[英语学习]应用化学专业英语考试必背
应用化学专业英语及答案

黄冈师范学院2009—2010学年度第一学期期末试卷考试课程:专业英语考核类型:考试A卷考试形式:闭卷出卷教师:杨一思考试专业:化学考试班级:应用化学200601 一、Translate the following into English(20 points)1.过滤2.浓缩3.结晶化4.吸附5. 蒸馏6.超临界的7.二氯甲烷8.热力学平衡9.亲电性10.表面张力11.共轭的12.酮13.平衡常数14.丙基15.丁基16.亚甲基18.环己酮19.同位素20.标准熵二、Translate the following into Chinese(20 points)1. methyl propanoate2. rate constant3. ethyl methyl ketone4. free energy5. radical intermediate6. isobutyl methyl ether7. 3-chloropropene8. primary radical9. n-propyl bromide10. bond energy 11. circulating electrons12. local magnetic fields13. tetramethylsilane14. mass to charge ratios15 phenylamine16 amide17. amine18. nucleophile19. perchlorate20. carbocation三、Translation the following into chinese (40 points)A卷【第1页共 3 页】1. We can see why benzene is stable: according to resonance theory, the more resonance forms a substance has, the more stable it is. Benzene, with two resonance forms of equal energy, is therefore more stable and less reactive than a typical alkene.2. Membranes can be defined essentially as barrier, which separates two phases and restricts transport of various chemicals in a selective manner. A membrane can be homogenous or heterogeneous, symmetric or asymmetric in structure, solid or liquid, can carry a positive or negative charge or be neutral or bipolar. Transport through a membrane can be effected by convection or by diffusion of individual molecules, induced by an electric field or concentration, pressure or temperature gradient. The membrane thickness may vary from as small as 100 micron to several mms.3. The most common industrial adsorbents are activated carbon, silica gel, and alumina, because they present enormous surface areas per unit weight.A surface already heavily contaminated by adsorbates is not likely to have much capacity for additional binding, but further heating will drive off these compounds to produce a surface with high adsorptive capacity.Temperature effects on adsorption are profound, and measurements are usually at a constant temperature. Graphs of the data are called isotherms. Most steps using adsorbents have little variation in temperature.A卷【第2页共 3 页】4. In the absence of peroxides, hydrogen bromide adds to peopene via the Markovnikov pathway to yield isopropyl bromide. In the presence of peroxides, however, the order of addition is reversed, and the product is n-propyl bromide; the addition in this case is said to be anti-Markovnikov. This is interpreted in terms of initiation of the addition reaction by bromine atom, rather than by a proton, as is the case for electrophilic addition.四、Translate the following paragraphs into Chinese(20 points)1.Benzene and its derivatives can be nitrated using a mixture of concentrated nitric and sulphuric acid. The temperature must be controlled to prevent more than one nitro-group going in.2. Benzene can be made to react with halogen derivatives using aluminium chloride as a catalyst. This is called a Friedel-Crafts reaction.can be sulphonated by reacting it with fuming sulphuric acid(oleum). The benzene reacts with sulphur trioxide in the oleum.benzene is converted into ethylbenzene by reacting it with ethene. The ethylbenzene (also called styrene) is used to make polystyrene.黄冈师范学院2009—2010学年度第一学期期末试卷参考答案及评分标准考试课程:专业英语考核类型:考试A卷考试形式:闭卷出卷教师:杨一思考试专业:化学考试班级:应用化学200601 一、Translate the following into English(20 points)2. concentrate 4. adsorption chlorideequilibriumtensionconstant14. propylmagneticresonanceentropy二、Translate the following into Chinese(20 points)1. 丙酸甲酯2. 速率常数3. 甲乙酮4. 自有能5. 自由基中间体6. 异丁基甲醚7. 3-氯丙烯8. 伯自由基9. 正丙基溴化10. 键能11.循环电子12. 局部电磁场13. 四甲基硅烷14. 质荷比15.苯胺16.氨基化合物17.胺18亲核试剂19.高氯酸盐20.碳正离子三、Translation the following into chinese (50 points)1.依据共振理论,物质具有的共振式越多就越稳定。
应用化学专业英语-Lesson-2..

• Elements are composed of extremely small particles called atoms. All atoms of a given element are identical. The atoms of one element are different from the atoms of all other elements.
• Compounds are composed of atoms of more than one element.
• Chemical reactions involve only the rearrangement of atoms; atoms are not created or destroyed in chemical reactions.
• Protons and neutrons are located in the nucleus of the atom, which is small. Most of the mass of the atom is due to the nucleus.
• Electrons are located outside of the nucleus. Most of the volume of the atom is due to electrons.
