Electrochemical behavior of Li3V2(PO4)3C composite cathode material for lithium-ion batteries

Electrochemical behavior of Li 3V 2(PO 4)3/C composite
cathode material for lithium-ion batteries
Anping Tang a,b ,Xianyou Wang a,?,Zhiming Liu a
a
School of Chemistry,Xiangtan University,Hunan 411105,China
b
School of Chemistry and chemical Engineering,Hunan University of Science and Technology,Hunan 411201,China
Received 31May 2007;accepted 21September 2007
Available online 29September 2007
Abstract
Monoclinic lithium vanadium phosphate was synthesized by a low temperature route.NH 4VO 3,LiOH,(NH 4)2HPO 4and sucrose were used as starting materials to prepare a precursor,and Li 3V 2(PO 4)3/C composite was finally obtained by sintering the precursor.X-ray diffraction results show that Li 3V 2(PO 4)3sample is monoclinic structure.The initial discharge capacities of Li 3V 2(PO 4)3/C composite materials are 125mA h g ?1in the voltage range of 3.0–4.3V ,164mA h g ?1in the voltage range of 3.0–4.8V ,respectively.The discharge capacity after 30cycles at two voltage windows was held to be 122and 142mA h g ?1,respectively.?2007Elsevier B.V .All rights reserved.
Keywords:Li 3V 2(PO 4)3/C;Composite materials;Electric properties
1.Introduction
Recently,lithiated transition metal phosphates,such as LiFePO 4and Li 3V 2(PO 4)3,have been proposed as a new class of cathode materials for lithium-ion batteries.The title material,Li 3V 2(PO 4)3exists in two forms:monoclinic form and rhombohedral phase.Two lithium ions per Li 3V 2(PO 4)3formula unit can be extracted from the rhombohedral form at 3.77V (vs.Li/Li +),and only 1.3can be reinserted;however,all the three Li ions in monoclinic form can be removed and reversibly intercalated [1–4],and then high capacity can be expected in this system.
Usually,monoclinic Li 3V 2(PO 4)3materials were obtained through high temperature solid-state reactions.In this investiga-tion,to obtain Li 3V 2(PO 4)3/C composite materials,we developed a low temperature route using LiOH and NH 4VO 3,(NH 4)2HPO 4and sucrose as reactants.
2.Experimental procedures
Precursor was prepared as follows.Firstly,stoichiometric NH 4VO 3was added to LiOH solution,with continued stirring at about 70°C until a clear solution formed.Secondly,above mixture was added to the solution which contained the stoichiometric (NH 4)2HPO 4and an appropriate amount of sucrose (28wt.%vs.Li 3V 2(PO 4)3).Finally,the obtained mixture was dried at about 80°C in an oven.
The precursor was ground for 1h and then pressed into pellets.Then they were transferred to a tube furnace equipped with a flowing argon atmosphere and heated to 300°C for 6h.After cooling to room temperature,the pellets were ground for 1h and pelletized again,then heated to 700°C and held at this temperature for 12h.
Structural and crystallographic analysis of the reaction product was taken from powder diffraction data obtained using diffractometer (D/MAX-3C)with Cu K αradiation.The surface morphology of the samples was observed using the Hitachi S-3500N scanning electron microscopy (SEM).
The Li 3V 2(PO 4)3cathode electrodes were made by mixing 10%acetylene black,10%polyvinylidene fluoride binder and 80%active material.The electrolyte consisted of 1M LiPF 6
Available online at https://www.360docs.net/doc/505603589.html,
Materials Letters 62(2008)1646–
1648
https://www.360docs.net/doc/505603589.html,/locate/matlet
?Corresponding author.Tel.:+867328293043;fax:+867328292282.E-mail address:wxianyou@https://www.360docs.net/doc/505603589.html, (X.Wang).
