CO2 -binding organic liquids (CO2 BOLs) for post-combustion CO2 capture
金属化多级孔密胺材料的催化性能

第1期捕获和转化CO2。
然而,MOF的应用受到其稳定性以及其他问题的影响[6-8]。
与MOF类似,多孔有机聚合物(POPs)继承了其结构中各种官能化单体所提供的优异的物理和化学可调性;此外,强共价键保证聚合物的化学稳定性,具有高比表面积的多孔聚合物结合特定的催化位点,更适用于CO2捕获/转化[9-11]。
研究表明,具有良好的CO2亲和性和高比表面积的多孔密胺(PMF)是理想的材料之一。
事实上,多孔PMF聚合物已经研究多年。
Dery覥o-Marczewska等[12]通过使用气相二氧化硅作为硬模板来合成介孔PMF树脂以引入多孔结构。
Kailasam等[13]报道了通过软模板法制备的介孔PMF聚合物以形成多孔结构。
然而,在这些工作中,所得到的聚合物由柔性交联分子链组成,具有低比表面积。
Tan等[14]通过形成刚性多孔有机骨架,制备了具有高比表面积的多孔PMF聚合物。
此前,本课题组通过以三聚氰胺和多聚甲醛为反应单体,以二甲基亚砜(DMSO)为连续相溶剂、Isopar M为分散相溶剂,泊洛沙姆F127为稳定剂,通过高内相乳液聚合,一步法制备出具有微孔、介孔、大孔的多级孔结构材料[15]。
但在高内相乳液制备过程中,需要额外加入大量长链烷烃作为内相乳液,不利于工业化生产。
本文提出了一种简易的多孔密胺制备方法,吸附铜离子后,用于氧化苯乙烯与CO2的环加成催化反应。
通过在三聚氰胺和多聚甲醛的共聚体系中加入致孔剂泊洛沙姆F127,利用水热法制备出了具有多孔结构的密胺材料,负载铜离子后作为环加成催化反应的催化剂,使其具有优秀的催化性能。
1实验部分1.1主要试剂及仪器主要试剂:三聚氰胺,分析纯,百灵威科技有限公司产品;多聚甲醛、醋酸铜、四丁基溴化铵、氧化苯乙烯、氘代氯仿(0.03v/v TMS),均为分析纯,上海阿拉丁生化科技有限公司产品;泊洛沙姆F127,分析纯,西格玛奥德里奇(上海)贸易有限公司产品;甲醇、四氢呋喃、丙酮,均为分析纯,天津科密欧化学试剂有限公司产品。
光学词汇-中英对照

A01-001 光学材料 Optical MaterialsA01-002 光学玻璃 Optical GlassA01-003 激光玻璃 Laser GlassA01-004 声光玻璃 Acousto-Optic GlassA01-005 红外线玻璃 Infrared GlassA01-006 红外线材料 Infrared MaterialsA01-007 紫外线材料 Ultraviolet MaterialsA01-008 石英镜片Fused Silica GlassA01-009 光学陶瓷 CeramicsA01-010 矽半导体材料Silicon Semiconductor MaterialsA01-011 化合物半导体材料Compound Semiconductor Materials A01-012 光纤材料Fiber Optic MaterialsA01-013 光纤预型体Fiber Optic PreformsA01-014 PLZT晶圆,钛酸锆酸铅晶圆 PLZT WafersA01-015 环氧树脂 EpoxiesA01-016 声光光学晶体 Acousto-Optic CrystalsA01-017 双折射/偏光晶体Birefringent and Polarizing Crystals A01-018 电光光学晶体 Electro-Optic CrystalsA01-019 红外线晶体 Infrared CrystalsA01-020 激光晶体(YAG) YAG Laser CrystalsA01-021 激光晶体(亚历山大) Alexandrite Laser CrystalsA01-022 激光晶体(GGG) GGG Laser CrystalsA01-023 激光晶体(GSGG,GSAG) GSGG GSAG Laser Crystals A01-024 激光晶体(YLF) YLF Laser CrystalsA01-025 激光晶体(其他) Other Laser CrystalsA01-026 非线性光学晶体 Nonlinear CrystalsA01-027 有机光学材料Organic Optical MaterialsA01-028 萤光放射晶体Fluorescent Emission CrystalsA01-029 结晶育成材料Crystals Growing MaterialsA01-030 镀膜材料 Coating MaterialsA01-031 光罩材料 Photomask MaterialsA01-032 真空蒸镀化学药品Vaccum Evaporation ChemicalsA01-033 感光剂 SensitizersA01-034 影像用材料Materials for ImagingA01-035 热色材料 Thermochromic MaterialsA01-036 光色材料 Photochromic MaterialsA01-037 稀土族材料Rare Earth MaterialsA01-038 光碟基板,基板材料Optical Disk Substrate Materials A01-039 光碟记录材料Optical Disk Data Storage MaterialsA02加工用其他材料:A02 加工用其他材料MATERIALS FOR PROCESSINGA02-001 光学用胶合剂/接著剂Optical Cements and Adhesives A02-002 光学用气体Gases for Optical ApplicationA02-003 激光用气体Gases for LasersA02-004 光学研磨材料(研磨布纸) Optical-Coated AbrasiveA02-005 光学研磨材料(砥粒) Optical-Powder or Grin AbrasiveA02-006 光学研磨材料(砥石) Optical-Wheel AbrasiveA02-007 研磨化合物 Polishing CompoundsA02-008 研磨衬垫及布Polishing Pads and ClothA02-009 全像底片及感光板Holographic Films and PlatesA02-010 红外线底片及感光板Infrared Films and PlatesA02-011 相片用化学药品 Photographic ChemicalsA02-012 折射率液Refractive Index LiquidsA02-013 显微镜浸液Microscope Immerison LiquidsA02-014 显微镜埋置用材料Microscope Imbedding MediaA02-015 激光用染料 Laser DyesA02-016 冷媒 CoolantsA02-017 拭镜纸 Lens TissueA03 显示器用材料:A03 显示器用材料MATERIALS FOR DISPLAYA03-001 液晶 Liquid CrystalsA03-002 导电膜玻璃基板ITO Glass SubstrateA03-003 彩色滤光片 Color FilterA03-004 偏光板/相位差板Polarizer/ Phase Shift LayerA03-005 显示面板用驱动IC Driver ICA03-006 背光源 BacklightA03-007 配向膜 Alignment FilmA03-008 间隔物SpacerB01 透镜:B01 透镜 LENSESB01-001 单透镜Simple (Single) LensesB01-002 球透镜 Ball LensesB01-003 歪像透镜 Anamorphic LensesB01-004 圆锥透镜 Conical LensesB01-005 柱状透镜,环形透镜Cylindrical & Toroidal LensesB01-006 非球面透镜 Aspheric LensesB01-007 反射折射透镜 Catadioptric LensesB01-008 绕射极限透镜 Diffraction-Limited LensesB01-009 GRIN透镜GRIN Lenses (Graduated Refractive Index Rod)B01-010 微小透镜阵列Micro Lens ArraysB01-011 准直透镜 Collimator LensesB01-012 聚光透镜 Condenser LensesB01-013 多影像透镜Multiple Image LensesB01-014 傅利叶透镜Fourier Lenses B01-015 菲涅尔透镜 Fresnel Lenses B01-016 替续透镜 Relay LensesB01-017 大口径透镜(直径150mm以上) Large Aperture Lenses (150mm)B01-018 复合透镜 Complex LensesB01-019 红外线透镜 Infrared LensesB01-020 紫外线透镜 Ultraviolet LensesB01-021 激光透镜 Laser LensesB01-022 望远镜对物镜Telescope Objectives LensesB01-023 显微镜对物镜Microscope Objectives LensesB01-024 接目镜 Eyepieces LensesB01-025 向场透镜 Field LensesB01-026 望远镜头 Telephoto LensesB01-027 广角镜头Wide Angle LensesB01-028 可变焦伸缩镜头Variable Focal Length Zoom LensesB01-029 CCTV镜头 CCTV LensesB01-030 影印机镜头Copy Machine LensesB01-031 传真机镜头 Facsimile LensesB01-032 条码扫描器镜头Bar Code Scanner LensesB01-033 影像扫描器镜头Image Scanner LensesB01-034 光碟机读取头透镜Pick-up Head LensesB01-035 APS相机镜头APS Camera LensesB01-036 数位相机镜头Digital Still Camera LensesB01-037 液晶投影机镜头Liquid Crystal Projector LensesB02 镜面:B02 镜面 MIRRORB02-001 平面镜 Flat MirrorsB02-002 球面凹面镜,球面凸面镜Spherical Concave and Convex Mirrors B02-003 抛物面镜,椭圆面镜Off-Axis Paraboloids and Ellipsoids Mirrors B02-004 非球面镜 Aspheric MirrorsB02-005 多面镜 Polygonal MirrorsB02-006 热镜 Hot MirrorsB02-007 冷镜 Cold MirrorsB02-008 玻璃,玻璃/陶瓷面镜Glass and Glass-Ceramic MirrorsB02-009 双色向面镜 Dichroic MirrorB02-010 金属面镜 Metal MirrorsB02-011 多层面镜 Multilayer MirrorsB02-012 半涂银面镜 Half-Silvered MirrorsB02-013 激光面镜 Laser MirrorsB02-014 天文用面镜 Astronomical MirrorsB02-099 其他面镜 Other MirrorsB03 棱镜:B03 棱镜 PRISMB03-001 Nicol棱镜 Nicol PrismsB03-002 Glan-Thomson棱镜 Glan-Thomson PrismsB03-003 Wollaston棱镜 Wollaston PrismsB03-004 Rochon棱镜 Rochon PrismsB03-005 直角棱镜Right-Angle; Rectangular PrismsB03-006 五面棱镜 Pentagonal PrismsB03-007 脊角棱镜 Roof PrismsB03-008 双棱镜 BiprismsB03-009 直视棱镜Direct Vision PrismsB03-010 微小棱镜 Micro PrismsB03-099 其他棱镜 Other PrismsB04 滤光镜:B04 滤光镜 FILTERB04-001 尖锐滤光镜Sharp Cut (off) FiltersB04-002 色温变换滤光镜,日光滤光镜Colour Conversion/Daylight Filters B04-003 干涉滤光镜 Interference FiltersB04-004 中性密度滤光镜Neutral Density FiltersB04-005 空间/光学匹配滤光镜Spatial/Optical Matched FiltersB04-006 双色向滤光镜 Dichroic FiltersB04-007 偏光滤光镜 Polarizing FiltersB04-008 排除频带滤光镜Rejection Band FiltersB04-009 可调式滤光镜 Turnable FilterB04-010 超窄频滤光镜Ultra Narrowband FiltersB04-011 色吸收滤光镜 Absorption FiltersB04-012 红外吸收/反射滤光镜Infrared Absorbing/Reflecting FiltersB04-013 红外透过滤光镜Infrared Transmitting FiltersB04-014 紫外吸收滤光镜Ultraviolet Absorbing FiltersB04-015 紫外透过滤光镜Ultraviolet Transmitting FiltersB04-016 针孔滤光镜 Pinhole FiltersB04-017 有色玻璃滤光镜 Colored-Glass FiltersB04-018 塑胶滤光镜 Plastic FiltersB04-019 照像用滤光镜 Photographic FiltersB04-020 全像滤光镜 Holographic FiltersB04-021 微小干涉滤光镜Micro Interference FiltersB06 激光:LASERS B06 激光 LASERSB06-100 气体激光 GAS LASERSB06-101 氦氖激光 He-Ne LasersB06-102 金属蒸气激光Metal Vapor LasersB06-103 氩离子激光 Argon LasersB06-104 氪离子激光 Krypton LasersB06-105 二氧化碳激光(气流型) CO2 (Gas Flow type) LasersB06-106 二氧化碳激光(脉冲,TEA型) CO2 (Pulsed,TEA) LasersB06-107 二氧化碳激光(密封型) CO2 (Sealed tube) LasersB06-108 二氧化碳激光(波导型) CO2 (Wave guide) LasersB06-109 一氧化碳激光 CO LasersB06-110 氦镉激光 He-Cd LasersB06-111 氮分子激光 Nitrogen LasersB06-112 准分子激光 Excimer LasersB06-113 氙分子激光 Xenon LasersB06-200 固体激光SOLID STATE LASERSB06-201 红宝石激光 Ruby LasersB06-202 玻璃激光 Glass LasersB06-203 Nd:YAG激光(脉冲式) Nd:YAG (Pulsed) LasersB06-204 Nd:YAG激光(连续式) Nd:YAG Laser (CW) LasersB06-205 Nd:YAG激光(半导体激光激发) Nd:YAG (LD Pumped) LasersB06-206 YLF激光 YLF LasersB06-207 亚历山大激光 Alexanderite LasersB06-208 铒固体激光 Erbium LasersB06-209 半导体激光激发式固态激光Solid State(LD pumped)LaserB06-210 其他固态激光 OthersB06-300 染料激光 DYE LASERSB06-301 染料激光(闪光灯激发) Dye (Flash lamp Pumped) LasersB06-302 染料激光(激光激发) Dye (Laser Pumped) LasersB06-400 半导体激光 SEMICONDUCTOR LASERSB06-401 半导体激光(1.55μm带) Semiconductor (1.55μm) LasersB06-402 半导体激光(1.30μm带) Semiconductor (1.30μm) LasersB06-403 半导体激光(0.85μm带) Semiconductor (0.85μm) LasersB06-404 半导体激光(0.78μm带) Semiconductor (0.78μm) LasersB06-405 半导体激光(0.60μm带) Semiconductor (0.60μm) LasersB06-406 半导体激光(其他波长带) Other Semiconductor LasersB06-407 半导体激光模组(长波长) Semiconductor (Long Wavelength) Laser Modules B06-408 半导体激光模组(短波长) Semiconductor (Short Wavelength) Laser Modules B06-409 半导体激光模组(可见光) Semiconductor (Visible) Laser ModulesB06-501 铁离子中心激光 F-Center LasersB06-502 化学激光(HF-DF) Chemical (HF-DF) LasersB06-503 平板激光 Slab LasersB06-504 远红外线激光 Far-Infrared LasersB06-505 真空紫外线激光Vacuum Ultraviolet LasersB06-506 多色激光Multi Colour LasersB06-507 稳频激光Frequency Stabilized LasersB06-508 自由电子激光Free Electron LasersB07 激光用元件:B07 激光用元件 LASER COMPONENTSB07-001 Q 开关 Laser Q-SwitchesB07-002 激光管Laser Tubes and BoresB07-003 激光棒 Laser RodsB07-004 激光板 Laser