drug delivery in inflammatory bowel disease

Review Article
Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease:Selective targeting to
diseased versus healthy tissue
Susan Hua,BPharm,PhD a,?,Ellen Marks,PhD a,b ,Jennifer J.Schneider,BPharm,PhD a ,
Simon Keely,PhD a,b
a
The School of Biomedical Sciences and Pharmacy,The University of Newcastle,Callaghan,NSW,Australia
b
Gastrointestinal Research Group,Hunter Medical Research Institute,
New Lambton Heights,NSW,Australia
Received 8September 2014;accepted 25February 2015
Abstract
Colon targeted drug delivery is an active area of research for local diseases affecting the colon,as it improves the efficacy of therapeutics and enables localized treatment,which reduces systemic toxicity.Targeted delivery of therapeutics to the colon is particularly advantageous for the treatment of inflammatory bowel disease (IBD),which includes ulcerative colitis and Crohn's disease.Advances in oral drug delivery design have significantly improved the bioavailability of drugs to the colon;however in order for a drug to have therapeutic efficacy during disease,considerations must be made for the altered physiology of the gastrointestinal (GI)tract that is associated with GI inflammation.Nanotechnology has been used in oral dosage formulation design as strategies to further enhance uptake into diseased tissue within the colon.This review will describe some of the physiological challenges faced by orally administered delivery systems in IBD,the important developments in orally administered nano-delivery systems for colon targeting,and the future advances of this research.
From the Clinical Editor:Inflammatory Bowel Disease (IBD)poses a significant problem for a large number of patients worldwide.Current medical therapy mostly aims at suppressing the active inflammatory episodes.In this review article,the authors described and discussed the various approaches current nano-delivery systems can offer in overcoming the limitations of conventional drug formulations.
?2015The Authors.Published by Elsevier Inc.This is an open access article under the CC BY-NC-ND license (https://www.360docs.net/doc/ad5902891.html,/licenses/by-nc-nd/4.0/).
Key words:Colon targeted drug delivery;Nano-delivery systems;Oral administration;Inflammatory bowel disease;Colitis
Inflammatory bowel disease (IBD)is the umbrella term for a group of chronic relapsing gastrointestinal (GI)diseases which include ulcerative colitis (UC)and Crohn's disease (CD).1While UC and CD are considered distinct conditions,they can share many clinical features and are both characterized by cycles of relapsing and remitting mucosal inflammation.For UC,this inflammation is confined to the colon,extends proximally from the rectum and is continuous,in some cases involving the entire colon (pancolitis).Crohn's inflammation can affect any region of the GI tract,with the terminal ileum and the colon commonly affected.The inflammation is generally discontinuous in manner.1Although the exact cause of disease is undefined,certain factors
have been suggested to play a role,such as genetics,microbiome,environmental stress and immune dysfunction.2
There is currently no cure for IBD,with therapeutic strategies aimed toward attaining and maintaining remission from inflam-matory episodes.Steroids are commonly prescribed for acute exacerbations of both UC and CD,but prolonged use can lead to undesirable systemic side-effects.3,4Other therapies for IBD include aminosalicylates,antibiotics,and immuno-suppressive agents.While these medications can temporarily induce and maintain remission,70%of IBD patients will require at least one surgical intervention in their lifetime.5,6A systematic review by Talley et al 5highlighted the variable performance of current IBD therapies across IBD phenotype,location,stage and severity of disease.
Conventional oral formulations are limited for use in IBD as they are generally designed to achieve systemic delivery of therapeutics,which results in adverse effects and toxicity following distribution of drug around the body.Oral formula-tions achieving a localized effect are preferred in rational
drug
Nanomedicine:Nanotechnology,Biology,and Medicine
11(2015)1117–
1132
https://www.360docs.net/doc/ad5902891.html,
The authors wish to thank The University of Newcastle for their funding support.
Conflict of Interest:All authors declare no conflict of interest.
?Corresponding author at:School of Biomedical Science and Pharmacy,The University of Newcastle,Callaghan 2038,Australia.
E-mail address:Susan.Hua@https://www.360docs.net/doc/ad5902891.html,.au (S.Hua).
https://www.360docs.net/doc/ad5902891.html,/10.1016/j.nano.2015.02.018
1549-9634/?2015The Authors.Published by Elsevier Inc.This is an open access article under the CC BY-NC-ND license (https://www.360docs.net/doc/ad5902891.html,/licenses/by-nc-nd/4.0/).Please cite this article as:Hua S,et al,Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease:Selective targeting to diseased versus healthy tissue.Nanomedicine:NBM 2015;11:1117-1132,https://www.360docs.net/doc/ad5902891.html,/10.1016/j.nano.2015.02.018
delivery design for IBD.This ensures that drug will be delivered to the site of action within the GI tract,but will not be absorbed or will be poorly absorbed.Current therapeutic approaches specifically indicated for IBD rely on conventional dosage forms such as delayed or controlled release mechanisms.Their design is based on exploiting physiological conditions in the GI tract,in particular the colon.7For example,prodrugs of 5-aminosalicylic acid (5-ASA),such as sulfasalazine or olsalazine,rely on the enzymatic activity of colonic bacteria to cleave the prodrug into active moieties.8Similarly non-starch polysaccharide coatings,such as the COLAL-PRED?(prednisolone sodium metasulfo-benzoate)system,and matrix formulations rely on enzymatic degradation that is specific to colonic bacteria.9Another approach involves the use of pH-specific soluble coatings and matrices,which rely on the pH gradient of the GI tract to activate release,7while time-dependent release systems use GI transit times as a guide to activate release of the drug.10
These approaches are associated with inconsistent efficacy and inter-patient variability.5One reason for varied efficacy of these conventional colon targeted delivery approaches may lie in the diverse physiological changes and variability in the GI tract that present with chronic and active inflammation in IBD patients —including pH,GI transit time and the colonic microbiome.5,11Attempts to overcome these issues have focused
on improved understanding of the physiology of the GI tract during active IBD and following GI tract resection,as well as rational design of oral formulations.These considerations not only improve biodistribution of therapeutics to the colon,but also confer specific accumulation and cellular uptake within diseased tissue.12,13Recent pharmaceutical advances have applied nanotechnology to oral dosage form design in an effort to overcome the limitations of conventional formulations.14This review will describe some of the physiological challenges faced by orally administered delivery systems in IBD,the important developments in orally administered nano-delivery systems for colon targeting,and the future direction of this research.
General physiological considerations for colonic drug delivery Drug delivery to the colon relies on a number of physiological factors to ensure optimal efficacy following oral administration (Figure 1).Considerations should be made during formulation design to the residence time of the formulation in the GI tract,how the GI environment affects the delivery of the formulation and dissolution of the drug at the site of action,the intestinal fluid volume,and the propensity of the formulation or drug to be metabolised in the GI tract through enzymatic or
microbial
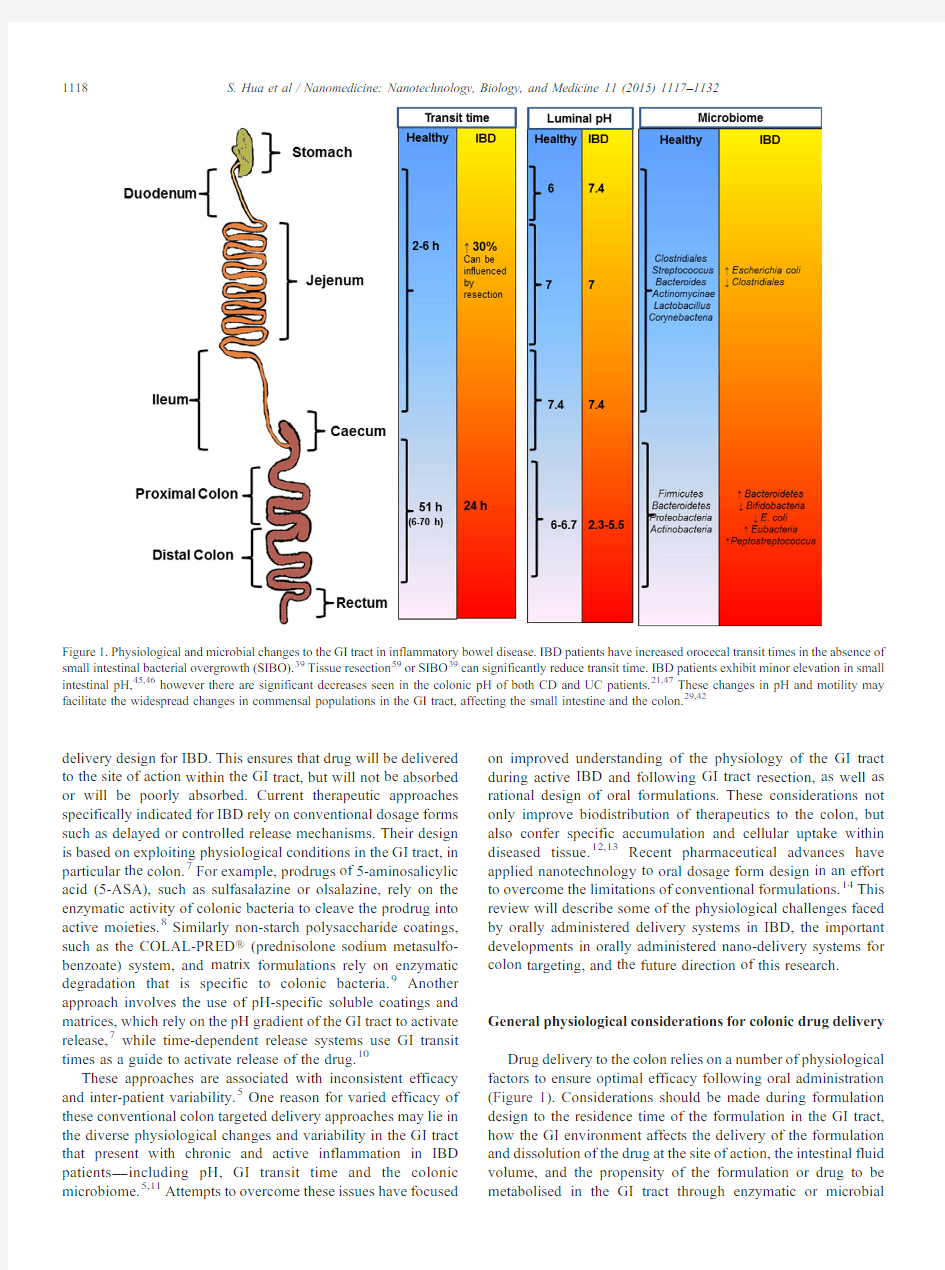
Figure 1.Physiological and microbial changes to the GI tract in inflammatory bowel disease.IBD patients have increased orocecal transit times in the absence of small intestinal bacterial overgrowth (SIBO).39Tissue resection 59or SIBO 39can significantly reduce transit time.IBD patients exhibit minor elevation in small intestinal pH,45,46however there are significant decreases seen in the colonic pH of both CD and UC patients.21,47These changes in pH and motility may facilitate the widespread changes in commensal populations in the GI tract,affecting the small intestine and the colon.29,42
1118S.Hua et al /Nanomedicine:Nanotechnology,Biology,and Medicine 11(2015)1117–1132
degradation.For instance,consideration of the formulation transit time through the GI tract is critical to ensuring delivery of the drug to the site of action.15Small intestinal transit time is generally accepted as4hours,16with individual variability ranging from2to6hours.In contrast,colonic transit times can vary significantly,with ranges from6to70hours reported.17,18 Additional confounders influencing GI transit time include gender,with females having significantly longer colonic transit times,19and the time of dosing with respect to an individual's bowel movements.20
Differences in pH along the GI tract have been exploited for the purposes of delayed release therapies.The highly acid stomach environment rises rapidly to pH6in the duodenum and increases along the small intestine to pH7.4at the terminal ileum.21,22Cecal pH drops below pH6and again rises in the colon reaching pH6.7at the rectum.23,24However,pH ranges can exhibit variability between individuals,with factors such as water and food intake as well as microbial metabolism being major determinants.25In addition to influencing pH,fluid:matter ratios may also affect the colonic delivery of drugs.For instance, free fluid volumes,bile salts and digestive enzyme levels in the GI tract are significantly altered following food intake.26,27 Intestinal fluid secretion also affects the viscosity of the mucous-gel layer,which may influence the ability of drugs to be taken up by cells at the site of action.28
Finally,we are now appreciating the importance of the intestinal microbiome in GI physiology.The GI tract plays host to over500distinct bacterial species,with many estimating the number of species to be close to2,000.29These bacteria play pivotal roles in both digestion and intestinal health,including digestion and metabolism of fatty acids,proteins and carbohydrates.30The majority of the intestinal microbiome resides in the anaerobic colon and fermentation of carbohydrates is the main source of nutrition for this population.30The relatively exclusive fermentation of non-starch polysaccharides by the colonic microbiome is exploited in formulations that use non-starch polysaccharide coatings.31While there appears to be considerable variation in the composition of the microbiome between individuals,which is influenced by both genetic and environmental factors,2the dominant Firmicutes,Bacteroidetes, Proteobacteria,and Actinobacteria species appear to be consistent and represent the majority of the colonic flora.32Despite this,the microbiome is exposed to temporal disruption(dysbiosis)by disease and medications(e.g.antibiotics),and is influenced by factors such as diet,lifestyle and geographical distinctions.2,33 It is therefore clear that within healthy individuals there is variability in the physiology of the GI tract.Adding to this complexity are the changes in physiology associated with GI disease,which will further affect the efficacy of orally administered formulations.These physiological factors are dynamic,inter-related and remain an important challenge in dosage form design.Depending on disease severity,gastroin-testinal pathologies can affect some or all of the physiological variables for oral drug delivery.Many acute GI infections will cause dysbiosis34and drive increased intestinal fluid secretion,35 and may increase or decrease bowel motility,36while more chronic conditions,such as IBD,can drastically and permanently alter the physiology of the GI tract.Changes in the physiology of the GI tract during active IBD An often overlooked confounder when considering oral delivery strategies in IBD is the change in the physiological condition of the GI tract associated with chronic inflammation. Mucosal inflammation in IBD causes pathophysiological changes,such as(i)a disrupted intestinal barrier due to the presence of mucosal surface alterations,crypt distortions and ulcers,(ii)increased mucus production and(iii)the infiltration of immune cells(e.g.neutrophils,macrophages,lymphocytes and dendritic cells).37,38During relapse of IBD,patients suffering from severe mucosal inflammation may exhibit altered GI motility and diarrhea,which in turn affects intestinal volume,pH and mucosal integrity.The inflammatory response at the mucosa, along with severe diarrhea,will also disrupt the resident microbiome,which can alter microbial metabolism in the GI tract.Thus active inflammation significantly alters the physiol-ogy of the GI tract(Figure1),which can affect the efficacy of conventional approaches to colon targeted drug delivery. Transit time and microbial considerations
Alterations to GI physiology in states of disease are often dynamic and inter-related,and therefore difficult to examine in isolation.For instance,orocecal transit time(OCTT),the time taken for the meal to reach the cecum,has been shown to be delayed in both CD and UC patients compared to healthy controls.39However,significantly faster OCTTs have been observed in IBD patients with the dysbiotic condition—small intestinal bacterial overgrowth(SIBO).These observations have been confirmed experimentally in humanized mice,following dietary manipulation of the gut microflora.40
Changes in the composition of the microbiome(dysbiosis)are common in GI disease,with alterations in physiology, inflammatory state,or as a result of treatment regimens.41 While it is generally accepted that the bacterial load is relatively static in IBD,the diversity of the microbiome is reduced with increases in major species such as Bacteroides,Eubacteria and Peptostreptococcus acting to the detriment of other populations.42It is not known what precipitates the initial dysbiosis,and whether dysbiosis precedes or is a symptom of disease.However,there is some evidence to suggest that physiological factors such as dysmotility and increased luminal fluid(diarrhea)may play a role.Studies in animal models have shown that prolonged water secretion into the bowel,leading to decreased colonic transit times,can alter the colonic microbiome.43,44Therefore,not only can the microbiome affect intestinal transit times,but conversely,transit times may also alter the intestinal microbiome.
In contrast to OCTT,colonic transit is significantly faster in IBD patients,likely due to the diarrhea that is a hallmark of the disease.1,11UC patients may exhibit transit times twice as rapid as a healthy individual,leading to difficulties in targeting specific regions of the colon with conventional formulations.Studies using conventional delayed release formulations have shown asymmetric biodistribution in the colon,with higher retention of drug in the proximal colon and significantly lower drug concentrations in the distal colon.11Thus transit time in itself
1119
S.Hua et al/Nanomedicine:Nanotechnology,Biology,and Medicine11(2015)1117–1132
may not be a reliable approach to colon-targeted drug delivery in IBD.