0167-577X/$-see front matter ?2007Elsevier B.V .All rights reserved.doi:10.1016/j.matlet.2007.09.064
solution in a mixture of ethylene carbonate and dimethyl carbonate with the volumetric ratio of 1:1.The cells were measured using a Neware battery tester.Cyclic voltammetry (CV)was performed with a CHI 660electrochemical instrument at scan rate of 0.05mV s ?1in the range of 3.0–4.3Vand 3.0–4.8V ,respectively.
3.Results and discussion
Fig.1shows the XRD pattern for the Li 3V 2(PO 4)3/C composite.All the peaks correspond to a single phase and are indexed with monoclinic structure with space group 14(P21/n ),consistent with the previously published results [1].Although the carbon content in the Li 3V 2(PO 4)3/C composite materials was 1.76(wt.)%validated by thermogravimetric analysis and chemical analysis,no diffraction peak related-carbon in Fig.1was observed.It indicates that the residual carbon is too small or the thickness of the residual carbon on the Li 3V 2(PO 4)3powders is too thin [5].The morphology of obtained composite is shown in Fig.2.The particles of Li 3V 2(PO 4)3/C composite are relatively small,most of which are between 0.5and 3μm.In addition,for Li 3V 2(PO 4)3/C composite,its surface is rough,and some small particles bound on the surface of Li 3V 2(PO 4)3were observed,which can be considered as small scattered carbon
particles.This observation is consistent with the carbon-coated LiCoO 2and LiFePO 4,in which sucrose was used as carbon source [6,7].
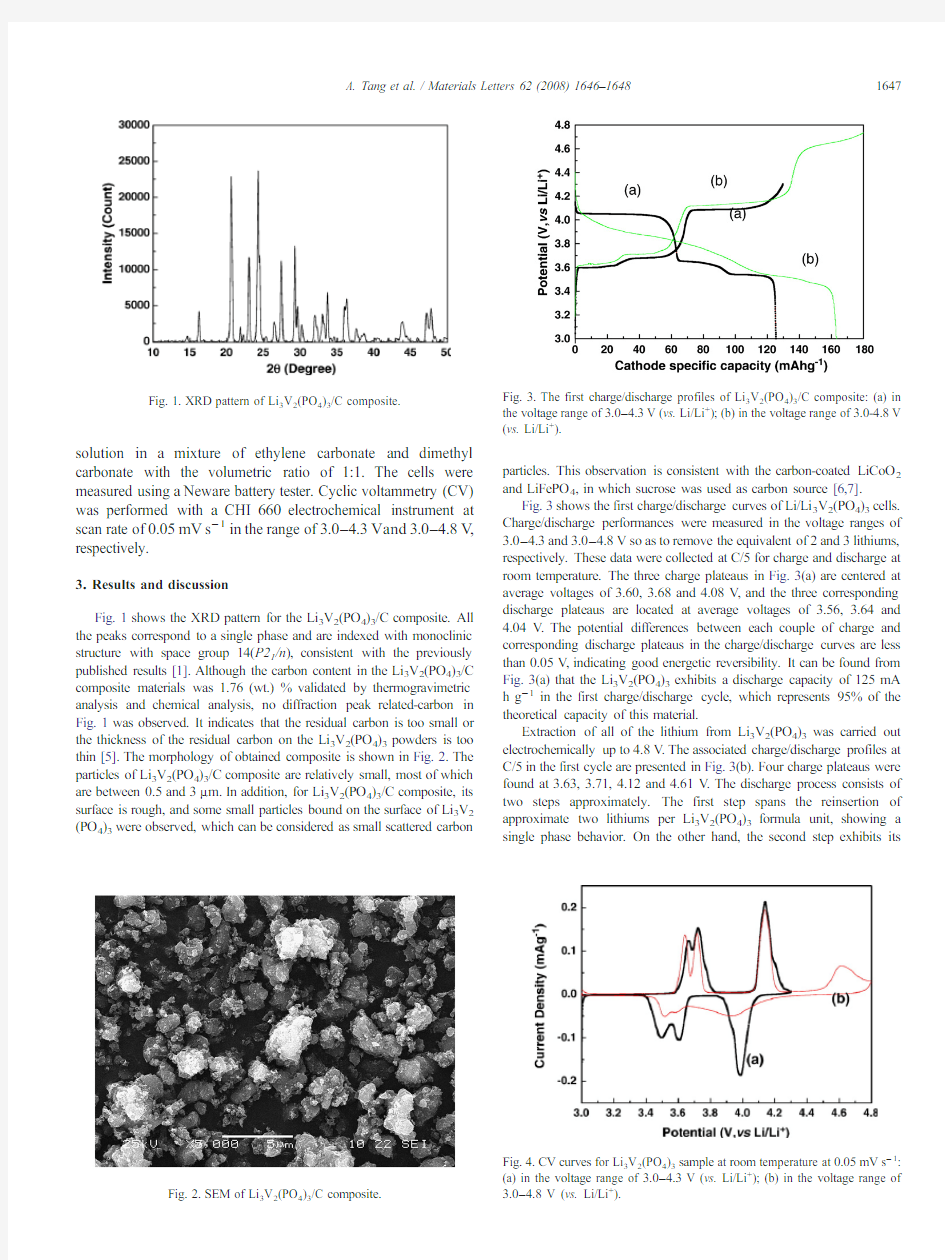
Fig.3shows the first charge/discharge curves of Li/Li 3V 2(PO 4)3cells.Charge/discharge performances were measured in the voltage ranges of 3.0–4.3and 3.0–4.8V so as to remove the equivalent of 2and 3lithiums,respectively.These data were collected at C/5for charge and discharge at room temperature.The three charge plateaus in Fig.3(a)are centered at average voltages of 3.60,3.68and 4.08V ,and the three corresponding discharge plateaus