Determination of the plant growth regulator chlormequat in food by
农产品中常用植物生长调节剂检测方法

农产品中常用植物生长调节剂检测方法任瑞;赵向东;李增良;刘青;李晓辉【摘要】该文概述了农产品中常用植物生长调节剂检测方法的研究进展,包括光谱法、免疫法、色谱法、色谱-质谱联用等分析方法及其优缺点,并对其发展趋势进行展望.【期刊名称】《河北林业科技》【年(卷),期】2016(000)003【总页数】3页(P80-81,90)【关键词】农产品;植物生长调节剂;检测方法【作者】任瑞;赵向东;李增良;刘青;李晓辉【作者单位】河北省林果桑花质量监督检验管理中心,河北石家庄050081;河北省林果桑花质量监督检验管理中心,河北石家庄050081;河北省林果桑花质量监督检验管理中心,河北石家庄050081;河北省林果桑花质量监督检验管理中心,河北石家庄050081;河北省林果桑花质量监督检验管理中心,河北石家庄050081【正文语种】中文【中图分类】S482.8植物生长调节剂是对植物的生长发育具有重要调节调控作用的一种小分子化合物,其检测方法受调节剂自身的化学性质、物理性质,以及机体干扰等因素影响。
目前,植物生长调节剂的检测方法主要有光谱法、免疫检测法、色谱法、色谱—质谱联用等。
1.1 光谱法光谱法主要有紫外、红外和荧光法的应用。
紫外分光和红外吸收光谱法早期是对经提纯的植物生长调节剂进行定性或结构分析,但存在专一性差的问题[1]。
近年来荧光法在植物生长调节剂检测方面得到了一定的应用,徐文婷等[2]用荧光分析法对西红柿、黄瓜和豆芽中的琢-NAA与6-BAP进行了测定,得到了较好的实验结果;张韶虹等[3]建立了化学发光直接检测IAA的新方法。
荧光法具有选择性好、用样量小、灵敏度高,以及简便快速等优点。
但由于生成物具有光不稳定性,对检测操作的及时性、熟练快速操作要求较高,荧光法的应用受到较大限制。
将荧光法与其他技术结合进行光谱分析是光谱法检测植物生长调节剂的发展趋势[4-5]。
1.2 免疫检测法放射免疫检测法(RIA)和酶联免疫吸附检测法(ELISA)是常见的两种植物生长调节剂的定量检测方法,因放射免疫存在一定的安全隐患现已很少使用。
保护植物作文英语

The Importance of Plant Conservation andOur ResponsibilityPlants are the foundation of all life on Earth. They provide oxygen, absorb carbon dioxide, and maintain the balance of our ecosystem. However, with the rapid pace of industrialization and urbanization, plant life is facing an unprecedented threat. It is our duty to conserve and protect plants for the sake of our planet and future generations.One of the main reasons for the decline in plant life is the loss of natural habitats. As cities expand and factories multiply, natural habitats like forests and wetlands are being destroyed at an alarming rate. This not only affects the plants but also disrupts the food chain, leading to a decline in biodiversity.Another factor contributing to plant conservation is climate change. Global warming is causing extreme weather conditions like droughts and floods, which are devastating for plant life. Plants are finding it increasinglydifficult to adapt to these changing conditions, leading to a decline in their numbers.To conserve plants, we need to take several measures. Firstly, we must protect their natural habitats. This involves stopping deforestation, wetland drainage, and other activities that destroy plant habitats. We should also promote afforestation and reforestation to restore damaged ecosystems.Secondly, we need to promote sustainable practices that are friendly to plants. This includes using organic fertilizers and pesticides instead of chemical ones, which are harmful to plants and soil health. We should also encourage the use of renewable energy sources to reduce the carbon footprint of human activities.Lastly, we need to raise awareness about plant conservation among people. Education and awareness campaigns can help create a culture of conservation, where people understand the importance of plants and are willing to protect them.In conclusion, plant conservation is crucial for the health of our planet and future generations. We must take action to protect plants by conserving their habitats, promoting sustainable practices, and raising awarenessamong people. Only then can we ensure a green and sustainable future for all.**植物保护的重要性与我们的责任**植物是地球上所有生命的基础。
Atrimmec 植物生长调节剂及其他产品说明说明书
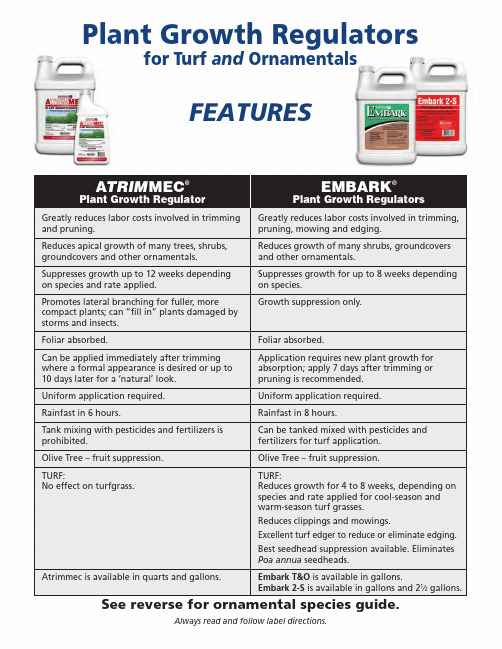
Plant Growth Regulators for Turf and OrnamentalsFEATURESA TRIM MEC®Plant Growth RegulatorEMBARK®Plant Growth RegulatorsGreatly reduces labor costs involved in trimming and pruning.Reduces apical growth of many trees, shrubs, groundcovers and other ornamentals.Suppresses growth up to 12 weeks depending on species and rate applied.Promotes lateral branching for fuller, morec ompact plants; can “fill in” plants damaged by storms and insects.Foliar absorbed.Can be applied immediately after trimming where a formal appearance is desired or up to 10 days later for a ‘natural’ look.Uniform application required.Rainfast in 6 hours.Tank mixing with pesticides and fertilizers isp rohibited.Olive Tree – fruit suppression.TURF:No effect on turfgrass.Atrimmec is available in quarts and gallons.Greatly reduces labor costs involved in trimming, pruning, mowing and edging.Reduces growth of many shrubs, g roundcovers and other ornamentals.Suppresses growth for up to 8 weeks depending on species.Growth suppression only.Foliar absorbed.Application requires new plant growth fora bsorption; apply 7 days after trimming or pruning is recommended.Uniform application required.Rainfast in 8 hours.Can be tanked mixed with pesticides andf ertilizers for turf application.Olive Tree – fruit suppression.TURF:Reduces growth for 4 to 8 weeks, depending on species and rate applied for cool-season and warm-season turf grasses.Reduces clippings and mowings.Excellent turf edger to reduce or eliminate edging. Best seedhead suppression available. Eliminates Poa annua seedheads.Embark T&O is available in gallons.Embark 2-S is available in gallons and 21⁄2gallons.Always read and follow label directions.See reverse for ornamental species guide.ORNAMENTAL SPECIES GUIDEFor growth regulation, apply Embark as a full cover spray withfoliage p enetration and to the drip point. Applications should bemade to healthy actively growing plants 7-14 days after pruning ortrimming. Once the plant resumes active growth, pruning andrepeat applications may be made.Always read and follow label directions.ATRIMMEC®and EMBARK®are registered trademarks of PBI-Gordon Corporation.© 2010, PBI-Gordon Corporation.01187。