Some Complex Ions
Name Carbonate Nitrate Phosphate Dihydrogen Phosphate Sulfate Sulfite Thiosulfate Perchlorate Chlorite Cyanide Chromate
应用化学专业英语lesson10ChemicalEquilibriumandkinetics
NH3
Add more NH3?
Reaction shifts to the left [N2] and [H2] inc
5 - 35
Le Chatelier’s principle
Adding Pressure affects an equilibrium with gases
N2(g) + 3 H2(g)
N2(g) + 3 H2(g)
Keq =
[ NH3 ] 2 [ N2 ] [ H2 ] 3
2 NH3(g)
5 - 33
Le Chatelier’s principle
Stress causes shift in equilibrium Adding or removing reagent
N2(g) + 3 H2(g)
Temperature: 2. Higher Temperature:
Faster cars More collisions
More Energy More collisions
Reacting molecules move faster, providing colliding molecules w/ Eact.
At this point, equilibrium is achieved. Time
5 - 26
Figure 9.8
2SO2(g) + O2(g)
At Equilibium
2SO3(g)
SO2(g)+O2(g)
Initially
SO3(g)
Initially
5 - 27
Figure 9.9
2SO2(g) + O2(g)
应用化学专业英语 -无机化学命名

(3) 基本元素有多种价态 酸:最低氧化态(次酸) 基础元素(前缀 hypo-, 后缀 -ous) +acid 较低氧化态(亚酸) 基础元素加后缀-ous + acid 较高氧化态 (正酸) 基础元素加后缀-ic + acid 最高氧化态(高酸) 基础元素(前缀 per-, 后缀 -ic) +acid 盐:最低氧化态 阳离子元素 + 基础元素(前缀 hypo-, 后缀 -ite) 较低氧化态 阳离子元素 + 基础元素加后缀-ite 较高氧化态 阳离子元素 + 基础元素加后缀-ate 最高氧化态 阳离子元素 + 基础元素(前缀 per-, 后缀 -ate)
16. K4[Fe(CN)6]; 17. CuSO4· 5H2O 18. Cu2(OH)2CO3 19. NaNH4SO4
1. (NH4)2CO3: ammonium carbonate 2. N2O: nitrogen(Ⅰ) oxide; nitrous oxide ; laughing gas 3. H2SO4: sulphuric acid 4. P4O6 diphosphorus trioxide 5. Al2O3 Aluminum oxide 6. SnCl4 tin(Ⅳ) chloride; stannic chloride; tin terachloride 7. KHSO4 Potassium hydrogen sulfate 8. Cu2S copper(I) sulphide; dicopper sulphide 9. HClO4 perchloric acid
含氧酸及其盐:
(1) 基本元素仅有一种氧化态
酸:基本元素加后缀-ic +acid 例:H2CO3 carbonic acid 盐:阳离子元素+基础元素加后缀-ate 例:Na2CO3 sodium carbonate (2) 基本元素有两种氧化态 酸:基础元素加后缀(-ous 低价态,-ic 高价态) + acid HNO2:nitrous acid HNO3: nitric acid 盐:阳离子元素+基础元素加后缀( -ite低价态,-ate 高价态) NaNO2: Co(NO3)2: sodium nitrite cobalt(II) nitrate or cobaltous nitrate
应用化学专业英语复习资料

一单词短语1.Molecule 分子molecular 分子的2.chemical process 化学过程element 元素3.a t o m原子a t t r a c t i o n吸引力4.repulsion 排斥力distillation 蒸馏、n5.distill 蒸馏v rectification 精馏position 构成structure 结构7.property 性质mass 质量8.atomicweight 原子量atomic number 原子序数9.ionization energy 电离能period 周期10.g r o u p族f a m i l y族11.transition group 过渡族main group 主族12.i o n离子s u b s t i t u t i o n取代反应13.el i mi na ti on消除反应nucl eoph i l i c 亲核的14.nucleophilie 亲核试剂electrophilie亲电试剂15.alkyl 烷基的functional group 官能团16.halides 卤素的leaving group 离去基团17.transition state过渡态intermediate 中间体18.r e a c t a n t反应物p r o d u c t生成物19.concentration 浓度rate equation 速率方程20.c o n s t a n t常数e t h e r醚21.endothermic 吸热的substrate 反应底物22.mechanism 机理reagen 试剂23.alkene 烯烃exothermic 放热的24.A n i o n阴离子n i t r o g e n氮气25.Hydrocarbon 碳氢化合物carbonhydrate 碳水化合物26.Alkane 烷烃substituent 取代基27.Isomerism 同分异构现象isomer 同分异构28.V i n y l乙烯基d e r i v a t i v e s衍生物29.acid halides 酰卤acid anhydrides 酸酐30.e s t e r s酯a m i d e酰胺31.ammonia NH3 Acetic anhydride乙酸酐32.phenol 芬acid—base titration 酸碱滴定33.precipitation沉淀analyses 化学分析员34.IR 红外UV紫外MS质谱GC色相色谱HPLC高效液相色谱TLC薄层色谱X—rayX射线衍射二选词填空1、We can now easily account for many things,which were thought to be mysterious by theancients2、the acid acts on the metal and a gas is givenoff.3、you should adapt yourself to new ways oflooking at matters4、electrolytes have more pronounced effect oncolligative properties than do nonelectrolytes. 