the fifth chapter hormone chemistry
化工专业英语翻译-华东理工大学-胡鸣版

Unit 1 Chemical Industry化学工业1.Origins of the Chemical IndustryAlthough the use of chemicals dates back to the ancient civilizations, the evolution of what we know as the modern chemical industry started much more recently. It may be considered to have begun during the Industrial Revolution, about 1800, and developed to provide chemicals roe use by other industries. Examples are alkali for soapmaking, bleaching powder for cotton, and silica and sodium carbonate for glassmaking. It will be noted that these are all inorganic chemicals. The organic chemicals industry started in the 1860s with the exploitation of William Henry Perkin’s discovery if the first synthetic dyestuff—mauve. At the start of the twentieth century the emphasis on research on the applied aspects of chemistry in Germany had paid off handsomely, and by 1914 had resulted in the German chemical industry having 75% of the world market in chemicals. This was based on the discovery of new dyestuffs plus the development of both the contact process for sulphuric acid and the Haber process for ammonia. The later required a major technological breakthrough that of being able to carry out chemical reactions under conditions of very high pressure for the first time. The experience gained with this was to stand Germany in good stead, particularly with the rapidly increased demand for nitrogen-based compounds (ammonium salts for fertilizers and nitric acid for explosives manufacture) with the outbreak of world warⅠin 1914. This initiated profound changes which continued during the inter-war years (1918-1939).1.化学工业的起源尽管化学品的使用可以追溯到古代文明时代,我们所谓的现代化学工业的发展却是非常近代(才开始的)。
Chemistry_Chemical_Change_Ch15_Types_of_Reactions

iiCopyright2007“Free High School Science Texts”Permission is granted to copy,distribute and/or modify this document under theterms of the GNU Free Documentation License,Version1.2or any later versionpublished by the Free Software Foundation;with no Invariant Sections,no Front-Cover Texts,and no Back-Cover Texts.A copy of the license is included in thesection entitled“GNU Free Documentation License”.Did you notice the FREEDOMS we’ve granted you?Our copyright license is different!It grants freedomsrather than just imposing restrictions like all those othertextbooks you probably own or use.•We know people copy textbooks illegally but we would LOVE it if you copied our’s-go ahead copy to your hearts content,legally!•Publishers’revenue is generated by controlling the market,we don’t want any money,go ahead,distribute our books far and wide-we DARE you!•Ever wanted to change your textbook?Of course you have!Go ahead,change ours,make your own version,get your friends together,rip it apart and put it back together the way you like it.That’s what we really want!•Copy,modify,adapt,enhance,share,critique,adore,and contextualise.Do it all,do it with your colleagues,your friends,or alone but get involved!Together we can overcome the challenges our complex and diverse country presents.•So what is the catch?The only thing you can’t do is take this book,makea few changes and then tell others that they can’t do the same with yourchanges.It’s share and share-alike and we know you’ll agree that is only fair.•These books were written by volunteers who want to help support education, who want the facts to be freely available for teachers to copy,adapt and re-use.Thousands of hours went into making them and they are a gift to everyone in the education community.ContentsI Introduction1 II Matter and Materials31Classification of Matter-Grade1051.1Mixtures (5)1.1.1Heterogeneous mixtures (6)1.1.2Homogeneous mixtures (6)1.1.3Separating mixtures (7)1.2Pure Substances:Elements and Compounds (9)1.2.1Elements (9)1.2.2Compounds (9)1.3Giving names and formulae to substances (10)1.4Metals,Semi-metals and Non-metals (13)1.4.1Metals (13)1.4.2Non-metals (14)1.4.3Semi-metals (14)1.5Electrical conductors,semi-conductors and insulators (14)1.6Thermal Conductors and Insulators (15)1.7Magnetic and Non-magnetic Materials (17)1.8Summary (18)2What are the objects around us made of?-Grade10212.1Introduction:The atom as the building block of matter (21)2.2Molecules (21)2.2.1Representing molecules (21)2.3Intramolecular and intermolecular forces (25)2.4The Kinetic Theory of Matter (26)2.5The Properties of Matter (28)2.6Summary (31)3The Atom-Grade10353.1Models of the Atom (35)3.1.1The Plum Pudding Model (35)3.1.2Rutherford’s model of the atom (36)vChapter15Types of Reactions-Grade11There are many different types of chemical reactions that can take place.In this chapter,we will be looking at a few of the more common reaction types:acid-base and acid-carbonate reactions, redox reactions and addition,elimination and substitution reactions.15.1Acid-base reactions15.1.1What are acids and bases?In our daily lives,we encounter many examples of acids and bases.In the home,vinegar(acetic acid),lemon juice(citric acid)and tartaric acid(the main acid found in wine)are common,while hydrochloric acid,sulfuric acid and nitric acid are examples of acids that are more likely to be found in laboratories and industry.Hydrochloric acid is also found in the gastric juices in the stomach.Evenfizzy drinks contain acid(carbonic acid),as do tea and wine(tannic acid)!Bases that you may have heard of include sodium hydroxide(caustic soda),ammonium hydroxide and ammonia.Some of these are found in household cleaning products.Acids and bases are also important commercial products in the fertiliser,plastics and petroleum refining industries.Some common acids and bases,and their chemical formulae,are shown in table15.1.Table15.1:Some common acids and bases and their chemical formulaeAcid FormulaHydrochoric acid NaOHH2SO4Potassium hydroxideNitric acid Na2CO3CH3COOH Calcium hydroxideCarbonic acid Mg(OH)2H2SO3AmmoniaPhosphoric acid NaHCO3Most acids share certain characteristics,and most bases also share similar characteristics.It is important to be able to have a definition for acids and bases so that they can be correctly identified in reactions.15.1.2Defining acids and basesA number of definitions for acids and bases have developed over the years.One of the earliest was the Arrhenius definition.Arrhenius(1887)noticed that water dissociates(splits up)into hydronium(H3O+)and hydroxide(OH−)ions according to the following equation:H2O⇔H3O++OH−Definition:Acids and basesAccording to the Bronsted-Lowry theory of acids and bases,an acid is a substance that gives away protons(H+),and is therefore called a proton donor.A base is a substance that takes up protons,and is therefore called a proton acceptor.Below are some examples:1.HCl(g)+NH3(g)→NH4++Cl−In order to decide which substance is a proton donor and which is a proton acceptor,we need to look at what happens to each reactant.The reaction can be broken down as follows:HCl→Cl−+H+andNH3+H+→NH+4From these reactions,it is clear that HCl is a proton donor and is therefore an acid,and that NH3is a proton acceptor and is therefore a base.2.CH3COOH+H2O→H3O++CH3COO−The reaction can be broken down as follows:CH3COOH→CH3COO−+H+andH2O+H+→H3O+In this reaction,CH3COOH(acetic acid)is a proton donor and is therefore the acid.In this case,water acts as a base because it accepts a proton to form H3O+.3.NH3+H2O→NH+4+OH−The reaction can be broken down as follows:Definition:AmphotericAn amphoteric substance is one that can react as either an acid or base.Examples of amphoteric substances include water,zinc oxide and beryllium hydroxide.15.1.3Conjugate acid-base pairsLook at the reaction between hydrochloric acid and ammonia to form ammonium and chloride ions:HCl+NH3⇔NH+4+Cl−Lookingfirstly at the forward reaction(i.e.the reaction that proceeds from left to right),the changes that take place can be shown as follows:HCl→Cl−+H+andNH3+H+→NH+4Looking at the reverse reaction(i.e.the reaction that proceeds from right to left),the changes that take place are as follows:NH+4→NH3+H+andCl−+H+→HClIn the forward reaction,HCl is a proton donor(acid)and NH3is a proton acceptor(base). In the reverse reaction,the chloride ion is the proton acceptor(base)and NH+4is the proton donor(acid).A conjugate acid-base pair is two compounds in a reaction that change into each other through the loss or gain of a proton.The conjugate acid-base pairs for the above reaction are shown below.HCl+NH3NH+4+Cl−acid1base2base1acid2conjugate pairconjugate pairThe reaction between ammonia and water can also be used as an example:Definition:Conjugate acid-base pairThe term refers to two compounds that transform into each other by the gain or loss of a proton.Exercise:Acids and bases1.In the following reactions,identify(1)the acid and the base in the reactantsand(2)the salt in the product.