化学专业英语5-experiment
化学中的英语学习

n-hexyl(或hexyl) n-undecyl(或undecyl)
烃中还有四个常用结构词头:
iso-(isomeric的缩写) (异):
例如:
iso-butyl(异丁基)iso-hexane(异己烷)
sec-(secondary的缩写) (仲):
tert-(tertiary的缩写)(叔)
八. octa十七. heptadeca
九. nona十八. octadeca
十九. nonadeca二十八. octacosa
二十. eicosa二十九. nonacosa
二十一. heneicosa三十. triaconta
二十二. docosa三十一. hentriaconta
二十三. tricosa三十二. dotriaconta
1.1.6、卤代酸根的构成
将卤代酸的词头hydro和acid去掉用ide代替词尾ic
例如:
氢氟酸hydrofluoric acid氢氟酸根(盐) fluoride
氢氯酸hydrochloric acid氢氯酸根(盐) chloride
氢溴酸hydrobromic acid氢溴酸根(盐) bromide
四十二烷. dotetracontane四十六烷. hexatetracontane
四十三烷. tritetracontane四十七烷. heptatetracontane
四十四烷. tetratetracontane四十八烷. octatetracontane
四十九烷. Nonatetracontane五十烷. Pentacontane
iso (isomeric)异
temp. (temperature)温度
化学实验知识点高考英语

化学实验知识点高考英语Chemistry Experiment Knowledge for the College Entrance ExaminationChemistry is a fundamental subject that plays a significant role in our daily lives. Not only does it help us understand the natural world, but it also has practical applications in various fields. For students preparing for the College Entrance Examination, commonly referred to as the Gaokao, having a good understanding of chemistry experiments is crucial. In this article, we will explore some important knowledge points related to chemistry experiments that are likely to appear in the Gaokao English paper.1. Laboratory SafetySafety should always be the top priority in any chemistry experiment. While conducting experiments in the laboratory, students must adhere to certain safety precautions. Wearing protective clothing such as lab coats and goggles, handling chemicals cautiously, and being aware of emergency exits and safety equipment are essential. It is vital to remember that prevention is better than cure, especially when it comes to handling hazardous substances or using fire.2. Lab EquipmentUnderstanding the different equipment used in chemistry experiments is essential for successfully conducting them. Some common lab equipment includes beakers, test tubes, flasks, pipettes, and Bunsen burners. Each piece of equipment has its specific purpose and usage. For instance, beakers are used to measure and mix liquids, while test tubes are employed for small-scale chemical reactions. Familiarizing oneself with the names and functions of these materials will help students comprehend the instructions and questions in the exam accurately.3. Chemical ReactionsChemical reactions are at the core of chemistry experiments. Students must be well-versed in various types of reactions, such as synthesis, decomposition, displacement, and redox reactions. Understanding the reactants, products, and balanced chemical equations for these reactions is essential. Additionally, knowing how to identify the speed of reactions, factors influencing the rate of reactions, and the concept of activation energy can help students answer relevant questions with ease.4. Solution PreparationPreparing solutions is a common task in chemistry experiments. Students should be aware of the correct method for preparing a solution with a desired concentration. This involves accurately measuring and dissolving the solute in a solvent. It is crucial to determine the balanced equation and the number of moles involved in order to calculate the mass of solute required. Furthermore, understanding concentration units such as molarity and percentage can help in both the preparation and dilution of solutions.5. StoichiometryStoichiometry is a key concept in chemistry that involves calculating the quantities of reactants and products in a chemical reaction. It is crucial to understand the concept of mole and how it relates to the Avogadro's number. By utilizing balanced equations and stoichiometric calculations, students candetermine the amounts of substances involved in a reaction. This knowledge is vital for solving problems related to limiting reactants, percent yield, and theoretical yield.6. Data AnalysisInterpreting and analyzing experimental data is an integral part of chemistry experiments. Students should be familiar with various methods of representing data, such as graphs, tables, and charts. They should also be able to identify trends, make comparisons, and draw conclusions based on the data provided. Moreover, understanding the concept of uncertainties and error analysis is crucial when evaluating the accuracy and reliability of experimental results.In conclusion, having a strong foundation in chemistry experiment knowledge is essential for students preparing for the Gaokao. By focusing on laboratory safety, understanding lab equipment, chemical reactions, solution preparation, stoichiometry, and data analysis, students can excel in the chemistry section of the exam. Remember, practice and thorough understanding are the keys to success in any subject, including chemistry.。
