HLCL-61_hydrochloride_COA_20611_MedChemExpress
仿制药参比制剂目录(第三十三批)
Eli Lilly and CO
未进口原研药品
美国橙皮书
33-45
注射用盐酸吉西他滨
Gemcitabine Hydrochloride for Injection/Gemzar
1g(以吉西他滨计)
Eli Lilly and CO
未进口原研药品
美国橙皮书
33-46
甲苯磺酸索拉非尼片
1mg(按西尼必利计)
Almirall, S.A.
国内上市的原研药品
原研进口
33-9
依普利酮片
Eplerenone Tablets/Inspra
25mg
GD Searle LLC
未进口原研药品
美国橙皮书
33-10
依普利酮片
Eplerenone Tablets/Inspra
50mg
GD Searle LLC
Loteprednol Etabonate Ophthalmic Suspension
0.2%(5ml:10mg;10ml:20mg)
Bausch & Lomb Incorporated
国内上市的原研药品
原研进口
33-6
卡铂注射液
Carboplatin Injection/Paraplatin
(伯尔定)
0.5mg/ml(5ml)
Allergan Pharmaceuticals Ireland
未进口原研产品
欧盟上市
33-27
利伐沙班细粒剂
Rivaroxaban Fine Granules/ Xarelto
10mg
バイエル薬品株式会社
未进口原研药品
日本上市
Healthy Blue SC会员手册说明书
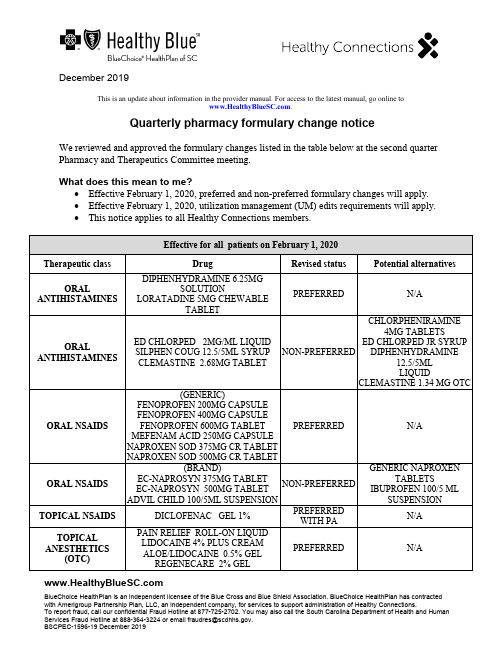
December 2019BlueChoice HealthPlan is an independent licensee of the Blue Cross and Blue Shield Association. BlueChoice HealthPlan has contracted with Amerigroup Partnership Plan, LLC, an independent company, for services to support administration of Healthy Connections.To report fraud, call our confidential Fraud Hotline at 877-725-2702. You may also call the South Carolina Department of Health and Human ************************************************************.BSCPEC-1596-19 December 2019This is an update about information in the provider manual. For access to the latest manual, go online to .Quarterly pharmacy formulary change noticeWe reviewed and approved the formulary changes listed in the table below at the second quarter Pharmacy and Therapeutics Committee meeting.What does this mean to me?• Effective February 1, 2020, preferred and non-preferred formulary changes will apply. • Effective February 1, 2020, utilization management (UM) edits requirements will apply. • This notice applies to all Healthy Connections members.Effective for all patients on February 1, 2020Therapeutic class DrugRevised status Potential alternativesORALANTIHISTAMINESDIPHENHYDRAMINE 6.25MGSOLUTIONLORATADINE 5MG CHEWABLETABLETPREFERREDN/AORALANTIHISTAMINESED CHLORPED 2MG/ML LIQUID SILPHEN COUG 12.5/5ML SYRUP CLEMASTINE 2.68MG TABLET NON-PREFERRED CHLORPHENIRAMINE4MG TABLETSED CHLORPED JR SYRUPDIPHENHYDRAMINE12.5/5MLLIQUIDCLEMASTINE 1.34 MG OTCORAL NSAIDS(GENERIC)FENOPROFEN 200MG CAPSULE FENOPROFEN 400MG CAPSULE FENOPROFEN 600MG TABLET MEFENAM ACID 250MG CAPSULE NAPROXEN SOD 375MG CR TABLET NAPROXEN SOD 500MG CR TABLETPREFERRED N/A ORAL NSAIDS(BRAND) EC-NAPROSYN 375MG TABLET EC-NAPROSYN 500MG TABLET ADVIL CHILD 100/5ML SUSPENSION NON-PREFERRED GENERIC NAPROXENTABLETSIBUPROFEN 100/5 ML SUSPENSIONTOPICAL NSAIDSDICLOFENAC GEL 1% PREFERREDWITH PAN/A TOPICALANESTHETICS(OTC)PAIN RELIEF ROLL-ON LIQUIDLIDOCAINE 4% PLUS CREAMALOE/LIDOCAINE 0.5% GELREGENECARE 2% GELPREFERRED N/ALIDODOSE 