(a)H2SO4+Ca(OH)2→CaSO4+2H2O(b)CuO+H2SO4→CuSO4+H2O(c)H2O+C6H5OH→H3O++C6H5O−(d)HBr+C5H5N→(C5H5NH+)Br−2.In each of the following reactions,label the conjugate acid-base pairs.(a)H2SO4+H2O⇔H3O++HSO−4(b)NH+4+F−⇔HF+NH3(c)H2O+CH3COO−⇔CH3COOH+OH−(d)H2SO4+Cl−⇔HCl+HSO−415.1.4Acid-base reactionsWhen an acid and a base react,they neutralise each other to form a salt.If the base contains hydroxide(OH−)ions,then water will also be formed.The word salt is a general term which applies to the products of all acid-base reactions.A salt is a product that is made up of the cation from a base and the anion from an acid.When an acid reacts with a base,they neutralise each other.In other words,the acid becomes less acidic and the base becomes less basic.Look at the following examples:1.Hydrochloric acid reacts with sodium hydroxide to form sodium chloride(the salt)andwater.Sodium chloride is made up of Na+cations from the base(NaOH)and Cl−anions from the acid(HCl).HCl+NaOH→H2O+NaCl2.Hydrogen bromide reacts with potassium hydroxide to form potassium bromide(the salt)and water.Potassium bromide is made up of K+cations from the base(KOH)and Br−anions from the acid(HBr).HBr+KOH→H2O+KBrVTherefore,n(HCl)=M×V(make sure that all the units are correct!)M=0.2mol.dm−3V=15cm3=0.015dm3Thereforen(HCl)=0.2×0.015=0.003There are0.003moles of HCl that reactStep3:Calculate the number of moles of sodium hydroxide in the reaction Look at the equation for the reaction.For every mole of HCl there is one mole of NaOH that is involved in the reaction.Therefore,if0.003moles of HCl react, we can conclude that the same quantity of NaOH is needed for the reaction.The number of moles of NaOH in the reaction is0.003.Step4:Calculate the molarity of the sodium hydroxideFirst convert the volume into dm3.V=0.025dm3.Then continue with the calculation.M=n0.025=0.12The molarity of the NaOH solution is0.12mol.dm3or0.12MWorked Example75:Titration calculationQuestion: 4.9g of sulfuric acid is dissolved in water and thefinal solution has a volume ing titration,it was found that20cm3of this solution wasM =4.9g0.22=0.23mol.dm−3Step3:Calculate the moles of sulfuric acid that were used in the neutrali-sation reaction.Remember that only20cm3of the sulfuric acid solution is used.M=n/V,therefore n=M×Vn=0.23×0.02=0.0046molStep4:Calculate the number of moles of sodium hydroxide that were neutralised.According to the balanced chemical equation,the mole ratio of H2SO4to NaOH is 1:2.Therefore,the number of moles of NaOH that are neutralised is0.0046×2= 0.0092mols.Step5:Calculate the concentration of the sodium hydroxide solution.M=n0.01=0.92M15.1.5Acid-carbonate reactionsActivity::Demonstration:The reaction of acids with carbonatesApparatus and materials:Small amounts of sodium carbonate and calcium carbonate(both in powder form);hydrochloric acid and sulfuric acid;retort stand;two test tubes;two rubber stoppers for the test tubes;a delivery tube;lime water.The demonstration should be set up as shown below.Definition:Oxidation and reductionOxidation is the loses of an electron by a molecule,atom or ion.Reduction is the gain of an electron by a molecule,atom or ion.Example:Mg+Cl2→MgCl2As a reactant,magnesium has an oxidation number of zero,but as part of the product magnesium chloride,the element has an oxidation number of+2.Magnesium has lost two electrons and has therefore been oxidised.This can be written as a half-reaction.The half-reaction for this change is:Mg→Mg2++2e−As a reactant,chlorine has an oxidation number of zero,but as part of the product magnesium chloride,the element has an oxidation number of-1.Each chlorine atom has gained an electron and the element has therefore been reduced.The half-reaction for this change is:Cl2+2e−→2Cl−Definition:Redox reactionA redox reaction is one involving oxidation and reduction,where there is always a change in the oxidation numbers of the elements involved.Activity::Demonstration:Redox reactionsMaterials:A few granules of zinc;15ml copper(II)sulphate solution(blue colour),glassbeaker.zinc granulescopper sulphatesolutionMethod:Add the zinc granules to the copper sulphate solution and observe what happens.What happens to the zinc granules?What happens to the colour of the solution?Results:•Zinc becomes covered in a layer that looks like copper.•The blue copper sulphate solution becomes clearer.Cu2+ions from the CuSO4solution are reduced to form copper metal.This is what you saw on the zinc crystals.The reduction of the copper ions(in other words, their removal from the copper sulphate solution),also explains the change in colour of the solution.The equation for this reaction is:Cu2++2e−→CuZinc is oxidised to form Zn2+ions which are clear in the solution.The equation for this reaction is:Zn→Zn2++2e−The overall reaction is:Cu2+(aq)+Zn(s)→Cu(s)+Zn2+(aq)Conclusion:A redox reaction has taken place.Cu2+ions are reduced and the zinc is oxidised.APPENDIX A.GNU FREE DOCUMENTATION LICENSEAPPENDIX A.GNU FREE DOCUMENTATION LICENSEAPPENDIX A.GNU FREE DOCUMENTATION LICENSEAPPENDIX A.GNU FREE DOCUMENTATION LICENSE。
《Organic Chemistry 5th Edition (L.G. Wade JR.)》wade03

Chapter 3 18
Reactions of Alkanes
• Combustion
2 CH3CH2CH2CH3 + 13 O2 heat 8 CO2 + 10 H2O
• Cracking and hydrocracking (industrial)
long-chain alkane s catalyst shorte r-chain alkane s
Chapter 3
=>
4
IUPAC Names
• Find the longest continuous carbon chain. • Number the carbons, starting closest to the first branch. • Name the groups attached to the chain, using the carbon number as the locator. • Alphabetize substituents. • Use di-, tri-, etc., for multiples of same substituent. =>
• Lower b.p. with increased branching • Higher m.p. with increased branching • Examples:
CH3
CH3 CH3 CH CH2 CH2 CH3 bp 60°C mp -154°C
CH3 CH3 CH CH CH3
CH3 C CH2 CH3 CH3 bp 50°C mp -98°C
• Melting points increase with increasing carbons (less for oddnumber of carbons).
Chemistry Chapter 5
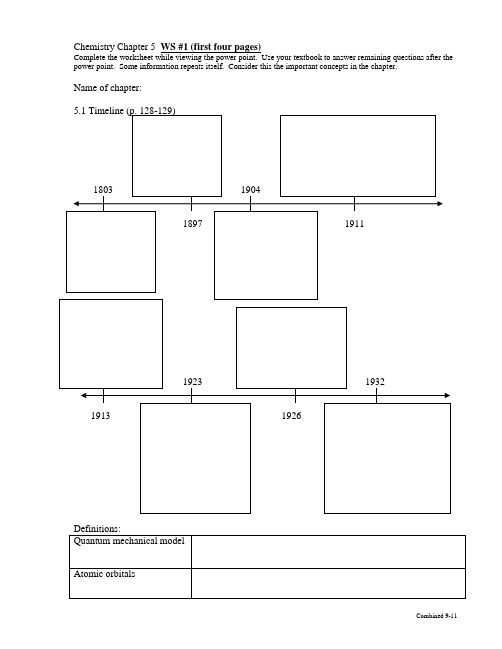
Chemistry Chapter 5 WS #1 (first four pages)Complete the worksheet while viewing the power point. Use your textbook to answer remaining questions after the power point. Some information repeats itself. Consider this the important concepts in the chapter.Name of chapter:5.1 The Development of Atomic Models1. Rutherford’s atomic model could not explaina. why _________________________________give off characteristic colors when heatedb. why objects ___________________________ first glow dull red, then yellow, then white.c. his model could not explain ____________________________________.The Bohr Model2. Bohr proposed that an electron is found only in specific circular paths, or _________, aroundthe nucleus.3. Each possible electron orbit in Bohr’s model has a fixed energy called ____________.4. A quantum of energy is the amount of energy required to move an electron_______________________________________________________________________.The Quantum Mechanical Model5. Erwin Shrodinger used math to propose the ___________________________ model.6. The quantum mechanical model determines the allowed energies an electron can have and how likely it is ___________________________________________________________________________________________________________________________.Atomic Orbitals7. An _______________________ is often thought of as a region of space in which there is a high probability of finding an electron. The s orbitals are _____________ shaped, and p orbitals are __________________ shaped.5.2 Electron Configurations8. The ways in which electrons are arranged in various orbitals around the nuclei of atoms are called ________________________.9. Aufbau principle: electrons occupy the orbitals of _________ energy first.Pauli exclusion principle: an atomic orbital may describe at most ____________. To occupy the same orbital, two electrons must have ______________; that is, the electron spins must be paired. Hund’s rule: electrons occupy orbitals of the ______________ in a way that makes the number of electrons with the _____________ direction as large as possible.Some actual electron configurations differ from those assigned using the aufbau principle because __________sublevels are not as stable as _________ sublevels, but they are more stable than other configurations.Exceptions to the aufbau principle are due to ___________________________________ in orbitals with very similar energies.5.3 Physics and the Quantum Mechanical Model—Light10. The __________________ of a wave is the wave’s height from zero to the crest. The______________________, represented by _________, is the distance between the crests. The _________________, represented by _______, is the number of wave cycles to pass a given point per unit of time. The SI unit for cycles per second is