AR-42_935881-37-1_DataSheet_MedChemExpress
SAS-1
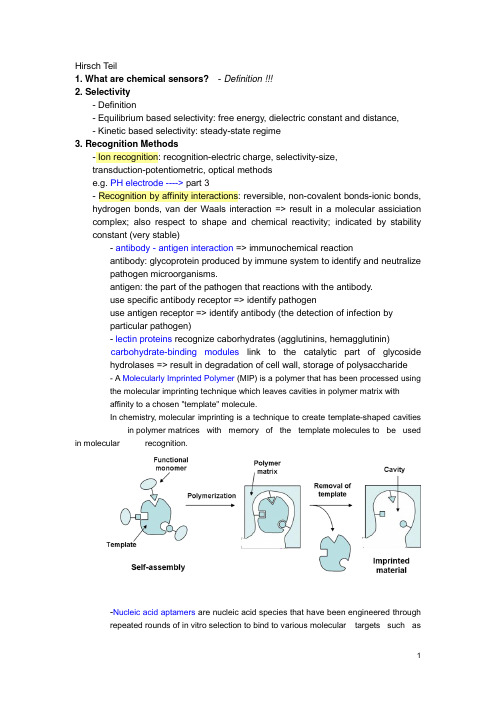
Hirsch Teil1. What are chemical sensors?- Definition !!!2. Selectivity- Definition- Equilibrium based selectivity: free energy, dielectric constant and distance,- Kinetic based selectivity: steady-state regime3. Recognition Methods- Ion recognition: recognition-electric charge, selectivity-size,transduction-potentiometric, optical methodse.g. PH electrode ----> part 3- Recognition by affinity interactions: reversible, non-covalent bonds-ionic bonds, hydrogen bonds, van der Waals interaction => result in a molecular assiciation complex; also respect to shape and chemical reactivity; indicated by stability constant (very stable)- antibody - antigen interaction => immunochemical reactionantibody: glycoprotein produced by immune system to identify and neutralizepathogen microorganisms.antigen: the part of the pathogen that reactions with the antibody.use specific antibody receptor => identify pathogenuse antigen receptor => identify antibody (the detection of infection byparticular pathogen)- lectin proteins recognize caborhydrates (agglutinins, hemagglutinin)carbohydrate-binding modules link to the catalytic part of glycosidehydrolases => result in degradation of cell wall, storage of polysaccharide- A Molecularly Imprinted Polymer (MIP) is a polymer that has been processed usingthe molecular imprinting technique which leaves cavities in polymer matrix withaffinity to a chosen "template" molecule.In chemistry, molecular imprinting is a technique to create template-shaped cavities in polymer matrices with memory of the template molecules to be used in molecular recognition.-Nucleic acid aptamers are nucleic acid species that have been engineered throughrepeated rounds of in vitro selection to bind to various molecular targets such assmall molecules, proteins, nucleic acids, and even cells, tissues and organisms.Aptamers are useful in biotechnological and therapeutic a pplications as they offer molecular recognition properties that rival that of the commonly used bimolecular antibodies.- Recognition by nucleic acids: hydrogen bonds between two distinct pairs of nucleobases => two complementary nucleic acids form a double strand association complex => called hybridizationnucleic acid sensors: short single strand NA as receptor to recognize a particular NA sequence in the analyte NA => detection of genetic anomalies and pathogen mircoorganism- Recognition by enzyme: dynamic processEnzyme: protein compound that function as catalysts in chemical reaction occurring in living system.- Recognition by cells and tissues: advantages of enzyme incorporated in biological materials => in their natural environmentsee part 3, Wegener - Recognition by gases and vapors: based on sorption at solid material => surface-adsorption, inner-absorption; purely physical phenomenon or chemical reaction.4. Transduction MetohdosChemical transduction: monitoring the change of chemical composition of the sensing element in response to the recognition process. => change in concentration/amount is measured => detect primary product -> secondary product or coreagent -> labeling productLABEL can be a simple molecular species or nanoparticals that can be detected by available physiochemical methods => enzyme, fluorescent dyes, luminescent dyes, electroactive compoundsPhysical transduction: a specific physical property of the sensing element that is affected by its interaction with the analyte is monitored. => mass, reflective index, dielectric properties, electrical resistivity => LABEL-FREE- Thermometric transductionRecognition of the analyte leads to change in temperature => only catalytical processes generate sufficient heat to the measurement => application: combustible gases react with O2 at the surface of a catalyst.- Transduction based on mechanical effectsRecognition leads to change in mass of the sensing element => monitored by mass tranducer based on quartz crystal microbalance (QCM)----------------------------------------------------------------------------------------------------------------- QCM, correct name: Thickness shear modePiezoelectric effect:generation of electrical charges on the surface of a solid by strain, pressure or torsion (mechanical deformation of solid) =>electricity resulting from pressureI nverse piezoelectric effect:application of charges to surfaces of piezoelectricsolid generates mechanical deformation (elongation, contraction, torsion)QCM is based on Inverse piezoelectric effect!# AT cut => 35`15`=> minimum temperature coefficient at 50~70 CIt makes the AT-cut well suited to applications requiring high degree of frequency stability over wide temperature ranges.## Electrodes are applied on both sides, and AC voltage applied.DC cannot flow across the crystal because it consists of an insulator material;however the crystal somewhat behaves as capacitor and allow an AC current to f low along the left-hand loop.AC voltage applied => leads to shear oscillation of crystal => when the voltage frequency matches the intrinsic vibration frequency of the crystal => the vibration amplitude is at maximal => the resonant => resonant frequency (f0) => depend on crystal thickness (e.g. d q= 330 um, f0= 5MHZ), density and elasticity of piezoelectric material### AT-cut resonator: thickness: ~0.2 mm, diameter of the active area: 5~20 mm #### Deposition of a homogenous mass film (a rigid overlay)Sauerbrey equation:Cf indicate sensitivity of QCMcondition of this equation: rigid deposited mass; △m<2% of crystal mass;operated in vacuum or in gaseous atmopphereIn liquid: the liquid breaks the vibration by friction => lessen f0Thickness of the layer must be greater than the wave decay lengththat is of 250 nm of 5 MHz resonator at water. ----> part 2!!!