Hydroquinone_123-31-9_DataSheet_MedChemExpress
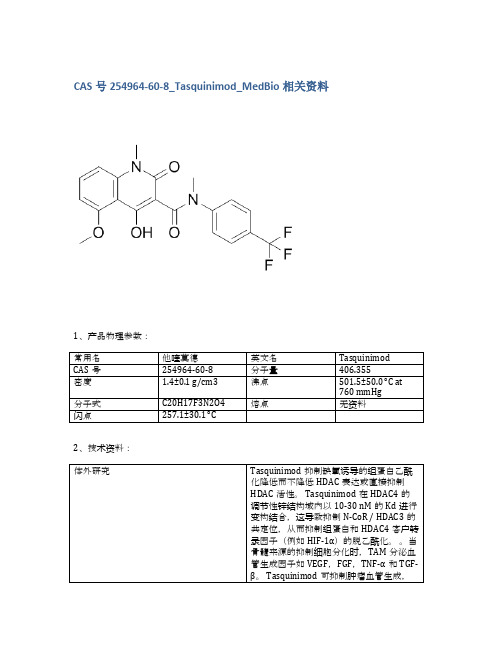
CAS号254964-60-8_Tasquinimod_MedBio相关资料
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11931
Cyclophosphamide
Cyclophosphamide
50-18-0
10mM (in 1mL DMSO)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12034
Mupirocin
包装
纯度
MedBio
MED11950
Resminostat hydrochloride
Resminostat hydrochloride
1187075-34-8
100mg
≥98%
品牌
CAS
包装
纯度
MedBio
MED12017
Raltitrexed
Raltitrexed
112887-68-0
10mM (in 1mL DMSO)
≥98%
体内研究
当通过管饲法或饮用水给予成年雄性小鼠(即C57B1 / 6J或无胸腺裸鼠)0.1-30mg / kg(即0.2-74μmoles/ kg)时,Tasquinimod的生物利用度和口服吸收是极好的。Tasquinimod的效力表示为抑制癌症生长50%的Tasquinimod每日口服剂量范围为0.1-1.0 mg / kg / d(即0.24-2.40μmoles/ kg /天),相对于一系列(n> 5)免疫缺陷小鼠中的人前列腺癌异种移植物。通过饮用水以5mg / kg /天的慢性剂量服用的Tasquinimod在免疫活性同系小鼠中产生> 80%的TRAMP-C2小鼠前列腺癌生长抑制(p <0.05)[2]。携带皮下LNCaP肿瘤的裸鼠用Tasquinimod治疗3周。在接种后第7天开始以1mg / kg /天和10mg / kg /天暴露于Tasquinimod。与接种后28天的未处理对照组相比,1 mg / kg /天和10 mg / kg /天的肿瘤重量均有统计学显着的剂量依赖性降低(p <0.001),说明Tasquinimod的抗肿瘤作用[3]。
Sarafloxacin hydrochloride_91296-87-6_DataSheet_MedChemExpress

Product Name:Sarafloxacin hydrochloride CAS No.:91296-87-6Cat.No.:HY-B0343A Product Data SheetCat. No.:HY B0343A MWt:421.83Formula:C20H18ClF2N3O3Purity :>98%DMSO 1mg/mL;Water <1mg/mL Solubility:Mechanisms:Biological Activity:S fPathways:Anti-infection; Target:Antibacterial DMSO 1 mg/mL; Water <1 mg/mLSarafloxacin hydrochloride is a quinolone antibiotic drug.Target: Antibacterial sarafloxacin hydrochloride is a fluoroquinolone antibiotic registered for use against poultry diseases.Sarafloxacin treatment demonstrated mineralization to 14CO2 amounting to 0.58%, 0.49%, and 0.57% in loam, silt loam, and sandy loam soils, respectively, at the termination of the test [1]. The inhibitory level of sarafloxacin for the tested bacteria was strain dependent. It appeared that in broth culture Escherichia coli isolates were sensitive to sarafloxacin concentrations 5‐fold lower than the concentrations present in the simulated gut model, suggesting that sarafloxacin may be partially unavailable due to absorption to organic matter in the model [2]Administering Sarafloxacin References:[1]. Marengo, J.R., et al., Aerobic biodegradation of (14C)‐sarafloxacin hydrochloride in soil.Environmental Toxicology and Chemistry, 1997. 16(3): p. 462-471.[2]. McConville, M.L., et al., Effects of sarafloxacin hydrochloride on human enteric bacteria under unavailable due to absorption to organic matter in the model [2]. Administering Sarafloxacinhydrochloride in the feed for 5 d at a dose of 10 or 12.5 mg/kg of fish proved efficacious in treating cha...simulated human gut conditions. Vet Q, 1995. 