化学实验英语作文

化学实验英语作文Title: A Chemistry Experiment: Synthesis of Aspirin。
Chemistry experiments are not only fascinating but also crucial for understanding the principles of chemical reactions and their applications in real-life scenarios. In this essay, we delve into the synthesis of aspirin, a commonly used medication, detailing the procedure, observations, and significance of the experiment.The synthesis of aspirin involves the reaction between salicylic acid and acetic anhydride in the presence of a catalyst, typically sulfuric acid. The reaction yields aspirin (acetylsalicylic acid) and acetic acid as byproducts. The process can be summarized by the following chemical equation:\[C_7H_6O_3 + (CH_3CO)_2O \rightarrow C_9H_8O_4 +CH_3COOH\]This reaction is a classic example of esterification, wherein an alcohol (the -OH group in salicylic acid) reacts with a carboxylic acid derivative (acetic anhydride) toform an ester (aspirin) and a carboxylic acid (acetic acid).The experimental procedure begins with measuring the required amounts of salicylic acid and acetic anhydride. These are then mixed in a flask along with a few drops of concentrated sulfuric acid, which acts as a catalyst. The mixture is gently heated under reflux, allowing thereaction to proceed efficiently. Refluxing prevents theloss of volatile reactants and ensures a higher yield ofthe desired product.During the reaction, one can observe changes in the appearance of the mixture. Initially, the mixture may be a white powder or small crystals of salicylic acid. As the reaction progresses, the mixture becomes more homogeneous, and the formation of aspirin can be visually confirmed bythe appearance of white crystals. The reaction is typically complete within a couple of hours.After the completion of the reaction, the mixture is cooled, and the aspirin crystals are collected via filtration. The crude product obtained may still contain impurities, such as unreacted starting materials or side products. Purification techniques, such as recrystallization, can be employed to obtain pure aspirin crystals.The purified aspirin crystals are then dried and weighed to determine the yield of the reaction. Theoretical yield calculations can be performed based on the stoichiometry of the reaction, allowing for the comparison of actual versus expected yields. Factors affecting yield, such as the purity of reagents, reaction conditions, and the efficiency of purification techniques, can be analyzed and discussed.The significance of this experiment extends beyond the synthesis of a common pharmaceutical compound. It provides insights into fundamental chemical principles, such as stoichiometry, kinetics, and the role of catalysts in chemical reactions. Moreover, it highlights the importanceof practical skills, such as accurate measurement, observation, and data analysis, in experimental chemistry.Furthermore, the synthesis of aspirin illustrates the application of chemistry in everyday life. Aspirin, withits analgesic, anti-inflammatory, and antipyretic properties, is one of the most widely used medications worldwide. Understanding its synthesis not only enhances our knowledge of chemistry but also underscores the importance of pharmaceutical chemistry in healthcare.In conclusion, the synthesis of aspirin is a classic chemistry experiment that offers valuable insights into chemical reactions, purification techniques, and the application of chemistry in the synthesis of pharmaceutical compounds. Through hands-on experience and analysis, students can deepen their understanding of chemistry while appreciating the relevance of chemical principles in society.。
化学专业课程中英文对照+化工装置常用词汇