SlabsB07-005 气体再生设备,气体填充设备Gas Recyclers and Gas Handling Equipment B07-006 激光控制设备Laser Control EquipmentB07-007 激光用盒 Laser CellsB07-008 参数振汤器 Parametric OscillatorsB07-009 光脉冲产生设备Optical Pulse GeneratorsB07-010 激光用共振腔Resonators for LasersB07-011 磁铁 MagnetsB07-012 激光用冷却设备Cooling Systems for LasersB07-013 激光护眼镜Safty Equipment; Goggles Glasses and FilmsB07-014 激光光吸收体Safty Equipment; Laser AbsorbersB07-015 激光用安全设备Safty Equipment; Protective HousingsB08 发光二极体:B08 发光二极体LIGHT-EMITTING DIODES; LEDB08-001 通信用1.55μm发光二极体 1.55μm LEDs for CommunicationB08-002 通信用1.30μm发光二极体 1.30μm LEDs for CommunicationB08-003 通信用0.85μm发光二极体 0.85μm LEDs for CommunicationB08-004 通信用长波长发光二极体模组Long Wavelength LED Modules for Communication B08-005 通信用短波长发光二极体模组Short Wavelength LED Modules for Communication B08-006 可见光发光二极体(红色) Visible (Red) LEDsB08-007 可见光发光二极体(黄色) Visible (Yellow,Orange) LEDsB08-008 可见光发光二极体(绿色,多色) Visible (Green,Multi-Color) LEDsB08-009 可见光发光二极体(蓝色) Visible (Blue) LEDsB08-010 红外线二极体(非通信用) Infrared (not for Communication) LEDsB08-011 文数字表示用发光二极体 Alpha-Numeric LEDsB08-012 发光二极体晶圆(通信用) LED Wafers for CommunicationB08-013 发光二极体晶圆(非通信用) LED Wafers not for CommunicationB08-014 发光二极体晶片、晶粒(通信用) LED Chips for CommunicationB08-015 发光二极体晶片、晶粒(非通信用) LED Chips not for CommunicationB09 光源设备:B09 光源设备 LIGHT SOURCESB09-001 标准光源Standard Light SourcesB09-002 安定化光源Stabilized Light SourcesB09-003 弧光灯Arc Light SourcesB09-004 氪灯Krypton Light SourcesB09-005 卤素灯Halogen Light SourcesB09-006 氙灯Xenon /Xenon Flashlamps Light SourcesB09-007 紫外线光源Ultraviolet Light SourcesB09-008 真空紫外线光源VUV Light SourcesB09-009 红外线光源Infrared Light SourcesB09-010 闪光光源Stroboscopic Light SourcesB09-011 小型光源Miniature Light SourcesB09-012 光纤光源Fiber Optic IlluminatorsB10 显示器元件:B10 显示器元件 DISPLAY PANELB10-001 发光二极体显示器 LED DisplaysB10-002 液晶显示器Liquid Crystal Display (LCD)B10-003 电浆显示器Plasma Display Panels(PDP)B10-004 电激发光显示器Electroluminescence Display (ELD)B10-005 电铬显示器Electrochromic Display (ECD)B10-006 真空萤光显示器Vacuum Fluorescent Display (VFD)B10-007 平面阴极射线管 Flat CRTsB10-008 场发射显示器Field Emitter Display(FED)B10-099 其他平面显示元件Other Flat Panel DisplaysB11 检光元件及光纤混成元件:B11 检光元件及光纤混成元件DETECTORS & FIBEROPTIC HYBRID DEVICESB11-001 通信用PIN光二极体PIN Photodiodes for CommunicationB11-002 通信用崩溃光二极体Avalanche Photodiodes for CommunicationB11-003 通信用(长波长)Ge和III-V族检光元件Long-wavelength Detectors for Communication B11-004 通信用PIN光二极体模组PIN Photodiode Modules for CommunicationB11-005 通信用崩溃光二极体模组Avalanche Photodiode Modules for CommunicationB11-006 通信用(长波长)Ge和III-V族检光模组Long-wavelength Decector Modules for CommunicationB11-007 光二极体(近红外光) Near-infrafed PhotodiodesB11-008 光二极体(可见光) Visible PhotodiodesB11-009 光二极体(紫外光) Ultraviolet PhotodiodesB11-010 光电晶体 PhototransistorsB11-011 光电管 PhototubesB11-012 光电子增倍管(PMT) PhotomultipliersB11-013 光导电池 Photoconductive CellsB11-014 热电偶检测器 Thermocouple DetectorsB11-015 热堆检测器 Thermopile DetectorsB11-016 微道板 Microchannel PlatesB11-017 热电检测器 Pyroelectroic DetectorsB11-018 辐射热测定器 BolometersB11-019 其他红外线检测器 Infrared DetectorsB11-020 摄像管 Camera TubesB11-021 线型检光元件One Dimension Detector ArraysB11-022 面型检光元件Two Dimension Detector ArraysB11-023 光电耦合器 Photo CouplerB11-024 光断续器 Photo InterrupterB11-025 光反射器 Photo ReflectorB11-026 光闸流晶体管 PhotocyristorsB11-027 光感测元件 Photosensing UnitsB11-028 内藏电路之光感测器Detectors with CircuitB11-029 民用用太阳电池Solar Cells for Consumer UseB11-030 产业用太阳电池Solar Cells for Power & Space UseB12 光纤及光缆:B12 光纤及光缆FIBER OPTIC FIBERS & CABLEB12-100 光纤FIBER OPTIC FIBERSB12-101 石英系多模态步阶式折射率型光纤Fiber Optic Fibers, Silica, Multimode, Step Index B12-102 石英系多模态渐近式折射率型光纤(50/125) Fiber Optic Fibers, Silica, Multimode, Graded Index,50/125B12-103 石英系多模态渐近式折射率型光纤(62.5/125) Fiber Optic Fibers, Silica, Multimode,Graded Index ,62.5/125B12-104 石英系多模态渐近式折射率型光纤(100/140) Fiber Optic Fibers, Silica, Multimode,Graded Index ,100/140B12-105 石英系单模态标准型光纤Fiber Optic Fibers, Silica, Single Mode,StandardB12-106 色散位移光纤Fiber Optic Fibers, Dispersion – ShiftedB12-107 偏振恒持光纤Fiber Optic Fibers, Polarization – MaintainingB12-108 其他单模态光纤Other Single Mode Optic FibersB12-109 石英系塑胶包覆光纤Fiber Optic Fibers, Plastic - Clad SilicaB12-110 塑胶光纤Fiber Optic Fibers, PlasticB12-111 石英系影像光纤Fiber Optic Bundles, Silica, ImagingB12-112 多成分影像光纤Fiber Optic Bundles, Non-silica, ImagingB12-113 光导管Fiber Optic LightguidesB12-199 其他集束光纤Other Fiber Optic BundlesB12-200 光缆FIBER OPTIC CABLEB12-201 单模态标准型松包悬空式光缆Fiber Optic Cable, Single Mode, Standard, Loosely Buffered, AerialB12-202 单模态标准型松包管路式光缆Fiber Optic Cable, Single Mode, Standard, Loosely Buffered, DuctB12-203 单模态标准型松包直埋式光缆Fiber Optic Cable, Single Mode, Standard, Loosely Buffered, Direct BuriedB12-204 单模态标准型紧包单心式光缆Fiber Optic Cable, Single Mode, Standard, Tightly Buffered, Single FiberB12-205 单模态标准型紧包多心式光缆Fiber Optic Cable, Single Mode, Standard, Tightly Buffered, MultifiberB12-206 光纤带 RibbonB12-207 色散位移光缆Fiber Optic Cable, Dispersion-ShiftedB12-208 偏振恒持光缆Fiber Optic Cable, Polarization – MaintainingB12-209 其他单模态光缆Other Single Mode Fiber Optic CableB12-210 多模态石英系(50/125)光缆Fiber Optic Cable, Multimode, Silica, 50/125B12-211 多模态石英系(62.5/125)光缆Fiber Optic Cable, Multimode, Silica, 62.5/125B12-212 多模态石英系(100/140)光缆Fiber Optic Cable, Multimode, Silica, 100/140B12-213 塑胶光缆Fiber Optic Cable, PlasticB12-214 石英系塑胶包覆光缆Fiber Optic Cable, Plastic-Clad SilicaB12-215 其他多模态光缆Other Multimode Fiber Optic CableB12-216 光纤保护用管Protect Tubes for Fiber Optic FiberB13 光被动元件/光控制元件:B13 光被动元件/光控制元件OPTICAL PASSIVE DEVICES/CONTROL DEVICESB13-001 单模态ST光纤连接器Fiber Optic Connectors, Single Mode, STB13-002 单模态Biconic光纤连接器Fiber Optic Connectors, Single Mode, BiconicB13-003 单模态FC/PC光纤连接器Fiber Optic Connectors, Single Mode, FC/PCB13-004 单模态APC光纤连接器Fiber Optic Connectors, Single Mode, APCB13-005 单模态FDDI光纤连接器Fiber Optic Connectors, Single Mode, FDDIB13-006 单模态SC光纤连接器Fiber Optic Connectors, Single Mode, SCB13-007 单模态D4光纤连接器Fiber Optic Connectors, Single Mode, D4B13-008 单模态光纤连接器插座(ST,FC/PC,SC,Biconic) Fiber Optic Connectors, Single Mode, Adapter(ST,FC/PC,SC,Biconic)B13-009 单模态多心光纤连接器(MT) Fiber Optic Connectors, Single Mode,Multi-Channel/MT B13-010 其他单模态光纤连接器Other Single Mode Fiber Optic ConnectorsB13-011 多模态ST光纤连接器Fiber Optic Connectors, Multimode, STB13-012 多模态FC/PC相容光纤连接器Fiber Optic Connectors, Multimode, FC/PCB13-013 多模态SMA光纤连接器Fiber Optic Connectors, Multimode, SMAB13-014 多模态FDDI光纤连接器Fiber Optic Connectors, Multimode, FDDIB13-015 多模态SC光纤连接器Fiber Optic Connectors, Multimode, SCB13-016 多模态D4光纤连接器Fiber Optic Connectors, Multimode, D4B13-017 多模态光纤连接器插座(ST,SMA,FC/PC) Fiber Optic Connectors,Multimode,Adapter(ST,SMA,FC/PC)B13-018 多模态多心光纤连接器Fiber Optic Connectors, Multimode, Multi-ChannelB13-019 其他多模态光纤连接器Other Multimode Fiber Optic ConnectorsB13-020 套筒 SleevesB13-021 金属箍(套管) Metal FerrulesB13-022 塑胶箍(套管) Plastic FerrulesB13-023 陶瓷箍(套管) Ceramic FerrulesB13-024 插座 ReceptaclesB13-025 插头 PlugsB13-026 光连接器(含光纤线) Optical Connectors with FiberB13-027 光纤耦合器(两分支) Optical Couplers, Tap/SplitterB13-028 光纤耦合器(树状分支) Optical Couplers, TreeB13-029 星状光纤耦合器(穿透形) Transmission Type Star Optical CouplersB13-030 星状光纤耦合器(反射形) Reflection Type Star Optical CouplersB13-031 其他光纤耦合器Other Optical CouplersB13-032 光分波合波器(两波长) Optical Couplers, WDM, Dual-WavelengthB13-033 光分波合波器(多波长) Optical Couplers, WDM, Over Two WavelengthB13-034 其他光分波合波器Other Optical WDM CouplersB13-035 光衰减器(固定) Fixed Optical AttenuatorsB13-036 光衰减器(可变) Adjustable Optical AttenuatorsB13-037 光隔离器(通信用) Optical Isolators for CommunicationB13-038 光隔离器(非通信用) Optical Isolators for Non-CommunicationB13-039 光环流器 Optical CirculatorsB13-040 光开关(机械式) Mechanical Optical SwitchesB13-041 光开关(非机械式) Non-mechanical Optical SwitchesB13-042 光纤光栅Fiber Bragg GratingB13-043 光移相器Optical Phase ShiftersB13-044 光共振器 Optical ResonatorsB13-045 空间调变元件Spatial Light ModulatorsB13-046 光影像转换元件(ITC) Incoherent to Coherent Devices(ITC)B13-047 光截波器,机械式光调变器Optical Choppers, Mechanical ModulatorsB13-048 磁光调变器 Maganeto-Optic ModulatorsB13-049 声光调变器 Acousto-Optic ModulatorsB13-050 电光调变器 Electro-Optic ModulatorsB13-051 波导形调变器,行波形调变器Optical Waveguide,Travelling-wave Modulators B13-052 类比/强度调变器 Analog/Intensity ModulatorsB13-053 数位调变器 Digital ModulatorsB13-054 其他调变器 Other ModulatorsB13-055 光弹性调变器 Photoelastic ModulatorsB13-056 