10-Byzantine Quorum Systems
【国家自然科学基金】_容错编码_基金支持热词逐年推荐_【万方软件创新助手】_20140801
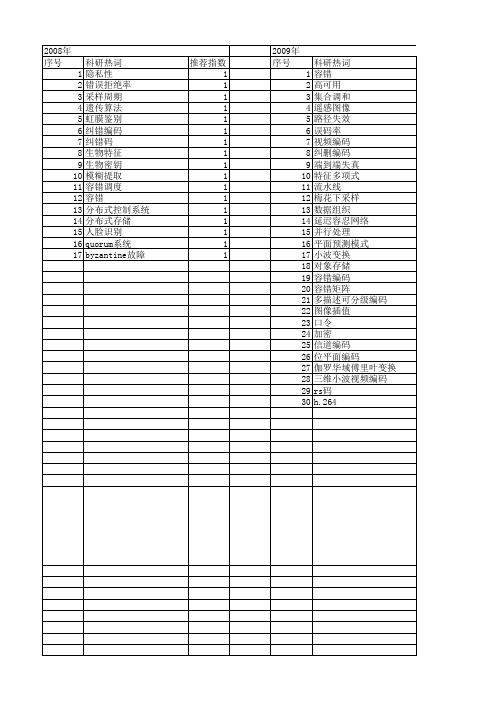
推荐指数 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
2009年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30
科研热词 容错 高可用 集合调和 遥感图像 路径失效 误码率 视频编码 纠删编码 端到端失真 特征多项式 流水线 梅花下采样 数据组织 延迟容忍网络 并行处理 平面预测模式 小波变换 对象存储 容错编码 容错矩阵 多描述可分级编码 图像插值 口令 加密 信道编码 位平面编码 伽罗华域傅里叶变换 三维小波视频编码 rs码 h.264
2011年 科研热词 视频编码 高误码率 阵列码 防篡改技术 链路容错 语音识别 网络编码 纠错输出编码 纠删码 码流提取 盲识别 片层 汉明重量 残差数据 无线传感器网络 数据处理 支持向量机 差错控制 层间结构 容错编码 容错矩阵分解 基数编码 卷积码 动态图水印 分簇 中颗粒度可分级 中粒度质量可伸缩 pyramid码 推荐指数 2 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
科研热词 容错编码 高容错性 阵列码 链路容错 配电网 遗传算法 运动跟踪 视觉敏感区域 芯片 联合信源信道编码 网络编码 纠错编码 神经网络集成 码流提取 直接纠错流水线 状态同步 无线传感器网络 数据布局 故障定位 提取误差 拜占庭错误 抗辐射 微处理器 帧内刷新 工程造价 容错 存储系统 多参数优化 图像隐藏 可靠性 单粒子效应 分簇 分支选择法 仿电磁学算法 不等差错保护 zfs x码 v阵列码 turbo码 rdp mgs编码 jpeg2000 evenodd码 erasure coding bp神经网络
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
苯乙烯吡啶盐阳离子荧光染料的染色性能

线符合 Ln m i模型 , ag ur 与普 通阳离子荧光染料荧光 黄 X 1G F相 比, 色速率较后 者略快 , 升性和反射率 一 F 0 染 提 在一定程度上优 于荧 光黄 X 1 G F .0 F 。
h a ig r t u v e t —a e c e,b i ig u r p ris。ma i u f o e c n e l c a c n o o e e e p o e n r ul n — p p o e te d x m m l r s e t r f t n e a d s n w r x l r d.Atr om e p r t r u e o t m e a u e, DHE ASP rC4wa ea ie y s a l n rd fe e t p au . W hl i ie i e p r t r B - s r lt l t be u de i r n H v ! e v f i w t a r n t m e a u e。s a it ft e p e a e — e h s t b l y o h r p r d DH i E P . d s e d d ob i sy w i h n r a e o H v l e AS BrC e c n e vou l t t e i c e s fp au .T e a s r t n o h h d o p i f DHE o ASP — on t e CDP f e s wa n a - BrC h i r s i c b c d w i h a g u rt p d o p i .C or t t e L n m i y e a s r t h on omp r d w i h l o e c n e lw - 0 a e t t e Fu r s e tY l h o X 1 GF ,DHE PB - h we l h l uc F AS rC4s o dsi t q i g y - k r y n a e.a l a e t rb i ig u r p t e n lo e c n e lc a c . e eig r t d s we I s b t e u l n — p p o er s a d fu r s e tr f t n e d i e Ke r s y i g;c t nc d e;f o e c n y y wo d :d e n ai i y o l r s e td e;s y e e p r ii m ;p le t rf e ;m o ic t n u t r n y i nu d oy s e i r b df a i i o
Quorum Systems

10/13/04 - Purdue University
Load and availability of quorum systems
Load Singleton Majorities Grid FPP CWLog 1 ½ O(1/√n) 1/√n 1/log n Resilience 0 (n-1)/2 Failure Probability p e(n)
The write operation is unchanged. However the read operation for a variable x is a pair {<vu, tu>}u∈Q that was returned by at least 2b+1 servers. It is proved that for any b-masking quorum system Q, L(Q) ≥ √( (2b+1) / n)
10/13/04 - Purdue University
10/13/04 - Purdue University
Measures (Availability)
Resilience
The resilience f of a quorum system Q is the largest k such that for every set K ⊆ U, |K| = k, there exists Q ∈ Q such that K ∩ any Q, L(Q)≥1/√n
Let a strategy w be given for a quorum system Q over a universe U. For an element u ∈ U, the load induced by w on u is lw(u) = Σ w(Qi) over all Qi such that u∈ Qi The load induced by w is Lw(Q) = maxu{lw(u)} The system load (or just load) on a quorum system Q is L(Q) = minw{Lw(Q)}
无人系统核心架构综述及标准化刍议

学术研讨无人系统核心架构综述及标准化刍议■ 孙梦男(武警研究院)摘 要:近些年来无人系统民用和军用领域发展突飞猛进,应用广泛。
本文从无人系统自主性、无人系统互操作性和无人系统协同三个方面对无人系统核心架构进行了梳理和总结。
首先对无人系统核心技术,即自主性和互操作性进行了概念内涵、关键技术、互操作性标准体系和能力评估等方面进行论述;其次,结合国内外相关的研究和应用介绍了无人系统协作的发展现状;最后从规范无人系统的发展和军民融合发展角度提出相关标准化建议和思考。
关键词:无人系统,自主性,互操作性,人机协同,跨域协同,标准化DOI编码:10.3969/j.issn.1002-5944.2023.17.011Overview of Core Architecture and Brief Discussion of Standardization ofUnmanned SystemSUN Meng-nan(Armed Police Force Research Institute)Abstract:In recent years, unmanned systems in civil and military fi elds are developing rapidly and widely applied. This paper sorts out and summarizes the core architecture of unmanned systems from three aspects: autonomy, interoperability and collaboration of unmanned systems. Firstly, the paper expounds core technologies of unmanned systems, namely autonomy and interoperability, in terms of concept connotation, key technologies, interoperability standards system, and capability evaluation. Secondly, the paper introduces the development status of unmanned system collaboration, based on relevant research and application at home and abroad. Finally, the paper puts forward relevant standardization suggestions from the perspective of regulating the development of unmanned systems and military-civilian integration. Keywords: unmanned system, autonomy, interoperability, human-machine collaboration, cross-domain collaboration, standardization0 引 言无人系统(Unmanned System,UMS)是指在主要部件上无需操作者的干预,完成指定任务的动力物理系统[1]。
碧云天生物技术产品说明书.pdf_1694034760.8424914