为什么植物很重要英语作文英汉互译

为什么植物很重要英语作文英汉互译全文共3篇示例,供读者参考篇1Why Plants are ImportantPlants are undoubtedly one of the most crucial elements of our ecosystem, playing a vital role in sustaining life on Earth. As a student, I have come to appreciate the significance of plants not only through my studies but also through observing the world around me. In this essay, I will explore the various reasons why plants are so essential, touching upon their ecological, economic, and cultural importance.First and foremost, plants are the primary producers in the food chain, harnessing the energy from the sun through photosynthesis. This process is the foundation of all life on our planet, as it generates oxygen and organic compounds that serve as food for other living organisms. Without plants, the intricate web of life would collapse, leaving our planet devoid of the sustenance necessary for survival.Moreover, plants play a crucial role in maintaining the balance of our atmosphere. Through photosynthesis, theyabsorb carbon dioxide and release oxygen, helping to regulate the levels of these gases in the air we breathe. This natural process mitigates the effects of greenhouse gases and helps combat climate change, a pressing issue that threatens thewell-being of our planet.The ecological importance of plants extends far beyond their role in the food chain and atmospheric regulation. They provide habitats for countless species of animals, insects, and microorganisms, contributing to the rich biodiversity of our world. The intricate relationships between plants and other living beings are truly remarkable, forming complex ecosystems that support life in all its forms.From an economic standpoint, plants are a invaluable resource. Agriculture, forestry, and countless other industries rely heavily on plant-based products. Plants provide us with food, fiber, fuel, medicine, and numerous other commodities that are essential for human survival and development. The cultivation and trade of plant-based goods have shaped civilizations throughout history and continue to be a driving force in the global economy.Furthermore, plants hold deep cultural and spiritual significance in many societies around the world. They are oftenrevered as symbols of life, growth, and renewal, playing a central role in various religious and cultural traditions. The beauty and diversity of plants have inspired countless works of art, literature, and poetry, enriching our lives with their aesthetic appeal and serving as a source of inspiration for generations.Despite their vital importance, the world's plant life is facing numerous threats, such as deforestation, urbanization, and climate change. It is our collective responsibility to protect and preserve these invaluable resources for the sake of our planet and future generations. By promoting sustainable practices, raising awareness about the importance of plants, and taking active steps to conserve and restore plant habitats, we can ensure that these essential organisms continue to thrive and support life on Earth.In conclusion, plants are truly indispensable to our existence. They provide us with the very air we breathe, the food we eat, and the resources we rely on for our daily lives. Beyond their practical applications, plants enrich our cultural heritage and serve as a source of inspiration and beauty. As students and global citizens, it is our duty to appreciate and protect these remarkable organisms, for they are the foundation upon which all life depends.植物的重要性植物无疑是我们生态系统中最关键的元素之一,在维持地球上的生命扮演着至关重要的角色。
Plant Seedling Root Tissue Specimen

Plant Seedling Root Tissue SpecimenPlant Seedling Root Tissue Specimen: Understanding the Importance of Root Tissue in Plant GrowthPlants are an essential component of the ecosystem, and their growth and development are dependent on various factors, including soil quality, water availability, light, and temperature. However, one aspect that is often overlooked is the role of root tissue in plant growth. Root tissue is a critical component of plant physiology, and its health and functionality can significantly impact a plant's growth and development. In this article, we will explore the importance of root tissue in plant growth from multiple perspectives.From a biological perspective, root tissue is responsible for several essential functions in plants. Firstly, it anchors the plant to the soil, providing stability and support. Secondly, it absorbs water and nutrients from the soil, which are essential for plant growth. Thirdly, it stores food and water for the plant, which can be used during periods of drought or other environmental stressors. Lastly, it produces hormones that regulate plant growth and development.From an ecological perspective, root tissue is essential for maintaining soil health and preventing erosion. The roots of plants help to bind soil particles together, making it more resistant to erosion caused by wind or water. Additionally, root tissue plays a vital role in the carbon cycle by storing carbon in the soil. This stored carbon can help to mitigate the effects of climate change by reducing the amount of carbon dioxide in the atmosphere.From an agricultural perspective, root tissue is critical for crop production. Healthy root tissue is essential for nutrient uptake, which is necessary for plant growth and development. Additionally, root tissue can help to prevent soil erosion and improve soil