Fosphenytoin_disodium_COA_25210_MedChemExpress
倍他米松磷酸钠 Betnesol-贝尔卡 低价高纯甾体激素原料药

外观 白色结晶粉末
分子式 C22H2量 516.4
规格一 10g
铝箔袋
价格 不开票 25/g
规格二 100g
铝箔袋
价格 不开票 16/g
规格三 500g
铝箔袋
价格 开票 10.5/g;不开票 9.5/g
规格四 1kg
铝听
价格 开票 9200/kg;不开票 8800/kg
缓解红、肿、痛等症状,且能抑制炎症晚期成纤维细胞的增生和肉芽组织的形成,减轻炎症引 起的瘢痕和粘连;糖皮质激素能提高机体对毒血症的耐受性,减轻细胞损伤,缓解毒血症状。 还能抑制中性粒细胞释放致热原和抑制下丘脑体温中枢,使热下降或防止发热。
主要用途 炎症、过敏性与自身免疫性疾病以及感染的综合治疗
产品应用 注射剂、滴剂等
公司主要生产基地位于通山,占地面积 3000 亩,主要生产雄性激素、雌性激素、孕激素、糖皮质激
素等甾体激素原料药。公司联合了武汉大学、华中科技大学、武汉工程大学等相关专家教授了组建了一批 技术专家团队,专门负责甾体合成工艺的优化和绿色生产工艺的开发,确保贝尔卡的生产技术处于国际领 先水平。贝尔卡生产的多个甾体产品已通过国家 GMP 认证、COS 认证,遍及亚洲、欧洲、北美、南美等
质检科长:高进
武汉贝尔卡生物医药有限公司创建于 2001 年,原名通山县医药原料厂,是国家级高新技术企业。2014 年,公司董事会从战略发展角度出发,决定响应打造国家级产业基地的号召,将公司总部迁入武汉市生物
贝尔卡生物医药
产品采购请点击
医药园。与此同时,公司借力园区政策优势,进行产研合作,引入外商投资,正式更名为"武汉贝尔卡生物 医药有限公司"。
贝尔卡生物医药
产品采购请点击
化学药品原研厂家

澳大利亚默沙东Merck Sharp & Dohme(Australia)Pty.Ltd 美国OSO BioPharmaceuticals Manufacturing, 注射用醋酸卡泊芬净 LLC 卡前列素氨丁三醇注射液 美国法玛西亚普强公司 注射用头孢呋辛钠 意大利GlaxoSmithKline Manufacturing S.p.A. 异氟烷 上海雅培制药有限公司 恩氟烷 上海雅培制药有限公司 克拉霉素片(薄膜衣) 上海雅培制药有限公司 英国雅培制药Abbott Laboratories Limited(上 盐酸特拉唑嗪片 海雅培制药分装) 盐酸美金刚片(薄膜衣) 德国Rottendorf Pharma GmbH 瑞士诺华制药有限公司Novartis Pharma 甲磺酸伊马替尼片(薄膜衣) Stein AG 瑞士诺华制药有限公司Novartis Pharma 甲磺酸伊马替尼胶囊 Stein AG 重酒石酸长春瑞滨注射液 法国皮尔法伯 盐酸阿莫罗芬搽剂 法国高德美制药公司Laboratoires Galderma 盐酸阿莫罗芬乳膏 法国高德美制药公司Laboratoires Galderma 丙酸氟替卡松乳膏 英国葛兰素史克有限公司 硝酸舍他康唑乳膏 西班牙FERRER INTERNACIONAL, S.A. 罗库溴铵注射液 荷兰欧加农公司anon 注射用盐酸阿柔比星(冻干) 深圳万乐药业有限公司 爱尔兰Allergan Pharmaceuticals 酒石酸溴莫尼定滴眼液 (Ireland)Ltd Inc 爱尔兰Allergan Pharmaceuticals 盐酸左布诺洛尔滴眼液 (Ireland)Ltd Inc 盐酸法舒地尔注射液 日本旭化成株式会社名古屋医药工厂 盐酸乐卡地平片(薄膜衣) 意大利Recordati S.P.A. 法国施维雅药厂LES LABORATOIRES SERVIER 地奥司明片(薄膜衣) INDUSTRIE(天津施维雅分装) 盐酸伊立替康注射液 澳大利亚Pfizer (Perth) Pty Limited 爱尔兰惠氏制药有限公司Wyeth Medica 盐酸文拉法辛缓释胶囊 Ireland(惠氏制药公司分装) 爱尔兰惠氏制药有限公司Wyeth Medica 结合雌激素片(糖衣) Ireland(惠氏制药公司分装) 注射用哌拉西林钠三唑巴坦钠(冻 美国Wyeth Piperacillin Div. of Wyeth 干) Holdings Corporation(惠氏制药有限公司分装) 盐酸米诺环素胶囊 惠氏制药有限公司 酒石酸伐尼克兰片(薄膜衣) 德国 Heinrich Mack Nachf.GmbH & Co.KG 盐酸齐拉西酮胶囊 澳大利亚Pfizer Australia Pty Limited 氟康唑注射液 法国辉瑞 重组人干扰素α 2b注射液 爱尔兰SP (Brinny) Company 多烯磷脂酰胆碱胶囊 赛诺菲安万特(北京)制药有限公司 奥美沙坦酯片(薄膜衣) 第一三共制药(上海)有限公司 普伐他汀钠片 第一三共制药(上海)有限公司 洛索洛芬钠片 第一三共制药(上海)有限公司 注射用胸腺肽α 1(冻干) 意大利Patheon Italia S.p.A 重组人促卵泡激素注射液 雪兰诺公司意大利制药厂Merck Serono S.p.A. 枸橼酸托瑞米芬片 芬兰奥立安大药厂(Orion Corporation) 注射用头孢他啶(含碳酸钠) 意大利GlaxoSmithKline Manufacturing S.p.A. 硫酸依替米星注射液 常州方圆制药有限公司 富马酸比索洛尔片(薄膜衣) 德国默克 (Merck KGaA) 磷酸西格列汀片(薄膜衣)
达格列净联合贝前列素钠治疗2_型糖尿病周围神经病变患者的效果及有效率评价

DOI:10.16658/ki.1672-4062.2024.04.183达格列净联合贝前列素钠治疗2型糖尿病周围神经病变患者的效果及有效率评价沈灿芳1,林莞蓉2,杨荣思1,王志伟11.石狮市总医院神经外科,福建石狮362700;2.石狮市总医院内分泌科,福建石狮362700[摘要]目的探究达格列净联合贝前列素钠治疗2型糖尿病周围神经病变患者的效果及有效率。
方法选取2021年9月—2023年10月石狮市总医院收治的2型糖尿病周围神经病变患者88例进行研究,经抽签法分组,对照组(44例)应用贝前列素钠治疗,观察组(44例)应用达格列净、贝前列素钠治疗,对比两组血糖、传导速度、临床疗效及不良反应。
结果用药4周后,观察组各项血糖水平均低于对照组,各项神经传导速度高于对照组,临床治疗有效率高于对照组,差异有统计学意义(P均<0.05)。
两组不良反应发生率比较,差异无统计学意义(P>0.05)。
结论达格列净、贝前列素钠联用可有效治疗2型糖尿病周围神经病变,血糖显著下降,神经传导速度明显加快,不良反应减少。