Changes in colonic pH
There is little evidence to suggest major alterations to small intestinal pH in IBD patients,45,46however colonic pH is significantly lower in both UC and CD patients.In the colon, intestinal pH is influenced by microbial fermentation processes, bile acid metabolism of fatty acids,bicarbonate and lactate secretions,and intestinal volume and transit times.23As all of these factors may be disrupted during active IBD,changes in luminal pH in the colon are not surprising.While normal colonic pH ranges from6.8in the proximal colon and rises to7.2in the distal colon,this can significantly vary in active UC patients from pH5.5to as low as2.3.21,47Similarly,reported colonic pH values for CD patients are approximately5.3,irrespective of disease activity.24These pH changes are likely to affect the composition of the colonic microbiome and thus colonic transit times,which can influence the release of drug from formulations requiring bacterial fermentation or enzymatic activity.Likewise, pH changes can affect the release of compounds from pH-dependant release coatings.
Intestinal volume
The composition of the intestinal biomass is altered in disease and is directly related to changes in microbial metabolism, intestinal transit time and luminal pH.In particular,increased fluid secretion and decreased reabsorption can dilute the digestive enzymes that control intestinal transit to allow nutrient absorption.48This in turn may influence the intestinal micro-biome,which can alter carbohydrate and polysaccharide digestion49as well as contribute to changes in intestinal transit times.48These changes in intestinal fluid volumes may alter the way conventional formulations are processed in the GI tract and the subsequent local delivery of drugs to the colon.
Mucosal integrity
The epithelial barrier selectively regulates transport from the lumen to the underlying tissue compartments,50restricting transport of smaller molecules across the epithelium,while virtually abolishing macromolecule transport.This selectivity is determined by apical transmembrane protein complexes known as tight junctions(TJ).These multi-protein complexes interact directly with underlying epithelium actomycin rings,influencing physiological and pathophysiological stimuli,such as ion transport,luminal glucose transport,water secretion and the transport of cytokines and leukocytes.51While these properties make TJ an attractive pharmacological target for enhancing drug absorption,52dysfunctional regulation of TJ complex formation is associated with a loss of epithelial integrity in intestinal inflammatory diseases,such as IBD.50Active inflammation not only alters intestinal mucosal integrity,but also significantly alters mucosal metabolism as the tissue attempts to limit further damage and repair.53For instance,in an attempt to compensate for the loss of intestinal epithelial integrity accompanying inflammation and tissue ischemia,a number of endogenous protective pathways are activated subtly altering the physiology of the mucosa.In order to augment intestinal barrier function, and perhaps compensate for the reduction in mucous-gel integrity with fluid secretion,the oxygen-sensing transcription factor,hypoxia-inducible factor(HIF),mediates increased expression of intestinal mucins and trefoil factors.53The viscosity of the mucous-gel layer is likely to affect the permeability of lipophilic drugs and mucoadhesive formulations.3In addition,HIF transcriptionally regulates multi-drug resistance gene1(MDR1),which codes for the xenobiotic drug efflux pump,P-glycoprotein(P-gp),that is involved in actively transporting substrate compounds back into the lumen.54For example,glucocorticoids are substrates for P-gp and have been shown to stimulate the expression of MDR1, potentially contributing to steroid resistance in IBD.55Interest-ingly,nano-delivery systems have been shown to target both drug and biological mechanisms to overcome multidrug resistance via P-gp inhibition and ATP depletion.56
Intestinal resection in IBD patients
Resection of bowel tissue is common among IBD sufferers, with over70%of IBD patients undergoing at least one surgery in their lifetime.6Removal of bowel tissue results in a shortening of the intestine and reduced transit distance through the GI tract, which potentially affects the way conventional oral formulations are processed.Beyond this,resection profoundly changes the physiology of the intestinal tract by altering pH,nutrient absorption,digestion and transit.57-59In particular,resection of the terminal ileum alters water absorption and dilutes residual bile acids in the colon,therefore reducing net colonic fatty acid concentrations.60,61This may profoundly alter microbial metab-olism of fatty acids by hydroxylation to produce ricinoleic acid analogues that can drive diarrhea.60,62Diarrhea significantly affects the therapeutic efficacy of conventional oral formulations.63The reduction in fatty acids also reduces the “ileal brake”—a nutrient feedback mechanism which slows transit times to allow nutrient absorption.48,64As fatty acids are the most potent stimulant of the ileal brake,a loss of both fatty acid receptors(from resected tissue)and fatty acids from digestion leads to a loss of the ileal brake65and a subsequent decrease in intestinal transit time.As a large proportion of IBD patients have undergone resection of the bowel,these physio-logical changes should be considered when devising targeted delivery strategies in the GI tract.
Current oral nano-delivery system strategies for drug delivery to inflamed colon
Improved oral drug delivery design has drastically improved the colonic bioavailability of drugs,that is,these formulations are effective at reaching and releasing drug specifically in the colon.However,in order for a drug to have therapeutic efficacy it must be localized to the site of action within the colon. Conventional oral formulations can be adversely affected during active IBD or following intestinal resection,and have limited efficacy and specificity for diseased colon tissue versus healthy colon tissue.66In addition,despite coverage of the colonic surface(including diseased tissue),there is no guarantee that the
1120S.Hua et al/Nanomedicine:Nanotechnology,Biology,and Medicine11(2015)1117–1132
drug is effectively taken up into the tissue and cells at the site of inflammation.12Pharmaceutical strategies utilizing nano-delivery systems as carriers for active compounds have shown promising results in addressing the physiological changes in IBD,and exploiting these differences to enhance specific delivery of drugs to diseased tissue.67,68Therefore the use of nanotechnology in formulation design may further improve the efficacy of therapeutics by allowing inflammation-specific targeting and uptake within the colon.
Nano-delivery systems have been designed to passively or actively target the site of inflammation.These systems have been shown to be more beneficial than conventional formulations, because their size leads to more effective targeting,better bioavailability at diseased tissues and reduced systemic adverse effects.Hence,nano-delivery systems have been found to have similar or improved therapeutic efficacy at lower drug concentrations in comparison to conventional formulations.67,68 Although size is an important factor in targeting the colon, additional strategies to enhance drug delivery to inflamed intestinal mucosa and achieve maximal retention time in tissues are being explored.This section will review current information on the effect of size and then characterize the different orally administered nano-delivery systems for IBD by their pharmaceutical strategy for targeted drug delivery to inflamed colonic tissue.
Size-dependent nano-delivery systems
Reducing the size of drug delivery carriers to the nanometer scale has been shown to improve colonic residence time in inflamed intestinal regions and provide additional benefits for IBD therapy(Figure2).This reduction in size enables enhanced and selective delivery of active molecules into the colitis tissue by exerting an epithelial enhanced permeability and retention (eEPR)effect,67,68and allows the preferential uptake of the nano-sized particles by immune cells that are highly increased in number at the inflamed regions.69By reducing the diameter of the particles,it is also possible to avoid rapid carrier elimination by diarrhea,which is a common symptom in IBD.70Nano-delivery systems avoid rapid carrier elimination by being readily taken up into inflamed tissue and cells.Conventional formula-tions do not have this advantage as they are generally designed to promote regional deposition of drug in the GI tract.Preferential accumulation in inflamed tissue increases the local concentration of therapeutics against IBD.Nanoparticles in the GI tract generally undergo cellular internalization by paracellular trans-port or endocytosis into epithelial cells in the GI tract.In IBD, specialized differentiated epithelial cells called M cells are involved in the predominant uptake of nanoparticles through transcytosis.Translocation of nanoparticles can also occur by persorption through gaps or holes at the villous tips.71-73 This accumulation is particle size dependent with an increasing effect for smaller particle diameters.74Lamprecht et al74investigated the significance of particle size on deposition in the inflamed colon in the trinitrobenzenesulfonic acid induced (TNBS)rat model of colitis.Studying fluorescent polystyrene particles ranging in size from0.1to10μm,administered orally for3days in vivo,highest binding to inflamed tissue was found for0.1μm particles(control healthy group: 2.2%±1.6%; colitis:14.5%±6.3%).The ratio of colitis/control deposition increased with smaller particle sizes.Interestingly,after removal of the mucus from the inflamed tissue through several washing steps,the total fluorescence in the tissue decreased by~39%for the0.1μm particles.This suggests that a high proportion of the particles were binding to the thick insoluble mucus layer rather than being taken up by macrophages.It should be noted that the nanoparticles in this study had a negative charge(negative zeta potential),which is a characteristic in itself that would have an influence on nanoparticle accumulation(discussed in Surface charge-dependent nano-delivery systems).
This particle size dependent effect has been shown to be independent of the nature of the carrier material itself,with a number of studies demonstrating accumulation of‘basic’nanocarriers within inflamed colon tissue.The term‘basic’is used to denote that no coating or conjugation mechanism or surface property alterations were utilized to enhance colon selectivity other than reducing particle size alone.The majority of these reports have been limited to in vitro cell studies,ex vivo tissue studies or in vivo animal studies following rectal administration.75While passive targeting with size enables longer retention time and enhanced permeability,there have been contradictory findings with regards to specificity to disease versus non-diseased tissue.For example,nanoparticles prepared with cetyltrimethylammonium bromide(CTAB)with a size of 200nm and a relatively neutral charge,significantly adhered to both non-inflamed colonic tissue in healthy controls as well as inflamed colonic tissue in the TNBS colitis model following rectal administration.76Conversely,polylactide–coglycolide nanoparticles(100nm)encapsulating the immunosuppressant drug,tacrolimus(FK506),was shown to significantly enhance drug penetration into inflamed tissue in both the TNBS and oxazolone(OXA)colitis model following rectal administration, with a reduction in both myeloperoxidase activity and colon/ body weight ratio.69The relative drug penetration into the inflamed tissue was approximately3-fold higher compared with healthy tissue with the use of nanoparticles as drug carriers ex vivo.The therapeutic effects of FK506-nanoparticles by the oral route were minor.This was potentially attributed to slow onset of drug release,degradation of the nanoparticle building matrix polyester by digestive enzymes in the upper GI tract,or systemic uptake and subsequent hepatic metabolism.
Interestingly,Schmidt et al77investigated the potential of nano-and microparticle uptake into the rectal mucosa of human IBD patients and found an obvious accumulation of micropar-ticles in active IBD,whereas nanoparticles were detectable only in traces in the mucosa of these patients.They demonstrated that microparticles exhibited accumulation and bioadhesion to the inflamed mucosal wall;however no absorption of these particles across the epithelial barrier was detected.Conversely,nanopar-ticles were translocated to the serosal compartment of IBD patients,possibly leading to systemic absorption.The study suggested that nanoparticles might not be required for local drug delivery to intestinal lesions in humans.The reason for the discrepancy of particle size between animal and human studies is unclear;however,it may be of major importance in the future treatment of human IBD.It should be noted that while particle
1121
S.Hua et al/Nanomedicine:Nanotechnology,Biology,and Medicine11(2015)1117–1132
Figure 2.Characteristic changes to the mucosal barrier in inflammatory bowel disease.The healthy mucosa (A)is protected by an inner adherent and outer mobile mucous-gel layer,which acts as a barrier to large molecules and hydrophobic compounds.28Underneath the mucous layer,a selectively permeable epithelial barrier allows nutrient absorption while excluding bacteria and luminal contents from the serosa.Antigen sampling is performed by Microfold (M)cells overlying lymphoid follicles.These M cells have a reduced or absent mucous-gel layer,28and may be targeted by nanoparticles.72,73Mucosal inflammation (B)is associated with a loss of both the inner adherent and outer mobile mucous-gel layers,loss of epithelial barrier integrity through enterocyte damage,and increased translocation of intestinal bacteria leading to a recruitment of immune cells to the mucosal tissue.These changes lead to a preferential accumulation and uptake of nanoparticles by both enterocytes and macrophages,including increased exposure of inflammatory receptor targets to nanoparticles.
1122S.Hua et al /Nanomedicine:Nanotechnology,Biology,and Medicine 11(2015)1117–1132
accumulation in ulcerated areas was statistically significant,the total fraction of particles penetrating into the mucosa was relatively low in the study.
While cumulative results to date confirm that reduction in particle size is essential for targeting colitis,the concept that ‘basic’nanoparticles when administered by the oral route are expected to accumulate specifically in inflamed colonic tissue over non-inflamed tissue has not been proven when taken into in vivo animal studies.To improve the effectiveness of nano-delivery systems administered orally,other mechanisms for maximising delivery of encapsulated therapeutics to areas of colitis,including altering surface properties,have been studied to enhance colon selectivity and diseased tissue specificity in vivo. Surface charge-dependent nano-delivery systems
Relatively little is known about how physicochemical parameters of drug carriers,other than particle size,influences adhesion to inflamed intestinal tissue.In particular,conflicting results have been reported on the influence of surface charge to colonic targeting,with results predominantly based on ex vivo tissue binding studies or in vivo studies following rectal administration.Modifying the surface charge of nano-delivery systems can influence the electrostatic interaction the nanocar-riers have with components in the GI tract and theoretically should confer selectivity to diseased tissue.It should be noted however,that there is a potential for electrostatic interactions and subsequent binding of these nanoparticles with other charge-modifying substances during GI transit(e.g.bile acids and soluble mucins).Therefore it is likely that additional pharma-ceutical strategies are needed,in addition to surface charge,in order to localize drug delivery specifically to diseased colitis tissue.
Positively charged nano-delivery systems—mucoadhesives Several studies have revealed a major influence by the cationic surface of nanoparticles on the deposition pattern and therapeutic efficiency in IBD.78,79Cationic nano-delivery systems adhere to the mucosal surface within inflamed tissue due to the interaction between the positively charged nanocarrier and the negatively charged intestinal mucosa.14Colonic mucins carry a negative charge since their carbohydrates are substituted with numerous sulfate and sialic acid residues.38,80Adhesion to the mucosa can be an advantage for GI tract targeting as it promotes better contact with the mucosal surface for cellular uptake and drug release.It can also reduce the clearance of nanocarriers when intestinal motility is increased,which is common in IBD.78,81An increase in mucus production is also observed in Crohn's disease,leading to a thicker mucus layer in particularly ulcerated areas,making mucoadhesion a promising strategy to increase targeting and retention of drug delivery systems in colitis.38,68
In support of mucoadhesive nano-delivery systems,Niebel et al82investigated the efficacy of rectally administered clodrona-te-loaded nanoparticles(120nm)comprising cationic poly-methacrylate(Eudragit RS)in the TNBS and OXA models of colitis.Although clodronate alone was ineffective in experimen-tal colitis therapy,its association with cationic nanoparticles enabled alleviation of the inflammatory response in both colitis models.Interestingly,it was observed that the therapeutic potential of Eudragit RS nanoparticles could have been greater if not for the nanoparticles being immobilized in the mucus, rather than penetrating the mucus layer and reaching and adhering to the inflamed mucosa for uptake into epithelial cells or immune cells.Interaction with mucin impeded the transport of cationic nanoparticles through the mucus layer and additionally risked premature drug release by an ion exchange mechanism. Similar results were seen by Lautenschlager et al83that assessed the ex vivo targeting potential of chitosan-functionalised poly(lactic-co-glycolic acid)(PLGA)nanoparticles(300nm)to human intestinal mucosa.The study showed that nanoparticles were able to adhere onto the tissue surface,however minimal particle translocation and deposition was detected in both inflamed(6.2%±2.6%)and healthy tissue(5.3%±2.3%). This was thought to be due to the strong electrostatic adhesion to the negative mucosal surface,thus preventing translocation into the tissue.In inflamed tissues,the particles showed a low particle penetration into the tissue,caused by adhesion into the mucosal gaps.This result is further supported by the ex vivo study conducted by Coco et al,14using mucoadhesive nanoparticles comprised of trimethylchitosan(TMC)on in-flamed mouse colon tissue.Therefore this approach might be useful for drugs that act on extracellular domains or only act after uptake into immune cells in active inflammation.
Conversely,research into chitosan-and pectin-coated lipo-somes have demonstrated enhanced drug uptake in vitro on Caco-2intestinal cells,ex vivo on excised intestinal tissue,and in vivo using animal models of colitis,compared to uncoated liposomes.78,84–87For example,Thirawong et al88evaluated the mucoadhesion and uptake of orally-administered pectin-liposome nanocomplexes(PLNs)in vivo in healthy Wistar rats that had been fasted for48hours.The study demonstrated increased residence of these nanoparticles in the GI tract mucosa, with very little colloidal aggregation;however the majority of the formulation was found to accumulate in the small intestine,with little uptake in the colon.Therefore additional pharmaceutical strategies are potentially needed in addition to surface charge in order to localize drug delivery specifically to diseased colitis tissue.