health, which can lead to increased crop yields. Understanding the importance of root tissue in crop production can help farmers to make informed decisions about soil management practices and crop selection.From a societal perspective, root tissue is essential for food security and environmental sustainability. As the global population continues to grow, the demand for food will increase, and the importance of sustainable agriculture practices will become even more critical. Understanding the role of root tissue in plant growth and development can help to ensure that we are using our natural resources in a responsible and sustainable manner.From a personal perspective, root tissue is fascinating and beautiful. Root systems are incredibly diverse, with different plant species having unique root structures and shapes. Additionally, the intricate network of roots that exists beneath the soil's surface is often hidden from view, making it a mystery to many people. Understanding the importance of root tissue in plant growth can help to deepen our appreciation for the natural world and the complex systems that exist within it.In conclusion, root tissue is a critical component of plant growth and development, with essential functions in biology, ecology, agriculture, society, and personal appreciation. By understanding the importance of root tissue, we can make informed decisions about soil management practices, crop selection, and environmental sustainability. Additionally, we can deepen our appreciation for the natural world and the complex systems that exist within it. As we continue to face environmental challenges, such as climate change and food insecurity, the importance of root tissue in plant growth will become even more critical, making it a topic worthy of further exploration and study.。
植物不停生长英文作文

植物不停生长英文作文英文回答:Plants are remarkable organisms that have the abilityto continuously grow and develop throughout their lifespan. This ongoing process is essential for their survival and adaptation to changing environmental conditions. The continuous growth of plants is driven by a combination of internal factors and external stimuli, including genetics, hormones, nutrients, and light.Internal Factors:Genetics: The genetic makeup of a plant determines its potential for growth and development. Each species has a specific set of genes that control the production ofvarious proteins and enzymes that regulate growth processes.Hormones: Hormones are chemical messengers thatregulate plant growth and development. Key growth hormonesinclude auxin, cytokinin, and gibberellin. Auxin promotes cell elongation and root development, while cytokinin stimulates cell division and shoot growth. Gibberellin is responsible for stem elongation and seed germination.External Factors:Nutrients: Plants require essential nutrients, such as nitrogen, phosphorus, and potassium, for growth. These nutrients are obtained from the soil or through fertilizers. Nitrogen is a critical component of proteins and nucleic acids, while phosphorus is involved in energy metabolismand cell division. Potassium plays a role in maintaining osmotic balance and regulating water transport.Light: Light is essential for photosynthesis, the process by which plants convert sunlight into energy. This energy is used to fuel growth and development. Different wavelengths of light have specific effects on plant growth. Blue light promotes leaf growth and development, while red light stimulates stem elongation and flowering.Growth Patterns:The growth pattern of plants varies depending on the species and environmental conditions. Some plants, such as trees and shrubs, exhibit indeterminate growth, meaning they can continue to grow in height and width indefinitely. Others, such as annuals and perennials, have determinate growth, which means they reach a fixed size and then stop growing.Growth Stages:Plants typically go through distinct growth stages, including seed germination, seedling growth, vegetative growth, reproductive growth, and senescence. Each stage is characterized by specific physiological and morphological changes.Seed Germination: The growth process begins with seed germination, which occurs when a seed absorbs water and begins to develop.Seedling Growth: Seedlings are young plants that have recently emerged from seeds. They are characterized byrapid growth and the development of simple leaves and roots.Vegetative Growth: During vegetative growth, plants focus on increasing their size and producing leaves and stems. This stage is primarily driven by auxin and cytokinin.Reproductive Growth: Reproductive growth occurs when plants develop flowers and seeds. This stage is triggeredby environmental cues, such as day length and temperature, and is regulated by gibberellin.Senescence: Senescence is the final stage of plant growth and development. It is characterized by the breakdown of tissues and a decline in physiological processes.Environmental Influences:In addition to internal and external factors,environmental conditions also play a significant role in plant growth. Factors such as temperature, water availability, and the presence of pathogens can influence growth rates and patterns.Overall, the continuous growth of plants is a complex and dynamic process that involves a combination of genetic, hormonal, nutritional, and environmental factors. By understanding these factors, scientists and horticulturists can optimize plant growth and development for various purposes, such as food production, landscaping, and environmental conservation.中文回答:植物是一种奇妙的生物,它们在其整个生命周期中都具有持续增长和发育的能力。