[关键词] 2型糖尿病周围神经病变;达格列净;贝前列素钠;血糖;有效率;不良反应[中图分类号] R4 [文献标识码] A [文章编号] 1672-4062(2024)02(b)-0183-04Evaluation of the Effectiveness and Efficacy of Dapagliflozin Combined with Beraprost Sodium in the Treatment of Patients with Peripheral Neu⁃ropathy in Type 2 Diabetes MellitusSHEN Canfang1, LIN Guanrong2, YANG Rongsi1, WANG Zhiwei11.Department of Neurosurgery, Shishi General Hospital, Shishi, Fujian Province, 362700 China;2.Department of Endo⁃crinology, Shishi General Hospital, Shishi, Fujian Province, 362700 China[Abstract] Objective To investigate the effect and efficiency of dapagliflozin combined with beraprost sodium in the treatment of patients with peripheral neuropathy in type 2 diabetes mellitus. Methods A total of 88 patients with type 2 diabetic peripheral neuropathy from Shishi General Hospital from September 2021 to October 2023 were selected for this study. They were divided by lot method. The control group (44 cases) was treated with beprost sodium, while the observation group (44 cases) was treated with dagaglizin and beprost sodium, and the blood glucose, conduction speed, clinical efficacy and adverse reactions of the two groups were compared. Results After 4 weeks of treatment, all blood glucose levels in the observation group were lower than those in the control group, all nerve conduction veloci⁃ties were higher than those in the control group, and the clinical treatment effectiveness was higher than that in the control group, the difference was statistically significant (all P<0.05). There was no statistically significant difference in the incidence of adverse reactions between the two groups (P>0.05). Conclusion Dapagliflozin and beraprost so⁃dium combination can effectively treat type 2 diabetic peripheral neuropathy, with a significant decrease in blood glu⁃cose, significantly accelerated nerve conduction velocity, with fewer adverse reactions.[Key words] Type 2 diabetes mellitus peripheral neuropathy; Dapagliflozin; Beraprost sodium; Blood glucose; Effec⁃tive rate; Adverse reaction[作者简介]沈灿芳(1988-),男,硕士,主治医师,研究方向为神经外科。
Xofigo 放射性治疗药剂指南说明书

UnitedHealthcare ® Medicare AdvantagePolicy GuidelineXofigo ® Radioactive Therapeutic AgentGuideline Number : MPG356.09Approval Date : November 8, 2023 Terms and ConditionsTable of Contents Page Policy Summary ............................................................................. 1 Applicable Codes .......................................................................... 1 References ..................................................................................... 2 Guideline History/Revision Information ....................................... 2 Purpose .......................................................................................... 3 Terms and Conditions . (3)See PurposeOverviewXofigo ® (radium Ra 223 dichloride) injection is an alpha particle-emitting radioactive therapeutic agent which mimics calcium and forms complexes with hydroxyapatite at areas of increased bone turnover, such as bone metastases.GuidelinesThe U.S. Food and Drug Administration (FDA) approved radium Ra 223 dichloride (Xofigo ® Injection, Bayer HealthCare Pharmaceuticals Inc.) for the treatment of patients with castration-resistant prostate cancer (CRPC), symptomatic bonemetastases and no known visceral metastatic disease.The recommended dose and schedule for Xofigo ® is 55 kBq/kg (1.49 microcuries/kg) administered by slow intravenous injection over 1 minute every 4 weeks for 6 doses.The following list(s) of procedure and/or diagnosis codes is provided for reference purposes only and may not be all inclusive. Listing of a code in this guideline does not imply that the service described by the code is a covered or non-covered health service. Benefit coverage for health services is determined by the member specific benefit plan document