F~ole and regulation of EGFR in actin remodeling in sperm :apacitation and the acrosome reactio
沙库巴曲缬沙坦治疗急性心肌梗死合并Ⅱ~Ⅲ期慢性肾脏病患者的短期效果

·临床研究·基金项目:安徽省安庆市科技局基金项目(2021Z2001)作者简介:沈国秀,主治医师,Email:1158710529@qq.com通信作者:项学军,主任医师,Email:guangf4508@163.com
沙库巴曲缬沙坦治疗急性心肌梗死合并Ⅱ~Ⅲ期慢性肾脏病患者的短期效果
沈国秀,王贤进,顾崇怀,项学军安庆市立医院心血管内科,安庆246000
[摘要] 目的 探讨沙库巴曲缬沙坦治疗急性心肌梗死合并Ⅱ~Ⅲ期慢性肾脏病患者住院期间的治疗效果。方法 回顾性分析2019年1月1日至2022年3月31日于安庆市立医院住院的急性心肌梗死合并Ⅱ~Ⅲ期慢性肾脏病患者病历资料,依据其使用不同种类肾素-血管紧张素-醛固酮系统(RAAS)抑制剂将其分组为沙库巴曲缬沙坦组96例,缬沙坦组126例,共计222例患者。分别记录入院及出院时估算的肾小球滤过率(eGFR)、左室射血分数(LVEF),疗效值及安全效应值分别为出院LVEF、eGFR水平与入院时比较的差值,同时使用1∶1倾向性评分匹配(PSM)比较均衡基线资料后效应量的改变。结果 2组出院LVEF、eGFR较入院时均有改善,差异有统计学意义(P<0.05),组间比较显示沙库巴曲缬沙坦组出院LVEF、eGFR改善程度更大,差异有统计学意义(P<0.05);
在进行PSM均衡基线后(匹配后2组各70例),沙库巴曲缬沙坦组出院LVEF、eGFR较入院时仍有改善,差异有统计学意义(P<0.05);缬沙坦组出院LVEF较入院时有改善,差异有统计学意义(P<0.05),eGFR水平出院与入院比较,差异无统计学意义(P>0.05);PSM后LVEF改善程度2组比较,差异无统计学意义(P>0.05)。结论 沙库巴曲缬沙坦能够改善急性心肌梗死合并Ⅱ~Ⅲ期慢性肾脏病患者住院期间心、肾功能,使用安全有效。[关键词] 心肌梗死;沙库巴曲缬沙坦;肾功能不全DOI:10.3969/J.issn.16726790.2022.03.017
G蛋白耦联胆汁酸受体激动剂INT777通过激活AMPK信号通路抑制施万细胞成髓鞘过程

南通大学学报(医学版)Journal of Nantong University (Medical Sciences) 2021 : 41 (1)6・D0I:10.16424/32-1807/r.2021.01.002G 蛋白耦联胆汁酸受体激动剂INT777通过激活AMPK 信号通路抑制施万细胞成髓鞘过程*关晋东:丁杰,刘晓宇,孙诚**** [基金项目]国家自然科学基金青年基金资助项目(81701222)** [作者简介]关晋东,男,汉族,生于1995年10月,山西省晋城市人,硕士在读,研究方向:外周神经发育及损伤修复机制的研究。
*** [通信作者] 孙诚,电话**************,E-mail: ********************.cn(南通大学教育部/江苏省神经再生重点实验室/神经再生协同创新中心,南通226001)[摘 要]目的:研究G 蛋白耦联胆汁酸受体(G-protein-coupled bile acid receptor 1, GPBAR1,同时也被称为TGR5) 特异性激动剂6琢-乙基-23(S)-甲基胆酸[6 alpha-ethyl-23(S)-methylcholic acid, INT777[对原代施万细胞髓鞘形成的影响并初步探讨其可能的作用机制遥方法:用5 滋mol/L 的INT777处理二丁酰环腺苷酸(dibutyryl cyclic adenoslne phosphate, dbcAMP )诱导分化原代施万细胞成髓鞘模型,用免疫印迹(Western Blot )方法检测髓磷脂蛋白表达量的变化遥同时,提取 施万细胞总核糖核酸后用定量聚合酶链式反应试验检测INT777对髓鞘形成过程相关分子基因表达的影响遥另外,用Western Blot 方法检测INT777对单磷酸腺苷活化蛋白激酶/核糖体蛋白S6激酶(adenosine 5'-monophosphate-activatedprotein kinase/ribosomal S6 kinase, AMPK/S6K )信号途径的影响遥结果:在dbcAMP 诱导分化原代施万细胞成髓鞘过程中,5 滋mol/L INT777的处理抑制了髓鞘早期生长因子20,八聚体结合转录因子6,髓磷脂蛋白的表达,且5滋mol/L INT777 处理激活了 AMPK 的活性,抑制了雷帕霉素作用靶点信号通路遥结论:INT777抑制dbcAMP 诱导的施万细胞成髓鞘过程,这种抑制作用可能是通过激活施万细胞AMPK 活性、抑制S6K 活性实现的遥[关键词]施万细胞曰髓鞘曰6琢-乙基-23(S)-甲基胆酸曰单磷酸腺苷活化蛋白激酶[中图分类号]R338.1 [文献标志码]A [文章编号]1674-7887(2021)01-0006-05G-protein-coupled bile acid receptor agonists INT777 inhibits myelination of Schwann cells byactivating AMPK signaling pathway*GUAN Jindong **, DING Jie, LIU Xiaoyu, SUN Cheng ***('Key Laboratory of Neuroregeneration of Jiangsu and Ministry ofEducation, Co-innovation Center of Neuroregeneration, Nantong University, Nantong 226001)[Abstract ] Objective: To investigate the effects of G-protein-coupled bile acid receptor 1(GPBAR1, also known as TGR5)specific agonist 6 alpha-ethyl-23(S)-methylcholic acid(INT777) on myelination in primary Schwann cells and the underlying mechanisms. Methods: Primary Schwann cells were treated with 5 滋mol/L INT777 and dibutiryl cyclic adenoslne phosphate (dbcAMP), and the changes of myelin protein zero were detected by Western Blot. Meanwhile, the total RNA was extractedfrom Schwann cells and quantitative real time polymerase chain reaction was employed for detecting myelin gene expression. In addition, the effect of INT777 on the adenosine 5' -monophosphate -activated protein kinase/ribosomal protein S6 kinase (AMPK/S6K) signaling pathway was examined by Western Blot. Results : Treatment with 5 滋mol/L INT777 inhibited theexpression of myelin early growth response -2, octamer -binding transcription factor 6, and myelin protein zero during dbcAMP-induced myelination of differentiated Schwann cells, and treatment with 5 滋mol/L INT777 activated AMPK activity and inhibited mTOR signaling pathway. Conclusion: INT777 attenuates dbcAMP-induced myelination of Schwann cells, whichmay be achieved by activating AMPK and inhibiting S6K activity.[Key words ] Schwann cell; myelination; 6 alpha-ethyl -23(S)-methylcholic acid; adenosine 5'-monophosphate-activated protein kinase外周神经系统(peripheral nervous system, PNS) 在机体内分布广泛且起到介导靶器官与中枢神经系 统信号传递的重要作用。
血管内皮间质样转分化遗传示踪小鼠模型的构建及其在肝纤维化研究中的应用

Establishment of a mouse model of vascular endothelial - mesenchymal transdifferentiation genetic tracing and its role
in liver fibrosis studies
, , , , , ( , XU Hao1 RUAN Bai1,2 LI Zhiwen1 FANG Zhiqiang1 WANG Lin1 DOU Kefeng1 . 1. Department of Hepatobiliary Pancreatic and , , , ’ , ; , Splenic Surgery Xijing Hospital Air Force Medical University Xi an 710032 China 2. Center of Clinical Aerospace Medicine School of , , ’ , ) Aerospace Medicine Air Force Medical University Xi an 710032 China : , ( : ) Corresponding author DOU Kefeng doukef@ fmmu. edu. cn ORCID 0000 - 0002 - 8857 - 5663
lected for immunofluorescent staining to observe the expression of the fluorescent proteins tdTomato and eGFP. Results After being induced
, by tamoxifen LSECs and liver tissue of Cdh5 - CreERT / Acta2 - KI genetic tracing mice expressed eGFP under the conditions for epithelial - , mesenchymal transdifferentiation established in vivo and in vitro while the control group without induction expressed tdTomato alone. Conclu
6.何慈江-controversie in podocytes-final3-翻译
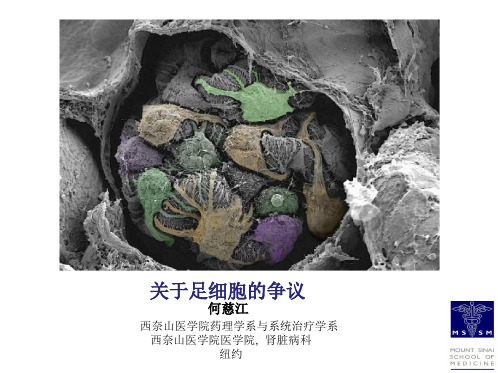
Western blot 验证不同足细胞系标志蛋白的相对表达
Chittiprol S et al. Am J Physiol Renal Physiol 2011;301:F660F671
©2011 by American Physiological Society
63X Nephrin
Actin DAPI
足细胞分化标志的改变
GAPDH对表达进行标准化
20.0 18.0 16.0 14.0 12.0 10.0
8.0 6.0 4.0 2.0 0.0
Glass
Channels
Synaptopodin Nephrin WT-1
Boxes
39
足细胞研究:转基因动物模型
足细胞体外研究
1. 体外培养的足细胞能否保持体内的表型? 2. 体外培养的最合适模型是什么? 3.足细胞原代培养是否可行? 4. 原代足细胞怎样分类?
电镜观察肾脏切片分离培养的足细胞