分析化学Analytical Chemistry有机化学Organic Chemistry物理化学Physical Chemistry谱学导论Introducton of Spectroscopy无机化学Inorganic Chemistry普通化学和分析化学实验 Experiments of General and Analytical Chemistry现在基础化学 The Principle of Mordern Chemistry现在基础化学实验Experiments of Modern Fundamental Chemistry有机化学实验 Experiments of Organic Chemistry仪器分析和物理化学实验 Experiments of Instrumental Analysis and Physical Chemistry 合成化学实验 Experiments of Synthetic Chemistry现代化学专题 Topic of Modern Chemistry化学综合实验 Experiments of Comprehensive Chemistry化工原理Principle of Chemical Engineering化工原理实验 Experiments of Chemical Engineering应用化学实验 Experiments of Applied Chemistry无机合成化学 Synthetic Inorganic Chemistry近代分析化学 Modern Analytical Chemistry分离分析化学 Separation Analytical Chemistry有机化合物波谱鉴定Spectrum Identification of Organic Compounds有机合成及反应机理Organic Synthesis and Mechanics化学反应工程 Chemical Reaction Engineering应用电化学 Applied Electrochemistry工业催化Industrial Catalysis环境化学 Environmental Chemistry环境监测 Environmental Monitoring化学科技英语 Scientific English for Chemistry数理方法在化学中的应用 Mathematical Statistics for Chemistry 化工制图Chemical Engineering Cartography计算机与化学测量实验 Computer and Chemical Measurement 化学信息学 Chemoinformatics or Chemical Informatics应用化学专题 Special Topics in Applied Chemistry化工装置常用词汇1一概论introduction方案(建议书) proposal可行性研究feasibility study方案设计concept design工艺设计process design基础设计basic design详细设计detail design开工会议kick—off meeting审核会议review meeting外商投资foreign investment中外合资joint venture中外合营joint venture补偿贸易compensation trade合同合同附件contract卖方vendor买方buyer顾客client承包商contractor供应范围scope of supply生产范围production scope生产能力production capacity项目project界区battery limit装置plant公用工程utilities工艺流程图process flow diagram工艺流程方块图process block diagram管道及仪表流程图piping and instrument drawing物料及热量平衡图mass &heat balance diagram蒸汽及冷凝水平衡图steam &condensate balance diagram 设备布置图equipment layout设备表equipment list成品(产品) product(final product)副产品by—product原料raw—material设计基础数据basic data for design技术数据technical data数据表data sheet设计文件design document设计规定design regulation现场服务site service项目变更project change用户变更client change消耗定额consumption quota技术转让technical transfer技术知识technical know—howtechnical knowledge技术保证technical guarantee咨询服务consultative services技术服务technical services工作地点location施工现场construction field报价quotation标书bidding book公司利润company profit固定价合同fixed price contract固定单价合同fixed unit price contract成本加酬金合同cost plus award fee contract定金mobilization银行保证书bank guarantee letter保留金retention特别承包人税special contractor's taxes城市和市政税city and municipal taxes工作手册work manual工作流程图work flow diagram质量保证程序QA/QC procedures采购计划procurement plan施工计划construction plan施工进度construction schedule项目实施计划project execution plan项目协调程序project coordination procedure 项目总进度计划project master schedule设计网络计划engineering network logic项目质量保证project quality assurance项目质量控制project quality control采购procurement采购周期procurement period会签the squad check计算书calculation sheets询价inquiry检验inspection运输transportation开车start up / commission验收inspection & acceptance校核check审核review审定approve版次version部门department专业specialty项目号project number图号drawing number目录contents序言foreword章chapter节section项itemMR material requisitionSPEC engineering specificationDATA SHEET(技术表)technical data sheet TBA(技术评标)technical bid analysis PDP preliminary design packagePM (项目经理)project managerLDE(专业负责人) lead discipline engineerMRQ(材料询价单)Material requisition for quotationMRP(材料采购单)material requisition for purchaseBEP(基础工程设计包) basic engineering packageP&ID(管道及仪表流程图)piping and instrument drawing(diagram) PFD process flow diagramNNF normally no flowFO failure openFC failure closeC/S/A civil/structure/architectureDDP(详细设计阶段)detail design phase化工装置词汇二。
化学专业英语实验设计方案范文

化学专业英语实验设计方案范文Experimental Design Proposal for a Chemical Process.Introduction.In the field of chemical engineering, the optimization of processes is crucial for achieving desired outcomes while minimizing resource waste and environmental impact. The proposed experiment aims to investigate the efficiency of a specific chemical reaction under varying conditions. The objective is to identify the optimal conditions that maximize the reaction yield while minimizing by-product formation and energy consumption. This experiment will provide valuable insights into the kinetics and mechanisms of the reaction, leading to improved process design and operation.Experimental Objectives.1. To study the effect of temperature on the rate andyield of the chemical reaction.2. To investigate the impact of catalyst concentration on the reaction kinetics.3. To evaluate the influence of reactant concentration on product selectivity and by-product formation.4. To determine the optimal reaction conditions that maximize the desired product yield.Materials and Methods.Materials.1. Reactants A and B (purified to ≥99% purity)。