and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claim payment. Other Policies and Guidelines may apply.CPT Code Description 79101 Radiopharmaceutical therapy, by intravenous administrationCPT ® is a registered trademark of the American Medical AssociationHCPCS Code DescriptionA9606 Radium RA-223 dichloride, therapeutic, per microcurieDiagnosis Code DescriptionC61Malignant neoplasm of prostateRelated Medicare Advantage Reimbursement Policy • Add-on Codes Policy, ProfessionalDiagnosis CodeDescriptionAnd at least one of the following:C79.51 Secondary malignant neoplasm of boneC79.52 Secondary malignant neoplasm of bone marrowCMS Local Coverage Determinations (LCDs) and ArticlesLCDArticleContractor Medicare Part A Medicare Part BN/A A54559 Billing and Coding: Xofigo Billing Instructions PalmettoAL, GA, NC, SC,TN, VA, WV N/AA55052 Billing and Coding: Radiopharmaceutical Agents Retired 12/29/2022WPSAK, AL, AR, AZ, CA, CO, CT, DE, FL, GA, HI, IA, ID, IL, IN, KS, KY, LA, MA, MD, ME, MI, MO, MS, MT, NC, ND, NE, NH, NJ, NM, NV, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VA, VT, WA, WI, WV, WYIA, IN, KS, MI, MO, NECMS Benefit Policy ManualChapter 15; § 50 Drugs and BiologicalsCMS Claims Processing ManualChapter 12; § 30.5 Payment for Codes for Chemotherapy Administration and Nonchemotherapy Injections and Infusions Chapter 14; § 10 General Ambulatory Surgical CenterChapter 17; § 90.2 Drugs, Biologicals, and RadiopharmaceuticalsOther(s)CGS Website (Submitting Claims for Xofigo/Radium 223)CMS HCPCS Codes for which ASP Reporting is in Units of Measure Other Than an NDC, Updated July 2023, CMS Website Xofigo Package Insert, Bayer Healthcare Pharmaceuticals WebsiteRevisions to this summary document do not in any way modify the requirement that services be provided and documented in accordance with the Medicare guidelines in effect on the date of service in question.Date Summary of Changes11/08/2023Policy Summary OverviewRemoved and relocated language pertaining to the U.S. Food and Drug Administration (FDA)approval of radium Ra 223 dichloride (Xofigo ® Injection, Bayer HealthCare Pharmaceuticals Inc.) usage (refer to the Guidelines section) GuidelinesRevised language to indicate:Date Summary of Changeso The U.S. Food and Drug Administration (FDA) approved radium Ra 223 dichloride (Xofigo®Injection, Bayer HealthCare Pharmaceuticals Inc.) for the treatment of patients with castration-resistant prostate cancer (CRPC), symptomatic bone metastases and no known visceralmetastatic diseaseo The recommended dose and schedule for Xofigo® is 55 kBq/kg (1.49 microcuries/kg)administered by slow intravenous injection over 1 minute every 4 weeks for 6 dosesSupporting InformationUpdated References section to reflect the most current informationArchived previous policy version MPG356.08The Medicare Advantage Policy Guideline documents are generally used to support UnitedHealthcare Medicare Advantage claims processing activities and facilitate providers’ submission of accurate claims for the specified services. The document can be used as a guide to help determine applicable:Medicare coding or billing requirements, and/orMedical necessity coverage guidelines; including documentation requirements.UnitedHealthcare follows Medicare guidelines such as NCDs, LCDs, LCAs, and other Medicare manuals for the purposes