17(1): p. 1-5.[3]. Johnson, M.R., K.L. Smith, and C.R. Boyle, Field efficacy trials of the antibacterial sarafloxacin-hydrochloride (A-56620) for treatment of Edwardsiella ictaluri infections in channel catfish. Journal ofaquatic animal health, 1992. 4(4): p. 244-251.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d ch e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
Medlife,1115-70-4,盐酸二甲双胍,技术规格说明书(SDS)

1115-70-4|盐酸二甲双胍,技术规格说明书(SDS)简介:盐酸二甲双胍,Metformin (hydrochloride)是FDA批准的用于治疗2型糖尿病的一线药物。
二甲双胍主要是通过对线粒体呼吸链复合物1的轻度和短暂抑制降低肝脏葡萄糖的产生。
盐酸二甲双胍物理化学性质:盐酸二甲双胍详细介绍:盐酸二甲双胍参考文献:[1]. Soraya H, et al. Acute treatment with metformin improves cardiac function following isoproterenol induced myocardial infarction in rats. Pharmacol Rep. 2012;64(6):1476-84.[2]. Quaile MP, et al. Toxicity and toxicokinetics of metformin in rats. Toxicol Appl Pharmacol. 2010 Mar 15;243(3):340-7.[3]. Xue J, et al. Metformin inhibits growth of eutopic stromal cells from adenomyotic endometrium via AMPK activation and subsequent inhibition of AKT phosphorylation: a possible role in the treatment of adenomyosis. Reproduction. 2013 Aug 21;146(4):397-406.[4]. Otto M, et al. Metformin inhibits glycogen synthesis and gluconeogenesis in cultured rat hepatocytes. Diabetes Obes Metab. 2003 May;5(3):189-94.[5]. Avci CB, et al. Therapeutic potential of an anti-diabetic drug, metformin: alteration of miRNA expression in prostate cancer cells. Asian Pac J Cancer Prev. 2013;14(2):765-8.[6]. Nie L, et al. The Landscape of Histone Modifications in a High-Fat Diet-Induced Obese (DIO) Mouse Model. Mol Cell Proteomics. 2017 Jul;16(7):1324-1334.[7]. Zhang D, et al. Metformin ameliorates BSCB disruption by inhibiting neutrophil infiltration and MMP-9 expression but not direct TJ proteins expression regulation. J Cell Mol Med. 2017 Jul 12.产品技术规格说明书由上海创赛科技有限公司收集整理,仅作参考使用。
Hydroquinone_CAS号123-31-9说明书_AbMole中国

分子量110.11溶解性(25°C )
DMSO 22 mg/mL 分子式C H O Water 22 mg/mL CAS 号123-31-9Ethanol 22 mg/mL
储存条件
3年 -20°C 粉末状
生物活性
Hydroquinone 是抑制剂和抗氧化剂,作为染料,发动机燃料,及油的合成中间体。
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA 指南)
小鼠
大鼠兔豚鼠仓鼠狗重量 (kg)0.020.15 1.80.40.0810体表面积 (m )0.0070.0250.150.050.020.5K 系数
3
6
12
8
5
20
动物 A (mg/kg) = 动物 B (mg/kg) ×
动物 B 的K 系数动物 A 的K 系数
例如,依据体表面积折算法,将白藜芦醇用于小鼠的剂量22.4 mg/kg 换算成大鼠的剂量,需要将22.4 mg/kg 乘以小鼠的K 系数(3),再除以大鼠的K 系数(6),得到白藜芦醇用于大鼠的等效剂量为11.2 mg/kg 。
参考文献
Rapid method for the quantification of hydroquinone concentration: chemiluminescent analysis. Chen TS, et al. Luminescence. 2015 Nov;30(7):947-9. PMID: 25693839.
