asp-Lys▼
羧肽酶
羧肽酶B可以切割C端的Lys或Arg;羧肽酶A可以切割C端除了Lys、Arg、Pro的氨基酸,但如果倒数第二个氨基酸为Pro两种羧肽酶均不能作用
1.胰蛋白酶属肽链内切酶,能把多肽链中Lys和Arg残基中的羧基侧切断。
2.胰凝乳蛋白酶(亦称糜蛋白酶)属肽链内切酶,主要切断多肽链中的芳香族氨基酸(Phe、Trp、Tyr)残基的羧基一侧。
LifeSensors
Ni-NTA (6His recomb. enzyme)
Kex-2
-Arg-X-Lys/Arg-Arg▼
Invitrogen – Life Technologies,
Ni-NTA (6His recomb. enzyme)
KEX2对arg的专一性高,要求最重要。
Arg前为lys效率最高,不切-Arg-lys,Pro影响KEX2切割
Ni-NTA (6His recomb. TEV)
PreScission
Leu-Glu-Val-Leu-Phe-Gln▼Gly-Pro
上海易恩化学技术有限公司正己醛化学品安全技术说明书

化学品安全技术说明书公司地址:上海化学工业区奉贤分区银工路28号E栋楼客服热线:400-133-2688 1 化学品及企业标识1.1 产品标识符化学品俗名或商品名:正己醛CAS No.:66-25-1别名:己醛;1.2 鉴别的其他方法无数据资料1.3 有关的确定了的物质或混合物的用途和建议不适合的用途仅供科研用途,不作为药物、家庭备用药或其它用途。
2 危险性概述2.1 GHS分类健康危害急性毒性(经口):AcuteTox.4严重损伤/刺激眼睛:EyeIrrit.2皮肤腐蚀/刺激:SkinIrrit.22.2 GHS 标记要素,包括预防性的陈述危害类型GHS02:易燃物;信号词 【警告】危险申明H226 易燃液体和蒸气。
警告申明无数据资料 无数据资料RSHazard symbol(s) XiR-phrase(s) R10;R38S-phrase(s) S16;S26;S392.3 其它危害物-无3 成分/组成信息3.1 物质分子式 - C6H12O分子量 - 100.164 急救措施4.1 必要的急救措施描述一般的建议请教医生。
向到现场的医生出示此安全技术说明书。
如果吸入用大量水彻底冲洗至少15分钟并请教医生。
在皮肤接触的情况下用肥皂和大量的水冲洗。
请教医生。
在眼睛接触的情况下无数据资料如果误服用水雾,耐醇泡沫,干粉或二氧化碳灭火。
4.2 最重要的症状和影响,急性的和滞后的最重要的症状和健康影响据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示如有必要,佩戴自给式呼吸器进行消防作业。
5 消防措施5.1 灭火介质火灾特征无数据资料灭火方法及灭火剂碳氧化物5.2 源于此物质或混合物的特别的危害使用个人防护装备。
避免吸入蒸气、气雾或气体。
保证充分的通风。
消除所有火源。
注意蒸气积累达到可爆炸的浓度,蒸气可蓄积在地面低洼处。
5.3 救火人员的预防喷水冷却未打开的容器。
甲磺酸瑞波西汀对照标化记录

名称
配制批号
氨试液
酚酞指示液
醋酸盐缓冲液(pH3.5)
标准铅贮备液
硫代乙酰胺试液
标准铅溶液取用量
ml
试验结果
乙管中显出的颜色甲管颜色
结论
符合规定()不符合规定()
检验人:检验日期:
对照品标化记录(5/5)
品名
甲磺酸瑞波西汀
批号
4.色谱纯度(附液相图谱)
仪器型号及编号
高效液相色谱仪,型号:编号:
对照品标化记录(1/5)
产品名称
甲磺酸瑞波西汀
批号
规格
收样日期
数量
检验日期
检品来源
有效期至
检验依据
国家食品药品监督管理局标准(试行)YBH02392008
甲磺酸瑞波西汀对照品质量标准
一、性状:
1.本品为_____________________。【应为白色或类白色结晶性粉末】
检验人:检验日期:
2.熔点
(应>1.5)
面积%=(应≥99.5%)
结论
符合规定()不符合规定()
检验人:检验日期:
复核人
复核日期
品名
甲磺酸瑞波西汀
批号
3.红外鉴别:取本品及甲磺酸瑞波西汀对照品适量,分别按SOP-QTY 004《红外分光光度法》测定。【红外光谱(KBr压片法)应与对照品谱图一致】(附红外图谱)
甲磺酸瑞波西汀对照品来源:批号:含量:
仪器型号及编号
傅立叶变换红外光谱仪,型号:编号:
电热鼓风干燥箱,型号:编号:
试验温度、湿度
品名
甲磺酸瑞波西汀
批号
二、鉴别:
1.理化鉴别
仪器型号及编号