碧云天生物技术/Beyotime Biotechnology订货热线:400-168-3301或800-8283301订货e-mail:******************技术咨询:*****************网址:碧云天网站微信公众号mVSMC-SV40 (小鼠永生化主动脉平滑肌细胞)产品编号产品名称包装C7419 mVSMC-SV40 (小鼠永生化主动脉平滑肌细胞) 1支/瓶产品简介:Organism Tissue Morphology Culture Properties Mus musculus (Mouse) Aorta/smooth muscle Fibroblast Adherent本细胞株详细信息如下:General InformationCell Line Name mVSMC-SV40 (Mouse Immortalized Aortic Smooth Muscle Cells)Synonyms -Organism Mus musculus (Mouse)Tissue Aorta/smooth muscleCell Type -Morphology FibroblastDisease -Strain -Biosafety Level* -Age at Sampling -Gender -Genetics -Ethnicity -Applications -Category -* Biosafety classification is based on U.S. Public Health Service Guidelines, it is the responsibility of the customer to ensure that their facilities comply with biosafety regulations for their own country.CharacteristicsKaryotype -Virus Susceptibility -Derivation -Clinical Data -Antigen Expression -Receptor Expression -Oncogene -Genes Expressed -Gene expressiondatabases -Metastasis -Tumorigenic -Effects -Comments This cell line was SV40 immortalized. If needed, blasticidin (1-2µg/ml, should be tested before adding) should be added directly to the cells in culture to maintain the immortalization.Culture Method Doubling Time -Methods for Passages Wash by PBS once then 0.05% trypsin-EDTA solution and incubate at room temperature (or at 37ºC), observe cells under an inverted microscope until cell layer is dispersed (usually within 1 to 5 minutes)2 / 5 C7419 mVSMC-SV40 (小鼠永生化主动脉平滑肌细胞)400-1683301/800-8283301 碧云天/BeyotimeMedium DMEM/F-12 (1:1)+10% FBSSpecial Remarks - Medium Renewal - Subcultivation Ratio - Growth Condition 95% air+ 5% CO 2, 37ºC Freeze medium DMEM (high glucose)+20% FBS+10% DMSO ,也可以订购碧云天的细胞冻存液(C0210)。
A Versatile Zero Background T-Vector System for Gene

Breakthrough TechnologiesA Versatile Zero Background T-Vector System for Gene Cloning and Functional Genomics1[C][W][OA]Songbiao Chen,Pattavipha Songkumarn,Jianli Liu,and Guo-Liang Wang*Department of Plant Pathology,The Ohio State University,Columbus,Ohio43210(S.C.,P.S.,J.L.,G.-L.W.); and Hunan Provincial Key Laboratory of Crop Germplasm Innovation and Utilization,Hunan Agricultural University,Changsha410128,China(G.-L.W.)With the recent availability of complete genomic sequences of many organisms,high-throughput and cost-efficient systems for gene cloning and functional analysis are in great demand.Although site-specific recombination-based cloning systems,such as Gateway cloning technology,are extremely useful for efficient transfer of DNA fragments into multiple destination vectors,the two-step cloning process is time consuming and expensive.Here,we report a zero background TA cloning system that provides simple and high-efficiency direct cloning of PCR-amplified DNA fragments with almost no self-ligation.The improved T-vector system takes advantage of the restriction enzyme Xcm I to generate a T-overhang after digestion and the negative selection marker gene ccdB to eliminate the self-ligation background after transformation.We demonstrate the feasibility andflexibility of the technology by developing a set of transient and stable transformation vectors for constitutive gene expression,gene silencing,protein tagging,protein subcellular localization detection,and promoter fragment activity analysis in plants.Because the system can be easily adapted for developing specialized expression vectors for other organisms, zero background TA provides a general,cost-efficient,and high-throughput platform that complements the Gateway cloning system for gene cloning and functional genomics.Rapid advances in genome sequencing technologies in the last few years have led to the complete decoding of many complex eukaryotic genomes and have stim-ulated large-scale analysis of gene functions in se-quenced genomes.In general,gene function can be elucidated using a variety of approaches,such as ectopic expression,gene silencing,protein subcellular localization examination,gene expression pattern analysis by promoter activity assay,structure-function analysis,and in vitro or in vivo biochemical assays (Hartley et al.,2000;Curtis and Grossniklaus,2003; Earley et al.,2006).Typically,all these approaches require the cloning of target genes,mutated fragments, or their promoter fragments into various specialized vectors for subsequent characterization.However,the traditional approach for engineering expression con-structs based on the restriction enzyme/ligase cloning method is extremely laborious and time consuming and is often hampered by lack of appropriate restric-tion sites;thus,the production of constructs is a significant technical obstacle for large-scale functional gene analysis in plants.In recent years,the Gateway cloning system from Invitrogen and the Creator cloning system from CLONTECH have been developed to facilitate large-scale production of gene constructs.The recombina-tional cloning systems are based on a two-step process (Marsischky and LaBaer,2004).The DNA fragment of interest isfirst cloned into a general donor plasmid. Subsequently,the DNA fragmentflanked by two site-specific recombination sites in the donor vector can be transferred precisely into a variety of expression vec-tors by site-specific recombination reactions.A great advantage of the recombinational cloning technologies is that once the DNA fragment has been engineered into a donor vector,the transfer of the DNA fragment into an expression destination vector is a simple reac-tion that requires no traditional restriction enzyme/ ligase cloning.The recombinational cloning systems, particularly the Gateway technology,have been widely used in the research community,and many Gateway-compatible open reading frame entry(do-nor)clone collections and expression vectors have been created for functional genomics in many organ-isms(Yashiroda et al.,2008),including plants(Karimi et al.,2007b).On the other hand,although extremely useful for the simple and efficient transfer of DNA fragments into multiple expression destination vec-tors,the usefulness of the Gateway cloning system is rather limited for many projects where only a single expression vector is required for a DNA fragment.The two-step cloning process of the Gateway technology is laborious and time consuming for the production of a1This work was supported by the National Science Foundation-Plant Genome Research Program(grant no.0605017).