are located at average voltages of 3.56,3.64and 4.04V .The potential differences between each couple of charge and corresponding discharge plateaus in the charge/discharge curves are less than 0.05V ,indicating good energetic reversibility.It can be found from Fig.3(a)that the Li 3V 2(PO 4)3exhibits a discharge capacity of 125mA h g ?1in the first charge/discharge cycle,which represents 95%of the theoretical capacity of this material.
Extraction of all of the lithium from Li 3V 2(PO 4)3was carried out electrochemically up to 4.8V .The associated charge/discharge profiles at C/5in the first cycle are presented in Fig.3(b).Four charge plateaus were found at 3.63,3.71,4.12and 4.61V .The discharge process consists of two steps approximately.The first step spans the reinsertion of approximate two lithiums per Li 3V 2(PO 4)3formula unit,showing a single phase behavior.On the other hand,the second step exhibits
its
Fig.1.XRD pattern of Li 3V 2(PO 4)3/C
composite.
Fig.2.SEM of Li 3V 2(PO 4)3/C
composite.
Fig.3.The first charge/discharge profiles of Li 3V 2(PO 4)3/C composite:(a)in the voltage range of 3.0–4.3V (vs.Li/Li +);(b)in the voltage range of 3.0-4.8V (vs.Li/Li +
).
Fig.4.CV curves for Li 3V 2(PO 4)3sample at room temperature at 0.05mV s ?1:(a)in the voltage range of 3.0–4.3V (vs.Li/Li +);(b)in the voltage range of 3.0–4.8V (vs.Li/Li +).
1647
A.Tang et al./Materials Letters 62(2008)1646–1648
characteristic two-phase behavior,corresponding to the reinsertion of the last lithium,Li 2V 2(PO 4)3→Li 3V 2(PO 4)3.Its associated plateaus are located at 3.50and 3.58V ,respectively.Still,the Li 3V 2(PO 4)3exhibits a charge capacity of 187mA h g ?1and a discharge capacity of 164mA h g ?1,indicating a moderate coulombic efficiency of 86.7%,which is less than that in the voltage range of 3.0–4.3V .The decrease in coulombic efficiency is probably attributed to electrolyte oxidation and a significant overpotential during this voltage window.