机械式偏折/扫瞄器(Galvanometer方式) Mechanical OpticalDeflectors/Scanners(Galvanometer Mirror)B13-057 声光偏折/扫瞄器Acousto-Optic Optical Deflectors/ScannersB13-058 电光偏折/扫瞄器Electro-Optic Optical Deflectors/ScannersB13-059 机械式扫瞄器(回转多面镜方式) Mechanical Optical Scanners(Polygonal Mirrors) B13-060 机械式扫瞄器(全像方式) Mechanical Optical Scanners(Holographic)B13-061 光纤跳接线Fiber Optic Patchcord PigtailB13-062 光纤终端箱Fiber Optic Distribution BoxB13-063 光纤接续盒Fiber Optic ClosureB13-099 其他光被动元件/控制元件Other Optical Passive Devices/Control DevicesB14 积体光元件:B14 积体光元件INTEGRATED OPTICAL DEVICESB14-001 光IC Optical ICB14-002 OEIC Optoelectronic ICB14-099 其他光电元件 Other DevicesC01 光通讯设备:C01 光通讯设备OPTICAL COMMUNICATION EQUIPMENTC01-100 电信用光通讯设备 OPTICAL COMMUNICATIONEQUIPEMNT(TELECOMMUNICATION)C01-101 同步光纤网路光波传输系统及多工机设备Lightwave/Transimission System and Multiplexer Equipment (SONET-Based)C01-102 同步光纤网路光数位回路载波机设备Optical/Digital Loop Carrier Equipment (SONET-Based)C01-103 同步光纤网路数位交换连接系统设备Digital Cross Connect System Equipment (SONET-based)C01-104 同步数位阶层光波传输系统及多工机设备Lightwave/Transmission System and Multiplexer Equipment (SDH-Based)C01-105 同步数位阶层光数位回路载波机设备Optical/Digital Loop Carrier Equipment(SDH-Based)C01-106 同步数位阶层数位交换连接系统设备Digital Cross Connect System Equipment (SDH-Based)C01-107 光纤网路单体ONU(Optical Network Unit)C01-108 非同步光通讯设备Asynchronous Optical Communication EquipmentC01-199 其他公众用光通讯设备Other Optical Communication Equipment (Telecommunication)C01-200 数据通讯光纤网路设备OPTICAL DATA COMMUNICATION NETWORK EQUIPMENT (PREMISES)C01-201 光纤分散式资料介面网路设备FDDI Network EquipmentC01-202 非同步传输模式网路设备ATM Network EquipmentC01-203 高速乙太网路设备Fast Ethernet Network EquipmentC01-204 光纤通道 Fiber ChannelC01-299 其他用户光数据通讯设备Other Optical Data Communication Network Equipment (Premises)C01-300 特殊用途光传输设备OPTICAL TRANSMISSION EQUIPMENT(SPECIAL PURPOSE)C01-301 有线电视光传输设备Optical Transmission Equipment, CATVC01-302 视讯/闭路监视光传输设备Optical Transmission Equipment, Video/CCTVC01-303 量测/控制信号光传输设备Optical Transmission Equipment, Measure/ControlC01-304 空间(无线)光传输设备Optical Transmission Equipment, Spatial (Wireless)C01-305 光放大器 Optical AmplifierC01-399 其他特殊用途光传输设备Other Optical Transmission Equipment (Special Purpose)C02 光测仪器设备:C02 光测仪器设备OPTICAL MEASURING EQUIPMENTC02-001 量测用标准光源Standard/Stabilized Light SourcesC02-002 光功率计(热转换型) Thermal Conversion Type Optical Power MetersC02-003 光功率计(光电转换型) Photoelectric Conversion Type Optical Power MetersC02-004 光谱分析仪Optical Spectrum AnalyzersC02-005 光波长计Optical Wavelength MetersC02-006 光谱幅宽量测器Spectral Width Measuring EquipmentC02-007 光时域反射计(OTDR) Optical Time-Domain Reflectometers(OTDR)C02-008 基频传输特性检测器Baseband Frequency Characteristics Evaluation EquipmentC02-009 波长色散量测器Wavelength Dispersion Measuring EquipmentC02-010 光纤测试设备Optical Fiber Test EquipmentC02-011 激光光束波形量测器Laser Beam Profile Measuring EquipmentC02-012 光纤尺寸量测器Optical Fiber Sizes Measuring EquipmentC02-013 光纤模态参数测试器Optical Fiber Mode Field Parameters Test EquipmentC02-014 光纤强度测试器Optical Fiber Strength Test EquipmentC02-015 其他光纤相关量测设备Other Optical Fiber Measurement EquipmentC02-016 光连接器尺寸量测器Optical Connector Sizes Measuring EquipmentC02-017 光碟测定检查设备(装置用) Optical Disk Drive Inspection EquipmentC02-018 光碟测定检查设备(碟片用) Optical Disk Inspection EquipmentC02-019 光度计 PhotometersC02-020 复光束光度计,复光束量测器Double Beam PhotometersC02-021 测微光度计 MicrophotometersC02-022 感光密度计 DensitometersC02-023 光泽度计 GrossmetersC02-024 照度计 Illuminance MetersC02-025 测距仪 RangefindersC02-026 曝光计 Exposure MetersC02-027 辉度计 Luminance MetersC02-028 比色计 Comparison ColorimetersC02-029 色彩计(分光型) Spectral ColorimetersC02-030 色彩计(光电型) Photoelectric ColorimetersC02-031 积分球 Integrating SpheresC02-032 折射计 RefractometersC02-033 椭圆计 EllipsometersC02-034 偏振光镜 PolariscopesC02-035 偏振计 PolarimetersC02-036 比较量测器 ComparatorsC02-037 焦距仪 FocometersC02-038 球径计 SpheremetersC02-039 OTF(光学转换函数)设备Optical Transfer Function InstrumentationC02-040 MTF分析/量测装置Modulation Transfer Function(MTF) Analysis/Measurement EquipmentC02-041 投影检查器 Profile ProjectorsC02-042 自动准直仪 AutocollimatorsC02-043 光弹性机器 Photoelastic InstrumentsC02-099 其他光(学)量测器Other Optical Measurement EquipmentC03 分光镜、干涉仪:C03 分光镜、干涉仪 SPECTROSCOPES, INTERFEROMETERSC03-001 分光计 SpectrometersC03-002 单色器 MonochromatorsC03-003 分光镜,干涉分光镜,摄谱仪 Spectroscopes, Interference Spectroscopes,Spectrographs C03-004 分光光度计,分光测光器 SpectrophotometerC03-005 Michelson干涉仪 Michelson InterferometersC03-006 Tywman Green干涉仪Tywman Green InterferometersC03-007 Mach-Zehnder干涉仪 Mach-Zehnder InterferometersC03-008 Fizeau干涉仪 Fizeau InterferometersC03-009 Fabry-Perot干涉仪 Fabry-Perot InterferometersC04 显微镜,望远镜,照像机:C04 显微镜,望远镜,照像机MICROSCOPES, TELESCOPES, CAMERASC04-001 放大镜 MagnifiersC04-002 单接物镜双眼显微镜 Binocular MicroscopesC04-003 双眼实体显微镜,立体显微镜 Stereo MicroscopesC04-004 金属显微镜 Metallurgical MicroscopesC04-005 偏光显微镜 Polarizing MicroscopesC04-006 相位差显微镜 Phase-Contrast MicroscpoesC04-007 干涉显微镜,微分干涉对比显微镜 Interferences/Differential Interference Contrast MicroscopesC04-008 萤光显微镜 Fluorescence MicroscopesC04-009 激光显微镜 Laser MicroscopesC04-010 量测用显微镜,工具显微镜 Measurement MicroscopesC04-011 显微镜光度计 Microscope PhotometersC04-012 折射望远镜,Galilean望远镜Galilean Refracting TelescopesC04-013 反射望远镜 Reflecting TelescopesC04-014 反射折射望远镜 Catadioptric TelescopesC04-015 35mm焦平面自动对焦相机35mm AF Focal Plane CamerasC04-016 35mm焦平面手动对焦相机35mm NON-AF Focal Plane CamerasC04-017 35mm镜头快门多焦点相机35mm Multi Focal Points Lens Shutter CamerasC04-018 35mm镜头快门单焦点相机35mm Single Focal Point Lens Shutter CamerasC04-019 中,大型照相机Medium and Large Size CamerasC04-020 VTR摄影机 VTR CamerasC04-021 电视摄影机 TV CamerasC04-022 高画质电视摄影机High Definition(HDTV) CamerasC04-023 CCTV摄影机 CCTV CamerasC04-024 全像照像机 Holographic CamerasC04-025 眼镜 EyeglassesC04-026 夜视设备Night Vision EquipmentC04-027 照像机用之日期显示模组 Date moduleC04-028 照像机用之底片计数器 Film counterC04-029 APS相机 APS CamerasC05 光感测器:C05 光感测器 OPTICAL SENSORSC05-001 光电开关,光电感测器Photo Switches, Photo SensorsC05-002 标记感测器Mark Photo SensorsC05-003 色彩标记感测器Color Mark Photo SensorsC05-004 色彩感测器Color Photo SensorsC05-005 光学式编码器,角度感测器Optical Encoders, Angle SensorsC05-006 光遥控器Optical Remote Control EquipmentC05-007 影像感测器式量测设备Image Sensor Type Measurement InstrumentsC05-008 显微镜式量测设备Microscope Type Measurement InstrumentsC05-009 精密长度干涉仪Precise Length InterferometersC05-010 光波测距装置Electronic Distance MetersC05-011 三角测量法距离感测器Triangulation Distance MetersC05-012 激光调变测距方式距离感测器Laser Modulation Distance MetersC05-013 脉冲测距方式距离感测器Pulse Distance MetersC05-014 激光外径测定器Laser Outer Diameter Measuring SensorsC05-015 激光厚度计Laser Thickness GaugesC05-016 激光拉伸计Laser Extension MeterC05-017 红外线厚度计Infrared Thickness GaugesC05-018 水平仪 LevelsC05-019 激光水平仪 Laser LevelsC05-020 经纬仪 Theodlites/TransitsC05-021 激光经纬仪 Laser Theodlites/TransitsC05-022 激光标线设备Laser Marking-off EquipmentC05-023 位置光电感测器Position Sensors, Pattern Edge SensorsC05-024 半导体位置感测器Position Sensitive Devices(PSDs)C05-025 激光指示器 Laser PointersC05-026 激光都卜勒测速计Laser Doppler VelocimetersC05-027 环形激光流速计,光纤陀螺仪Ring Laser Velocimeters, Optical Fiber Laser Gyros C05-028 转速仪Rotational Speed MetersC05-029 激光都卜勒转速仪Laser Doppler Rotational Speed MetersC05-030 全像方式图样量测设备Holographic Method Pattern Measurement EquipmentsC05-031 激光移位计Laser Displacement MetersC05-032 激光指纹检测器Laser Fingerprint DetectorsC05-033 光学水质污染检测设备Optical Water Pollution Measurement and Detection EquipmentC05-034 光学大气污染检测设备Optical Air Pollution Measurement and Detection Equipment C05-035 红外线气体浓度感测器Infrared Gas Density MetersC05-036 光电式烟检知器Photo Smoke DetectorsC05-037 激光粉尘监视器,粒径量测器Laser Dust MonitorsC05-038 距离测定用激光雷达Rang-finding Lidar SystemsC05-039 环境监测用激光雷达Environment Monitoring Lidar SystemsC05-040 激光表面检查设备Laser Surface Inspection EquipmentC05-041 平面度测定系统 Flatness TestersC05-042 斑点图形量测设备Speckle Method Pattern Measurement EquipmentC05-043 云纹图形量测设备Moire Method Pattern Measurement EquipmentC05-044 影像分析仪 Image AnalyzersC05-045 激光缺陷检查设备Laser Defect Inspection EquipmentC05-046 红外线辐射温度感测器 Infrared ThermometersC05-047 人体检知感测器,激光保全设备Laser Security/Surveillance EquipmentsC05-048 光计数器 Photo CountersC05-049 激光公害检测设备Laser Pollution Detective DevicesC05-050 激光热常数量测设备Laser Thermal Constants Measurement EquipmentC05-051 全像非破坏检查设备Holographic Nondestructive Testing EquipmentC06 光纤感测器:C06 光纤感测器FIBER OPTIC SENSORSC06-001 光纤光电开关/感测器Fiber Optic Photo Switches/ SensorsC06-002 光纤式标记感测器Fiber Optic Mark Photo SensorsC06-003 光纤式色彩标记感测器Fiber Optic Color Mark Photo SensorsC06-004 光纤温度感测器Fiber Optic Temperature SensorsC06-005 光纤压力感测器Fiber Optic Pressure SensorsC06-006 光纤声波感测器Fiber Optic Acoustic SensorsC06-007 光纤变形感测器Fiber Optic Strain SensorsC06-008 光纤振动感测器Fiber Optic Vibration SensorsC06-009 光纤移位感测器Fiber Optic Displacement SensorsC06-010 光纤陀螺仪感测器Fiber Optic Gyro SensorsC06-011 光纤速度感测器Fiber Optic Velocity SensorsC06-012 光纤磁通量感测器Fiber Optic Magnetic Flux SensorsC06-013 光纤磁场感测器Fiber Optic Magnetic Field SensorsC06-014 光纤电流感测器Fiber Optic Current SensorsC06-015 光纤电场感测器Fiber Optic Electric Field SensorsC06-016 光纤浓度、成份感测器Fiber Optic Density,Constituent SensorsC06-017 光纤油膜感测器Fiber Optic Oil Film SensorsC06-018 光纤液位感测器Fiber Optic Liquid Surface Level SensorsC06-019 光纤光分布/放射线感测器Fiber Optic Light Distribution/Radiation Sensors C06-020 光纤显微镜Fiber Optic FiberscopesC06-021 光纤光栅应变感测器Fiber Grating Strain SensorC07 光储存装置:C07 光储存装置OPTICAL STORAGE PRODUCTC07-100 消费性光碟机CONSUMER OPTICAL DISC PLAYERSC07-101 激光唱盘Compact Disc (CD) PlayersC07-102 激光音响组合Products Incorporated CD(CD-Radio-Cassette Tape Recorders) C07-103 LD 影碟机Laser Disc (LD) PlayersC07-104 影音光碟机Video CD PlayersC07-105 DVD DVD 影碟机Digital Versatile Disc (DVD) PlayersC07-106 迷你音碟机Mini Disc (MD) PlayersC07-200 资讯用仅读型光碟机READ-ONLY OPTICAL DISC DRI597VESC07-201 CD-ROMCD-ROM光碟机 CD-ROM DrivesC07-202 DVD-ROM DVD-ROM 光碟机 DVD-ROM DrivesC07-300 资讯用仅写一次型光碟机RECORDABLE OPTICAL DISC DRIVESC07-301 CD-R CD-R 光碟机 CD-R DrivesC07-399 其他仅写一次型光碟机Other Recordable Optical Disc DrivesC07-400 资讯用可覆写型光碟机REWRITABLE OPTICAL DISC DRIVESC07-401 3.5" MO 光碟机3.5" MO Disc DrivesC07-402 5.25" MO 光碟机5.25" MO Disc DrivesC07-403 PD 光碟机 PD DrivesC07-404 CD-RW光碟机 CD-RW DrivesC07-499 其他可覆写型光碟机Other Rewritable Optical Disc DrivesC07-500 光碟机零组件DEVICES OF OPTICAL DISC DRIVESC07-501 光学头,光学读取头Optical Heads , Pick-up HeadsC07-502 光学头伺服装置,伺服用IC模组Optical Head Controllers, Control ICs/Modules C07-503 光学头驱动装置Optical Head ServomotorsC07-504 光碟匣Optical Disc CartridgesC07-505 主轴马达 Spindle MotorC07-600 光碟片 OPTICAL DISCSC07-601 CD 音碟片 Compact DiscsC07-602 LD 影碟片 Laser DiscsC07-603 影音光碟片 Video CDsC07-604 DVD光碟片Digital Versatile Discs : DVDsC07-605 迷你音碟片Mini Discs : MDsC07-606 CD-ROM 光碟片 CD-ROMsC07-607 DVD-ROM光碟片 DVD-ROMsC07-608 CD-R 光碟片 CD-RsC07-609 其他可写仅读型光碟片Other Recordable Optical DiscsC07-610 3.5" MO 光碟片3.5" MO DiscsC07-011 5.25" MO 光碟片5.25" MO DiscsC07-612 PD 光碟片 PD DiscsC07-613 CD-RW 光碟片 CD-RW DiscsC07-699 其他可复写型光碟片Other Rewritable Optical DiscsC08 光输出入装置:C08 光输出入装置OPTICAL INPUT &OUTPUT DEVICESC08-100 数位相机Digital Still CameraC08-200 光学印表机 OPTICAL PRINTERSC08-201 彩色激光印表机Laser Color PrintersC08-202 单色激光印表机Laser Monochrome PrintersC08-203 彩色LED印表机LED Color PrintersC08-204 单色LED印表机LED Monochrome PrintersC08-299 其他光学式印表机Other Optical PrintersC08-300 影印机 COPY MACHINESC08-301 彩色激光数位影印机Laser Digital Color Copy MachinesC08-302 单色激光数位影印机Laser Digital Monochrome Copy MachinesC08-400 传真机 FACSIMILESC08-401 热感纸传真机Termal Paper FacsimilesC08-402 热转写传真机Thermal Transfer FaxC08-403 喷墨普通纸传真机Inkjet Plain Paper FacsimilesC08-404 发光二极体传真机 LED FacsimilesC08-405 激光传真机 Laser FacsimilesC08-406 多功能复合事务机Multi-Function Products (MFPs)C08-500 条码扫描器BAR CODE SCANNERSC08-501 手持式激光条码扫描器Hand-Held Laser Bar Code ScannersC08-502 固定式激光条码扫描器Fixed Laser Bar Code ScannersC08-503 CCD 条码扫描器CCD Bar Code Scanners。
尿素与苯胺合成N,N硕士论文

湘潭大学硕士学位论文尿素与苯胺合成N,N-二苯基脲的清洁生产工艺研究姓名:游志敏申请学位级别:硕士专业:环境工程指导教师:戴友芝李会泉20080519 尿素与苯胺合成N N’-二苯基脲的清洁生产工艺研究摘要N N′-二苯基脲是一种重要的有机中间体,主要用于制备磺胺类药物和异氰酸酯等化学品。
其传统合成方法以剧毒光气为直接或间接原料,易造成重大环境污染和生态危害,必将被淘汰。
作为光气法替代工艺而开发的工业尿素法,以尿素和苯胺为原料,水为溶剂,盐酸为催化剂,N N′-二苯基脲产率只有40%左右,废物产量大,排放大量苯胺废水和氯化氢废气,后处理困难,环境效益低。
由于尿素价格低廉,又是重要的CO2 资源化工业产品,因此,尿素法生产N N′-二苯基脲具有较高的经济和环保开发潜能。
然而,如何达到原料最大化利用、废物最小化或零排放,还有一些值得环保工作者深入研究的问题。
本文以尿素和苯胺为原料,研究了在非水溶剂、非盐酸催化体系中合成NN′-二苯基脲的工艺;在此基础上,进一步研究了无溶剂非催化合成N N′-二苯基脲的清洁工艺及其强化方法,并对反应机理进行了探讨。
主要研究内容和结果如下:(1)比较研究了在不同溶剂体系中和不同催化剂作用下尿素与苯胺合成NN′-二苯基脲的产率,发现二甲苯为溶剂具有副反应少、产率较高的特点,乙酸锌和对甲苯磺酸-乙酸铅复合催化剂具有最好的催化活性。
最佳工艺条件为:n苯胺:n 尿素: n二甲苯3: 1: 2,乙酸锌和对甲苯磺酸-乙酸铅催化,144 ℃反应 1.5 h,N N′-二苯基脲产率分别为94.0%和92.9%。
(2)研究了无溶剂非催化条件下,以尿素与苯胺合成N N′-二苯基脲的清洁工艺,考察了物料比、温度、时间、搅拌速率等因素对产率的影响。
产物的红外、核磁共振、质谱、紫外等分析结果表明,反应得到的产物是N N′-二苯基脲。
当苯胺与尿素摩尔比为5: 1 时,180 ℃,反应1.5 h,N N′-二苯基脲产率达到92.1%。
消防安全知识资料英文

消防安全知识资料英文Fire Safety KnowledgeIntroduction:Fire safety is a crucial aspect of our daily lives, as fires can rapidly spread and cause significant damage to property and endanger lives. Understanding fire safety measures and implementing them correctly can greatly reduce the risk of fire incidents and ensure the safety of individuals and communities. This article aims to provide comprehensive knowledge on fire safety, including fire prevention, evacuation procedures, and the correct usage of fire extinguishers.Section 1: Fire Preventiona) Tips for Fire Prevention at Home:1. Keep flammable materials away from heat sources: Store flammable liquids such as gasoline, propane, and paint in a cool and well-ventilated area away from any heat sources.2. Properly dispose of cigarette butts: Ensure that cigarette butts are completely extinguished and dispose of them in designated containers. Do not discard them in plant pots, garbage bins, or on the ground.3. Install smoke detectors and regularly test them: Place smoke detectors in every room and regularly check their batteries to ensure they are in good working condition. Test these alarms every month to ensure they are functioning properly.4. Practice safe cooking habits: Never leave cooking unattended. Keep flammable objects, such as towels and curtains, away from the stove. Install a fire extinguisher in the kitchen for immediate use in case of a small fire.5. Electrical safety precautions: Use electrical appliances, cords, and outlets correctly. Do not overload outlets or extension cords, and replace damaged cords immediately. Avoid using multiple adapters or extension cords plugged into one outlet.b) Fire Prevention in Public Places:1. Follow the "No Smoking" policy: Respect and adhere to "No Smoking" signs in public places, including restaurants, theaters, airports, and government buildings.2. Familiarize yourself with emergency exits: Whenever you entera public building, take note of the nearest emergency exits and escape routes. In case of a fire, follow these routes to evacuate safely.3. Report fire hazards: If you notice any potential fire hazards in public areas, promptly report them to the relevant authorities to ensure quick action is taken.4. Regular maintenance of fire safety equipment: Public places should have fire safety equipment, such as fire alarms, sprinkler systems, and fire extinguishers. Ensure that these are frequently inspected and in good working condition.Section 2: Evacuation Proceduresa) Home Evacuation Plan:1. Create a fire escape plan: Develop a fire escape plan for your home, identifying primary and secondary escape routes. Practice this plan with all family members regularly.2. Know the location of fire exits: Become familiar with the locations of all exits in your home, including windows and doors. Keep these pathways clear of obstacles for quick and easy evacuation.3. Establish a meeting point: Choose a safe location outside your home where all family members can meet after evacuating. This helps ensure everyone has escaped and assists in accounting for all individuals during an emergency.4. Call emergency services: Once outside, immediately call the emergency services (fire department) using your mobile phone or a neighbor's phone. Provide them with your location and details of the incident.b) Evacuation in Public Places:1. Stay calm and follow instructions: In the event of a fire alarm or emergency announcement, remain calm and listen carefully to instructions provided by public address system operators or emergency response personnel.2. Emergency exits should be prioritized: When evacuating a public place, prioritize the use of emergency exits rather than elevators or escalators.3. Help the elderly or disabled: If you are able, assist elderly or disabled individuals in reaching safety. Offer guidance and support throughout the evacuation process.4. Stay low when necessary: If the area you are