##### QCM in practice => see p.41----------------------------------------------------------------------------------------------------------------- - Resistive and capacitive transductionRecognition leads to changes in the electrical property of this materialResistive transduction: gases interact with MOS => change in electrical resistivity Capactive transduction => dielectric constant- Electrochemical transductionsee part 2, Matysik - Optical transductionOptical transduction can be based on light emission or light absorption, also by physical quantity (reflective index) and light scattering.5. Sensor Configuration and Fabrication- Lateral flow assayA typical test strip consists of the following components:1. Sample pad – an absorbent pad onto which the test sample is applied2. Conjugate pad –this contains antibodies specific to the target analyte;conjugated to coloured particles (e.g. gold nanoparticles)3. Reaction membrane –typically a hydrophobic nitrocellulose or celluloseacetate membrane onto which anti-target analyte antibodies are immobilized in a line across the membrane as a capture zone or test line, and a control zonecontaining antibodies specific for the conjugate antibodies.4. Wicking pad –a further absorbent pad designed to draw the sample acrossthe reaction membrane by capillary action and collect it.Double antibody sandwich assays: the sample migrates from the sample pad through the conjugate pad where any target analyte present will bind to the c onjugate.=> The sample then continues to migrate across the membrane until it reaches the test line where the target or conjugate complex will bind to the immobilized antibodies producing a visible line on the membrane. => The sample then migrates further along the strip until it reaches the control line, where excess conjugate will bind and producea second visible line on the membrane.This control line indicates that the sample has migrated across the membrane as intended. Two clear lines on the membrane is a positive result. A single line in the control zone is a negative result. Double antibody sandwich assays are most suitable for larger analytes, such as bacterial pathogens and viruses, with multiple antigenic sites. 6. Methods and Material in Sensor Preparation- Immobilization at solid surface => integration of a transducer with the receptor Physical adsorption at a solid supportNon covalent immobilization at solid surface => hydrophobic interaction, hydrogen bonding, electrostatic attraction; monolayer; no restrict access; not stable; Langmuir isotherm -> equilibrium interactionSupport material: silica, cellulose acetate, PVCCovalent bonding to the solid supportCovalent conjugation => stable, covalent bond, time consuming, expensiveCommon reactive group: -OH, -NH2, -C=O, -SH- Carboxylic acid with DCC- Glutaraldehyde reacts with the a.a. of lysine in protein => widely used Support: porous material => high specific area, high density of immobilized compounds => hydrogel: immobilized by entrapment/covalent corsslink - Natural polymers: Cellulose, Dextran- Synthetic polymers: Polystyrene- Active polymers: Epoxide (without preliminary activation) -->DNA array !!!- Inactive Polymers: Vicinal hydroxyls actived by CNBr- Inorganic support: Silica, AL2O3, TiO2 => stable at extreme PH- Metal support: noble metals, thiols on golds --> self assembled monolayers!Affinity reaction: avidin-biotin !!!Thin molecular layers: one or several molecular layers in solid support - Self-assembly of amphiphilic compounds: preparation of liposome andmicelles; liposome can be used of entrapment of molecular- Bilipid layer membranes: Langmuir-Blodgett technique- Layer by Layer assembly- Sol-Gel chemistry methods: silica gel => -O-Si-O-- Hydrogels: Xerogel, aerogel- Conducting polymers: Polyacetylene, polyaniline --> gas senor based on CP (----> part 3 !!!); also as entrapment matrix for biological receptors- Mesoporous materials: porous materials with pore (diameter: 2-50 nm,close to protein) => enzyme immobilization by entrapment (crosslinking withglutaraldehyde)- Deposition of polymers onto solid surfaces: dip coating, drop coating, spin coating ----> part 2 !!!Perm-selective memberanes: Nafin ----> Clark oxygen electrode Support-free crosslinkingEntrapment in a polymer networkEncapsulation7. Microfabrication Methodes- Spot Arraying: Contact-based & Noncontact-based; DNA microarray !!!!!Pros & Cons- Thick-film Technology: screen-printing technique (5-50 um thick layer)- Thin-film Technology: Photolithography (2 um)- Softlithography ----> experiment !!!!- Microcontact printing ----> experiment !!!!8. Optical Sensors- Electromagnetic RadiationOptical sensor => interaction of electromagnetic radiation with sensor layer - frequency; wavelength; photon energy (definition)- Structure: integration with wavelength-selection (optical filters) device and light sources (lasers), light detectors (phototransistors)- Optical Waveguides- Optical FibersOptical fibers' structuretotal internal reflection => evanescent wave- Spectrochemical Transduction MethodsSpectrochemical method analysis => light absorption or emission by sample => optical label performs absorption or emission (organic dye or metal complexes) - Light absorption: absorbance => concentration; sensitivity => thickness, absorpyivity, absorptivity => wavelength- Diffuse reflectance spectrometry: refelctance => concentration; suitable forsolid in near IR- Luminescence: Fluorescence spectromerty => fluorophore (label, organic dye or metal complexes, luminescent nanparticle ); steady-statefluorescence measurement, Time-resolved fluormetry; fluorescencequenching; resonance energy transfer (FRET); chemical- andbioluminescence => luminol; electrochemicaluminescence; Ramanspetrometry- Surface Plasmon Resonance Spectroscopy (SPR)。
Trigonox B(滴苷但羊水)产品数据表单说明书

Product Data SheetTrigonox BDi-tert-butyl peroxideTrigonox® B is a pure peroxide in liquid form.CAS number110-05-4EINECS/ELINCS No. 203-733-6TSCA statuslisted on inventory Molecular weight 146.2Active oxygen contentperoxide10.94%SpecificationsAppearance Clear liquidAssay≥ 99.0 %ApplicationsTrigonox® B (Di-tert-butyl peroxide) can be used for the market segments: polymer production, polymer crosslinking and acrylics production with their different applications/functions. For more information please check our website and/or contact us.Half-life dataThe reactivity of an organic peroxide is usually given by its half-life (t½) at various temperatures. For Trigonox® B in chlorobenzene half-life at other temperatures can be calculated by using the equations and constants mentioned below:0.1 hr at 164°C (327°F)1 hr at 141°C (286°F)10 hr at 121°C (250°F)Formula 1kd = A·e-Ea/RTFormula 2t½ = (ln2)/kdEa153.46 kJ/moleA 4.20E+15 s-1R8.3142 J/mole·KT(273.15+°C) KThermal stabilityOrganic peroxides are thermally unstable substances which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition may occur with a substance in the packaging as used for transport is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT80°C (176°F)Method The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria - United Nations, NewYork and Geneva).StorageDue to the relatively unstable nature of organic peroxides, a loss of quality will occur over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max. ) for each organic peroxide product.Ts Max.40°C (104°F) andTs Min.