the ___________. The speed of light is _________________. Draw a picture of a wave and label the parts.11. ___________________________________ include radio waves, microwaves, infrared waves, visible light, ultraviolet waves, X-rays, and gamma rays.12. When sunlight passes through a prism, the different frequencies separate into a______________ of colors. The lowest frequency and longest wavelength is _____________. The other colors in order are _____________________________________________________. Atomic Spectra and Explanation of Atomic Spectra13. When atoms absorb energy, __________ move into higher energy levels. These electrons then lose energy by ________________ when they return to lower energy levels.14. The frequencies of light emitted by an element separate into discrete lines to give the_____________________ of the element.15. When the electron has its lowest possible energy, the atom is in its _______________.16. Excitation of the electron by absorbing energy raises the atom from the ground state to an______________.17. A quantum of energy in the form of light (photon) is emitted when the electron drops back toa ___________________.18. The light emitted by an electron moving from a higher to a lower energy level has a frequency __________ proportional to the energy change of the electron.Quantum Mechanics19. Einstein proposed that light could be described as quanta of energy called ______________. The quanta behave as if they were ____________________, resulting in a dual wave-particle behavior.20. In 1924, De Broglie developed an equation that predicts that all moving objects have___________________ behavior.21. The older theory of classical mechanics, adequately describes __________________________________________________________, while the newer theory of quantum mechanics describes ___________________________________________________________________. 22. The ___________________ uncertainty principle states that it is impossible to know exactly both the velocity and the position of a particle at the same time. The very act of measuring the position of an electron changes it velocity, and makes its velocity uncertain.Review…5.2 Electron Configurations (pages 133–135)1. The ways in which electrons are arranged around the nuclei of atoms arecalled ___________________________________________ .2. In the shorthand method for writing an electron configuration, what does asuperscript stand for? What do the sum of the superscripts equal?5.3 Light (pages 138–140)1. Match each term describing waves to its definition._______ amplitude a. the distance between two crests_______ wavelength b. the wave’s height from the origin to the crest_______ frequency c. the number of wave cycles to pass a given point per unit of time Atomic Spectra (page 141)4. What happens when an electric current is passed through the gas or vapor ofan element?5. Is the following sentence true or false? Explain. The emission spectrum of an elementcan be the same as the emission spectrum of another element.An Explanation of Atomic Spectra (pages 142–143)6. What is the lowest possible energy of an electron called?7. Only electrons moving from ______________________ to______________________ energy levels lose energy and emit light.WS #2 (last two pages)Chemistry—Ch. 5 textbook problems p. 149-151 (22-26, 30, 34-35, 40, 43, 61-65, 68)22. What was inadequate about Rutherford’s model of the atom? Which subatomic particles didThomson include in the plum-pudding model of the atom?23. What did Bohr assume about the motion of electrons?24. Describe Rutherford’s model of the atom and compare it with the model proposed by hisstudent Niels Bohr.25. What is the significance of the boundary of an electron cloud?26. What is an atomic orbital?30. How many electrons are in the highest occupied energy level of these atoms?c)barium b) sodium c) aluminum d) oxygen34. Give electron configurations for atoms of these elements:a) Na b) S c) Mgd) Ne e) K35. Which of these orbital designations are invalid?a)4s b) 2d c) 3f d) 3d40. List the colors of the visible spectrum in order of increasing wavelength.43. Explain the difference between the energy lost or gained by an atom according to the laws ofclassical physics and according to the quantum model of an atom.61. Pieces of energy are known asa) isotopes b) particles c) quanta d) line spectra62. The lowest sublevel in each principal energy level is represented by the symbola) f b) p c) s d) d63. Which electron transition results in the emission of energy?a)3p to 3s b) 3p to 4p c) 2s to 2p d) 1s to 2s64. Which is the ground state configuration of a magnesium atom?a) 1s22s22p63s2b) 1s22s22p63s1 c) 1s22s23s22p6 d) 1s22s22p43s265. Explain the difference between an orbit in the Bohr model and an orbital in the quantum mechanical model of the atom.68. Orbital diagrams for the ground states of two elements are shown below. Each diagramshows something that is incorrect. Identify the error in each diagram and then draw thecorrect diagram.a. Nitrogenb. MagnesiumCh. 4 review questions_______________ discovered the electron in 1897. He sealed gases in a tube and put electricity through the tube. The result was a glowing beam, or________________ ray. By using a magnet, he showed that there were tiny_______________ charged particles moving at high speeds (corpuscles or electrons). He found that the charge-to-mass ratio of electrons did not depend on the kind of gas used or type of metal plates. Thus, electrons must be part of the atoms of all elements.Thomson’s ___________________ model suggested that atoms were a_______________ mass with _______________ charged particles within similarto a plum pudding. His student, ___________________, disproved this theory by his ____________________ experiment. He found that most alpha particles went straight through showing that an atom is mostly _______________ space. He also concluded that there is a small, dense, positively charged _______________ at the center of the atom.Ch. 5 concept questionOuter _______________ gain a quantum of energy from high temperature or high _______________ and move from the ground state to an excited state. The_______________ are not stable in this higher energy level, so they emit energy called _______________ and move back to the lower energy, or ground state. We see colors because the energy emitted is in our _______________ spectrum, which is between ________ nm and __________nm. The colors in this spectrum from lowest energy are _________________________________________________________________________________________________________________.。
化学及化工专业词汇英语翻译

化学及化工专业词汇英语翻译18 electron rule 18 电子则abbe refractometer 阿贝折射计abbreviated analysis 简略分析abderhalden's dryer 阿布德尔哈尔登干燥器abderhalden's reaction 阿布德尔哈尔登反应abegg's rule 阿贝格规则abel closed tester 阿贝尔氏密闭实验机abel pensky tester 阿贝尔彭斯基试验器abel tester 阿贝尔试验器abelite 阿贝立特aberration 像差abies oil 松节油abietate 松香酯abietic acid 松香酸abietin 松香素abiochemistry 无生化学;无机化学ablation 消融ablution 洗净abnormal setting 反常凝结abnormality 反常abradant 磨料abrader 磨损试验机abrasion 磨耗abrasion loss 磨损量abrasion resistance 耐磨能力abrasion test 磨耗试验abrasion testing machine 磨损试验机abrasive 磨料abrasive grain 磨料颗粒abrasive industry 磨料工业abrasive paper 砂纸abrasiveness 磨损性abrasives 研磨剂abs resin abs 尸abs resins abs尸abscisic acid 阿伯喂酸absinthe oil 洋艾油absolute activity 绝对活性度absolute alcohol 无水酒精absolute calibration 绝对校准absolute configuration 绝对构型absolute dry condition 绝对干燥状态absolute dry weight 绝对干重absolute error 绝对误差absolute humidity 绝对湿度absolute measurement 绝对测量absolute reaction rate 绝对反应速度absolute sensitivity 绝对灵敏度absolute specific gravity 真比重absolute temperature 绝对温度absolute unit 绝对单位absolute value 绝对值absolute viscosity 绝对粘度absolute zero 绝对零度absorbability 吸收性absorbance 吸光度absorbed dose 吸收线量absorbent 吸收剂absorbent paper 吸收纸absorber 吸收器吸收体absorbing power 吸收能力absorptiometer 吸光测定计absorptiometric analysis 吸光分析absorptiometry 吸收分光光度法absorption 吸收absorption band 吸收带absorption cell 吸收池absorption coefficient 吸收系数absorption column 吸收塔absorption curve 吸收曲线absorption edge 吸收端absorption factor 吸收因子absorption heat 吸收热absorption intensity 吸收强度absorption line 吸收线absorption maximum 最大吸收absorption method 吸收法absorption oil 吸收油absorption pipet 吸收管absorption refrigerator 吸收式冷冻器absorption spectrophotometry 吸收分光光度法absorption spectrum 吸收光谱absorption tower 吸收塔absorption tube 吸收管absorptive power 吸收能力absorptivity 吸收率abukumalite 钇硅磷灰石abysmal deposit 深海沉积物abyssal deposit 深海沉积物ac polarography 交莲谱法acacia 阿拉伯屎acaricide 杀螨剂acaroid resin 禾木尸accelerant 促进剂accelerated aging 加速老化accelerated aging test 加速老化试验accelerated weathering test 加速风化试验accelerating agent 促进剂acceleration globulin 促凝血球蛋白acceleration of gravity 重力加速度accelerator 促进剂acceptor 接受体accessory constituent 副成分accetyl value number 