Negatively charged nano-delivery systems—bioadhesives Anionic nano-delivery systems were designed to preferen-tially adhere to inflamed tissue via electrostatic interaction with the higher concentration of positively charged proteins in inflamed regions.In particular,high amounts of eosinophil cationic protein and transferrin have been observed in inflamed colon sections of IBD patients.89-91However in order to reach the inflamed tissue,the drug delivery system would need to penetrate the thicker mucus layer overlaying the inflamed areas. Irrespective of surface charge,smaller particles tend to show improved adherence to the mucus layer due to an easier penetration into the layer with respect to their relatively small size.74,92Rather than immobilization following binding to the mucus(as seen with cationic nanoparticles),anionic nanoparti-cles are able to interdiffuse among the mucus network due to less electrostatic interaction with the mucus.
1123
S.Hua et al/Nanomedicine:Nanotechnology,Biology,and Medicine11(2015)1117–1132
An ex vivo comparison of cationic,anionic and neutral multilamellar liposomes(800±50nm)on inflamed tissue from the dinitrobenzene sulfonic acid(DNBS)-induced colitis model showed a preferential adherence of negatively charged liposomes to inflamed tissue,with a2-fold higher adherence compared to neutral or cationic liposomes.93The adherence of negatively charged liposomes was dependent on the concentration of DSPG used in the liposomal formulation and hence the negative charge density.Cationic and neutral particles showed no significant binding to the inflamed intestinal areas;however three times as many cationic liposomes adhered to the healthy colonic mucosa than neutral or anionic liposomes.Conversely,Lautenschlager et al83assessed the ex vivo targeting potential of negatively charged poly(lactic-co-glycolic acid)(PLGA)nanoparticles (300nm)to inflamed human intestinal mucosa.These anionic nanoparticles adhered onto the tissue surface and showed similar bioadhesion to inflamed(9.4%±5.2%)and healthy tissue (7.4%±6.3%)as compared to positively charged chitosan-functionalized nanoparticles.It should be noted that both of these studies examined the specificity of binding of nanoparticles under ex vivo conditions,which may not be comparable to an in vivo situation in IBD.
In vivo studies have shown promising results for anionic nano-delivery systems in IBD.For example,Beloqui et al70 demonstrated that anionic nanostructured lipid carriers(NLCs) loaded with budesonide(200nm)significantly reduced inflam-mation in the dextran-sulfate(DSS)-induced colitis model following oral gavage.NLCs are considered second-generation solid lipid nanoparticles(SLN)with higher stability and drug loading capacity.94The budesonide-loaded NLCs were able to decrease neutrophil infiltration,decrease the levels of pro-inflammatory cytokines in the colon and reduce histological disease in the colon.Even after DSS challenge in mice and in mice subjected to severe diarrhea,higher amounts of NLCs were observed in the colon12h after administration.It should be noted that overall,high amounts of the fluorescent-labeled NLCs were also detected in the small intestine of DSS-treated mice and in the colon of healthy control mice.The NLCs were reported to penetrate the mucosae in DSS-treated mice,in comparison to accumulating at the surface of the villi in healthy control mice. Recently,an in vivo study investigating the fate of negatively charged NLCs(150nm)following oral administration in healthy animals determined the pharmacokinetics and biodistribution of the NLC nano-delivery carrier.95The study showed localization mainly in the small intestine and retention of the nanocarriers in the underlying epithelium,allowing further uptake by epithelial cells.
In addition,Meissner et al96showed that the in vivo therapeutic efficacy and reduction in adverse effects of tacrolimus(FK506)-loaded PLGA nanoparticles were similar to tacrolimus-loaded pH-sensitive nanoparticles(composed of Eudragit P-4135F)(both450nm)in the DSS-induced colitis https://www.360docs.net/doc/ad5902891.html,pared to PLGA nanoparticles,pH-sensitive nano-particles exhibited a lack of specificity;however they had comparatively lower drug leakage and higher total amount of tacrolimus delivered to the colon.Conversely,PLGA nanopar-ticles increased drug concentration specifically inside the inflamed tissue,with a lower total amount of drug delivered.Degradation of PLGA nanoparticles during passage in upper parts of the intestine may enhance the potential for adverse effects.Similar alleviation of colitis was reported by Lamprecht et al92following oral administration of rolipram-loaded PLGA nanoparticles in the TNBS-induced colitis model.The nanopar-ticle system enabled the drug to accumulate in the inflamed tissue with higher efficiency than when given as a solution, which allowed continued reduction in inflammation following cessation of treatment.Although the results to date are promising for the specificity of anionic nano-delivery systems to diseased tissue,it appears that additional approaches may be required to improve bioavailability into the colon.
PEGylation-dependent nano-delivery systems
The use of poly(ethylene glycol)(PEG)on the surface of nanoparticles creates a hydrophilic surface chemistry that reduces interaction of the PEG-functionalized nanoparticles with the intestinal environment,therefore enabling an almost unhindered diffusion through the disturbed epithelium.97-99PEG is a hydrophilic and uncharged molecule that has properties which minimize a strong interaction with the mucus constituents, and increases particle translocation through the mucus as well as mucosa.97In particular,low molecular weight PEG has been shown to provide an effective shield of the hydrophobic core of the particles,while minimizing interpenetration or intermolec-ular interactions between PEG polymers and luminal surrounding.83,98This hydrophilic surface provides an acceler-ated translocation into the leaky inflamed intestinal epithelium, which is ideal for colitis targeted drug delivery.83
Lautenschlager et al83assessed the ex vivo targeting potential of PEG-functionalized PLGA nanoparticles(300nm)and micropar-ticles(3000nm)to inflamed human intestinal mucosa.Surface modification of nanoparticles with PEG demonstrated significantly enhanced particle translocation and deposition in inflamed mucosal tissues compared to chitosan-and non-functionalized PLGA particles.PEG-functionalized microparticles showed significantly increased translocation through inflamed mucosa (3.33%)compared to healthy mucosa(0.55%,P=0.045),and significantly increased particle deposition in inflamed mucosa (10.8%)compared to healthy mucosa(4.1%,P=0.041). Interestingly,PEG-functionalized nanoparticles showed the high-est translocation through inflamed(5.27%)and healthy mucosa (2.31%,P=0.048).Particle deposition was also higher in comparison to PEG-functionalized microparticles,however there was no significant difference between depositions in inflamed mucosa(16.7%)compared to healthy mucosa(13.7%).
In addition,Vong et al100designed a novel nitroxide radical-containing nanoparticle(RNP O),which possesses anti-oxidative nitroxide radicals in the core for treatment of mice with DSS-induced colitis.In several experimental models,antioxidant compounds and free radical scavengers have improved colitis; however are associated with significant bioavailability and retention issues.101-103Therefore these nanoparticles were designed to deliver antioxidant compounds specifically to diseased tissue for treatment of IBD.RNP O contains a new redox polymer,methoxy-poly(ethylene glycol)-b-poly(4-[2,2,6,6-tetramethyl-piperidine-1-oxyl]oxymethylstyrene
1124S.Hua et al/Nanomedicine:Nanotechnology,Biology,and Medicine11(2015)1117–1132
(MeO-PEG-b-PMOT),which is an amphiphilic block copolymer with stable nitroxide in a hydrophobic segment as a side chain via an ether linkage,and forms40nm sized core shell-type micelles(RNP O)by self-assembly in aqueous environments regardless of pH.RNP O showed significant accumulation in the colonic mucosa,especially the inflamed mucosal tissue, in comparison to control4-hydroxyl-2,2,6,6-tetramethyl-piperidine-1-oxyl(TEMPOL)or polystyrene latex particles, and did not undergo systemic absorption despite its long-term retention in the colon.Accumulation of RNP O in the colon was observed to be almost50times higher than that of TEMPOL. Mice with DSS-induced colitis had significantly lower disease activity index and less inflammation following7days of oral administration of RNP O compared to mice given TEMPOL or mesalamine.The accumulation of RNP O in the colon was dependent on both the size and PEGylated character of the nanoparticles.The PEGylated character of RNP O was thought to protect nitroxide radicals in the hydrophobic core from harsh conditions in the GI tract after oral administration,resulting in significant accumulation in the colon area.104Furthermore,PEG chains may achieve mucoadhesion due to their ability to interdiffuse among the mucus network and polymer entangle-ment with mucin,which is composed of glycoprotein.99Despite a limited number of studies,those to date support PEGylation as a promising pharmaceutical strategy for accumulation in inflamed colonic mucosa in IBD.
pH-dependent nano-delivery systems
This pharmaceutical strategy takes advantage of the difference in pH in various regions of the GI tract.The pH in the terminal ileum and colon is generally higher than in any other region of the GI tract21,22;therefore a dosage form that disintegrates preferentially at high pH levels has potential for site-specific delivery into the colon.One of the simplest ways to modify dosage forms for pH-dependent drug delivery is to coat them with pH-sensitive biocompatible polymers.105In addition to trigger-ing release at specific pH range,the enteric-coating protects the incorporated active agents against the harsh GI tract environment (e.g.gastric juice,bile acid and microbial degradation),and creates an extended and delayed drug release profile to specific GI tract regions to enhance therapeutic efficiency.
The most commonly used pH-dependent coating polymers for oral delivery are methacrylic acid copolymers(Eudragit?). By varying their side-group composition Eudragits?can be manipulated to alter the pH at which they are soluble.Eudragit L100and Eudragit S100,which dissolve at pH6and7 respectively,are commonly used in combination in various ratios to manipulate drug release within the pH6to7range.15 Eudragit FS30D is one of the more recently developed polymers and dissolves at pH above6.5.It is an ionic co-polymer of methyl acrylate,methyl methacrylate and methacrylic acid and is increasingly used for colon targeted drug delivery.15In addition to its pH-dependent release strategy,Eudragit?coatings have also been suggested to have mucoadhesive properties.Karn et al106demonstrated that liposomes coated with Eudragit?have superior mucoadhesion characteristics in freshly extracted pig intestinal tissue,compared to other commonly investigated polymer coatings such as chitosan and carbopol.The results suggest that Eudragit?coatings may enable pH-dependent release and possibly reduce formulation clearance to enhance colon targeted drug delivery.
Eudragit?-coated nano-delivery systems have demonstrated favorable pH-dependent release characteristics in vitro.107,108 For example,Barea et al109reported a significant reduction in drug release from Eudragit?-coated liposomes in solutions designed to simulate the pH conditions of the stomach and small intestine.Drug release was equivalent to the uncoated control at pH7.8,indicating that the formulation displayed appropriate pH responsive release characteristics.A further assay tested the stability of the Eudragit-coated liposomes in simulated small intestine fluid with the addition of biologically relevant quantities of bile salts.The coating layer was not able to withstand the additional challenge of bile salts,which would potentially adversely affect its stability in vivo,causing premature degradation of the liposomes and release of the drug in the duodenum.
The potential instability of liposomes in the GI tract has led to development of polymer-based carriers for colon-specific drug delivery.A number of in vivo studies have investigated the use of pH-dependent polymer-based nano-delivery systems for colon targeting in IBD.Makhlof et al110investigated budesonide-loaded pH-sensitive nanospheres in the TNBS colitis model.The nanospheres(260-290nm)were prepared using polymeric mixtures of PLGA and Eudragit?S100.In vivo experiments demonstrated superior therapeutic efficacy of budesonide-loaded nanospheres in alleviating colitis compared to that of conventional enteric-coated microparticles(1.97±0.78μm).Nanospheres showed higher colon levels and lower systemic bioavailability, as well as specific adhesion to the ulcerated and inflamed mucosal tissue of the rat colon.Similarly,Kshirsagar et al111formulated polymeric nanocapsules of prednisolone with Eudragit?S100 (567nm)and demonstrated pH-dependent release in vitro over a time period corresponding to normal physiological GI transit. Nanocapsules have a polymeric wall enveloping an oil core and generally have a lower polymer content and higher loading capacity for lipophilic drugs in comparison to nanospheres.112 Pharmacokinetic studies in healthy rats did not show a rise in plasma concentration for up to3h,and the increase in drug concentration thereafter indicates dissolution of the nanocapsules and release of the drug in the colon.
Several studies have thoroughly evaluated the clinical outcomes following treatment with pH-dependent nano-delivery systems in colitis,giving a good indicator of translational applicability other than biodistribution.For example,Ali et al113 showed that budesonide-loaded PLGA nanoparticles coated with Eudragit?S100(~240±14.7nm)were able to significantly alleviate inflammation and demonstrated signs of regeneration in the DSS,TNBS and OXA in vivo models.More specifically,the general endoscopic appearance of the groups treated with the coated PLGA nanoparticles was similar to the healthy mice groups,with no severe signs of inflammation as opposed to the remaining control groups(inflamed control,budesonide solution and plain PLGA NP).The coated PLGA nanoparticles also showed significant down-regulation of pro-inflammatory cyto-kines expression(TNF-α,IL-6,IL-1β,IFN-γ)in the colons.
1125
S.Hua et al/Nanomedicine:Nanotechnology,Biology,and Medicine11(2015)1117–1132
More recently,Beloqui et al114evaluated the local delivery of curcumin using pH-sensitive polymeric nanoparticles(166nm) composed of PLGA and Eudragit?S100(CC-NPs)both in vitro and in https://www.360docs.net/doc/ad5902891.html,-NPs significantly enhanced drug permeation across Caco-2cell monolayers when compared to free drug in suspension.In addition,CC-NPs significantly reduced neutro-phil infiltration,TNF-αsecretion and histological disease in the colon of DSS-treated mice following oral gavage.It should be noted that a higher accumulation of curcumin was seen in both healthy and DSS-treated mice when encapsulated in nanoparti-cles compared to the free drug suspension.Similarly,Lamprecht et al115designed tacrolimus(FK506)-loaded PLGA nanoparti-cles entrapped into pH-sensitive microspheres(NPMS)to achieve greater selectivity to the colon.Nanoparticles were coating with Eudragit?P-4135F.In vivo studies in the TNBS colitis model demonstrated significant reduction in the colon/ body weight index and myeloperoxidase activity only in the NPMS group(colitis control:21.94±4.97;FK506solution: 15.81±3.42;FK506-NP:17.03±5.52;FK506-MS:15.17±7.81;and FK506-NPMS:10.26±7.76U/mg tissue),which indicates a reduction in the severity of inflammation.The NPMS system also showed reduced systemic absorption,thus confer-ring a local and selective delivery of NP in the colon.
Although preclinical studies of pH-dependent nano-delivery systems for colon targeting have been promising,a major concern has been the inherent inter-individual and intra-individual variability of pH and emptying times from the GI tract,as well as the change in luminal pH due to disease state.A colonic delivery system that is based only on GI transit time or pH of the GI tract would simply not be reliable for IBD.Studies in human volunteers have shown that since the pH drops from7.0at the terminal ileum to6.0of ascending colon,such systems sometimes fail to release the drug.15The potential for degradation of the Eudragit?coating by bile acids in the duodenum also requires further investigation.
Biodegradable nano-delivery systems in the colon
Having an understanding of the physiological variability in IBD,such as pH and GI transit time,biodegradable nano-delivery systems were devised to take advantage of other factors that are known to be more consistent in IBD patients to allow efficient colon-targeted drug https://www.360docs.net/doc/ad5902891.html,roui et al13developed a hydrogel that is specifically degraded by enzymes in the colon at pH6.2,using ions(Ca2+and SO42?)that cross-link chitosan and alginate.The hydrogel was embedded with nanoparticles containing an anti-inflammatory tripeptide Lys–Pro–Val(KPV) (400nm).Under the protection of the hydrogel,particles were able to pass through the stomach and upper small intestine,and were degraded in the inflamed colon.Encapsulated KPV-loaded nanoparticles in hydrogel,administered by oral gavage, efficiently reduced the severity of colitis in the DSS colitis model,as shown by a reduction in myeloperoxidase activity and histologic https://www.360docs.net/doc/ad5902891.html,ing this improved oral nanoparticle-based drug delivery system,a1200-fold lower dose was sufficient to ameliorate mucosal inflammation in vivo compared to KPV in free solution.It should be noted that the biomaterial used in the study was composed of chitosan,which is a cationic polymer with mucoadhesive properties.This may have increased the therapeutic efficacy of the formulation following enhanced delivery into the colon by the novel hydrogel.