3% GELREGENECARE SPRAYALOCANE 4% GELAFTERBURN 2.5% GELXOLIDO 2% CREAM BURN RELIEF 0.5% AEROSAL ASPERCREME 4% SPRAYLIDOCAINE 3% CREAMLIDOCAINE 4% CREAMLIDOCAINE 5% CREAMAFTERSUN 0.5% GELLIDOCAINE 4% PADTOPICAL ANESTHETICS(RX)LIDOCAINE 3% CREAMLIDOCAINE 5% OINTMENT NON-PREFERREDOTC LIDOCAINEPRODUCTSRX LIDOCAINE5% PATCH(PA REQUIRED)MISCELLANEOUS ANTICONVULSANTSPREGABALIN 25MG CAPSULEPREGABALIN 50MG CAPSULEPREGABALIN 75MG CAPSULEPREGABALIN 100MG CAPSULEPREGABALIN 150MG CAPSULEPREGABALIN 200MG CAPSULEPREGABALIN 225MG CAPSULEPREGABALIN 300MG CAPSULEPREGABALIN SOL 20MG/MLPREFERREDWITH NO PRIORAUTHORIZATION(PA)N/AATOPICDERMATITIS PIMECROLIMUS 1% CREAMPREFERREDWITH STEPTHERAPY (ST)N/AFIBRATESFENOFIBRATE 130MG CAPSULEFENOFIBRATE 145MG TABLETFENOFIBRIC 35MG TABLETFENOFIBRIC 105MG TABLETFENOFIBRIC 135MG DR CAPSULENON-PREFERREDWITH STFENOFIBRATE134MG, 160MG, 200MG,43MG, 48MG,54 MG,67 MGFENOFIBRIC ACID 45 MGALCOHOL SWABS (MANUFACTURERS) GLOBAL DIABETICRITE AID NON-PREFERREDMANUFACTURERSBD DIABETESDYNAREXHEALTH MARTULTIMEDALCOHOL SWABS (MANUFACTURERS) BD DIABETESDYNAREXHEALTH MARTULTIMEDPREFERRED N/AIRON SUPPLEMENTS (GENERIC OTC)IRON 45MG TABLETSLOW-RELEASE FE 45MG TABLETHEMAX TABLETGENTLE IRON 28MG CAPSULEHIGH POTENCY FE 27MG TABLETNU-IRON 150 150MG CAPSULEABATRON AF TABLETSLOW IRON 50MG TABLETPREFERRED N/AFERGON 27MG TABLETIRON SUPPLEMENTS(BRAND OTC)FOLITAB 500 TABLET IRON 28MG TABLETFERROUS GLUC 324MG TABLETEZFE 200MG CAPSULEFERROUS GLUC TAB 324MGFERROUS SULF 324MG EC TABLETFERRETTS 325MG TABLETFERREX 150MG CAPSULEFERREX 28 MIS FERREX 150 PLUS CAPSULE FERREX 150 FORTE PL CAPSULECHEWABLE IRONPEDIATRIC IRON CHEWABLEFERROUS SUL 220/5ML LIQUIDFERROUS SULF 300/5ML SYRUPFEOSOL 200MG TABLETSLOW RELEASE FE 143MG CRTABLETNON- PREFERRED OTC GENERIC IRONSUPPLEMENTSRX PRODUCTS:HEMATOGEN FA CAPSULEHEMETAB TABLETMULTIGEN TABLETMULTIGEN PLS TABLETMULTIGEN FOLICTABLETFERRAPLUS 90 TABLETTARON FORTE CAPSULEFOLIVANE-F CAPSULEFOLIVANE-PLS CAPSULECENTRATEX CAPSULEIRON SUPPLEMENTS(PRESCRIPTIONSTRENGTH)IFEREX 150 FORTE CAPSULE HEMATOGEN CAPSULE HEMATOGEN FORTE CAPSULE TRICON CAPSULE MYFERON 150 FORTE CAPSULE FERROCITE PLUS TABLET FEROCON CAPSULE PUREVIT DUA FE PLUS CAPSULE HEMATINIC PL VIT/MIN TABLET HEMATINIC/FA TABLET POLY-IRON 150 FORT CAPSULE CORVITA 150 TABLET TRIGELS-F FORTE CAPSULE TL ICON CAPSULE SE-TAN PLUS CAPSULE NON- PREFERRED OTC GENERIC IRON SUPPLEMENTSRX PRODUCTS:HEMATOGEN FA CAPSULE HEMETAB TABLET MULTIGEN TABLET MULTIGEN PLS TABLET MULTIGEN FOLIC TABLET FERRAPLUS 90 TABLETTARON FORTE CAPSULE FOLIVANE-F CAPSULEFOLIVANE-PLS CAPSULE CENTRATEX CAPSULEUM edits — effective for all members no later than February 1, 2020 No changes in preferred/non-preferred status revision or addition to UM edit onlyANDROGENS*JATENZO