-30°C (-22°F) to prevent crystallizationNote When stored according to these recommended storage conditions, Trigonox® Bwill remain within the Nouryon specifications for a period of at least 6 months afterdelivery.Packaging and transportIn North America Trigonox® B is packed in non-returnable, five gallon polyethylene containers of 30 lb net weight and steel drums of 100 or 340 lb net weight. In other regions the standard packaging is a 30-liter HDPE can (Nourytainer®) for 20 kg peroxide. Delivery in a 200 l steel drum for 150 kg peroxide is also possible in a number of countries. Both packaging and transport meet the international regulations. For the availability of other packed quantities consult your Nouryon representative. Trigonox® B is classified as Organic peroxide type E; liquid, Division 5. 2; UN 3107.Safety and handlingKeep containers tightly closed. Store and handle Trigonox® B in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalis and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for detailed information on the safe storage, use and handling of Trigonox® B. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsAcetone, Methane, tert-ButanolAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Trigonox® and Nourytainer are registered trademarks of Nouryon Functional Chemicals B.V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2022-6-30© 2022Polymer crosslinking Trigonox B。
hss-p-5.75.09 - hyaluronic acid derivatives说明书

5.75.09Section:Prescription DrugsEffective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject:Hyaluronic Acid DerivativesPage:1 of 7Last Review Date:March 13, 2020Hyaluronic Acid DerivativesDescriptionDurolane, Euflexxa, GelSyn-3, GenVisc 850, Hyalgan , SodiumHyaluronate, Supartz , Synojoynt*, Triluron, TriVisc, Visco-3 (sodium hyaluronate)Gel-ONE , Hymovis, Monovisc, Orthovisc (hyaluronan)Synvisc, Synvisc-One (hylan G-F 20)Bolded medications are the preferred products*These medications are included in this policy but are not available in the market as of yetBackgroundOsteoarthritis of the knee is a disease in which the elastoviscous property of the synovial fluid in the knee joint becomes diminished, resulting in less protection and shock absorption. Durolane, Euflexxa, Gel-One, GelSyn-3, GenVisc 850, Hyalgan, Hymovis, Monovisc, Orthovisc, Sodium Hyaluronate, Synvisc, Synvisc-One, Supartz, Synojoynt, Triluron, TriVisc, Visco-3 are hyaluronan derivatives that are injected into the knee joints to increase the elastoviscous properties of arthritic joint fluid and slow its outflow from the joint . The goal of therapy is torestore the viscoelasticity in the affected joints, thereby decreasing pain, improving mobility, and restoring the natural protective functions (1).The American College of Rheumatology (ACR) updated its guidelines for the treatment of osteoarthritis (OA) of the knee in 2012. In mild symptomatic OA, treatment may be limited toFederal Employee Program® 1310 G Street, N.W.Washington, D.C. 20005 202.942.1000Fax 202.942.1125Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 2 of 7patient education, physical and occupational therapy and other non-pharmacologic modalities. Nonpharmacologic modalities strongly recommended for the management of knee OA were aerobic, aquatic, and/or resistance exercises as well as weight loss for overweight patients. Nonpharmacologic modalities conditionally recommended for knee OA included medial wedge insoles for valgus knee OA, subtalar strapped lateral insoles for varus knee OA, medially directed patellar taping, manual therapy, walking aids, thermal agents, tai chi, self-management programs, and psychosocial interventions. Pharmacologic modalities conditionally recommended for the initial management of patients with knee OA included acetaminophen, oral and topical NSAIDs, tramadol, and intraarticular corticosteroid injections (1).Regulatory StatusFDA-approved indication: Hyaluronic acid derivatives are indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy, simple analgesics (e.g., acetaminophen), NSAIDs, tramadol, or intra-articular steroid injections (2-18).The hyaluronic acid derivatives are contraindicated for use in patients with known hypersensitivity to hyaluronan (sodium hyaluronate) preparations. Orthovisc lists hypersensitivity to gram positive bacterial proteins as an additional contraindication (4). Caution should be exercised when Gel-One, Hyalgan, Visco-3, Synvisc, Synvisc-One, Supartz, and Triluron are administered to patients with allergies to avian proteins, feathers, and egg products (3-8, 18).Hyaluronic acid derivatives are contraindicated to treat patients with knee joint infections, infections or skin diseases in the area of the injection site (2-17).A treatment cycle for most of the hyaluronan derivatives typically involves multiple weekly injections. Euflexxa, GelSyn-3, Sodium Hyaluronate, Synvisc, Triluron, TriVisc, and Visco-3 are given for a total of three injections. Orthovisc is given for three or four injections. GenVisc 850, Supartz and Hyalgan are given for a total of three or five injections. Durolane, Gel-One, Synojoynt, and Synvisc-One differ from the other hyaluronan derivatives in that it only requires one injection. Repeat courses of hyaluronan derivatives may be administered if symptoms return (2-18).Upon the basis of high quality supporting evidence, the American Academy of Orthopedic Surgeons cannot recommend using hyaluronic acid for patients with symptomatic osteoarthritis of the knee (19).Related policiesSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 3 of 7Hyaluronate PowderPolicyThis policy statement applies to clinical review performed for pre-service (Prior Approval, Precertification, Advanced Benefit Determination, etc.) and/or post-service claims.Hyaluronic acid derivatives may be considered medically necessary for the treatment of osteoarthritis of the knee and if the conditions indicated below are met.Hyaluronic acid derivatives may be considered investigational for all other indications.Prior-Approval RequirementsAge18 years or older (22 or older for Synvisc, Synvisc-One, and