PLANTGROWTHREGULATORS:植物生长调节剂

KNOWLEDGE EXPECTATIONS FOR PEST CONTROL ADVISERS:PLANT GROWTH REGULATORS1.Be familiar with the general uses and classification of the following plant growth regulators:a.Auxins: i.1-naphthalenacetic acid (NAA)ii.2,4-Diii.3-indoleacetaldehyde acid (IAld)iv.3-indoleacetic acid (IAA)v.3-indolepyruvic acid (IPA)vi.indolebutanoic acid (IBA)b.Gibberellins (GA):i.GA4GA7ii.GA3c.Cytokinins: i.CPPUii.kinetind.Ethylene/Ethylene releasersi.ethephonii.ethylenee.Inhibitors/Retardants:i.abscisic acid (ABA)ii.ancymidoliii.carbaryliv.chlormequatv.chloro IPCvi.daminozidevii.flurprimidolviii.hydrogen cyanamide (H2CN2)ix.mefluididex.mepiquat chloridexi.paclobutrozolxii.prohexadione calciumxiii.succinic acid (SADH)I. PLANT GROWTH REGULATORS1.Define plant growth regulator.2.List the common classes of plant growth regulators. (auxins, gibberellins, cytokinins, growth retardants/inhibitors, ethylene, others)3.List the plant growth regulators that play a major role in:a.abscission;b.dormancy;c.fruit abscission;d.fruit ripening;e.fruit set;f.leaf expansion [ethylene];g.plant senescence;h.root initiation;i.seed germination;j.stem elongation.4.Recognize that plant growth regulators can act at low concentrations.5.Recognize that plant growth regulators can have undesirable effects when applied at improper rates or times.6.Describe how environmental conditions, the plant developmental stage, and plant condition (e.g., stress, fruit load), on their own or in combination, can affect the activity of plant growth regulators.pare/contrast the ability of a plant growth regulator or plant hormone to stimulate growth and retard growth in different situations.8.Differentiate between a plant growth regulator and a plant hormone (plant growth substance).9.Define: a.plant hormone;b.abscisic acid (ABA).10.List the “classical” five naturally occurring plant hormone groups. (auxins, cytokinins, ethylene, the gibberellins, abscisic acid)11.Describe how each type of plant growth regulator affects:a.seed dormancy;b.seed growth;c.vegetative growth;d.flower and fruit growth;an abscission.12.Describe the primary physiological processes in plants that are regulated by:a.auxins;b.cytokinins;c.ethylene;d.gibberellins;e.growth retardants/inhibitors.13.Recognize that plant growth regulators interact with other organic compounds (hormones and other growth regulating substances) in plants.Auxins14.Define: a.auxin;b.3-indoleacetic acid (IAA).15.Describe the effect of auxins on plant growth.16.List the primary uses of auxins as plant growth regulators and identify the crops on which they are used. (Reduces fruit drop, increases fruit drop, delays maturation, blossom thinning agent, sets fruit, enhances adventitious root formation, delays color development)17.List the auxins contained in plant tissues. [3-indoleacetic acid (IAA), 3-indoleacetaldehyde (IAld), 3-indolepyruvic acid (IPA), 3-indoleacetonitrile (IAN), ethyl ester of indoleacetic acid (IAE)]18.Describe the effect of auxin on ethylene and how leaf sensitivity changes as leaves age. [Younger leaves are less sensitive to ethylene than older leaves due in part to higher auxin levels in younger leaves.]19.Recognize that auxins are also used as herbicides and give an example.▪Gibberellins20.Define gibberellins (GA).21.Describe the effect of gibberellin on plant growth.22.List the primary uses of gibberellins as plant growth regulators and identify the crops on which they are used. (cell elongation, cell division, overcoming dormancy, overcoming or breaking bud dormancy, increases or reduces fruit set, affects fruit shape, fruit maturation, delay of flowering in fruit trees, stimulates flowering and bolting in biennials, delays senescence)23.Describe how gibberellins stimulate plants to overcome dormancy.24.Recognize that there are over 100 different chemical structures of gibberellins but only a few are used commercially.pare/contrast GA3 and GA4GA7.26.Identify the primary gibberellins used.27.Identify the primary crop and use of GA4GA7.28.Identify the primary use of GA3 in citrus.▪Cytokinins29.Define cytokinins.30.Describe the effects of cytokinins on plant growth.▪Ethylene and ethylene releasers31.Define ethylene.32.Recognize that ethylene is a gas.33.Understand the relationship of ethephon to ethylene.34.Describe the effect of ethylene and ethephon on plant growth.35.List the primary uses of ethylene and ethephon for the crops on which they are used. a.citrus (fruit elimination, thinning agent, and postharvest degreening of fruit)b.cotton (increases lint strength, hybrid seed production, and boll opening)c.grain crops (induces fruit ripening, induces flowering, accelerates fruit andleaf abscission, promotes lateral branching, promotes shortened stems)d.pome fruit treese.tomato and table grapes (advances ripening and accelerates pigmentdevelopment or color accumulation)f.walnuts▪Growth retardants and inhibitors36.Define plant growth inhibitor (retardant).37.List the materials that are primarily used as growth retardants and inhibitors, identify the crops on which they are used, and describe how they inhibit plant growth. (paclobutrazol, flurprimidol, prohexadione calcium, ancymidol, chlormequat, mepiquat