of determining coverage. It is expected providers retain or have access to appropriate documentation when requested to support coverage. Please utilize the links in the References section above to view the Medicare source materials used to develop this resource document. This document is not a replacement for the Medicare source materials that outline Medicare coverage requirements. Where there is a conflict between this document and Medicare source materials, the Medicare source materials will apply.The Medicare Advantage Policy Guidelines are applicable to UnitedHealthcare Medicare Advantage Plans offered by UnitedHealthcare and its affiliates.These Policy Guidelines are provided for informational purposes, and do not constitute medical advice. Treating physicians and healthcare providers are solely responsible for determining what care to provide to their patients. Members should always consult their physician before making any decisions about medical care.Benefit coverage for health services is determined by the member specific benefit plan document* and applicable laws that may require coverage for a specific service. The member specific benefit plan document identifies which services are covered, which are excluded, and which are subject to limitations. In the event of a conflict, the member specific benefit plan document supersedes the Medicare Advantage Policy Guidelines.Medicare Advantage Policy Guidelines are developed as needed, are regularly reviewed and updated, and are subject to change. They represent a portion of the resources used to support UnitedHealthcare coverage decision making. UnitedHealthcare may modify these Policy Guidelines at any time by publishing a new version of the policy on this website. Medicare source materials used to develop these guidelines include, but are not limited to, CMS National Coverage Determinations (NCDs), Local Coverage Determinations (LCDs), Medicare Benefit Policy Manual, Medicare Claims Processing Manual, Medicare Program Integrity Manual, Medicare Managed Care Manual, etc. The information presented in the Medicare Advantage Policy Guidelines is believed to be accurate and current as of the date of publication and is provided on an "AS IS" basis. Where there is a conflict between this document and Medicare source materials, the Medicare source materials will apply.You are responsible for submission of accurate claims. Medicare Advantage Policy Guidelines are intended to ensure that coverage decisions are made accurately based on the code or codes that correctly describe the health care services provided. UnitedHealthcare Medicare Advantage Policy Guidelines use Current Procedural Terminology (CPT®), Centers for Medicare andMedicaid Services (CMS), or other coding guidelines. References to CPT® or other sources are for definitional purposes only and do not imply any right to reimbursement or guarantee claims payment.Medicare Advantage Policy Guidelines are the property of UnitedHealthcare. Unauthorized copying, use, and distribution of this information are strictly prohibited.*For more information on a specific member's benefit coverage, please call the customer service number on the back of the member ID card or refer to the Administrative Guide.。
N2神经细胞生长添加剂

Shelf Life and Storage
N2 Supplement is stable for 1 year when stored at ≤-15°C.