*Corresponding author;e-mail wang.620@.The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors()is: Guo-Liang Wang(wang.620@).[C]Somefigures in this article are displayed in color online but in black and white in the print edition.[W]The online version of this article contains Web-only data.[OA]Open access articles can be viewed online without a sub-scription./cgi/doi/10.1104/pp.109.137125single expression vector.This is particularly true when a large number of plasmids must be cloned.Although a one-step recombinational cloning method was de-scribed to eliminate the production of an entry clone (Fu et al.,2008),the approach is rather limited in scope because long primers containing the specific attach-ment site(att)and two-step PCR are required(Fu et al., 2008).TA cloning is routinely used for cloning of PCR-amplified fragments.This technique exploits the ter-minal transferase activity of some DNA polymerases that add a3#-A overhang to each end of the PCR product.PCR products can be easily cloned into a linearized vector with3#-T overhangs compatible with 3#-A overhangs.Because it is difficult to generate a high-quality TA cloning vector in individual laborato-ries,many TA cloning kits are available in the market. Many of them use blue/white screening for recombi-nants,and the DNA fragments can only be cloned into the TA vector provided in the kit.To meet the need for high-throughput cloning of DNA fragments into di-verse expression vectors,we have developed a signif-icantly improved TA cloning vector system by taking advantage of the negative selection gene marker ccdB to eliminate the self-ligation background after trans-formation.We refer to this new method as the zero background TA cloning system(ZeBaTA).Numerous cloning tests in our laboratory have shown that ZeBaTA provides very high cloning efficiency with almost no self-ligation.Moreover,the ZeBaTA technology can be flexibly adapted for developing specialized expression vectors allowing single-step assembly of PCR-ampli-fied genes or fragments.We demonstrate the feasibility andflexibility of the technology by developing a set of 12transient and12stable transformation vectors for constitutive gene expression,gene silencing,protein tagging,protein subcellular localization,and promoter fragment activity analysis for rice(Oryza sativa)and Arabidopsis(Arabidopsis thaliana).Our results suggest that ZeBaTA technology can also be easily used to develop expression vectors for other organisms(e.g. Escherichia coli,yeast[Saccharomyces cerevisiae],insect, and mammal),thereby providing a novel and general high-throughput platform for functional genomics of target genes.RESULTSConstruction of the ZeBaTA SystemTwo different strategies were used to produce T-vectors,i.e.adding a single thymidine at the3# blunt ends of a linearized vector(Holton and Graham, 1991;Marchuk et al.,1991)and generating single3#-T overhangs of a linearized vector by restriction endo-nuclease digestion(Kovalic et al.,1991;Mead et al., 1991;Ichihara and Kurosawa,1993;Chen et al.,2006a). Although the former has been used to produce com-mercial cloning kits like the pGEM-T system,we selected the restriction endonuclease digestion-mediated strategy to develop a TA cloning vector system because this approach is easy to use for indi-vidual laboratories.Previous publications have de-scribed the use of restriction enzyme Xcm I(Kovalic et al.,1991;Mead et al.,1991)or Ahd I/Eam1105I (Ichihara and Kurosawa,1993;Chen et al.,2006a)to produce intermediate T-vectors.We chose Xcm I as the digestion enzyme to develop the ZeBaTA cloning system because it had a better digestion efficiency than AhdI.Figure1.Construction of the ZeBaTA system.A,Schematic represen-tation of direct cloning of PCR product using the ZeBaTA vector system. The linker of the vector(in gray)is removed after Xcm I digestion yielding a linearized T-vector.B,TA cloning tests of the ZeBaTA system.(1)Self-ligation of Xcm I-digested pGXT using T4DNA ligase from Promega.(2)Ligation of Xcm I-digested pGXT with the PCR product of the rice blast fungus M.oryzae gene MGG_07986.5using T4DNA ligase from Promega.