TriVisc)DiagnosisPatient must have the following:Osteoarthritis of the kneeAND ALL of the following:1. Inadequate response to TWO or more of the following conservative non-pharmacologic therapy:a. Cardiovascular (aerobic) activity, such as: walking, biking, stationarybike, aquatic exerciseb. Resistance exercisec. Weight reduction (for persons who are overweight)d. Participation in self-management programse. Wear of medially directed patellar tapingf. Wear of wedged insolesg. Thermal agentsh. Walking aidsi. Physical therapyj. Occupational therapy2. Inadequate response, intolerance, or contraindication to TWO or more of thefollowing:Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 4 of 7a. Acetaminophenb. Oral NSAIDsc. Topical NSAIDs3. Inadequate response, intolerance, or contraindication to intra-articularsteroid injections in which efficacy lasted less than 8 weeks4. Radiologic confirmation of Kellgren-Lawrence Scale score of grade 2 orgreater5. NO dual therapy with another hyaluronic acid injectable6. Non-preferred medications only: Patient MUST have tried at least TWO ofthe preferred products unless the patient has a valid medical exception (e.g.inadequate treatment response, intolerance, contraindication)Prior – Approval Renewal RequirementsAge18 years or older (22 or older for Synvisc, Synvisc-One, and TriVisc)DiagnosisPatient must have the following:Osteoarthritis of the kneeAND ALL of the following:1. Documentation of improvement in pain with previous course of treatment2. At least 12 months has elapsed since last injection of the prior treatmentcycle3. Documentation of reduction of dosing of NSAIDs or other analgesicsduring the 12 month period following the last injection of the prior treatmentcycle4. NO dual therapy with another hyaluronic acid injectable5. Non-preferred medications only: Patient MUST have tried at least TWOof the preferred products unless the patient has a valid medical exception(e.g. inadequate treatment response, intolerance, contraindication) Policy GuidelinesPre - PA AllowanceNoneSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 5 of 7Prior - Approval LimitsDuration12 monthsQuantity One course of therapy for each kneePrior – Approval Renewal LimitsSame as aboveRationaleSummaryOsteoarthritis of the knee is a disease in which the elastoviscous property of the synovial fluid in the knee joint becomes diminished, resulting in less protection and shock absorption. Durolane, Euflexxa, Gel-One, GelSyn-3, GenVisc 850, Hyalgan, Hymovis, Monovisc, Orthovisc, Sodium Hyaluronate, Synvisc, Synvisc-One, Supartz, Synojoynt, Triluron, TriVisc, Visco-3 are hyaluronan derivatives that are injected into the knee joints to increase the elastoviscous properties of arthritic joint fluid and slow its outflow from the joint. The goal of therapy is to restore the viscoelasticity in the affected joints, thereby decreasing pain, improving mobility, and restoring the natural protective functions (1-18).Prior approval is required to ensure the safe, clinically appropriate and cost effective use of the hyaluronic acid derivatives while maintaining optimal therapeutic outcomes.References1. American College of Rheumatology, Subcommittee on Osteoarthritis Guidelines.Recommendations for the medical management of osteoarthritis of the hip and knee:2012 update. Arthritis Care & Research 2012; 64(4):465-474.2. Euflexxa [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc.; July 2016.3. Hyalgan [package insert]. Parsippany, NJ: Fidia Pharma USA Inc.; May 2014.4. Orthovisc [package insert]. Woburn, MA: Anika Therapeutics; June 2005.5. Supartz [package insert]. Durham, NC: Bioventus LLC; April 2015.6. Synvisc [package insert]. Ridgefield, NJ: Genzyme Corp.; December 2014.7. Synvisc-One [package insert]. Ridgefield, NJ: Genzyme Corp.; September 2014;8. Gel-One [package insert]. Warsaw, IN: Zimmer Inc.; May 2011.9. Monovisc [package insert]. Bedford, MA: Anika Therapeutics; December 2013.10. Hymovis [package insert]. Parsippany, NJ: O Fidia Pharma USA Inc.; October 2015.Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 6 of 711. GenVisc 850 [package insert]. Doylestown, PA: OrthogenRx Inc.; January 2015.12. GelSyn-3 [package insert]. Durham, NC: Bioventus LLC; January 2016.13. Durolane [package insert]. Durham, NC: Bioventus LLC; November 2017.14. Visco-3 [package insert]. Warsaw, IN: Zimmer, Inc.; May 2017.15. Sodium Hyaluronate [package insert]. North Wales, PA: Teva Pharmaceuticals USA,Inc.; March 2019.16. Synojoynt [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc.;September 2019.17. TriVisc [package insert]. Doylestown, PA: OrthogenRx, Inc.; September 2018.18. Triluron [package insert]. Florham Park, NJ: Fidia Pharma USA Inc.; March 2019.19. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee.Evidence-based guideline 2nd edition. May 2013.Policy HistoryDate Action ReasonJanuary 2012 Added minimum age - only approved for adultsDecember 2012 Annual editorial review and reference updateDecember 2013 Annual editorial review and reference updateMarch 2014 Annual editorial reviewAddition of examples of non-pharmacological agents and agents of priorfailure medications.April 2014 Line-Addition of Monovisc to PAMarch 2015 Annual criteria review and reference updateMarch 2016 Change from one tried and failed to two tried and failed non-pharmacologic and pharmacologic therapies and addition of the tried and failed of intra-articular steroid and radiologic confirmation of Kellgren-Lawrence Scalescore of grade 2 or greaterAddition of HymovisPolicy # change from 5.11.04 to 5.75.09May 2016 Addition of GelSyn-3 and GenVisc 850December 2016 Annual editorial review and reference updateAdded: no dual therapy with another hyaluronic acid injectableMarch 2017 Bolded preferred products in the title pageJuly 2017 GelSyn-3 has been changed to preferredSeptember 2017 Annual reviewDecember 2017 Addition of Durolane and Visco-3March 2018 Annual editorial reviewRemoval of Tramadol from the T/F listSeptember 2019 Annual review and reference update. Addition of Sodium Hyaluronate,Synojoynt, and TriViscSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 7 of 7December 2019 Annual review. Addition of requirement to trial preferred products January 2020 Addition of TriluronMarch 2020 Annual reviewKeywordsThis policy was approved by the FEP® Pharmacy and Medical Policy Committee on March 13, 2020 and is effective on April 1, 2020.。
AROTEC_RNP_68K_P...