Hydroquinone 目录号M5697
化学数据
6622m m m m m。
HA14-1_DataSheet_MedChemExpress
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:HA14–1 is a non–peptidic ligand of a Bcl–2 surface pocket with IC50 of ~9 μM.IC50 Value: 9 μMTarget: Bcl–2in vitro: HA14–1 is a small molecule and nonpeptidic ligand of a Bcl–2 surface pocket with IC50 of approximately 9 μM. HA14–1induces the apoptosis of HL–60 cells in a dose–dependent manner via caspase activation. HA14–1 shows cytotoxic effects on HF1A3,HF4.9 and HF28RA follicular lymphoma B cell lines with LC50 of 4.5 μM, 12.6 μM and 8.1 μM respectively. HA14–1 induces apoptosis of HF1A3, HF4.9 and HF28RA cells via caspase and ROS.in vivo: HA14–1(400 nM) treatment did not have any significant effect on the growth of glioblastoma tumors in immunodeficient mice.But HA14–1 (400 nM) increases the effect of the DNA–damaging agent etoposide (2.5 mg/Kg) on glioblastoma growth in vivo.PROTOCOL (Extracted from published papers and Only for reference)Cell assay [5]IGROV1, OAW42, A2780, and SKOV3 cell lines were established from human ovarian adenocarcinomas. Resistant IGROV1–R10 and OAW42–R cells were obtained by reiterating treatments with increasing concentrations of cisplatin. These cell lines were grown.Exponentially growing cells were exposed for 2 h to 20 μg/mL CDDP in serum–free medium or with HA14–1 dissolved in DMSO in complete medium. For HA14–1 treatment, control cultures received the same dose of DMSO as treated counterparts.References:[1]. Doshi et al (2006) Structure–activity relationship studies of ethyl 2–amino–6–bromo–4–(1–cyano–2–ethoxy–2–oxoethyl)–4H–chromene–3–carboxylate (HA 14–1), an antagonist for antiapoptotic Bcl–2 proteins to overcome drug resistance in cancer. J.Med.Chem. 49 7731[2]. Nyhan MJ, O'Donovan TR, Elzinga B, Crowley LC, O'Sullivan GC, McKenna SL.The BH3 mimetic HA14–1 enhances 5–fluorouracil–induced autophagy and type II cell death in oesophageal cancer cells.Br J Cancer. 2012 Feb 14;106(4):711–8.[3]. Weyland M, Manero F, Paillard A, Grée D, Viault G, Jarnet D, Menei P, Juin P, Chourpa I, Benoit JP, Grée R, Garcion E.Mitochondrial targeting by use of lipid nanocapsules loaded with SV30, an analogue of the small–molecule Bcl–2 inhibitor HA14–1.J Control Release. 2011 Apr 10;151(1):74–82. Epub 2010 Dec 5.[4]. Moon DO, Kim MO, Kang SH, Choi YH, Park SY, Kim GY.HA14–1 sensitizes TNF–alpha–induced apoptosis via inhibition of the NF–kappaB signaling pathway: involvement of reactive oxygen species and JNK.Cancer Lett. 2010 Jun 1;292(1):111–8. Epub 2009 Dec 22.[5]. Simonin K, Brotin E, Dufort S, Dutoit S, Goux D, N'diaye M, Denoyelle C, Gauduchon P, Poulain L.Mcl–1 is an important determinant of the apoptotic response to the BH3–mimetic molecule HA14–1 in cisplatin–resistant ovarian carcinoma cells.Mol Cancer Ther. 2009 Nov;8(11):3162–70. Epub 2009 Nov 3.Product Name:HA14–1Cat. No.:HY-12011CAS No.:65673-63-4Molecular Formula:C 17H 17BrN 2O 5Molecular Weight:409.23Target:Bcl–2 Family Pathway:Apoptosis Solubility:10 mM in DMSOCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
cas923565-22-4_GSK962040 hydrochloride_MedBio技术参数

1、产品物理参数:
常用名
盐酸Camicinal
英文名
GSK962040 (hydrochloride)
CAS号
923565-22-4
分子量
461.02
密度
无资料
沸点
无资料
分子式
C25H34ClFN4O
熔点
无资料
闪点
无资料
2、技术资料:
体外研究
Camicinal(GSK962040)在一系列其他受体(包括生长素释放肽),离子通道和酶中没有显着活性。在兔胃窦中,Camicinal(GSK962040)300 nmol L1-10μmolL1导致胆碱能介导的收缩幅度的延长促进,在3μmolL1时最大为248±47%。胃动素的pEC50值,红霉素和Camicinal(GSK962040)分别为10.4±0.01(n = 770),7.3±0.29(n = 4)和7.9±0.09(n = 17)[1]。Camicinal(GSK962040)激活狗胃动素受体(pEC50 5.79;内在活性0.72,与[Nle13] -肌动蛋白相比)[2]。Camicinal(GSK962040)是首选,因为它在CYP3A4的初始IC50值显着高于我们优选的10μM阈值[3]。