in is filled with smoke, crawl on your hands and knees to minimize inhalation of smoke. Cover your mouth and nose with a cloth if possible.Section 3: Using Fire Extinguishersa) Understanding Different Types of Fire Extinguishers:1. Water fire extinguishers (Class A): These are primarily for fires involving paper, wood, textiles, and other organic materials.2. Carbon dioxide (CO2) fire extinguishers (Class B and C): These are suitable for fires involving flammable liquids, such as oil and gasoline, as well as electrical fires.3. Dry powder fire extinguishers (Class D): These are designed for fires involving metals, such as magnesium, lithium, and titanium.4. Foam fire extinguishers (Class A and B): These are effective for fires involving flammable liquids and solid combustibles.b) Proper Usage of Fire Extinguishers:1. Assess the situation: Before attempting to use a fire extinguisher, evaluate if it is safe to do so. If the fire is spreading rapidly or blocking your escape route, evacuate immediately and call emergency services.2. Remember the PASS technique: Pull the pin, aim the nozzle at the base of the fire, squeeze the handle, and sweep the nozzle from side to side to extinguish the flames.3. Keep a safe distance: Stand at least 6 to 8 feet away from the fire while using the fire extinguisher. This will prevent you from being exposed to heat and flames.4. Evacuate if necessary: If the fire grows too large or becomes unmanageable, abandon your efforts and evacuate immediately. Your safety is the highest priority.Conclusion:Fire safety is everyone's responsibility. By following proper fire prevention measures, being aware of evacuation procedures, and learning how to use fire extinguishers correctly, individuals can minimize the risk of fires and ensure the safety of themselves and those around them. Always remember that early detection, quick action, and a calm approach are the key factors in successfully preventing and managing fire incidents.。
化工进展标准关键词库
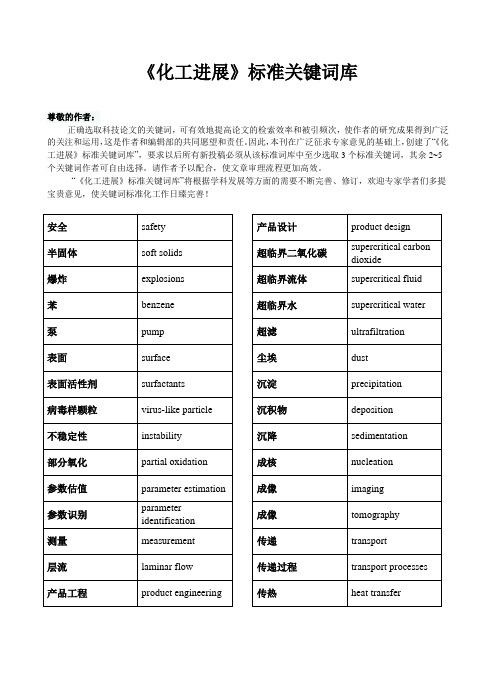
《化工进展》标准关键词库尊敬的作者:正确选取科技论文的关键词,可有效地提高论文的检索效率和被引频次,使作者的研究成果得到广泛的关注和运用,这是作者和编辑部的共同愿望和责任。
因此,本刊在广泛征求专家意见的基础上,创建了“《化工进展》标准关键词库”,要求以后所有新投稿必须从该标准词库中至少选取3个标准关键词,其余2~5个关键词作者可自由选择。
请作者予以配合,使文章审理流程更加高效。
“《化工进展》标准关键词库”将根据学科发展等方面的需要不断完善、修订,欢迎专家学者们多提宝贵意见,使关键词标准化工作日臻完善!安全safety半固体soft solids爆炸explosions苯benzene泵pump表面surface表面活性剂surfactants病毒样颗粒virus-like particle不稳定性instability部分氧化partial oxidation参数估值parameter estimation参数识别parameter identification测量measurement层流laminar flow产品工程product engineering 产品设计product design超临界二氧化碳supercritical carbondioxide超临界流体supercritical fluid 超临界水supercritical water 超滤ultrafiltration尘埃dust沉淀precipitation沉积物deposition沉降sedimentation成核nucleation成像imaging成像tomography传递transport传递过程transport processes 传热heat transfer传质mass transfer纯化purification醇alcohol催化(作用)catalysis催化剂catalyst催化剂活化catalyst activation催化剂载体catalyst support萃取extraction存埋sequestration代谢metabolism单克隆抗体monoclonal antibody 弹性elasticity蛋白质protein蛋白质变性protein denaturation 蛋白质复性protein refolding蛋白质稳定性protein stability滴流床反应器trickle-bed reactor 电化学electrochemistry电解electrolysis电解质electrolytes电渗透electro-osmosis 电泳electrophoresis电子材料electronic materials 动力效应模型DEM动力学kinetics动力学理论kinetic theory动力学模型kinetic modeling动量传递momentum transfer 动态仿真dynamic simulation 动态建模dynamic modeling 动态学dynamics对流convection多尺度multiscale多孔介质porous media多相反应multiphase reaction 多相反应器multiphase reactor 多相流multiphase flow二氧化硅silica二氧化碳carbon dioxide二氧化碳捕集CO2 capture二元混合物binary mixture发酵fermentation反应reaction反应动力学reaction kinetics反应工程reaction engineering 反应精馏reactive distillation 反应器reactors放大scale-up非牛顿流体non-Newtonian fluids非线性动力学nonlinear dynamics 废水waste water废物处理waste treatment沸石zeolite分布distributions分离separation分立元件建模discrete element modeling分散,分散系dispersion 分形fractals分子工程molecular engineering分子合成molecular synthesis 分子模拟molecular simulation 分子筛moleclar sieves分子生物学molecular biology 粉碎crushing粉体powders粉体技术powder technology 风能wind energy浮选flotation辐射radiation腐蚀corrosion复合材料composites复杂流体?complex fluids干燥drying公式化formulation共沸(混合)物azeotrope鼓泡反应器bubble columnreactor鼓泡塔bubble column固定床fixed-bed固定化immobilization固体力学solid mechanics光化学photochemistry过程控制process control过程系统process systems过渡transition过滤filtration焓enthalpy合成synthesis合成气syngas合成生物学synthetic biology 核磁共振NMR化学反应chemical reaction 化学反应器chemical reactors 化学分析chemical analysis 化学过程chemical processes 还原reduction环境environment回收recovery混沌chaos混合blend混合mixing混合物mixtures混凝coagulation活度系数activity coefficient 活化(作用)activation活性reactivity 活性碳activated carbon火用exergy机械性能mechanicalproperties集成integration挤出extrusion计算化学computationalchemistry计算机模拟computer simulation 计算流体力学computational fluiddynamics,CFD加工制造fabrication加氢hydrogenation加氢脱硫HDS甲烷methane间歇式batchwise浆料slurry降解degradation胶体colloid焦化coking搅拌容器stirred vessel结垢fouling结晶crystallization解吸,脱附desorption介尺度mesoscale界面interface界面流变学interfacial rheology 界面张力interfacial tension 浸取leaching经济economics静态混合器static mixer聚合polymerization聚合物polymers聚合物加工polymer processing 聚集(作用)aggregation聚结coalescence均化(作用)homogenization抗体antibody颗粒过程particulate processes 颗粒流granular flow颗粒物料granular materials 可持续性sustainability空隙率voidage控制control扩散diffusion 离析segregation离心分离centrifugation离子交换ion exchange离子液体ionic liquids粒度分布particle sizedistribution粒度分布size distribution 粒子particle粒子图像测速PIV粒子形成particle formation 两相流two-phase flow 流变学rheology流动flow流化床fluidized-bed流态化fluidization流体动力学hydrodynamics 流体力学fluid mechanics 流域flow regimes煤燃烧coal combustion 酶enzyme蒙特卡罗模拟Monte Carlosimulation模拟simulation模塑Molding模型model模型简化model reduction模型预测控制model-predictive control模制moulding膜film膜membranes磨损attrition纳滤nanofiltration 纳米材料nanomaterials 纳米技术nanotechnology 纳米结构nanostructure 纳米粒子nanoparticles 黏度viscosity凝胶gels凝结condensation 排水drainage泡沫foam配合物complexes平衡equilibrium曝气aeration 气含率gas holdup气化gasification气力输送pneumaticconveying气泡bubble气溶胶aerosol气体gas气液两相流gas-liquid flow 汽化vaporization汽液平衡vapor liquidequilibria氢Hydrogen燃料fuel燃料电池fuel cells热传导heat conduction 热解pyrolysis热力学thermodynamics 热力学过程thermodynamicsprocess热力学性质thermodynamicproperties溶剂solvents溶剂萃取solvent extraction 溶解dissolution溶解性solubility溶液solution乳液emulsions色谱chromatography 熵entropy上升管riser烧结sintering设计design神经网络neural networks 渗透permeation渗透率permeability渗透蒸发pervaporation 生产production生化工程biochemical engineering生物柴油biodiesel生物催化biocatalysis 生物反应器bioreactors 生物分离bioseparation生物分子工程biomolecular engineering生物工程biological engineering生物过程bioprocess生物技术biotechnology 生物模板biotemplating生物膜biofilm生物能源bioenergy生物燃料biofuel生物医学工程biomedicalengineering生物质biomass失活deactivation石油petroleum实验验证experimentalvalidation食品加工food processing数学模拟mathematicalmodeling数值分析Numerical analysis 数值模拟numerical simulation 水合物hydrate水解hydrolysis水热hydrothermal水溶液aqueous solution瞬态响应transient response算法algorithm塔器column太阳能solar energy肽peptide碳氢化合物hydrocarbons 天然气natural gas 填充床packed bed停留时间分布residence time distribution统计热力学statistical thermodynamics透析dialysis湍动turbulence湍流turbulent flow 团聚agglomeration 脱盐desalination脱氧核糖核酸DNA烷烃alkane微尺度microscale微电子学microelectronics 微反应器microreactor微流体学microfluidics微通道microchannels 温室气体greenhouse gas 稳定性stability稳态steady state 污染pollution吸附(作用)adsorption吸附剂adsorbents吸附剂sorbents吸收absorption系统工程systems engineering 细胞工程cell engineering细胞生物学cell biology下游加工过程downstreamprocessing显微结构microstructure相变phase change相平衡phase equilibria形态学morphology修复remediation需氧aerobic悬浮系suspensions选择催化还原SCR选择性selectivity循环流化床circulating fluidizedbed压缩机compressor烟道气flue gas厌氧anaerobic氧化oxidation氧化铝alumina药物pharmaceuticals液化liquefaction一氧化碳carbon monoxide 仪器,仪表instrumentation移动床moving bed遗传算法genetic algorithm 优化optimization优化设计optimal design有机化合物organic compounds 预测prediction载体support再生regeneration再生能源renewable energy 造粒granulation增湿humidification 蒸发evaporation蒸馏distillation整体器件monolith整体优化global optimization 酯化esterification制备preparation制氢hydrogen production 制造manufacture种群平衡population balance 种群平衡公式population balanceequations主元分析principal componentanalysis状态方程equation of state自催化autocatalysis自由基radical组织工程学tissue engineering。
211051752_咪唑鎓溴盐催化CO2与氧化苯乙烯的环加成反应

4
5
6
7
8
0 5
3
3
3
3
4
100
100
100
90
80
100
碳酸苯乙烯酯产率 / %
化合物 1
化合物 2
24
18
54
24
83
86
20
24
24
24
0
40
70
78
2
72
注:反应条件为氧化苯乙烯 6 8 mmolꎬCO2 球压.
