福建省厦门外国语学校高三第一次月考
2024-2025学年福建省泉州市泉港区第一中学、厦门外国语学校石狮分校高三上学期第一次联考历史试卷

2024-2025学年福建省泉州市泉港区第一中学、厦门外国语学校石狮分校高三上学期第一次联考历史试卷1. 贾湖遗址位于河南省舞阳县,C.14测年结果显示其距今约9000―7500年。
该遗址出土的遗物十分丰富,其中最引人注目的是刻符龟甲、猪骨等动物遗骸、骨笛、稻作遗存等。
对上述考古发现,解读正确的是()A.该地区具备了国家的初始形态B.黄河中上游是粟的发源地C.古人具有一定的精神生活追求D.原始农耕和畜牧开始出现2. 管仲认为,工匠应“群萃而州处,相良材……相语以事,相示以功,相陈以巧,相高以知。
旦夕从事于此,以此教其子弟”;而商人应“群萃而州处……相语以利,相示以赖,相陈以知贾”。
管仲的这一主张()A.将国家治理寓于职业教化之中B.意在全面限制人口的流动C.致力于对工商业实行官府垄断D.强调了基层治理因地制宜3. 汉代,今朝鲜半岛的玄菟、乐浪“郡初取吏于辽东”;云贵高原的群制、永吕、越满郡太守多为巴蜀籍;长沙出土的“逃阳令印”表明岭南地区的一些郡县官吏来自湖南。
由此可知,汉代()A.长途贸易较为发达B.民族交融程度加深C.加强对边疆的统治D.官僚政治日益完善4. 如表展示了两汉时期史学领域代表性成就,由此可知两汉时期()C.文化氛围自由宽松D.史学体例日趋完备5. 汉武帝时“罢黜百家,尊崇儒术”的同时,积极派兵北击匈奴,南征闽越;而宋太祖确立了“兴文教,抑武事”的基本国策,实行守内虚外的方针。
这种变化说明了()A.政治风气日趋保守B.儒学重新回归主流地位C.儒学思辩与哲学化D.人文教化倾向内省收敛6. 清朝前期采用当时世界上先进的经纬度测绘技术在全国范围内测绘,绘成《皇舆全览图》;乾隆时期绘成更为详细的全国地图《乾隆内府舆图》。
清朝还进行了三次全国地理总志《大清一统志》的编纂,各级地方政府也定期编修地方志。
这主要反映了()A.清朝社会治理能力的全面提升B.中央对地方的控制力加强C.统一多民族国家的发展与巩固D.西学东渐的风潮已经兴起7. 1932—1933年。
福建省厦门外国语学校2011届高三11月月考 数学文
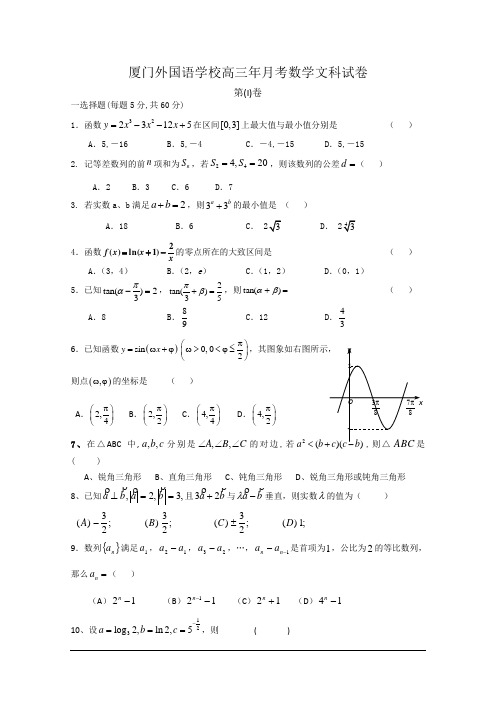
厦门外国语学校高三年月考数学文科试卷第(I)卷一选择题(每题5分,共60分)1.函数3223125y x x x =--+在区间[0,3]上最大值与最小值分别是 ( )A .5,-16B .5,-4C .-4,-15D .5,-152. 记等差数列的前n 项和为n S ,若244,20S S ==,则该数列的公差d =( )A .2B .3C .6D .73. 若实数a 、b 满足2a b +=,则33a b +的最小值是 ( )A .18B .6C .D .4.函数xx x f 2)1ln()(-+=的零点所在的大致区间是( )A .(3,4)B .(2,e )C .(1,2)D .(0,1) 5.已知tan()23πα-=,2tan()35πβ+=,则=+)tan(βα( )A .8B .98C .12D .34 6.已知函数()sin y x =ω+ϕ0,02π⎛⎫ω><ϕ≤ ⎪⎝⎭则点(),ωϕ的坐标是( )A .2,4π⎛⎫ ⎪⎝⎭B .2,2π⎛⎫ ⎪⎝⎭C .4,4π⎛⎫ ⎪⎝⎭D .4,2π⎛⎫⎪⎝⎭7、在△ABC 中,c b a ,,分别是C B A ∠∠∠,,的对边,若2(a b < ( )A 、锐角三角形B 、直角三角形C 、钝角三角形D 、锐角三角形或钝角三角形8、已知,3,2,==⊥b a b a 且b a 23+与b a-λ垂直,则实数λ的值为( ))(A ;23- )(B ;23 )(C ;23± )(D ;19.数列{}n a 满足1a ,12a a -,23a a -,…,1--n n a a 是首项为1,公比为2的等比数列,那么=n a ( )(A )12-n(B )121--n (C )12+n (D )14-n10、设123log 2,ln 2,5a b c -===,则 ( )(A )a b c << (B )b c a << (C )c a b << (D )c b a << 11、设b 3是a -1和a +1的等比中项,则b a 3+的最大值为( )A 、1B 、2C 、3D 、412.数列}{n a 的各项均为正数,n S 为其前n 项和,对于任意的*N n ∈,总有2,,n n n a S a 成等差数列,又记32121++⋅=n n n a a b ,数列}{n b 的前n 项和T n =( )A .96+n n B .69+n n C .96+n nD.6+n n二填空题(每题4分,共16分)13.已知点)2,1(),1,0(),1,2(),0,1(--D C B A ,则与的夹角大小为 . 14 三个不同的实数c b a ,,成等差数列,且b c a ,,成等比数列,则::a b c =_________ 15.已知等差数列{a n }的前13项之和39,则a 6+a 7+a 8=_______.16.已知()()()()2cos sin 4cos sin 3=+---++απαααπ,则αtan = ;三解答题(17至21题每题12分,22题14分,共74分)17.(12分)已知函数()2sin (sin cos )f x x x x =+ (1)求()f x 的最小正周期和最大值; (2)将()f x 的图像向右平移8π个单位得到函数()g x 的图像,求()g x 在[0,]π上的零点。
【KS5U推荐】考点05 金属及其化合物(拔高专练)-2019领军高考化学考点必练 Word版含解析

Evaluation Only. Created with Aspose.Words. Copyright 2003-2016 Aspose Pty Ltd.考点五金属及其化合物拔高专练1.(2019届江西省红色七校高三第一次联考)N A为阿伏伽德罗常数的值,下列叙述正确的是()A. 4.0g由H218O与D216O组成的混合物中所含中子数为2N AB.标准状况下,22.4 L己烷含有的共价键数目为19 N AC. 1L pH=13的NaOH溶液中含有OH-的数目一定为0.1 N AD. 1mol的 Na2O和BaO2混合物中含有的阴、阳离子总数为3N A【答案】A2.(2019届云南省师范大学附属中学高三上学期第二次月考)下列反应的离子方程式不正确的是A.向Ba(OH)2溶液中滴加稀硫酸:Ba2++2OH-+2H++SO42-=BaSO4↓+2H2OB.向NaHCO3溶液中加入稀HCl:HCO3-+H+=CO2↑+H2OC.向AlCl3溶液中加入过量稀氨水:Al3++4NH3·H2O=AlO2-+4NH4++2H2OD.酸性介质中KMnO4氧化H2O2:2MnO4-+5H2O2+6H+=2Mn2++5O2↑+8H2O【答案】C【解析】A. 向Ba(OH)2溶液中滴加稀硫酸的离子反应为Ba2++2OH-+2H++SO42-=BaSO4↓+2H2O,所以A选项是正确的;B. NaHCO3在溶液中电离出碳酸氢根离子,碳酸氢根离子不能拆开,故B正确;C.氢氧化铝不溶于弱碱氨水中,正确的是Al3++3NH3·H2O= Al(OH)3↓+3NH4+,故C不正确;D. 酸性介质中KMnO4氧化H2O2的离子反应为2MnO4-+5H2O2+6H+=2Mn2++5O2↑+8H2O,所以D选项是正确的。
3.(2019届河北省邯郸市永年区第二中学高三9月月考)某Mg-Al合金放入足量HCl溶液中,生成H2的体积在标准状况下是2 240 mL;同质量该Mg-Al合金放入足量NaOH溶液中,生成H2的体积在标准状况下是2 016 mL,则合金中Mg、Al的质量之比是 ( )A. 1﹕1 B. 6﹕1 C. 1﹕6 D. 4﹕27【答案】D再根据Mg与HCl(aq)的反应求出Mg的质量:Mg+2HCl══MgCl2+H2↑24 g 22.4Lm(Mg) 2.240 L-2.016 Lm(Mg)=0.24g。
2021高考生物一轮习题:第五单元 重点强化练42 突破遗传系谱图的推断与概率计算(含解析)