化学实验报告 英文

化学实验报告英文Chemistry Experiment ReportIntroduction:In the field of science, experiments play a crucial role in deepening our understanding of various phenomena. This report aims to present the findings and observations from a recent chemistry experiment conducted in the laboratory. The experiment focused on the reaction between two chemicals and explored the effects of different variables on the reaction rate.Experimental Procedure:The experiment began by carefully measuring and preparing the required chemicals: sodium hydroxide (NaOH) and hydrochloric acid (HCl). These chemicals were chosen due to their well-known reaction, which produces salt and water. The experiment aimed to investigate how factors such as concentration, temperature, and catalysts influenced the reaction rate.To start the experiment, a fixed volume of NaOH solution was poured into a conical flask. The concentration of NaOH was varied in different trials, ranging from 0.1 M to 1.0 M. The flask was placed on a magnetic stirrer to ensure uniform mixing. Then, a burette was used to add a fixed volume of HCl solution to the flask. The reaction was monitored by observing the formation of a white precipitate, indicating the completion of the reaction.Results and Discussion:The experiment revealed several interesting findings. Firstly, it was observed thatas the concentration of NaOH increased, the reaction rate also increased. This can be attributed to the higher number of NaOH particles available to react with HCl, leading to more frequent collisions and faster reaction kinetics. Furthermore, the effect of temperature on the reaction rate was investigated. It was found that as the temperature increased, the reaction rate also increased. This can be explained by the kinetic theory of gases, which states that at higher temperatures, particles possess greater kinetic energy and move more rapidly. Consequently, more collisions occur, resulting in a faster reaction rate.The influence of catalysts on the reaction rate was also examined. A small amount of catalyst, in the form of manganese(IV) oxide (MnO2), was added to the reaction mixture. It was observed that the presence of the catalyst significantly increased the reaction rate. Catalysts provide an alternative reaction pathway with lower activation energy, allowing the reaction to proceed more rapidly.Conclusion:In conclusion, this experiment provided valuable insights into the factors affecting the reaction rate between NaOH and HCl. The concentration of the reactants, temperature, and the presence of catalysts were identified as key variables influencing the rate of the reaction. Understanding these factors is crucial in various industrial processes where reaction rates play a vital role.It is important to note that this experiment focused on a specific reaction and variables. Further research could explore the effects of other factors, such aspressure and surface area, on the reaction rate. Additionally, investigating the reaction kinetics using mathematical models could provide a more comprehensive understanding of the underlying mechanisms.Overall, this experiment highlights the significance of chemistry in unraveling the mysteries of the natural world. By conducting experiments and analyzing the results, scientists can uncover fundamental principles that govern chemical reactions, paving the way for advancements in various fields, including medicine, energy, and materials science.。
(完整版)化学专业英语
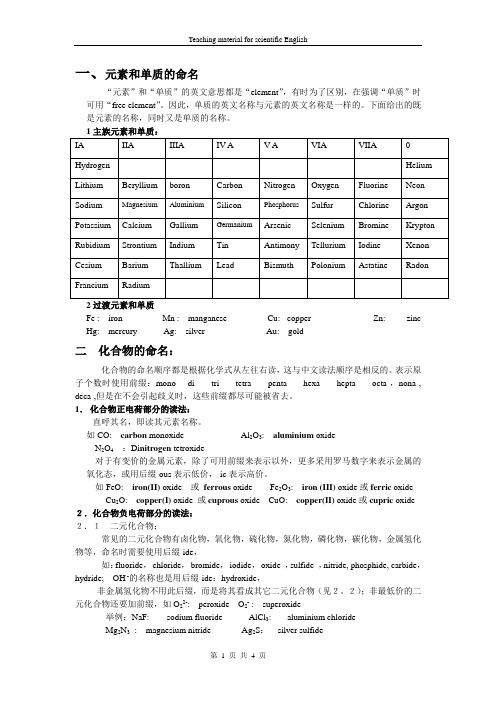
一、元素和单质的命名“元素”和“单质”的英文意思都是“element”,有时为了区别,在强调“单质”时可用“free element”。
因此,单质的英文名称与元素的英文名称是一样的。
下面给出的既是元素的名称,同时又是单质的名称。
2过渡元素和单质Fe : iron Mn : manganese Cu: copper Zn: zinc Hg: mercury Ag: silver Au: gold二化合物的命名:化合物的命名顺序都是根据化学式从左往右读,这与中文读法顺序是相反的。
表示原子个数时使用前缀:mono-di -tri- tetra -penta- hexa-hepta- octa-,nona-, deca-,但是在不会引起歧义时,这些前缀都尽可能被省去。
1.化合物正电荷部分的读法:直呼其名,即读其元素名称。