CAPSULE ADD ST WITH QUANTITY LIMITS (QL)58 MG AND 198 MG QL: 4 PER DAY 237 MG QL: 2 PER DAY ANTICONVULSANTSNAYZILAM SPRAY 5MG ADD PA WITH QLQL: 50 MG PER 30 DAYS ANTICONVULSANTSOXTELLAR XR 150 MGOXTELLAR XR 600 MGREVISED QL LIMIT:150 MG: 3 TABLETS PER DAY 600 MG: 4 TABLETS PER DAYANTINEOPLASTICAGENTSPIQRAY 200 MG TABLETSPIQRAY 250 MG TABLETSPIQRAY 300 MG TABLETSADD PA WITH QL QL: 1 CARTON PER 28 DAYS ANTINEOPLASTICAGENTSXPOVIO PAK 60MGXPOVIO PAK 80MGXPOVIO PAK 100MGADD QL 1 CARTON PER 28 DAYSANTINEOPLASTICAGENTSNUBEQA 300MG TABLET ADD QL 4 TABLETS PER DAY ANTINEOPLASTICAGENTS TURALIO CAP 200MG ADD QL 4 TABLETS PER DAY ANTINEOPLASTICAGENTS PIQRAY 200MG TAB DOSE PIQRAY 300MG TAB DOSE PIQRAY 250MG TAB DOSE REVISE QL1 CARTON PER 28 DAYS CHOLESTEROLAGENTS EZALLOR SPRINKLE 5 MG CAP EZALLOR SPRINKLE 10 MG CAP EZALLOR SPRINKLE 20 MG CAP EZALLOR SPRINKLE 40 MG CAP ADD PA AND QLQL: 1 TABLET PER DAY COPD AGENTS DUAKLIR 400/12 INHALER ADD ST AND QLQL: 1 INHALER PER 30 DAYSCYSTIC FIBROSISAGENTSKALYDECO PAK 25MG ADD QL2 PACKETS PER DAYCYSTIC FIBROSISAGENTSORKAMBI GRANULES ADD QL2 PACKETS PER DAY HIVDOVATO TABLET EDURANT 25 MG TABLET DELSTRIGO TABLET COMPLERA TABLET ODEFSEY TABLET JULUCA TABLET ADD PA FOR NEW STARTS AND ADD QLQL: 1 PER DAY HIVINTELENCE TABLET ADD PA FOR NEW STARTS AND ADD QLQL:200 MG- 2 TABLETS PER DAY 400 MG- 4 TABLETS PER DAY 25 MG – 16 TABLETS PER DAYHIVATRIPLA TABLET BIKTARVY TABLET CIMDUO TABLET DESCOVY TABLETEMTRIVA 200 MG CAPSULE EPIVIR 300 MG TABLET EPZICOM TABLET EVOTAZ TABLET GENVOYA TABLET PIFELTRO 100 MG TABLET PREZCOBIX TABLET PREZISTA 800 MG TABLET REYATAZ 300 MG CAPSULESTRIBILD TABLET SUSTIVA 600 MG TABLETSYMFI TABLET SYMFI LO TABLET SYMTUZA TABLET TRIUMEQ TABLET TRUVADA TABLET TYBOST 150 MG TABLET VIDEX EC 400 MG CAPSULE VIDEX EC 250 MG CAPSULE VIRAMUNE XR 400 MG TABLETADD QL 1 PER DAYTEMIXYS TABLETHIVREYATAZ 200 MG CAPSULE REYATAZ 150 MG CAPSULE VIDEX EC 200 MG CAPSULE ZERIT 40 MG CAPSULE ZERIT 30 MG CAPSULE COMBIVIR TABLET DUTREBIS TABLET EPIVIR 150 MG TABLET ISENTRESS HD 600 MG TABLET PREZISTA 600 MG TABLET RETROVIR 300 MG TABLET SELZENTRY 75 MG TABLET TIVICAY 10 MG, 25 MG AND 50 MGTABLETTRIZIVIR TABLETVIRAMUNE 200 MG TABLET ZIAGEN 300 MG TABLET ADD QL 2 PER DAYHIV ISENTRESS 100 MG GRANULE PACKET FOR SUSPENSION ADD QL2 PACKETS PER DAYHIVVIDEX EC 125 MG CAPSULE VIRAMUNE XR 100MG TABLET ADD QL 3 PER DAYHIVAPTIVUS 250 MG CAPSULE INVIRASE 500 MG TABLET ISENTRESS 400 MG TABLET KALETRA 200 MG-50 MG TABLETLEXIVA 700 MG TABLET SELZENTRY 300 MG TABLET SELZENTRY 150 MG TABLET SUSTIVA 200 MG CAPSULE VIRACEPT 625 MG TABLET ZERIT 20 MG CAPSULE ZERIT 15 MG CAPSULEADD QL 4 PER DAYHIVREYATAZ 50 MG POWDER FORSUSPENSIONADD QL5 PACKETS PER DAYHIVCRIXIVAN 400 MG CAPSULE PREZISTA 