5、if water in these lakes evaporated at the samerate as fresh water ,both would nearly dryup in a matter of year.6、both laks evaporated very slow compared with afresh lake or even the ocean.7、a property that depends only on the relativeamounts of solute and solvent is know as acolligative property.8、for example ,both NaCl (ionic) and HCl (polarcovalent)are classified as electrolytes becausethey form ions in aqueous solution.9、when compounds such as NaCl and HCl aredissolved in water ,the effect is obvious.10、if the wires is cut ,the light goes out becausethe circuit is broken.11、when wires are attached to a charged batteryand then to a light bulb ,the light shinesbrightly.12、glass and wood as well as pure water areexamples or nonconductors of electricity.13、other substances resist the flow of electricityand are known as nonconductors orinsulators.14、it has long been known that the presence of asolute in water may affect its ability toconduct electricity.15、when the collection of papers was first broughtout,it was well received by the reviewers.16、in the same way the dozen or so mostcommon kinds of kinds of atoms can be put together in many millions of different ways tomake molecules .17、elements are made up of tiny fundamentalparticles called atoms. Fundamental, as it is usedhere ,means that they cannot be furtherdivided by any chemical metheods.18、each element has atoms that is different fromthe atoms of other elements.19、it would not be quite round; on the contraryit would consist of three parts represented byspheres.20、it is not to be summed up in a singleproduct or word ,but in an idea or basicconcept.21、the chemical symbol of an element may standthe element for.22、the rate of a chemical reaction is influencedby several factors such as temperature ,concentration of reagents , particle size ,light ,and catalyst.23、all forms of life in earth are very dependenton chemical reactions or chemical changes.24、a chemical reaction occurs when elements andcompounds react together to produce differentcompounds , or when compounds break down into simpler compounds or elements.三无机物的命名H Hydrogen Li Lithium Na Sodium K Potassium Mg Magnesium Ca CalciumMn manganese Cu copper Zn zinc Fe iron Hg mercury Ag silver Au gold C Carbon Si Silicon Pb Lead Al Aluminium F Fluorine Cl Chlorine Br Bromine I IodineO Oxygen S Sulfur N Nitrogen P Phosphorus1.直呼其名,即读其元素名称+ ion如:Na+ sodium ionK+ potassium ion2.对于有变价的金属元素,除了可用前缀来表示以外,更多采用罗马数字来表示金属的氧化态,或用后缀-ous 表示低价,-ic 表示高价如:Cu+ copper (Ⅰ) ion 或cuprous ion Cu2+ copper (Ⅱ) ion 或cupric ionFe2+ iron (Ⅱ) ion 或ferrous ionFe3+ iron (Ⅲ) ion 或ferric ion3.含氢酸根:酸根中的H读做hydrogen,氢原子的个数用希腊前缀表示:mono- di - tri- tetra - penta- hexa-hepta- octa- nona- deca-举例:CO32-carbonate ionHCO3-hydrogen carbonate ionPO43- phosphate ionHPO42hydrogencarbonate ionH2PO4- dihydrogenphosphate ion4.