1.(2019·山东德州夏津第一中学高三上学期第一次月考)下图为人类某种单基因遗传病的系谱图,Ⅱ-4为患者。
下列相关叙述错误的是()A.该病属于隐性遗传病,可代表红绿色盲的遗传B.若Ⅰ-2是携带者,则该病是常染色体隐性遗传病C.该病不易受环境影响,Ⅱ-3有可能是纯合子D.若Ⅰ-2不携带致病基因,则Ⅰ-1的一个初级卵母细胞中含1个致病基因2.(2019·四川攀枝花高三第一次统考)下图是某种单基因遗传病的遗传系谱图,控制该病的基因位于X染色体与Y染色体的同源区段上。
不考虑基因突变和交叉互换。
据图分析,下列叙述正确的是()A.若该致病基因是隐性基因,则1的基因型为X A Y a或X a Y AB.仅考虑该致病基因,2可能为纯合子,也可能为杂合子,但3一定为纯合子C.若5为该致病基因的携带者,6是一个男孩,则6可能不患病D.若4和5所生女儿一定患病,则该病为隐性基因控制的遗传病3.(2020·河北秦皇岛调研)已知人类白化病是常染色体隐性遗传病,红绿色盲是伴X染色体隐性遗传病。
下列对如图三个遗传系谱图的叙述错误的是(不考虑基因突变及其他变异)()A.甲和丙不能表示红绿色盲的遗传,乙可以表示红绿色盲遗传B.甲、乙、丙都可表示白化病的遗传,丙中的子代一定是杂合子C.若表示白化病遗传,甲、乙中患者的患病概率因性别而不同D.若乙表示红绿色盲遗传,患病男孩的致病基因一定来自母亲4.(2019·福建厦门外国语学校高三1月月考)下图为某单基因遗传病的家族系谱图,已知Ⅰ-2为携带者。
下列说法正确的是()A.该病为伴X染色体隐性遗传病B.一定为携带者的是Ⅱ-3C.Ⅱ-4是携带者的概率为1/2D.Ⅲ-2是纯合子的概率为1/35.(2019·江西五市八校联盟体高三第一次模拟)下图为与甲、乙两种病有关的遗传系谱图,其中有一种病为伴性遗传病。
下列有关说法中不正确的是()A.甲病为伴X染色体隐性遗传病,乙病为常染色体显性遗传病B.Ⅲ-13的致病基因来自Ⅱ-8C.Ⅱ-5为杂合子的概率是1/2D.Ⅲ-12和一个正常的男性结婚,生患病孩子的概率为1/46.如图所示为具有甲种遗传病(基因为A或a)和乙种遗传病(基因为B或b)的家庭系谱图,其中一种遗传病基因位于X染色体上。
精品解析:福建省厦门外国语学校2022-2023学年高三上学期10月月考物理试题(解析版)

A.需要用天平测出砂和砂桶的总质量
B.小车靠近打点计时器,先接通电源,再释放小车,打出一条纸带,同时记录拉力传感器的示数
C.选用电磁打点计时器比选用电火花计时器实验误差小
D.为减小误差,实验中一定要保证砂和砂桶的质量远小于小车的质量
(2)实验得到如图乙所示的纸带,已知打点计时器使用的交流电源的频率为 ,相邻两计数点之间还有四个点未画出,由图中的数据可知,小车运动的加速度大小是______ 。(计算结果保留三位有效数字)
C.根据能量守恒定律,加速过程电梯对人做的功
C错误;
D.扶梯匀速运动时,支持力等于重力,因此支持力做功的功率
D正确。
故选AD。
4.一质量为2kg的物体受到水平拉力F作用,在粗糙水平面上做加速直线运动时的a-t图像如图所示,t=0时其速度大小为2m/s,滑动摩擦力大小恒为2N,则( )
A.在t=6s时刻,物体的速度为18m/s
厦门外国语学校2023届高三10月月考
物理试题
本试卷分选择题和非选择题两部分,共6页,满分为100分。考试用时75分钟。
注意事项:
1.答卷前,考生务必用黑色字迹的钢笔或签字笔将自己的姓名和准考证号填写在答题卡相应的位置上,用2B铅笔将自己的准考证号填涂在答题卡上。
2.选择题每小题选出答案后,用2B铅笔把答题卡上对应题目的答案标号涂黑;如需改动,用橡皮擦干净后,再选涂其他答案;在试卷上做答无效。
物块A与物块B碰撞时间极短,根据动量守恒定律
解得
故A正确;
B.根据动能定理有
由上述可知 图像的斜率代表物体所受的合外力,由图乙可知,物块A与物块B碰撞后,在 处合外力最大,即加速度最大。故B正确;
2023-2024学年福建省厦门第一中学高三上学期10月月考英语试题

2023-2024学年福建省厦门第一中学高三上学期10月月考英语试题GET A BIRD’S-EYE VIEW OF THE WORLD’S MOST ATTRACTIVE FEATHERED ANIMALS WITH THESE BOOKSFlamingo (火烈鸟)Biologist and photographer Claudio Contreras Koob spent 20 years travelling deep into the wet lands and forests of his native Mexico—and beyond—to feed his flamingo attraction. This book offers a unique window into the behavior and life of red-feathered birds, with more than 120 show-stopping shots displaying their beauty. teNeues, £35.Around the World in 80 BirdsInspiring secrets, national pride or scientific discoveries, every bird has a story to tell, from the weaver bird building multi-nest “apartment blocks” in Namibia to the bar-headed goose taking on a twice-yearly trans-Himalayan journey at an extreme altitude. Mike Unwin’s tour is accompanied by beautiful illustrations from Ryuto Miyake. Laurence King Publishing, £22.A World on the WingPulitzer-shortlisted Weidensaul, who’s at the forefront of research into bird migration, here tracks some of nature’s most remarkable journeys. He sails through the stormy Bering Sea, encounters trappers in the Mediterranean and visits former headhunters in northeast India, where a bird migration crisis has become a conservation success story. Pan Macmillan, £9.99.Galapagos Crusoes: A Year Alone with the BirdsExplore this updated version of the 1968 title, Galapagos: Islands of Birds, by late bird expert Bryan Nelson, with previously unpublished material from his wife, June. The couple spent a year living on two Galapagos islands, studying birds, including the Galapagos albatross (信天翁). This is their clever and amusing account. Bradt Guides, £11.99.1. By whom is the second costliest book illustrated?A.Claudio Contreras Koob. B.Mike Unwin.C.Ryuto