To bypass the degradative effects of components in the GI tract,recent studies have also embedded nano-delivery systems in hydrogel(chitosan/alginate)that selectively degrades in the vicinity of the inflamed colon.For example,Laroui et al116used hydrogel to embed siRNA in nanoparticles for IBD therapy.Oral administration of CD98siRNA/polyethyleneimine(PEI)-loaded nanoparticles(~480nm)encapsulated in hydrogel reduced CD98expression in mouse colonic tissues and decreased DSS-induced colitis in a mouse model.Flow cytometry showed that CD98was effectively down-regulated in the intestinal epithelial cells and intestinal macrophages of treated mice. Similarly,Xiao et al117used hydrogel(chitosan/alginate)to deliver CD98-targeted nanoparticles loaded with CD98siRNA (discussed in Active targeting-dependent nano-delivery systems).
Another unique colon-targeted nano-delivery system is the nanoparticle-in-microparticle oral delivery system (NiMOS).118-120NiMOS are designed for oral administration of plasmid and siRNA by encapsulating them in type B gelatin nanoparticles,which are further entrapped in poly(epsilon-caprolactone)(PCL)microspheres.PCL is a synthetic hydro-phobic polyester that is resistant to degradation by acid,therefore protecting nanoparticles during transit through the stomach.In addition,the coated microparticles are able to inhibit protein/ enzyme adsorption,thereby avoiding the harsh environment of the GI tract.Release of the payload carrying nanoparticles occurs over time at inflamed sites in the intestine,via controlled degradation of the outer PCL layer by action of lipases abundantly present at this location,after which they can be endocytosed by enterocytes or other cells at these sites.120This multicompartmental biodegradable polymer-based nano-delivery system has been used to deliver TNF-αsiRNA,118as well as a combination of siRNA duplexes specifically targeted against TNF-αand cyclin D1(Ccnd1).119NiMOS loaded with siRNA demonstrated successful gene silencing and significantly reduced inflammation in the DSS-induced colitis model.
In addition,silica nanoparticles(SiNP)have been modified to have selective drug delivery toward inflamed tissue in chronic inflammatory diseases of the intestine.121SiNPs have been widely used in the biomedical field as a pharmaceutical excipient for oral drug delivery.Typically when used as medication carriers,drugs are adsorbed onto the surface of the nanoparticles and release triggered by simple desorption kinetics;which are poorly controllable in complex biological media(e.g.blood and GI juices).122To combat premature release of drug compounds that are physically entrapped or adsorbed to nano-delivery systems,Moulari et al121covalently bound the anti-inflammatory drug,5-aminosalicylic acid(5ASA),to the surface of modified SiNP(Me5ASA-SiNP)(140nm).The resulting chemical bond is biodegradable and intended to considerably delay drug release. Me5ASA–SiNP for drug delivery in IBD combines therapeutic approaches of passive drug targeting by nanoparticles,and the triggered release from a prodrug retaining the entrapped drug following selective accumulation in the inflamed tissue.The chemical modification of the SiNP surface involves the integration of hydrophobic chemical entities,which makes the
1126S.Hua et al/Nanomedicine:Nanotechnology,Biology,and Medicine11(2015)1117–1132
surface less accessible for enzymes.A second aspect is the density of Me5ASA on the surface,which itself may act to inhibit accessibility to enzymes for steric reasons.These modifications delay drug release until the nanoparticles accumulate within inflamed regions of the colon,where drug release is then triggered following enzymatic degradation of the peptide bonds between the SiNP and Me5ASA.It has been suggested that the enzymatic cleavage of Me5ASA into5ASA is potentially triggered by esterases;however under in vivo conditions various other mechanisms may be involved.In vivo studies in the TNBS-induced colitis model demonstrated selective accumulation in the inflamed colonic tissues following oral administration,with a 6-fold higher adhesion compared to healthy control groups. Me5ASA–SiNP also significantly reduced inflammation at a much lower dose compared to5ASA-solution.
Redox nano-delivery systems
This pharmaceutical strategy for targeted drug delivery to diseased colonic tissue takes advantage of the abnormally high levels of reactive oxygen species(ROS)produced at the site of intestinal inflammation.For example,biopsies taken from patients suffering from ulcerative colitis have a10-to100-fold increase in mucosal ROS concentrations,which are confined to sites of disease and correlate with disease progression.123,124The unusually high concentrations of ROS localized to sites of intestinal inflammation are generated by activated phagocytes.125 Taking advantage of this increased ROS concentration in diseased tissue in IBD,Wilson et al126synthesized thioketal nanoparticles(TKNs)as a delivery vehicle for https://www.360docs.net/doc/ad5902891.html,Ns are formulated from a polymer,poly(1,4-phenyleneacetone dimethy-lene thioketal)(PPADT)that degrades selectively in response to ROS.PPADT was used to encapsulate TNF-αsiRNA complexed with the cationic lipid,1,2-dioleoyl-3-trimethylammonium-propane(DOTAP),to form https://www.360docs.net/doc/ad5902891.html,plexing siRNA with cationic species,such as DOTAP,enhances siRNA transfection by increasing siRNA stability,mucosal transport, cellular internalisation and endosomal escape.127,128Further-more,incorporating DOTAP confers nanoparticles with a positive surface charge,which can increase particle uptake by phagocytes129and adhesion to the negatively charged intestinal mucosa.38TKNs showed selective localization of orally delivered siRNA,against the proinflammatory cytokine TNF-α(0.23mg siRNA/kg/day),to sites of intestinal inflammation in the DSS-induced colitis model.These nanoparticles significantly suppressed mRNA levels of TNF-αand several other pro-inflammatory cytokines(IL-1,IL-6and IFNγ)in colon tissues, while also reducing colonic inflammation as measured by histology. In contrast,mice receiving DSS and treated with controls,including TNF-α-PLGA(anionic)and TNF-α-DOTAP(cationic),showed all of the characteristics of DSS-induced inflammation as measured by histology,high levels of myeloperoxidase activity and significant weight loss.These results support the ability of TKNs to target inflamed tissues as an important factor for their in vivo efficacy. Active targeting-dependent nano-delivery systems
Active targeting approaches using ligands coupled to the surface of nano-delivery systems may increase therapeutic efficiency and reduce adverse reactions,by further improving selective drug accumulation at inflamed sites within the colon.This approach has been used to exploit disease-induced changes in the expression of receptors,adhesion molecules and proteins on the cellular surface of tissues affected by disease.12,130The vast majority of research in active targeting-based nano-delivery systems has been studied using the parenteral route of adminis-tration to target a multitude of conditions,such as cancers, infections and sites of inflammation.131,132With such positive outcomes,it was inevitable for researchers to attempt this pharmaceutical strategic approach for orally administered nano-delivery systems.Monoclonal antibodies and peptides are commonly used as targeting moieties,as they have been shown to have high specificity in targeting and potential mucopenetrative properties.133It should be noted that oral administration of antibody and peptide-based formulations encounter many obsta-cles in the GI tract,in particular degradation by the stomach acid as well as by enzymes;therefore these nano-delivery systems may require further formulation design.This pharmaceutical strategy is based on the concept that interactions between targeting ligands and specific receptors expressed predominantly at inflamed sites would improve bioadhesion of the drug formulation to specific cells and increase the extent for endocytosis.
An increasing number of targeting ligands have been studied for oral colon-specific drug delivery strategies.Mane and Muro134evaluated the biodistribution and cellular uptake of polystyrene nanoparticles coated with anti-ICAM-1antibodies in the GI tract,following oral administration,in wild-type C57BL/6 mice using fluorescence and radiolabeling.As expected, approximately60%of the antibody dose administered(1.1mg antibody per kg)was degraded,which was attributed mainly to GI tract enzymes.The nanoparticles were deposited mainly in the stomach and duodenum,which suggest more upper GI tract targeting.Transmission electron microscopy and energy disper-sive X-ray spectroscopy were used to show endocytosis of the radiolabeled anti-ICAM-1nanoparticles within duodenal tissue via ICAM-1.It should be noted that biodistribution of these targeted nanoparticles was not evaluated in an animal model of colitis.ICAM-1is known to be significantly upregulated in inflamed regions of the colon in IBD,135-138with most prominent expression on the surface of inflamed intestinal mucosal tissues and microvasculature.139,140
Macrophage receptors have been another target for orally administered nano-delivery systems for IBD.Mannose receptors and macrophage galactose-type lectin(MGL)are highly expressed by activated macrophages under inflammatory conditions.141,142Xiao et al143synthesized a mannosylated bioreducible cationic polymer(PPM)that was formed into nanoparticles with sodium triphosphate(TPP)and TNF-αsiRNA by electrostatic interaction(TPP-PPM/siTNF NPs).The nanoparticles showed an enhanced siRNA condensation capac-ity,a desirable size distribution of~240nm,and a significant macrophage-targeting ability.TPP-PPM/siTNF NPs significant-ly inhibited TNF-αsynthesis and secretion in tissue samples from the DSS-induced colitis model ex vivo.Importantly, TPP-PPM/siTNF NPs were efficiently taken up by macrophages, with flow cytometry analysis demonstrating29.5%uptake by colon macrophages and insignificant uptake by epithelial cells.
1127
S.Hua et al/Nanomedicine:Nanotechnology,Biology,and Medicine11(2015)1117–1132
The high penetration ability and macrophage-targeting efficiency of TPP–PPM/siTNF NPs was suggested to reflect the combined effect of the small particle size,surface PEGylation,and the conjugation of mannose ligands.Similarly,Coco et al14 demonstrated in an ex vivo study that mannose-grafted PLGA nanoparticles had the highest accumulation in inflamed colon tissue from the DSS-induced colitis model,in comparison to trimethylchitosan(TMC),PLGA and Eudragit?S-100nanopar-ticles.It should be noted that both of these studies did not evaluate the formulation in an in vivo model of colitis—the results of which would be of great interest.
To target macrophage galactose-type lectin(MGL)on activated macrophages,Zhang et al144prepared galactosylated trimethyl chitosan-cysteine(GTC)nanoparticles for oral delivery of mitogen-activated protein kinase kinase kinase kinase4 (Map4k4)siRNA(si Map4k4),which is a key upstream mediator of TNF-αproduction.siRNA loaded GTC nanoparticles were prepared based on ionic gelation of GTC with anionic cross-linkers,such as tripolyphosphate(TPP).Cellular uptake in activated macrophages was significantly higher for GTC/TPP nanoparticles compared to trimethyl chitosan-cysteine(TC)/TPP nanoparticles,owing to galactose receptor-mediated endocyto-sis.In vitro and in vivo studies showed effective inhibition of TNF-αproduction and selective biodistribution of siRNA in ulcerative tissue.Daily oral administration of si Map4k4loaded GTC/TPP nanoparticles significantly improved DSS-induced colitis,as measured by body weight,histology,and myeloper-oxidase activity.
A recent study by Laroui et al145demonstrated that TNFαsiRNA can be efficiently loaded into nanoparticles made of poly(lactic acid)poly(ethylene glycol)block copolymer(PLA–PEG),and that grafting of a macrophage-specific ligand(Fab′portion of the F4/80Ab—Fab'-bearing)onto the nanoparticle surface,via maleimide/thiol group-mediated covalent bonding, increases the specificity of targeting to intestinal macrophages. TNFα-siRNA-loaded nanoparticles significantly improved DSS-induced colitis in vivo,following oral administration, more efficiently when the nanoparticles were covered with Fab ′-bearing ligands compared to non-conjugated nanoparticles. Grafting of Fab′-bearing ligands also improved nanoparticle endocytosis as well as macrophage targeting ability,as indicated by flow cytometry.It should be noted that this study did load the nanoparticles into a colon-specific biodegradable hydrogel (chitosan/alginate),which also enhanced its specific delivery to the colon and protected the grafted ligand during GI transit (discussed in Biodegradable nano-delivery systems).
The transferrin receptor(TfR)is another target that is overexpressed in inflamed colon tissue,with elevated expression in both the basolateral and apical membranes of enterocytes.89 This increase was observed in both colon biopsies from IBD patients and excised colon tissue from colitis-induced rat models of IBD.89,146TfR levels are also elevated in activated immune cells,including lymphocytes and macrophages.146Harel et al147 investigated the ex vivo adhesion capacity of immunoliposomes with anti-TfR antibodies conjugated to its surface to inflamed mucosal tissue.The study reported mucopenetration of the targeted formulation,with a4-fold increase in uptake in inflamed colon tissue from the TNBS-induced colitis model,compared to non-inflamed colon tissue.This study does suggest a role for mucosal transferrin receptor targeting in IBD;however additional formulation measures would be required to protect the lipid-based immunoliposomes from premature degradation in the GI tract prior to evaluating the targeted nano-delivery system in vivo.
Epithelial CD98,a type II membrane glycoprotein heterodi-mer,has been suggested as another promising target for IBD,as it has been shown to play a vital role in intestinal inflammation. Overexpression of CD98on the surface of colonic epithelial cells and macrophages promote the development and progression of IBD.148-150Recently,Xiao et al117developed an orally delivered hydrogel that releases CD98siRNA loaded nanoparticles with single-chain CD98antibodies conjugated onto the surface (200nm).In mice with DSS-induced colitis and colitis induced by transfer of CD4+CD45Rb high T cells to Rag?/?mice,oral administration of the targeted nanoparticles significantly reduced the overexpression of CD98by colonic epithelial cells and macrophages.Approximately24%of colonic macrophages in the mice had taken up the targeted nanoparticles within12hours of administration.Severity of colitis was also significantly reduced compared to the control groups,based on loss of body weight,myeloperoxidase activity,inflammatory cytokine pro-duction,and histological analysis.Overall,these studies demonstrate that active targeting is a very promising approach to enhancing drug accumulation and uptake into inflamed tissue in IBD;however further in vivo studies are required to assess the different targeting ligands and formulations for efficacy and stability in animal models of colitis.
Future advances in colon targeted drug delivery
The design of nano-delivery systems has significantly advanced the future for IBD therapy by improving the selective targeting of active agents to sites of inflammation.Contrary to most therapeutic regimens'utilizing oral administration,sys-temic absorption is an undesirable delivery feature for these drugs.Disease localization dictates the need for maximal intestinal tissue drug exposure while systemic delivery should be minimized to avoid unwanted side effects.This drug delivery approach has been shown to increase therapeutic efficacy,lower the therapeutically effective dose,reduce systemic side effects, and has allowed the use of novel compounds with poor physicochemical properties for oral delivery.This has been achieved through specific biodistribution and accumulation in the inflamed intestinal regions.
In order for the translational use of these carrier systems to the clinic,several issues still have to be addressed.Firstly,the safety of the different nano-delivery carriers following uptake need to be explored further.Studies focused on the nanotoxicology of these delivery systems in the human GI tract in IBD have been limited,and is likely to vary according to the nanoparticle material(e.g.polymer,lipids)and nanoparticle size.73Secondly, structural stability during GI transit would need to be further optimized to prevent premature release in the stomach and small intestine.Although in vitro and ex vivo stability,binding and uptake studies provide valuable information for the nano-delivery systems,these same parameters need to be validated in vivo using well-established colitis models.Thirdly,increased
1128S.Hua et al/Nanomedicine:Nanotechnology,Biology,and Medicine11(2015)1117–1132
drug residence time in regions of diseased tissue would serve to further optimize this therapy.Based on the results to date,it is very likely that a combination of pharmaceutical strategies that have been discussed in this review is required for optimal targeting to inflamed colon.Finally from a commercial development point of view,simplification of drug delivery design is required to allow efficient and reliable large-scale manufacturing.In translating these findings from animals to humans,we need to determine how to modify these formulations so that they are appropriate for human administration.These in vivo studies have been done in animal models of IBD,which place limitations on the size and consistency of the dosage form that can be administered orally.The practicability of designing dosage forms that are both acceptable to humans and efficacious needs to be further explored.
References
1.Podolsky DK.Inflammatory bowel disease.N Engl J Med
2002;347(6):417-29.
2.Sartor RB.Genetics and environmental interactions shape the intestinal
microbiome to promote inflammatory bowel disease versus mucosal homeostasis.Gastroenterology2010;139(6):1816-9.
3.Keely S,Ryan SM,Haddleton DM,Limer A,Mantovani G,Murphy
EP,et al.Dexamethasone–pDMAEMA polymeric conjugates reduce inflammatory biomarkers in human intestinal epithelial monolayers.J Control Release2009;135(1):35-43.
4.Podolsky DK.The future of IBD treatment.J Gastroenterol
2003;38(Suppl15):63-6.
5.Talley NJ,Abreu MT,Achkar JP,Bernstein CN,Dubinsky MC,
Hanauer SB,et al.An evidence-based systematic review on medical therapies for inflammatory bowel disease.Am J Gastroenterol 2011;106(Suppl1):S2-S25[quiz S6].