结晶水读做hydrate ,结晶水的个数用希腊前缀表示:mono-di - tri- tetra - penta- hexa- hepta- octa- nona- deca-CuSO4·5H2O copper(Ⅱ) sulfate pentahydrateAlCl3 ·6H2O aluminum chloride hexahydrate5.测试Mg(OH)2magnesium hydroxide AlCl3aluminum chlorideFeBr2 iron(II) bromide CaSO4calcium sulfateZnCO3zinc carbonate HF hydrofluoric acidH3PO4phosphoric acid NO2nitrogen dioxideCuO copper(II) oxide Al2O3aluminum oxideNaHSO3sodium hydrogen sulfiteKMnO4potassium permanganateNaClO sodium hypochloride四有机物的命名1)命名正烷基时,只需把烷烃的词尾“-ane换成“-yl”,加在相应的烷烃的字首后2)字母规则:Butyl>Ethyl>Isopropyl>Methyl>Neopentyl>tert-Pentyl >Propyl3)环烷烃:只需在所对应的烷烃前加上cyclo-即可4)有些结构较复杂的烷基,需添加词头5)烯烃和炔烃命名时将相应的烷烃的词尾“烷”(ane)改为“烯”(ene)或“炔”(yne),后缀前加上不饱和键的编号即可。
东华大学应用化学专业英语总结

专业英语重点总结单词Toxic chemicals:有毒化学品 Chemical pollution:化学污染Physical property :物性 Isolate:分离Determine:测定 Synthesize:合成Fundamental principles:基本原理 Investigation:研究Utilize:利用 Catalyst 催化剂Enzyme 酶 Biosphere 生物圈Heterogeneous catalyst 非均相催化剂 Nanotechnology 纳米技术Carbon monoxide 一氧化碳 Chemical formulas:化学式anion: 阴离子 Oxidation number:氧化值sulphate: 硫酸盐 Hydrides: 氢化物Sodium:钠 cation: 阳离子Covalent bond:共价键 electroneutral: 电中性的Electronegative atom:电负性原子 trivial names:俗名Oxidation:氧化 Peroxides:过氧化物Superoxide:超氧化物 Periodic table:周期表Noble gases: 惰性气 vacant orbital:空轨道Coordination (complex) compound: 配位化合物Unshared pair of electrons:未共用电子对oxidation state:氧化态 hydroxides:氢氧化物caustic soda solution:苛性钠溶液 vacant orbital:空轨道Formula 分子式 Common name 俗名Derivative 衍生物 Acid salt 酸式盐Hydrate 水合物 Anhydrous 无水的Oxidizing agent 氧化剂 Reducing agent 还原剂Oxidation reduction reaction氧化还原反应Electrochemistry 电化学 Electrolysis 电解Strong acid 强酸 Weak base 弱碱Acid-base indicator 酸碱指示剂 Distilled water 蒸馏水Buffer solution 缓冲溶液 Common ion effect 同离子效应Equivalence point 等效点 Neutralization 中和Dissociation 离解度 Anhydride 脱水物Periodic law: 元素周期率 periods (rows):周期group (columns):族 protons:质子Valence electrons:价电子 Halogens: 卤素Atomic radius: 原子半径 alkaline earths:碱土金属attractive force: 吸引力 electronegativity: 电负性electropositive:正电性 univalent ion: 一价离子electron shell: 电子层 bonding force 结合力monatomic 单原子的 Neutrons:中子hydrogen bond 氢键 conduct electricity 导电Electrically neutral 电中性的 Electrostatic 静电的isomerism :异构现象 Reversible :可逆的Chirality :手性 Charge:电荷mirror image:镜像 mirror image:镜像Hydrocarbons:碳氢化合物 Methane: 甲烷propane :丙烷 butane :丁烷-ane:-烷 -ene:烯-yne:炔 -ol:醇-one :酮 -al:醛-aldehyde: 醛 -acetal:缩醛alkoxy-:烷氧基 -amide: 酰胺-amino:氨基的 -amine : 胺Aquo- 含水的 azo- 偶氮Polythene (PE): 聚乙烯 Benzene:苯Ethene:乙烯 Propene:丙烯double bond:双键 triple bond:三键Valence bond theory 价键理论 skeleton:骨架methyl:甲基 ethyl:乙基alkyl :烷基 side chains: 侧链substituent:取代基Meth-:甲 eth-:乙propyl-:丙 but-:丁Pent(a)-:戊 hex-:己hepta-:庚 oct-:辛non-:壬 deca-:葵di-:二 tri- : 三tetra- : 四 penta-: 五primary carbon:伯碳 secondary carbon:仲碳tertiary carbon:叔碳 allyl :烯丙基substitution reaction :取代反应 free radical:自由基nucleophilic substitution:亲核取代 carbocation :碳正离子electrophilic substitution:亲电取代 carbanion :碳负离子addition reaction :加成反应elimination reaction:消去反应acid anhydride:酸酐 alcohol:乙醇Thermodynamics 热力学 quantum chemistry 量子化学statistical mechanics 统计力学 Kinetics 动力学Equilibrium 平衡 deposition 沉积sublimation 升华 Condensation 冷凝evaporation 蒸发 Melting 熔化Freezing 冷冻 Work 功the states of system系统状态Irreversible 不可逆的 surrounding 环境open system 开放系统 closed system 封闭系统isolated system 孤立系统 impermeable 不可渗透的Adiabatic 绝热的 steady state稳态tap water 自来水 rinse 润洗fine chemicals 精细化学品 Buchner funnel 布氏漏斗bunsen burners 煤气喷灯 tripod supports 三角支架wash bottles 洗瓶 dropper 滴管transfer pipette 移液管 hot plate 轻便电炉wire gauzes 石棉网 test tube brush 试管刷test tube rack 试管架filter paper 滤纸ring stand with rings 带环环架iron support 铁架台utility clamp 铁试管夹 clamp holder 夹柄buret clamp 滴定管夹 extension clamp 万能夹ring clamp 环形夹子 pinchcock 弹簧夹pinch clamp 弹簧节流夹 tubing clamp 管夹hose clamp 软管夹 test tube clamp 试管夹cork stopper 软木塞 rubber stopper 橡胶塞laboratory jack 实验升降台 spatulas 刮刀beaker tongs 烧杯钳 crucible tongs 坩埚钳tweezer 镊子 watch glasses 表面皿goggles 护目镜fume hoods 通风橱capillary melting point tube 毛细熔点管 Caliper 卡尺table balance 托盘天平 analytical balance 分析天平top pan balance 市秤 magnetic stirrers 电磁搅拌器Fahrenheit thermometer 华氏温度计 celsius 摄氏度magnetic stir bar 磁搅拌子 alcohol lamp 酒精灯Spectrophotometers 分光光度计 connection tube 连接管rubber tube 橡皮管 adapter 接合器Socket 套接口 ball joint 球形接头Stopper 塞子 adaptor 转接口splash heads 防溅头 thermometer pocket温度计插孔air leak tube 空气渗漏管 distill head 蒸馏头melting point tube 熔点管 burettes 滴定管Stopcock 活塞 volumetric flask 容量瓶measuring cylinder 量筒 centrifuge tube 离心管graduated pipette刻度吸量管 filter funnel 过滤漏斗chromatography colum 层析柱dropping funnel 滴液漏斗pressure equalizing funnel 均压漏斗 separating funnel 分液漏斗rotary evaporator 旋转蒸发仪 petri dishes 培养皿spectrophotometer 分光光度计 drying tube 干燥管evaporating dishes 蒸发皿 condenser 冷凝管Reflux 回流 erlenmeyer flask 锥形烧瓶round bottom flask 圆底烧瓶 distillation flask 蒸馏烧瓶filtration flask 过滤瓶 reagent bottle 试剂瓶glass desiccator 玻璃干燥器Beaker 烧杯distilling receiver 蒸馏接收器dry tower 干燥塔vaccume dessicator 真空干燥器 extractor 萃取器无机化合物及化学式的命名·二元化合物:氧化物,盐,酸(1)阴离子元素加后缀–ide(2)多价态元素加前缀:mono-, di-, tri-, tetra-, penta-, hexa(3)低价氧化态后缀–ous,高价氧化态后缀–ic·酸:基础元素(前缀 hydro-, 后缀-ic)+ acid·氢氧化物(碱):金属元素(价态)+ hydroxide·含氧酸及其盐(1)基本元素仅有一种氧化态酸:基础元素加后缀-ic + acid盐:阳离子元素+基础元素加后缀-ate(2)基本元素有二种氧化态酸:基础元素加后缀( -ous低价态,-ic高价态) + acid盐:阳离子元素 + 基础元素加后缀( -ite低价态, -ate高价态)(3)基本元素有多种氧化态酸:最低氧化态基础元素(前缀 hypo-, 后缀-ous)+ acid较低氧化态基础元素加后缀-ous+ acid较高氧化态基础元素加后缀-ic + acid最高氧化态基础元素(前缀 per-, 后缀-ic)+ acid 盐:最低氧化态阳离子元素 + 基础元素(前缀 hypo-, 后缀-ite)较低氧化态阳离子元素 + 基础元素加后缀-ite较高氧化态阳离子元素 + 基础元素加后缀-ate最高氧化态阳离子元素 + 基础元素(前缀 per-, 后缀-ate)·不同水分子含量的酸较低水含量前缀 meta较高水含量前缀 ortho·不同基本元素形成的酸前缀 di-, pyro·含硫的酸:源于含氧酸中的氧被硫取代,使用前缀 thio·含氢盐(酸式盐):源于含有1个以上氢原子酸中的氢原子被金属离子取代,形成酸式盐,氢原子以及金属离子使用前缀 di-, (bi),tri·配位化合物的命名阳离子 + [ 配体及中心原子] (氧化数)前缀:prefix·二元化合物,原子个数前缀1—mono-(可省略);2—di-;3—tri-;4—tetra-;5—penta-;6—hexa- Eg:·Oxide-氧化物CaO:calcium oxide(quicklime-生石灰)N2O:nitrogen(Ⅰ) oxide = dinitrogen oxide = nitrous oxide(laughing gas) NO:nitrogen(Ⅱ) oxide = nitrogen oxide = nitric oxideN 2O3:nitrogen(Ⅲ) oxide = dinitrogen