Miyake. D.Weidensaul.2. Which book best suits those concerned about the survival of migratory birds?A.Flamingo . B.Around the World in 80 Birds .C.A World on the Wing . D.Galapagos: Island of Birds.3. What feature may Galapagos Crusoes: A Year Alone with the Birds have?A.Its humorous description. B.Its romantic style.C.Its vivid imagination. D.Its moving plot.In the 1940s, young male Royal Air Force pilots held needles as they waited for their next mission. Wartime pilots suffered a lot and knitting helped rebuild dexterity (灵巧) in wounded arms while also helping to settle wounded minds.Today, millions of people around the world employ the same techniq ue. “I know that if I haven’t knitted for a few days, I really miss it. It’s like meditation.” says Janine Smith, who owns a store in Sydney selling supplies for knitting.Research supports Smith’s statement. Physiotherapist Betsan Corkhill and occupationa l therapist Jill Riley were part of a team from Cardiff University that, ten years ago, surveyed more than 3,500 knitters and found that the more frequently people knitted, the calmer and happier they felt.Or as Jannie Smith puts it, “That rhythm of making stitch after stitch is like deep breathing. It’s a flow where you don’t have to stress about it, you’ve got the rhythm happening.”“Flow” is a concept first named by psychologist Mihaly Csikszentmihalyi. As he wrote in his book, “The best moments in our lives are neither the passive nor relaxing times. The best moments usually occur if a person’s body or mind is stretched to its limits in a voluntary effort to accomplish something difficult and worthwhile.”The Cardiff research team found that many respondents described feeling calmer and in a better mood after knitting, and the majority of respondents who suffered from depression “perceived that knitting made them feel happier.” For respondents who suffered from chronic pain, almost nine out of ten said that knitting gave them a sense of accomplishment and a means of coping with their pain. Interestingly, more than half of those surveyed said that knitting pushed them to develop other skills, like building furniture. Because knitting is so accessible — at its heart it’s two sticks and one stitch — it helps people build confidence in their abilities. After all, if you make a mistake, you can just pull it all out and start again.4. Why does the author mention young male Royal Air Force pilots in paragraph 1?A.To give an example of a knitter.B.To show the heavy burden on pilots.C.To demonstrate the technique of pilots.D.To introduce the healing effect of knitting.5. Which can be called as the best moment according to Mihaly Csikszentmihalyi?A.Watching TV together with friends. B.Taking physical exams.C.Playing chess with a great opponent. D.Reading funny comic books.6. According to a research of Cardiff University, which statement about knitting is true?A.It motivates people to stretch their bodies.B.It enables people to build furniture.C.It helps people to get rid of chronic pain.D.It allows people to build confidence to learn other skills.7. What would be the best title for the text?A.Active Hands, Calm Minds B.Healthier Attitude, Longer LifeC.Easy Knitting, Skill Building D.Mind Calming, Flow FindingIn 2012, James Cameron, creator of Avatar and Titanic, became the first person to reach the Challenger Deep. When he arrived at the deepest spot on Earth at 7 miles below sea level, he spent hours mapping the region and taking photos and samples.