如CO: carbon monoxide Al2O3: aluminium oxideN2O4:Di nitrogen tetroxide对于有变价的金属元素,除了可用前缀来表示以外,更多采用罗马数字来表示金属的氧化态,或用后缀-ous表示低价,-ic表示高价。
如FeO: iron(II) oxide 或ferrous oxide Fe2O3: iron (III) oxide或ferric oxide Cu2O: copper(I) oxide 或cuprous oxide CuO: copper(II) oxide或cupric oxide 2.化合物负电荷部分的读法:2.1二元化合物:常见的二元化合物有卤化物,氧化物,硫化物,氮化物,磷化物,碳化物,金属氢化物等,命名时需要使用后缀-ide,如:fluoride,chloride,bromide,iodide,oxide ,sulfide ,nitride, phosphide, carbide,hydride; OH -的名称也是用后缀-ide:hydroxide,非金属氢化物不用此后缀,而是将其看成其它二元化合物(见2。
化学实验报告常见英文

Experiment Title: Synthesis of Ethanol from EthanolamineDate: [Date]Objective:The objective of this experiment was to synthesize ethanol from ethanolamine using the dehydration reaction. Ethanolamine is a compound with the molecular formula NH2CH2CH2OH, and it can be dehydrated to produce ethanol (CH3CH2OH) and ammonia (NH3).Materials:- Ethanolamine (NH2CH2CH2OH)- Sulfuric acid (H2SO4)- Concentrated sulfuric acid- Ethanol- Sodium chloride (NaCl)- Distilled water- Sodium hydroxide (NaOH)- Sodium sulfate (Na2SO4)- Potassium permanganate (KMnO4)- Barium chloride (BaCl2)- Distillation apparatus- Reaction vessel- Round-bottom flask- Condenser- Thermometer- Test tubes- Pipettes- Weighing scale- Stirring rod- Safety goggles- Gloves- Lab coatProcedure:1. Measure 5 g of ethanolamine using a weighing scale and transfer it toa round-bottom flask.2. Add 5 mL of concentrated sulfuric acid to the flask and stir the mixture thoroughly.3. Place the flask in a water bath and heat it to 60°C for 2 hours. This will facilitate the dehydration reaction.4. After 2 hours, remove the flask from the water bath and allow it to cool to room temperature.5. Transfer the reaction mixture to a distillation apparatus. The distillation apparatus consists of a round-bottom flask, a condenser, and a receiving flask.6. Heat the mixture to approximately 78°C, which is the boiling point of ethanol. Ethanol will vaporize and be collected in the receiving flask.7. Collect the distillate and transfer it to a test tube. Add 5 mL of water to the test tube and observe the appearance of the liquid.8. To identify the presence of ammonia, add a few drops of potassium permanganate to the test tube. If the solution turns brown, it indicates the presence of ammonia.9. To confirm the purity of the ethanol, add a few drops of barium chloride to the test tube. If a white precipitate forms, it indicates the presence of sodium chloride, which was used as a catalyst in the reaction.10. Dispose of the waste products and clean the equipment.Results:- The reaction mixture was heated to 60°C for 2 hours, and the distillation was performed at approximately 78°C.- Ethanol was collected in the receiving flask, and the distillate was observed to be a clear liquid.- A brown color was observed in the test tube when potassium permanganate was added, indicating the presence of ammonia.- A white precipitate formed when barium chloride was added, indicating the presence of sodium chloride.Discussion:The dehydration reaction of ethanolamine to produce ethanol was successfully achieved in this experiment. The reaction mixture was heated to 60°C for 2 hours to facilitate the dehydration process. Ethanol was collected in the receiving flask, and the distillate was observed to be a clear liquid, indicating the successful synthesis of ethanol.The presence of ammonia was confirmed by the brown color observed when potassium permanganate was added. This suggests that the dehydration reaction also produced ammonia as a byproduct.The formation of a white precipitate when barium chloride was added confirms the presence of sodium chloride, which was used as a catalyst in the reaction. The sodium chloride did not affect the purity of the ethanol product.Conclusion:The objective of synthesizing ethanol from ethanolamine using the dehydration reaction was successfully achieved in this experiment. Ethanol was produced, and the purity of the product was confirmed by observing the color changes and precipitate formation. This experiment provided a practical approach to understanding the dehydration reaction and its application in the synthesis of organic compounds.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
dropwise to a solution of 1,3-propanediol (29.9 g,
0.393 mol) and ethyl chloroformate (85.4 g, 0.786
mol) in 2L of THF at 0 oC over a period of 30 min.