chloride, mefluidide, AVG–aminoethoxyvinylglycine) [AVG is used on apples—delays fruit maturity to reduce preharvest fruit drop and improved fruit quality; pears—help maintain fruit firmness; and ornamentals—reduce flower senescence and flower bud abscission during shipping]38.Describe the use of carbaryl as a plant growth regulator.II. PLANT GROWTH CONCEPTS1.Define: a.abscission;b.apical dominance;c.apical meristem;d.bioassay;e.cambium;f.cultivars;g.dormancy;h.endogenous;i.locules;j.meristem;k.parthenocarpy;l.phenotypic;m.phloem;n.rachis;o.rest period;p.senescence;q.xylem.III. APPLICATION TECHNOLOGY1.Define the following terms and describe their importance when using plant growth regulators:a.calibration;b.parts per million.2.Describe the relationship between dosage, volume and efficacy when applying plant growth regulators.3.Describe the importance of the solution’s pH when using plant growth regulators.4.Describe how to determine the need for a surfactant when using plant growth regulators.5.Describe how to avoid drift in the application of plant growth regulators.6.Recognize that plant growth regulators can be incompatible with other chemicals when combined in a tank mix.7.Describe how the following factors affect the appropriate dosage when using plant growth regulators:a.humidity;b.pH;c.plant growth stage;d.plant condition (e.g., fruit load, water or disease stress);e.rainfall;f.sunlight;g.temperature.8.Recognize the importance of reading and understanding the language on the label ofa plant growth regulator.9.Be able to interpret all terms and concepts on a plant growth regulator label.10.Recognize that specific hazards are associated with some formulations of the following plant growth regulators:a.corrosive – ethephon;b.flammable – ethephon, gibberellin;c.eye injury – ethephon, gibberellin;d.skin irritant, may be fatal if swallowed or through contact with skin –hydrogen cyanamide;e.hazard to bees – carbaryl;f.potential to drift and undesirably harm target and nontarget plants – all.。
英语作文how to plant

英语作文how to plantPlanting is a fundamental activity that has been practiced by humans for thousands of years. Whether it is growing crops for sustenance, cultivating flowers for aesthetic pleasure, or tending to trees for environmental benefits, the act of planting is a crucial part of our relationship with the natural world. In this essay, we will explore the step-by-step process of planting, from selecting the right plant to caring for it in the long term.The first and perhaps most important step in planting is to choose the right plant for your specific needs and environmental conditions. This involves considering factors such as the climate, soil type, available sunlight, and the intended use of the plant. For example, if you are planting a vegetable garden, you would need to select crops that are well-suited to the local climate and can thrive in the type of soil you have. On the other hand, if you are planting a decorative garden, you might focus more on choosing plants that complement each other aesthetically and create a visually appealing landscape.Once you have selected the appropriate plant, the next step is toprepare the planting area. This typically involves clearing the area of any weeds, debris, or other obstacles, and loosening the soil to a depth of several inches. Depending on the type of plant, you may also need to amend the soil with compost, fertilizer, or other organic matter to improve its fertility and drainage.After the planting area is prepared, it is time to plant the actual plant. The specific method for planting will vary depending on the type of plant, but there are some general guidelines to follow. For example, when planting a tree or shrub, you should dig a hole that is slightly wider than the root ball and only as deep as the root ball itself. This will ensure that the plant is not planted too deeply, which can lead to issues such as rot or stunted growth.When planting seeds or seedlings, you should carefully follow the instructions on the seed packet or plant label. This will typically involve planting the seeds or seedlings at the appropriate depth and spacing, and then gently covering them with soil. It is important to water the soil thoroughly after planting to help the plant establish its roots.After the plant has been planted, the next step is to provide it with the necessary care and attention to help it thrive. This may include regular watering, fertilizing, pruning, and protecting the plant from pests or diseases. The specific care requirements will vary dependingon the type of plant, but the general goal is to create an environment that allows the plant to grow and flourish.One of the most important aspects of plant care is watering. Different plants have different water requirements, and it is important to monitor the soil moisture and adjust the watering schedule accordingly. Overwatering can be just as harmful as underwatering, so it is important to strike a balance.Fertilizing is another important aspect of plant care. Different plants have different nutrient requirements, and it is important to choose a fertilizer that is appropriate for the type of plant you are growing. Organic fertilizers, such as compost or manure, can be a great way to provide plants with the nutrients they need while also improving the overall health of the soil.Pruning is another important aspect of plant care, as it can help to shape