6.Byrne CM,Solomon MJ,Young JM,Selby W,Harrison JD.Patient
preferences between surgical and medical treatment in Crohn's disease.
Dis Colon Rectum2007;50(5):586-97.
7.Yang L,Chu JS,Fix JA.Colon-specific drug delivery:new approaches
and in vitro/in vivo evaluation.Int J Pharm2002;235(1-2):1-15.
8.Sandborn WJ.Rational selection of oral5-aminosalicylate formulations
and prodrugs for the treatment of ulcerative colitis.Am J Gastroenterol 2002;97(12):2939-41.
9.Hanauer SB,Sparrow M.COLAL-PRED Alizyme.Curr Opin Investig
Drugs2004;5(11):1192-7.
10.Steed KP,Hooper G,Monti N,Benedetti MS,Fornasini G,Wilding IR.
The use of pharmacoscintigraphy to focus the development strategy for
a novel5-ASA colon targeting system(“TIME CLOCK(R)”system).J
Control Release1997;49(2-3):115-22.
11.Hebden JM,Blackshaw PE,Perkins AC,Wilson CG,Spiller RC.
Limited exposure of the healthy distal colon to orally-dosed formulation is further exaggerated in active left-sided ulcerative colitis.
Aliment Pharmacol Ther2000;14(2):155-61.
12.Hua S.Orally administered liposomal formulations for colon targeted
drug delivery.Front Pharmacol2014;5:1-4[Opinion].
https://www.360docs.net/doc/ad5902891.html,roui H,Dalmasso G,Nguyen HT,Yan Y,Sitaraman SV,Merlin D.
Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model.Gastroenterology 2010;138(3):843-853.e1-2.
14.Coco R,Plapied L,Pourcelle V,Jerome C,Brayden DJ,Schneider YJ,
et al.Drug delivery to inflamed colon by nanoparticles:comparison of different strategies.Int J Pharm2013;440(1):3-12.
15.Asghar LF,Chandran S.Multiparticulate formulation approach to
colon specific drug delivery:current perspectives.J Pharm Pharm Sci 2006;9(3):327-38.16.Hu Z,Mawatari S,Shibata N,Takada K,Yoshikawa H,Arakawa A,et al.
Application of a biomagnetic measurement system(BMS)to the evaluation of gastrointestinal transit of intestinal pressure-controlled colon delivery capsules(PCDCs)in human subjects.Pharm Res 2000;17(2):160-7.
17.Coupe AJ,Davis SS,Wilding IR.Variation in gastrointestinal transit of
pharmaceutical dosage forms in healthy subjects.Pharm Res 1991;8(3):360-4.
18.Rao KA,Yazaki E,Evans DF,Carbon R.Objective evaluation of small
bowel and colonic transit time using pH telemetry in athletes with gastrointestinal symptoms.Br J Sports Med2004;38(4):482-7.
19.Buhmann S,Kirchhoff C,Ladurner R,Mussack T,Reiser MF,
Lienemann A.Assessment of colonic transit time using MRI:a feasibility study.Eur Radiol2007;17(3):669-74.
20.Sathyan G,Hwang S,Gupta SK.Effect of dosing time on the total
intestinal transit time of non-disintegrating systems.Int J Pharm 2000;204(1-2):47-51.
21.Fallingborg J,Christensen LA,Jacobsen BA,Rasmussen SN.Very low
intraluminal colonic pH in patients with active ulcerative colitis.Dig Dis Sci1993;38(11):1989-93.
22.Bratten J,Jones MP.New directions in the assessment of gastric
function:clinical applications of physiologic measurements.Dig Dis 2006;24(3-4):252-9.
23.Nugent SG,Kumar D,Rampton DS,Evans DF.Intestinal luminal pH in
inflammatory bowel disease:possible determinants and implications for therapy with aminosalicylates and other drugs.Gut2001;48(4):571-7.
24.Sasaki Y,Hada R,Nakajima H,Fukuda S,Munakata A.Improved
localizing method of radiopill in measurement of entire gastrointestinal pH profiles:colonic luminal pH in normal subjects and patients with Crohn's disease.Am J Gastroenterol1997;92(1):114-8.
25.Ibekwe VC,Fadda HM,McConnell EL,Khela MK,Evans DF,Basit
AW.Interplay between intestinal pH,transit time and feed status on the in vivo performance of pH responsive ileo-colonic release systems.
Pharm Res2008;25(8):1828-35.
26.Nyhof RA,Ingold-Wilcox D,Chou CC.Effect of atropine on digested food-
induced intestinal hyperemia.Am J Physiol1985;249(6Pt1):G685-90. 27.Fatouros DG,Mullertz A.In vitro lipid digestion models in design of
drug delivery systems for enhancing oral bioavailability.Expert Opin Drug Metab Toxicol2008;4(1):65-76.
28.Keely S,Feighery L,Campion DP,O'Brien L,Brayden DJ,Baird AW.
Chloride-led disruption of the intestinal mucous layer impedes Salmonella invasion:evidence for an‘enteric tear’mechanism.Cell Physiol Biochem2011;28(4):743-52.
29.Sartor RB.Microbial influences in inflammatory bowel diseases.
Gastroenterology2008;134(2):577-94.
30.Macfarlane GT,Macfarlane S.Fermentation in the human large
intestine:its physiologic consequences and the potential contribution of prebiotics.J Clin Gastroenterol2011;45:S120-7[Suppl.].
31.Sinha VR,Kumria R.Polysaccharides in colon-specific drug delivery.
Int J Pharm2001;224(1-2):19-38.
32.Frank DN,St Amand AL,Feldman RA,Boedeker EC,Harpaz N,Pace
NR.Molecular–phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases.Proc Natl Acad Sci U S A2007;104(34):13780-5.
33.Albenberg LG,Wu GD.Diet and the intestinal microbiome:
associations,functions,and implications for health and disease.Gas-troenterology2014;146(6):1564-72.
34.Britton RA,Young VB.Role of the intestinal microbiota in resistance
to colonization by Clostridium difficile.Gastroenterology 2014;146(6):1547-53.
35.Patel S,McCormick BA.Mucosal inflammatory response to Salmo-
nella typhimurium infection.Front Immunol2014;5:311.
36.Grover M,Kanazawa M,Palsson OS,Chitkara DK,Gangarosa LM,
Drossman DA,et al.Small intestinal bacterial overgrowth in irritable bowel syndrome:association with colon motility,bowel symptoms,and psychological distress.Neurogastroenterol Motil2008;20(9):998-1008.
1129
S.Hua et al/Nanomedicine:Nanotechnology,Biology,and Medicine11(2015)1117–1132
37.Li AC,Thompson RP.Basement membrane components.J Clin Pathol
2003;56(12):885-7.
38.Antoni L,Nuding S,Wehkamp J,Stange EF.Intestinal barrier in
inflammatory bowel disease.World J Gastroenterol2014;20(5):1165-79.
39.Rana SV,Sharma S,Malik A,Kaur J,Prasad KK,Sinha SK,et al.
Small intestinal bacterial overgrowth and orocecal transit time in patients of inflammatory bowel disease.Dig Dis Sci 2013;58(9):2594-8.
40.Kashyap PC,Marcobal A,Ursell LK,Larauche M,Duboc H,Earle KA,
et https://www.360docs.net/doc/ad5902891.html,plex interactions among diet,gastrointestinal transit,and gut microbiota in humanized mice.Gastroenterology2013;144(5):967-77.
41.Sartor RB.Therapeutic correction of bacterial dysbiosis discovered by
molecular techniques.Proc Natl Acad Sci U S A2008;105(43): 16413-4.
42.Linskens RK,Huijsdens XW,Savelkoul PH,Vandenbroucke-Grauls
CM,Meuwissen SG.The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics.Scand J Gastroenterol Suppl2001;234:29-40.
43.Keely S,Kelly CJ,Weissmueller T,Burgess A,Wagner BD,Robertson
CE,et al.Activated fluid transport regulates bacterial–epithelial interactions and significantly shifts the murine colonic microbiome.
Gut Microbes2012;3(3):250-60.
44.Musch MW,Wang Y,Claud EC,Chang EB.Lubiprostone decreases
mouse colonic inner mucus layer thickness and alters intestinal microbiota.Dig Dis Sci2013;58(3):668-77.
45.Barkas F,Liberopoulos E,Kei A,Elisaf M.Electrolyte and acid–base
disorders in inflammatory bowel disease.Ann Gastroenterol 2013;26(1):23-8.
46.Lucas ML,Cooper BT,Lei FH,Johnson IT,Holmes GK,Blair JA,et al.
Acid microclimate in coeliac and Crohn's disease:a model for folate malabsorption.Gut1978;19(8):735-42.
47.McConnell EL,Fadda HM,Basit AW.Gut instincts:explorations in
intestinal physiology and drug delivery.Int J Pharm2008;364(2): 213-26.
48.Van Citters GW,Lin HC.Ileal brake:neuropeptidergic control of
intestinal transit.Curr Gastroenterol Rep2006;8(5):367-73.
49.Yang L.Biorelevant dissolution testing of colon-specific delivery
systems activated by colonic microflora.J Control Release 2008;125(2):77-86.
https://www.360docs.net/doc/ad5902891.html,ukoetter MG,Nava P,Nusrat A.Role of the intestinal barrier in
inflammatory bowel disease.World J Gastroenterol2008;14(3):401-7.
51.Cunningham KE,Turner JR.Myosin light chain kinase:pulling the
strings of epithelial tight junction function.Ann N Y Acad Sci 2012;1258:34-42.
52.Wang X,Maher S,Brayden DJ.Restoration of rat colonic epithelium
after in situ intestinal instillation of the absorption promoter,sodium caprate.Ther Deliv2010;1(1):75-82.
53.Goggins BJ,Chaney C,Radford-Smith GL,Horvat JC,Keely S.
Hypoxia and integrin-mediated epithelial restitution during mucosal inflammation.Front Immunol2013;4:272.
https://www.360docs.net/doc/ad5902891.html,erford KM,Wallace TJ,Karhausen J,Louis NA,Montalto MC,
Colgan SP.Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance(MDR1)gene.Cancer Res2002;62(12):3387-94.
55.Creed TJ,Probert CS.Review article:steroid resistance in inflamma-
tory bowel disease—mechanisms and therapeutic strategies.Aliment Pharmacol Ther2007;25(2):111-22.
56.Dong X,Mattingly CA,Tseng MT,Cho MJ,Liu Y,Adams VR,et al.
Doxorubicin and paclitaxel-loaded lipid-based nanoparticles overcome multidrug resistance by inhibiting P-glycoprotein and depleting ATP.
Cancer Res2009;69(9):3918-26.
57.Schmidt T,Pfeiffer A,Hackelsberger N,Widmer R,Meisel C,Kaess H.
Effect of intestinal resection on human small bowel motility.Gut 1996;38(6):859-63.
58.Spiller RC,Trotman IF,Adrian TE,Bloom SR,Misiewicz JJ,Silk DB.
Further characterisation of the‘ileal brake’reflex in man—effect of ileal infusion of partial digests of fat,protein,and starch on jejunal
motility and release of neurotensin,enteroglucagon,and peptide YY.
Gut1988;29(8):1042-51.
59.Fallingborg J,Pedersen P,Jacobsen BA.Small intestinal transit time
and intraluminal pH in ileocecal resected patients with Crohn's disease.
Dig Dis Sci1998;43(4):702-5.
60.Gracie DJ,Kane JS,Mumtaz S,Scarsbrook AF,Chowdhury FU,Ford
AC.Prevalence of,and predictors of,bile acid malabsorption in outpatients with chronic diarrhea.Neurogastroenterol Motil 2012;24(11):983-e538.
61.Thompson JS,Quigley EM,Adrian TE,Path FR.Role of the ileocecal
junction in the motor response to intestinal resection.J Gastrointest Surg1998;2(2):174-85.
62.Ammon HV,Phillips SF.Inhibition of ileal water absorption by
intraluminal fatty acids.Influence of chain length,hydroxylation,and conjugation of fatty acids.J Clin Invest1974;53(1):205-10.
63.Watts PJ,Barrow L,Steed KP,Wilson CG,Spiller RC,Melia CD,et al.
The transit rate of different-sized model dosage forms through the human colon and the effects of a lactulose-induced catharsis.Int J Pharm1992;87:215-21.
64.Van Citters GW,Lin HC.The ileal brake:a fifteen-year progress report.
Curr Gastroenterol Rep1999;1(5):404-9.
65.Lin HC,Prather C,Fisher RS,Meyer JH,Summers RW,Pimentel M,
et al.Measurement of gastrointestinal transit.Dig Dis Sci 2005;50(6):989-1004.
66.Malayandi R,Kondamudi PK,Ruby PK,Aggarwal D.Biopharmaceu-
tical considerations and characterizations in development of colon targeted dosage forms for inflammatory bowel disease.Drug Deliv Transl Res2014;4:187-202.
67.Xiao B,Merlin D.Oral colon-specific therapeutic approaches toward
treatment of inflammatory bowel disease.Expert Opin Drug Deliv 2012;9(11):1393-407.
68.Collnot EM,Ali H,Lehr CM.Nano-and microparticulate drug carriers
for targeting of the inflamed intestinal mucosa.J Control Release 2012;161(2):235-46.
https://www.360docs.net/doc/ad5902891.html,mprecht A,Yamamoto H,Takeuchi H,Kawashima Y.Nanoparticles
enhance therapeutic efficiency by selectively increased local drug dose in experimental colitis in rats.J Pharmacol Exp Ther2005;315(1):196-202.
70.Beloqui A,Coco R,Alhouayek M,Solinis MA,Rodriguez-Gascon A,
Muccioli GG,et al.Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis.Int J Pharm 2013;454(2):775-83.
71.Hillyer JF,Albrecht RM.Gastrointestinal persorption and tissue
distribution of differently sized colloidal gold nanoparticles.J Pharm Sci2001;90(12):1927-36.
72.des Rieux A,Ragnarsson EG,Gullberg E,Preat V,Schneider YJ,
Artursson P.Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium.Eur J Pharm Sci 2005;25(4-5):455-65.
73.Pichai MV,Ferguson LR.Potential prospects of nanomedicine for
targeted therapeutics in inflammatory bowel diseases.World J Gastroenterol2012;18(23):2895-901.
https://www.360docs.net/doc/ad5902891.html,mprecht A,Sch?fer U,Lehr C-M.Size-dependent bioadhesion of
micro-and nanoparticulate carriers to the inflamed colonic mucosa.
Pharm Res2001;18(6):788-93.
https://www.360docs.net/doc/ad5902891.html,mprecht A.IBD:selective nanoparticle adhesion can enhance colitis
therapy.Nat Rev Gastroenterol Hepatol2010;7(6):311-2.
76.Wachsmann P,Moulari B,Beduneau A,Pellequer Y,Lamprecht A.
Surfactant-dependence of nanoparticle treatment in murine experimen-tal colitis.J Control Release2013;172(1):62-8.
77.Schmidt C,Lautenschlaeger C,Collnot EM,Schumann M,Bojarski C,
Schulzke JD,et al.Nano-and microscaled particles for drug targeting to inflamed intestinal mucosa:a first in vivo study in human patients.J Control Release2013;165(2):139-45.
78.Han HK,Shin HJ,Ha DH.Improved oral bioavailability of alendronate
via the mucoadhesive liposomal delivery system.Eur J Pharm Sci 2012;46(5):500-7.
1130S.Hua et al/Nanomedicine:Nanotechnology,Biology,and Medicine11(2015)1117–1132
79.Liu L,Fishman ML,Hicks KB,Kende M.Interaction of various pectin
formulations with porcine colonic tissues.Biomaterials2005;26(29):5907-16.
https://www.360docs.net/doc/ad5902891.html,rsson JM,Karlsson H,Sjovall H,Hansson GC.A complex,but
uniform O-glycosylation of the human MUC2mucin from colonic biopsies analyzed by nanoLC/MSn.Glycobiology2009;19(7):756-66.
81.Urayama S,Chang EB.Mechanisms and treatment of diarrhea in
inflammatory bowel diseases.Inflamm Bowel Dis1997;3(2):114-31.
82.Niebel W,Walkenbach K,Beduneau A,Pellequer Y,Lamprecht A.
Nanoparticle-based clodronate delivery mitigates murine experimental colitis.J Control Release2012;160(3):659-65.
https://www.360docs.net/doc/ad5902891.html,utenschlager C,Schmidt C,Lehr CM,Fischer D,Stallmach A.