(3)Ligation of Xcm I-digested pGXT with the PCR product of MGG_07986.5using T4DNA ligase from USB Corporation. C,Samples of restriction digestion analysis of the randomly selected colonies derived from ligation of Xcm I-digested pGXT with the PCR product of MGG_07986.5using T4DNA ligase from Promega.pGXT contains two Bam HI recognition sites outside the two Xcm I recognition sites(Supplemental Fig.S1),and MGG_07986.5contains one internal Bam HI site.All samples(lanes1–20)digested by Bam HI released two bands as expected.M,1-kb DNA ladder.Chen et al.The schematic illustration of the improved T-vector system for PCR-amplified gene/fragment cloning is shown in Figure 1A.A pair of Xcm I recognition sites,CCAATACT/TGTATGG,was introduced in the vec-tors,which allowed the generation of a single thymi-dine residue at both 3#ends of the vector when digested with Xcm I.To eliminate the potential self-ligation due to incomplete Xcm I digestion of the vec-tor,the ccdB gene (Bernard and Couturier,1992;Miki et al.,1992),which inhibits growth of E .coli strains by expressing a protein to interfere with its DNA gyrase,was introduced between the two Xcm I sites.Hence,any self-ligation transformants containing the ccdB gene will be eliminated.To test the cloning efficiency of the T-vector system,an intermediate vector pGXT was generated based on the backbone of the pGEM-T easy vector.After Xcm I digestion,ligation reactions of the resulting T-vector alone and T-vector with the PCR-amplified product of the rice blast fungus Mag-naporthe oryzae gene MGG_07986.5were set up follow-ing the standard protocol of the Promega pGEM-T easy vector system.Transformation tests showed that ligation of the T-vector with the MGG_07986.5frag-ments yielded a large number of colonies,whereas ligation of the T-vector alone yielded only a few colonies (Fig.1B).Restriction digestion screening con-firmed that the plasmids yielded from ligation of theT-vector with the PCR product were true recombinants (Fig.1C).To establish a general guide for consistently successful cloning,several factors,such as Xcm I over-digestion for generating a T-vector,insert-to-vector molar ratios,and different T4DNA ligases,were tested to determine their effect on cloning efficiency.Surprisingly,we observed that T4DNA ligases could have a significant impact on cloning efficiency.Liga-tions using Promega T4DNA ligase,the same product used by the pGEM-T easy vector system,consistently gave very high cloning efficiency with almost no self-ligation background.However,regular T4DNA li-gases from USB Corporation usually gave very low ligation efficiency for this TA cloning system (Fig.1B).Although the ligation efficiencies were a little higher at insert-to-vector molar ratios of 4:1to 8:1with the T-vector generated by standard digestion,ligations from vectors with 10-or 20-fold overdigestion and ligations with insert-to-vector molar ratios of 1:1,4:1,8:1,and 12:1all yielded good cloning efficiency when Promega T4DNA ligase was used (data not shown).Set of Expression ZeBaTA Vectors for PlantsUsing ZeBaTA,we developed a set of transient and stable expression vectors for different applications in both dicot and monocot plants.The backbone ofallFigure 2.Site-specific mutagenesis of the maize ubiqutin-1promoter (A)and the backbone of the binary vector pCAMBIA1300(B)in which three Xcm I recognition sites were deleted.The nucleotides represented in lowercase italic letters are the positions where deletions or mutations were made.Kan,Kanamycin resistance gene;LB,T-DNA left border;RB,T-DNA right border.C,Comparison of the levels of GUS expression mediated by the original and modified maize ubiquitin-1promoter in transiently transfected rice protoplasts.GUS activities are represented as a ratio of relative GUS/LUC.The experiment was repeated three times with similar results.1,Protoplast sample transfected with pUbiGUS;2,protoplast sample transfected with pXUN-GUS.A Zero Background Vector Systemtransient expression vectors is derived from pBlue-script II KS (),a high-copy-number cloning vector that can facilitate the isolation of a large amount of plasmid DNA for transient ex-pression.The backbone of all stable expression vectors is derived from pCAMBIA1300(),an Agrobacterium tumefaciens binary vector widely used for transformation in both dicot and monocot plants.Two different promoters,a cauliflower mosaic virus 35S promoter (Odell et al.,1985)and a maize (Zea mays )ubiquitin-1promoter (Christensen et al.,1992)were used to drive expression of genes of interest in dicots and monocots,respectively.The 35S promoter is more efficient in dicots,whereas the maizeubiquitin-1Figure 3.ZeBaTA-based expression vectors for gene overexpression/silencing,protein tagging,protein subcellular localization,and promoter analysis in plants.A,Schematic structures of the transient expression vectors generated by Xcm I digestion.B,Schematic structures of the Agrobacterium -mediated stable transformation vectors generated by Xcm I digestion.LB,T-DNA left border;RB,T-DNA right border.Chen et al.promoter is more efficient in monocots(Christensen et al.,1992).The original maize ubiquitin-1promoter and pCAMBIA1300vector,however,contain one and three Xcm I recognition sites,respectively(Fig.2,A and B).To facilitate the construction of the ZeBaTA-based expression vectors,the Xcm I recognition sites of the maize ubiquitin-1promoter