“As human beings, we’re drawn to absolutes—the deepest, the highest, the coldest, the farthest,” he says. “And as a storyteller and curious monkey, I just wanted to see what was there.” The answer is obvious—plastic and more. “Our so-called civilization is using the ocean as its toilet,” Cameron says. “Unless this changes, ocean ecosystems are going to continue their rapid collapse.”Despite decades of environmental studies, the impact of plastic and other forms of pollution on oceans are not entirely understood. Initial studies appear to indicate that ingesting(摄取) them—either directly or indirectly—could cause disease. Plastics can also release poisonous substances into the water, which could potentially impact animal populations.But plastic is just one of the problems facing oceans that have yet to be fully understood. “Plastic waste in the ocean is horrifying but is only the most obvious of our many deadly waste streams, which include car bon that’s heating the atmosphere and making the ocean acidic, and the run-off nutrients from all the world’s agriculture, which is causing anoxic(缺氧的) dead zones the size of countries,” Cameron says.Oceans, like the rest of the world, are impacted by the burning of fossil fuels and the release of greenhouse gases like carbon dioxide—about 30 percent of which is absorbed by the sea. This absorption causes ocean acidification, where the pH level is altered to become more acidic. As a result, it’s harder for some creatures to form shells and skeletons and countless species at the base of the food web can struggle to survive, which, scientists say, has the potential to cause huge disruptions to entire ecosystems. Indeed, ocean acidification is thought to have pl ayed an important role in Earth’s worst-ever mass extinction event 252 million years ago.The effect of climate change on the world’s oceans will likely worsen in coming decades. Last June, scientists announced carbon dioxide levels had reached the highest levels since human records began. The last time carbon dioxide levels were this high was during the Pliocene era, between 3 and 5 million years ago, when global temperatures were about 4 degrees Celsius warmer than they are today. Current climate models suggest that if greenhouse gas emissions continue on their current trend, we may be on course to see 4 degrees of warming by 2100.As a result, understanding the role oceans have on global systems is becoming more and more important.8. What are the first two paragraphs mainly about?A.The author’s feelings to the ocean.B.Cameron’s movies and remarks.C.The author’s discoveries under the sea.D.Cameron’s observation and concern.9. What can we infer from the passage?A.Several countries are suffering from anoxic dead zones.B.More concern should have been given to the pollution on oceans.C.Plastic is supposed to be the most serious environmental problem.D.Ocean acidification removes the nutrients from agricultural products.10. What does the underli ned word “disruptions” in Paragraph 5 probably mean?A.Decreases. B.Destruction.C.Diseases. D.Discrimination.11. Why does the author mention the mass extinction event 252 million years ago?A.To call on people to protect sea animals.B.To compare current situations with the past.C.To explain how serious the ocean problem is.D.To prove pollution to be the cause of acidification.A snake-robot designer, a technologist, an extradimensional physicist and a journalist walk into a room. The