150 MG TABLET RESCRIPTOR 200 MG TABLET RETROVIR 100 MG CAPSULE ISENTRESS 100 MG CHEWABLEADD QL 6 PER DAY HIV SELZENTRY 25 MG TABLET ADD QL 8 PER DAY HIV TROGARZO 150MG/ML VIAL ADD QL8 VIALS PER 28 DAYSHIVINVIRASE 200 MG CAPSULE KALETRA 100 MG-25 MG TABLETPREZISTA 75 MG TABLET VIRACEPT 250 MG TABLET ADD QL 10 PER DAY HIVCRIXIVAN 200 MG CAPSULE NORVIR 100 MG TABLET NORVIR 100 MG CAPSULEADD QL 12 PER DAYNORVIR 100 MG ORAL POWDERPACKETRESCRIPTOR 100 MG TABLET SUSTIVA 50 MG CAPSULEHIV APTIVUS 100 MG/ML SOLUTION ADD QL 13 ML PER DAYHIV PREZISTA 100 MG/ML SUSPENSION ADD QL 14 ML PER DAY HIV KALETRA 400 MG-100 MG/5 MLORAL SOLUTIONNORVIR 80 MG/ML ORAL SOLUTION ADD QL 16 ML PER DAY HIV ISENTRESS 25 MG CHEWABLE ADD QL24 TABLETS PER DAYHIV EMTRIVA 10 MG/ML SOLUTION ADD QL 29 ML PER DAYHIVEPIVIR 10 MG/ML ORAL SOLUTION ZIAGEN 20 MG/ML SOLUTION ADD QL 32 ML PER DAY HIVVIDEX 4 GM PEDIATRIC ORALSOLUTIONVIDEX 2 GM PEDIATRIC ORALSOLUTIONVIRAMUNE 50 MG/5 MLSUSPENSION ADD QL 40 ML PER DAY HIV VIRACEPT 50 MG/G POWDERADD QL 53 GM PER DAYHIV FUZEON 90 MG VIAL ADD QL60 VIALS PER 30 DAYSHIV LEXIVA 50 MG/ML SUSPENSION ADD QL 60 ML PER DAYHIV SELZENTRY 20 MG/ML ORALSOLUTION ADD QL 62 ML PER DAYHIV RETROVIR 10 MG/ML SYRUP ADD QL 64 ML PER DAYHIVZERIT 1 MG/ML SOLUTION ADD QL 80 ML PER DAY IRRITABLE BOWEL SYNDROME (IBS)AGENTSZELNORM 6MG TABLET ADD PA AND QL QL 2 TABLETS PER DAY LAMBERT-EATON MYASTHENIC SYNDROME AGENTSRUZURGI 10MG TABLET ADD PA AND QL QL 10 TABLETS PER DAYNARCOTIC ANTAGONISTS SUBLOCADE 100/0.5 INJECTION SUBLOCADE 300/1.5 INJECTION REMOVE PANARCOTIC ANTAGONISTS VIVITROL 380MG INEJCTION REMOVE PA AND ADD QL QL 1 VIAL PER 28 DAYSNARCOTIC ANTAGONISTS ZUBSOLV 2.9-0.71 SUB REVISE QL QL 5 PER DAY ORAL DIABETICAGENTS*QTERNMET XR TABLETADD ST AND QLQL:5 MG/5 MG/1000 MG, 10 MG/5 MG/1000 MG:1 TABLET PER DAY2.5 MG/2.5 MG/1000 MG, 5 MG/2.5 MG/10000MG: 2 TABLETS PER DAYORAL DIABETICAGENTS QTERN 5-5MG TABLET ADD QL1 TABLET 28 DAYSINJECTABLE DIABETIC AGENTSOZEMPIC 2/1.5ML INJECTION ADD QL 1 PER 28 DAYSPRENATAL VITAMINS DUET DHADUET DHA BALANCEDNESTABS ABC NESTABS DHA OBTREX DHA SELECT-OB+DHATHERANATAL COMPLETEVITAFOL FE+ VITAFOL-OB+DHABAL-CARE DHA ESSENTIAL ADD QL 2 PER DAYPRENATAL VITAMINS CITRANATAL B-CALMADD QL 3 PER DAYTOPICAL ANTIPRURITICS DOXEPIN HCL 5% CREAM,ZONALON 5% CREAM, PRUDOXIN5% CREAM ADD PA AND QLQL 1 TUBE PER FILL; 1 FILL PER 3 MONTHSTOPICAL ANESTHETIC COMBINATIONSLIDOCAINE/PRILOCAINE CREAMREVISE QL30 GM PER 30 DAYS* Clinical edits will be put in place as these new drugs to come market.What action do I need to take?Please review these changes and work with your Healthy Connections members to transition them to formulary alternatives. If you determine preferred formulary alternatives are notclinically