体内研究
Camicinal(GSK962040)(5 mg游离碱kg 1)在给药后2小时内总粪便重量也增加(21.2±4.5 g; P <0.05)[1]。Camicinal(GSK962040)诱导阶段性收缩,其持续时间与剂量相关(3和6 mg kg 1为48和173分钟),由平均血浆浓度>1.14μmolL驱动1.Camicinal(GSK962040)的作用消退后,迁移运动复合体(MMC)活动返回。迁移的运动复合体恢复不受3 mg kg 1 Camicinal(GSK962040)的影响,但在6 mg kg 1时,MMC在给药后253 min返回,而生理盐水后为101 min(每组n = 5)[2]。他发现Camicinal(GSK962040)的口服生物利用度(Fpo)为48(13%.Camicinal(GSK962040)与短寿命效应相比,显示出长效(T1 / 2)46.9(在3μM时为5.0分钟)[Nle13]胃动素(T1 / 2)11.4(0.3μM1.5分钟)[3] .Camicinal(GSK962040)强烈促进胃窦中的胆碱能活性,仅在眼底和小肠中活性较低[4]。
Determination of the binding parameter constants of

Journal of Pharmaceutical and Biomedical Analysis29(2002)195–201Determination of the binding parameter constants ofRenagel®capsules and tablets utilizing the Langmuirapproximation at various pH by ion chromatographyRonald A.Swearingen*,Xi Chen,John S.Petersen,Kristine S.Riley,Donghui Wang,Eugene ZhorovGelTex Pharmaceuticals,153Second A6enue,Waltham,MA02451,USAReceived30July2001;received in revised form11January2002;accepted29January2002AbstractSevelamer hydrochloride is a cross-linked polymeric amine;it is the active ingredient in Renagel®capsules and tablets.Sevelamer hydrochloride is indicated for the control of hyperphosphatemia in patients with end-stage renal disease.The binding parameter constants of sevelamer hydrochloride were determined using the Langmuir approxi-mation for three different dosage forms at pH4.0,5.5and7.0.The three dosage forms were Renagel®403mg capsules,Renagel®400mg tablets and Renagel®800mg tablets.The results demonstrate the equivalency of all three dosage forms at each pH.The results also demonstrate a shift in the binding mechanism from pH4.0to7.0.©2002 Elsevier Science B.V.All rights reserved.Keywords:Sevelamer hydrochloride;Ion chromatography;Langmuir;Quaternary amines;Hydrogel;Renagel®/locate/jpba1.IntroductionSevelamer hydrochloride is the active ingredient in Renagel®capsules and tablets.Sevelamer hy-drochloride,a cross-linked poly(allylamine hydro-chloride),is a novel phosphate binder used for the reduction of serum phosphate levels in patients with end-stage renal disease(ESRD)[1–4].The advantage of sevelamer hydrochloride for ESRD over existing therapies,such as calcium or alu-minum supplementation,is that it is non-ab-sorbed,leading to an improved safety profile. An important aspect of the analytical charac-terization of sevelamer hydrochloride tablets is to demonstrate equivalency to the capsule dosage form.The structure of sevelamer hydrochloride is shown in Fig.1.The amines in sevelamer hydro-chloride may bind phosphate ionically and through hydrogen bonding.This paper describes the methodology and pro-cedures for the determination of the binding con-stants at three different pH levels utilizing the Langmuir approximation.A comparison of these binding constants demonstrates the equivalency of*Corresponding author.Tel.:+1-781-434-3515;fax:+1-781-672-5823.E-mail address:rswearingen@(R.A.Swearingen).0731-7085/02/$-see front matter©2002Elsevier Science B.V.All rights reserved. PII:S0731-7085(02)00007-9R.A.Swearingen et al./J.Pharm.Biomed.Anal.29(2002)195–201 196the tablet dosage form to the capsule dosage form at each pH studied.The binding of the dibasic phosphate anion and monobasic phosphate anion is also discussed.2.Materials and methods2.1.ChemicalsSevelamer hydrochloride was obtained from GelTex Pharmaceuticals,Inc.