分离后
培养1-2天
Bertram et al. 1989 Cell Tissue Res
体外培养使用离体的肾小球
Type I Col gene
or
TRE
Pod rtTA3
GFP
shRNAmir
pA
Sirt 4/13-1 line DOX 0 0 + + + Sirt1
GFP
GAPDH
CAGs;ColTGM
Sirt 1/7-3 line
1.5
+ ++
1.0
* 0.5
Sirt1/GAPDHdensityrel.toNoDox
组蛋白泛素化及其与甲基化的关系

DNA , ò¦ x×S£ ö、 ²³。o£öBC{ @ X ·¸ A å L N 7 6 ? X [ 5] 15 ~ 38 V·ÉUDÉ 。 2 pqrs#^
[ 6]
Ubiquitination of Histone and Its Relationship with Methylation GAO Chen-lin, XU Yong. ( Department of Endocrinology, Affiliated Hospital of Luzhou Medical College, Luzhou 646000 , China) Abstract : The ubiquiห้องสมุดไป่ตู้ination of histone is a crucial part of histone modifications. The core histone ubiqubiquitin protein is conjugated to histone by sequential action of three uitination is based on core octamer, ubiquitin-related enzymes. The study mainly focused on ubiquitylation of histone H2A and H2B. The relationship between histone ubiquitination and methylation is complicated, furthermore, different organisms can speak different languages in the " cross-talk " between ubiquitination and methylation on different histones. The ubiquitination of H2A could reduce dimethylation and trimethylation of H3 at lysine 4 ( H3K4 ) . In conubiquitylates H2B is required for methylation of H3K4 in yeast and mammalian cells, however, this relatrast , tionship does not exist in tetrahymena. Key words: Epigenetic;Histone;Ubiquitination;Methylation
mTOR通路

Seminars in Cell &Developmental Biology 36(2014)79–90Contents lists available at ScienceDirectSeminars in Cell &DevelopmentalBiologyj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /s e m c dbGrowing knowledge of the mTOR signaling networkKezhen Huang a ,Diane C.Fingar a ,b ,∗aDepartment of Cell and Developmental Biology,University of Michigan Medical School,Ann Arbor,MI 48109-2200,United StatesbDivision of Metabolism,Endocrinology,and Diabetes (MEND),Department of Internal Medicine,University of Michigan Medical School,Ann Arbor,MI 48109-2200,United Statesa r t i c l ei n f oArticle history:Available online 19September 2014Keywords:mTOR mTORC1mTORC2InsulinAmino acids Energya b s t r a c tThe kinase mTOR (mechanistic target of rapamycin)integrates diverse environmental signals and trans-lates these cues into appropriate cellular responses.mTOR forms the catalytic core of at least two functionally distinct signaling complexes,mTOR complex 1(mTORC1)and mTOR complex 2(mTORC2).mTORC1promotes anabolic cellular metabolism in response to growth factors,nutrients,and energy and functions as a master controller of cell growth.While significantly less well understood than mTORC1,mTORC2responds to growth factors and controls cell metabolism,cell survival,and the organization of the actin cytoskeleton.mTOR plays critical roles in cellular processes related to tumorigenesis,metabolism,immune function,and aging.Consequently,aberrant mTOR signaling contributes to myriad disease states,and physicians employ mTORC1inhibitors (rapamycin and analogs)for several pathological conditions.The clinical utility of mTOR inhibition underscores the important role of mTOR in organismal physiology.Here we review our growing knowledge of cellular mTOR regulation by diverse upstream signals (e.g.growth factors;amino acids;energy)and how mTORC1integrates these signals to effect appropriate downstream signaling,with a greater emphasis on mTORC1over mTORC2.We highlight dynamic sub-cellular localization of mTORC1and associated factors as an important mechanism for control of mTORC1activity and function.We will cover major cellular functions controlled by mTORC1broadly.While signif-icant advances have been made in the last decade regarding the regulation and function of mTOR within complex cell signaling networks,many important findings remain to be discovered.©2014Elsevier Ltd.All rights reserved.Contents 1.Introduction ..........................................................................................................................................802.Growth factor sensing by the TSC–Rheb axis ........................................................................................................812.1.Insulin-PI3K-Akt signaling ....................................................................................................................812.2.EGF-Ras-MAPK signaling......................................................................................................................822.3.mTORC2regulation ...........................................................................................................................822.4.Negative feedback signaling ..................................................................................................................823.Amino acid sensing by the Rag–Ragulator axis and other emerging factors ........................................................................823.1.The Rag GTPases ..............................................................................................................................833.2.The Ragulator complex .......................................................................................................................833.3.The v-ATPase ..................................................................................................................................833.4.Emerging amino acid sensing factors .........................................................................................................844.The lysosome as a critical platform