乙酰值accidental error 偶然误差acclimatization 驯化accommodation 适应accommodation coeffieient 适应系数accumulator 蓄电池accumulator acid 蓄电池酸液accuracy 准确度acenaphthene 威杀灵acenaphthene quinone 苊醌acenaphthenone 二氢苊酮acenaphthylene 萘嵌戊烯acenocoumarol 苊香豆醇acephate 乙酰甲胺磷acetal 乙缩醛acetal phosphatide 缩醛磷脂acetal resin 缩醛尸acetaldehydase 乙醛酶acetaldehyde 乙醛acetaldehyde ammonia 乙醛合氨acetaldehyde reductase 醇脱氢酶acetaldol 3 羟基丁醛acetaldoxime 乙醛肟acetamide 乙酰胺acetamidine 乙脒acetanilide 乙酰苯胺acetarsol 乙酰胂胺acetate 醋酸盐acetate dye 醋酸染料acetate fiber 醋酸纤维acetate film 醋酸纤维胶片acetate rayon 醋酸丝acetazolamide 乙酰唑胺acetic acid 醋酸acetic acid fermentation 乙酸发酵acetic acid glacial 冰醋酸acetic aldehyde 乙醛acetic anhydride 醋酐acetic bacteria 醋酸菌acetic ester 醋酸酯acetification 醋化酌acetimeter 醋酸计acetine 醋精acetoacetanilide 乙酰乙酰替苯胺acetoacetate 乙酰醋酸盐acetoacetic acid 乙酰醋酸acetoin 醋偶姻acetol 丙酮醇acetolactic acid 乙酰乳酸acetolysis 乙酸水解acetomeroctol 醋汞辛酚acetometry 醋酸测定法acetone 丙酮acetone alcohol 丙酮醇acetone body 酮体acetone butanol fermentation 丙酮丁醇发酵acetone chloroform 三氯叔丁醇acetone cyanhydrin 丙酮合氰化氢acetone dicarboxylic acid 丙酮二羧酸acetone fermentation 丙酮发酵acetone sugar 丙酮糖acetonic acid 醋酮酸acetonitrile 乙腈acetonyl acetone 丙酮基丙酮acetophenetidin n 乙酰乙氧基苯胺acetophenone 苯乙酮acetopurpurin 乙酰替红紫acetoxime 丙酮肟acetoxyl group 乙酰氧基acetoxylation 乙酸化aceturic acid 乙酰甘氨酸acetyl bromide 乙酰溴acetyl chloride 乙酰氯acetyl hydroperoxide 过乙酸acetyl iodide 碘化乙酰acetyl ketene 二酮acetyl peroxide 过氧化乙酰acetyl propionyl 乙酰丙酰acetyl value 乙酰值acetylacetone 乙酰丙酮acetylase 乙酰酯酶acetylating agent 乙酰剂acetylation 乙酰化acetylbenzoyl peroxide 乙酰过氧化苯甲酰acetylcellulose 乙酰纤维素acetylcholine 乙酰胆碱acetylene 乙炔acetylene black 乙炔炭黑acetylene burner 乙炔燃烧器acetylene chemistry 乙炔化学acetylene chloride 乙炔基氯acetylene complex 乙炔络合物acetylene generator 乙炔发生器acetylene linkage 炔键acetylene polymer 乙炔聚合物acetylene tetrachloride 四氯乙炔acetylene welding 气焊acetylenic hydrocarbon 乙炔属烃类acetylide 乙炔化合物acetylisoeugenol 乙酰异丁子香酚acetylphenylhydrazine 乙酰苯肼acetylsalicylic acid 乙酰水杨酸acetylurea 乙酰脲achirality 非手胀achroite 无色电气石achromatic lens 消色差透镜aci form 针形acicular crystal 针状结晶acid 酸acid acceptor 受酸体acid albumin 酸蛋白acid alizarine 酸性茜素acid amide 酸胺acid ammonium sulfate 硫酸氢铵acid ammonium tartrate 酒石酸氢铵acid anhydride 酸酐acid azid 酰基叠acid azo dye 酸性偶氮染料acid base catalysis 酸碱催化acid base equilibrium 酸碱平衡acid base indicator 酸碱指示剂acid base pair 酸碱对acid base titration 酸碱滴定acid bath 酸浴acid black 酸性黑acid carbonate 酸性碳酸盐l acid 醇酸alcohol dehydrogenase 醇脱氢酶alcohol fuel 酒精燃料alcohol lamp 酒精灯alcohol of crystallization 结晶醇alcohol thermometer 酒精温度计alcohol varnish 醇溶清漆alcoholase 醇酶alcoholate 烃氧基金属alcoholic compound 醇化合物alcoholic extract 酒精提出物alcoholic fermentation 酒精发酵alcoholic potash 钾碱醇液alcoholic solution 醇溶液alcoholism 酒中毒alcoholmeter 酒精比重计alcoholometry 酒精测定alcoholysis 醇解alcosol 醇溶胶alcoxyl 烷氧aldehyde 醛aldehyde acid 醛酸aldehyde alcohol 醛醇aldehyde ammonia 醛氨aldehyde dehydrogenase 醛脱氢酶aldehyde resin 聚醛尸aldimine 亚胺醛aldoheptose 庚醛糖aldohexose 乙醛糖aldoketene 醛烯酮aldol 羟醛aldol condensation 醛醇缩合aldol reaction 醇醛缩合反应aldolase 醛缩酶aldolization 缩醛反应aldonic acid 醛糖酸aldopentose 戊醛糖aldose 醛糖aldosterone 醛甾酮aldotriose 丙醛糖aldox process 羰醇法aldoxime 醛肟aldrin 艾氏剂alexandrite 翠绿宝石alfin catalyst 阿尔芬催化剂alfin polymer 阿尔芬聚合物alfin polymerization 阿尔芬聚合algae 藻类algin 藻酸alginate 藻蛋白酸盐alginate fiber 藻酸纤维alginic acid 海藻酸algol blue 阿果蓝algol color 阿果染料algorithm 算法alicyclic 脂环族的alicyclic compound 脂环化合物aliesterase 脂族酯酶aliphatic 无环的aliphatic acid 脂族酸aliphatic alcohol 脂族醇aliphatic amine 脂族胺aliphatic base 脂族碱aliphatic compound 脂族化合物aliphatic ether 脂族醚aliphatic hydrocarbon 脂族烃aliphatic series 脂族系aliphatic unsaturated carboxylic acid 脂族不饱羧酸alite 阿里特alizarin 茜素alizarin blue 茜素蓝alizarin brown 茜素棕alizarin dye 茜素染料alizarin lake 茜素色淀alizarin yellow 茜黄alizarine 茜素alkali 碱alkali blue 碱性蓝alkali cellulose 碱纤维素alkali fusion 碱熔融alkali ion diode 碱离子二极管alkali lignin 碱木素alkali liquor 碱液alkali metal 碱金属alkali resistance 耐碱性alkali rock 碱性岩alkali salt 碱金属盐alkalimeter 碱量计alkalimetry 碱量滴定法alkaline accumulator 减蓄电池alkaline bath 碱浴alkaline cell 碱性电池alkaline cleaner 碱性清洗剂alkaline earth metal 碱土金属alkaline earths 碱土族alkaline hydrolysis 加碱水解alkaline reaction 碱性反应alkaline solution 碱性溶液alkaline storage battery 减蓄电池alkalinity 碱度alkalization 碱化alkaloid 生物碱alkaloid reagent 生物碱试剂alkalosis 碱中毒alkamine 氨基醇类alkane 链烷alkannin 紫草素alkanolamine 烷烃醇胺alkansulfonic acid 链烷磺酸alkene 烯烃alkine 链炔alkyd paint 醇酸涂料alkyd resin 醇酸尸alkyd resin varnish 醇酸清漆alkyl 烷基alkyl cellulose 烷基纤维素alkyl cyanide 烷基氰alkyl group 烷基alkyl halide 烷基卤alkyl sulfate 烷基硫酸盐alkyl sulfide 烷基硫alkyl sulfonic acid 烷基磺酸alkylarsine 烷基胂alkylarsonic acid 烷基胂酸alkylate 烷基化产物alkylating agent 烷化剂alkylation 烷基化alkylbenzene 烷基苯alkylbenzene sulfonate 烷基苯磺酸盐alkylene 烷撑alkylidene 次烷基alkylmagnesium halide 烷基镁化卤alkylnaphthalene 烷基萘alkyne 炔烃alkynol 炔醇allanite 褐帘石allantoic acid 尿囊酸allantoinase 尿囊素酶allantoxanic acid 尿囊毒酸allanturic acid 尿囊脲酸allelochemical 变异化学的allelochemistry 变异化学allene 丙二烯allergy 过敏反应allethrin 丙烯拟除虫菊酯allicin 蒜辣素alligator pear oil 鳄梨油allobarbital 二烯丙巴比妥allochromatic crystal 羼质色晶体allocinnamic acid 别肉桂酸alloisomerism 立体异构现象allomerism 异质同晶allophane水铝英石allophanic acid 脲基甲酸alloprene 阿洛波林allopurinol 别嘌呤醇allose 阿洛糖allosteric effect 别构效应allosteric enzyme 变构酶allosteric transition 变构转变allostery 变构性allothreonine 别苏氨酸allotrope 同素异形体allotropism 同素异形allotropy 同素异形allowable error 容许误差allowed transition 容许跃迁alloxan 阿脲alloxanic acid 阿脲酸alloxazine 咯嗪alloy 合金alloy analysis 合金分析alloy steel 合金钢allulose 阿卢糖alluvial gold 砂金allyl acetate 醋酸丙烯酯allyl alcohol 烯丙醇allyl amine 烯丙胺allyl bromide 烯丙基溴allyl chloride 烯丙基氯allyl complex 烯丙基络合物allyl compound 烯丙基化合物allyl cyanide 烯丙基腈allyl iodide 烯丙基碘allyl isothiocyanate 异硫氰酸烯丙酯allyl mercaptan 烯丙硫醇allyl resin 烯丙尸allyl sulfide 烯丙基硫allylene 丙炔allylic rearrangement 烯丙重排allylmustard oil 烯丙基芥子油allylthiourea 烯丙基硫脲almandine 铁铝榴石almandite 铁铝榴石almond oil 扁桃仁油aloe 芦荟aloin 芦荟素alpha brass 黄铜alpha cellulose 纤维素alpha counter 粒子计数器alpha iron 铁alpha naphthol 萘酚alpha position 位alpha ray spectrometer 射线能谱仪alpha rays 射线alstonine 鸡骨常山碱alternant hydrocarbon 交替烃alternating copolymer 交替共聚物alternating current 交流alternating current polarography 交莲谱alternation 交替altimeter 测高仪altitude 高度altrose 阿卓糖alum 茂alumel 镍基锰合金alumina 氧化铝alumina brick 矾土砖alumina bubble brick 泡沫矾土砖alumina cement 矾土水泥alumina fiber 氧化铝纤维alumina gel 铝凝胶alumina silica refractory 硅酸铝耐火材料aluminate 铝酸盐aluminium 铝aluminium acetate 乙酸铝aluminium alloy 铝合金aluminium ammonium sulfate 硫酸铝铵aluminium boride 硼化铝aluminium bromide 溴化铝aluminium bronze 铝青铜合金aluminium carbide 碳化铝aluminium chlorate 氯酸铝aluminium chloride 氯化铝aluminium ethylate 乙醇铝aluminium fluoride 氟化铝aluminium foil 铝箔aluminium hydroxide 氢氧化铝aluminium nitrate 硝酸铝aluminium oleate 油酸铝aluminium oxide 氧化铝aluminium plate 铝板aluminium potassium sulfate 硫酸铝钾aluminium powder 铝粉aluminium resinate 尸酸铝aluminium silicate 硅酸铝aluminium sulfate 硫酸铝aluminon 铝试剂aluminosilicate 硅铝酸盐aluminothermit process 铝热法aluminothermy 铝热法aluminum 铝alumite 茂石alumstone 茂石alundum 刚铝石alunite 茂石amalgam 汞齐amalgam cell 汞齐电池amalgam electrode 汞齐电极amalgamation 汞齐化amalgamation process 汞齐化过程amanitin 鹅膏菌素amaranth 蓝光酸性红amatol 阿马图amber 琥珀amber glass 琥珀玻璃amberite 琥珀炸药americium 镅americyl ion 镅酰离子amethopterin 氨甲喋呤amicron 次微粒amidase 酰胺酶素amidation 酰胺化amide 酰胺amide chloride 二氯代酰胺amidine 脒amidine hydrochloride 盐酸脒amidohydrolase 氨基水解酶amidone 美沙酮amination 胺化amine 胺amine formaldehyde 胺甲醛amine oxidase 胺氧化酶amino acid 氨基酸amino acid sequence 氨基酸顺序amino compound 氨基化合物amino nitrogen 氨基氮amino plastic resin 氨基塑料尸amino resins 氨基尸amino sugar 氨基糖amino terminal 氨基末端aminoacetaldehyde 氨基乙醛aminoacetone 氨基丙酮aminoalcohol 氨基醇aminobenzoic acid 氨基苯甲酸aminobutyric acid 氨基丁酸aminocaproic acid 氨基己酸aminodiborane 氨基乙硼烷aminoglutaric acid 氨基戊二酸aminoglycoside antibiotics 氨基糖苷类抗生物素aminogram 氨基图aminoisovaleric acid 氨基异戊酸aminolysis 氨基分解aminonaphthol 氨基萘酚aminonaphthol sulfonic acid 氨基萘磺酸aminopeptidase 氨基胜胨酵素aminophenol 氨基苯酚aminophenylarsonic acid 氨基苯胂酸aminophosphorylase 淀粉磷酸化酶aminophylline 氨苯碱aminopolypeptidase 氨基多胜酵素aminoprotease 氨蛋白酶aminopterin 氨基蝶呤aminopyridine 氨基吡啶aminopyrin 氨基吡啉aminoquinoline 氨基喹啉aminosalicylic acid 氨基水杨酸aminosuccinic acid 氨基琥珀酸aminosulfonic acid 氨基磺酸aminotoluene 氨基甲苯ammeter 电另ammonia 氨ammonia compressor 氨气压缩机ammonia gas 氨气ammonia poisoning 氨中毒ammonia still 氨气塔ammonia synthesis 氨合成ammonia water 氨水ammoniacal brine 氨盐水ammoniacal fermentation 氨发酵ammoniacal latex 氨胶乳ammoniameter 氨量计ammoniasoda process 氨碱法ammoniated superphosphate 含铵过磷酸钙ammoniator 氨化器ammoniometry 氨量测定法ammonite 阿芒炸药ammonium 铵ammonium acetate 乙酸铵ammonium alum 铵茂ammonium benzoate 安息香酸铵ammonium bifluoride 氟化氢铵ammonium borate 硼酸铵ammonium carbamate 氨基甲酸铵ammonium carbonate 碳酸铵ammonium chloride 氯化铵ammonium chromate 铬酸铵ammonium cyanate 氰酸铵ammonium dichromate 重铬酸铵ammonium fluoride 氟化铵ammonium formate 甲酸铵ammonium hydrogen carbonate 碳酸氢铵ammonium hydroxide 氢氧化铵ammonium iodate 碘酸铵ammonium iron sulfate 硫酸铁铵ammonium metavanadate 偏钒酸铵ammonium molybdate 