(Waltham,MA). N,N-Bis(hydroxyethyl)-2-aminoethanesulfonic acid(BES)was obtained from Sigma Chemical Company(St.Louis,MO).Potassium phosphate, monobasic(KH2PO4)and1N aqueous sodium hydroxide were obtained from Aldrich Chemical Company,Inc.(Milwaukee,WI).Sodium chloride and sodium hydroxide pellets were from VWR Scientific Products(West Chester,PA).All chemi-cals were of ACS grade or higher and were used without further purification.Deionized water was obtained from an in-house Barnstead Nanopure System(Barnstead/Thermolyne Corporation, Dubuque,IA).2.2.ApparatusA Dionex(Dionex Corporation,Sunnyvale, CA)DX-500IC system was used for phosphate analysis.This system consists of an AS50au-tosampler,GP50quaternary gradient pump, CD20conductivity detector,and PeakNet soft-ware control and data acquisition(version5.10d). Separations were performed using a Dionex AS-11analytical column(4×250mm2)and an AG-11guard column(4×40mm2).Suppression was achieved with an ASRS-II anion self regenerating suppressor from Dionex.Samples were shaken using a Labline Heated Orbital Shaker Model No. 4628(Labline Instruments,Melrose Park,IL). Injection loop volume was100m l with a full injection setting.2.3.Sample preparationThree individual sets of aqueous phosphate so-lutions were prepared at the following concentra-tions:38.7,30.0,14.5,10.0,7.5,5.0,2.5and1.0 mM.Each set of phosphate solutions were pre-pared so that afinal pH of4.0,5.5and7.090.3 was obtained after the addition of Renagel®cap-sules and tablets,as described below.All solutions contained100mM BES and80mM NaCl.For the403mg capsule and400mg tablet,one unit dose was placed into150ml of each phos-phate solution.For the800mg tablet,one unit dose was placed into300ml of each phosphate solution in order to keep the phosphate to poly-mer ratio constant.The solutions at pH 4.0were prepared by adding the capsules and tablets to a set of phos-phate solutions,which had no prior pH adjust-ments.Upon their disintegration,approximately3 ml of1N HCl was added to all solutions with the exception of300ml solutions(800mg tablets)at 38.7and30.0mM phosphate in which approxi-mately6and5ml were added,respectively.The pH of thefinal solutions was approximately pH 4.0.All of the samples were prepared in duplicate. The solutions at pH5.5were prepared by ad-justing the pH of a set of phosphate solutions to pH4.0,volumetrically,with1N NaOH.Approx-imately2–4ml of1N NaOH was added per literFig.1.Structure of sevelamer hydrochloride;(a,b=number of primary amine groups)a+b=9;(c=number of cross-link-ing groups)c=1;(n=fraction of protonated amines)n=0.4; (m=large number to indicate extended polymer network).R .A .Swearingen et al ./J .Pharm .Biomed .Anal .29(2002)195–201197Fig.2.Chromatogram of typical phosphate standard (see text for conditions).curve was generated at each pH to produce a total of three separate 8point calibration curves for quantitation at pH 4.0,5.5and 7.0.The area of the phosphate peak versus the concentration was plotted and the coef ficient of determination values for each curve was greater than 0.998.The average of the two phosphate binding val-ues was used to generate the phosphate binding isotherms and Langmuir plots.2.4.Chromatographic conditionsStandards and samples were analyzed for phos-phate levels by ion chromatography using the columns described above [5].The mobile phase was 25mM NaOH pumped at 1ml /min.Suppres-sion was performed in the recycle mode,with an applied current of 100mA.The injection volume was 100m l with a full injection setting.A