PEG-functionalized microparticles selectively target inflamed mu-cosa in inflammatory bowel disease.Eur J Pharm Biopharm 2013;85(3Pt A):578-86.
84.Gradauer K,Barthelmes J,Vonach C,Almer G,Mangge H,Teubl B,et al.
Liposomes coated with thiolated chitosan enhance oral peptide delivery to rats.J Control Release2013;172(3):872-8.
85.Manconi M,Nacher A,Merino V,Merino-Sanjuan M,Manca ML,
Mura C,et al.Improving oral bioavailability and pharmacokinetics of liposomal metformin by glycerolphosphate–chitosan microcomplexa-tion.AAPS PharmSciTech2013;14(2):485-96.
86.Takeuchi H,Matsui Y,Yamamoto H,Kawashima Y.Mucoadhesive
properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to rats.J Control Release2003;86(2-3):235-42.
87.Gradauer K,Vonach C,Leitinger G,Kolb D,Frohlich E,Roblegg E,et al.
Chemical coupling of thiolated chitosan to preformed liposomes improves mucoadhesive properties.Int J Nanomedicine2012;7:2523-34.
88.Thirawong N,Thongborisute J,Takeuchi H,Sriamornsak P.Improved
intestinal absorption of calcitonin by mucoadhesive delivery of novel pectin–liposome nanocomplexes.J Control Release2008;125(3):236-45.
89.Tirosh B,Khatib N,Barenholz Y,Nissan A,Rubinstein A.Transferrin
as a luminal target for negatively charged liposomes in the inflamed colonic mucosa.Mol Pharm2009;6(4):1083-91.
90.Carlson M,Raab Y,Peterson C,Hallgren R,Venge P.Increased
intraluminal release of eosinophil granule proteins EPO,ECP,EPX, and cytokines in ulcerative colitis and proctitis in segmental perfusion.
Am J Gastroenterol1999;94(7):1876-83.
91.Peterson CG,Eklund E,Taha Y,Raab Y,Carlson M.A new method for
the quantification of neutrophil and eosinophil cationic proteins in feces: establishment of normal levels and clinical application in patients with inflammatory bowel disease.Am J Gastroenterol2002;97(7):1755-62.
https://www.360docs.net/doc/ad5902891.html,mprecht A,Ubrich N,Yamamoto H,Schafer U,Takeuchi H,
Maincent P,et al.Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease.J Pharmacol Exp Ther2001;299(2):775-81.
93.Jubeh T,Barenholz Y,Rubinstein A.Differential adhesion of normal
and inflamed rat colonic mucosa by charged liposomes.Pharm Res 2004;21(3):447-53.
94.Muchow M,Maincent P,Muller RH.Lipid nanoparticles with a solid
matrix(SLN,NLC,LDC)for oral drug delivery.Drug Dev Ind Pharm 2008;34(12):1394-405.
95.Beloqui A,Solinis MA,Delgado A,Evora C,Isla A,Rodriguez-
Gascon A.Fate of nanostructured lipid carriers(NLCs)following the oral route:design,pharmacokinetics and biodistribution.J Micro-encapsul2014;31(1):1-8.
96.Meissner Y,Pellequer Y,Lamprecht A.Nanoparticles in inflammatory
bowel disease:particle targeting versus pH-sensitive delivery.Int J Pharm2006;316(1-2):138-43.
97.Cu Y,Saltzman WM.Controlled surface modification with poly(ethylene)
glycol enhances diffusion of PLGA nanoparticles in human cervical mucus.Mol Pharm2008;6:173-81.
98.Tang BC,Dawson M,Lai SK,Wang YY,Suk JS,Yang M,et al.
Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier.Proc Natl Acad Sci U S A2009;106(46):19268-73.
https://www.360docs.net/doc/ad5902891.html,i SK,Wang YY,Hanes J.Mucus-penetrating nanoparticles for drug
and gene delivery to mucosal tissues.Adv Drug Deliv Rev 2009;61(2):158-71.
100.Vong LB,Tomita T,Yoshitomi T,Matsui H,Nagasaki Y.An orally administered redox nanoparticle that accumulates in the colonic mucosa and reduces colitis in mice.Gastroenterology 2012;143(4):1027-1036.e3.
101.Ju J,Hao X,Lee MJ,Lambert JD,Lu G,Xiao H,et al.A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice.Cancer Prev Res(Phila)2009;2(2):143-52.
102.Jin Y,Kotakadi VS,Ying L,Hofseth AB,Cui X,Wood PA,et al.
American ginseng suppresses inflammation and DNA damage associated with mouse colitis.Carcinogenesis2008;29(12):2351-9. 103.Kotakadi VS,Jin Y,Hofseth AB,Ying L,Cui X,Volate S,et al.
Ginkgo biloba extract EGb761has anti-inflammatory properties and ameliorates colitis in mice by driving effector T cell apoptosis.Carci-nogenesis2008;29(9):1799-806.
104.Tobio M,Sanchez A,Vila A,Soriano II,Evora C,Vila-Jato JL,et al.
The role of PEG on the stability in digestive fluids and in vivo fate of PEG–PLA nanoparticles following oral administration.Colloids Surf B:Biointerfaces2000;18(3-4):315-23.
105.Ashford M,Fell JT,Attwood D,Sharma H,Woodhead PJ.An in vivo investigation into the suitability of pH dependent polymers for colonic targeting.Int J Pharm1993;95(1-3):193-9.
106.Karn PR,Vani?Z,Pepi?I,?kalko-Basnet N.Mucoadhesive liposomal delivery systems:the choice of coating material.Drug Dev Ind Pharm 2011;37(4):482-8.
107.Haznedar S,Dortunc B.Preparation and in vitro evaluation of Eudragit microspheres containing acetazolamide.Int J Pharm 2004;269(1):131-40.
108.Barea MJ,Jenkins MJ,Lee YS,Johnson P,Bridson RH.Encapsulation of liposomes within pH responsive microspheres for oral colonic drug delivery.Int J Biomater2012:1-8[458712].
109.Barea MJ,Jenkins MJ,Gaber MH,Bridson RH.Evaluation of liposomes coated with a pH responsive polymer.Int J Pharm 2010;402(1-2):89-94.
110.Makhlof A,Tozuka Y,Takeuchi H.pH-Sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model.Eur J Pharm Biopharm2009;72(1):1-8.
111.Kshirsagar SJ,Bhalekar MR,Patel JN,Mohapatra SK,Shewale NS.
Preparation and characterization of nanocapsules for colon-targeted drug delivery system.Pharm Dev Technol2012;17(5):607-13. 112.Legrand P,Barratt G,Mosqueira V,Fessi H,Devissaguet JP.Polymeric nanocapsules as drug delivery systems.STP Pharm Sci1999;9:411-8. 113.Ali H,Weigmann B,Neurath MF,Collnot EM,Windbergs M,Lehr CM.Budesonide loaded nanoparticles with pH-sensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases.J Control Release2014;183:167-77.
114.Beloqui A,Coco R,Memvanga PB,Ucakar B,des Rieux A,Preat V.
pH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease.Int J Pharm2014;473(1-2):203-12. https://www.360docs.net/doc/ad5902891.html,mprecht A,Yamamoto H,Takeuchi H,Kawashima Y.A pH-sensitive microsphere system for the colon delivery of tacrolimus containing nanoparticles.J Control Release2005;104(2):337-46. https://www.360docs.net/doc/ad5902891.html,roui H,Geem D,Xiao B,Viennois E,Rakhya P,Denning T,et al.
Targeting intestinal inflammation with CD98siRNA/PEI-loaded nanoparticles.Mol Ther2014;22(1):69-80.
117.Xiao B,Laroui H,Viennois E,Ayyadurai S,Charania MA,Zhang Y,et al.
Nanoparticles with surface antibody against CD98and carrying CD98 small interfering RNA reduce colitis in mice.Gastroenterology 2014;146(5):1289-1300.e1-19.
118.Kriegel C,Amiji M.Oral TNF-alpha gene silencing using a polymeric microsphere-based delivery system for the treatment of inflammatory bowel disease.J Control Release2011;150(1):77-86.
1131
S.Hua et al/Nanomedicine:Nanotechnology,Biology,and Medicine11(2015)1117–1132
119.Kriegel C,Amiji MM.Dual TNF-alpha/cyclin D1gene silencing with an oral polymeric microparticle system as a novel strategy for the treatment of inflammatory bowel disease.Clin Transl Gastroenterol 2011;2:e2.
120.Bhavsar MD,Amiji MM.Gastrointestinal distribution and in vivo gene transfection studies with nanoparticles-in-microsphere oral system (NiMOS).J Control Release2007;119(3):339-48.
121.Moulari B,Pertuit D,Pellequer Y,Lamprecht A.The targeting of surface modified silica nanoparticles to inflamed tissue in experimental colitis.Biomaterials2008;29(34):4554-60.
122.Li ZZ,Wen LX,Shao L,Chen JF.Fabrication of porous hollow silica nanoparticles and their applications in drug release control.J Control Release2004;98(2):245-54.
123.Lih-Brody L,Powell SR,Collier KP,Reddy GM,Cerchia R,Kahn E,et al.
Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease.Dig Dis Sci1996;41(10):2078-86. 124.Simmonds NJ,Allen RE,Stevens TR,Van Someren RN,Blake DR, Rampton DS.Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease.Gastroenterology 1992;103(1):186-96.
125.Mahida YR,Wu KC,Jewell DP.Respiratory burst activity of intestinal macrophages in normal and inflammatory bowel disease.Gut 1989;30(10):1362-70.
126.Wilson DS,Dalmasso G,Wang L,Sitaraman SV,Merlin D,Murthy N.
Orally delivered thioketal nanoparticles loaded with TNF-alpha-siRNA target inflammation and inhibit gene expression in the intestines.Nat Mater2010;9(11):923-8.
127.Leirdal M,Sioud M.Gene silencing in mammalian cells by preformed small RNA duplexes.Biochem Biophys Res Commun2002;295(3):744-8. 128.Zhang S,Zhao B,Jiang H,Wang B,Ma B.Cationic lipids and polymers mediated vectors for delivery of siRNA.J Control Release 2007;123(1):1-10.
129.Thiele L,Rothen-Rutishauser B,Jilek S,Wunderli-Allenspach H,Merkle HP,Walter E.Evaluation of particle uptake in human blood monocyte-derived cells in vitro.Does phagocytosis activity of dendritic cells measure up with macrophages?J Control Release2001;76(1-2):59-71. 130.Hua S.Targeting sites of inflammation:intercellular adhesion molecule-1as a target for novel inflammatory therapies.Front Pharmacol2013;4:127.
131.Sofou S,Sgouros G.Antibody-targeted liposomes in cancer therapy and imaging.Expert Opin Drug Deliv2008;5(2):189-204.
132.Willis M,Forssen E.Ligand-targeted liposomes.Adv Drug Deliv Rev 1998;29(3):249-71.
133.Saltzman WM,Radomsky ML,Whaley KJ,Cone RA.Antibody diffusion in human cervical mucus.Biophys J1994;66(2Pt1):508-15. 134.Mane V,Muro S.Biodistribution and endocytosis of ICAM-1-targeting antibodies versus nanocarriers in the gastrointestinal tract in mice.Int J Nanomedicine2012;7:4223-37.
135.Sans M,Panes J,Ardite E,Elizalde JI,Arce Y,Elena M,et al.VCAM-1 and ICAM-1mediate leukocyte–endothelial cell adhesion in rat experimental colitis.Gastroenterology1999;116(4):874-83.136.Danese S,Semeraro S,Marini M,Roberto I,Armuzzi A,Papa A,et al.
Adhesion molecules in inflammatory bowel disease:therapeutic implications for gut inflammation.Dig Liver Dis2005;37(11):811-8. 137.Binion DG,West GA,Volk EE,Drazba JA,Ziats NP,Petras RE,et al.
Acquired increase in leucocyte binding by intestinal micro-vascular endothelium in inflammatory bowel https://www.360docs.net/doc/ad5902891.html,ncet 1998;352(9142):1742-6.
138.Binion DG,West GA,Ina K,Ziats NP,Emancipator SN,Fiocchi
C.Enhanced leukocyte binding by intestinal microvascular
endothelial cells in inflammatory bowel disease.Gastroenterol-ogy1997;112(6):1895-907.
139.Bernstein CN,Sargent M,Gallatin WM,Wilkins J.Beta2-integrin/ intercellular adhesion molecule(ICAM)expression in the normal human intestine.Clin Exp Immunol1996;106(1):160-9.
140.Salmi M,Granfors K,MacDermott R,Jalkanen S.Aberrant binding of lamina propria lymphocytes to vascular endothelium in inflammatory bowel diseases.Gastroenterology1994;106(3):596-605.
141.Wileman TE,Lennartz MR,Stahl PD.Identification of the macrophage mannose receptor as a175-kDa membrane protein.Proc Natl Acad Sci U S A1986;83(8):2501-5.
142.van Vliet SJ,Saeland E,van Kooyk Y.Sweet preferences of MGL:carbohydrate specificity and function.Trends Immunol 2008;29(2):83-90.
143.Xiao B,Laroui H,Ayyadurai S,Viennois E,Charania MA,Zhang Y,et al.
Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-alpha RNA interference for IBD therapy.Biomaterials 2013;34(30):7471-82.
144.Zhang J,Tang C,Yin C.Galactosylated trimethyl chitosan–cysteine nanoparticles loaded with Map4k4siRNA for targeting activated macrophages.Biomaterials2013;34(14):3667-77.
https://www.360docs.net/doc/ad5902891.html,roui H,Viennois E,Xiao B,Canup BS,Geem D,Denning TL,et al.
Fab′-bearing siRNA TNFalpha-loaded nanoparticles targeted to colonic macrophages offer an effective therapy for experimental colitis.
J Control Release2014;186:41-53.
146.Tacchini L,Gammella E,De Ponti C,Recalcati S,Cairo G.Role of HIF-1and NF-kappaB transcription factors in the modulation of transferrin receptor by inflammatory and anti-inflammatory signals.J Biol Chem2008;283(30):20674-86.
147.Harel E,Rubinstein A,Nissan A,Khazanov E,Nadler Milbauer M, Barenholz Y,et al.Enhanced transferrin receptor expression by proinflammatory cytokines in enterocytes as a means for local delivery of drugs to inflamed gut mucosa.PLoS One2011;6(9):e24202. 148.Xiao B,Yang Y,Viennois E,Zhang Y,Ayyadurai S,Baker M,et al.
Glycoprotein CD98as a receptor for colitis-targeted delivery of nanoparticle.J Mater Chem B Mater Biol Med2014;2(11):1499-508. 149.Kucharzik T,Lugering A,Yan Y,Driss A,Charrier L,Sitaraman S,et al.
Activation of epithelial CD98glycoprotein perpetuates colonic https://www.360docs.net/doc/ad5902891.html,b Invest2005;85(7):932-41.
150.Nguyen HT,Merlin D.Homeostatic and innate immune responses:role of the transmembrane glycoprotein CD98.Cell Mol Life Sci 2012;69(18):3015-26.