for upstream signal integration and mTORC1activation ......................................................855.Energy and stress sensing ............................................................................................................................856.Major cellular functions controlled by mTORC1.....................................................................................................86∗Corresponding author at:Department of Cell and Developmental Biology,University of Michigan Medical School,Ann Arbor,MI 48109-2200,United States.Tel.:+17347637541.E-mail address:dfingar@ (D.C.Fingar)./10.1016/j.semcdb.2014.09.0111084-9521/©2014Elsevier Ltd.All rights reserved.80K.Huang,D.C.Fingar/Seminars in Cell&Developmental Biology36(2014)79–907.Future directions (87)Acknowledgments (87)References (87)1.IntroductionAll cells from single-celled organisms to those comprising mul-ticellular organisms sense and respond rapidly tofluctuations in their nutritional and energetic environments in order to modulate cell metabolism appropriately and maintain cellular homeostasis. Consequently,cells coordinate nutritional and energetic supply and demand tightly to prevent engagement in ATP-consuming anabolic processes when environmental resources become limited. During evolution,multicellular organisms acquired the additional ability to sense and respond to long-range systemic signals(i.e. hormones;growth factors;mitogens;cytokines)(referred to col-lectively as“growth factors”here)to enable communication between tissues and organ systems.mTOR,the mechanistic tar-get of rapamycin,functions as a critical integrator of these diverse environmental cues by integrating them into appropriate cellu-lar responses.mTOR,an evolutionarily conserved serine/threonine protein kinase,belongs to the phosphatidylinositol-3kinase(PI3K)-related kinase(PIKK)superfamily.mTOR represents the functional target of a natural macrolide antibiotic called rapamycin(clini-cally known as sirolimus).Rapamycin,produced by the bacterium Streptomyces hygroscopicus,was discovered in soil samples from Easter Island(known as Rapa Nui to the native population)in the 1970s[1,2].Rapamycin reduces eukaryotic cell proliferation to var-ious degrees,with immune cells showing strong sensitivity.To identify the target of rapamycin,Hall and colleagues performed an elegant genetic screen in1991in the budding yeast Saccha-romyces cerevisiae.Mutations in three genes,Fpr1(an orthologue of FKBP12[FK506-binding protein12]),Tor1and Tor2,conferred resis-tance to rapamycin[3](and reviewed in[2]).Today we understand that rapamycin associates with an endogenous cellular protein, FKBP12,and this complex docks to the FRB(FKBP12rapamycin binding)domain located immediately N-terminal to the C-terminal mTOR kinase domain(see the accompanying article for greater detail regarding mTOR structure),resulting in allosteric inhibi-tion of mTOR activity and signaling[4–6].By affinity purification of FKPB12-rapamycin binding proteins,several groups identified the mammalian orthologue of budding yeast Tor1/2in1994–1995 [7–9].mTOR constitutes the catalytic core of two known signal-ing complexes,mTOR complex1(mTORC1)and mTOR complex2 (mTORC2)[10,11].These mTOR complexes(mTORCs)possess dis-tinct substrates,cellular functions,and sensitivity to rapamycin. Acute rapamycin treatment inhibits cellular mTORC1but not mTORC2signaling while longer-term rapamycin treatment sup-presses mTORC2function by compromising complex integrity to variable degrees depending on cell type[12].Rapamycin fails to inhibit the phosphorylation of all mTORC1substrates equally [13,14].It completely inhibits phosphorylation of S6K1(ribosomal protein S6kinase1)but only partially inhibits phosphorylation of 4EBP1(eukaryotic initiation factor4E binding protein1).The devel-opment of ATP-competitive mTOR catalytic inhibitors(i.e.Torin1; Ku-0063794)revealed that mTORC1phosphorylates substrates in both rapamycin-sensitive and-insensitive manners[15–18],pos-sibly due to differential substrate access to the kinase active site controlled by the mTOR FRB domain[19]and/or due to differen-tial substrate quality conferred by phosphorylation site consensus sequence[20,21].The exclusive mTOR partner raptor(regulatory-associated pro-tein of mTOR)defines mTORC1[22,23]while the exclusive mTOR partner rictor(rapamycin-insensitive companion of mTOR)defines mTORC2[24,25](Fig.1).In addition to raptor,mTORC1contains mLST8/GL(mammalian lethal with Sec13protein8/G-protein -protein subunit like)[26],PRAS40(Akt/PKB substrate40kDa) [27,28],and deptor(DEP-domain-containing mTOR interacting protein)[29].Raptor serves a scaffolding role,functioning to recruit substrates to the mTOR kinase through their TOS(TOR signal-ing)motifs[30,31].Global deletion of raptor in mice results in early embryonic lethality(e5.5)[32],similar to the global knock-out of mTOR[33].PRAS40and deptor function as both suppressors and targets of mTORC1,likely by acting as competitive sub-strates[29,34],while mLST8/GL is not essential for mTORC1 function[32].In addition to rictor[24,25],mTORC2contains mSIN1 (mammalian stress-activated protein kinase interacting protein1) [35,36],protor1/2(protein observed with rictor1/2)(aka PRR5) [37],mLST8/GL[24,25],and deptor[29].Thus,mTORC1and mTORC2contain distinct and shared partner proteins.Similar to raptor within mTORC1,rictor and mSin1serve as critical scaffolds that control mTORC2integrity,regulation by upstream signals, and