journalist turns to the crowd and asks: Should we build houses on the ocean? Like a think-tank panel, members of the team dream up far-out answers to the crucial problem, such as self-driving housing units that could park on top of one another in the coastal city center.The setting is X, the enterprise which considers more than 100 ideas each year, in areas ranging from clean energy to artificial intelligence. Although only a tiny percentage become “projects” with far-reaching creativity, these projects exist, ultimately, to change the world, like Waymo, the biggest self-driving-car company.In the past 60 years, something strange has happened. As the academic study of creativity has thrived (蓬勃发展), the label innovation may have covered every tiny change of a soda can or a toothpaste flavor, but the rate of productivity growth has been mostly declining since the 1970s. John Fernald, an economist, points out that the notable exception to the post-1970 decline in productivity occurred when businesses throughout the economy finally figured out the breakthrough technology-information technology. John Fernald says, “It’s possible that productivity took off, because we picked all the low-hanging fruit from the IT wave.” Actually. the world economy continues to harvest the benefits of IT. But where will the next technology shock come from?Breakthrough technology results from two distinct activities—invention and innovation. Invention is typically the work of scientists and researchers in labs, while innovation is an invention put to commercial use. Seldom do the two activities occur successfully under the same roof. They tend to thrive in opposite conditions; while competition and consumer choice encourage innovation, invention has historically progressed in labs that are protected from the pressure to generate profit.Allowing well-funded and diverse teams to try to solve big problems is what gave us the computer and the Internet. Today, we fail to give attention to planting the seeds of this kind of ambitious research, whi le complaining about the harvest. “Companies are really good at combining existing breakthroughs in ways that consumers like. But the breakthroughs come from patient and curious scientists, not the rush to market,” says John Gertner, the author of The Idea Factory.“Technology is a tall tree,” John Fernald said. “But planting the seeds of invention and harvesting the fruit of innovation are entirely distinct skills, often mastered by different organizations and separated by many years.” As for me, both of t hem are essential for technology, although they are relatively independent. “I don’t think X is a planter or a harvester, actually. I think of X as building taller ladders. They reach where others cannot.” he added. Several weeks later, his words were repe ated to several X employees. “That’s perfect,” they said. “That’s so perfect.” Nobody knows for sure what, if anything, the employees at X are going to find up on those ladders. But they’re reaching. At least someone is.12. What is the main purpose of the first two paragraphs?A.To present the process of group discussion. B.To illustrate X’s worry about big problems.C.To reveal the importance of the crazy ideas. D.To stress the varied backgrounds of the team.13. What can we learn from the Paragraph 3-4?A.Breakthroughs must stand the test of the market.B.Innovation on necessities can promote productivity.C.Invention develops slowly under the pressure of profit.D.The harvest of innovation lies in some ambitious research.14. What’s X employee’ attitude regarding John Fernald’s view on technology?A.Ironic. B.Uninterested. C.Conservative. D.Supportive. 