typical phosphate standard chromatogram is shown in Fig.2.2.5.CalculationsThe unbound phosphate concentration remain-ing in each sample was calculated from the linear regression generated from a plot of the area of the phosphate peak versus the concentration of phos-phate (mM)using the following equation:Unbound phosphate concentration (mM)=area phosphate −interceptslopeFrom the known initial concentration of phos-phate in each solution before the addition of sevelamer hydrochloride (i.e.38.7,30.0,14.5,10.0,7.5,5.0,2.5and 1.0mM),the bound con-centration was calculated by subtracting the un-bound concentration from the initial concentration.Bound phosphate concentration (mM)=initial concentration−unbound phosphate concentration (mM)The phosphate binding capacity,in mmol of phosphate /g of polymer,was calculated as follows:of solution.After the addition of the capsules and tablets,the pH of all solutions was approximately 5.5.The samples were prepared in duplicate.The solutions at pH 7.0were prepared by ad-justing the pH of each solution to pH 7.0with 1N NaOH [5].Approximately 50ml of 1N NaOH was volumetrically added per liter of solution.After the addition of the capsules and tablets,the pH of the solution was approximately pH 7.0.The pH of this solution does not change because the p K a of BES is 7.1and thus provides excellent buffering capacity in this pH range.BES was utilized throughout this experiment so that a di-rect comparison of all results is possible.It has been demonstrated that BES,in concentrations from 60to 120mM,does not affect the phosphate binding (R.Swearingen et.al.,unpublished results).All samples were then placed on a Labline Orbital Shaker at 37°C for 2h.The samples were removed,filtered through a 25mm,0.2m m nylon syringe filter (Pall Corp.,Ann Arbor,MI).The samples were diluted with deionized water in the appropriate ratios.The dilutions ranged from 1:100to undiluted for the 1mM phosphate concentration.The phosphate standards were prepared by di-luting each set of phosphate solutions,1:50with deionized water to generate an eight point calibra-tion curve.This dilution was performed prior to the addition of capsules and tablets.A calibrationR.A.Swearingen et al./J.Pharm.Biomed.Anal.29(2002)195–201198Phosphate binding capacity(mmol/g)=bound phosphate concentration(mM)×V sweight(g)where V s is the volume of solution,approximately0.15l for the403mg capsules and the400mgtablets and approximately0.30l for the800mgtablets.The weight(g)is the weight of sevelamerhydrochloride.The phosphate binding constants were calcu-lated from the Langmuir approximation.TheLangmuir approximation describes themonomolecular adsorption of an adsorbate(phos-phate)from solution,at constant temperature,onto an adsorbent(sevelamer hydrochloride)[6].This process is described by the Langmuirequation:C eqx/m=1k1k2+C eqk2where C eq is the concentration,in mM,of phos-phate remaining in solution at equilibrium or theunbound concentration.x/m is the amount ofphosphate bound per weight of polymer in mmol/g.The constant k1is the affinity constant and isrelated to the magnitude of the forces,which areinvolved in binding.The constant k2is the Lang-muir capacity constant and is the maximumamount of phosphate that can be bound per unitweight of sevelamer hydrochloride.The affinity and Langmuir capacity constantswere calculated by performing linear regressionon a plot of the unbound(mM)/bound(mmol/g)versus the unbound(mM)concentrations.The k1value is calculated by