substrate choice[24,25,35,36].Unlike mTORC1,mLST8/GL is required for mTORC2function;like rictor,its deletion in mice causes embryonic lethality(e10.5)[32].The role of protor remains unclear.It is important to note that mTOR also assembles into relatively homologous TORC1and TORC2complexes in bud-ding andfission yeast,underscoring the ancestral origin of the TORCs.While TORC1in yeast responds simply to environmental nutri-ents and energy,mTORC1in higher eukaryotes responds to a broader array of upstream signals,integrating cues from growth factors(i.e.insulin;IGF;EGF;cytokines)to modulate cellular func-tions appropriately(Fig.1)[10,11].mTORC1function absolutely requires sufficient levels of amino acids such that their withdrawal inactivates mTORC1signaling rapidly and renders mTORC1acti-vation refractory to virtually all other inputs,including growth factors.To limit cellular engagement in energy costly anabolic pro-cesses,nutrient and growth factor withdrawal as well as diverse types of cell stress(i.e.low energy;hypoxia;ER stress;ROS(reac-tive oxygen species))downregulate mTORC1signaling[38].Upon activation,mTORC1signaling drives cap-dependent protein syn-thesis,cell growth,and cell proliferation through phosphorylation of the ribosomal protein S6kinases(S6K1/2)and the eukaryotic ini-tiation factor4E(eIF4E)binding proteins1–3(4EBP1-3)at least in part[10,11,39].While the current set of direct mTORC1substrates remains somewhat limited(e.g.S6Ks;4EBPs;IRS-1;ULK1;Lipin1; TFEB;Grb10),quantitative phosphoproteomic screens identified a large number of downstream mTORC1effectors,many of which likely represent bonafide mTORC1substrates[40,41](Fig.1). mTORC1promotes other anabolic processes including lipid and nucleotide synthesis and suppresses autophagy,a degradative pro-cess in which autophagosomes break down macromolecules and organelles during nutrient and energy starvation.Thus,mTORC1 drives anabolic and suppresses catabolic cellular processes.Our understanding of the regulation and function of mTORC2lags far behind that of mTORC1due to its more recent discovery[24,25] and the lack of mTORC2-specific inhibitors.Growth factors activate mTORC2,which phosphorylates a limited set of known substrates including Akt(aka PKB),PKC␣(protein kinase C␣),and SGK1(serum and glucocorticoid-induced protein kinase).mTORC2modulates cell metabolism and the organization of the actin cytoskeleton and enhances cell survival,due to its activation of the survival kinase Akt[42,43](Fig.1).K.Huang,D.C.Fingar /Seminars in Cell &Developmental Biology 36(2014)79–9081Fig.1.Regulation of the mTORC1and mTORC2signaling network by diverse upstream inputs.Growth factors such as insulin or EGF activate mTORC1through either the PI3K-Akt or the Ras-MAPK (ERK)-RSK axes,respectively.Growth factor-mediated activation of mTORC1absolutely requires sufficient levels of amino acids,which are sensed through a variety of factors,as indicated.mTORC1action also requires sufficient levels of energy (i.e.ATP)and/or oxygen,which are sensed by AMPK,REDD1,and TCA cycle metabolites (i.e.␣KG).The TSC complex integrates diverse upstream signals to regulate mTORC1action.TSC suppresses the conversion of Rheb-GDP to Rheb-GTP,a small GTPase that activates mTORC1.mTORC1phosphorylates a limited known set of bona fide substrates to drive anabolic and suppress catabolic cellular processes and to mediate negative feedback toward PI3K.Growth factors (i.e.insulin)also activate mTORC2through poorly defined signaling intermediates.Aberrant mTORC1function contributes to myriad patho-logic conditions including cancer and benign tumor syndromes,metabolic disorders (e.g.type II diabetes;obesity),cardiovascu-lar disorders,inflammatory disorders,and neurological disorders [10,11].Consequently,clinicians employ rapamycin (aka sirolimus)and rapamycin analogs (rapalogs)(i.e.everolimus;temsirolimus)for immunosuppression following renal transplantation and for treatment of renal cell carcinoma,neuroendocrine tumors of pan-creatic origin,tuberous sclerosis complex (TSC,a benign tumor syndrome),and cardiac restenosis following angioplasty [6].The role of mTOR in pathophysiology of disease combined with the utility of mTORC1inhibitors in clinical medicine underscores the importance of elucidating the regulation and function of mTORC1at the cellular level [44].2.Growth factor sensing by the TSC–Rheb axisGrowth factors,in particular insulin/IGF (insulin-like growth factor)and EGF (epidermal growth factor),represent the best understood inputs that lead to activation of mTORC1upon converg-ing on the TSC (tuberous sclerosis complex)/Rheb axis [45].TSC,composed of TSC1(aka hamartin),TSC2(aka tuberin),and a morerecently discovered third subunit,TBC1D7(TBC [Tre2-Bub2-Cdc16]1domain family member 7),functions as a tumor suppressor that inhibits mTORC1[46](Fig.1).Inactivating mutations in either TSC1or TSC2increases mTORC1signaling and causes an autosomal dominant disease in which benign tumors form in various organs including brain,kidney,and heart [47].TSC inhibits mTORC1by inhibiting Rheb (Ras homolog enriched in brain),a small Ras-like GTPase essential for mTORC1activation by all upstream signals.2.1.Insulin-PI3K-Akt signalingInsulin/IGF binding to its cognate cell surface tyrosine kinase receptor leads to tyrosine phosphorylation of IRS (insulin receptor substrate)proteins,which recruits and activates PI3K (phos-phatidylinositol 3-kinase)[48](Fig.1).Increased production of the