the plant, encourage healthy growth, and remove any dead or damaged parts. The specific pruning techniques will vary depending on the type of plant, but the general goal is to maintain the plant's overall health and appearance.Finally, it is important to protect plants from pests and diseases. This may involve using organic pest control methods, such as introducing beneficial insects or using natural repellents, or using moretraditional pesticides if necessary. It is also important to monitor the plant regularly for any signs of disease or infestation, and to take action quickly to address any issues that arise.In conclusion, planting is a complex and multifaceted activity that requires careful planning, attention, and ongoing care. By following the steps outlined in this essay, you can successfully plant and care for a wide variety of plants, whether they are for practical purposes or aesthetic enjoyment. Remember, the key to successful planting is to tailor your approach to the specific needs of the plant and the environmental conditions in which it will be growing.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Journal of Chromatography A,878(2000)77–86/locate/chromaDetermination of the plant growth regulator chlormequat in food byliquid chromatography–electrospray ionisation tandem massspectrometry*¨JorgHau,Sonja Riediker,Natalia Varga,Richard H.Stadler ´Department of Quality and Safety Assurance ,NestleResearch Center ,Nestec Ltd .,Vers -chez -les -Blanc ,CH -1000Lausanne 26,SwitzerlandReceived 18September 1999;received in revised form 11February 2000;accepted 24February 2000AbstractA confirmatory method for the determination of trace levels of chlormequat in a variety of different food matrices was developed.It entails a single clean-up step over a solid-phase cation exchange resin and subsequent liquid chromatography–electrospray ionisation tandem mass spectrometry using a stable isotopically labelled internal standard.Mass spectral 3537acquisition was done in selected reaction monitoring mode,selecting the transitions from both the Cl and the Cl isotope of chlormequat.Recoveries after extraction and clean-up,determined with radio-labelled chlormequat and averaged over the 21spiking range (16–65m g kg )in four different commodities,were within 88–96%,with a coefficient of variation better´than 8%.The method can be applied to pears,pear juice concentrates,fruit purees,and cereal products,with typical limits of 21detection for chlormequat estimated at 2–5m g kg .A survey of different food commodities revealed that chlormequat was detectable —albeit at very low levels —in many of the food samples analysed,with the highest concentration recorded in 21pears purchased in Switzerland and of South African origin (5.5mg kg ).Measurements were also conducted on two LC–MS instruments and demonstrate the versatility and robustness of the method and its applicability to instruments of different ion source design.©2000Elsevier Science B.V .All rights reserved.Keywords :Food analysis;Chlormequat;Pesticides1.Introductionproves fruit setting in pears,almonds,vines,olives and tomatoes,and prevents premature fruit drop of Chlormequat chloride [(2-chloroethyl)trimethyl-pears,apricots and plums [1].Chlormequat is em-ammonium chloride,also known as chlorocholine ployed extensively in agriculture,primarily in food chloride,Cycocel,and often abbreviated as CCC]is grains and on pears.Data published by national a plant growth regulator and is used to reduce the pesticide surveys in Europe clearly reflect the intense risk of lodging and increase yields of wheat,rye,oats usage of this compound,particularly in wheat and and barley.It also promotes flower formation,im-rye [2].In the case of pears,residues may be found at significant levels even if treatment is carried out under Good Agricultural Practice.Supervised trials *Corresponding author.Tel.:141-21-785-8360;fax:141-21-with pears in The Netherlands led to residue levels 785-8553.21E -mail address :richard.stadler@ (R.H.Stadler)ranging from 3.5to 8mg kg [3],whereas the0021-9673/00/$–see front matter ©2000Elsevier Science B.V .All rights reserved.PII:S0021-9673(00)00286-778J.Hau et al./J.Chromatogr.A878(2000)77–86present Codex Alimentarius Maximum Residue Furthermore,identical samples were analysed on two21Limit(MRL)for pears is set at3mg kg[4].different LC–MS instruments allowing us to assess Chlormequat is a strongly polar,cationic molecule the robustness and versatility of the method and to and lacks any chromophore,making its detection by compare the performance of two different LC–MS conventional analytical techniques rather cumber-interface designs.some.Earlier methods for the determination ofchlormequat include thin-layer chromatography[5],headspace gas chromatography[6],gas chromatog- 2.Experimentalraphy after derivatisation with sodium benzenethiol-ate[7]and colorimetry[8].The disadvantages of 2.1.Materials and reagentsthese methods have been addressed in recent publi-cations[9,10],the major criticisms being the lack of All solvents were of analytical grade and were specificity and the presence of other quaternary purchased from Merck(Dietikon,Switzerland). ammonium compounds that may interfere in the Water was either purified in-house using a Millipore analysis and thus lead to false-positive li-Q water purification system(Millipore,Vol-Furthermore,in the case of gas chromatographic