and pCAMBIA1300vec-tor were eliminated by site-specific deletion or site-specific mutation(Fig.2,A and C).The designed expression vectors were all engineered with the cas-sette of the Xcm I-ccdB-Xcm I fragment(Supplemental Fig.S1).Figure3,A and B,illustrates the structural maps of the12transient and12stable transformation T-vectors.All vectors have been tested for cloning at least one time,and the results showed that these ZeBaTA expression vectors,including those binary vectors that are relatively large in size(.10kb), consistently yielded high cloning efficiency(Supple-mental Fig.S2).Because the maize ubiquitin-1promoter used in this system was modified to block its original Xcm I recog-nition site by deleting a single base(Fig.2A)at the position of nucleotide2480,a gus gene(Jefferson et al., 1987)was amplified by PCR and then cloned into the Xcm I-digested pXUN vector to produce an expression construct to test the expression activity of the modified maize ubiquitin-1promoter.The derived constructpXUN-GUS and a control construct pUbi-GUS(Chen et al.,2006b),of which a gus gene is driven by the original maize ubiquitin-1promoter,were tested tran-siently in the transfected rice protoplasts.Transient expression assays showed that the levels of GUS activity in rice protoplasts transfected with these two constructs were similar(Fig.2C),indicating that the deletion of nucleotide2480does not affect the activity of the maize ubiquitin-1promoter.Testing of Tagged Protein ExpressionEpitope tagging is a widely used method for the rapid and effective characterization,purification,and in vivo localization of the protein products of cloned genes.To facilitate gene cloning for epitope tagging in plants,a total of12epitope-tagging vectors(Fig.3,A and B)were constructed using ZeBaTA.These vectors contain a35S promoter or a maize ubiquitin-1pro-moter,allowing direct cloning of genes of interest into expression vectors to express a translational fusion of target protein with three commonly used epitope tags in plants(i.e.FLAG,HA,or Myc;Earley et al.,2006). To determine the feasibility of this epitope-tagging system,a gfp gene was cloned into the pXUN-HA vector to fuse with the HA tag.The resulting expres-sion construct pXUN-HA-GFP was transiently ex-pressed in rice protoplasts.As shown in Figure4,A and B,protoplasts transfected with pXUN-HA-GFP showed strong GFPfluorescence,and HA-tagged GFP protein was detected in protein extracts of trans-fected protoplasts but not in the nontransfected con-trol,demonstrating the potential application of this system for functional study of target proteins in plants.Gene Silencing by Hairpin RNAi or Artificial MicroRNA In plants,a typical and efficient approach to induce gene silencing is to use an inverted-repeat construct to express hairpin RNA(hpRNA;Waterhouse et al.,1998; Smith et al.,2000).However,a major limitation of the hpRNA interference(hpRNAi)approach for high-throughput gene functional analysis is the cumber-some cloning procedure for generating hpRNAi constructs(Helliwell and Waterhouse,2003).The gen-eration of a hpRNAi construct using conventional restriction enzyme digestion and DNA ligation meth-ods usually requires several cloning steps.Although Gateway cloning technology has been adapted to gen-erate hpRNAi constructs(Helliwell and Waterhouse, 2003;Miki and Shimamoto,2004),it still requires two cloning steps.With the ZeBaTA system,hpRNAi con-structs can be made by a single-step cloning procedure (Fig.5A).Instead of making an inverted-repeat cas-sette by DNA recombination techniques,we designed a new approach to assemble the hpRNAi cassette by overlapping PCR.Briefly,a target fragment with an additional3#-terminal sequence complementary to both the5#-and3#-terminal ends of a designed spacer fragment is amplified as afirst step.The overlapping fragments are then fused together in a subsequent PCR reaction,and the resulting inverted-repeat is cloned directly into a ZeBaTA expression vector(Fig.5A).To test the feasibility of this approach,an RNAiconstruct Figure4.Transient expression and protein-tagging detection of the ZeBaTA vectors in rice protoplasts.A,Fluorescence microscopy of the expression of HA-tagged GFP in rice protoplasts.B,Detection of HA-tagged GFP by western ne1,Nontransfected control protoplast sample;lanes2to4,independent protoplast samples transfected with pXUN-HA-GFP.