phospho-lipid PI(3,4,5)P3on lipid membranes by PI3K recruits Akt via its PH (pleckstrin homology)domain,leading to PDK1-mediated phosphorylation of Akt on its activation loop (T308)[49].It is important to note that additional phosphorylation of Akt on its hydrophobic motif (S473)by PI3K-controlled mTORC2boosts Akt activity several fold further [49,50].Activated Akt then phosphorylates TSC2on several sites (S939;T1462)to82K.Huang,D.C.Fingar/Seminars in Cell&Developmental Biology36(2014)79–90suppress the inhibitory effect of the TSC complex toward mTORC1, thus leading to increased mTORC1signaling[51,52].TSC2pos-sesses a GAP(GTPase activating protein)domain that hydrolyzes active Rheb-GTP to inactive Rheb-GDP.Thus,in response to insulin/PI3K signaling,Akt phosphorylates and inactivates TSC2, which increases Rheb-GTP loading and mTORC1kinase activity [27].While Rheb-GTP interacts with the mTOR kinase domain [27,53],the underlying molecular mechanism by which Rheb acti-vates mTORC1remains unclear.Other parallel mechanisms contribute to activation of mTORC1 by insulin/IGF.Akt and mTORC1phosphorylate PRAS40,an mTORC1inhibitory partner(on T246and S183/S212/S221,respec-tively),inducing the dissociation of PRAS40from mTORC1and thus relieving PRAS40-mediated substrate competition[27,28,34,54]. Insulin/PI3K signaling also leads to mTORC1-mediated phospho-rylation of raptor(on S863)to promote mTORC1signaling[55,56]. Moreover,phosphorylation of mTOR itself(on S1261,S2159,and T2164)by unknown kinases[57,58]and on S1415by IKK␣[59] contributes to increased mTORC1signaling.While mTOR autophos-phorylation on S2481plays no known role in mTORC1function, it serves as a biomarker for mTORC1and mTORC2catalytic activ-ity in intact cells[5].Many phospho-proteomic studies agree that mTOR and its partner proteins undergo phosphorylation on many sites[40,41,60].Consequently,a challenge for the future will be to identify the kinases that act on these sites directly and to deci-pher the regulation and functional significance of complex mTORC phosphorylation.2.2.EGF-Ras-MAPK signalingEGF activates mTORC1signaling independently of the PI3K/Akt axis.EGF binding to its cell surface tyrosine kinase receptor acti-vates the Ras GTPase,which leads to activation of c-Raf,MEK (MAPK/ERK kinase),MAPK(mitogen activated protein kinase) (aka ERK)and RSK(p90ribosomal S6kinase(Fig.1).Similar to Akt,MAPK and RSK phosphorylate TSC2on different sites (S540/S644and S1798,respectively)to suppress the inhibitory action of TSC2toward Rheb[61,62].By a parallel pathway,the Ras/MAPK pathway converges on raptor.MAPK phosphorylates raptor(on S8/S696/S863)and RSK phosphorylates raptor(on S719/S721/S722)[63,64]to promote mTORC1signaling.2.3.mTORC2regulationInsulin/PI3K signaling leads to mTORC2-mediated phosphory-lation of Akt on its hydrophobic motif(HM)site,S473(Fig.1)as well as the HM sites of other AGC kinases,PKC␣(on S657),and SGK1(on S422)[25,50,65].Insulin increases the kinase activity of mTORC2in vitro in a manner sensitive to cellular treatment with the PI3K inhibitor wortmannin[66].Thus,insulin/PI3K signaling activates mTORC2;it is important to note,however,that the sig-naling intermediates that link PI3K to mTORC2remain virtually unknown.Interestingly,while TSC inhibits mTORC1,TSC activates mTORC2[66].MEFs lacking TSC2display reduced mTORC2kinase activity toward Akt in vitro and decreased Akt S473phosphoryla-tion in intact cells in a manner independent of the well-known mTORC1-mediated negative feedback loop that attenuates PI3K signaling(discussed below)[66].In addition,TSC associates with mTORC2.These data suggest quite different regulation of mTORC2 compared to mTORC1.On the other hand,the Rac1GTPase interacts with mTOR and provides an activating signal to both mTORC1and mTORC2in response to growth factors in a PI3K/Akt independent manner,suggesting that common upstream inputs co-regulate both mTORCs[67].There is no question that important discoveries await regarding mTORC2regulation.As mTORC2mediates Akt S473phosphorylation,it would seem that mTORC2lies upstream of mTORC1.While such epistasis may hold true in certain cellular contexts,genetic knockout or knockdown of core mTORC2components(i.e.rictor;mSin1)in many cultured cell types has no effect on TSC2phosphoryla-tion and mTORC1signaling[32,68].Thus,mTORC2function is not required for mTORC1action.On the other hand,mTORC2function is required for Akt-mediated phosphorylation of other substrates (i.e.FoxO1/3a)[32,68].These data can be explained by the known essential requirement for activation loop site(T308)but not HM site(S473)phosphorylation for Akt kinase activity;Akt S473phos-phorylation boosts Akt activity further and may modulate substrate specificity[32,49,68].Thus,in many cellular contexts Akt phospho-rylation on its activation loop without HM-site phosphorylation provides sufficient activity to mediate downstream signaling to mTORC1.Interestingly,mTORC2associates with ribosomes in a growth factor sensitive manner[69,70].Structurally intact ribosomes,but not protein synthesis itself,are required for mTORC2kinase activity in vitro and signaling in intact cells[70].Thus,a direct interaction of mTORC2with ribosomes may play a role in insulin/PI3K-meditated mTORC2activation.mTORC2also promotes turn-motif (TM)site phosphorylation of Akt(T450)and several conventional PKCs(PKC␣T638and PKCT641)in a co-translational manner independently of growth factor status,functioning to increase