appropriate for specific members, you will need to obtain prior authorization (PA) to continue coverage beyond the applicable effective date.What if I need assistance?We recognize the unique aspects of member cases. If your Healthy Connections member cannot be converted to a formulary alternative for medical reasons, call our Pharmacy department at 866-902-1689 and follow the voice prompts for pharmacy PA.You can find the Preferred Drug List on our website at > Providers > Pharmacy Information. If you need assistance with any other item, contact the Customer Care Center at 866-757-8286.。
盐酸法舒地尔说明书

分类:化学药品类别:3.1【药品名称】通用名:盐酸法舒地尔注射液曾用名:商品名:英文名:Fasudil Hydrochloride Injection汉语拼音:Yansuan Fashudier Zhusheye剂型:注射剂【成分】化学名称:六氢—1—(5—磺酰基异喹啉)—1(H)—1,4—二氮杂卓盐酸盐。
化学结构式:分子式:C14H17N3O2S?HCl分子量:327.83【性状】本品为白色、类白色或微黄色的结晶性粉末。
无臭,味微苦。
有引湿性。
本品在水中极易溶,在甲醇中溶解,在乙醇中略溶,在氯仿中极微溶,在乙醚中几乎不溶。
【药理毒理】盐酸法舒地尔是一种蛋白激酶抑制剂即细胞内钙离子拮抗剂。
血管平滑肌的收缩是由于平滑肌细胞内Ca2+浓度显著增高激活了关键酶的缘故。
当CA2+ 达到一定浓度时,与CA2+结合蛋白钙调素结合,激活肌球蛋白轻链磷酸化酶,将肌球蛋白轻链磷酸化,引起肌肉收缩。
蛛网膜下腔出血时,血管中释放出的各种血管收缩物质参与血管痉挛,最终通过肌球蛋白轻链磷酸化造成血管收缩。
盐酸法舒地尔通过阻断血管收缩过程的最终阶段,肌球蛋白轻链磷酸化,来扩张血管,抑制血管痉挛。
急性毒性:小鼠、大鼠口服给药的LD50分别为:小鼠雄性为273.9 mg/kg;雌性为277.3 mg/kg;大鼠雄性为335 mg/kg;雌性为348 mg/kg。
小鼠静脉给药的LD50 为69.5mg/kg。
亚急性毒性:以大鼠、猴静脉内给药1个月,无毒性剂量为:大鼠12.5mg/kg,猴3.125mg/kg。
慢性毒性:以大鼠、猴静脉内给药6个月,无毒性剂量为:大鼠9mg/kg,猴3.125mg/kg。
致突变性实验:细菌回复突变实验及啮齿类动物微核试验均为阴性。
哺乳类细胞染色体试验证明在体内无致突变性。
生殖毒性试验:妊娠前及妊娠初期的大鼠及大鼠胎仔器官形成期生殖和发育的毒性研究,剂量分别为1.56,6.25,25和1.6,8.0,40mg/kg。
国际化妆品原料标准中文名称、INCI名、CAS号查询表(2010年版)

序号
1681 5894 10462 12033 1493 1494 1682 1679 1685 8598 10441 5892 5893 5896 5897 5895 8599 6505 6506 8241 8602 6523 66 71 660 14686 8209 8210 1683 3847 8600 3852 3851 3854 3855 3856 3857 3848 4272 8206 14846 4080 3860 3858 3859
中文名称
1,2-丁二醇 1,2-己二醇 1,2-戊二醇 1,3-丙二醇 1,3-双-(2,4-二氨基苯氧基)丙烷 1,3-双-(2,4-二氨基苯氧基)丙烷 HCl 1,4-丁二醇 1,4-丁二醇/琥珀酸/己二酸/HDI 共聚物 1,4-丁二醇二(甲基丙烯酸)酯 1,5-萘二酚 1,5-戊二醇 1,6-己二胺 1,6-己二醇 1,6-己二醇二水杨酸酯 1,6-己二醇二硬脂酸酯 1,6-己二醇蜂蜡酸酯 1,7-萘二酚 10-羟基癸酸 10-羟基癸烯酸 1-甲基乙内酰脲-2-酰亚胺 1-萘酚 1-羟乙基-4,5-二氨基吡唑硫酸盐 1-乙酰萘 1-乙酰氧基-2-甲基萘 2-(2-氨基乙氧基)乙醇 2,2'-硫代双(4-氯苯酚) 2,2'-亚甲基双 4-氨基苯酚 2,2'-亚甲基双-4-氨基苯酚 HCl 2,3-丁二醇
国际化妆品原料标准中文名称目录(2010年版)
序号 INCI名称 中文名称
酸 (C10-18 脂酸甘油三酯类聚甘油-3酯类) 磷酸酯类 (动物)肝水解产物 (动物)睾丸水解产物 (动物)脊髓索带提取物 (动物)脊髓索带脂质 (动物)脊髓脂质提取物 (动物)脐带提取物 (动物)气管水解产物 (动物)乳房提取物 (动物)神经提取物 (动物)胎盘蛋白 (动物)胎盘酶 (动物)胎盘脂质 (动物)心脏水解产物 (动物)心脏提取物 (动物)胸腺水解产物 (动物)胸腺提取物 (镁/钾/硅)(氟化物/氢氧化物/氧化 物) (牛)肝提取物 (牛)睾丸提取物 (牛)骨髓类脂质 (牛)骨髓提取物 (牛)肌肉提取物 (牛)卵巢提取物 (牛)脾提取物 (牛)网膜类脂质 (牛/猪)脑提取物 (日用)香精 (三油酰氧甲基)甲氨基乙醇硫酸酯 (神经)鞘磷脂 (神经)鞘脂类 (四丁氧基)丙基三硅氧烷 (天冬氨酸/谷氨酸)金 (辛/癸)酸异辛酯 (椰油/葵花籽油)酰胺丙基甜菜碱 (月桂/肉豆蔻)基二醇羟丙基醚 1-(3,4-二甲氧基苯基)-4,4-二甲基1,3-戊二酮 1,10-癸二醇 1,2,4-苯三酚三乙酸酯 1,2,4-三羟基苯 1,2,6-己三醇 1
莱士雅苷酸衍生品说明书