CV curves shown in Fig.4were recorded for the Li 3V 2(PO 4)3/C sample,using Li metal as counter and reference electrodes in stimulant cell configuration.In the voltage range of 3.0–4.3V ,there are three oxidation peaks and three reduction peaks in Fig.4(a).The three oxidation peaks are located around 3.67,3.73and 4.14V ,while three reduction peaks are located around 3.50,3.61and 3.99V .The symmetrical feature of the CV data shown in Fig.4(a)further indicates the good reversibility of the insertion processes between voltage limits of 3.0and 4.3V .
For the CV curves in the voltage range of 3.0–4.8V ,there are four oxidation peaks and three reduction peaks in Fig.4(b).As mentioned earlier,the first two lithiums are extracted below 4.3V .The fourth oxidation peak is located around 4.60V ,associated with the V 4+/V 5+redox couple.The reduction reactions occurred at 3.49,3.59and 3.91V .The fourth reduction current peak corresponding to V 4+/V 5+redox couple was not detected in Fig.4(b),but the third reduction peak in Fig.4(b)is apparently broadened in comparison with Fig.4(a),indicating the two reduction current peaks were overlapped.
The cyclic performances of the Li 3V 2(PO 4)3/C composite were evaluated at room temperature in the cell configuration Li/Li 3V 2(PO 4)3.As shown in Fig.5,the initial discharge capacities of Li 3V 2(PO 4)3/C composite materials are 125mA h g ?1in the voltage range of 3.0–4.3V
and 164mA h g ?1in the voltage range of 3.0–4.8V ,respectively.The discharge capacity after 30cycles at two voltage windows was held to be 122(97.6%of initial capacity)and 142mA h g ?1(86.5%of initial capacity),respectively.These results showed that no obvious capacity fading was observed after 30cycles in the voltage range of 3.0–4.3V ,while the discharge capacity decreases relatively quickly in the voltage range of 3.0–4.8V .Efforts to improve its cycling capability between voltage limits of 3.0and 4.8V are underway in our group.
4.Conclusions
Li 3V 2(PO 4)3/C composite can be synthesized by a low temperature route using NH 4VO 3,LiOH and (NH 4)2HPO 4as reactants and using sucrose as the carbon source.XRD results show a pure single phase with monoclinic structure.When charge/discharge performances were measured between 3.0and 4.3V under C/5rate,an initial specific capacity of 125mA h g ?1and a discharge capacity of 122mA h g ?1after 30cycles were observed.The reversible specific capacities for Li 3V 2(PO 4)3in voltage range of 3–4.8V with C/5rate are up to 164mA h g ?1in the first cycle and 142mA h g ?1at the 30th cycle,respectively.It was confirmed that Li 3V 2(PO 4)3/C composite had a good reversibility between voltage limits of 3.0–4.3V charge/discharge from the first charge/discharge curves of the Li 3V 2(PO 4)3electrode and CV curves.Acknowledgement
This work was funded by the Natural Science Foundation of Hunan Province under project No.06JJ50078.References
[1]M.Y .Saidi,J.Barker,H.Huang,J.L.Swoyer,G.Adamson,Electrochem.
Solid State Lett .5(2002)A149.
[2]H.Huang,S.C.Yin,T.Kerr,Nicholas Taylor,Linda F.Nazar,Adv.Mater.4
(2004)1525.
[3]P.Fu,Y .M.Zhao,Y .Z.Dong,X.N.An,G.P.Shen,Electrochimica Acta 52
(2006)1003.
[4]M.Ren,Z.Zhou,Y .Z.Li,X.P.Gao,J.Yan,J.Power Sources 162(2006)
1357.
[5]K.F.Hsu,S.Y .Tsay,B.J.Hwang,J.Mater.Chem.14(2004)2690.
[6]Q.Cao,H.P.Zhang,G.J.Wang,Q.Xia,Y .P.Wu,H.Q.Wu,Electrochem.
Commun.9(2007)1228.
[7]X.Z.Liao,Z.F.Ma,Y.S.He,X.M.Zhang,L.Wang,Y.Jiang,
J.Electrochem.Soc.152(2005)
A1969.