15. What can be inferred about X from the passage?A.It will focus on innovation. B.It will have its outcome soon.C.It may bring an encouraging outlook. D.It may give in to its fruitless reality.You will be leaving this school at the end of this year and the unavoidable question many people will ask you is, “So, what do you want to be?” 16 But many of you still don’t have a clue! Here are some things you might want to consider.First, does the career you are considering have staying power? Will it be in demand in 20 years? Rapid technological change is disruptive (破坏性的). 17 While many occupations are being taken over by new technology, jobs that require high-level of critical thinking, emotional intelligence and human interaction, remain in high demand. These jobs are more adaptable and not easily replaced by machines or technology.Second, choose a career that interests you. Some people might think this is unimportant, but if you are truly passionate about your job then going to work every day won’t seem a chore. It’s also likely that you will stay and grow in this career. Before making a choice, you should examine your values, skills and personality type. 18Third, of course you will want to consider your chosen profession’s earning power! Although high pay plays a part, you should know that a job with a big salary will likely require more time to get to the top, much more effort and a higher level of stress. 19 If you have interests outside of work, or are struggling with other demands in your life, you might want to choose a less taxing line of work.20 Let me leave you with an old saying: “Choose a job you love, and you will neve r have to work a day in your life. “On a cold winter night, Andrew, a 22-year-old Canadian, suffered a heart attack and collapsed to the floor, unconscious.______, his dog, a 4-year-old Husky named Koda, didn’t______. Instead, the clever dog sprang into action and called 911.Koda ______the emergency number on a cell phone. The 911 operator heard______on the other end of the line and sent a police officer to conduct a(n) ______ . When the officer arrived at the house, he found Koda barking at the front door, ______to lead him inside.The officer quickly realized that something was______and followed Koda into the bedroom, where he found Andrew______on the floor. The officer called an ambulance and Andrew was rushed to the hospital, where he received timely______ .Andrew said th at he had______Koda to call 911 by pressing his nose against the phone’s screen. He never thought that Koda would______use the skill in a real emergency.The story of Koda’s_______act has gone viral (疯传), with people around the world praising the dog’s______and loyalty. This heartwarming tale is a(n)______of the special bond between humans and their pets. Koda may not be able to speak, but he communicated in a way that______a life —and that’s something truly remarkable.21.A.Besides B.However C.Therefore D.Otherwise22.A.panic B.bite C.care D.escape 23.A.stored B.remembered C.dialed D.chose24.A.silence B.sighs C.screams D.barks25.A.negotiation B.investigation C.experiment D.survey 26.A.pretending B.refusing C.trying D.deciding 27.A.missing B.different C.wrong D.strange 28.A.lying B.sitting C.resting D.struggling 29.A.advice B.information C.support D.treatment 30.A.trained B.persuaded C.allowed D.warned 31.A.gradually B.actually C.occasionally D.immediately 32.A.adventurous B.selfless C.considerate D.heroic 33.A.honesty B.creativity C.patience D.intelligence 34.A.reminder B.explanation C.test D.prediction 35.A.spared B.changed C.saved D.created阅读下面短文,在空白处填入1个适当的单词或括号内单词的正确形式。