Hyaluronic Acid Derivatives:Euflexxa™, Gel-One®(Intra-articular)Document Number: MH-0061 Last Review Date: 10/01/2019Date of Origin: 01/01/2012Dates Reviewed: 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013, 09/2013, 12/2013, 03/2014, 06/2014, 09/2014, 12/2014, 03/2015, 06/2015, 12/2015, 03/2016, 06/2016, 09/2016, 12/2016, 03/2017, 06/2017, 09/2017, 11/2017, 12/2017, 03/2018, 06/2018, 07/2018, 10/2018, 07/2019, 10/2019I.Important Note:a.Medica ONLY covers Gel-One (preferred) and Euflexxa. All other Hyaluronic AcidDerivatives will not be covered. This requirement is applicable to non-Medicarerequests only.II.Length of AuthorizationCoverage will be provided for six months and may be renewed.III.Dosing LimitsA.Quantity Limit (max daily dose) [Pharmacy Benefit]:B.Max Units (per dose and over time) [Medical Benefit]:*IV.Initial Approval CriteriaCoverage is provided in the following conditions:Osteoarthritis of the knee †∙Documented symptomatic osteoarthritis of the knee; AND∙Trial and failure of conservative therapy (including physical therapy, pharmacotherapy [e.g., non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen (up to 1 g 4 times/day)and/or topical capsaicin cream]) has been attempted and has not resulted in functionalimprovement after at least 3 months; AND∙The patient has failed to adequately respond to aspiration and injection of intra-articular steroids; AND∙Patient has not received therapy with intra-articular long-acting corticosteroid type drugs(i.e. Zilretta, etc.) within the previous 6 months of therapy; AND∙The patient reports pain which interferes with functional activities (e.g., ambulation, prolonged standing); AND∙There are no contraindications to the injections (e.g., active joint infection, bleeding disorder) †FDA Approved Indication(s)V.Renewal CriteriaCoverage can be renewed based upon the following criteria:∙The medical record demonstrates a reduction in the dose of NSAIDS (or other analgesics or anti-inflammatory medication) during the 6-month period following the previous series ofinjections; AND∙The medical record objectively documents significant improvement in pain and functional capacity as the result of the previous injections; AND∙Absence of unacceptable toxicity from the previous injections. Examples of unacceptable toxicity include: severe joint swelling and pain, severe infections, anaphylactic oranaphylactoid reactions, etc.VI.Dosage/Administration (per knee per 180 days)VII.Billing Code/Availability InformationJcode & NDC:VIII.References1.Euflexxa [package insert]. Parsippany, NJ; Ferring Pharmaceuticals; July 2016. AccessedJune 2019.2.Gel-One [package insert]. Warsaw, IN; Zimmer; May 2011. Accessed August 2018.3.