钼酸铵ammonium nitrate 硝酸铵ammonium nitrate explosive 硝铵炸药ammonium nitrate fertilizer 硝铵肥料ammonium oxalate 草酸铵ammonium perchlorate 高氯酸铵ammonium persulfate 过硫酸铵ammonium phosphate 磷酸铵ammonium phosphite 亚磷酸铵ammonium phosphomolybdate 磷钼酸铵ammonium picrate 苦味酸铵ammonium polysulfide 多硫化铵ammonium rhodanide 硫氰酸铵ammonium salt 铵盐ammonium selenate 硒酸铵ammonium stearate 硬脂酸铵ammonium sulfate 硫酸铵ammonium sulfite 亚硫酸铵ammonium thiocyanate 硫氰酸铵ammonium thiosulfate 硫代硫酸铵ammonium uranate 铀酸铵ammonium vanadate 钒酸铵ammonobase 氨基金属ammonolysis 氨解ammophos 安福粉amobarbital 戊巴比妥amodiaquine 阿莫待喹amorphism 无定形amorphous carbon 无定形碳amorphous graphite 无定型石墨amorphous material 无定形材料amorphous metal 无定形金属amorphous phosphorus 无定形磷amorphous polymer 非晶态聚合物amorphous state 无定形状态amorphous sulfur 无定形硫ampere 安amperemeter 电另amperometric titration 电廖定amperometry 电廖定amphetamine 苯异丙胺amphibole 闪石amphipathic molecule 两亲水脂分子amphiphilic molecule 两亲水脂分子ampholyte 两性电解质ampholytic active agent 两性表面活性剂ampholytic surfactant 两性表面活性剂ampholytoid 两性胶体amphoteric 两性的amphoteric character 两性特征amphoteric colloid 两性胶体amphoteric compound 两性化合物amphoteric ion 两性离子amphoteric oxide 两性氧化物amphoteric resin 两性尸amphotericeledrolyte 两性电解质amplifier 放大器ampule 安瓿amygdalin 扁桃苷amyl 戊基amyl acetate 醋酸戊酯amyl alcohol 戊醇amyl bromide 戊基溴amyl butyrate 丁酸戊酯amyl ether 戊醚amyl formate 甲酸戊酯amyl mercaptan 戊硫醇amyl nitrite 亚硝酸戊酯amyl oleate 油酸戊酯amyl propionate 丙酸戊酯amylamine 戊胺amylase 淀粉酶amylbenzene 戊基苯amylene 戊烯amylo process 淀粉发酵法amylodextrin 淀粉糊精amyloid 淀粉状朊amylolysis 淀粉分解amylopectin 支链淀粉amylopsin 胰淀粉酶amylose 直链淀粉amytal 戊巴比妥anabasine 安纳巴松anabolism 同化酌anaerobe 厌氧微生物anaerobic glycolysis 无氧糖酵解analcime 方沸石analgesic 镇痛药analog digital conversion 模拟数字转换analog signal 模拟信号analogue 类似analogue computer 模拟计算机analysis 分析analysis line 分析线analysis with ion selective electrodes 离子选择电极分析法analyte 分析物analytic function 解析函数analytical balance 分析天平analytical chemistry 分析化学analytical extraction 分析抽出analytical method 分析法analytical reaction 分析反应analytically pure 分析纯anapaite 斜磷钙铁矿anaphoresis 阴离子电泳anatase octahedrite 锐钛矿anchor agitator 锚式搅拌器anchor stirrer 锚式搅拌器andalusite 红柱石andesite 安山岩andreasen pipet 安德烈森型吸管androsin 雄素androstane 雄烷androstendione 雄烯二酮androsterone 雄酮andrussow process 安德卢梭法anelasticity 滞弹性anemometer 风速计anemonin 白头翁脑aneroid barometer 空盒气压计anesthesin 氨基苯甲酸乙酯anesthetic 麻醉剂anethole 茴香脑aneurin 硫胺素angelica lactone 当归内酯angelica oil 当归油angiotensin 血管紧张肽angle of polarization 偏振光角angle of refraction 折射角angle of repose 休止角anglesite 硫酸铅矿angstrom 埃angular momentum 角动量anhalonine 老头掌碱anhydride 酐anhydrite 硬石膏anhydrone 无水高氯酸镁anhydrous 无水的anhydrous acid 无水酸anhydrous alcohol 无水酒精anhydrous ammonia 无水氨anhydrous salt 无水盐anileridine 氨苄哌替啶anilide 酰替苯胺aniline 苯胺aniline black 苯胺黑aniline blue 苯胺蓝aniline dye 苯胺染料aniline formaldehyde resin 苯胺甲醛尸aniline hydrochloride 盐酸苯胺aniline point 苯胺点aniline red 苯胺红aniline resin 苯胺尸aniline yellow 苯胺黄anilol 酒精苯胺混合液animal biochemistry 动物生化学animal charcoal 骨炭animal chemistry 动物化学animal dye 动物染料animal fat 动物脂animal fiber 动物纤维animal glue 动物胶animal oil 动物油anime 硬尸anion 阴离子anion active agent 阴离子表面活性剂anion exchange 阴离子交换anion exchange resin 阴离子交换尸anion exchanger 阴离子交换剂anionic polymerization 阴离子聚合anionic surfactant 阴离子表面活性剂anionoid reagent 类阴离子试剂anionotropy 阴离子移变现象anisaldehyde 茴香醛anise oil 茴香油anisic acid 茴香酸anisic alcohol 茴香醇anisidine 茴香胺anisole 茴香醚anisometric crystal 不等轴晶体anisotropic body 蛤异性体anisotropic liquid 蛤异性液体anisotropic membrane 蛤异性膜anisotropy 蛤异性anisoyl chloride 茴香酰氯anisyl acetate 醋酸茴香酯anisyl alcohol 茴香醇ankerite 铁白云石annabergite 镍华annealing 退火annealing furnace 退火窑annealing temperature 退火温度annulene 环轮烯anode 阳极anode effect 阳极效应anode process 阳极过程anode slime 阳极淀渣anodic oxidation 阳极氧化anodic polarization 阳极极化anodic reaction 阳极反应anodization 阳极化anodizing 阳极化anolyte 阳极电解液anomalous dispersion 异常弥散anomalous magnetic moment 异常磁矩anomalous skin effect 反常囚效应anomer 异头物anone 环己酮anorthoclase 钠斜微长石antagonism 拮抗酌antazoline 安他唑啉anthelmintics 驱肠虫剂anthocyan 花青素anthocyanidin 花色素anthocyanin 花色素苷anthophyllite 直闪石anthracene 蒽anthracene oil 蒽油anthracite 无烟煤anthracite duff 无烟煤粉anthralin 蒽啉anthranil 氨茴内酐anthranilate 邻氨基苯甲酸盐anthranilic acid 邻氨基苯酸anthranol 蒽酚anthranone 蒽酮anthrapurpurin 蒽红紫anthraquinone 蒽醌anthraquinone dye 蒽醌染料anthrarufin 蒽绛酚anthraxylon 结焦素anthrone 蒽酮anti allergic drug 抗过敏性药anti fouling paint 防污涂料anti tack agent 防粘剂antiacid 解酸药antiacid additive 抗酸添加剂antiager 抗老剂antiaromaticity 反芳香性antibiosis 抗生antibiotics 抗生物质antibody 抗体antibonding orbital 反键轨道anticarcinogen 抗癌物anticatalyst 抗催化剂anticathode 对阴极antichlor 脱氯剂anticholinesterase 抗胆碱酯酶剂anticoagulant 抗凝剂anticoagulating action 阻凝酌anticonvulsant 镇痉剂anticorrosion 抗腐蚀anticorrosive agent 防腐蚀剂anticorrosive paint 防腐涂料antidetonant 抗爆剂antidote 解毒剂antienzyme 抗酶antifertilizin 抗受精介体antifibrinolysin 抗纤维蛋白酶antifoamer 抗泡剂antifoaming agent 抗泡剂antifouling paint 防污漆antifreezing agent 阻冻剂antigen 抗原antihistamine 抗组胺剂antihistaminic agent 抗组胺剂antiknock agent 抗爆剂antiknock gasoline 抗爆汽油antiknocking fuel 抗爆燃料antimetabolite 抗代谢物antimonate 锑酸盐antimonial lead 锑铅antimonic acid anhydride 锑酸酐antimonide 锑化物antimonite 亚锑酸盐antimony 锑antimony chloride 氯化锑antimony electrode 锑电极antimony hydride 氢化锑antimony oxide 氧化锑antimony pentachloride 五氯化锑antimony potassium tartrate 酒石酸锑钾antimony red 锑红antimony sulfate 硫酸锑antimony sulfide 硫化锑antimony trisulfide 三硫化二锑antimony vermillon 锑朱antimony white 锑白antineuralgic 治神经痛药antinucleon 反核子antioxidant 抗氧化剂antiozonant 抗臭氧剂antiparticle 反粒子antipode 对映体antiproton 反质子antipyretic and analgesic 解热镇痛药antipyrine 安替吡啉antiscorbutic vitamin 抗坏血病维生素antiscorcher 防焦剂antiscorching agent 防焦剂antisepsis 防腐antiseptics 防腐剂antispasmodic 镇痉剂antistat 抗静电剂antistatic agent 抗静电剂antitermination factor 抗终止因素antithrombin 抗凝血酶antitoxin 抗毒素antivitamin 抗维生素apatite 磷灰石aphthitalite 硫酸钾石apiin 芹实苷apiose 洋芹糖aplysiopurpurin 海螺紫apocodeine 阿朴可特因apoenzyme 酶朊apoferritin 脱铁铁蛋白apomorphine 阿朴吗啡apoprotein 脱辅基蛋白apozymase 酒化酶原apparatus 装置apparent activation energy 表观活化能apparent density 表观密度apparent equilibrium 表观平衡apparent specific gravity 表观比重apparent viscosity 表观粘度applied chemistry 应用化学applied thermodynamics 应用热力学approximate calculation 近似计算approximate value 近似值aprotic solvent 非质子溶剂aqua ion 水合离子aqua regia 王水aquagel 水凝胶aquametry 测水法aqueous emulsion 水乳状液aqueous medium 水介质aqueous phase 水相aqueous solution 水溶液aqueous vapor 水蒸汽arabic acid 阿糖酸arabic gum 阿拉伯胶arabinose 阿拉伯糖arabitol 阿糖醇arabonic acid 阿糖酸arachic acid 花生酸arachidonic acid 花生四烯酸arachis oil 花生油aragonite 霰石aralkyl 芳烷arbutin 熊果苷arc furnace 电弧炉arc process 电弧法arc spectrum 弧光谱arch brick 拱砖archeochemistry 考古化学arecoline 槟榔素areometer 比重计areometry 比重测定法argentite 辉银矿argentometry 银量滴定argillaceous sand 粘质砂土argillite 泥质板岩arginase 精氨酸酶arginine 精氨酸argol 粗酒石argon 氩aristolochic acid 马兜铃酸arnicin 由金车苦素aroma 香味aromatic acid 芳族酸aromatic aldehyde 芳族醛aromatic amine 芳香胺aromatic compound 芳族化合物aromatic hydrocarbon 芳香烃aromatic nucleus 芳香环aromatic series 芳香系aromaticity 芳香度aromatization 芳香化aromatization reaction 芳香化反应aroylation 芳酰基化arrhenius equation 阿雷尼厄斯方程arsanilic acid 阿散酸arsenate 砷酸盐arsenazo i 偶氮胂arsenblende 雄黄arsenic 砷arsenic acid 砷酸arsenic butter 三氯化砷arsenic glass 砷玻璃arsenic hydride 砷化三氢arsenic mirror 砷镜arsenic sulfide 硫化砷arsenic trichloride 三氯化砷arsenic trioxide 三氧化二砷arsenic trisulfide 三硫化二砷arsenide 砷化物arsenite 亚砷酸盐arseno compound 偶砷化合物arsenobenzene 偶砷苯arsenometry 亚砷酸滴定法arsenopyrite 砷黄铁矿arsenous anhydride 亚砷酸酐arsine 胂arsonic acid 胂酸arsonium 氢化砷arsonium compound 胂化合物arsphenamine 胂凡纳明art glass 艺术玻璃art paper 加工印刷纸artemisin 蒿属素arthropodin 节肢蛋白artiad 偶价元素artificial abrasive 人造磨料artificial aging 人工老化artificial almond oil 人造扁桃油artificial asphalt 人造地沥青artificial atmospher 人工气氛artificial butter 人造奶油artificial camphor 人造樟脑artificial corundum 人造金刚砂artificial diamond 人造金刚石artificial dye 人造染料artificial fertilizer 人造肥料artificial fiber 人造纤维artificial intelligence 人工智能artificial lattice 人工晶格artificial leather 人造革artificial musk 人造香artificial perfume 人造香料artificial radioactivity 人工放射性artificial resin 人造尸artificial rubber 人造橡胶artificial silk 人造丝artificial stone 人造石aryl compound 芳基化合物aryl halide 芳基卤arylamine 芳基胺arylation 芳基化arylide 芳基化物aryloxy compound 芳氧基化合物arylsulphonate 芳基磺酸盐asarin 细辛脑asarone 细辛脑asbestine 滑石棉asbestos 石棉asbestos board 石棉纸板asbestos cement 石棉水泥asbestos cloth 石棉布asbestos felt 石棉毛毯asbestos fiber 石棉纤维asbestos filter 石棉滤器asbestos insulation 石棉绝热体asbestos paper 石棉纸asbestos powder 石棉粉asbestos slate 石棉板asbestos wire gauze 石棉衬网asbestos yarn 石棉丝asbolane 钴土矿asbolite 钴土矿ascaridol 驱蛔脑ascending method 上行法ascorbic acid 抗坏血酸asepsis 防腐ash 灰ash bath 灰浴ash collector 除尘器ash content 灰分含量ash ejector 灰喷射器ash pit door 灰坑门ash softening point 灰熔温度ashing 灰化ashless filter paper 无灰滤纸asparaginase 天门冬酰胺酶asparagine 天门冬酰胺aspartase 天门冬氨酸酶aspartate 天冬氨酸盐aspartic acid 天冬氨酸aspartokinase 天冬氨酸激酶aspartyl phosphate 天冬氨酰磷酸aspergillic acid 曲霉酸asphalt 沥青asphalt cement 沥青膏asphalt emulsion 地沥青乳液asphalt mastic 地沥青砂胶asphalt varnish 沥青油漆asphaltene 