RNP 68K ANTIGEN (Sm-FREE)AROTEC_RNP_68K_Product_Info.pdf Version/Date: B/04.05.20 ATR04-02 RNP 68K antigen 0.20 mgATR04-10 RNP 68K antigen 1.0 mg_________________________________________________________________________________Description of the ProductPurified from bovine thymus. After coating onto ELISA plates the product will bind autoantibodies to RNP 68K antigen.Purity: The RNP 68K autoantigen is more than 90% pure, as assessed by SDS gel electrophoresis.Concentration: 0.1-1.0 mg protein/ml.Storage: The product is stabilised with 0.1% Micr-O-protect TM. Store at -20 o C or below (long term) or at +4o C (short term). Avoid repeated freezing and thawing. Mix thoroughly before use.Clinical and Biochemical DataAutoantibodies directed against the snRNP (small nuclear ribonucleoprotein) autoantigens referred to as RNP and Sm were originally detected in the sera of systemic lupus erythematosus (SLE) patients1,2. Anti-RNP antibodies were subsequently found in the sera of mixed connective tissue disease (MCTD) patients3, and it is now known that when these antibodies occur at high titre and in the absence of Sm, they are a good marker for MCTD3,4. Such autoantibodies are also known to occur in the sera of patients with a range of other rheumatic diseases including progressive systemic sclerosis, rheumatoid arthritis, discoid lupus erythematosus, Sjögren's syndrome and various overlapping conditions5-10.The snRNPs are a group of nuclear particles comprised of several polypeptides associated with a small nuclear RNA molecule11. The most abundant snRNPs are involved in pre-mRNA-splicing11. At least 13 different snRNAs have been identified in mammalian cells11. Whereas autoantibodies directed against Sm are able to precipitate a wide range of snRNAs, RNP autoantibodies are only able to precipitate one particular type, referred to as U1snRNA. Anti-RNP antibodies react with the U1 snRNP-specific polypeptides12 (68K, A and C antigens) whereas anti-Sm antibodies react with polypeptides associated with U1, U2, U5 and U4/U6 snRNAs (B,B', D, E, F and G antigens)12,13.Current ELISA methods for the detection of autoantibodies to RNP often require the simultaneous determination of reactivity to both RNP/Sm and Sm antigens. The reactivity with Sm is then subtracted from that with RNP/Sm, the difference being considered to be due to RNP-specific autoantibodies. The availability of RNP antigen in the absence of Sm would avoid the necessity for a dual assay to detect anti-RNP antibodies and assays have been reported using isolated U1-RNP specific subunits14,15. Since the 68K U1-RNP polypeptide constitutes the major MCTD RNP autoantigen16,17, efforts to produce a recombinant RNP antigen have focussed on this subunit18-20, and not the U1-RNP specific subunits A and C which are known to share sequence homology and cross-reactivity with the Sm antigen B/B’21.Native RNP 68K subunit (present as a 33/35K doublet) is the primary component of AroTec's RNP 68K antigen (Sm-free). Although the human RNP 68K sequence is known22, there is currently no data available for the bovine antigen. However the complete identity between human22 and mouse23 sequences would indicate that such antigens are highly conserved between mammalian species. MethodologyThe following is an ELISA procedure which can be used to detect anti-RNP 68K autoantibodies in human serum using the ATR04 purified antigen:1. Dilute the purified antigen to 0.5-1.0 μg/ml in PBS (10 mM Potassium phosphate, pH 7.4, 0.15 M NaCl).2. Coat ELISA plates with 100 μl of diluted antigen per well. Cover and incubate 24 hours at +4o C.3. Empty the plates and remove excess liquid by tapping on a paper towel.4. Block excess protein binding sites by adding 200 μl PBS containing 1% BSA per well. Cover and incubate at +4o C overnight.5. Empty plates and apply 100 μl of serum samples diluted 1:100 in PBS / 1% BSA / 1% casein / 0.1% Tween® 20. Incubate at room temperature for 1 hour.6. Empty plates and add 200 μl PBS / 0.1% Tween® 20 per well. Incubate 5 minutes then empty plates. Repeat this step twice.7. Apply 100 μl anti-human IgG-enzyme conjugate (horseradish peroxidase or alkaline phosphatase) diluted in PBS / 1% BSA / 1% casein / 0.1% Tween® 20 per well and incubate for 1 hour.8. Repeat step 6.9. Add enzyme substrate and stop the reaction when appropriate.10. Read absorbance in an ELISA spectrophotometer. References1. Tan, E.M. & Kunkel, H.G. (1966) J. Immunol. 96, 4642. Mattioli, M. & Reichlin, M. (1971) J. Immunol. 107, 12813. Sharp, G.C. et al. (1972) Am. J. Med. 52, 1484. Sharp, G.C. et al. (1976) New Engl. J. Med. 295, 11495. Notman, D.D. et al. (1975) Ann. Intern. Med. 83, 4646. Tan, E.M. et al. (1988) Clin. Immunol. Pathol. 47, 1217. Tan, E.M. (1989) Adv. Immunol. 