Instructions for use
N2 Supplement is a 100 fold concentrate. Dilute N2 Supplement (100x) in the base medium 1:100. The final concentration of N2 Supplement corresponds to 1x. For preparation of 100 ml medium add 1 ml N2 Supplement (100x) into 99 ml of the appropriate base medium.
Cell culture vessels must be coated with poly-D-lysine (0.05 mg/ml). Fibronectin must be added at a final concentration of 5-10 µg/ml directly to the medium.
n2神经细胞生长添加剂n2supplement编号体积n2supplement100x浓度f0010031ml应用?无血清培养?血清替代品?神经细胞瘤生长?各种cns和pns神经元的理想配方?与神经元基础培养基联用特点?神经元瘤细胞的特定生长因子?优化的激素组成产品规格ph7075内毒素已测定无菌已检测保存期1年贮存条件ห้องสมุดไป่ตู้5c配方?gmlhumantransferrinholo50000insulinbovine50000progesterone063putrescine161100sodiumselenite052reagentseriespr
美索巴莫注射液标准

美索巴莫注射液标准美索巴莫注射液是一种中枢性肌肉松弛剂,主要用于治疗急性骨骼肌疼痛或不适症状。
以下是关于美索巴莫注射液的一些标准信息:1. 中文通用名:美索巴莫注射液2. 英文通用名:Methocarbamol Injection3. 标准号:ws-10001-(hd-0269)-20024. 药品名称:美索巴莫注射液5. 药品英文名:Methocarbamol Injection6. 主要成分:本品为美索巴莫的灭菌聚乙二醇,400水溶液。
含美索巴莫(c11h15no5)应为标示量的95.0%~105.%。
7. 处方:具体处方应根据医生建议和患者病情来确定。
8. 性状:本品为无色或几乎无色略带黏稠的澄明液体。
9. 鉴别:通过化学分析和物理测试等方法来鉴别美索巴莫注射液的真伪和质量。
10. 作用类别:中枢性肌肉松弛剂11. 药理毒理:本品对中枢神经系统有选择作用,特别对脊椎中神经元作用明显。
抑制与骨骼肌痉挛有关的神经突触反射,有抗士的宁和电刺激所致惊厥的作用,并有解痉、镇痛和抗炎作用。
其作用机制主要是阻断脊髓内中枢神经元从而使骨骼肌松弛。
12. 药代动力学:美索巴莫注射液在体内的吸收、分布、代谢和排泄过程。
13. 适应症:主要用于急性骨骼肌疼痛或不适症状的辅助治疗。
14. 用法用量:美索巴莫注射液的用法主要是采用静脉滴注或者是静脉推注的方式给药。
如果是用于静脉推注时,患者在静卧的条件下缓慢推注,给药的速度每分钟不可以超过3ml,注射之后应该至少休息10~15分钟。
如果是用于静脉滴注时,将药品配在9%的氯化钠注射液或者是5%的葡萄糖注射液当中,滴注的速度不宜过快。
使用剂量和次数根据病情和治疗效果来决定。
成人一次使用剂量为1.0g,一日最大剂量为3.0g,连续使用不得超过3天。
轻度病例静注后应改为口服给药以维持治疗。
15. 不良反应:使用美索巴莫注射液可能出现的不良反应,如头痛、恶心、呕吐、皮疹等。
16. 禁忌症:对美索巴莫过敏者、肝肾功能不全者、哺乳期妇女禁用。
代谢示意图
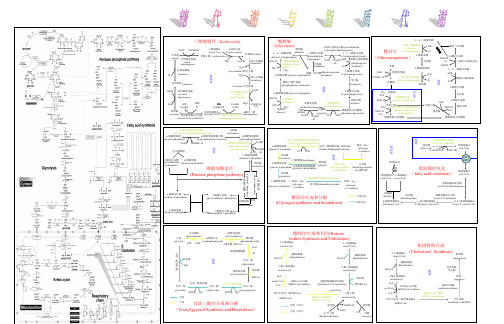
胞 液
甘油一酯 (glyceride)
甘油二酯脂肪酶 甘油二酯 (diglyceride)
甘油三酯脂肪酶
甘油三酯
CoA
脂肪酸 分解
H2O