[See online article for color version of thisfigure.]A Zero Background Vector Systemwas generated by overlapping PCR in which the sense and antisense 217-bp fragments of the Arabidopsis phytoene desaturase gene (PDS )were separated by a 420-bp stuffer fragment derived from the gus gene.The resulting fragment was cloned into the pCXSN vector (Fig.3B)to generate the expression construct pCXSN-atPDS-RNAi.The RNAi construct was introduced into Arabidopsis by the floral-dip method.Over 80%of transgenic plants had a clear albino phenotype (Fig.6A),a typical visible phenotype caused by silencing of the PDS gene (Guo et al.,2003;Miki and Shimamoto,2004).Recently,the artificial microRNA (amiRNA)ap-proach has been introduced for highly specific gene silencing in both dicot and monocot plants (Niu et al.,2006;Schwab et al.,2006;Ossowski et al.,2008;Warthmann et al.,2008).Typically,the amiRNA is generated by site-directed mutagenesis on precursors of endogenous miRNAs to exchange the natural miRNA sequences with those of amiRNAs using overlapping PCR (Ossowski et al.,2008).The same ZeBaTA-based vector system developed for ectopic gene expression and hpRNAi can also be used for making amiRNA expression constructs by simpleTAFigure 5.Schematic illustration of the construction of hpRNAi or amiRNA constructs by single-step cloning.A,Generation of hpRNAi constructs by overlapping PCR approach.The target gene fragment and the stuffer sequence fragment are amplified in the first-round PCR.Primers P2,P3,and P4introduce complementary adapters (indicated by vertically lined boxes)to the amplified fragments.The two amplified fragments are fused together as an inverted-repeat cassette in the second-round PCR by using single P1primer.The resulting fragment is then directly cloned into the plant expression T-vector.B,Generation of amiRNA constructs by overlapping PCR approach.C,Generation of amiRNA constructs for rice genes by single-step PCR.The expression vectors pXUN-osaMIR528and pCXUN-osaMIR528were preassembled with 5#and 3#stemloop backbone sequences of a rice miRNA precursor Osa-MIR-528(Warthmann et al.,2008).Thus,making amiRNA constructs for rice target genes only requires an amiRNA-amiRNA*fragment generated from single-step PCR.The nucleotides represented in lowercase letters are the positions where mutations were made to introduce two Xcm I recognition sites.Chen et al.cloning (Fig.5B),thus bypassing the time-consuming two-step procedure for the regular restriction enzyme digestion-mediated cloning or the Gateway cloning (Ossowski et al.,2008).We further developed a ZeBaTA-amiRNA system to simplify the generation of rice amiRNA constructs because our lab is focusing on rice functional genomics.The new ZeBaTA-amiRNA vector was designed based on the stemloop backbone derived from Osa-MIR528,an endogenous rice miRNA precursor that has been used to efficiently express amiRNAs for highly specific silencing of targeted genes in rice (Warthmann et al.,2008).By site-directed muta-genesis of a single base on the 5#and 3#stemloop backbones of Osa-MIR528,respectively,a cassette of 5#Osa-MIR528stemloop backbone-Xcm I-ccdB -Xcm I-3#Osa-MIR528was assembled and cloned into the expres-sion vectors where the expression of amiRNA is under the control of the maize ubiquitin-1promoter.Figure 5C illustrates the structural maps of the Osa-MIR528-based vectors pXUN-osaMIR528and pCXUN-osaMIR528.The vectors allow for high-throughput generationof rice amiRNA constructs by cloning the amiRNA-amiRNA*fragment generated from a single-step PCR into the ZeBaTA vector with the preassembled Osa-MIR528stemloop backbone (Fig.5C;Supplemental Fig.S3),thus avoiding the time-consuming overlapping PCR.The modified vector was evaluated by expression of the amiRNA for silencing of the OsPDS gene.The two constructs pCXUN-amiPDS and pCXUN528-PDS,which contain original or modified Osa-MIR528stem-loop backbone with amiRNA sequence targeting OsPDS ,respectively ,were introduced into rice cv Nip-ponbare by Agrobacterium -mediated transformation.Consistent with a previous study (Warthmann et al.,2008),70.1%of the primary transgenic lines transformed with pCXUN-amiPDS had a bleaching PDS silencing phenotype (Fig.6B;Table I).Similarly,77.1%of the primary transgenic lines transformed with pCXUN528-PDS had the same albino phenotype,suggesting that the mutagenesis on the Osa-MIR528stemloop backbone does not affect the biogenesis of the amiRNA for silenc-ing of the PDS gene.Protein Subcellular Localization/Colocalization and Promoter Activity AssayTo investigate the subcellular localization or colo-calization of particular proteins,a set of ZeBaTA vectors (i.e.pXDG,pXDR,pCXDG,and pCXDR)was devised for transient or stable expression of protein fusions with GFP or red fluorescent protein.The vectors contain a 35S promoter-driven gfp or DsRed cassette that has been used to visualize protein local-ization in both dicot and monocot plants (Goodin et al.,2002;Chen et al.,2006b).As shown in Figure 3,A and B,PCR products of genes of interest can be simply engineered into the vectors to fuse with the gfp or DsRed gene.To confirm whether the vectors can be used for detecting protein localization in plant cells,the rice Spin1gene encoding a putative RNA-binding protein previously shown to be nuclear targeted(Vega-Sa´nchez et al.,2008)was cloned into vectors pXDG and pXDR to fuse in-frame with gfp and DsRed ,respectively.Transient expression of the constructs pXDG-Spin1and pXDR-Spin1in rice protoplasts dem-onstrated that the GFP-and DsRed-SPIN1fusion proteins were targeted to the nuclear region as pre-dicted (Fig.7A).For promoter activity assays,two reporters,gus and gfp ,were used for constructing pXGUS-P/pCXGUS-P and pXGFP-P/pCXGFP-P ,respectively.The linear T-vectors of pXGUS-P/pCXGUS-P orpXGFP-P/Figure 6.Silencing of the PDS gene in Arabidopsis and rice by the ZeBaTA-based hpRNAi or amiRNA approaches.A,Arabidopsis plants transformed with the hpRNAi construct pCXSN-atPDS-RNAi showing the PDS silencing albino phenotype.