protein stability and folding[71,72].In addition to interacting with ribosomes(likely those associated with ER engaged in pro-tein translation),mTORC2associates with an ER sub-compartment called MAM(mitochondrial-associated ER membrane)in a growth factor stimulated manner.mTORC2inactivation decreases MAM integrity,mitochondrial metabolism,and cell survival[73].2.4.Negative feedback signalingSeveral negative feedback mechanisms modulate the mTOR signaling network,as signal attenuation limits signal amplitude and duration critical for homeostatic control of complex biolog-ical systems.Cellular TSC loss leads to elevated and constitutive mTORC1signaling independent of growth factor status and atten-uates PI3K signaling,thus producing a state of cellular insulin resistance[74,75].S6K1and mTORC1phosphorylate IRS-1directly to induce its degradation,thus uncoupling the insulin receptor from PI3K.mTORC2also limits PI3K signaling by inducing IRS-1 degradation[76].mTORC2phosphorylates and stabilizes Fbw7,an ubiquitin ligase subunit that targets IRS-1for degradation[76]. Grb10was identified as a direct mTORC1substrate in phospho-proteomic screens[40,76].mTORC1-mediated phosphorylation of Grb10,a growth factor receptor-bound adaptor that limits growth factor signaling,stabilizes Grb10and attenuates both PI3K and MAPK/ERK signaling.Depending on cellular context,either S6K1 or Akt phosphorylate mSin1directly(on T86and T398),a crit-ical mTORC2partner,dissociating mSin1from the complex and decreasing mTORC2signaling[40].Along similar lines,several groups reported that S6K1phosphorylates rictor directly(T1135) [77–80],which may reduce mTORC2signaling to Akt[78,79].These data reveal that both mTORC1and mTORC2engage in negative feedback to maintain proper signaling by growth factor receptors and the mTORCs.3.Amino acid sensing by the Rag–Ragulator axis and other emerging factorsSufficient levels of amino acids,particularly leucine,are essen-tial for basal mTORC1signaling from yeast to mammals and for robust activation of mTORC1in response to growth factor signalsK.Huang,D.C.Fingar/Seminars in Cell&Developmental Biology36(2014)79–9083Fig. 2.Rag heterodimers recruit mTORC1to lysosomal membranes for Rheb-mediated activation.Activation of mTORC1by amino acids through Rag GTPase heterodimers involves the v-ATPase,Ragulator complex,and Rag regulatory factors. The Ragulator complex,which acts as a GEF toward RagA/B GTPases,induces forma-tion of active RagA/B GTP–RagC/D GDP heterodimers.The GATOR1complex functions as a GAP(GTPase activating protein)for RagA/B(thus inhibiting Rag heterodimers) while folliculin(FLCN)and its associated proteins(FNIP1/2)functions as a GAP for Rag C/D(thus promoting a Rag heterodimer active state).The GATOR2complex sup-presses GATOR1.Active RagA/B GTP–RagC/D GDP heterodimers bind mTORC1through raptor to recruit mTORC1to the lysosomal surface where Rheb resides.When loaded with GTP,Rheb activates mTORC1through a poorly defined mechanism.An“inside-out”model proposes that the v-ATPase and Ragulator respond to amino acid levels inside the lysosomal lumen to control Rag nucleotide binding state.in higher eukaryotes[81,82].How cells sense amino acid levels remains poorly defined,but great progress has been made in recent years identifying the machinery that propagates amino acid sens-ing proximal to mTORC1.While several signaling molecules that link amino acid sensing to mTORC1have been identified(see text below),the Rag GTPases,the Ragulator complex,and the v-ATPase represent the best-characterized links between amino acid sensing and mTORC1.3.1.The Rag GTPasesThe evolutionarily conserved family of Rag GTPases function as obligate heterodimers in which Rag A or Rag B dimerizes with RagC or RagD(Fig.2).Upon amino acid stimulation,RagA/B loads with GTP and binds raptor while RagC/D loads with GDP[83,84]. Expression of dominant-active RagA/B mutants(loaded consti-tutively with GTP)promote mTORC1signaling in the absence of cellular amino acids while expression of dominant-negative RagA/B mutants(nucleotide-free)suppress mTORC1signaling in the presence of amino acids.Thus,heterodimers composed of RagA/B GTP–RagC/D GDP form during amino acid sufficiency to promote mTORC1signaling and heterodimers composed of RagA/B GDP–RagC/D GTP form during amino acid withdrawal[83,84]. As exogenous expression of RagA/B GTP–RagC/D GDP heterodimers more strongly activate mTORC1in amino acid deprived cells than exogenous expression of dominant-active RagA/B alone,these data suggest that the nucleotide-bound state of RagC/D as well as that of RagA/B indeed contributes to mTORC1signaling in response to amino acids.How do Rags control amino acid-mediated activation of mTORC1?While Rheb-GTP provided in vitro increases mTORC1 kinase activity directly[27],active RagA/B GTP–RagC/D GDP het-erodimers provided in vitro are insufficient[83].Cellular amino acid stimulation induces the translocation of mTOR and raptor from a poorly defined cytoplasmic compartment to LAMP1/2-positive lysosomal membranes,a site to which the Rags and Rheb also localize[83,85].Importantly,mTORC1translocation requires the Rags[83].These data suggest a model in which the amino acid-Rag axis activates mTORC1by controlling mTORC1subcel-lular localization:amino acid signaling drives the formation of active RagA/B GTP–RagC/D GDP heterodimers,which bind raptor to recruit mTORC1to the lysosomal surface where mTORC1receives an essential activating input from