Fig.5.Cycling performance of Li 3V 2(PO 4)3/C composite under C/5rate at room temperature.
1648 A.Tang et al./Materials Letters 62(2008)1646–1648
Electrochemical supercapacitors for energy storage and delivery
Editorial Electrochemical supercapacitors for energy storage and delivery:Advanced materials,technologies and applications q 1.Introduction In the effort to achieve a clean and sustainable world,energy storage and delivery have become one of today’s most important topics in globe research and development.In this regard,electro-chemical energy technologies such as batteries,fuel cells,and elec-trochemical supercapacitors have been recognized as the most important portion of the various energy storage and delivery tech-nologies.Among these technologies,supercapacitors,emerging as one of the most important energy storage and delivery devices for the 21st century,are particularly the most reliable and safe devices with extremely high power density and cycling stability in many applications including portable electronics,automobile vehicles,stationary power stations and energy storage devices,backup power supplies,etc.However,its challenges of low energy density as well as this low energy density induced high cost are the major drawbacks.To overcome these challenges,in recent years,there are tremendous efforts focusing on the development of new and cost-effective electrodes and electrolyte materials as well as electrode con?guration to improve the capacitance,the energy density of the next generation of supercapacitors. In order to benchmark the state of research in this area at this time,Applied Energy organized a special issue dedicated to recent research development in Supercapacitors.The articles in this spe-cial issue cover the most recent progress of supercapacitor research and development in terms of both fundamentals and applications with focuses on cutting edge research on new materi-als for system designs and practical deployment investigations.2.Background of supercapacitors Normally,the energy storage mechanism of supercapacitors is electrochemical in nature,but differs from that of batteries or fuel cells that rely on the coupling of Faradaic redox reactions.Instead,supercapacitors store and discharge energy dominantly through what is called electric double layer capacitance (EDLC).This occurs by employing two high surface area electrodes,that when charged,form a Helmholtz double layer at the interface between the con-ductive electrode materials and the electrolyte,as illustrated in Fig.1.The electrodes have opposing charges,and in this fashion energy is stored by the electrostatic charge separation.As a result of this energy storage mechanism,supercapacitors can charge and discharge in a matter of seconds while delivering immense power densities (typically in the 10–1000KW/kg).Their operational life-times are also an attractive feature,boasting in excess of 100,000charge and discharge cycles before needing replacement (ref).Owing to these advanced properties,supercapacitors have gained commercial success in certain applications that include dynamic load levelling and uninterruptable power supplies.However,due to their low energy density,large untapped markets remain,including the use of supercapacitors in electric vehicles,large scale energy storage units (grid),and small scale portable electronics.For supercapacitors to effectively penetrate these markets,further technological advances,such as those reported in this special issue are required. At the current state of research,a large body of research has been focused on increasing the energy density of supercapacitors by developing novel high surface area carbon materials,such as graphene,with unprecedented electric double layer capacitance.It is expected that the viability of supercapacitors could also be extended towards a wide range of applications if energy densities can be improved to more closely resemble those of batteries.To accomplish this,psuedocapacitors (or hybrid supercapacitors)are an emerging trend of research that includes compositing conven-tional EDLC electrodes with materials that contribute redox beha-viour during charge and discharge.These materials,with a high capacity to store energy must also be integrated into operational supercapacitor cell designs.These systems may then have their performance investigated and validated towards new,promising applications. 3.Research covered in this special issue In this special issue,13papers have been included covering the areas of electric double layer capacitance materials,psuedocapac-itor material,system design and application deployment.3.1.New high surface area EDLC materials Developing novel nano-structured electrode materials are one of the most active approaches in supercapacitors.For example,Article [1]reports the use of rice husks,a renewable resource,to prepare high surface area porous carbons that were used as EDLC https://www.360docs.net/doc/505603589.html,/10.1016/j.apenergy.2015.05.0540306-2619/ó2015Published by Elsevier Ltd. q This paper is included in the Special Issue of Electrochemical Supercapacitors for Energy Storage and Conversion,Advanced Materials,Technologies and Applications edited by Dr.Jiujun Zhang,Dr.Lei Zhang,Dr.Radenka Maric,Dr.Zhongwei Chen,Dr.Aiping Yu and Prof.Yan.