1132S.Hua et al/Nanomedicine:Nanotechnology,Biology,and Medicine11(2015)1117–1132
施工报批合同(一式6份)
爆破工程专业分包合同合同编号:GDBP20150908-BP 工程名称:广州伟腾黄边商住楼基坑石方爆破工程总包单位: 广州市伟腾建筑工程有限公司 分包单位: 广东爆破工程有限公司 签订日期: 二0一五年九月 合同签订地点:广州市白云区
爆破工程专业分包合同 合同编号:GDBP20150908-BP 发包人:广州市伟腾建筑工程有限公司(以下简称甲方) 承包人:广东爆破工程有限公司(以下简称乙方) 依据《中华人民共和国合同法》、《爆破安全规程》、《民用爆炸物品安全管理条理》及其他法律、法规,遵循平等、自愿、公平和诚实信用的原则,结合本工程的具体情况,双方就本工程施工事项协商一致,同意订立本合同,并共同信守合同各项条款。 第一条:工程概况 1、工程名称:广州伟腾黄边商住楼基坑石方爆破工程 2、工程地点:广州市白云区黄边 3、工程范围:广州伟腾黄边商住楼基坑土石方挖运过程中需处理的石方,含基坑石方爆破、承台石方爆破。 4、工期:约两年。 5、质量标准:符合《爆破安全规程》(GB6722-2014)的相关规定及满足甲方的施工质量要求,块度以发包方要求为准。 第二条:承包内容 提供符合广州市爆破施工要求的爆破设计及施工组织设计方案,岩石爆破的钻孔、装药、填塞、联线、安全防护、警戒、起爆、爆破后的安全检查及盲炮处理。 第三条:合同价款 1、双方按照合同范围内容实行单价包干计价: (1)基坑石方爆破(不包括沟槽、承台)爆破单价27.0元/m3 (每立方米贰拾柒元整,不包含税金); (2)承台石方爆破单价228.0元/m3 (每立方米贰佰贰拾捌元整,不包含税金); 2、本合同工程价格采用固定综合单价,为完成本合同工程所需的人工费、材料费、水电费、机械费、劳保费、安全风险费、安全文明施工措施费、保险(工程一切险和第三方责任险除外)、缺陷修复、合同备案以及各类临时设施费、调遣费、协调费、管理费、利润、可能存在的施工干扰而增加的窝工费等一切费用均已包含在合同综合单价内。综合单价不因市场价格、工期的变化、天气影响、地下管线拆除影响以及工程量增减等其他任何因素而调整。 双方应充分考虑市场影响,本合同在履行期间由于国家和地方法令、法规、政策、物价
古代晋灵公不君、齐晋鞌之战原文及译文
晋灵公不君(宣公二年) 原文: 晋灵公不君。厚敛以雕墙。从台上弹人,而观其辟丸也。宰夫胹熊蹯不熟,杀之,寘诸畚,使妇人载以过朝。赵盾、士季见其手,问其故而患之。将谏,士季曰:“谏而不入,则莫之继也。会请先,不入,则子继之。”三进及溜,而后视之,曰:“吾知所过矣,将改之。”稽首而对曰:“人谁无过?过而能改,善莫大焉。诗曰:‘靡不有初,鲜克有终。’夫如是,则能补过者鲜矣。君能有终,则社稷之固也,岂惟群臣赖之。又曰:‘衮职有阙,惟仲山甫补之。’能补过也。君能补过,衮不废矣。” 犹不改。宣子骤谏,公患之,使鉏麑贼之。晨往,寝门辟矣,盛服将朝。尚早,坐而假寐。麑退,叹而言曰:“不忘恭敬,民之主也。贼民之主,不忠;弃君之命,不信。有一于此,不如死也!”触槐而死。 秋九月,晋侯饮赵盾酒,伏甲将攻之。其右提弥明知之,趋登曰:“臣侍君宴,过三爵,非礼也。”遂扶以下。公嗾夫獒焉。明搏而杀之。盾曰:“弃人用犬,虽猛何为!”斗且出。提弥明死之。 初,宣子田于首山,舍于翳桑。见灵辄饿,问其病。曰:“不食三日矣!”食之,舍其半。问之,曰:“宦三年矣,未知母之存否。今近焉,请以遗之。”使尽之,而为之箪食与肉,寘诸橐以与之。既而与为公介,倒戟以御公徒,而免之。问何故,对曰:“翳桑之饿人也。”问其名居,不告而退。——遂自亡也。 乙丑,赵穿①攻灵公于桃园。宣子未出山而复。大史书曰:“赵盾弑其君。”以示于朝。宣子曰:“不然。”对曰:“子为正卿,亡不越竟,反不讨贼,非子而谁?”宣子曰:“呜呼!‘我之怀矣,自诒伊戚。’其我之谓矣。” 孔子曰:“董狐,古之良史也,书法不隐。赵宣子,古之良大夫也,为法受恶。惜也,越竞乃免。” 译文: 晋灵公不行君王之道。他向人民收取沉重的税赋以雕饰宫墙。他从高台上用弹弓弹人,然后观赏他们躲避弹丸的样子。他的厨子做熊掌,没有炖熟,晋灵公就把他杀了,把他的尸体装在草筐中,让宫女用车载着经过朝廷。赵盾和士季看到露出来的手臂,询问原由后感到很忧虑。他们准备向晋灵公进谏,士季说:“如果您去进谏而君王不听,那就没有人能够再接着进谏了。还请让我先来吧,不行的话,您再接着来。”士季往前走了三回,行了三回礼,一直到屋檐下,晋灵公才抬头看他。晋灵公说:“我知道我的过错了,我会改过的。”士季叩头回答道:“谁能没有过错呢?有过错而能改掉,这就是最大的善事了。《诗经》说:‘没有人向善没有一个开始的,但却很少有坚持到底的。’如果是这样,那么能弥补过失的人是很少的。您如能坚持向善,那么江山就稳固了,不只是大臣们有所依靠啊。
药学英语第五版第三单元
Biochemistry Seeks to Explain Life in Chemical Terms The molecules of which living organisms are composed conform to all the familiar laws of chemistry, but they also interact with each other in accordance with another set of principles, which we shall refer to collectively as the molecular logic of life. These principles do not involve new or yet undiscovered physical laws or forces. Instead, they are a set of relationships characterizing the nature, function, and interactions of biomolecules. If living organisms are composed of molecules that are intrinsically inanimate, how do these molecules confer the remarkable combination of characteristics we call life? How is it that a living organism appears to be more than the sum of its inanimate parts? Philosophers once answered that living organisms are endowed with a mysterious and divine life force, but this doctrine (vitalism) has been firmly rejected by modern science. The basic goal of the science of biochemistry is to determine how the collections of inanimate molecules that constitute living organisms interact with each other to maintain and perpetuate life. Although biochemistry yields important insights and practical applications in medicine, agriculture, nutrition, and industry, it is ultimately concerned with the wonder of life itself. All Macromolecules Are Constructed from a Few Simple Compounds Most of the molecular constituents of living systems are composed of carbon atoms covalently joined with other carbon atoms and with hydrogen, oxygen, or nitrogen. The special bonding properties of carbon permit the formation of a great variety of molecules. Organic compounds of molecular weight less than about 500, such as amino acids, nucleotidase, and monosaccharide, serve as monomeric subunits of proteins, nucleic acids, and polysaccharides,
药学专业英语药学词汇
6-磷酸葡萄糖脱氢酶 glucose-6-phosphate dehydrogenase Janbon综合症 Janbon's syndrome PPB浓度 parts per billion concentration pphm浓度 parts per hundred million concentration PPH浓度 parts per hundred concentration ppm浓度 parts per million concentration 安全范围 safety range 安全试验法 innocuity test method 安全系统 safety coefficient 安慰剂 placebo 螯合剂 chelating agent 靶细胞 target cell 白蛋白微球制剂 albumin microballoons 百分浓度 percentage concentration 半合成抗生素 semisynthetic antibiotics 半抗原 haptene 半数致死剂量 half lethal dose ; median lethal dose; LD50 半衰期 half-life period; half life time 包衣片 coated tablet 薄膜衣 film-coating 饱和溶液 saturated solution 贝克勒尔 Becquerel 被动免疫 passive immunity 被动转运 passive transport
崩解度 disintegration 崩解剂 disintegrants 必需氨基酸 essential aminoacid 必需脂肪酸 essential fatty acid 变态反应 allergy; allergic reaction 表面活性 surface activity 表面张力 surface tension 丙种射线 gamma rays 补体 complement 补体系统 complement system 不良反应 adverse reaction 不完全抗原 incomplete antigen 搽剂 liniments 长期毒性实验 long term toxicity test 长效制剂 prolonged action preparation 肠肝循环 enterohepatic circulation 肠溶控释片 enteric controlled release tablets 肠溶衣 enteric coating 处方 prescription;recipe 穿透促进剂 penetration enhancers 磁性控释制剂 magnetic controlled release dosage form 磁性药物制剂 magnetic medicinal preparations 大分子 macromolecule 单克隆抗体 monoclonal antibody
协议一式几份官方写法 (8页)
本文部分内容来自网络整理,本司不为其真实性负责,如有异议或侵权请及时联系,本司将立即删除! == 本文为word格式,下载后可方便编辑和修改! == 协议一式几份官方写法 篇一:协议的写法 协议的写法 1.含义 协议又称协议书,它是国家机关、社会团体、企事业单位之间,为了统一计划、分工负责、协同一致地完成某一共同议定的事项而签订的一种契约性文书。 协议书与合同具有相同的功能,但在使用中有一些细微的区别,其区别主要是: (1)协议书的内容比较原则、单纯,往往是共同协商的原则性意见;而合同内容具体、详细,各方面的问题全面周到。 (2)协议书的适用范围广泛,可以是共同商定的各方面的事务;而合同主要是经济关系方面的事项。 (3)合同一次性生效,而协议书签订以后,往往就有关具体问题还需要签订合同加以补充、完善。 2作用 协议作为契约的一种,将双方经过洽谈商定的有关事项记载下来,作为检查信 用的凭证,一经订立,对签订各方具有约束作用。 它确定了各自的权利与义务,双方各执一张,作为凭据,互相监督、互相牵制,以保证合作的正常进行。 3.内容 协议的内容根据不同的情况有时原则一些,有时相对具体一些,但基本内容主 要是: (1)协议事项。在合同中协议事项称为标的,即双方当事人要求实现的结果,共同指向的事物。如货物、劳务、工程项目等。协议事项同样是指这些内容。
(2)数量、质量、价金等。 (3)协议要求与违约责任。 4.结构 协议的结构主要由四个方面组成: (1)标题 一般按协议事项的性质写出名称。 (2)称谓 要写明签订协议的双方(或多方)单位名称和代表人姓名。为了行文方便,习惯上规定一方为甲方,另一方为乙方,如有第三方,可简称为丙方。在协议中不能用我方、你方、他方作为代称。 (3)正文 主要由两部分组成:一是开头。开头主要写明双方签订协议的依据、目的和双方”信守” 的表态。二是协议的主要条款,一般分条列项具体说明。 (4)结尾 结尾包括三个方面:一是署名,二是签订协议的日期;三是附项,即附加的有关材料予以注明,最后还要写清双方的地址、电话、开户行、账号、电报挂号等。 5.撰写 由于协议是一种契约活动,一旦签订,就具有法律效力,因此内容必须遵守国家法律、法令、符合国家政策要求,任何单位和个人都不能以协议为名进行违法活动。 平等互利、协商一致、等价有偿的原则。协议必须是出于当事人的真正自愿,在双方自由表达意志的基础上,经过充分协商而达成协议。同时要体现协作的精神,遵循等价有偿的原则,符合价值规律的要求。 窗体底端。 家教协议书 为规范广大中、小学家教的教学活动,山西家教网特立此协议书。予以书面保证。请家教双方自觉遵守此协议条款。甲方:乙方(家长):
工程施工合同
工 程 施 工 合 同发包方(甲方):
承包方(乙方): 合同编号: 公装工程施工合同条款 发包方(以下简称甲方): (公司全称) 办公地址: 法人代表:联系电话: 签约代理人:联系电话: 施工代理人:联系电话: 承包方(以下简称乙方): 办公地址: 签约代理人:(营业部经理): 联系电话: 施工代理人:1 工程部经理: 联系电话: 施工代理人:2 设计师: 联系电话: 依照《中华人民共和国合同法》、《建筑安装工程承包合同条例》、《中华人民共和国消费者权益保护法》、《中华人民共和国价格法》、《咸宁市保护消费者合法权益条例》、及其它有关法律的规定,甲、乙双方在平等、自愿、协商致的期础上,就乙方承包甲方的公装工程(以下简称工程)的有关事宜,达成如下协议; 第一条: 工程概况 1.1 工程名称: 1.2 工程地点: 1.3 工程建筑面积: 1.4 工程内容及做法(见图纸施工内容单及《报价单》) 1.5 工程承包方式,双方商定采取下列第种承包方式;
(1) 包工、包施工材料、包部分(或全部)内饰物品(以:“甲方委托乙方代购内)饰物品明细表”为准); (2)包工、包施工材料,甲方提供内饰物品(见“甲方提供内饰物品明细表”); 1.6 工程期限工作日; 开工日期为甲方预收款到乙方第四即2011年月日)(或另行约定为2012年月日); 竣工日期为开工后第工作日; 1.7 合同价款: 本合同工程造价为2008年最新定额预算结算工程款,以2010年行情差价补贴《如人工、材料差价等》) 第二条: 工程监理 若本工程实行工程监理,甲方与监理公司另行签订《工程监理合同》,并将监理工程师的姓名、单位、联系方式及监理工程师的职责等书面通知乙方。 第三条: 装修范围 3.1、 2、3、4号集中宿舍楼所有室内外装修由乙方负责装修(如水电工程、地面铺贴工程、吊顶工程、卫生间、房门、楼梯铺贴及扶手等) 3.2、 1-13号宿舍楼所有室内外装修由乙方负责装修(如水电工程、地面铺贴工程、吊顶工程、卫生间、房门、楼梯铺贴及扶手等) 第四条: 施工图纸 双方商定施工图纸设计采取下列第种方式提供; 1.甲方自行设计并提供施工图纸,图纸一式三份,甲方执一份,乙方执两份; 2.甲方委托乙方设计施工图纸,图纸一式两份,甲方、乙方各执一份(须双方在每页图纸上签字方为有效)。
如何翻译古文
如何翻译古文 学习古代汉语,需要经常把古文译成现代汉语。因为古文今译的过程是加深理解和全面运用古汉语知识解决实际问题的过程,也是综合考察古代汉语水平的过程。学习古代汉语,应该重视古文翻译的训练。 古文翻译的要求一般归纳为信、达、雅三项。“信”是指译文要准确地反映原作的含义,避免曲解原文内容。“达”是指译文应该通顺、晓畅,符合现代汉语语法规范。“信”和“达”是紧密相关的。脱离了“信”而求“达”,不能称为翻译;只求“信”而不顾“达”,也不是好的译文。因此“信”和“达”是文言文翻译的基本要求。“雅”是指译文不仅准确、通顺,而且生动、优美,能再现原作的风格神韵。这是很高的要求,在目前学习阶段,我们只要能做到“信”和“达”就可以了。 做好古文翻译,重要的问题是准确地理解古文,这是翻译的基础。但翻译方法也很重要。这里主要谈谈翻译方法方面的问题。 一、直译和意译 直译和意译是古文今译的两大类型,也是两种不同的今译方法。 1.关于直译。所谓直译,是指紧扣原文,按原文的字词和句子进行对等翻译的今译方法。它要求忠实于原文,一丝不苟,确切表达原意,保持原文的本来面貌。例如: 原文:樊迟请学稼,子曰:“吾不如老农。”请学为圃。子曰:“吾不如老圃。”(《论语?子路》) 译文:樊迟请求学种庄稼。孔子道:“我不如老农民。”又请求学种菜蔬。孔子道:“我不如老菜农。”(杨伯峻《论语译注》) 原文:齐宣王问曰:“汤放桀,武王伐纣,有诸?”(《孟子?梁惠王下》) 译文:齐宣王问道:“商汤流放夏桀,武王讨伐殷纣,真有这回事吗?(杨伯峻《孟子译注》) 上面两段译文紧扣原文,字词落实,句法结构基本上与原文对等,属于直译。 但对直译又不能作简单化理解。由于古今汉语在文字、词汇、语法等方面的差异,今译时对原文作一些适当的调整,是必要的,并不破坏直译。例如: 原文:逐之,三周华不注。(《齐晋鞌之战》) 译文:〔晋军〕追赶齐军,围着华不注山绕了三圈。
药学英语第五版原文翻译 (2)(2020年7月整理).pdf
Introduction to Physiology Introduction Physiology is the study of the functions of living matter. It is concerned with how an organism performs its varied activities: how it feeds, how it moves, how it adapts to changing circumstances, how it spawns new generations. The subject is vast and embraces the whole of life. The success of physiology in explaining how organisms perform their daily tasks is based on the notion that they are intricate and exquisite machines whose operation is governed by the laws of physics and chemistry. Although some processes are similar across the whole spectrum of biology—the replication of the genetic code for or example—many are specific to particular groups of organisms. For this reason it is necessary to divide the subject into various parts such as bacterial physiology, plant physiology, and animal physiology. To study how an animal works it is first necessary to know how it is built. A full appreciation of the physiology of an organism must therefore be based on a sound knowledge of its anatomy. Experiments can then be carried out to establish how particular parts perform their functions. Although there have been many important physiological investigations on human volunteers, the need for precise control over the experimental conditions has meant that much of our present physiological knowledge has been derived from studies on other animals such as frogs, rabbits, cats, and dogs. When it is clear that a specific physiological process has a common basis in a wide variety of animal species, it is reasonable to assume that the same principles will apply to humans. The knowledge gained from this approach has given us a great insight into human physiology and endowed us with a solid foundation for the effective treatment of many diseases. The building blocks of the body are the cells, which are grouped together to form tissues. The principal types of tissue are epithelial, connective, nervous, and muscular, each with its own characteristics. Many connective tissues have relatively few cells but have an extensive extracellular matrix. In contrast, smooth muscle consists of densely packed layers of muscle cells linked together via specific cell junctions. Organs such as the brain, the heart, the lungs, the intestines, and the liver are formed by the aggregation of different kinds of tissues. The organs are themselves parts of distinct physiological systems. The heart and blood vessels form the cardiovascular system; the lungs, trachea, and bronchi together with the chest wall and diaphragm form the respiratory system; the skeleton and skeletal muscles form the musculoskeletal system; the brain, spinal cord, autonomic nerves and ganglia, and peripheral somatic nerves form the nervous system, and so on. Cells differ widely in form and function but they all have certain 生理学简介 介绍 生理学是研究生物体功能的科学。