Rheb(and possibly from other inputs)(Fig.3).While Rheb docks to internal membranes by a farnesyl lipid moiety,Rag GTPases do not possess lipid-anchoring motifs.3.2.The Ragulator complexA pentameric complex called the Ragulator,consisting of p18 (LAMTOR1),p14(LAMTOR2),MP1(LAMTOR3),c7orf59(LAMTOR4), and HBXIP(hepatitisB virus X interacting protein)(LAMTOR5),was found to anchor the Rags to lysosomal membranes through p18 myristoylation[85,86](Fig.3).The Ragulator complex not only tethers the Rags to lysosomal membranes,it possesses GEF activity toward RagA/B to enable exchange of GDP for GTP,thus converting Rag heterodimers to an active state[85].Conversely,a complex of proteins termed GATOR1,which contains proteins DEPDC5,Nprl2, and Nprl3,binds Rag heterodimers and possesses GAP activity for RagA/B,thus converting Rag heterodimers to an inactive state[87].A complex called GATOR2,which contains proteins Mios,Wdr24, Wdr59,Seh1L,and Sec13,suppresses GATOR1.Indeed,inactivation of GATOR1subunits renders mTORC1resistant to amino acid depri-vation while inactivation of GATOR2subunits suppresses mTORC1 signaling[87].RagC/D not only function as obligate binding partners for RagA/B,their nucleotide binding state also participates in amino acid controlled mTORC1function.Folliculin(FLCN)and its inter-acting partners FNIP1/2are required for amino acid-stimulated translocation of mTORC1to lysosomes,where they dock to Rag GTPases in the absence of amino acids,poised to convert RagC/D GTP to RagC/D GDP upon docking of mTORC1to the lysosomal sur-face upon amino acid addition[88,89](Fig.2).FLCN possess GAP activity toward RagC/D but not RagA/B and thus converts Rag heterodimers from an inactive RagA/B GDP–RagC/D GTP to an active RagA/B GTP–RagC/D GDP nucleotide-bound state that stabilizes mTORC1docking[89].The discovery that a spatial-temporal mech-anism governs amino acid-mediated mTORC1signaling explained a long-standing mystery in thefield regarding why growth factors fail to activate mTORC1in the absence of amino acids:if mTORC1local-izes within the cell to the wrong place at the wrong time,mTORC1 cannot be activated by upstream inputs.3.3.The v-ATPaseHow do cells sense amino acid levels?The discovery that the v-ATPase(vacuolar H+-adenosine triphosphatase)interacts with the Ragulator complex and Rag GTPases on the lysosomal surface and senses amino acids levels,possibly from within the lysoso-mal lumen,begins to elucidate these important but poorly resolved questions[90].v-ATPase subunits and its ATP hydrolyzing catalytic activity are required for generation of active RagA/B GTP–RagC/D GDP heterodimers,for amino acid-mediated localization of mTORC1to lysosomes,and for activation of mTORC1(Fig.2).Its classical func-tion as a proton pump that lowers luminal pH of lysosomes is not required for mTORC1activation,however[90].Amino acids modulate interactions between the v-ATPase,Ragulator,and Rags [90],and amino acid accumulation within the lysosomal lumen correlates with recruitment of mTORC1to lysosomal membranes. Thus,mTORC1appears to sense intra-lysosomal amino acids by an“inside-out”mechanism.It is important to note,however, that the overall importance of intra-lysosomal amino acids for mTORC1recruitment remains unclear.In this proposed model,。
以FXR为核心的胆汁酸代谢机制研究进展

Hans Journal of Biomedicine 生物医学, 2018, 8(4), 62-68Published Online October 2018 in Hans. /journal/hjbmhttps:///10.12677/hjbm.2018.84008Advanced Progression in the Mechanism of Bile Acid Metabolism Targeting FXRXiuli Yang1, Sicong Tian1, Bo Pang1, Baolong Li2, Yujuan Shan1*1Department of Food Science and Engineering, Harbin Institute of Technology, Harbin Heilongjiang2Center of Drug Safety Evaluation, Heilongjiang University of Chinese Medicine, Harbin HeilongjiangReceived: Oct. 4th, 2018; accepted: Oct. 19th, 2018; published: Oct. 26th, 2018AbstractBile acids are important physiological factors that facilitate the digestion & absorption of dietary lipids and fat-soluble vitamins in the gut. In addition, they also act as signaling molecules to regu-late glucose homeostasis, lipid metabolism and energy expenditure. Disorders of bile acid meta-bolism can lead to a series of diseases. The nuclear receptor farnesoid X receptor (FXR) is a spe-cific bile acid receptor which plays an important role in the metabolism of bile acids through the regulation of multiple metabolic pathways and of corresponding target genes. Consequently, FXR is targeted to be a new drug for the therapy of disorders related to bile acid metabolism. This ar-ticle reviews the recent progressions of FXR in regulating bile acid metabolism and its mechanism, which aims to provide scientific strategies for the prevention/treatment of bile acid metabolic disorders, and new drugs exploration.KeywordsBile Acids, Farnesoid X Receptor, Bile Acid Metabolism以FXR为核心的胆汁酸代谢机制研究进展杨修利1,田思聪1,庞博1,李宝龙2,单毓娟1*1哈尔滨工业大学食品科学与工程系,黑龙江哈尔滨2黑龙江中医药大学药物安全性评价中心,黑龙江哈尔滨收稿日期:2018年10月4日;录用日期:2018年10月19日;发布日期:2018年10月26日*通讯作者。
抑制还是转导:信号分子调节机体健康与疾病

生命科学Chinese Bulletin of Life Sciences第22卷 第3期
2010年3月Vol. 22, No. 3
Mar., 2010
文章编号 :1004-0374(2010)03-0240-08抑制还是转导:信号分子调节机体健康与疾病裴 钢1,2
(1 同济大学,上海 200092;2 中科院上海生命科学研究院生物化学与细胞生物学研究所,上海200031)