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012recommendations for the use of nonpharmacologic and pharmacologic therapies inosteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012 Apr;64(4):465-74.4.McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgicalmanagement of knee osteoarthritis. Osteoarthritis Cartilage. 2014 Mar;22(3):363-88. doi:10.1016/j.joca.2014.01.003. Epub 2014 Jan 24.5.Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee:evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013 Sep;21(9):577-9. doi:10.5435/JAAOS-21-09-577.6.Cooper C, Rannou F, Richette P, et al. Use of intra-articular hyaluronic acid in themanagement of knee osteoarthritis in clinical practice. Arthritis Care Res (Hoboken). 2017Jan 24.7.Bhadra AK, Altman R, Dasa V, et al. Appropriate use criteria for hyaluronic acid in thetreatment of knee osteoarthritis in the United States. Cartilage. 2016 Aug 10.8.National Institute for Health and Care Excellence. NICE 2014. Osteoarthritis-Care andmanagement in adults. Published Feb 2014. Clinical guideline CG177.https:///guidance/cg177/evidence/full-guideline-pdf-191761309. AccessedAugust 2018.9.Strand V, Baraf H, Lavin P, et. al. Effectiveness and Safety of a Multicenter Extension andRetreatment Trial of Gel-200 in Patients with Knee Osteoarthritis. Cartilage. 2012 Oct;3(4): 297–304.10.Novitas Solutions, Inc. Local Coverage Determination (LCD): Hyaluronan Acid Therapiesfor Osteoarthritis of the Knee (DL35427). Centers for Medicare & Medicaid Services, Inc.Updated on (proposed draft) with effective date (proposed draft). Accessed June 2019.11.Palmetto GBA. Local Coverage Determination (LCD): Hyaluronate Polymers (L33432).Centers for Medicare & Medicaid Services, Inc. Updated on 12/13/2018 with effective date01/1/2019. Accessed June 2019.12.First Coast Service Options, Inc. Local Coverage Determination (LCD):Viscosupplementation Therapy for Knee (L33767). Centers for Medicare & MedicaidServices, Inc. Updated on 02/01/2019 with effective date 01/08/2019. Accessed June 2019.13.National Government Services, Inc. Local Coverage Article: Hyaluronans (e.g. Hyalgan ®,Supartz ®, Euflexxa™, Synvisc ®, Synvisc-One™, Orthovisc ®, Gel-One® ), Intra-articular Injections of - Related to LCD L33394 (A52420). Centers for Medicare & Medicaid Services, Inc. Updated on 12/19/2018 with effective date 1/1/2019. Accessed June 2019.14.Novitas Solutions, Inc. Local Coverage Article: HYALURONAN Acid Therapies forOsteoarthritis of the Knee- Related to LCD L35427 (A55036).Centers for Medicare &Medicaid Services, Inc. Updated on 3/29/2019 with effective date 5/20/2019. Accessed June 2019.Appendix 1 – Covered Diagnosis CodesAppendix 2 – Centers for Medicare and Medicaid Services (CMS)Medicare coverage for outpatient (Part B) drugs is outlined in the Medicare Benefit Policy Manual (Pub. 100-2), Chapter 15, §50 Drugs and Biologicals. In addition, National Coverage Determination (NCD) and Local Coverage Determinations (LCDs) may exist and compliance with these policies is required where applicable. They can be found at: /medicare-coverage-database/search/advanced-search.aspx. Additional indications may be covered at the discretion of the health plan.Medicare Part B Covered Diagnosis Codes (applicable to existing NCD/LCD):。