Nanomaterial-based electrochemical
Nanomaterial-based electrochemical biosensors Joseph Wang DOI:10.1039/b414248a The unique properties of nanoscale materials offer excellent prospects for interfacing biological recognition events with electronic signal transduction and for designing a new generation of bioelectronic devices exhibiting novel functions.In this Highlight I address recent research that has led to powerful nanomaterial-based electrical biosensing devices and examine future prospects and challenges.New nanoparticle-based signal amplification and coding strategies for bioaffinity assays are discussed,along with carbon-nanotube molecular wires for achieving efficient electrical communication with redox enzyme and nanowire-based label-free DNA sensors. 1.Why nanomaterials? The buzzword‘‘nanotechnology’’is now around us everywhere.Nanotechnology has recently become one of the most exciting forefront fields in analytical chemistry.Nanotechnology is defined as the creation of functional materials, devices and systems through control of matter at the1–100nm scale.A wide variety of nanoscale materials of differ-ent sizes,shapes and compositions are now available.1The huge interest in nanomaterials is driven by their many desirable properties.In particular,the ability to tailor the size and structure and hence the properties of nanomaterials offers excellent prospects for designing novel sensing systems and enhancing the performance of the bioanalytical assay. The goal of this article is to highlight recent advances in nanomaterials for such electrical sensing devices. 2.Nanoparticles,nanowires and nanotubes Research efforts on metal and metal semiconductor nanoparticles have flour-ished in recent years.2,3Metal nano-particles are generally defined as isolable particles between1and50nm in size,that are prevented from agglo-merating by protecting shells.Owing to their small size such nanoparticles have physical,electronic and chemical proper-ties that are different from those of bulk metals.Such properties strongly depend on the number and kind of atoms that make up the particle.Several reviews have addressed the synthesis and proper- ties of nanoparticles.2,3Typically,such particles are prepared by chemical reduc- tion of the corresponding transition metal salts in the presence of a stabilizer (capping agent such as citrate or thiol) which binds to their surface to impart high stability and rich linking chemistry and provide the desired charge and solubility properties.Designer particles, including colloidal gold or inorganic nanocrystals have found broad applica- tions in many forms of biological tagging schemes.For example,colloidal quan- tum dots have been widely used for optical bioassays because their light emitting properties can be broadly tuned through size variation.4Recent years have witnessed the development of powerful electrochemical bioassays based on nanoparticle labels and ampli- fication platforms. One-dimensional(1-D)nanostruc- tures,such as carbon nanotubes(CNT) and semiconductor-or conducting- polymer nanowires,are particularly attractive for bioelectronic detection. Because of the high surface-to-volume ratio and novel electron transport pro- perties of these nanostructures,their elec- tronic conductance is strongly influenced by minor surface perturbations(such as those associated with the binding of macromolecules).Such1-D materials thus offer the prospect of rapid(real- time)and sensitive label-free bioelectro- nic detection,and massive redundancy in nanosensor arrays.The extreme smallness of these nanomaterials would allow packing a huge number of sensing elements onto a small footprint of an array device.Metal and conducting polymer nanowires can be readily prepared by a template-directed electro- chemical synthesis involving electro- deposition into the pores of a membrane template.5Carbon nanotubes (CNT)are particularly exciting1-D nanomaterials that have generated a considerable interest owing to their unique structure-dependent electronic and mechanical properties.6CNT can be divided into single-wall carbon- nanotubes(SWCNT)and multi-wall carbon-nanotubes(MWCNT).SWCNT possess a cylindrical nanostructure (with a high aspect ratio),formed by rolling up a single graphite sheet into a tube.SWCNT can thus be viewed as molecular wires with every atom on the surface.MWCNT comprise of an array of such nanotubes that are concentrically nested like rings of a tree trunk.The remarkable properties of CNT suggest the possibility of developing superior electrochemical sensing devices,ranging from amperometric enzyme electrodes to label-free DNA hybridization bio- sensors.7The tailored electronic con- ductivity of conducting polymers, coupled with their ease of processing/ modification and rich chemistry,make them extremely attractive as1-D sensing materials.Newly introduced CNT/ conducting-polymer nanowire mate- rials,8based on incorporating oxidized CNT as the charge-balancing dopants i-SECTION:HIGHLIGHT https://www.360docs.net/doc/505603589.html,/analyst|The Analyst