44, 938. van Venrooij, W.J. & Pruijn, G.J.M. (1995) Curr. OpinionImmunol. 7, 8199. Guldner, H.H. (1986) Clin. Immunol. Immunopathol. 40, 53210. Williams, D.G. et al. (1988) J. Immunol. Meth. 113, 2511. Lührmann, R. et al. (1990) Biochim. Biophys. Acta 1087, 26512. Petersson, I. et al. (1984) J. Biol. Chem. 259, 590713. Reuter, R. et al. (1990) Eur. J. Immunol. 20, 43714. Takeda, Y. et al. (1989) Clin. Immunol. Immunopathol. 50, 21315. Williams, D.G. et al. (1988) J. Immunol. Meth. 113, 2516. Combe, B. et al. (1989) Clin. Exp. Immunol. 75, 1817. Francoeur, A.M. (1989) J. Clin. Immunol. 9, 25618. Nyman, U. et al. (1990) Clin. Exp. Immunol. 81, 5219. Ter-Borg, E.J. et al. (1991) J. Rheumatol. 18, 36320. Frorath, B. et al. (1991) BioTechniques 11, 36421. Habets, W.J. et al. (1989) Proc. Natl. Acad. Sci. USA 86, 467422. Theissen, H. et al. (1986) EMBO J. 5, 320923. Kawai, J. et al. (2001) Nature 409, 685Micr-O-protect TM is from Roche Diagnostics GmbH (Mannheim, Germany).Tween® 20 is a registered trademark of ICI Americas Inc. NOTE: No patented technology has been used by AroTecduring the preparation of this product.。
FreeRadicBiolMed7197-203
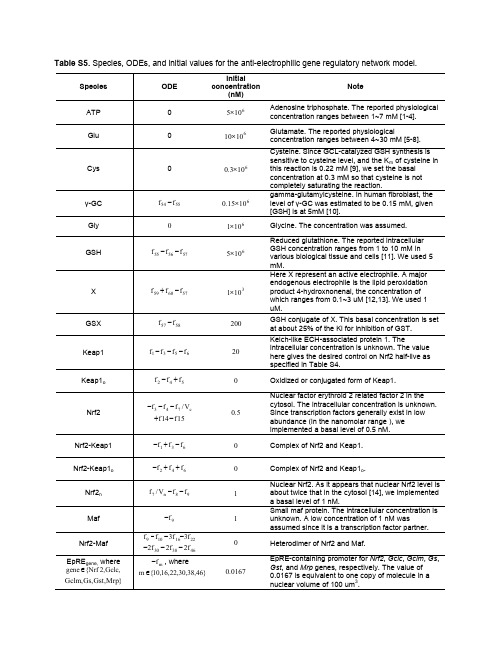
Table S5. Species, ODEs, and initial values for the anti-electrophilic gene regulatory network model.Species ODE Initialconcentration(nM) NoteATP 0 6105× Adenosine triphosphate. The reported physiologicalconcentration ranges between 1~7 mM [1-4]. Glu 0 61010×Glutamate. The reported physiologicalconcentration ranges between 4~30 mM [5-8]. Cys 0 6103.0×Cysteine. Since GCL-catalyzed GSH synthesis is sensitive to cysteine level, and the K m of cysteine in this reaction is 0.22 mM [9], we set the basal concentration at 0.3 mM so that cysteine is not completely saturating the reaction.γ-GC 5554f f − 61015.0× gamma-glutamylcysteine. In human fibroblast, the level of γ-GC was estimated to be 0.15 mM, given [GSH] is at 5mM [10].Gly0 6101× Glycine. The concentration was assumed. GSH575655f f f −−6105×Reduced glutathione. The reported intracellular GSH concentration ranges from 1 to 10 mM in various biological tissue and cells [11]. We used 5 mM.X576059f f f −+ 3101×Here X represent an active electrophile. A major endogenous electrophile is the lipid peroxidation product 4-hydroxnonenal, the concentration of which ranges from 0.1~3 uM [12,13]. We used 1 uM.GSX5857f f −200GSH conjugate of X. This basal concentration is set at about 25% of the Ki for inhibition of GST. Keap16531f f f f −−−20Kelch-like ECH-associated protein 1. Theintracellular concentration is unknown. The value here gives the desired control on Nrf2 half-live as specified in Table S4.Keap1o542f f f +− 0Oxidized or conjugated form of Keap1.Nrf2c 743V /f f f −−−15f 14f −+ 5.0Nuclear factor erythroid 2 related factor 2 in the cytosol. The intracellular concentration is unknown. Since transcription factors generally exist in low abundance (in the nanomolar range ), we implemented a basal level of 0.5 nM. Nrf2-Keap1 631f f f −+− 0 Complex of Nrf2 and Keap1. Nrf2-Keap1o642f f f ++− 0 Complex of Nrf2 and Keap1o .Nrf2n 98n 7f f V /f −−1 Nuclear Nrf2. As it appears that nuclear Nrf2 level is about twice that in the cytosol [14], we implemented a basal level of 1 nM.Maf 9f − 1Small maf protein. The intracellular concentration is unknown. A low concentration of 1 nM wasassumed since it is a transcription factor partner. Nrf2-Maf 2216109f 3f 3f f −−−463830f 2f 2f 2−−− 0Heterodimer of Nrf2 and Maf.EpRE gene, where ,Gclc ,2Nrf {gene ∈ }Mrp ,Gst ,Gs ,Gclmm f −, where }46,38,30,22,16,10{m ∈0167.0EpRE-containing promoter for Nrf2, Gclc , Gclm , Gs , Gst , and Mrp genes, respectively. The value of 0.0167 is equivalent to one copy of molecule in a nuclear volume of 100 um 3.Nrf2-Maf-EpRE gene ,where,Gclc ,2Nrf {gene ∈ }Mrp ,Gst ,Gs ,Gclm m f , where }46,38,30,22,16,10{m ∈Complex of Nrf2-Maf dimer and EpRE-containing promoter for Nrf2, Gclc , Gclm , Gs , Gst , and Mrp genes, respectively.Gene off , where ,Gclc ,2Nrf {gene ∈ }Mrp ,Gst ,Gs ,Gclm n f −, where }47,39,31,23,17,11{n ∈0167.0Inactive state of Nrf2, Gclc , Gclm , Gs , Gst , and Mrp genes, respectively. The value of 0.0167 isequivalent to one copy of gen in a nuclear volume of 100 um 3.Gene on , where ,Gclc ,2Nrf {gene ∈ }Mrp ,Gst ,Gs ,Gclm n f , where }47,39,31,23,17,11{n ∈Active state of Nrf2, Gclc , Gclm , Gs , Gst , and Mrp genes, respectively.Gene mRNA, where ,Gclc ,2Nrf {Gene ∈ }Mrp ,Gst ,Gs ,Gclmq c n p f V /V f −, where}48,40,32,24,18,12{p ∈}49,41,33,25,19,13{q ∈mRNA of Nrf2, Gclc , Gclm , Gs , Gst , and Mrp genes, respectively.GCLC 282120f f f −− 3107.1× Glutamate cysteine ligase catalytic subunit. See the GCLM and GCL entries in the same table.GCLM282726f f f −−31036.0×Glutamate cysteine ligase