trioxideNO2:nitrogen(Ⅳ) oxide = nitrogen dioxideN 2O5:nitrogen(Ⅴ)oxide = dinitrogen pentaoxideCu2O: copper(Ⅰ) oxide = dicopper oxide = cuprous oxide-低价态CuO: copper(Ⅱ) oxide = cupric oxide-高价态·Salt-盐ZnF2: znic fluorideSnCl2: tin(Ⅱ) chloride = stannous chlorideHg2Cl2: mercury(Ⅰ) chloride = mercurous chloride (calomel-甘汞)HgCl2: mercury(Ⅱ) chloride = mercuric chloride (sublimate) KCN: potassium cyanide·Acids-酸HBr: hydrobromic acid (aqueous solution)HCN: hydrocyanic acidH2S: hydrosulphuric acid·Hydroxides(bases)-碱NaOH: sodium hydroxide (sodium lye, caustic soda solution) KOH: potassium (hydroxid potassium lye, caustic potash)Fe(OH)2: iron(Ⅱ) hydroxide (ferrous hydroxide)Fe(OH)3: iron(Ⅲ) hydroxide (ferric hydroxide)·Oxo acids and their salts-含氧酸及其盐H2CO3: carbonic acid Na2CO3: sodium carbonateH3BO3: boric acid K3BO3: potassium borate·The basic element is in two oxidation statesHNO2: nitrous acid NaNO2: sodium nitriteHNO3: nitric acid Co(NO3)2: cobalt(Ⅱ) nitrate = cobaltous nirate·The basic element is in more than two oxidation states HClO: hypochlorous acid KClO: potassium hypochloritaHClO2: chlorous acid KClO2: potassium chloriteHClO3: chloric acid KClO3: potassium chlorateHClO4: perchloric acid KClO4: potassium perchlorateHMnO4: permanganic acid AgMnO4:sliver permanganate·The hydrogen salt-含氢盐Na3PO4: trisodium phosphate (tertiary phosphate)Na2HPO4: disodium hydrogen phosphate (secondary phosphate)NaH2PO4: sodium dihydrogen phosphate (primary phosphate)NaHCO3: sodium hydrogen carbonate (sodium bicarbonate) ·Give formulas for the following1.ammonium sulfate: (NH4)2 SO42.barium iodide: BaI23.iron(Ⅱ) sulfate:Fe2SO44.potassium permanganate:KMnO4 5.copper(Ⅱ)oxide:CuO6.carbonic acid:H2CO3科技论文写作Introduction section-研究的是什么问题?Key sections·Materials and Methods section-这个问题是怎么研究的?·Results and Discussion sections-发现了什么?Conclusion section-这些发现意味着什么?1.ABSTRACT (摘要)研究动机、研究方法、主要结果、简要结论摘要应言简意赅,因此以上各项内容争取用一句话说明一项,每项最多不要超过三句话,即:为什么做这个研究?用了什么方法?取得了什么结果?结论是什么?这些问题逐一回答了,摘要就写完整了。
应用化学专业英语 -化合物命名
樊海梅
LOGO
有机化合物的命名
链 烃
饱和烃:烷烃 不饱和烃:烯烃,炔烃 脂环烃 芳香烃
烃
有 机 物
环 烃
卤代烃
烃 的 衍 生 物
醇
含 氧 衍 生 物
酚 醛 酮 羧酸
酯等
烷烃(alkanes) 直链烷烃:英文名称除了含1到4个碳原子以外,其余均用希腊
90 alkane:nonacontane
100 alkane:hectane
含支链烷烃和烷基 烷基:只需要把烷烃的后缀ane换成-yl加在相应烷烃的字 首后 如:CH3- methyl CH3-CH2- ethyl CH3-(CH2)9-CH2 undecyl
还有一些烷基也可以在相应的烃名前加iso-(异)、sec-仲、tert-叔、
和拉丁文的数词加上相应的词尾来命名(烷烃:ane;烯烃: ene;炔 烃:yne),10个碳原子以上的则在数词前加前缀un、do、 tri、 tetra、 penta等。 例如:甲烷:methane 乙烷:ethane 丙烷:propane 丁烷:butane 庚烷:heptane 癸烷:decane 具体来说:11~19:数字前缀-decane 十一烷:undecane 十二烷:dodecane 十三烷:tridecane 十四烷:tetradecane 戊烷:pentane 己烷:hexane 辛烷:octane 壬烷:nonane
丁二烯 butadiene
丁三烯 butatriene
同时还有双键和三键的烷类成为烯炔,命名时烯在前,炔在后, 双键的编号写在前面,三键的定位号写在表示炔烃词尾前
CH3-CH=CH-C ≡ CH
应用化学专业英语 复习总结
英译汉:1.First, electrons are added one at a time moving from left to right across a period……首先,从左向右横跨一个周期时每次增加一个电子。
当这种情况发生时,最外层电子将受到逐渐增强的核引力,所以电子将更接近原子核而受到其更紧密的束缚力。
其次,在周期表中从上向下移动一列,最外层电子受到核的束缚力将变弱。
这是因为主能级数(屏蔽最外层电子受到核的吸引)在每族向下移动时增加。
这些趋势解释了通过观察元素的原子半径、电离能、电子亲和力和电负性而得到的元素性质的周期性规律。
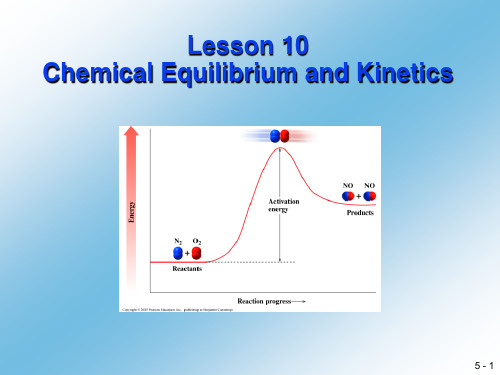