uncivilized behavior
Uncivilized behavior of Chinese tourists “You see, it’s common, most people didn’t care”said by one female tourist who is just throwing trash on the San Ya beach which has already been reduced to a dumping ground. When the beautiful island meets holiday crowd, under the hustling and bustling scene, we absolutely startled by the carving “Dao ci yi you”, dumbling beer bottles, spilling peels and half-burned branches. These facts are still far from enough.Uncivilized behavior makes headlines in the National Day holidays. At the memorable flag-raising ceremony at Tiananmen Square on October 1, over 120,000 participants left five tons of trash behind after showing their respect to the country. Sometimes, you could not find a place to sit –men take up all the benches in the park, lying full-length and blissfully snoring the afternoon away.Spitting, talking loudly and random littering are frequent scenes in tourist attractions. Countless visitors allow their children to use the city streets as a toilet, even though there is a toilet nearby. Newsstand and bookshop proprietors also suffer from bad-mannered people who tear the wrappers off magazines and read the contents without buying them. Bookstore visitors ignore the signs of asking them to rest only in designated areas instead of sitting on the stairwell. Similar stories are testing the tolerance of public opinion almost every day. We could be denied those undesirable manner. Nevertheless, it will be more persuasive to point out that China's tourism industry is not developing in a gradual way, but in a blowout manner. Growing wealth enables more Chinese people to seek leisure and fulfillment from traveling. Considering China's large population, some problems and challenges must emerge at the same time.We might be disappointed but not be desperate. Objectively speaking, before the national holiday, civilized traveling aroused surges of attention such as Wechat hot debate, media focus, screen exposure, which is beyond the past several years.Under the supervision of public opinion, the index of civilized golden week presents positive improvement. However,it’s still a serious short board that reflects on Chinese social moral deficiency. Not only should we take it seriously, but also wake up the consciousness of citizen responsibility and turn it in to action to make up. Forming harmonious social morality as if human’s maturation. We need time. I do believe the enlightened public moral education combined with perfected facilities will take several generations to nurture civilized behavior and display a positive image of Chinese tourists.
TPO4-leture1-animal behavior
Listen to part of a lecture in a biology class. The class is discussing animal behavior. Okay Ok , the next kind of animal behavior I want to talk about might be familiar to you. You may have seen, for example, a bird that's in a the middle of a mating ritual. and suddenly it stops and prines preens . You know, takes a few moments to straight straighten its feathers. and then returns to the mating ritual. This kind of behavior, this doing something that seems completely out of place. It's is what we call a displacement activity. Displacement activities are activities that animals engaged animal's engaging in when they have conflicting drives. If we take our example for from a minute ago if the bird is afraid of its mate, it's conflicted. It wants to mate, but it's also afraid and wants to run away. So instead, it starts grooming itself. So the displacement activity the grooming, the straightening of its feathers seems to be an irrelevant behavior. So what do you think another example of the a displacement activity might be? How about an animal that , um, instead of fighting its enemy or running away, it attacks a plant or a bush. That's a really good suggestion, Carol Karl . But that's called redirecting. The animal is redirecting its behavior to another object. In this case, the plant or the bush. But that's not an irrelevant or inappropriate behavior. The behavior makes sense. it's appropriate under the circumstances, but what doesn't make sense is the object behaviors direct the behavior's directed towards. Okay Ok , who else? Carol? I think I've read in another class about an experiment were where an object that the animal was afraid of was put next to its food. next to the animal's food. And the animal, it was conflicted between confronting the object and eating the food, so instead