福建省厦门外国语学校2024-2025学年高一上学期第一次月考 化学试题(无答案)

秘密★启用前厦门外国语学校2024级高一第一次月考化学试题2024.10.11本试卷分选择题和非选择题两部分,共8页,满分为100分。
考试用时75分钟。
注意事项:1.答卷前,考生务必用黑色字迹的钢笔或签字笔将自己的姓名和准考证号填写在答题卡相应的位置上,用2B铅笔将自己的准考证号填涂在答题卡上。
2.选择题每小题选出答案后,用2B铅笔把答题卡上对应题目的答案标号涂黑;如需改动,用橡皮擦干净后。
再选涂其他答案;在试卷上作答无效。
3.非选择题必须用黑色字迹的钢笔或签字笔在答题卡上作答,答案必须写在答题卡上各题目指定区域内的相应位置上,超出指定区域的答案无效;如需改动,先划掉原来的答案,然后再写上新的答案;不使用涂改液。
不按以上要求作答的答案无效。
4、考生必须保持答题卡的整洁和平整。
可能用到的相对原子质量H-1 C-12 N-14 O-16 Na-23 S-32一、选择题(前5题每小题2分,后15题每小题3分,共55分。
每小题只有一个选项符合题目要求)l.生活因化学更美好。
下列对化学物质在生活中的用途的知识解读正确的是( )选项生活中的用途知识解读A自来水厂用氯气消毒氯气有毒,能杀死病毒和细菌B用石灰石处理酸性废水石灰石属于强碱,具有碱性C面包师用小苏打作发泡剂烘焙面包小苏打不稳定、受热易分解D碳酸氢钠加入的量过多会使蒸出的馒头发黄碳酸氢钠是淡黄色固体A.A B.B C.C D.D2.哈工大研究团队设计了一种使用磁性材料Fe3O4纳米粒子的微型纳米生物机器人,下列关于研究Fe3O4性质的基本程序中排列正确的是( )a.观察Fe3O4的颜色、状态b.设计并进行实验,观察实验现象c.解释现象、得出结论d.预测Fe3O4的性质A.abcd B.adbc D.dbac D.dacb3.我国女科学家屠呦呦发现青蒿素(青蒿素的化学式:C15H22O5),成为我国获得诺贝尔科学奖的第一人。
下列关于青蒿素的叙述错误的是( )A.lmol青蒿素中含有42N A个原子B.青蒿素中碳元素的质量分数约为63.8%C.青蒿素的相对分子质量为282 D.0.lmol青蒿素的泰尔质量为28.2g/mol4.用下列装置或操作进行相应实验,能达到实验目的的是( )A.图甲装置用Na2O2固体粉敖"随开随用、随关随停”制氧气B.图乙装置可用于除去CO2中的HCl(g)C.图丙装置验证Na和水反应是否为放热反应D.图丁装置用于探究Na2CO3和NaHCO3的热稳定性5.下列各物质所含原子数目,按由大到小顺序排列的是( )①常温常压下,34gNH3 ②标准状况下22.4LHe ③4℃9mL水④0.2molH3PO4A.①④②③B.④③②①C.②③④①D.①④③②6.数字化实验将传感器、数据采集器和计算机相连,可利用信息技术对化学实验进行数据的采集和分析。
2009届高考历史各省月考试题“三新”精选(1):中国古代先秦时期(旧人教版)省月考试题“三新”精选(10

高考历史08—09届各省月考试题“三新”精选(01)中国古代先秦时期一.选择题:(一)第一节:远古社会和传说时代(未列入考纲)(二)第二节:夏商西周的更替和制度1.《尚书•牧誓》记载“王朝至于商郊牧野,王曰‘今商王受,惟妇言是用,暴虐于百姓’”。
材料中所反映的战争性质是 CA.诸侯兼并战争B.诸侯争霸战争C.反抗暴政的战争D.地方叛乱战争2.(黑龙江省哈师大附中2009届高三二次月考)《史记》中:“纣师虽众,皆无战之心,……武王驰之,纣兵皆叛纣”的内容反映了 BA.商汤灭夏桀B.周灭商C.国人暴动D.犬戎攻破镐京3.(黑龙江省哈师大附中2009届高三二次月考)《孟子•告子》载:“天子适诸侯,曰巡狩,诸侯朝于天子曰述职。
……一不朝,则贬其爵,再不朝,则削其地,三不朝,则六师移(讨伐)。
”这段材料反映的是 AA.西周初年的分封制B.西周末年周王室的衰落C.春秋时期的诸侯争霸D.战国时期的群雄并立4.(黑龙江省哈师大附中2009届高三二次月考)与图中生产力水平相适应的土地所有制形式是 BA.氏族公社土地公有制B.奴隶社会土地国有制C.封建社会土地私有制D.庄园经济土地国有制石刀耒耜青铜铲5.(福建省厦门外国语学校2009届高三一次月考)孔子说:“周因与殷礼,所损益,可知也。
”这句话所表达的主要意思是 DA.夏商制度区别很大B.商周制度有本质区别C.商周制度完全相同D.商周制度有继承关系6.(河北省正定中学2009届高三适应性考试)孔子曰:“殷因于夏礼,所损益可知也;周因于殷礼,所损益可知也。
”这表明 DA.夏商周三代统治的时间都不长B.夏商周三代的礼仪完全不同C.夏商周三代的制度毫无共同之处D.夏商周三代的主要制度是相沿袭的7.(福建省福州三中2009届高三一次月考)周王将商代贵族也分封为诸侯,其根本目的是D A.保护贵族的世袭血统,打破旧有附属国的界限B.调合统治阶级内部矛盾C.加强对奴隶和平民的控制D.巩固刚建立的西周政权8.(福建省福州三中2009届高三一次月考)《左传•昭公七年》:“天有十日,人有十等。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
20XX年中学测试中学试题试卷科目:年级:考点:监考老师:日期:20XX 届福建省厦门外国语学校高三第一次月考化学试卷第Ⅰ卷(选择题,共63分)一、选择题(每题只有一个正确选项,共13×3=39分)1.科学家对原子结构的认识顺序正确的是()①道尔顿原子模型②汤姆生原子模型③卢瑟福原子模型④电子云模型A .①③②④B .④①②③C .④②③①D .①②③④2.下面的排序不正确的是()A.晶体熔点的高低:>B .硬度由大到小:金刚石>碳化硅>晶体硅C.熔点由高到低:Na>Mg>AlD.晶格能由大到小: NaF> NaCl> NaBr>NaI3.下列关于晶体的说法正确的是()A .构成分子晶体的微粒之间可能含有离子键B .构成原子晶体的物质一定是单质C .离子晶体发生状态变化时,需要克服离子键D .碘晶体升华时,分子中共价键发生断裂4.有关化学用语正确的是()A .乙炔的最简式C 2H 2B .乙醛的结构简式 CH 3COHC .水的电子式+-⋅⋅⋅⋅+H :]O [:H 2D .硝酸钾的化学式 KNO 3 5.最近意大利罗马大学的FulvioCacace 等人获得了极具理论研究意义的N 4分子。
N 4分子结构如图所示,已知断裂1moIN-N 吸收167kJ 热量,生成1molN≡N 放出942kJ 。
根据以上信息和数据,下列说法正确的是()A 、N 4属于一种新型的化合物B 、N 4与N 2互为同素异形体C 、N 4沸点比P 4(白磷)高D 、1mo1N 4气体转变为N 2将吸收882kJ 热量6.下列说法正确的是()A.离子化合物中一定不含共价键B.共价化合物中一定不含离子键C.金属离子一定满足最外层电子数为2或8D.H2O是一种非常稳定的化合物,这是由于氢键所致7.下表中列出了有关晶体的说明,有错误的是()选项 A B C D 晶体名称碘化钾氩白磷冰醋酸组成晶体微粒名称阴、阳离子原子分子分子分子间晶体内存在的结合力离子键共价键范德华力作用力8.下列表达方式或说法正确的是()A.B.氯化铵的电子式:C.硫离子的核外电子排布式1s22s22p63s23p4D.NH3、H2O、CO2、HCl四分子中孤电子对最多的是CO29. 电子由3d能级跃迁至4p能级时,可通过光谱仪直接摄取()A.电子的运动轨迹图像B.原子的吸收光谱C.电子体积大小的图像D.原子的发射光谱10. 第3周期元素的原子,其最外层3p亚层上有一个未成对电子,它的最高价氧化物对应水化物的酸根离子是()A.RO-5B.RO-3C.RO-24D.RO-411. 关于元素周期律和元素周期表的下列说法,正确的是()A.目前发现的所有元素占据了周期表里全部位置,不可能再有新的元素被发现B.元素的性质随着原子序数的增加而呈周期性变化C.俄国化学家道尔顿为元素周期表的建立做出了巨大贡献D.同一主族的元素从上到下,金属性呈周期性变化12.氨气分子空间构型是三角锥形,而甲烷是正四面体形,这是因为()A .两种分子的中心原子杂化轨道类型不同,NH 3为sp 2型杂化,而CH 4是sp 3型杂化。