0
68
62
21
1
81
化合物 1 和化合物 2 催化 CO2 与氧化苯乙
3 87 ( sꎬ 6H ) IR ( KBr ) ꎬ σ / cm - 1 : 3426ꎬ 3009ꎬ
2 00 g) 、 N - 溴 代 丁 二 酰 亚 胺 ( NBS ) ( 0 01
mmolꎬ0 04 g) 加入到 50 mL 的 CCl4 溶液中ꎬ加
( sꎬ1H) ꎬ8 46( dꎬJ = 1 65 Hzꎬ1H) ꎬ8 41 ( dꎬJ =
咪唑鎓溴盐催化 CO2 与氧化苯乙烯的环加成反应
郭 杰ꎬ 徐 颖ꎬ 李金融ꎬ 由立新ꎬ 孙亚光
( 沈阳化工大学 理学院ꎬ 辽宁 沈阳 110142)
摘 要: 采用 4 - 甲基间苯二甲酸、三甲基硅咪唑和苯并咪唑为原料ꎬ设计合成了两种咪唑鎓溴
盐ꎬ即 1ꎬ3 - 双(3ꎬ5 - 二甲氧羰基苯甲基) 咪唑鎓溴盐( 化合物 1) 和 1ꎬ3 - 双( 二甲氧羰基苯甲基)
应时间为 24 h 时ꎬ碳酸苯乙烯酯产率达到最高
剂催 化 所 得 碳 酸 苯 乙 烯 酯 产 率 均 有 所 降 低
咪唑类离子液体分析测试方法汇总
咪唑类离子液体分析测试方法汇总(1)反相高效液相色谱法测定离子液体及其中的高沸点有机物姜晓辉,孙学文,赵锁奇,等. 反相高效液相色谱法测定离子液体及其中的高沸点有机物[J]. 分析测试技术与仪器,2006,12(4):195-198摘要: 建立了反相键合相液相色谱分析离子液体咪唑类离子液体[bmim]PF6、[bmim]BF4、吡啶类离子液体[bupy]BF4的纯度及其中高沸点有机物的方法.以缓冲溶液控制流动相pH值,显著改善了峰形.保留时间定性,外标法定量.关键词: 离子液体;高沸点有机物;高效液相色谱法离子液体[1]也称室温融盐,是近年来新兴的溶剂.一些有关离子液体相平衡的基础数据[2~4],主要是通过紫外分光光度法[5]和折射率法测得的[6],这两种方法各有一定的局限性.另外,如何测定离子液体的纯度,目前也尚无简便可靠的方法.本文建立了在离子液体与杂质,高沸点有机物与离子液体完全分离的情况下测定离子液体及其中的高沸点有机物含量的高效液相色谱分析方法,比现有的两种方法具有更高的准确度,更短的分析时间.参考文献:[1] Welton T. Room-temperature ionic liquids: solvents for synthesis and catalysis[J]. Chem Rev, 1999, 99:2 071-2 083.[2] Blanchard L A, Hancu D, Beckman E J,etal. Green processing using ionic liquids and CO2[J]. Nature(London), 1999, 399: 28-29.[3] Huddleston J G, Willauer H D, Swatloski R P,et al. Room temperature ionic liquids as novel media for 'clean' liquid2liquidextraction[J]. Chem Commun,1998, (16): 1 765-1 766.[4] Blanchard L A, Hancu D, Beckman E J,etal. Green processing using ionic liquids and CO2[J]. Nature(London), 1999, 399: 28-29.[5] Lynnette A Blanchard, Joan F Brennecke. Recovery of organic products from ionic liquids using supercritical carbon dioxide[J].Ind Eng Chem Res,2001; 30: 287-437.[6] 叶天旭,张予辉,刘金河,等.烷基咪唑氟硼酸盐离子液体的合成与溶剂性质研究[J].石油大学学报(自然科学版),2004,28(4):105-107.(2)反相高效液相色谱法直接测定离子液体中咪唑杂质含量薛洪宝,马春辉,刘庆彬,等. 反相高效液相色谱法直接测定离子液体中咪唑杂质含量[J]广东化工,2006,33(12): 83-85 [摘要]研究了高效液相色谱法测定离子液体中的杂质(4-甲基咪唑)含量的测定方法。
超临界二氧化碳中合成环碳酸酯的催化剂研究进展
CHEMICAL INDUSTRY AND ENGINEERING PROGRESS 2017年第36卷第8期·2924·化 工 进展超临界二氧化碳中合成环碳酸酯的催化剂研究进展范芳君,张治国,邢华斌,杨启炜,鲍宗必,杨亦文,任其龙(浙江大学化学工程与生物工程学院,生物质化工教育部重点实验室,浙江 杭州 310027)摘要:超临界二氧化碳(scCO 2)是一种环境友好型溶剂,它作为传统有机溶剂的替代品已被广泛应用于绿色化学过程的开发。
其中,环氧化合物在超临界二氧化碳中制备环碳酸酯是实现二氧化碳高附加值转化的有效途径之一。
本文回顾了近年来在超临界二氧化碳中合成环碳酸酯的研究进展,着重介绍了不同种类的催化剂,包括金属配合物、季盐、二元催化体系、离子液体、金属氧化物、有机小分子及其他类型催化剂在该反应体系中的应用,突出了超临界二氧化碳既作为溶剂,又作为反应物的双重优势。
从经济和环境角度考虑,离子液体或季盐等有机催化剂具有更好的工业化应用前景。
同时,指出了高效和绿色催化体系设计和创制是该研究领域的关键。
关键词:超临界二氧化碳;环碳酸酯;环氧化物;催化中图分类号:TQ072 文献标志码:A 文章编号:1000–6613(2017)08–2924–10 DOI :10.16085/j.issn.1000-6613.2016-2446Progress in synthesis of cyclic carbonates under supercritical carbondioxideF AN Fangjun ,ZHANG Zhiguo ,XING Huabing ,YANG Qiwei ,BAO Zongbi ,YANG Yiwen ,REN Qilong(Key Laboratory of Biomass Chemical Engineering of Ministry of Education ,College of Chemical and BiologicalEngineering ,Zhejiang University ,Hangzhou 310027,Zhejiang ,China )Abstract :Supercritical carbon dioxide (scCO 2)has been widely used as an environmentally friendly solvent to replace the conventional organic solvent in green chemical processes. Among those applications reported ,the synthesis of cyclic carbonates from epoxides in supercritical CO 2 has received a great interest. This review covered the recent progress in the synthesis of cyclic carbonates under supercritical carbon dioxide ,with an emphasis on the state of the art of different kinds of catalysts employed in this reaction ,including metal complex ,quaternary onium salts ,binary catalytic systems ,ionic liquids ,metal oxide ,organocatalysts ,et al. Furthermore ,the advantages of supercritical carbon dioxide both as solvent and as reactant for this transformation were highlighted. From the economic and environmental point of view ,organic catalysts ,such as ionic liquids and quaternary onium salts ,show promising potential application in the future development of chemical industry. It was also pointed out that the design and preparation of efficient and green catalysts are critical to this research field. Key words :supercritical carbon dioxide ;epoxides ;cyclic carbonates ;catalysisCO 2性质稳定,无毒,不燃,价廉易得,是主要的温室气体之一,同时也是目前地球上储量最为丰富的C 1资源[1-2]。
功能化离子液体用于CO2吸收和分离的研究进展
功能化离子液体用于CO2吸收和分离的研究进展李翠娜;贺高红;李祥村;李皓;赵薇【期刊名称】《化工进展》【年(卷),期】2011(30)4【摘要】Ionic liquids can be readily modified structurally to have desired chemical and physical properties by the incorporation of specific functional groups. Functionalized ionic liquid can be used in separation, electrochemistry, catalysis, organic synthesis and other fields, and has become a hot research subject in the ionic liquid area. In this paper, research progress of functionalized ionic liquid with fluoroalkyl, amine and other polar groups for CO2 absorption is summarized, and the CO2 separation performance of supported functionalized ionic liquid membranes is also introduced. The problems to be resolved in the future researches are presented.%离子液体的结构具有高度可调性,可通过改变阴阳离子的结构改变其物理、化学性能,实现离子液体的功能化.功能化离子液体可用于分离、电化学、催化剂.有机合成等方面,已成为离子液体领域的研究热点.本文综述了含氟、含氨基和其它极性基团的功能化离子液体吸收CO2的研究进展,介绍了功能化离子液体支撑液膜分离CO2的研究,指出了今后研究需解决的问题.【总页数】6页(P709-714)【作者】李翠娜;贺高红;李祥村;李皓;赵薇【作者单位】大连理工大学精细化工国家重点实验室,膜科学与技术研究开发中心,辽宁,大连,116024;大连理工大学精细化工国家重点实验室,膜科学与技术研究开发中心,辽宁,大连,116024;大连理工大学精细化工国家重点实验室,膜科学与技术研究开发中心,辽宁,大连,116024;大连理工大学精细化工国家重点实验室,膜科学与技术研究开发中心,辽宁,大连,116024;大连理工大学精细化工国家重点实验室,膜科学与技术研究开发中心,辽宁,大连,116024【正文语种】中文【中图分类】X701【相关文献】1.用于氢同位素分离的置换色谱分离材料的研究进展 [J], 邓潇君;罗德礼;钱晓静2.皮下分离面积大小对腔镜甲状腺切除术CO2吸收的影响 [J], 吴有军;仇明;江道振;郑向民;刘晟;单成祥;姜治国;史晓辉3.功能化离子液体在二氧化碳吸收分离中的应用 [J], 崔国凯; 吕书贞; 王键吉4.功能化离子液体分散液-液微萃取分离水中Cu(Ⅱ) [J], 吕瑞;江雪梅;杨家成;崔书亚5.用于红外碳硫分析仪的气体干燥剂和CO2吸收剂 [J],因版权原因,仅展示原文概要,查看原文内容请购买。
非常规有机化学反应介质简介
非常规有机化学反应介质简介陈 婷(中国地质大学材料科学与化学工程学院 湖北武汉 430074)摘 要 基于绿色化学理念,简要介绍了水、超临界流体、离子液体、氟相介质等作为非常规有机化学反应介质的研究进展。
关键词 有机化学反应 反应介质 超临界流体 离子液体绝大多数的有机化学反应都是在有机溶剂中进行的。
传统的有机溶剂有甲醇、乙醇、丙酮、苯、甲苯、DM F、四氢呋喃、三氯甲烷等。
很多有机溶剂都有不同程度的毒性,大量有机溶剂的使用,容易造成环境污染。
近年来,随着人们环境保护意识的增强,“绿色化学”的理念已经深入人心[1],越来越多的科学家将有机合成的研究重点放在绿色合成上。
绿色合成要求合成过程中采用无毒的试剂、溶剂或催化剂,反应过程中所排放的污染物最少,最好是“零排放”。
如何寻找传统有机反应溶剂的替代品,使化学反应高效、有序的进行已经成为有机化学的一个研究热点[2]。
目前,研究得较多的非常规有机化学反应介质主要有水、超临界流体、离子液体、氟相介质等。
1 水水应该说是一种非常理想的绿色溶剂。
是人类生命存在的基础,因而对环境是最为友好的。
水价格低廉,无毒,不易燃烧,不易爆炸,水作为介质可以控制反应的p H,是取代传统挥发性有机溶剂和助剂的理想替代品。
然而,水却是有机化学很少使用的溶剂,最简单的原因就是由于疏水效应的影响,绝大多数有机化合物不能溶解在水中。
另外,很多有机化合物和中间体(如SOCl2、格氏试剂等)能和水反应,需要使用无水有机溶剂。
所以,虽然早期的科学家就发现有些有机化学反应可以在水中进行,但没有对水介质的有机化学反应做深入的研究。
直到20世纪80年代,Breslow[3,4]重新研究了水作为介质的有机反应,才再次激起人们对于这种反应介质研究的兴趣。
目前,水相有机化学反应的研究正在受到越来越多的关注[5]。
水相有机化学反应的研究已涉及多个反应类型,如:周环反应、亲核加成和取代反应、金属参与的有机反应、路易斯酸和过渡金属试剂催化的有机反应、聚合反应、氧化和还原反应、加氢反应、水相中的自由基反应等。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Energy Procedia 00 (2008) 000–000Energy Procedia/locate/XXXGHGT-9CO 2-binding organic liquids (CO 2BOLs) for post-combustion CO 2capture.David J. Heldebrant a *, Clement R. Yonker a , Philip G. Jessop b , Lam Phan ba Pacific Northwest National Laboratory, Energy and Efficiency Division, 902 Battelle Boulevard, Richland, WA 99352Queens University, 9 Bader Lane, Kingston, Ontario, CanadaElsevier use only: Received date here; revised date here; accepted date hereAbstractCO 2-binding organic liquids (CO 2BOLs) chemically bind and release CO 2 more efficiently than aqueous alkanolamine systems. CO 2BOLs are comprised of alcohols and organic amidine or guanidine bases, which chemically bind CO 2 as liquid amidinium or guanidinium alkylcarbonate salts. CO 2BOLs have high CO 2 binding capacities (19% by weight, 147 g CO 2/L) compared to that of 30% monoethanolamine solution in water (7% by weight, 108 g CO2/L) because they are liquid with or without bound CO 2 and do not require any added solvent such as water.The dissolution of CO 2 into and out of the liquid phase limits the rate of CO 2 capture and release. Absorption of CO 2 is selective over nitrogen in both concentrated and dilute gas streams making these systems applicable to post- and pre-combustion CO 2 capture. The free energy of CO 2 binding in these systems is small and is independent of the choice of alcohol. The free energies of these systems are dependent on the choice of base; -9 kJ/mol for diazabicyclo[5.4.0]undec-7-ene (DBU) and Barton’s base and +2 kJ/mol for 1,1,3,3-tetramethylguanidine. The specific heats of the organic CO 2BOLs are over 50% lower than that of water, resulting in a 50% reduction in the energy needed to strip out CO 2 as compared to aqueous alkanolamine solutions. CO 2BOLs have been recycled for five cycles without losing activity or selectivity towards CO 2.© 2008 Elsevier Ltd. All rights reservedKeywords: CO 2 capture; ionic liquid; organic; alkylcarbonate; amidine; guanidine.1. IntroductionThe primary chemical CO 2-scrubbing agents in post-combustion systems are aqueous alkanolamines. Whilealkanolamines effectively bind CO 2 in systems where the CO 2 concentrations rarely exceed 5-15 volume %, these systems have inherent drawbacks such as corrosion, suboptimal gravimetric and the volumetric CO 2 capacity, solvent loss, and the high specific heat of water.1 In the case of monoethanolamine (MEA) systems, the concentration of MEA is limited by system* Corresponding author. Tel.: 509-372-6359; fax: 509-375-2186.E-mail address : david.heldebrant@.Available online at c 2009Elsevier Ltd.All rights reserved.Energy Procedia 1(2009)1187–/locate/procedia doi:10.1016/j.egypro.2009.01.1562 Author name / Energy Procedia 00 (2008) 000–000corrosion and rarely exceeds 30 wt %..2 The 30 wt% loading of the CO 2 absorbing MEA limits the maximum CO 2 volumetric ( 108 g/L) and gravimetric capacity ( 7 wt%) of the aqueous MEA based CO 2 scrubbers.2 The 30% MEA loading limit necessitates a large excess of water as a solvent to dissolve the MEA, the CO 2 carrier. The pumping and heating of this excess water greatly increases the energy requirements for CO 2 scrubbing. The energy requirements for CO 2 stripping from aqueous MEA systems are also increased by the high specific heat of water (4 Jg -1K 1).3 Thermal stripping of CO 2 from aqueous systems at temperatures greater than 100 ˚C leads to large evaporative losses of solvent, which has to be recovered. Switching to organic, high-boiling, liquid compounds that chemically bind CO 2 would reduce solvent loss during CO 2 stripping, reduce the high specific heats associated with water, and reduce system corrosion as well as improve volumetric and gravimetric capacity for CO 2 capture compared to aqueous MEA systems.We recently reported an innovative CO 2-selective solvent system, known as CO 2 binding organic liquids (CO 2BOLs).4 CO 2BOLs are liquid mixtures of an alcohol and a strong amidine or guanidine base that chemically bind CO 2 to form the respective liquid amidinium or guanidinium alkylcarbonate salt (Figure 1).5-8 CO 2BOLs are liquids when CO 2 is bound or not; no superfluous inert solvent is needed to dissolve the CO 2 carrier. The first CO 2BOL in our study (DBU:1-hexanol) is capable of chemically binding 1 mol of CO 2 per mol DBU (15 wt. %) with an additional physical adsorption of 0.3 mol of CO 2 per mol of DBU (4 wt. %) bringing the theoretical maximum CO 2 capacity to 19 wt. % and 147 gCO 2/L liquid. The chemical and physical binding of CO 2 gives CO 2BOLs potentially twice the CO 2 gravimetric capacity and a 36 % increase in volumetric capacity than current 30% MEA solutions in water (7 wt. %, 108 g/L liquid).CO 2BOLs chemically bind CO 2 as a liquid alkylcarbonate in contrast to the aqueous alkanolamines that bind CO 2 as a bicarbonate or carbamate salt..2 Aqueous carbamate and bicarbonate salts have high hydrogen bonding which increases the binding