dividing the slope of theregression line by the intercept,the k2value isequal to the inverse of the slope.The results arelisted in Figs.3–5and in Table1.3.Results and discussionThe results demonstrate that at each individualpH,all three dosage forms exhibit very similarbinding properties.The increase in the k2valuesfrom pH7.0to 5.5may be attributed to thedecrease in the ionic strength of the solutions asprepared at pH 5.5.The non-linearity of the ngmuir plot of Renagel®403mg capsules,400mg tablets and800mg tablets at pH4.0.ngmuir plot of Renagel®403mg capsules,400mg tablets and800mg tablets at pH5.5.R .A .Swearingen et al ./J .Pharm .Biomed .Anal .29(2002)195–201199ngmuir plot of Renagel ®403mg capsules,400mg tablets and 800mg tablets at pH 7.0.Langmuir plot and the order of magnitude de-crease in the af finity constants at pH 4.0can be explained by examining the fraction of each phos-phate ion present as a function of pH in dilute solution.This is accomplished by taking into ac-count the hydronium ion concentration at each pH,the p K a of each phosphate ion and the equi-librium reaction.The results are shown in Fig.6.The value at which one fraction of an ion is equal to another,corresponds to the p K a value of the phosphoric acid ions in dilute aqueous solution (i.e.2.1,7.2and 12.4).At a pH range of approximately 6–8,monoba-sic phosphate is in equilibrium with dibasic phos-phate.It has been demonstrated that at pH 7,the molar ratio of amine to bound phosphate is ap-proximately 2.The results are listed in Table 2.This may indicate that the dibasic phosphate is the predominately bound species at pH 7,as has been previously suggested [7].At pH 5.5–6.0,the fraction of monobasic ion increases.The small decrease in the binding af finity constants (k 1)demonstrates that the bind-ing forces are weaker at pH 5.5–6.0This is due to the decrease in the amount of dibasic phosphate bound and increase in the amount of the monoba-sic bound.The linearity of the Langmuir plots indicates monomolecular binding.At pH 4,the monobasic phosphate ion is pre-dominately present.The af finity constants are an order of magnitude lower than the af finity con-stants at pH 7.0.These results suggest that the monobasic ion,which has only one site for bind-ing,is more weakly bound than the dibasic ion,which has two sites for binding.The relativeTable 1The af finity and Langmuir capacity constants calculated at three pH levelsTablets 800Capsules 403Tablets 400mgmgmgpH 7.00.13Intercept 0.140.12Slope 0.160.160.170.9990.9990.999R 2k 1 1.2 1.41.2 6.16.2 6.0k 2pH 5.50.15Intercept 0.150.16Slope 0.140.140.140.9990.9980.998R 2k 10.940.950.897.27.07.1k 2pH 4.0 1.5Intercept 1.7 1.7Slope 0.140.150.160.939R 20.9150.946k 10.110.090.086.1k 26.67.0Fig.6.Fraction of phosphate ion present as a function of pH.R .A .Swearingen et al ./J .Pharm .Biomed .Anal .29(2002)195–201200Table 2Molar amine to phosphate ratio Total amines (mmol /g)Sevelamer sample number Amine /Phosphate ratio Phosphate (mmol /g)11.741 2.135.52 5.511.56 2.10311.525.7 2.0211.695.6 2.094 5.7511.63 2.0411.79 2.146 5.511.545.5 2.107 5.5811.632.11Average 2.10.04S.D.