cas1158279-20-9_HLCL-61_参考资料MedBio

1、HLCL-61 (hydrochloride)物理参数:
常用名
HLCL-61盐酸盐
英文名
HLCL-61 (hydrochloride)
CAS号
1158279-20-9
分子量
380.910
密度
无资料
沸点
无资料
分子式
C23H25ClN2O
熔点
无资料
闪点
无资料
2、HLCL-61 (hydrochloride)同类产品列表:
MED11654
CYT387 sulfate salt
CYT387 sulfate salt
1056636-06-6
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11731
TCS 401
TCS 401
243967-42-2
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
PFI-1 (PF-6405761)
PFI-1 (PF-6405761)
1403764-72-6
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11691
Chaetocin
Chaetocin
28097-03-2
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11660
Pyridone 6
确证化学结构的试验资料及文献资料样版

9盐酸帕洛诺司琼确证化学结构的试验资料及文献资料目录9.1新药名称、化学结构式、分子式及分子量9.2供确证化学结构用样品的纯度及其检查方法9.3确证化学结构的方法9.4综合解析9.5参考文献9.1、新药名称、化学结构式、分子式及分子量通用名:盐酸帕洛诺司琼英文名:Palonosetron Hydrochloride汉语拼音:Yan Suan Pa Luo Nuo Si Qiong结构式:多了2,3a 位的双键分子式:C19H25ClN2O分子量:332.87CAS登录号:135729-61-2中文化学名:(3aS)-[2-[(S)-1-氮杂双环[2.2.2]辛-3-基]-2,3,3a,4,5,6-六氢-1H-苯并[de]异喹啉-1-酮盐酸盐英文化学名:(3aS)-[2-[(S)-1-Azabicyclo[2.2.2]oct-3-yl]-2,3,3a,4,5,6-hexahydro -1H- benz[de]isoquinolin-1-one Hydrochloride通用名:盐酸帕洛诺司琼(Palonosetron Hydrochloride)其它名称:Aloxi、Onicit9.2、供确证化学结构用样品的来源与批号1、供确证化学结构用样品的精制和批号批号:盐酸帕洛诺司琼样品:20040705盐酸帕洛诺司琼精制品:20040707取帕洛诺司琼盐酸盐样品25g,无水乙醇重结晶2次,最后得白色帕洛诺司琼盐酸盐精制品12g。
具体精制方法见“资料编号8”。
盐酸帕洛诺司琼样品(批号:20040705)和精制品(批号:20040707)以归一化法测得含量为99.75%,有关物质≤0.3 %。
具体方法参见资料10中“含量测定”项。
2、来源:公司9.3、确证化学结构的方法9.3.1 理化性状1 外观性状【1】本品为白色或微黄色粉末状晶体,无臭,无味。
2 溶解性易溶于水,溶于丙二醇,微溶于乙醇和2-丙醇。
3 熔点≥290℃,与文献报道相符(m.p. ≥290℃)【1】)4 绝对构型4.1 比旋度的测定α=-88~ -92,(c=1,CHCl3)盐酸帕洛诺司琼样品测量值:[]20Dα=-88~ -92,(c=1,CHCl3)请各选定盐酸帕洛诺司琼精制品测量值:[]20D一个检测值并附上检测报告。
CAS1204313-51-8_Icotinib Hydrochloride_MedBio相关资料

在体外激酶测定中,将2.4 ng /μLEGFR蛋白与32 ng /μLCrk在含有1μM冷ATP和1μCi32P-γ-ATP的25μL激酶反应缓冲液中混合。将混合物与Icotinib在0,0.5,2.5,12.5或62.5nM下在冰上温育10分钟,然后在30℃温育20分钟。用SDS样品缓冲液在100℃猝灭4分钟后,通过在10%SDS-PAGE凝胶中电泳分离蛋白质混合物。然后暴露干燥的凝胶以检测放射性。量化由软件[1]执行。
CAS
1、产品物理参数:
常用名
凯美纳
英文名
Icotinib Hydrochloride
CAS号
1204313-51-8
分子量
427.881
密度
无资料
沸点
无资料
分子式
C22H22ClN3O4
熔点
无资料
闪点
无资料
2、技术资料:
体外研究
与Iconitib在0.5μM孵育导致激酶活性抑制分别为91%,99%,96%,61%和61%。 Iconitib抑制A431和BGC-823 A549,H460和KB细胞系的增殖,IC50分别为1,4.06,12.16,16.08,40.71μM。当用88种激酶进行分析时,Icotinib仅对EGFR及其突变体显示出有意义的抑制活性。 Icotinib阻断人表皮样癌A431细胞系中EGFR介导的细胞内酪氨酸磷酸化(IC50 = 45 nM)并抑制肿瘤细胞增殖[1]。
10mM (in 1mL DMSO)
≥98%
1172133-28-6
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