B .NH 3分子中N 原子形成三个杂化轨道,CH 4分子中C 原子形成4个杂化轨道。
C .NH 3分子中有一对未成键的孤对电子,它对成键电子的排斥作用较强。
D .以上说法都不正确。
13.下列说法中错误的是 ( )A .当中心原子的配位数为6时,配合单元常呈八面体空间结构B .[Ag(NH 3)2]+中Ag +空的5S 轨道和5P 轨道以sp 杂化成键C .配位数为4的配合物均为正四面体结构D .已知[Cu(NH 3)2]2+的中心原子采用sp 杂化,则它们的空间构型为直线型二、选择题(每题只有1个正确选项,共6×4=24分)14.已知X 、Y 元素同周期,且电负性X >Y ,下列说法错误..的是() A .X 与Y 形成化合物时,X 显负价,Y 显正价B .第一电离能Y 一定小于XC .最高价含氧酸的酸性:X 对应的酸性强于Y 对应的酸性D .气态氢化物的稳定性:H m Y 小于H m X15.元素处于基态时的气态原子获得一个电子成为-1价阴离子时所放出的能量叫做该元素的第一电子亲合能。
-1价阴离子再获得一个电子的能量变化叫做第二电子亲合能。
下表中给出了几种元素或离子的电子亲合能数据:下列说法正确的是()A .电子亲合能越大,说明越难得到电子B .一个基态的气态氟原子得到一个电子成为氟离子时放出327.9kJ 的能量C .氧元素的第二电子亲合能是-780kJ·mol -1D .基态的气态氧原子得到两个电子成为O 2-需要放出能量16.最近科学家成功制成了一种新型的碳氧化合物,该化合物晶体中每个碳原子均以四个共价单键与氧原子结合为一种空间网状的无限伸展结构,下列对该晶体叙述错误的是A .该晶体类型是原子晶体()B .该晶体中碳原子和氧原子的个数比为1∶2 元素Li Na K O O - F 电子亲合能/kJ·mol -159.8 52.7 48.4 141 -780 327.9C.晶体中碳原子数与C—O化学键数之比为1∶2D.晶体的空间最小环共有12个原子构成17.下列有机化合物分子中含有手性碳原子,且与H2发生加成反应后仍含有手性碳原子的是()A.CH3CH2CHO B.OHCCH(OH)CH2OHC.D.18.下列分子中C原子是以sp3杂化轨道与其他原子成键的是()A.金刚石B.石墨C.苯D.甲醛19.据报道,某种合金材料有较大的储氢容量,其晶体结构的最小单元如图所示。
则这种合金的化学式为()A.LaNi6B.LaNi3C.LaNi4D.LaNi5第Ⅱ卷(非选择题,共87分)三、填空题:20.(8分)下表是元素周期表的一部分。
表中所列的字母分别代表某一化学元素。
(1)下列(填写编号)组元素的单质可能都是电的良导体。
①a,c,h ②b,g,k ③c,h,1 ④d,e,f(2)如果给核外电子足够的能量,这些电子便会摆脱原子核的束缚而离去。
核外电子离开该原子或离子所需要的能量主要受两大因素的影响:一是原子核对核外电子的吸引力;二是形成稳定结构的倾向。
下表是一些气态原子失去核外不同电子所需的能量(kJ·mol-1):①通过上述信息和表中的数据分析为什么锂原子失去核外第二个电子时所需的能量要远远大于失去第一个电子所需的能量。
②表中X可能为以上13种元素中的(填写字母)元素。
③Y是周期表中族元素。
21.(8分)水是生命之源,它与我们的生活密切相关。
在化学实验和科学研究中,水也是一种常用的试剂。
(1)写出与H2O分子互为等电子体的微粒__________(填1种)。
(2)水分子在特定条件下容易得到一个H+,形成水合氢离子(H3O+)。
下列对上述过程的描述不合理的是_____A.氧原子的杂化类型发生了改变B.微粒的形状发生了改变C.微粒的化学性质发生了改变D.微粒中的键角发生了改变(3)下列是钠、碘、金刚石、干冰、氯化钠晶体的晶胞图(未按顺序排序)。
与冰的晶体类型相同的是_____(请用相应的编号填写)。
22. (16分)有A、B、C、D、E 5种元素,它们的核电荷数依次增大,且都小于20。
其中C、E是金属元素;A和E属同一族,它们原子的最外层电子排布为n s1。
B和D也属同一族,它们原子最外层的p能级电子数是s能级电子数的两倍,C原子最外层上电子数等于D原子最外层上电子数的一半。
请回答下列问题:⑴A是________,E是_________。
⑵写出C元素基态原子的电子排布式_________________________。
⑶用轨道表示式表示D元素原子的价电子构型____________________。
⑷元素B与D的电负性的大小关系是___________,C与E的第一电离能的大小关系是___________。
(填“>”、“<”或“=”)⑸E位于周期表的第周期,第族;其原子结构示意图为。
23.(14分)镁、铜等金属离子是人体内多种酶的辅因子。
工业上从海水中提取镁时,先制备无水氯化镁,然后将其熔融电解,得到金属镁。
(1)以MgCl2为原料用熔融盐电解法制备镁时,常加入NaCl、KCl或CaCl2等金属氯化物,其主要作用除了降低熔点之外还有。
(2)已知MgO的晶体结构属于NaCl型。
某同学画出的MgO晶胞结构示意图如下图所示,请改正图中错误:。
(用文字表述)(3)用镁粉、碱金属盐及碱土金属盐等可以做成焰火。
燃放时,焰火发出五颜六色的光,请用原子结构的知识解释发光的原因:。
(4)Mg是第三周期元素,该周期部分元素氟化物的熔点见下表:氧化物NaF MgF2SiF4熔点/K 1266 1534 183解释表中氟化物熔点差异的原因:。
(5)人工模拟是当前研究的热点。
有研究表明,化合物X可用于研究模拟酶,当其结合或Cu(I)(I表示化合价为+1)时,分别形成a和b:①a中连接相邻含N杂环的碳碳键可以旋转,说明该碳碳键具有键的特性。
②微粒间的相互作用包括化学键和分子间相互作用,比较a和b中微粒间相互作用力的差异。
24.(16分)X、Y、Z、Q、E五种元素中,X原子核外的M层中只有两对成对电子,Y原子核外的L层电子数是K层的两倍,Z是地壳内含量(质量分数)最高的元素,Q的核电荷数是X与Z的核电荷数之和,E在元素周期表的各元素中电负性最大。
请回答下列问题:(1)X、Y的元素符号依次为、;(2)XZ2与YZ2分子的立体结构分别是和,相同条件下两者在水中的溶解度较大的是(写分子式),理由是;(3)Q的元素符号是,它属于第周期,它的核外电子排布式为,在形成化合物时它的最高化合价为;25.(6分)有两种化合物,A:C O Cl3·5NH3·H2O;B:C O Cl3·5NH3·H2O,根据下面的实验结果,确定它们的络离子、中心离子和配体。
⑴分别取A和B的固体于试管中微热,A中未见明显现象,B中试管口出现少量水珠。
⑵向A和B的溶液中加入AgNO3溶液后均有AgCl沉淀。
⑶沉淀过滤后,分别向滤液中加AgNO3溶液均无变化,但加热煮沸,B溶液中又有AgCl 沉淀生成,其沉淀量为原来B溶液中AgCl的一半。
又已知该化合物中心离子配位数均为6,试按照“[Ag(NH3)2] OH”的书写格式写出A、B 的化学式:A ____________,B ______________。
26.(7分)过渡金属元素氧化物的应用研究是目前科学研究的前沿之一。
2021年诺贝尔物理学奖为法国科学家阿尔贝·费尔和德国科学家彼得·格林贝格尔共同获得,以表彰他们在巨磁电阻效应(CMR效应)研究方面的成就。