沥青烯asphaltic road oil 沥青质铺路油asphaltogenic acid 沥青酸asphaltous acid 沥青酸asphyxia 窒息asphyxiant 窒息剂asphyxy 窒息aspirator 吸气器aspirin 阿司匹林assay 试金assay balance 试金天平assay flask 试验瓶assayer's tongs 试金钳assili cotton 阿嘻棉assimilation 同化assimilation starch 同化淀粉assistant 助剂associated liquid 缔合液体association 缔合assortment 分类astacin 虾红素astatine 砹astaxanthin 虾青素astringency 收敛性astringent 收敛剂astrochemical 天体化学的astrochemist 天体化学家astrochemistry 天体化学astrogeochemical 天体地球化学的astrogeochemistry 天体地球化学asymmetric atom 不对称原子asymmetric carbon atom 不对称碳原子asymmetric oxidation 不对称氧化asymmetric structure 不对称结构asymmetric synthesis 不对称合成asymmetric system 不对称系asymmetry 不对称asymptotic freedom 渐近自由性atactic 无规立构的atactic polymer 无规聚合物atebrine 疟涤平atmolysis 微孔分气法atmosphere 大气atmospheric air 大气空气atmospheric corrosion 大气腐蚀atmospheric nitrogen 大气氮atmospheric pressure 大气压atom 原子atomic absorption spectrometry原子吸收分光光度法atomic arrangement 原子排列atomic battery 原子电池atomic beam 原子束atomic bomb 原子弹atomic bond 原子键atomic charge 原子电荷atomic clock 原子钟atomic core 原子核atomic dispersion 原子分散atomic energy 原子能atomic fluorescence spectrometry 原子荧光光谱法atomic form factor 原子散射因子atomic group 原子团atomic heat 原子热atomic hydrogen 原子氢atomic hydrogen welding 原子氢焊接atomic hypothesis 原子假说atomic lattice 原子晶格atomic magnetism 原子磁性atomic mass 原子质量atomic mass unit 原子质量单位atomic model 原子模型atomic molecular theory 原子分子论atomic nucleus 原子核atomic number 原子序atomic orbital 原子轨道atomic polarization 原子极化atomic properties 原子特性atomic radius 原子半径atomic refraction 原子折射atomic scattering factor 原子散射因子atomic spectrum 原子光谱atomic structure 原子结构atomic susceptibility 原子磁化率atomic symbol 原子符号atomic theory 原子论atomic unit 原子单位atomic volume 原子体积atomic weight 原子量atomicity 原子数atomism 原子论atomistics 原子论atomization 喷雾atomizer 喷雾器atophan 阿托方atrazine 阿特拉津atropic acid 阿托酸atropine 阿托品atropine sulfate 硫酸阿托品atropisomer 阿托异构体attachment 附件attrition 磨损aufbau principle 构造原理augmentation distance 扩增距离auramine 金胺aurantia 金橙黄aurantin 橙色菌素aurate 金酸盐aureomycin 金霉素aureusidin 金色草素auric acid 金酸auric compound 正金化合物auric oxide 氧化金auric salt 正金盐aurin 金精aurin tricarboxylic acid 铝试剂auripigment 雄黄aurothioglucose 金硫葡萄糖aurous chloride 氯化亚金aurous compound 亚金化合物aurous oxide 氧化亚金aurous salt 亚金盐austenite 奥氏体auto condensation 自动缩合autocatalysis 自动催化autocatalyst 自动催化剂autocatalytic reaction 自动催化反应autoclave 压热器autocomplex 自动合成物autocorrelation function 自相关函数autofermentation 自动发酵autogenous ignition 自动着火autoionization 自电离autolysis 自溶酌autolytic enzyme 自溶酶automatic analyser 自动分析计automatic balance 自动天平automatic buret 自动滴定管automatic control自动控制automatic regulation 自动控制automatic temperature controller 自动温度控制器automatic thermoregulator 自动温度控制器automatic titration 自动滴定automatic weighing machine 自动秤automation 自动化autometer 汽车速度表autopolymerization 自动聚合autoprotolysis 自质子解autoracemization 自动外消旋autotetraploid 同源四倍体autotransformer 单卷变压器autovulcanization 自动硫化autoxidation 自氧化autunite 钙铀云母auxiliary air 辅助空气auxiliary electrode 辅助电极auxiliary unit 辅助单位auxiliary valency 副价auximone 茁长激素auxin 茁长素auxochrome 助色团availability 有效性available chlorine 有效氯available energy 有效能available phosphoric acid 有效磷酸avenin 燕麦蛋白average boiling point 平均沸点average degree of polymerization 平均聚合度average error 平均误差average life 平均寿命average mean molecular weight 平均分子量average molecular weight 平均分子量average particle diameter 平均粒子直径average sample 平均试样average speed 平均速度average value 平均值aviation gasoline 航空汽油aviation mix 航空汽油抗爆液avidin 抗生物素蛋白avocado oil 鳄梨油avogadro number 阿伏伽德罗数avogadro's hypothesis 阿伏伽德罗假说avogadro's law 阿伏伽德罗定律axial bond 贮axial flow pump 轴撩axiomatic quantum field theory 公理的量子场理论axis 轴axis of rotation 旋转轴azaserine 重氮丝氨酸azelaic acid 杜鹃花酸azeotrope 共沸混合物azeotropic copolymer 共沸共聚物azeotropic distillation 共沸蒸馏azeotropic mixture 共沸混合物azeotropic point 共沸点azeotropy 共沸性azide 叠氮化物azimuthal 方位的azimuthal quantum number 角量子数azine 吖嗪azine dye 吖嗪染料aziridine 氮杂环丙烷azlactone 吖内酯azlon 人造蛋白质纤维azo compound 偶氮化合物azo coupling 偶氮耦合azo dye 偶氮染料azo group 偶氮基azobenzene 偶氮苯azodicarbonamide 偶氮甲酰胺azoimide 叠氮化氢azole 唑azolitmin 石蕊精azotometer 氮素计azoxy compound 氧化偶氮化合物azoxybenzene 氧化偶氮苯azulene 甘菊环烃azurite 蓝铜矿b stage resin b 阶尸baby dryer 小烧缸bacillus 杆菌bacitracin 杆菌肽back bond 反向键back flow condenser 回龄凝器back mixing 逆向混合back pressure 反压back reaction 逆反应back sweetening 返回脱硫法back titration 回滴定backfire 回火backflash 反闪backscattering 后方散射backward motion 反向运动backwash 回洗bacteria 细菌bacterial fertilizer 细菌肥料bacterial incubator 细菌培育箱bactericide 杀细菌剂bacteriochlorophyll 菌叶绿素bacteriolysis 溶菌酌bacteriostasis 抑菌酌baddeleyite 斜锆石baeyer reaction 拜尔反应baeyer reagent 拜尔试药baeyer villiger rearrangement 拜尔维利格重排baffle 挡板bag filter 袋滤器bagasse 甘蔗渣bakelite 酚醛塑料baking 烧制baking enamel 烘烤搪瓷baking powder 发粉baking varnish 烤漆balance 平衡balance bar 平衡杆balance beam 平衡杆balance pan 天平盘balance rider 游码balata 巴拉塔矢ball clay 块状粘土ball hardness 钢球硬度。
药学英语第五版第三单元

Biochemistry Seeks to Explain Life in Chemical TermsThe molecules of which living organisms are composed conform to all the familiar laws of chemistry, but they alsointeract with each other in accordance with another set of principles, which we shall refer to collectively as the molecularlogic of life. These principles do not involve new or yet undiscovered physical laws or forces. Instead, they are a set ofrelationships characterizing the nature, function, and interactions of biomolecules.If living organisms are composed of molecules that are intrinsically inanimate, how do these molecules confer theremarkable combination of characteristics we call life? How is it that a living organism appears to be more than the sum ofits inanimate parts? Philosophers once answered that living organisms are endowed with a mysterious and divine life force,but this doctrine (vitalism) has been firmly rejected by modern science. The basic goal of the science of biochemistry is todetermine how the collections of inanimate molecules that constitute living organisms interact with each other to maintainand perpetuate life. Although biochemistry yields important insights and practical applications in medicine, agriculture,nutrition, and industry, it is ultimately concerned with the wonder of life itself.All Macromolecules Are Constructed from a Few Simple CompoundsMost of the molecular constituents of living systems are composed of carbon atoms covalently joined with other carbonatoms and with hydrogen, oxygen, or nitrogen. The special bonding properties of carbon permit the formation of a greatvariety of molecules. Organic compounds of molecular weight less than about 500, such as amino acids, nucleotidase, andmonosaccharide, serve as monomeric subunits of proteins, nucleic acids, and polysaccharides, respectively. A single proteinmolecule may have 1,000 or more amino acids, and deoxyribonucleic acid has millions of nucleotides.Each cell of the bacterium Escherichia coli (E. coli) contains more than 6,000 different kinds of organic compounds,including about 3,000 different proteins and a similar number of different nucleic acid molecules. In humans there may betens of thousands of different kinds of proteins, as well as many types of polysaccharides (chains of simple sugars), avariety of lipids, and many other compounds of lower molecular weight.To purify and to characterize thoroughly all of these molecules would be an insuperable task, it were not for the factthat each class of macromolecules (proteins, nucleic acids, polysaccharides) is composed of a small, common set of monomericsubunits. These monomeric subunits can be covalently linked in a virtually limitless variety ofsequences, just as the 26letters of the English alphabet can be arranged into a limitless number of words, sentiments, or books.Deoxyribonucleic acids (DNA) are constructed from only four different kinds of simple monomeric subunits, thedeoxyribonucleotides, and ribonucleic acids (RNA) are composed of just four types of ribonucleotides. Proteins are composedof 20 different kinds of amino acids. The eight kinds of nucleotides from which all nucleic acids are built and the 20different kinds of amino acids from which all proteins are built are identical in all living organisms.Most of the monomeric subunits from which all macromolecules are constructed serve more than one function in livingcells. The nucleotides serve not only as subunits of nucleic acids, but also as energy-carrying molecules. The amino acidsare subunits of protein molecules, and also precursors of hormones, neurotransmitters, pigments, and many other kinds ofbiomolecules.From these considerations we can now set out some of the principles in the molecular logic of life: All living organismshave the same kinds of monomeric subunits. There are underlying patterns in the structure of biological macromolecules. Theidentity of each organism is preserved by its possession of distinctive sets of nucleic acids and