modifier subunit. At this concentration, the GCLC/GCL ratio is 3 at the basal condition, as specified in reaction 28 in Table S4. GCL2928f f − 3106.0×Glutamate cysteine ligase holoenzyme. Thisconcentration, together with that of GCLC, gives a basal GSH synthesis rate of 386 nM/s, which is close to 360 nM/s measured in B16M melanoma cells given a cell volume of 1000 um 3 [15] GS mono 363534f 2f f −− 0 Glutathione synthetase monomer. GST mono 444342f 2f f −− 0 Glutathione S-transferase monomer.MRP mono525150f 2f f −−Multidrug resistance-associated protein monomer. GS3736f f − 31096.0×Glutathione synthetase dimer. This concentration keeps γ-GC at 0.15 mM at the basal condition. See the γ-GC entry in the same table for the choice of 0.15 mM.GST 4544f f − 310185.0× Glutathione S-transferase dimer. This concentration keeps X at 1 uM at the basal condition. See the X entry in the same table for the choice of 1 uM. MRP5352f f −31022.7×Multidrug resistance-associated protein. This concentration keeps GSX at 0.2 uM at the basal condition.References1. Ko SH, Lee SK, Han YJ, Choe H, Kwak YG, et al. (1997) Blockade of myocardial ATP-sensitive potassium channels by ketamine. Anesthesiology 87: 68-74.2. Gribble FM, Loussouarn G, Tucker SJ, Zhao C, Nichols CG, et al. (2000) A novel method for measurement of submembrane ATP concentration. J Biol Chem 275: 30046-30049.3. Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P (1997) Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med 185: 1481-1486.4. Marcussen M, Larsen PJ (1996) Cell cycle-dependent regulation of cellular ATP concentration, and depolymerization of the interphase microtubular network induced by elevated cellular ATP concentration in whole fibroblasts. Cell Motil Cytoskeleton 35: 94-99.5. Goldstein L (1966) Relation of glutamate to ammonia production in the rat kidney. Am J Physiol 210: 661-666.6. Geerts WJ, Jonker A, Boon L, Meijer AJ, Charles R, et al. (1997) In situ measurement of glutamateconcentrations in the periportal, intermediate, and pericentral zones of rat liver. J Histochem Cytochem 45: 1217-1229.7. Divino Filho JC, Hazel SJ, Furst P, Bergstrom J, Hall K (1998) Glutamate concentration in plasma,erythrocyte and muscle in relation to plasma levels of insulin-like growth factor (IGF)-I, IGF binding protein-1 and insulin in patients on haemodialysis. J Endocrinol 156: 519-527.8. Huang CS, Chang LS, Anderson ME, Meister A (1993) Catalytic and regulatory properties of the heavysubunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem 268: 19675-19680.9. Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP (2005) Glutamate cysteine ligase catalysis:Dependence on ATPand modifier subunit for regulation of tissue glutathione levels. J Biol Chem.10. Ristoff E, Hebert C, Njalsson R, Norgren S, Rooyackers O, et al. (2002) Glutathione synthetasedeficiency: is gamma-glutamylcysteine accumulation a way to cope with oxidative stress in cells with insufficient levels of glutathione? J Inherit Metab Dis 25: 577-584.11. Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52: 711-760.12. Esterbauer H, Zollner H (1989) Methods for determination of aldehydic lipid peroxidation products.Free Radic Biol Med 7: 197-203.13. Esterbauer H, Eckl P, Ortner A (1990) Possible mutagens derived from lipids and lipid precursors.Mutat Res 238: 223-233.14. Pi J, Qu W, Reece JM, Kumagai Y, Waalkes MP (2003) Transcription factor Nrf2 activation byinorganic arsenic in cultured keratinocytes: involvement of hydrogen peroxide. Exp Cell Res 290: 234-245.15. Benlloch M, Ortega A, Ferrer P, Segarra R, Obrador E, et al. (2005) Acceleration of glutathione effluxand inhibition of gamma-glutamyltranspeptidase sensitize metastatic B16 melanoma cells toendothelium-induced cytotoxicity. J Biol Chem 280: 6950-6959.。
体外诊断生化试剂应用参数

CHEMISTRY ACP ALB ALP ALT AM 货号270010036008201110060180006051000503601850111018018001803601870111050018005003600930TEST ACP ALB ALP ALT AM SAMPLE VOL.20371025 DILUTION0101000 REAGENT VOL.1-STEP250300270290250 DILUTION00000 2-STEP50[N] DILUTION00000 WAVE-LENGTH-1410600410340340 WAVE-LENGTH-2450700480410700 METHOD RATE END RATE RATE END REACTION+++--FIRST-POINT-160439 LAST-POINT-1166161616 FIRST-POINT-2N/A N/A N/A N/A N/A LAST-POINT-2N/A N/A N/A N/A N/A NO LAG-TIME YES NO YES YES NO TURBIDITY NO NO NO NO NO PROZONE CHECK NO NO NO NO NO REAGENT OD L-0.5-0.5-0.5-0.5-0.5 H 2.5 2.5 2.5 2.5 2.5 SERUM BLANK L N/A N/A N/A N/A N/A REAGENT OD H N/A N/A N/A N/A N/A MIN-OD-0.5-0.5-0.5-0.5-0.5 MAX-OD 2.0 2.0 2.0 2.0 2.0 LINEARITYDYNAMIC RANGE L00000 H8060100010001180 UNIT U/L g/L U/L U/L umol/L CORRELATION FACTORA=11111 B=00000 COUNT11111 DILUTION VOLUME00000 CONDENSE VOLUME00000 MONITOR SPAN11111 AGC POINT00000 K.FACTOR1308.42628.260780上述参数仅供参考,详情请参阅试剂说明及仪器说明。