(triacylglycerol) 脂肪酸 H2O
3羟3甲基戊二酸单酰CoA (HMG CoA) 利用(肝外) 生成(肝内) HMG CoA裂解酶 (HMA CoA lyase) 脱氢酶
变位酶 2-磷酸甘油酸 GDP+P i CO2 烯醇化酶
葡萄糖 (glucose)
乳酸脱氢酶 丙酮酸激酶 (pyruvate kinase) (lactate dehydrogenase) 丙酮酸 乳酸 磷酸烯醇式丙酮酸 (lactate) (pyruvate) (phosphoenolpyruvate) + ATP ADP NAD NADH +H+
β 羟丁酸 (βhydroxybutyrate)
GDP + Pi
CO2 NADH+H
+
dehydrogena se) Mg2+ NADH+H CoAS + + CO2 NAD H
线 CO2 丙酮酸 粒 ATP 丙酮酸羧化酶 体 (pyruvate carboxylase) ADP+Pi 草酰乙酸 GTP 磷酸烯醇式丙酮酸羧激酶 (phosphoenolpyruvate GDP+P carboxykinase) i CO2 磷酸烯醇式丙酮酸 草酰乙酸
醛羧酶 3-phosphate dehydrogenase) ) 1,6-二磷酸果糖 (aldolaes) 3-磷酸甘油醛 1,3-二磷酸甘油酸 (glyceraldehyde (1,3-bisphosphoglycerate) (frutose 1,6-bisphosphate) 3-phosphate) + NADH ADP 磷酸甘油酸激酶 NAD ADP+Pi 6-磷酸果糖激酶1 +H+ (phosphoglycer异构酶 (6-phosphofructoate kinase) (isomerase) ATP -kinase-1) ATP 3-磷酸甘油酸 (dihydroxyacetone 磷酸二羟丙酮 (3-phosphoglycerate) 6-磷酸果糖 (fructose 6-phosphate) phosphate) 磷酸己糖异构酶 (phosphoglucose isomerase) (glucose 6-phosphate) 6-磷酸葡萄糖 ADP+Pi ATP 己糖激酶 (hexokinase) Mg2+ 变位酶 (Isomerase) Mg2+
药物Fosnetupitant(福奈妥匹坦)合成检索总结报告

操作方法二
Charged 2-chloro-4-methoxy-5-nitro pyridine1(100 g) N-methyl piperazine (175 mL) and tetrahydrofuran (1200 mL) and stirred for 10 hrs at room temperature. Charged reaction mixture in 720 mL of water and extracted in ethyl acetate. Organic layer washed with brine solution and treated with sodium sulphate. Distilled off organic layer under reduced pressure; crystallisation with diisopropyl ether gave 1-(4-methoxy-5-nitropyridin-2-yl)-4-methylpiperazine2(115.0 gms).
药物Fosnetupitant(福奈妥匹坦)合成检索总结报告
一、Fosnetupitant(福奈妥匹坦)分子结构式
英文名称:Fosnetupitant英文名称:Fosnetupitanthydrochloride
中文名称:福奈妥匹坦中文名称:福奈妥匹坦盐酸盐
二、Fosnetupitant(福奈妥匹坦)合成路线
US2015/315149; (2015); (A1) English
(二) Fosnetupitant(福奈妥匹坦)中间体3的合成
合成方法
实验步骤