Electrochemical supercapacitors Energy storage
GENERAL ARTICLES Electrochemical supercapacitors: Energy storage beyond batteries A. K. Shukla*, S. Sampath and K. Vijayamohanan Recently, a new class of reversible electrochemical energy storage systems have been developed that use: (a) the capacitance associated with charging and discharging of the electrical double-layer at the electrode–electrolyte interface and are hence called electrical double-layer capaci-tors (EDLCs), and (b) the pseudocapacitance with electrosorption or surface redox reactions which are referred as pseudocapacitors. While EDLCs with capacities of many tens of farads per gram of the electrode material have been achieved employing high surface-area carbon powders, fibres, or felts, much higher capacitance values are accomplished with pseudocapacitors employ-ing certain high surface-area oxides or conducting polymers. These electrochemical capacitors are being envisaged for several applications to complement the storage batteries. This article provides a brief introduction to scientific fundamentals and technological applications of electro-chemical supercapacitors. It is also stressed that there is a substantial scope for technology de-velopment in this newly emerging area, where materials science and polymer technology will have a pivotal role in conjunction with electrochemistry. A S the concern grows over fossil fuel usage, in terms of global warming and resource depletion, there will be a progressive swing to renewable energy. This will neces-sitate the development of improved methods for storing electricity when it is available and retrieving when it is needed. Electrical energy can be stored in two funda-mentally different ways: (i) indirectly, in batteries as potentially available chemical energy requiring faradaic oxidation and reduction of the electroactive reagents to release charges that can perform electrical work when they flow between two electrodes having different elec-trode potentials, and (ii) directly, in an electrostatic way as negative and positive electric charges on the plates of a capacitor by a process termed as non-faradaic electri-cal energy storage. A storage battery has two different types of active materials entrapped in a suitably conduc-tive matrix as anodes and cathodes to sustain the net cell reactions, while a capacitor comprises a dielectric sandwiched between two identical electrodes. During the storage of electrochemical energy in a bat-tery, chemical inter-conversions of the electrode mate-rials occur usually with concomitant phase changes. Although the overall energy changes can be conducted in a relatively reversible thermodynamic route, the A. K. Shukla is in the Solid State and Structural Chemistry Unit, and S. Sampath is in the Department of Inorganic and Physical Chemis-try, Indian Institute of Science, Bangalore 560 012, India; K. Vijayamohanan is in the Physical and Materials Chemistry Labora-tory, National Chemical Laboratory, Pune 411 008, India. *For correspondence. (e-mail: shukla@sscu.iisc.ernet.in) charge and discharge processes in a storage battery of-ten involve irreversibility in inter-conversions of the chemical electrode-reagents. Accordingly, the cycle-life of storage batteries is usually limited, and varies with the battery type. By contrast, with energy storage by a capacitor, only an excess and a deficiency of electron charges on the capacitor plates have to be established on charge and the reverse on discharge, and no chemical changes are involved. Accordingly, a capacitor has an almost unlimited recyclability, typically between 105 and 106 times. But, unlike storage batteries, capacitors can store only a very small amount of charge unless they are large. As a result, capacitors have a substan-tially low energy-density. However, charged electrode/ solution interfaces contain double layers that have ca-pacitances of 16–50 μFcm–2, and with sufficiently large accessible surface-electrode-areas realizable with high surface-area (1000–2000 m2/g) carbon powders, felts, and aerogels, capacitances as large as ~ 100 F/g can be achieved. In recent years, the practical realization of this possibility has led to the development of a new type of capacitors termed as electrochemical supercapacitors or ultracapacitors. At present, these capacitors are pro-gressing as energy devices to complement the storage batteries1–12. A schematic comparison between the charge–discharge profiles of a battery and a superca-pacitor is presented in Figure 1. In this article, we briefly describe the types of super-capacitors and origin of their capacitances, their charac-teristics vis-à-vis a rechargeable battery along with their envisaged applications.