of proteins.ATP Is the Universal Carrier of Metabolic Energy, Linking Catabolism and AnabolismCells capture, store, and transport free energy in a chemical form. Adenosine triphosphate (ATP) functions as the majorcarrier of chemical energy in all cells. ATP carries energy among metabolic pathways by serving as the shared intermediatethat couples endergonic reactions to exergonic ones. The terminal phosphate group of ATP is transferred to a variety ofacceptor molecules, which are thereby activated for further chemical transformation. The adenosine diphosphate (ADP) thatremains after the phosphate transfer is recycled to become ATP, at the expense of either chemical energy (during oxidativephosphorylation) or solar energy in photosynthetic cells (by the process of photophosphorylation). ATP is the majorconnecting link (the shared intermediate) between the catabolic and anabolic networks of enzyme-catalyzed reactions in thecell. These linked networks of enzyme-catalyzed reactions are virtually identical in all living organisms.Genetic Continuity Is Vested in DNA MoleculesPerhaps the most remarkable of all the properties of living cells and organisms is their ability to reproduce themselveswith nearly perfect fidelity for countless generations. This continuity of inherited traits impliesconstancy, over thousandsor millions of years, in the structure of the molecules that contain the genetic information. Very few historical records ofcivilization, even those etched in copper or carved in stone, have survived for a thousand years. But there is good evidencethat the genetic instructions in living organisms have remained nearly unchanged over much longer periods; many bacteria havenearly the same size, shape, and internal structure and contain the same kinds of precursor molecules and enzymes as thosethat lived a billion years ago.Hereditary information is preserved in DNA, a long, thin organic polymer so fragile that it will fragment from the shearforces arising in a solution that is stirred or pipetted. A human sperm or egg, carrying the accumulated hereditaryinformation of millions of years of evolution, transmits these instructions in the form of DNA molecules, in which the linearsequence of covalently linked nucleotide subunits encodes the genetic message. Genetic information is encoded in the linearsequence of four kinds of subunits of DNA. The double-helical DNA molecule has an internal template for its own replicationand repair.The Structure of DNA Allows for Its Repair and Replication with Near-Perfect FidelityThe capacity of living cells to preserve their genetic material and to duplicate it for the next generation results fromthe structural complementarity between the two halves of the DNA molecule. The basic unit of DNA is a linear polymer of fourdifferent monomeric subunits, deoxyribonucleotides, arranged in a precise linear sequence. It is this linear sequence thatencodes the genetic information. Two of these polymeric strands are twisted about each other to form the DNA double helix,in which each monomeric subunit in one strand pairs specifically with the complementary subunit in the opposite strand. Inthe enzymatic replication or repair of DNA, one of the two strands serves as a template for the assembly of another,structurally complementary DNA strand. Before a cell divides, the two DNA strands separate and each serves as a template forthe synthesis of a complementary strand, generating two identical double-helical molecules, one for each daughter cell. Ifone strand is damaged, continuity of information is assured by the information present on the other strand.The Linear Sequence in DNA Encodes Proteins with Three-Dimensional StructuresThe information in DNA is encoded as a linear (one-dimensional) sequence of the nucleotide units of DNA, but theexpression of this information results in a three-dimensional cell. This change from one to threedimensions occurs in twophases. A linear sequence of deoxyribonucleotides in DNA codes (through the intermediary, RNA) for the production of aprotein with a corresponding linear sequence of amino acids. The protein folds itself into a particular three-dimensionalshape, dictated by its amino acid sequence. The precise three-dimensional structure (native conformation) is crucial to theprotein’s function as either catalyst or structural element. This principle emerges: The linear sequence of amino acids in a protein leads to the acquisition of a unique three-dimensional structure by aself-assembly procession.Once a protein has folded into its native conformation, it may associate noncovalently with other proteins, or withnucleic acids or lipids, to form supramolecular complexes such as chromosomes, ribosomes, and membranes. These complexes arein many cases self-assembling. The individual molecules of these complexes have specific, high-affinity binding sites foreach other, and within the cell they spontaneously form functional complexes.Individual macromolecules with specific affinity for other macromolecules self-assemble into supramolecular complexes.Noncovalent Interactions Stabilize Three-Dimensional StructuresThe forces that provide stability and specificity to the three-dimensional structures of macromolecules andsupramolecular complexes are mostly noncovalent interactions. These interactions, individually weak but collectively strong,include hydrogen bonds, ionic interactions among charged groups, van der Waals interactions, and hydrophobic interactionsamong nonpolar groups. These weak interactions are transient; individually they form and break in small fractions of a second.The transient nature of noncovalent interactions confers a flexibility on macromolecules that is critical to their function.Furthermore, the large numbers of noncovalent interactions in a single macromolecule makes it unlikely that at any givenmoment all the interactions will be broken; thus macromolecular structures are stable over time.Three-dimensional biological structures combine the properties of flexibility and stability.The flexibility and stability of the double-helical structure of DNA are due to the complementarity of its two strandsand many weak interactions between them. The flexibility of these interactions allows strand separation during DNAreplication; the complementarity of the double helix is essential to genetic continuity.Noncovalent interactions are also central to the specificity and catalytic efficiency of enzymes. Enzymes bindtransition-state intermediates through numerous weak but precisely oriented interactions. Because the weak interactions areflexible, the complex survives the structural distortions as the reactant is converted into product.The formation of noncovalent interactions provides the energy for self-assembly of macromolecules by stabilizing nativeconformations relative to unfolded, random forms. The native conformation of a protein is that in which the energeticadvantages of forming weak interactions counterbalance the tendency of the protein chain to assume random forms. Given aspecific linear sequence of amino acids and a specific set of conditions (temperature, ionic conditions, pH), a protein willassume its native conformation spontaneously, without a template or scaffold to direct the folding.The Physical Roots of the Biochemical WorldWe can now summarize the various principles of the molecular logic of life:A living cell is a self-contained, self-assembling, self-adjusting, self-perpetuating isothermal system of molecules thatextracts free energy and raw materials from its environment.The cell carries out many consecutive reactions promoted by specific catalysts, called enzymes, which it produces itself.The cell maintains itself in a dynamic steady state, far from equilibrium with its surroundings. There is great economyof parts and processes, achieved by regulation of the catalytic activity of key enzymes.Self-replication through many generations is ensured by the self-repairing, linear information-coding system. Geneticinformation encoded as sequences of nucleotide subunits in DNA and RNA specifies the sequence of amine acids in each distinctprotein, which ultimately determines the three-dimensional structure and function of each protein.Many weak (noncovalent) interactions, acting cooperatively, stabilize the three-dimensional structures of biomoleculesand supramolecular complexes.。
chapter 05 The study of chemical reactions

Free Energy Change
• DG = free energy of (products - reactants), amount of energy available to do work. • Negative values indicate spontaneity. • DGo = -RT(lnKeq) where R = 1.987 cal/K-mol and T = temperature in kelvins • Since chlorination has a large Keq, the free energy change is large and negative.