血清C_肽、尿微量白蛋白与糖化血红蛋白应用于糖尿病肾病诊断中的效果分析

血清C肽、尿微量白蛋白与糖化血红蛋白应用于糖尿病肾病诊断中的效果分析陈丹,胡丽,王彬阶麻城市人民医院检验科,湖北麻城438300[摘要]目的探究血清C肽(C-PR)、尿微量白蛋白(mAlb)与糖化血红蛋白(HbA1c)应用于糖尿病肾病(dia‐betic nephropathy, DN)的诊断效果。
方法回顾性分析2021年4月—2022年4月麻城市人民医院检验科收治的95例糖尿病(diabetes mellitus, DM)患者的临床资料,依据是否合并肾病划分为DM组(50例)、DN组(45例),基于相同时期选取到本院接受健康体检的志愿者60名作为对照组,均接受C-PR、mAlb、HbA1c检测,分析联合检测效果。
结果DN组C-PR水平最低,对照组最高,DN组mAlb、HbA1c水平最高,对照组最低,差异有统计学意义(P<0.05)。
联合检测诊断灵敏度97.78%、特异度93.33%,均显著高于C-PR、mAlb或HbA1c单一检测结果,差异有统计学意义(P<0.05)。
结论在DN患者中实施C-PR、mAlb、HbA1c联合检测具有较高的诊断价值,便于患者尽早得到有效治疗。
[关键词] 血清C肽;尿微量白蛋白;糖化血红蛋白;糖尿病肾病;糖尿病[中图分类号] R587.2;R692.9 [文献标识码] A [文章编号] 1672-4062(2023)06(b)-0181-04 Analysis of the Effect of Serum C Peptide, Urine Microalbumin and Gly⁃cated Hemoglobin Applied to the Diagnosis of Diabetic NephropathyCHEN Dan, HU Li, WANG BinjieDepartment of Laboratory, Macheng People′s Hospital, Macheng, Hubei Province, 438300 China[Abstract] Objective To explore the diagnostic effect of serum C-peptide (C-PR), urinary microalbumin (mAlb) and glycated hemoglobin in diabetes nephropathy (DN). Methods Retrospective analysis was made on the clinical data of 95 patients with diabetes mellitus (DM) admitted to the Laboratory Department of Macheng People′s Hospital from April 2021 to April 2022. They were divided into DM group (50 cases) and DN group (45 cases) according to whether they were complicated with kidney disease or not. Based on the same period, 60 volunteers who received physical ex‐amination in our hospital were selected as the control group, all of whom received C-PR, mAlb, and HbA1c tests, and the effect of the joint test was analyzed. Results The C-PR level in the DN group was the lowest, while the control group was the highest, the levels of mAlb and HbA1c in the DN group were the highest, while the control group was the lowest, the difference was statistically significant (P<0.05). The diagnostic sensitivity and specificity of the com‐bined test were 97.78% and 93.33%, which were significantly higher than those of the single test results of C-PR, mA1b, or HbA1c, and the difference was statistically significant (P<0.05). Conclusion The implementation of com‐bined C-PR, mAlb, and HbA1c assay in DN patients has high diagnostic value and facilitate patients to get effective treatment as early as possible.[Key words] Serum C peptide; Urine microalbumin; Glycated hemoglobin; Diabetic nephropathy; Diabetes mellitus糖尿病(diabetes mellitus, DM)作为我国较为常见的一种慢性代谢性疾病,据流行病学调查显示,我国每11个人里面就有1例糖尿病,患病率高达30.2%,且近年来随着人们生活方式及饮食习惯的改变,患病率呈现出逐年递增趋势,引起了社会各界的广泛重视。
Cas号41575-94-4_Carboplatin分子式MedBio使用方法
20mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11964
AR-42 (OSU-HDAC42)
AR-42 (OSU-HDAC42)
935881-37-1
10mM (in 1mL DMSO)
≥98%
品牌
货号
中文名称
英文Байду номын сангаас称
CAS
包装
纯度
MedBio
MED12042
MedBio
MED11946
Deferasirox Fe3+ chelate
Deferasirox Fe3+ chelate
554435-83-5
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12058
Puromycin dihydrochloride
Puromycin dihydrochloride
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11934
Epirubicin HCl
Epirubicin HCl
56390-09-1
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12072
Trioxsalen
Trioxsalen
3902-71-4
250mg
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Mechanisms:
Pathways:Cell Cycle/DNA Damage; Target:HDAC Pathways:NF-KB; Target:HDAC
g y Biological Activity: AR-42 is a novel HDAC inhibitor with an IC50 of 0.61 μM for acute lymphoblastic leukemia (697) cell lines.
Product Data SFra bibliotekeetAR-42 935881-37-1 HY-13265 312.36 C18H20N2O3 >98%
Product Name: CAS No.: Cat. No.: MWt: Formula: Purity :
Solubility:
DMSO ≥60mg/mL Water <1.2mg/mL Ethanol ≥60mg/mL
[4]. Zimmerman B, Sargeant A, Landes K, Fernandez SA, Chen CS, Lairmore MD.Efficacy of novel histone deacetylase inhibitor, AR42, in a mouse model of, human T-ly...
References: [1]. Lin TY et al. AR-42, a novel HDAC inhibitor, exhibits biologic activity against malignant mast cell lines via down-regulation of constitutively activated Kit. Blood. 2010 May 27;115(21):4217-25. [2]. Shuhong Zhang et al. The novel histone deacetylase inhibitor, AR-42, inhibits gp130/Stat3 pathway p y and induces apoptosis p p and cell cycle y arrest in multiple p myeloma y cells International Journal of Cancer Volume 129, Issue 1, pages 204-213, 1 July 2011 [3]. Lu YS, Chou CH, Tzen KY, Gao M, Cheng AL, Kulp SK, Cheng JC.Radiosensitizing effect of a phenylbutyrate-derived histone deacetylase inhibitor in hepatocellular carcinoma.Int J Radiat Oncol Biol Phys. 2012 Jun 1;83(2):e181-9. Epub 2012 Feb 28.
Caution: Not fully tested. For research purposes only Medchemexpress LLC
m o c . s s e r p x e m e h c d e m . w w w : b e AW Sm U o ,c 0 . 4s 5s 8e 0r p Jx Ne ,m n e oh t c e cd ne i m r P @ o ,f y n a i Wl : ni a om s E n i k l i W 8 1