摘 要:细胞内的信号转导网络是由多条功能特异且彼此关联的信号通路所构成,它们赋予了细胞功能的多样性和可塑性,同时也必须受到精细严谨的调控。一些功能广泛的信号调节因子,如β-抑制蛋白(β-arrestin),在细胞信号转导网络完整性的维持中扮演着重要的角色。β-arrestin分子的经典功能是终止G-蛋白偶联受体(G-protein-coupled receptors)下游信号转导,即受体脱敏,但最近许多研究证据表明,这种脱敏功能(负调控)还可以针对其他的信号转导途径。例如,β-arrestin能够通过不同的机制负调控三条重要的NF-κB激活通路,该功能异常则导致NF-κB持续激活以及下游炎性因子的过度分泌。此外,近年来发现β-arrestin还能作为支架蛋白介导功能性信号复合物的形成。例如,在特定外界信号刺激下,β-arrestin 1能够转移至细胞核内并与组蛋白乙酰化酶p300相互作用而调控基因表达。该机制的生理意义之一反映在多发性硬化症的小鼠模型中,β-arrestin1在发病小鼠中较正常小鼠表达上调并能够显著加重病情。与之相反,在细胞质中富集的β-arrestin2参与了胰岛素激活时InsR/Akt/β-arrestin2/Src信号复合体的形成,它的缺失能够导致胰岛素耐受和2型糖尿病的发生。因此,在特定的条件下,β-arrestin对于胞内信号的传递究竟是抑制还是激活,已成为细胞信号转导中的关键问题,并在机体健康和疾病状态的相互转化中的起着重要作用。关键词:β-arrestin;受体脱敏;信号转导;NF-κB信号通路;核定位;胰岛素信号复合体中图分类号:R329.2;R363.2
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Asian Journal of Andrology(201 1)13,106-1 10 @2011 AJA.SIMM&SJTU.All rights reservedi008-682 ̄Ii¥32.O0
REVIEW www.natu re.com/aja
Role and regulation of EGFR in actin remodeling in sperm capacitation and the acrosome reaction
Hahn Breitbart and Nir Etkovitz To bind and fertilize the e器,the spermatozoon should undergo few biochemicaI and motility changes in the female reproductive tract collectively called capacitation.The capacitated spermatozoon binds to the egg zona pellucida,and then undergoes the acrosome reaction (AR),which allows its penetration into the e器.The mechanisms regulating sperm capacitation and the AR are not completely understood. In the present review。we summarize some data regarding the role and regulation of the epidermal growth factor receptor(EGFR)in these processes.In the capacitation process,the EGFR is partially activated by protein kinase A(PKA).resulting in phospholipase D(PLD) activation and actin polymerization.Protein kinase C alpha(PKCa),which is already activated at the beginning of the capacitation。also participates in PLD activation.Further activation of the EGFR at the end of the capacitation enhances intracellular Ca concentration leading to F-actin breakdown and allows the AR to take place.Under/n vivo conditions.the EGFR can be directly activated by its known ligand epidermal growth factor(EGF).and indirectly by activating PKA or by transactivation mediated by G protein.coupled receptors (GPCRs)activation or by ouabain.Under physiological conditions,sperm PKA is activated mainly by bicarbonate,which activates the soluble adenylyl cyclase to produce cyclic adenosine monophosphate(cAMP).the activator of PKA。The GPCR activators angiotensin ll or lysophosphatidic acid。as welI as ouabain and EGF are physiological cornponents present in the female reproductive tract. Asian Journaf ofAndrology(2011)13,1O6卜II10;doi:10.1038/aja.2010.78:published online 18 Octoher 2O10
Keywords:acrosome reaction;capacitation;PI3K:PKA;PKC;spermatozoa
I NTRODUCT10N Ejaculated mammalian spermatozoa should reside in the female genital tract for several hours before gaining the ability to fertilize the egg.In human sperm,however,sperm must move out of the seminal plasma immediately after ejaculation and appear in the fallopian tube within minutes.As soon as sperm are moving out of the ejaculate and passing the cervical mucus,tl1ev undergo several biochemical changes collectively called capacitation(renewed in Florman and Dudbella and Gadella and Visconti ).These changes are still not clear,but it seems certain to involve molecules absorbed on,or integrated into,the sperm plasma membrane during epididyrnal mamration,and on contact of spermatozoa with the seminal plasma they render the spermatozoa capable offertilization.The removal or alteration of tllese molecules prepares the sperm toward successEd binding to the egg and fertilization.It was shown by Yanagimachi and Chang3 that capacitation can be mimicked in vitro, making the analysis of capacitation mechanisms considerably easier. During mammalian fertilization,the capacitated spermatozoon penetrates the cumulus oophrous of the ovum,and then binds to the zona pellucida with its plasma membrane intact.After binding to the。gg zona pellucida,the spermatozoon undergoes an exocytotic process called the acrosome reaction(AR)(reviewed in Yanagimachi a1., Roldan and Shi,’Florman et a1.。and Breitbart,1.This event is required for fertilization,because it enables passage of the spermato— zoon through the zona pellucida and its subsequent fusion with the egg oolema.Therefore,elucidation of the mechanisms regulating the AR is important for understanding the process of mammalian fert— ilization.In our laboratory and others it was shown that variety of agonists can trigger the AR via receptor—mediated mechanisms. ’ 一 ” Mthough zona pellucida—derived glycoproteins are thought to be the physiological inducers of the AR, 1,12 the reaction can be induced
vitro by various constituents ofthe female reproductive tract including progesterone, 。’ prostaglandins, atrial natriuretic peptide, epi.
dermal growth factor(EGF), , 。, ouabain and other ligands.
These agonists may have a direct and/or synergistic effect with other constituents of the female reproductive or on the zona pellucida.14
The question regarding the physiological role ofthese factors under in vivo conditions is still an open question.Assuming that acrosome— reacted sperm cannot bind and fertilize the egg,we suggest that pre— mature AR before reaching the egg zona pellucida,might be a way of selection in which the‘bad’sperm will undergo the so—called non. specific AR and would not be able to fertilize,whereas the‘best’ selected sperm will reach the egg in its intact morphology and will fertilize it.Thus,to study the selection mechanism,it is very important to understand the mechanism of action of the various physiological factors that induce the AR.One ofthese mechanisms,the EGF receptor (EGFR)system is described in this review.