2.It is important to note that at equilibrium the rates of reaction,rate r and rate f are equilibriummixture are usually not equal……值得注意的是,在化学平衡时的反应速率,正反应速率和你反应速率相等但反应物和生成物的摩尔浓度在平衡混合态时一般不相等。
但是,事实上每种反应物和生成物在平衡时其浓度为定值,因为每种物质在一个反应中的消耗速率与其在相应你反应正的生成速率相等。
在化学平衡提出之前,这种系统被称为动力学平衡状态。
3.This is a mathematical expression of the law of chemical equilibrium which may be stated as follows:When a reversible…………这是化学平衡定律的数学表达式,它可以通过如下所述:当一个可逆反应在给定温度下达到平衡时,在方程式中箭头右边物质的摩尔浓度的积除以左边物质摩尔浓度的积(每种物质浓度的幂等于反应方程式中每种物质的分子数)为定值,4.Analytical chemistry,or the art of recognizing different substances and determining theirconstituents, takes a prominent position among分析化学或鉴定不同物质并测定其成分的技术,因为可以解决每当化学过程被用于科学的或技术性的目的是产生的问题,而在科学应用领域中占显著地位。
东华大学应用化学专业英语总结
东华大学应用化学专业英语总结专业英语重点总结单词Toxic chemicals:有毒化学品Chemical pollution:化学污染Physical property :物性 Isolate:分离Determine:测定 Synthesize:合成Fundamental principles:基本原理 Investigation:研究Utilize:利用 Catalyst 催化剂Enzyme 酶Biosphere 生物圈Heterogeneous catalyst 非均相催化剂Nanotechnology 纳米技术Carbon monoxide 一氧化碳Chemical formulas:化学式anion: 阴离子 Oxidation number:氧化值sulphate: 硫酸盐 Hydrides: 氢化物Sodium:钠 cation: 阳离子Covalent bond:共价键electroneutral: 电中性的Electronegative atom:电负性原子 trivial names:俗名Oxidation:氧化Peroxides:过氧化物Superoxide:超氧化物Periodic table:周期表Noble gases: 惰性气vacant orbital:空轨道Coordination (complex) compound: 配位化合物Unshared pair of electrons:未共用电子对oxidation state:氧化态 hydroxides:氢氧化物caustic soda solution:苛性钠溶液vacant orbital:空轨道Formula 分子式 Common name 俗名Derivative 衍生物 Acid salt 酸式盐Hydrate 水合物 Anhydrous 无水的Oxidizing agent 氧化剂Reducing agent 还原剂Oxidation reduction reaction氧化还原反应Electrochemistry 电化学 Electrolysis 电解Strong acid 强酸 Weak base 弱碱Acid-base indicator 酸碱指示剂Distilled water 蒸馏水Buffer solution 缓冲溶液Common ion effect 同离子效应Equivalencepoint 等效点 Neutralization 中和Dissociation 离解度 Anhydride 脱水物Periodic law: 元素周期率 periods (rows):周期group (columns):族 protons:质子Valence electrons:价电子 Halogens: 卤素Atomic radius: 原子半径alkaline earths:碱土金属attractive force: 吸引力electronegativity: 电负性electropositive:正电性univalent ion: 一价离子electron shell: 电子层 bonding force 结合力monatomic 单原子的 Neutrons:中子hydrogen bond 氢键conduct electricity 导电Electrically neutral 电中性的Electrostatic 静电的isomerism :异构现象Reversible :可逆的。
化学专业英语
unite 1. Inorganic chemistry1.1 what is chemistry(1). 重点专业词汇讲解:Chemical: adj . 化学的、化学药品Transformation: 变化,化学转变,转化Dye: n. 染料染色,或者vt. 染Charcoal: ['t ? ck??l] 木炭Cellulose : 纤维素细胞的['selj?l??z; ] Fat:n. 脂肪肥肉adj . 肥大的alkalis :碱adj . 碱性的glycerin: 甘油丙三醇alkalis: n. 碱金属alloy: 合金使成合金bronze:青铜色的n. 青铜(铜和锡的合金)brass:[br a s] n.黄铜(铜和锌)要求学生会区别黄铜及青铜的不同翻译Poison:毒物毒药t.毒害放毒下毒Proton:n. 质子Nulei: n.核(nucleus 的复数形式)['njukl ??s]Identical : adj . 同一的Chirality n.手性手征和Handeness的区别Amino acid :n. 氨基酸Ala nine: n.丙氨酸2. 课文中重点词组(phrase)Chemical change:化学变化physical change:物理变化Explore: 探险研究research investigate studyIsolate: 分离chemical bonds 化学键chemical reaction :化学反应Natural substance 天然物质Coke : 焦炭carbon monoxide 一氧化碳Carbon Dioxide 二氧化碳Chemical bond 化学键fundamental principle 基本原理The periodic table of elements :元素周期表numbers of protons 质子数atomic number 原子序数covalent bonds 共价键positive 正阳性negative 负阴性3. 课文中重点句子The first and most important principle is that chemical substances are made up of molecules in which atoms of various elements are linked in well-defined ways. 需要着重给学生讲解第一条也是最重要的原理是化学物质是有分子组成的,分子中的不同元素的原子是以一定的方式连接在一起的。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