ketswil,Switzerland)or was HPLC-grade(Merck). methods,the efficacy of the derivatisation step in a Ammonium acetate was from Merck;the scintillation complex food matrix is difficult to assess.fluid was Ultima Gold from Packard Bioscience Due to the polar and non-volatile nature of the(Groningen,The Netherlands).14molecule,separation by liquid chromatography(LC)[Methyl-C]-chlormequat chloride was custom or capillary electrophoresis and detection by mass synthesised by Moravek Biochemicals(Brea,CA, spectrometry(MS)are the most promising strategies USA)with a radiochemical purity.99.9%and a21for confirmatory analysis and quantitation at low specific activity of30mCi mmol.Non-labelled 21m g kg levels.The use of capillary electrophoresis chlormequat chloride was purchased from Dr.Ehrens-coupled to mass spectrometry for the separation and dorfer(Augsburg,Germany).d-Chlormequat9detection of quaternary ammonium herbicides has chloride isotopic purity.98%,was custom syn-,been described,but analyses were performed either thesized by Deutero(Kastellaun,Germany).Stock at high concentrations in formulation products[11]solutions of chlormequat were prepared at0.121or only in water[12].mg ml in water and stored at148C if not used; So far,two LC–MS methods using electrospray they were found to be stable over a period of at least ionisation(ESI)have been published to determine3months.Working standards were prepared fresh chlormequat residues at trace levels in grain[9]and with every batch of analyses by adequate dilution of pears[10].The latter method employs concertedly the stock solution in water.Pears were purchased in selected ion monitoring(SIM)and selected reaction various groceries in Switzerland and in France.Pear´monitoring(SRM)techniques for quantification,but and apple–pear purees were of different brands and, without the use of a stable isotopically labelled according to the declaration on the labels,contained 13internal standard.A C-enriched internal standard92–95%fruit.Ready-to-eat cereals and dried fruit of was introduced in the former method but suffered various brands were purchased off-the-shelf.Sam-from the apparent interference of matrix components ples of pear juice concentrates with incurred amounts´and an impure internal standard.of chlormequat were obtained from Nestle Germany. The aim of the present study was to develop aconfirmatory,quantitative method for the analysis of 2.2.Sample extraction and clean-up21chlormequat that can achieve low m g kg detectionlevels in a variety of food commodities(pears,pear 2.2.1.Cereals,pear juice concentrates,and fruit ´´puree,pear juice concentrate,and cereals).Radio-pureeslabelled chlormequat chloride was used to optimise If required,cereals were ground in a laboratory the clean-up step,and an isotope labelled internal mill(Model3303,Perten Instr.,Hamburg,Germany) standard was used for quantitative determination.to achieve a particle size of0.5–1mm.A test portionJ.Hau et al./J.Chromatogr.A878(2000)77–8679 (10g)of the sample material was weighed in a150-samples were fortified with the radio-labelled analyte21ml screw-capped bottle and afixed amount of d-(40m l,5.27ng m l)to achieve afinal spiking level9chlormequat chloride in water was added to achieve of16.3m g chlormequat cation per kg.Extraction21afinal concentration of the cation of31m g kg.was then performed as described above.Typically, The sample material was suspended in approximately the volume of each column effluent was determined 75ml of a methanol–water mix(1:1,v/v)and and an aliquot(500m l)analysed in a10-ml liquid stirred with a magnetic stirrer for15min at ambient scintillation cocktail to determine recovery.Radioac-temperature.tivity was measured with a1219Rackbeta liquidscintillation counter(LKB-Wallac,Turku,Finland).2.2.2.PearsUnpeeled pears(three to six pears,suspected 2.4.Liquid chromatography and mass spectrometry homogeneous lot,approx.500g)were cut into slicesand homogenised for|10s at room temperature in a Two different triple quadrupole mass spectrome-Buechi440laboratory mixer(Buechi,Flawil,Swit-ters were used in this study,a Finnigan TSQ700 zerland).An aliquot of the sample(10g)was taken(Finnigan,San Jose,CA,USA),and a Micromass and prepared as described above.Quattro-LC(Micromass,Manchester,UK).On both The suspensions were made up to100ml with instruments,LC separations were performed under methanol–water(1:1,v/v).An aliquot of10ml,almost identical conditions by ion exchange chroma-equivalent to1g of sample,was applied to solid-tography on a Spherisorb SCX column(15032mm phase extraction(SPE)cartridges(LiChrolut SCX,with guard column3032mm;Bischoff,Leonberg, 500mg,Merck)that were positioned on a Supelco Germany),injecting10m l of sample.All runs were Visiprep vacuum manifold(Supelco,Buchs,Switzer-performed under isocratic conditions at aflow-rate of2121land)and preconditioned with consecutively two bed0.3ml min and0.25ml min for the TSQ70021volumes each of methanol,water,and10mmol l and Quattro-LC,respectively,using methanol–water hydrochloric acid.If necessary,particularly with1:1(v/v)containing afinal concentration of5021solid food samples,the10ml aliquot was transferred mmol l ammonium acetate(pH6.8,not adjusted). to conical polypropylene tubes(15ml)and cen-The column temperature was set to358C,and the LC trifuged at3600g for5min in a benchtop centrifugeflow was introduced into the ion source of the MS (MSE Scientific