enthalpy of CO 2. The reduced hydrogen bonding in organic alkylcarbonate salts decreases the enthalpy, meaning that less energy is needed for thermal stripping of CO 2. Certain CO 2BOLs have been shown to release chemically bound CO 2 slowly even at room temperature.5,6The alcohol and base components can be changed to produce a CO 2BOL with the desired physical and chemicalproperties, such as specific weight capacity, volumetric capacity, CO 2 stripping temperature, or any other desired physical properties.. All linear alcohols and certain secondary alcohols are suitable for CO 2BOL systems. Organic bases such as amidines, guanidines, phosphazines, and possibly some amines are suitable base choices. The structures of the four bases discussed in this study are shown in Figure 2.Figure 1. Reversible binding of CO 2 with an amidine (DBU) and alcohol.4 Reproduced by permission of The RoyalSociety of ChemistryN NO R+ ROH CO 2N N H 33H 33C 3H 3CH 3I II III IV1188D.J.Heldebrant et al./Energy Procedia 1(2009)1187–1195D.J.Heldebrant et al./Energy Procedia1(2009)1187–11951189Author name / Energy Procedia 00 (2008) 000–000 3 Figure 2. Bases investigated in this study. I. Diazabicyclo[5.4.0]-undec-7-ene (DBU), II. 1,1,3,3 Tetramethylguanidine (TMG), III Barton’s base, IV Hunig’s base.4 Reproduced by permission of The Royal Society of Chemistry2.Results and discussionCO2BOLs are liquids before CO2 is present and trap CO2 as liquid amidinium or guanidinium alkylcarbonate salts and do not require superfluous solvents to dissolve the solid CO2 absorbents like aqueous MEA based systems. The first CO2BOL; DBU:1-Hexanol is capable of chemically capturing one mole of CO2 per mol DBU (15 wt. %) at room temperature, but it canalso physically adsorb 0.3 equivalents (4 wt. %) bringing the theoretical capacity to 19 wt % and 147 gCO2/L liquid. This combined chemical and physical adsorption captures more CO2 by weight and volume than a 30% aqueous MEA system (7 wt. %, 108 g/L liquid). The weight and volumetric capacity of CO2BOLs can be increased by switching to smaller alcohols such as methanol and lighter bases such as TMG.The uptake of CO2 by these CO2BOL mixtures is mildly exothermic and appears to be dependent on the rate of CO2 diffusion into the CO2BOL liquid. Initial kinetic investigations of CO2 uptake were performed using changes in the conductivity of an acetonitrile solution of an amidine or guanidine bases and an alcohol CO2BOL over time. CO2BOLs are non-conductive until CO2 is present; the resulting amidinium or guanidinium alkylcarbonate salt produces conductivity change (Equation (????) Figure 1). 5-7CO2(g) + DBU + ROH [DBUH+][ROCO2-] (1)The rate of CO2 uptake at 28 ˚C was complete within 20 seconds regardless of the choice of base (DBU or TMG) or the choice of alcohol (ethanol, 1-propanol, 1-butanol, 1-pentanol, or 1-hexanol). However, the rate of uptake was strongly dependent on the stirring rate, indicating that the reaction was limited by the rate of mass-transfer of CO2 from the gas phase into the solution rather than by the reaction of the dissolved CO2 with the base and alcohol. We attempted to increase the rate of mass-transfer of CO2 into solution by exclusively using liquids pre-saturated with CO2 and by increasing the stir-rate to 500 rpm. butthe rate remained dependent on the stir-rate. The process is as rapid as mass transport (mixing) will allow, which is clearly promising for CO2 capture applications.CO2BOLs are selective for CO2 binding in the presence of concentrated and dilute nitrogen. Our previous investigations showed that DBU:1-hexanol was able to capture CO2 selectively from a mixture of CO2 and N2 at 1 atm.9 The ability to capture CO2 at reduced partial pressures of CO2 showss that CO2BOLs have potential for post-combustion CO2 capture. Capture of CO2 in concentrated gas streams was demonstrated by placing a 1:1 molar ratio of DBU and 1-hexanol under 50 psiN2 in a stainless steel pressure vessel. The solution was stirred at a constant rate and was monitored for changes in temperature and pressure as well as conductivity to indicate chemical capture of CO2. There was no decrease in pressure or change in conductivity until 1 molar equivalent (50 psi) of CO2 was introduced to the system bringing the total pressure to 100 psi. A pressure drop of 50 psi (Figure 3) was observed with a concurrent spike in the conductivity of the solution indicating that CO2was being consumed and the [DBUH+][ROCO2-] salt was formed. The demonstrated selective capture of CO2 in 50% CO2/N2 pressurized streams suggests CO2BOLs can be designed for pre-combustion CO2 capture.1190 D.J.Heldebrant et al./Energy Procedia1(2009)1187–11954Author name / Energy Procedia 00 (2008) 000–000Figure 3. Selectivity of DBU:1-hexanol for CO2 in an N2/CO2 mixture.4 Reproduced by permission of The Royal Society of ChemistryCO2 ReleaseCO2 stripping from CO2BOLs (equation 2) was studied on an automated burette system. Pre-CO2-saturated CO2BOLs were plunged into a pre-heated oil bath under agitation and examined for CO2 evolution. Decarboxylations at 90 ˚C were nearly complete within 1 minute at 250 rpm. from DBU and TMG CO2BOLs with 1-hexyl, 1-pentyl, and 1-butyl alcohols. CO2 evolution from all CO2BOLs was determined to be first order with respect to the CO2BOL salt concentration in solution. The chain length of the linear alcohols (ethanol, 1-propanol, 1-butanol, 1-pentanol and 1-hexanol)did not affect the rate of CO2 evolution from DBU CO2BOLs.. The CO2 evolution did appear to be dependent on the stirring rate of the solution, suggesting mass-transfer limitations of CO2 escaping from solution, instead of limitation due to the decarboxylation of the alkylcarbonate anion. The Arrhenius plot of the first order rate constant yielded an E act for CO2 release of between 23-33 kJ/mol.[BaseH+][ROCO2-] CO2 + Base + ROH (2)The total volume of CO2 released in the burette was correlated to the stripping temperature. At room temperature,CO2BOLs do not decarboxylate under a static atmosphere.9 Gentle heating or sparging with an inert gas is required to remove CO2 from CO2BOLs because of the thermodynamic equilibrium between dissolved and gaseous CO2.5 On average, the DBU and TMG CO2BOLs with 1-hexyl, 1-pentyl, and 1-butyl alcohols evolved 0.25 equivalents of CO2 at 50 ˚C, 0.50 equivalents at 70 ˚C, 0.65 equivalents at 90 ˚C, and up to 1 equivalent near 130 ˚C.Nearly 60% of the energy for CO2 scrubbing from power plants involves the thermal stripping of CO2 from the scrubber.2 The high energy requirements for alkanolamine solutions are due to the high specific heat of the solvent water (4.18 J g-1 deg-1).10 The specific heat of the organic DBU/1-hexanol CO2BOL is 1.5 J g-1 deg-1. This is similar to other ionic liquids such as 3-ethyl-1-methyl-imidazolium tetrafluoroborate (1.28 J g-1 deg-1) and 3-butyl-1-methyl-imidazolium tetrafluoroborate (1.66 Jg-1 deg-1).11 The low specific heats associated with CO2BOLs mean that CO2 stripping at comparable temperatures to MEA,require up to 60% less energy, reducing the energy needed for CO2 scrubbing.LifetimeAuthor name / Energy Procedia 00 (2008) 000–000 5The DBU/1-hexanol CO 2BOL was exposed to five capture and release cycles of CO 2 using an automated gas burettesystem to verify the robustness and reproducibility of the CO 2BOL system. The DBU/1-hexanol was loaded in a flask and CO 2 was sparged through the liquid for 5 minutes, making the [BaseH +][ROCO 2-] salt. After CO 2 uptake had ceased, the flask was connected to the burette system and then decarboxylated by dipping the flask into a pre-heated oil bath at 90 ˚C. (Figure 4). After CO 2 evolution had ceased, the flask was cooled to 25 ˚C and the flask was disconnected from the burette and then promptly carboxylated again by sparging CO 2 through the liquid for 5 minutes. This process was performed a total of 5 times, with no observable loss of CO 2 binding capacity. Formal measurements are underway to determine the lifetime of CO 2BOLs on mock flue gas streams.Figure 4. Lifetime/repeated CO 2 release from DBU and 1-Hexanol at 90 ˚C. Heating begins at 1 minute.4 Reproduced by permission of The Royal Society of ChemistryEvaporative losses of solvent with aqueous MEA systems 2 will not be observed with CO 2BOLs as long as high boiling alcohols and bases are employed. Even if the CO 2 is stripped from CO 2BOLs at the same temperature as MEA systems (117 ˚C) this is far below the boiling point of both 1-hexanol (159 ˚C) and DBU (256 ˚C). The volatility of the CO 2BOL components can also been reduced by switching to even higher boiling alcohols such as 1-octanol and 1-decanol.In our initial report, we showed that while the introduction of water with the CO 2 stream could produce large amounts of the bicarbonate salt, the bicarbonate salt could be stripped at the same temperature as an MEA system. Any water in the CO 2 stream competes with the alcohol. A large excess of alcohol can drive the formation of the CO 2BOL and not the bicarbonate.. The thermally stable CO 2BOL bicarbonate salt, [DBUH +][HCO 3-] rather than the CO 2BOL alkylcarbonate[DBUH +][ROCO 2-] will be produced if large amounts of water are present. In our previous studies, the CO 2 source had minimal water content. Industrial gas streams can contain up to 15% water.12 CO 2 can be stripped from the [DBUH +][HCO 3-] bicarbonate salt at 121 ˚C, which is comparable to temperatures used for stripping CO 2 from MEA systems. The [DBUH +][HCO 3-] bicarbonate salt was measured to have a specific heat of 1.5 J g -1 deg -1, which is 60% less than that of water, meaning that even stripping of CO 2 from the [DBUH +][HCO 3-] bicarbonate salt is more energy efficient than stripping of aqueous MEA systems. ThermodynamicsApproximate thermodynamic data for fifteen CO 2BOLs were determined by 1H NMR spectroscopic measurements of the CO 2 binding equilibrium (equation 1, Table 2). The concentrations of CO 2BOLs with and without CO 2bound were measured D.J.Heldebrant et al./Energy Procedia 1(2009)1187–119511916Author name / Energy Procedia 00 (2008) 000–000in d-MeCN because the viscosity of the neat CO2BOLs results in very broad 1H NMR peaks. Placing CO2BOLs into an organic solvent results in a slightly lower CO2 capacity13 than neat systems7. The -hydrogens of the free RCH2OH and boundRCH2OCO2were evaluated over a temperature range of 24-60 ˚C, and the reaction enthalpy was extrapolated from the slope of the Van’t Hoff plot of the measured equilibrium. Barton’s base was measured over 25-50 ˚C because of curvature of the Van’t Hoff plot, attributed to the temperature range being too large.The data show very clear trends (Table 2.). The H and G values are almost independent of the choice of alcohol. Linear alcohols have almost identical pKa values in d-MeCN, so the similarities were expected. Iso-propanol has slightly larger G values because of its steric bulkiness which destabilizing the alkylcarbonate anion. Tert-butanol and other tertiary alcohols are unable to form detectable amounts of [BaseH+][ROCO2-], almost certainly due to steric crowding which destabilizes the alkylcarbonate anion.Table 2. Thermodynamics of the capture of CO2 by select CO2BOLs in MeCN, estimated by NMR spectroscopic determination of equilibrium constants.a 4 Reproduced by permission of The Royal Society of ChemistryBase/Alcohol pairH, kJ/mol a