(1)Control plant;(2and 3)two examples of transgenic Arabidopsis plants.B,Rice plants transformed with the amiRNA vectors showing the albino phenotype.(1)Control plant;(2)example of pCXUN-amiPDS-transformed plants;and (3)example of pCXUN528-PDS-transformed plants.C,RT-PCR analysis of PDS suppression transgenic rice plants.Five independent primary plants (1,2,3,4,and 5)transformed with pCXUN-amiPDS and five independent primary plants (6,7,8,9,and 10)transformed with pCXUN528-PDS were selected for the analysis.CK,Wild-type Nip-ponbare plant used as the control.Table I.PDS silencing frequency of transgenic rice mediated by the ZeBaTA-amiRNA systemamiRNA VectorTotal Independent TransformantsAlbino PhenotypeEfficiency%pCXUN-amiPDS 553970.1pCXUN528-PDS 352777.1A Zero Background Vector SystempCXGFP-P (Fig.3,A and B)allow direct cloning of PCR-amplified promoter fragments located in front of the reporter genes.As proof of concept,the 35S pro-moter was cloned into pCXGUS-P to drive expression of the reporter gene gus .Arabidopsis plants stably transformed with the construct pCX-35S-GUS showed constitutive GUS expression in the whole plants (Fig.7B),confirming the feasibility of the system for assay-ing promoter activity.DISCUSSIONWith the rapid development of the next-generation sequencing technology,more plant genomes will be sequenced in the near future.How to rapidly deter-mine the function of the identified genes on a large scale is a daunting challenge.The ability to efficiently make constructs to transiently and stably express specific genes in cells,tissues,or whole plants is a fundamental aspect and bottle neck of plant functional genomics research.Traditionally,the cloning vectorsfor plant research carry a multicloning site (MCS)within their target gene expression cassettes.The restriction sites in the MCS are rather limited,making cloning of most target genes difficult.Although TA cloning vectors have been widely used for cloning of PCR-amplified fragments,the system has not yet been incorporated in the cloning vectors for transient and stable expression of target genes because of the tech-nical challenge of generating low-background TA cloning vectors.The Gateway system has been a popular choice for generating various constructs be-cause it allows the gene of interest to be easily cloned into specifically designed plasmids without DNA re-striction digestions.The two-step cloning and expen-sive reagents,however,make the Gateway system impractical for large-scale cloning in most individual laboratories when the entry clone collections are not available.The ZeBaTA system described here over-comes the limitations of both the TA and Gateway cloning systems.After two Xcm I recognition sites have been introduced into the MCS,any PCR fragments with a T-overhang can be easily cloned into a ZeBaTA vector.With the introduction of the negative selection marker gene ccdB between the two Xcm I sites,any self-ligation transformants are ing this tech-nology,we constructed a set of 12transient and 12stable transformation vectors for plant gene expres-sion studies and tested the vectors in rice or Arabi-dopsis in our laboratories.These vectors can be used in a wide range of functional genomics projects in plants and will be distributed to the research community upon request.Under certain conditions,cloning with T-vectors generated by digestion with Ahd I or Xcm I gave low efficiency and the T residue of the insert-vector junc-tion in the recombinant clones is often missing (Mead et al.,1991;Chen et al.,2006a).Chen et al.(2006a)speculated that this may be due to the presence of unknown factors that,during digestion and prepara-tion of the T-vectors,influence the stability of 3#-T overhangs.In this study,we found that the main factor affecting successful cloning is the use of an appropri-ate T4DNA ligase.We tested the Promega T4DNA ligase,which is included in the pGEM-T easy vector system,and the T4DNA ligase from USB Corporation.The ligations using Promega T4DNA ligase consis-tently gave a very high cloning efficiency;most of the ligations using USB Corporation T4DNA ligases yielded low efficiency.Many of the recombinant plas-mids from the latter ligations missed a T residue in the insert-vector junction,consistent with observations by Mead et al.(1991)and Chen et al.(2006a).The T residue is missing mainly because regular commercial T4DNA ligases contain exonuclease activities that can remove the 3#-T tails from the vector,as reported in the technical manual of the pGEM-T and pGEM-T easy vector systems (/tbs/tm042/tm042.pdf);removal of the 3#-T tails from the vector results in very low cloning efficiency.When the Promega T4DNA ligase was used for ligation,weFigure 7.Protein subcellular localization and promoter activity anal-ysis using the ZeBaTA vectors.A,Fluorescence microscopy of the coexpression of GFP and DsRed,or GFP-SPIN1and DsRed-SPIN1fusions in rice protoplasts.Scale bar =20m m.The RNA binding nuclearprotein SPIN1was used as a tester (Vega-Sa´nchez et al.,2008).B,GUS staining of Arabidopsis transformed with pCX-35S-GUS,where the 35S promoter was cloned into the vector pCXGUS-P to test the system.CK,Plant transformed with control vector pCAMBIA1300();pCX-35S-GUS-1and pCX-35S-GUS-2,two independent primary transgenic plants.Chen et al.。