about the same dense
7)hardness hard soft ductile malleable
8)toxicity
toxic poisonous
9)melting
point boiling point
High
low
4)solubility
soluble
insoluble
slightly soluble
very soluble
5)observations
brisk effervescence
precipitate
aqueous solution
milky
6)density heavy light
less dense denser
3、 读 法
高温,高压
• 3.1 Nitrogen reacts with hydrogen to form ammonia at high temperature and pressure with the presence of a catalyst.
• 1 mol nitrogen reacts with 3 mol hydrogen to form 2 mol ammonia at high temperature and pressure with the presence of a catalyst.
《专业英语》教案 Teaching Plan on Specialized English Course for Applied Chemistry
课程类型:化学系专业选修课
Lesson Type: Specialized Course for Applied Chemistry
Teaching Plan on Specialized English Course for Applied Chemistry
2
•
Zinc treated with hydrochloric acid forms hydrogen and zinc chloride
3.4
Calcium carbonate when heated produces calcium oxide and carbon dioxide • Calcium carbonate is heated to yield calcium oxide and carbon dioxide • Calcium carbonate decomposes to calcium oxide and carbon dioxide when it is heated
Part7 A brief introduction to scientific writing in English
Part 1 Physical Properties
物 理 性 质 1)Colour 〖颜色〗
colourless
red-brown
violet-black
purple-black
3.2
• Nitrogen combines with hydrogen to form ammonia at high temperature and pressure with the presence of a catalyst.
பைடு நூலகம்
•
Ammonia decomposes to nitrogen and hydrogen at high temperature and pressure with the presence of a catalyst.
2、反应条件
• • • • • • heat ; burn ignite/ignition (点燃) electrolyze/electrolysis(电解) under/at ambient/room temperature under standard pressure with/in the prescence of catalyst
Part 1 Physical Properties
Part 2 Chemical Equations
Part 3 Chemical Calculation
Part 4 Nomenclature Of Inorganic Chemicals Part 5 Some Basic Chemical Theories Part6 Translation
10)conductivity
electronic conductivity
thermal conductivity
conductor semiconductor
insulator
Part 2 Chemical Equations
1.反应名称: 化 学 方 程 式
• Disproportionation(歧化反应) • neutralization; hydrolysis(中和反应, 水解反应) • exothermic reaction(放热反应) • endothermic reaction(吸热反应) • reversible reaction(可逆反应) • forward reaction(正向反应) • reverse reaction(逆反应) • spontaneous reaction(自发的反应) • nonspontaneous reaction (非自发反应)
pale yellow dark brown
2)state
solid
liquid
gas
gaseous
crystalline molten
oily
uncrystalline fused
3)smell
odourless
pungent
penetrating
choking
offensive
sour sweet bitter
3.3
• Reaction between nitrogen and hydrogen at high temperature and pressure with the presence of a catalyst gives ammonia.
• At high temperature and pressure, reaction of nitrogen with hydrogen in the presence of a catalyst takes place.