S, J/molK b G, kJ/mol c% CO2 absorption in MeCN at 25 ˚CDBU/HexOH -140 -440 -9.4 87DBU/PentOH -120 -390 -7.5 82DBU/BuOH -140 -450 -9.7 88DBU/ PrOH -130 -420 -7.8 83DBU/i-PrOH -140-450-5.7 76 DBU/linear alcohol d -136 -425 -8.6 - TMG/PentOH -210-7100.7 47TMG/BuOH -180-5902.4 38TMG/PrOH -170-5902.3 39TMG/i-PrOH -160-5505.5 25TMG/linear alcohol d -180 -610 1.7 -Barton’s/HexOH -83 -250-11 90 Barton’s/PentOH -52 -150-8.7 85 Barton’s/BuOH -60 -180-8.0 83 Barton’s/PrOH -53 -160-9.0 86 Barton’s/i-PrOH -76 -240-7.7 82 Barton’s/linear alcohol d -72 -210 -9.2 -a Data rounded to two significant figures.b Calculated at 25 ˚C from NMR integrations using G = -R*T*lnKeq, K eq =[BaseH+][ROCO2-]/P CO2[Base][ROH]. c Calculated at 25 ˚C using G = H – T S. d Average of the unrounded values for PrOH, BuOH, PentOH, HexOHThe CO2 binding energies are strongly correlated to the choice of base. Averages for the three bases paired with linear alcohols are listed in Table 2. The dependence of the enthalpy of the reaction with respect to base is correlated to the order of decreasing exothermicity: TMG > DBU > Barton’s base. TMG was the weakest Bronsted base of the three chosen in this study with a pK aH (of the conjugate acid, meaning the pK a of the conjugate acid of TMG in MeCN) of 23.314,15 CO2 binding with TMG and linear alcohols had the most favorable reaction enthalpy because of greater hydrogen bonding in [TMGH+][ROCO2-] than in the corresponding DBU and Barton’s CO2BOLs (see discussion below and Figure 5). This greater hydrogen bonding in the TMG CO2BOLs would also be expected to lower the entropy of the reaction. The S term is the major factor for the positive G and the resulting weakest capture of CO2 of the three bases. DBU was the intermediate Bronsted base (pK aH in MeCN is 24.3).18,19 Barton’s base was the strongest of the three Bronsted bases (estimated pKaHin MeCN is 25.3).16 DBU and Barton’s base were very close in their capability to bind CO2. DBU had a much more favorable reaction enthalpy (-140 kJ/mol for DBU vs. -83 1192 D.J.Heldebrant et al./Energy Procedia1(2009)1187–1195Author name / Energy Procedia 00 (2008) 000–000 7 kJ/mol for Barton’s) due to greater hydrogen bonding capability, compared to Barton’s base, which has a more favorable entropy (-390 J/mol•K for DBU vs. -185 J/mol•K for Barton’s base), effects which are attributed to steric repulsion between the cation and anion. For comparison, the enthalpy of Barton’s base is comparable to that of a 30 wt. % MEA solution in water at 40 ˚C (-80 kJ/mol CO 2).17 The entropy and enthalpy terms contribute to the overall reaction energetics but they are not linearly correlated to the pK aH of the bases used to bind CO 2 with linear alcohols.Hydrogen bonding contributes significantly to the stabilization of the CO 2BOL alkyl carbonate salt structures. Toomuch hydrogen bonding (as shown in the case of TMG) can decrease the G of CO 2 binding by a larger than desired decrease in the entropy of the system. The selection of a suitable base for a CO 2BOL must consider the availability and strength of hydrogen bonds when CO 2 is bound. As shown in Figure 5, the alkylcarbonate salts of DBU and Barton’s base appear to have less hydrogen bonding compared to TMG because [TMGH +] has two hydrogen-bond donor sites while DBU and Barton’s base have only one. The 6-member ring of the TMG alkylcarbonate salt is entropically favorable and similar to other 6 member rings of carboxylates and amidines.18 The highly delocalized cations of amidines and guanidine bases in general are weak hydrogen bond donors and acceptors 19 compared to localized amines such as triethylamine. Hydrogen bonds in the anion could also explain the preference for amidines and guanidines to bind CO 2 with water rather than alcohols. Crystal structures of the bicarbonate[DBUH +][HOCO 2-]20 have more extensive hydrogen bonding than the methylcarbonate [DBUH +][CH 3OCO 2-] and is CO 2 binding is enthalpically more favorable.7Figure 5. Proposed hydrogen bonding of cation with anion for salts made from DBU, TMG and Barton’s base withROH and CO 2.4 Reproduced by permission of The Royal Society of Chemistry+ ROH RO N N N H NN 3H 3C CH 33N 3H 333H R O + ROH t-Bu NN CH 3H 3C CH 33+ ROH N t-Bu 3R O NH 2C CH 3CH 33H 3C CH 3+ ROH 2CH H 3H 3C R O D.J.Heldebrant et al./Energy Procedia 1(2009)1187–119511931194 D.J.Heldebrant et al./Energy Procedia1(2009)1187–11958Author name / Energy Procedia 00 (2008) 000–000The hydrogen bonding of the salts can also explain the reaction entropies. The S term for Barton’s base is less negative because the bulky tert-butyl group forces the alkylcarbonate anion farther away from the Barton H+ cation, reducing available hydrogen bonding. The decreased steric crowding around the protonated nitrogen in DBU compared to Barton’s base results in a more ordered system and in turn a more negative S term. The S term is most negative for TMG, due to the highly ordered 6-member hydrogen-bonding ring that is entropically unfavorable.This data clearly shows that the pK aH of bases inCO2BOLs is not a valid predictor of the strength of CO2 binding; the hydrogen bonding and the reaction entropy must be included in the selection process.The thermodynamics confirm that choice of a linear alcohol with a chain length greater than two carbons has a minimal effect of the binding energies of CO2. We have shown that while alcohol choice does not alter the thermodynamic properties of CO2BOLs, the choice of alcohol can affect physical properties such as melting point and viscosity.5,6CO2BOLs can be molecularly tuned to meet desired physical properties because of the ability to change the alcohol without major effects on CO2 binding.Triethylamine and Hünig’s base were not able to form CO2BOLs under ambient conditions, and we anticipate that this is true for all tertiary amines. Hünig’s base and other tertiary amines should be basic enough (pK aH’s in MeCN ~18.1 - 18.8)21,22 to be protonated by the alkylcarbonic acid in CO2BOLs, but it remains unclear because there are no known pK a’s of alkylcarbonic acids. Tertiary amines can form CO2BOLs at high pressures. Triethylamine in methanol produces the[NEt3H+][CH3OCO2-] salt at high pressures, but the salt is rapidly decarboxylated when the pressure is reduced to atmospheric conditions.23 It is possible that the high pressure formation of a tertiary amine CO2BOL could be used for precombustion CO2 capture.The delocalized amidinium and guanidinium alkylcarbonate salts result in weakened interactions between the cation and anion of the ionic liquid. We hypothesize that the delocalization of both the cation and anion are key to the chemical and physical properties of these liquids. Formal investigations into the effect of the delocalization of the cation and anion on properties such as viscosity, melting point, as well as thermodynamic properties binding and release of CO2 are underway.3.ConclusionsCO2BOLs are a new class of liquid, organic, CO2 capture agents that can continually bind and release CO2 with a high gravimetric and volumetric CO2 capacity up to 19 % by weight and 147 g/L liquid. CO2BOLs are neat mixtures of alcohols and strong organic bases that require no solvent. The rate of CO2 binding and stripping from CO2BOLs appears to be mass-transfer limited by the rate of CO2 movement into and out of the liquid phase. CO2 uptake was selective in both dilute and concentrated streams, suggesting CO2BOLs are applicable to post- or pre-combustion scrubbing systems. CO2BOLs chemically bind CO2 as alkylcarbonate salts with binding energies of less than 10 kJ/mol, which are weaker than the binding energies of the bicarbonate and carbamate salts in aqueous systems. The organic CO2BOLs have low specific heat compared to aqueous systems, resulting in less energy required for stripping of CO2 when compared to aqueous alkanolamine systems. If water is present in the gas stream, CO2BOL bicarbonate salts will be formed, however they can be broken down at the same temperatures as MEA systems, but over 50% more efficiently due to the lower specific heat of CO2BOLs and CO2BOL bicarbonates than water and MEA.The energetics of CO2 binding are unrelated to choice of alcohol, but they are dependent on the choice of base, however they are not linearly correlated to the pK aH of the base. CO2BOLs can be fine tuned to produce the desired chemical and physical properties by appropriate selection of the alcohol and base components. All of these properties suggest CO2BOLs are energy efficient CO2 capture systems.D.J.Heldebrant et al./Energy Procedia1(2009)1187–11951195Author name / Energy Procedia 00 (2008) 000–000 94.References(1) Aaron, D.; Tsouris, C. Separation Science and Technology2005, 40, 321-348.(2) Peeters, A. N. M.; Faaij, A. P. C.; Turkenburg, W. C. International Journal of Greenhouse Gas Control2007, 1, 396-417.CRC Handbook of Chemistry and Physics; 84 ed.; CRC Press: Boca Raton, Florida,R.(3)D.Lide,2003.(4) David J. Heldebrant, C. R. Y., Philip G. Jessop, Lam Phan Energy and Environmental Science 2008, 1, 487-493.(5) Jessop, P. G.; Heldebrant, D. J.; Li, X. W.; Eckert, C. A.; Liotta, C. L. Nature2005, 436, 1102-1102.(6) Liu, Y. X.; Jessop, P. G.; Cunningham, M.; Eckert, C. A.; Liotta, C. L. Science2006, 313, 958-960.(7) Phan, L.; Chiu, D.; Heldebrant, D. J.; Huttenhower, H.; John, E.; Li, X. W.; Pollet, P.; Wang, R. Y.; Eckert, C. A.; Liotta, C. L.; Jessop, P. G. Industrial & Engineering Chemistry Research2008, 47, 539-545.(8) Phan, L.; Andreatta, J. R.; Horvey, L. K.; Edie, C. F.; Luco, A. L.; Mirchandani, A.; Darensbourg,D. J.; Jessop, P. G. Journal of Organic Chemistry2008, 73, 127-132.(9) Jessop, P. G. 2005.R.RC Handbook of Chemistry and Physics, 84th ed; CRC Press: Bocca Raton, Florida,D.Lide,(10)2003.(11) Wilkes, J. S. Journal of Molecular Catalysis a-Chemical2004, 214, 11-17.(12) Schurmann, H.; Monkhouse, P. B.; Unterberger, S.; Hein, K. R. G. Proceedings of the Combustion Institute2007, 31, 1913-1920.(13) Y. Hori, Y. N., J. Nakau, J. Nakau Chem. Express1986, 1, 173-176.(14) Kaljurand, I.; Rodima, T.; Leito, I.; Koppel, I. A.; Schwesinger, R. Journal of Organic Chemistry 2000, 65, 6202-6208.Acid-Base Dissociation Constants in Dipolar Aprotic Solvents; Blackwell Science, 1990.Klzutsu(15)(16) Raczynska, E. D.; Maria, P. C.; Gal, J. F.; Decouzon, M. Journal of Physical Organic Chemistry 1994, 7, 725-733.(17) I. Kim, H. S. Ind. Eng. Chem. Res.2007, 46, 5803-5809.(18) Kraft, A.; Peters, L.; Johann, S.; Reichert, A.; Osterod, F.; Frohlich, R. Materials Science & Engineering C-Biomimetic and Supramolecular Systems2001, 18, 9-13.(19) Galezowski, W.; Jarczewski, A.; Stanczyk, M.; Brzezinski, B.; Bartl, F.; Zundel, G. Journal of the Chemical Society-Faraday Transactions1997, 93, 2515-2518.(20) Perez, E. R.; Santos, R. H. A.; Gambardella, M. T. P.; de Macedo, L. G. M.; Rodrigues, U. P.; Launay, J. C.; Franco, D. W. Journal of Organic Chemistry2004, 69, 8005-8011.(21) Kaljurand, I.; Kutt, A.; Soovali, L.; Rodima, T.; Maemets, V.; Leito, I.; Koppel, I. A. Journal of Organic Chemistry2005, 70, 1019-1028.(22) Room, E. I.; Kutt, A.; Kaljurand, I.; Koppel, I.; Leito, I.; Koppel, I. A.; Mishima, M.; Goto, K.; Miyahara, Y. Chemistry-a European Journal2007, 13, 7631-7643.(23) Munshi, P.; Main, A. D.; Linehan, J. C.; Tai, C. C.; Jessop, P. G. Journal of the American Chemical Society2002, 124, 7963-7971.。