Conclusions
• • • • • • With increasing Ea, rate decreases. With increasing temperature, rate increases. Fluorine reacts explosively. Chlorine reacts at a moderate rate. Bromine must be heated to react. Iodine does not react (detectably).
Bond Dissociation Energy
• Bond breaking requires energy (+BDE) • Bond formation releases energy (-BDE) • Table 4.2 gives BDE for homolytic cleavage of bonds in a gaseous molecule.
Problem
• Given that -X is -OH, the energy difference for the following reaction is -1.0 kcal/mol. • What percentage of cyclohexanol molecules will be in the equatorial conformer at equilibrium at 25°C?
激素化学——精选推荐

第五章激素化学一、是非题(正确的划“+”,错误的划“–”)1、甾体激素首先与细胞质膜表面的受体结合,然后导致腺苷酸环化酶的激活及其它一系列生化反应。
()2、各种激素都需通过细胞膜表面受体的结合作用才能产生生物效应。
()3、睾酮与雌酮都是甾体激素,在化学组成上前者少一个碳原子。
()4、甲状腺素是从甲状腺蛋白分解下来的酪氨酸,然后被酶催化碘化而成的。
()5、哺乳动物的激素只能由内分泌腺所产生,通过体液或细胞外液运送到特定作用部位,从而引起特殊的激动效应。
()6、雌激素和雄激素虽然都是胆固醇的衍生物,但在机体内不能互相转变。
()7、孕酮能加强催产素的分娩胎儿和排乳作用。
()8、促性腺激素先作用于靶细胞内的受体,然后激活腺苷酸环化酶。
()9、肾上腺素能促进肝脏中肝糖原和骨骼肌、心肌中的肌糖原的分解,两者分解后都变成血糖()10、胰岛素在体内是先分别合成A、B两条链,然后再通过正确匹配的二硫键连接而成。
()11、促肾上腺皮激素(ACTH)是一种多肽激素,而生长激素(GH)则是一种蛋白质激素。
()12、激素按其化学本质来说是有特定生理功能的多肽或蛋白质。
()二、填空题1、促黄体生成素释放激素是分泌的激素。
2、肾上腺素的结构式为。
3、雌酮、雌二醇和雌三醇均属雌性激素,它们中活泩最强的是,其次是,最弱的是。
4、甲状腺产生两种具有激素活性的氨基酸衍生物,其结构式分别为:和。
5、大多数多肽激素是通过激活靶细胞膜中酶,增加的合成,从而激活。
6、脑下垂体前叶分泌三种属于糖蛋白的激素:,,。
7、雌二醇的生物活性与其分子中的两种重要结构,即和有密切关系。
8、多肽或蛋白质激素的受体主要分布于靶细胞的,而甾体激素的受体主要分布于靶细胞的。
9、催产素和加压素的结构稳定性取决于分子中的,它们由分泌。
10、糖皮质激素的主要生理功能是影响糖和蛋白质的代谢,可促使转化为。
11、性激素与靶细胞内受体结合后,进入细胞核与作用,从而调节基因表达。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
第五章激素化学
一、是非题(正确的划“+”,错误的划“–”)
1、甾体激素首先与细胞质膜表面的受体结合,然后导致腺苷酸环化酶的激活及其它一系列生化反应。
()
2、各种激素都需通过细胞膜表面受体的结合作用才能产生生物效应。
()
3、睾酮与雌酮都是甾体激素,在化学组成上前者少一个碳原子。
()
4、甲状腺素是从甲状腺蛋白分解下来的酪氨酸,然后被酶催化碘化而成的。
()
5、哺乳动物的激素只能由内分泌腺所产生,通过体液或细胞外液运送到特定作用部位,从而引起特殊的激动效应。
()
6、雌激素和雄激素虽然都是胆固醇的衍生物,但在机体内不能互相转变。
()
7、孕酮能加强催产素的分娩胎儿和排乳作用。
()
8、促性腺激素先作用于靶细胞内的受体,然后激活腺苷酸环化酶。
()
9、肾上腺素能促进肝脏中肝糖原和骨骼肌、心肌中的肌糖原的分解,两者分解后都变成血糖()
10、胰岛素在体内是先分别合成A、B两条链,然后再通过正确匹配的二硫键连接而成。
()
11、促肾上腺皮激素(ACTH)是一种多肽激素,而生长激素(GH)则是一种蛋白质激素。
()
12、激素按其化学本质来说是有特定生理功能的多肽或蛋白质。
()
二、填空题
1、促黄体生成素释放激素是分泌的激素。
2、肾上腺素的结构式为。
3、雌酮、雌二醇和雌三醇均属雌性激素,它们中活泩最强的是,其次是,最
弱的是。
4、甲状腺产生两种具有激素活性的氨基酸衍生物,其结构式分别为:和。
5、大多数多肽激素是通过激活靶细胞膜中酶,增加的合成,从而激
活。
6、脑下垂体前叶分泌三种属于糖蛋白的激素:,,。
7、雌二醇的生物活性与其分子中的两种重要结构,即和有密切关系。
8、多肽或蛋白质激素的受体主要分布于靶细胞的,而甾体激素的受体主要分布于靶细
胞的。
9、催产素和加压素的结构稳定性取决于分子中的,它们由分泌。
10、糖皮质激素的主要生理功能是影响糖和蛋白质的代谢,可促使转化为。
11、性激素与靶细胞内受体结合后,进入细胞核与作用,从而调节基因表达。
12、胰岛素除可降低血糖之外,还能增高肌肉、肝脏等组织的糖原合成、脂肪酸合成和合
成的速度。
13、激素作用的共同特征是。
14、激素的调节途径有、、、、。
三、选择题
1、性激素的受体蛋白位于()
A、细胞核
B、细胞质膜
C、细胞质
D、微粒体
2、昆虫的保幼激素是属于()类型的化合物。
A、类固醇
B、不饱和脂肪酸衍生物
C、萜类
D、多肽
3、肾上腺激素的作用是通过()
A、蛋白质的别构作用
B、激活基因
C、第二信使cAMP
D、组蛋白的乙酰化作用
4、具有二硫键的二十环多肽激素是()
A、加压素
B、胃泌素
C、胆囊收缩素
D、降钙素
5、激素对靶组织的专一性效应主要取决于()
A、受体的有无
B、受体的活化
C、受体量的多少
D、受体的结构
6、胸腺激素的主要生理功能是()
A、促使蛋白质转化
B、促进机体的生长和发育
C、维持血钙水平
D、增强机体免疫力
7、给正常动物注射肾上腺皮质激素对代谢的影响是()
A、加强蛋白质合成
B、抑制脂肪合成
C、血糖增加
D、水盐代谢混乱
8、通过cAMP提高肝糖原磷酸化酶活性,从而促进糖原分解,但不促使肌肉的糖原分解的是()
A、肾上腺素
B、胰岛素
C、胸腺素
D、胰高血糖素
9、雄激素对物质代谢最显著的一项重要作用是()
A、促进肝脏糖原分解
B、增加脂肪贮存
C、加强蛋白质的同化作用
D、钾、钙、磷等物质的吸收
10、甾体激素对机体的影响是激素与受体结合后通过什么而实现的()
A、cAMP第二信使的作用
B、磷酸肌醇的级联反应
C、激活酪氨酸激酶
D、直接调节基因的转录
四、问答题
1、哺乳动物内分泌系统的激素分泌受到三级水平的调节,其主要内容是什么?
2、简述 -肾上腺素促进糖原降解大致途径,并扼要说明蛋白激酶在该过程中的作用及生理调节
意义。
3、哪类激素一般可口服用?哪类激素一般只能注射用?
4、用对或不对回答下列问题。
如果不对,请说明原因
(1)所有动物激素都是机体自身产生的。
(2)下丘脑所分泌的激素都是肽类激素。
(3)甲状腺素是由蛋白质水解产生的酪氨酸经碘化后生成的。
(4)激素对代谢的调节,多数是促进性的,少数是抑制性的。
(5)脑下垂体(腺垂体)分泌激素均受下丘脑激素的控制。
五、翻译本章学习要点
HORMONE CHEMISTRY
Hormone is active a substance which is secreted by special differentiation cells of animal and plant or some tissue cells. The quantity of hormone is very little but the function of it is very important. Hormone can
keep the harmony and balance of metabolism of tissues and organs. The way by which hormone bring into play is as such: After hormone was secreted from special cells, it was transported by blood circulation and then brought into play in corresponding target cell. One of the characteristics of hormone is tissue specificity and effect specificity. Different hormones have different physiology functions.
Hormone is classified into animal hormone and plant hormone. Animal hormone is classified into senior animal hormone and insect hormone.
1. Hormones of senior animal include nitrogenous hormone and steroid hormone. Apart from these hormones, prostaglandin is included in hormone because its function is closely related to hormone.
Nitrogenous hormones include derivatives of amino acid, peptide and protein hormones. Derivatie of amino acid mainly are catecholamines which is derivates from tyrosine. They include thyroxine, epinephrine and noradrenaline, etc. Hormones that are secreted by hypothalamus, hypophysis, parathyroid gland and mucous membrane of gastrointestinal, are peptide and protein hormones.
Steroid hormones include adrenocortical hormone and sex hormone.
Prostaglandin is derivative of fatty acid. It is transformed from arachidonic acid (have 20 carbon atoms). Prostaglandins have many kinds and extensive physiology functions.
2. Insect hormones is an active substance which regulate the growth, development, metamorphosis and ecdysis of insects. Apart from these functions, insect hormones are related to sex behaviors and changing colors. Researches of insect hormone have important use in agriculture.
3. Plant hormone include auxin, gibberellin, cytokinin, abscisic acid and ethane, They are regulated substances of growth, development, blossom and bear fruit of plants. There are many artificial compounds that are imitated natural hormones and have applied in agri。