%R.S.D.2.0Fig.7.Phosphate binding as a function of pH.non-linearity of the Langmuir plots at pH 4.0may indicate non-monomolecular binding as a result of the monobasic ion.The change in the binding of the dibasic anion to the monobasic anion can be seen by plotting the phosphate bind-ing isotherms as a function of pH.The isotherm are presented in Fig.7.The graph shows the dibasic binding of phos-phate at pH 7.0and 5.5,and the monobasic binding of phosphate at pH 4.0.The lower slope of the curve at pH 4.0also demonstrates the lower binding af finity of the monobasic ion.These results show the differences in the binding of the dibasic and monobasic ion.The lower af finity constants values at pH 4.0are not pharmacologi-cally signi ficant as binding occurs in the intestine where the pH range is typically 6–7.A possible explanation for the similarities of the isotherms at pH 7.0and 5.5is the apparent p K a values.Fig.6shows the fraction of each ion atvarious pH in dilute solution with only phospho-ric acid present in water.Sevelamer hydrochloride has an internal charge and hence its own internal ionic strength due to the amines,which are present.This intermolecular charge of sevelamer hydrochloride may shift the p K a of the dibasic anion from 7.2to a slightly lower value when in solution and in contact with sevelamer hydrochlo-ride.This would cause the fraction of the dibasic ion,at pH 5.5,to be substantially more than predicted by Fig.6.It has also been shown that there are more protoanated amines at pH 5.5than at pH 7.0[7].4.ConclusionThe phosphate binding constants,k 1and k 2,were determined at pH 7.0,5.5and 4.0for the following dosage forms of sevelamer hydrochlo-R.A.Swearingen et al./J.Pharm.Biomed.Anal.29(2002)195–201201ride:403mg capsules,400mg tablets,and800mg tablets.The results demonstrate that the binding constants and affinity constants at pH7.0,5.5and 4.0for all three dosage forms exhibit similar binding properties at each pH.The differences in the affinity constants at pH 4.0,as compared to the affinity constants at pH 7.0,are due to the fact that at pH 4.0the monobasic ion is predominately bound and at pH 7.0the dibasic ion is predominately bound.The relative non-linearity of the Langmuir plot at pH 4and the linearity of the Langmuir plot at pH7 indicate a shift in the binding mechanism from pH4to7.References[1]D.Rosenbaum,S.Holmes-Farley,W.Mandeville,M.Pitruzello, D.Goldberg,Nephrol.Dial.Transplant.12 (1997)961–964.[2]S.Burke, D.Goldberg,J.Bonventere, E.Slatopolsky,Nephrol.Dial.Transplant.11(1996)A41.[3]G.Chertow,S.Burke,zarus,Am.J.Kidney Dis.1(1997)66–71.[4]S.Burke, E.Slatopolsky, D.Goldberg,Nephrol.Dial.Transplant.12(1997)1640–1644.[5]J.Mazzeo,R.Peters,M.Hanus,X.Chen,K.Norton,J.Pharm.Biomedical.Anal.19(1999)911–915.[6]W.Johns,T.Bates,J.Pharm.Sci.58(1969)179–183.[7]S.Randy-Holmes Farley,W.Harry Mandeville,K.L.Miller,Pure.Appl.Chem.A36(7and8)(1999)1085–1091.。
关于批准焦磷酸一氢三钠等5种食品添加剂新品种的公告(卫生部公告2012年第15号)附件

附件1焦磷酸一氢三钠等5种食品添加剂新品种一、焦磷酸一氢三钠英文名称:Trisodium monohydrogen diphosphate功能:水分保持剂(一)使用范围和使用量1.生产工艺以焦磷酸二氢二钠和氢氧化钠为原料反应后制得焦磷酸一氢三钠。
2.技术要求2.1感官要求:应符合表1的规定表1 感官要求2.2 技术要求:应符合表2 的规定。
附录A检验方法A.1 安全警示本试验方法中使用的部分试剂具有毒性或腐蚀性,操作时须小心谨慎!必要时,需在通风橱中进行。
如溅到皮肤上应立即用水冲洗,严重者应立即治疗。
A.2 一般规定本标准所用试剂和水,在没有注明其他要求时,均指分析纯试剂和GB/T 6682—2008规定的三级水。
试验中所需标准溶液、杂质标准液、制剂及制品,在没有注明其他规定时,均按GB/T 601、GB/T 602、GB/T 603之规定制备。
所用溶液在未指明溶剂时,均指水溶液。
A.3 鉴别试验A.3.1 试剂和材料A.3.1.1盐酸。
A.3.1.2 硝酸溶液:1+1。
A.3.1.3 喹钼柠酮溶液。
A.3.2 分析步骤A.3.2.1钠离子鉴别称取1g 试样,加20 mL 水溶解,用铂丝环蘸盐酸润湿后,在火焰上燃烧至无色。
再蘸取试液在火焰上燃烧,火焰应呈亮黄色。
A.3.2.2 焦磷酸根离子鉴别将0.1g试样溶于100mL硝酸溶液中。
向30mL喹钼柠酮溶液中滴入0.5mL试样溶液,不产生黄色沉淀;另取0.5mL此溶液于95℃水浴中加热10min,滴入30mL喹钼柠酮溶液,立即形成黄色沉淀。
A.4 焦磷酸一氢三钠含量的测定A.4.1 方法提要焦磷酸一氢三钠与盐酸反应生成焦磷酸二氢二钠,向溶液中加入硫酸锌,定量生成焦磷酸锌沉淀和硫酸,用氢氧化钠标准滴定溶液滴定生成的硫酸,再根据氢氧化钠标准滴定溶液的消耗量计算出焦磷酸一氢三钠的含量。
A.4.2 试剂和材料A.4.2.1 盐酸溶液:1+20。
A.4.2.2 硫酸锌溶液:125 g/L;将125 g 硫酸锌(ZnSO4·7H2O)溶解于水,用水稀释至1 L,在pH计上,根据显示的pH,用硫酸溶液(1+500)或氢氧化钠溶液(6 g/L)将pH调至3.8。