Generally, an experimental section mainly consists
three parts, that is:
Chemicals (Materials), Synthesis (preparation),
and Characterization
Experimental
PVA is poly (vinyl alcohol) 聚乙烯醇
一、原材料的来源及准备(Chemicals or Materials)
Hale Waihona Puke All materials, such as potassium hexacyano-cobaltate (III) (K3Co(CN)6), zinc fluoride (ZnF2), zinc chloride (ZnCl2), zinc bromide (ZnBr2), zinc iodide (ZnI2) and tertiary butyl alcohol (tBuOH) were purchased from Aldrich and used without further purification.
一、原材料的来源及准备(Chemicals or Materials) 2. 直接购买的普通溶剂或辅助药品只需简单提及或略 过
All other materials were commercially available and used as received unless otherwise noted. All other chemicals were of analytical grade and were used as received.
Experimental
Preparation of catalysts
A solution of ZnCl2 (220 mmol) in deionized water(115 mL) and tBuOH (21 g) is taken in the first beaker (solution 1). A solution of K3Co(CN)6 (3.15 g, 9.5 mmol) in deionized water (42 mL) is taken in the second beaker (solution 2). The third beaker contains a mixture of deionized water (1 mL), tBuOH (20 mL) and PTMEG (3.5 g) (solution 3). Solution 2 is added to solution 1 dropwise for a period of over 60 min at 50 oC with continuous stirring using a mechanical stirrer. Solution 3 is then added and the mixture is stirred for an additional 3 min. The mixture is then centrifuged. The resulting catalyst (DMC-Cl) cake is dried at 60 oC under vacuum (30 inHg) to a constant weight. The catalysts using ZnF2 (DMC-F), ZnBr2 (DMC-Br) and ZnI2 (DMC-I) were prepared by similar procedures.
二、阐述目前该领域的研究现状﹑存在问题﹑ 待解决的问题
Although … has been investigated extensively, very few literatures reported …
However, … have so far not been studied. Unfortunately, the current method suffers from certain limitations. though, whereas, on the other hand, but … Thus, there is a need to …
化学实验中使用的药品按照纯度的不同常可以分为: industrial grade 工业级 ; analytical grade 分析级 ; spectral grade 光谱级
一、原材料的来源及准备 (Chemicals or Materials) 3. 需经纯化处理的药品一般需说明纯化方法
Bisphenol A was recrystallized from chlorobenzene. Dichloromethane was distilled prior to use and stored over molecular sieves. Molecular sieves were activated at 350oC for 10 h under N2 before use.
synthesized
following a procedure described elsewhere.25 The product was recrystallized twice from
diethyl ether. White crystal was obtained in
55% yield: (mp 45°C). The 1H-NMR spectrum (4H, 4.50 ppm; 2H, 2.21 ppm) was consistent with that reported previously.25
chlorine —Cl2 bromine —Br2 iodine —I2
Polymerization grade of poly( tetramethylene ether glycol) (PTMEG, molecular weight =1800) was donated by BASF Korea Ltd.
under vacuum. The residue was recrystallized three
times from THF-ether.
Experimental
Synthesis of trimethylene carbonate <小标题>
Trimethylene
carbonate
was
Herein we demonstrated that …
专业英语课件
Part 6
Experimental
The format of an experimental section should be
in accordance with the requirement of the journal you choose.
H2N
N
2-(2-amino-ethyl)pyridine
ZnEt2 (diethylzinc)was purchased from Aldrich and was used as received.
Amino acid 氨基酸 amine 胺 ammonia 氨 Ammonium 铵 pyridine 吡啶
二.实验过程
研究对象为化学合成的产物要说明具体合成方法:
1.若参考已知的方法,此部分应指明合成方法的出处
…was prepared by the method of … [10]. …was synthesized according to Ref. [4].
…were synthesized according to
专业英语课件
Part 5
Introduntion
一、介绍研究背景(background)﹑ 研究意义
二、阐述目前该领域的研究现状﹑存在问题﹑
待解决的问题
三、指出本文的研究内容﹑目的及其独到之处
Introduction
一、介绍研究背景(background)﹑ 研究意义
Since the early 1960s, …has attracted much attention due to … In recent years, more and more attention has been paid
HO N
2-hydroxypyridine
一、原材料的来源及准备(Chemicals or Materials)
The starting materials were Li3PO4 (99%, Aldrich) and Fe3(PO4)· 2O (99%, Aldrich). 8H An appropriate amount of PVA (degree of polymerization 1500, Junsei) as the carbon source was added to the raw materials.
to …
Very recently, it was reported that …
…have recently evoked a lot of interest.