【国家自然科学基金】_quorum系统_基金支持热词逐年推荐_【万方软件创新助手】_20140803
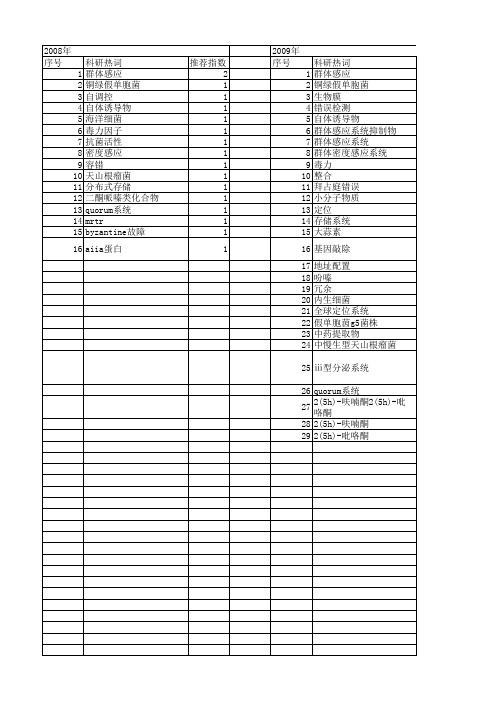
推荐指数 4 3 3 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
2010年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51
2008年 序号
科研热词 群体感应 铜绿假单胞菌 自调控 自体诱导物 海洋细菌 毒力因子 抗菌活性 密度感应 容错 天山根瘤菌 分布式存储 二酮哌嗪类化合物 quorum系统 mrtr byzantine故障 aiia蛋白
53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70
厌氧氨氧化菌 1 化学合成 1 六型分泌系统 1 假单胞菌 1 信号肽 1 信号分子ai-2 1 信号传递 1 优雅降级 1 交会 1 n-己酰高丝酰胺内酯(c6-hsl) 1 mvav 1 mvat 1 luxs 1 latic acid bacteria, quorum sensing, 1 co-culture, hrp基因 1 dsf 1 4-二乙酰基间苯三酚 1 2 1
推荐指数 2 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
2009年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29
科研热词 群体感应 铜绿假单胞菌 生物膜 错误检测 自体诱导物 群体感应系统抑制物 群体感应系统 群体密度感应系统 毒力 整合 拜占庭错误 小分子物质 定位 存储系统 大蒜素 基因敲除 地址配置 吩嗪 冗余 内生细菌 全球定位系统 假单胞茵g5菌株 中药提取物 中慢生型天山根瘤菌 ⅲ型分泌系统 quorum系统 2(5h)-呋喃酮2(5h)-吡咯酮 2(5h)-呋喃酮 2(5h)-吡咯酮
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
BQS
Byzantine Quorum Systems, 1998
D. Malkhi, M. Reiter Distributed Computing 11: 203–213
复制/写入/读取
分布式网络环境 利用复制技术,排除攻击者的影响
攻击者“完全地”控制Server,Byzantine Failure
BQS的异步假设
Reliable but Asynchronous
保证可以传输到达、但不对传输时间做保证
异步
不预先假定信道传输速度、不预先服务器的处 理速度 对于正常的服务器/信道,经过时间t后响应请求
时间t可以接近于无穷大的任意数
不是无穷大 无穷大就是等于“服务器死机”
上述信道的实际实现
Authenticated and Reliable Channel Asynchronous 信道实现
也有足够的q台正确服务器会回应/确认
BQS提出的安全条件-一致性
一致性Consistency
读出的结果,是最近一次写入的结果 Qw和Qr的交集足够大,足于用来排除失效服务器的影响
最好情况下,Qw和Qr交集=q
完全重合
最差情况下,Qw和Qr交集=q-(n-q)
q-(n-q)中,至少有f+1台正确服务器 正确服务器要比失效多 才能排除其影响 q-(n-q) >= f+1+f = 2f+1
即,在部分Server错误/被攻击的情况下,系统 如何继续正常运行、提供正确结果 暂不讨论“错误检测” 不讨论如何去检测/发现哪些服务器是错误的
入侵检测的研究内容 事实上,目前不存在100%检测率和0%虚警率的 入侵检测系统
假定,Client无法知道哪些Server是失效的
BQS的基本原理
最多f台服务器失效 复制/冗余
假定信道有无限小带宽,即速度没有任何保证 带宽不能为零,否则不能通信 Authenticated,要求发送方对消息数字签名 Reliable,要求接收方发送确认 确认也由接收方数字签名 不断地发送信息,直至收到确认为止 后面课程,我们讲解COCA会谈到
BQS的服务
以上是信道假设 在上述的网络环境中,BQS提供了安全的存 储服务
异步通信假设
BQS的假设
Authenticated and Reliable Channel
A收到B发送的消息M,当且仅当B发送了消息M
B发送消息M,A肯定能够收到
Reliable 必须成立,否则无论什么方案都无法解决问题
A收到B的消息,肯定不是别人假冒的
Authenticated 可以通过Authentication技术来实现 例如,口令鉴别协议密钥、消息加上MAC校验
下面我们讲解BQS的解决方案,如下方面
Byzantine Quorum System
系统模型
数据格式 Client操作 Server操作
讨论n/q/f的关系
数据格式
对于变量x
数据格式是[x, v, t] 其中,v是变量x的值、t是时戳timestamp 时戳timestamp可以理解为版本号
新的值有更大的版本号 从而使得能够选出新数据
Compromised Servers QwClBiblioteka ent操作ReadQr
向所有Server发出请求;不断发送请求,直至得到q台 Server(某个Quorum )的回应 从q个回应[x, v, t]中选出合适的结果 注意,[x, v, t]可能不同
Write
先读取变量,得到当前时戳t’ 选择更大时戳t > t’,向所有Server写[x, v, t] 直至收到q台Server(某个Quorum )的确认
对应的、取值没有限制的数据,我们称为普通数据
Generic Data
自验证数据的实际意义
有实际意义 存在着实在的自验证数据,例如常用的PKI数字证书 失效的存储服务器,不能产生合法的数字证书 只有特定的CA,可以签发证书
引入自验证数据对于BQS的影响
对于自验证数据
事实上,相当于是“限制了失效服务器的能力” 失效服务器可以 不回复 回复“旧数据” 对于Generic Data,失效服务器可以 不回复 回复“伪造数据”/“旧数据”
所以,引入自验证数据,事实上,相当于是带来 “新的攻击/失效模型”
Dissemination BQS
现在,我们假定BQS系统存储自验证数据
数据由Client产生
基于自验证数据的BQS系统,被称为 Dissemination BQS 安全条件
可用性Availability
同样,n-f >= q
一致性Consistency
读不出任何结果,可一定时间后再读
我们也说明Server操作的原因
Write 当收到Write请求时,回应确认消息 同时,比较Write请求中的时戳,如果比自己存储的更 大,则更新;否则不更新 考虑到并发写,只保留时戳大的Write请求
BQS的几种变形
上述的系统配置,被称为Masking Quorum System
Compromised Servers Qw
Qr
BQS的配置
综合n-f >= q和q-(n-q) >= 2f+1,解方程
要求n至少是4f+1、q至少是3f+1
下面,我们以n=4f+1/q=3f+1进行讨论 当Client收到q=3f+1份回应[x, vi, ti],采取如下 方式,从中选出正确结果
问题
读写并发的问题
假设写操作进行了一半,会发生什么? 原值是x=20,正在写入的值是x=17
正确的结果
应该是x=17;当然,x=20也可以接受 但是,不应该是其它值
可能发生的问题,收到2f+1结果中
f=3为例 3个[x=13],2个[x=17],2个[x=20] 认为正确结果是x=13?
复制/Quorum问题并不容易解决
提供数据读写
Client向Server请求读写
读出的结果,是最后一次写入的结果
容错要求
Failure-Tolerant
容错
首先,当所有服务器都失效时,不可能找到 可行的解决方案
服务器全部死机,什么也不能存储、不能读写
我们假设:最多有f台服务器失效
能不能找到合适的可行方案?
容错要求
注意,我们的系统是讨论“容错”
读出结果:只有1台服务器回复17、其它服务器随便乱 回复 甚至他们是共同有意地都回复x=16 如何找出正确的数据? 错误地判断为x=16?
改进
我们要求读出的结果数量至少是2f+1
即,至少读2f+1台服务器 “读Quorum”是2f+1
读出的结果中
有可能就包括了f台失效服务器的错误结果
例如,f个x=16
Dissemination BQS一致性条件
一致性Consistency
Qw和Qr的交集足够大,足于用来排除失效服务器的影响
最差情况下,Qw和Qr交集=q-(n-q)
q-(n-q)中,至少有1台正确服务器 1台就足够 失效服务器顶多是回复“旧数据” Compromised 有1个最新的足矣 Servers q-(n-q) >= f+1
Masking BQS
变形
对于自验证数据,有Dissemination Quorum System
Dissemination BQS
同步环境中
Synchronous BQS
什么是自验证数据
自验证数据
Self-verifying Data 从自身就可以验证其有效性的数据,只有特定的、具有 Write权限的实体,可以生成有效的数据 失效服务器不能生成“能通过验证”的数据
先排除“出现次数不大于f”的结果 排除失效服务器的影响 想要“出现次数大于f”,则其中必有正确服务器 从剩余的结果中,选出时戳最大的结果 最新的结果,时戳最大
并发操作的情况
当“读写并发”时,可能选不出任何正确结果
先排除“出现次数不大于f”的结果 可能所有结果都被排除 例如3f+1回应中:f个失效服务器回应、f个旧值、f个当前值、 1个正在被写入的值 从剩余的结果中,选出时戳最大的结果
但是会有一定的相交/覆盖
利用覆盖的部分,排除失效服务器的影响
使得Client能获得正确结果 Compromised
Servers Qw
Qr
BQS提出的安全条件-可用性
可用性Availability
任何情况下,总是会有Quorum来配合Client 操作 即:n-f >= q,即使所有f台失效服务器都故意 不回应/确认
Qw
结论 共需要n=3f+1台服务器 每次读写q=2f+1台服务器
Qr
选择正确结果的过程
当Client收到q=2f+1份回应[x, vi, ti],采取如下 方式,从中选出正确结果
先排除验证不通过的结果 去掉失效服务器的影响,剩下“旧数据的影响” 从剩余的结果中,选出时戳最大的结果 最新的结果,时戳最大
Synchronous BQS
Byzantine Quorum Systems
信息安全系统技术原理 林璟锵
冗余/备份/容错
在RAID中,给出了冗余/备份/容错技术在 存储服务中的应用 RAID的假设
同步假设
磁盘响应时间,有最大延迟假设 超过该延迟,则定义为“坏磁盘/失效的磁盘”
Crash Failure假设
不是Byzantine Failure 坏磁盘不会“有意识地”修改数据、而且使得校验和 正确
网络存储服务中,RAID假设是否成立?
分布式网络存储
应该如何复制?
……