它研究生物体如何进行各种活动,如何饮食,如何运动,如何适应不断改变的环境,如何繁殖后代。这门学科包罗万象,涵盖了生物体整个生命过程。生理学成功地解释了生物体如何进行日常活动,基于的观点是生物体好比是结构复杂而灵巧的机器,其操作受物理和化学规律控制。 尽管从生物学整个范畴看,生物体某些活动过程是相似的——如基因编码的复制——但许多过程还是某些生物体群组特有的。鉴于此有必要将这门学科分成不同部分研究,如细菌生理学、植物生理学和动物生理学。 要研究一种动物如何活动,首先需要了解它的构成。要充分了解一个生物体的生理学活动就必须掌握全面的解剖学知识。一个生物体的各部分起着什么作用可通过实验观察得知。尽管我们对志愿者进行了许多重要的生理调查,但是实验条件需要精确控制,所以我们当前大多生理知识还是源于对其它动物如青蛙,兔子,猫和狗等的研究。当我们明确大多数动物物种的特定生理过程存在共同之处时,相同的生理原理适用于人类也是合理的。通过这种方法,我们获得了大量的知识,从而让我们对人类生理学有了更深入的了解,为我们有效治疗许多疾病提供了一个坚实的基础。 机体的基本组成物质是细胞,细胞结合在一起形成组织。组织的基本类型有上皮组织,结缔组织,神经组织和肌组织,每类组织都有各自的特征。许多结缔组织中细胞量相对较少,但是有大量的细胞外基质。相比而言,光滑的肌组织由大量密密麻麻的肌细胞通过特定的细胞连接组成。各种器官如脑,心脏,肺,小肠和肝等由不同种类的组织聚集而成。这些器官是不同生理系统的组成部分。心脏和血管组成心血管系统;肺,器官,支气管,胸壁和膈肌组成呼吸系统;骨骼和骨骼肌组成骨骼肌系统;大脑,脊髓,自主神经和神经中枢以及
药学英语专业词汇word精品
药学名词(中-英)6-磷酸葡萄糖脱氢酶glucose-6-phosphate dehydrogenase Janbon综合症Janbon's syndrome PPB浓度parts per billion concentration pphm 浓度parts per hundred million concentration PPH浓度parts per hundred concentration ppm 浓度parts per million concentration 安全范围safety range 安全试验法innocuity test method 安全系统safety coefficient 安慰剂placebo 螯合剂chelating agent 靶细胞target cell 白蛋白微球制剂albumin microballoons 百分浓度percentage concentration 半合成抗生素semisynthetic antibiotics 半抗原haptene 半数致死剂量half lethal dose ; median lethal dose; LD50 半衰期half-life period; half life time 包衣片coated tablet 薄膜衣film-coating 饱和溶液saturated solution 贝克勒尔Becquerel 被动免疫passive immunity 被动转运passive transport 崩解度disintegration 崩解剂disintegrants 必需氨基酸essential aminoacid 必需脂肪酸essential fatty acid 变态反应allergy; allergic reaction 表面活性surface activity 表面张力surface tension 丙种射线gamma rays 补体complement 补体系统complement system 不良反应adverse reaction 不完全抗原incomplete antigen 搽剂liniments 长期毒性实验long term toxicity test 长效制剂prolonged action preparation 肠肝循环enterohepatic circulation 肠溶控释片enteric controlled release tablets 肠溶衣enteric coating 处方prescription;recipe 穿透促进剂penetration enhancers 磁性控释制剂magnetic controlled release dosage form 磁性药物制剂magnetic medicinal preparations 大分子macromolecule 单克隆抗体monoclonal antibody 胆碱酯酶cholinesterase 当量equivalent weight 当量定律equivalent law 当量浓度normality 当量溶液normal solution 等张溶液isotonic solution 低聚糖oligosaccharides 低密度脂蛋白low density lipoprotein 滴定titration 滴定曲线titration curve 滴丸剂 pill 递质transmitter 电解electrolyzation 电解质electrolyte 酊剂tincture 定向药物制剂directed pharmaceutical preparations 毒理学toxicology 毒性反应toxic response; toxic reaction 短期致癌实验short term carcinogenic test 对因治疗etiological treatment 对映体antipode 对症治疗symptomatic treatment 多功能酶multifunctional enzyme 多剂量给药multiple dose administration 多糖polyose 多肽polypeptide 儿茶酚胺catecholamine 二重感染superinfection 发酵fermentation 法定处方official formula
签订招标代理合同时需要几份合同
竭诚为您提供优质文档/双击可除签订招标代理合同时需要几份合同 篇一:审查招标代理合同有哪些重点 想学法律?找律师?请上 https://www.360docs.net/doc/ad5902891.html,审查招标代理合同有哪些重点 核心内容:审查招标代理合同有哪些重点?招标代理合同的审查重点有注意选用招标代理合同格式,注意甲乙双方名称、帐号信息,注意通用合同条款的引用和专用合同条款的对照,注意法律适用错误的问题,注意详列委托事项与合同价款等等。法律快车小编为您详细介绍。 专业化、资质综合化、分支机构扩大化已经成为招标代理行业的发展方向,因此,规范化管理成为综合型招标代理机构发展的内在要求,越来越多的公司设置了专职质量控制岗位。那么对于招标代理合同的审查,哪些方面是其重点呢? 注意招标代理合同格式的选用。对于工程建设招标代理项目,应当选用原建设部和国家工商行政管理总局20XX年 制定的标准合同格式。对于其他代理项目,当地监管部门有代理合同格式的,应优先选用,没有的,建议在招标人和招
标代理机构之间优先选用代理机构统一编制的合同格式。代理机构自行制定的合同条款应尽量避免歧义。 注意甲乙双方名称、帐号信息。综合型招标代理机构往往设有众多分支机构,在公司住所地以外进行工商注册、银行开户。出于管理便利,公司往往会根据风险大小授权一些分支机构直接与招标人签订合同。审查时应注意乙方名称填写时是公司还是公司的分支机构,核对银行帐户及帐户信息。 注意通用合同条款的引用和专用合同条款的对照。监管部门发布的标准合同格式中的通用合同条款应当原文引用,一般不允许进行修改;专用合同条款应当注意与通用合同条款的对照。 注意法律适用错误的问题。如工程建设项目走政府采购流程、政府采购货物和服务走招标投标流程,此类行为既可能是招标人规避监管的故意行为,也可能是操作人员对法律法规掌握不全产生的随意行为。审查人员应当充分考虑上述行为给公司带来的风险。 注意详列委托事项与合同价款。关于招标代理服务事项与服务费的收取,目前有3个规范性文件:《招标代理服务 收费管理暂行办法》(计价格〔20XX〕1980号)、《国家发展 改革委办公厅关于招标代理服务收费有关问题的通知》(发 改办价格[20XX]857号)和《国家发 有法律问题,上法律快车/retype/zoo
齐晋鞌之战原文和译文
鞌之战选自《左传》又名《鞍之战》原文:楚癸酉,师陈于鞌(1)。邴夏御侯,逢丑父为右②。晋解张御克,郑丘缓为右(3)。侯日:“余姑翦灭此而朝食(4)”。不介马而驰之⑤。克伤于矢,流血及屦2 未尽∧6),曰:“余病矣(7)!”张侯曰:“自始合(8),而矢贯余手及肘(9),余折以御,左轮朱殷(10),岂敢言病吾子忍之!”缓曰:“自始合,苟有险,余必下推车,子岂_识之(11)然子病矣!”张侯曰:“师之耳目,在吾旗鼓,进退从之。此车一人殿之(12),可以集事(13),若之何其以病败君之大事也擐甲执兵(14),固即死也(15);病未及死,吾子勉之(16)!”左并辔(17) ,右援拐鼓(18)。马逸不能止(19),师从之,师败绩。逐之,三周华不注(20) 韩厥梦子舆谓己曰:“旦辟左右!”故中御而从齐侯。邴夏曰:“射其御者,君子也。”公曰:“谓之君子而射之,非礼也。”射其左,越于车下;射其右,毙于车中。綦毋张丧车,从韩厥,曰:“请寓乘。”从左右,皆肘之,使立于后。韩厥俛,定其右。逢丑父与公易位。将及华泉,骖絓于木而止。丑父寝于轏中,蛇出于其下,以肱击之,伤而匿之,故不能推车而及。韩厥执絷马前,再拜稽首,奉觞加璧以进,曰:“寡君使群臣为鲁、卫请,曰:‘无令舆师陷入君地。’下臣不幸,属当戎行,无所逃隐。且惧奔辟而忝两君,臣辱戎士,敢告不敏,摄官承乏。” 丑父使公下,如华泉取饮。郑周父御佐车,宛茷为右,载齐侯以免。韩厥献丑父,郤献子将戮之。呼曰:“自今无有代其君任患者,有一于此,将为戮乎”郤子曰:“人不难以死免其君,我戮之不祥。赦之,以劝事君者。”乃免之。译文1:在癸酉这天,双方的军队在鞌这个地方摆开了阵势。齐国一方是邴夏为齐侯赶车,逢丑父当车右。晋军一方是解张为主帅郤克赶车,郑丘缓当车右。齐侯说:“我姑且消灭了这些人再吃早饭。”不给马披甲就冲向了晋军。郤克被箭射伤,血流到了鞋上,但是仍不停止擂鼓继续指挥战斗。他说:“我受重伤了。”解张说:“从一开始接战,一只箭就射穿了我的手和肘,左边的车轮都被我的血染成了黑红色,我哪敢说受伤您忍着点吧!”郑丘缓说:“从一开始接战,如果遇到道路不平的地方,我必定(冒着生命危险)下去推车,您难道了解这些吗不过,您真是受重伤了。”daier 解张说:“军队的耳朵和眼睛,都集中在我们的战旗和鼓声,前进后退都要听从它。这辆车上还有一个人镇守住它,战事就可以成功。为什么为了伤痛而败坏国君的大事呢身披盔甲,手执武器,本来就是去走向死亡,伤痛还没到死的地步,您还是尽力而为吧。”一边说,一边用左手把右手的缰绳攥在一起,用空出的右手抓过郤克手中的鼓棰就擂起鼓来。(由于一手控马,)马飞快奔跑而不能停止,晋军队伍跟着指挥车冲上去,把齐军打得打败。晋军随即追赶齐军,三次围绕着华不注山奔跑。韩厥梦见他去世的父亲对他说:“明天早晨作战时要避开战车左边和右边的位置。”因此韩厥就站在中间担任赶车的来追赶齐侯的战车。邴夏说:“射那个赶车的,他是个君子。”齐侯说: “称他为君子却又去射他,这不合于礼。”daier 于是射车左,车左中箭掉下了车。又射右边的,车右也中箭倒在了车里。(晋军的)将军綦毋张损坏了自己的战车,跟在韩厥的车后说: “请允许我搭乗你的战车。”他上车后,无论是站在车的左边,还是站在车的右边,韩厥都用肘推他,让他站在自己身后——战车的中间。韩厥又低下头安定了一下受伤倒在车中的那位自己的车右。于是逢丑父和齐侯(乘韩厥低头之机)互相调换了位置。将要到达华泉时,齐侯战车的骖马被树木绊住而不能继续逃跑而停了下来。(头天晚上)逢丑父睡在栈车里,有一条蛇从他身子底下爬出来,他用小臂去打蛇,小臂受伤,但他(为了能当车右)隐瞒了这件事。由于这样,他不能用臂推车前进,因而被韩厥追上了。韩厥拿着拴马绳走到齐侯的马前,两次下拜并行稽首礼,捧着一杯酒并加上一块玉璧给齐侯送上去,
药学英语第五版原文翻译
IntroductiontoPhysiology Introduction Physiologyisthestudyofthefunctionsoflivingmatter.Itisconcernedwithhowanorganismperformsitsv ariedactivities:howitfeeds,howitmoves,howitadaptstochangingcircumstances,howitspawnsnewgenerati ons.Thesubjectisvastandembracesthewholeoflife.Thesuccessofphysiologyinexplaininghoworganismsp erformtheirdailytasksisbasedonthenotionthattheyareintricateandexquisitemachineswhoseoperationisgo vernedbythelawsofphysicsandchemistry. Althoughsomeprocessesaresimilaracrossthewholespectrumofbiology—thereplicationofthegenetic codefororexample—manyarespecifictoparticulargroupsoforganisms.Forthisreasonitisnecessarytodivid ethesubjectintovariouspartssuchasbacterialphysiology,plantphysiology,andanimalphysiology. Tostudyhowananimalworksitisfirstnecessarytoknowhowitisbuilt.Afullappreciationofthephysiolog yofanorganismmustthereforebebasedonasoundknowledgeofitsanatomy.Experimentscanthenbecarriedo uttoestablishhowparticularpartsperformtheirfunctions.Althoughtherehavebeenmanyimportantphysiolo gicalinvestigationsonhumanvolunteers,theneedforprecisecontrolovertheexperimentalconditionshasmea ntthatmuchofourpresentphysiologicalknowledgehasbeenderivedfromstudiesonotheranimalssuchasfrog s,rabbits,cats,anddogs.Whenitisclearthataspecificphysiologicalprocesshasacommonbasisinawidevariet yofanimalspecies,itisreasonabletoassumethatthesameprincipleswillapplytohumans.Theknowledgegain edfromthisapproachhasgivenusagreatinsightintohumanphysiologyandendoweduswithasolidfoundation fortheeffectivetreatmentofmanydiseases. Thebuildingblocksofthebodyarethecells,whicharegroupedtogethertoformtissues.Theprincipaltype softissueareepithelial,connective,nervous,andmuscular,eachwithitsowncharacteristics.Manyconnective tissueshaverelativelyfewcellsbuthaveanextensiveextracellularmatrix.Incontrast,smoothmuscleconsists https://www.360docs.net/doc/ad5902891.html,anssuchasthebrain,theh eart,thelungs,theintestines,andtheliverareformedbytheaggregationofdifferentkindsoftissues.Theorgans arethemselvespartsofdistinctphysiologicalsystems.Theheartandbloodvesselsformthecardiovascularsyst em;thelungs,trachea,andbronchitogetherwiththechestwallanddiaphragmformtherespiratorysystem;thes keletonandskeletalmusclesformthemusculoskeletalsystem;thebrain,spinalcord,autonomicnervesandgan glia,andperipheralsomaticnervesformthenervoussystem,andsoon. Cellsdifferwidelyinformandfunctionbuttheyallhavecertaincommoncharacteristics.Firstly,theyareb oundedbyalimitingmembrane,theplasmamembrane.Secondly,theyhavetheabilitytobreakdownlargemol eculestosmalleronestoliberateenergyfortheiractivities.Thirdly,atsomepointintheirlifehistory,theyposses sanucleuswhichcontainsgeneticinformationintheformofdeoxyribonucleicacid(DNA). Livingcellscontinuallytransformmaterials.Theybreakdownglucoseandfatstoprovideenergyforother activitiessuchasmotilityandthesynthesisofproteinsforgrowthandrepair.Thesechemicalchangesarecollect ivelycalledmetabolism.Thebreakdownoflargemoleculestosmalleronesiscalledcatabolismandthesynthes isoflargemoleculesfromsmalleronesanabolism. Inthecourseofevolution,cellsbegantodifferentiatetoservedifferentfunctions.Somedevelopedtheabil itytocontract(musclecells),otherstoconductelectricalsignals(nervecells).Afurthergroupdevelopedtheabi litytosecretedifferentsubstancessuchashormonesorenzymes.Duringembryologicaldevelopment,thispro cessofdifferentiationisre-enactedasmanydifferenttypesofcellareformedfromthefertilizedegg. Mosttissuescontainamixtureofcelltypes.Forexample,bloodconsistsofredcells,whitecells,andplatele ts.Redcellstransportoxygenaroundthebody.Thewhitecellsplayanimportantroleindefenseagainstinfection 生理学简介 介绍 生理学是研究生物体功能的科学。它研究生物体如何进行各种活动,如何饮食,如何运动,如何适应不断改变的环境,如何繁殖后代。这门学科包罗万象,涵盖了生物体整个生命过程。生理学成功地