Instruments,Leicestershire,UK).without a split.The clear supernatant was charged onto the columnas described above.After penetration of the extract 2.4.1.TSQ70021(flow-rate|0.5ml min),the column was rinsed The TSQ700was equipped with a Finnigan‘‘ESI with4ml of each methanol and acetonitrile.The II’’electrospray ion source.The MS was coupled to analyte was then eluted with5ml of a1:1mixture a Waters(Rupperswil,Switzerland)LC system,21(v/v)of methanol and0.25mol l aqueous am-consisting of a type757autosampler,a600-MS monium acetate(pH6.8),prepared fresh before use.pump with system controller and column oven and a Care was taken that the columns did not run dry type486-MS UV detector.A column-switching during the clean-up procedure.Methanol was re-valve(Valco ECM5625,from a Finnigan TSP-II moved in vacuo at358C,and the samples were Thermospray interface)was used after the LC col-lyophilised overnight.The residues were redissolved umn and theflow directed to waste during the initial in methanol(0.5ml)and stored at2208C until phase of the run prior to elution of the analyte.MS analysed by LC–MS–MS as described below.conditions were as follows:electrospray voltage,4.2kV;transfer capillary temperature,2008C;sheath gas 2.3.Determination of extraction recovery using(nitrogen),0.52MPa;auxiliary gas,20‘‘units’’.The radio-labelled standard capillary and lens voltages were set to3.9and41.4V for chlormequat and to21.0V and58.9V for the 14A solution of C-chlormequat chloride in ethanol isotope-labelled standard,respectively.MS–MS data21(51m Ci ml)was diluted1:100in water,and were acquired at a collision energy of235eV in the80J.Hau et al./J.Chromatogr.A878(2000)77–86laboratory framework using argon at a pressure of4the internal standard.All quantitative calculations hPa(3.0mTorr)as the collision gas.Quantitation were done using robust statistics[13].data were acquired in neutral loss mode using a cycletime of0.50s and a tolerance of60.5mass units.For non-labelled chlormequat,the transitions m/z 3.Results and discussion122→58and m/z124→58were observed;for thedeuterated analogue,the transitions m/z131→66 3.1.Sample extraction and clean-upand m/z133→66were observed.Data acquisitionwas performed on a DECstation5100running under A major challenge in this study was to develop a Ultrix4.4(Digital Equipment,Maynard,MA,USA)common clean-up method for the food commodities using the Finnigan software package ICIS2,Ver.of interest(pears,pear juice concentrates,pear´8.3.0SP1.purees,and cereals).Thus,radio-isotope dilution,which allows determination of the absolute recovery142.4.2.Quattro-LC of the C-labelled tracer,was employed to screen a The Quattro-LC mass spectrometer,equipped with number of disposable SPE cartridges.Surprisingly, a‘‘Z-Spray’’electrospray ion source,was coupled to the only resin that performed consistently well with a Waters2690‘‘Alliance’’separation module.The all food matrices was LiChrolut SCX.Elution of the chromatographic conditions were described above.analyte was found best with afinal concentration of21Instrument control and data processing were per-0.125mol l ammonium acetate in the solvent formed using MassLynx NT software,Ver. 3.3mixture.(Micromass).The needle voltage was typically set to Food samples without detectable levels of chlor-12.77kV,the cone voltage to39V,and the RF lens mequat,as confirmed by techniques described in to0.12V.Source block and desolvation temperatures[14],were chosen to determine recovery.However, were set at1508C and4008C;nebuliser and desolva-in the case of pear juice concentrates,no chlor-21tion gasflows to97and620l N h,respectively.mequat-‘‘free’’samples were available,so recovery2The ion energy of thefirst and second quadrupole experiments were done on the sample with the21was0.6V and 1.0V.Chlormequat was detected lowest chlormequat levels(|0.1mg kg).As illus-using the same SRM channels as described above,trated in Table1,recoveries of chlormequat in all except that a third transition using m/z122→63was four food commodities and at three spiking levels observed,thus giving added confidence to analyte were above80%,with a coefficient of variation identification.All data were acquired at a collision(C.V.)better than10%.When averaged over the energy of230eV except for the transition m/z whole spiking range for each food matrix,recoveries 122→63whose optimum was220eV.Argon was are within the range88–96%,and the C.V.is better used as collision gas at a pressure of2.5hPa(1.9than8%.These results demonstrate that this SPE mTorr).The dwell time was set to1s.Table12.5.Quantitation Recoveries6C.V.(%)of C-chlormequat in various foods after14asample extraction and solid-phase clean-up on LiChrolut SCX Calibration curves were established using spiked Food commodity Spiking level of chlormequat matrix standards.Concentrations between 3.9and(m g kg)2121780m g kg of non-labelled chlormequat were used16.332.665.2 together with afixed amount of d-chlormequat as921Pears8365.39869.29467.6 internal standard(31m g kg).Each sample was bPear juice concentrate9267.98865.58465.0 prepared in duplicate,and each injected at least three´Pear puree9569.69164.510363.5 times in an arbitrary order during a series of analy-Cereals9468.09467.68365.3 ses,which included samples,standards and blanks.aEntries represent an average of three extractions,performed on Quantitation data were obtained using the transition different days and each with two independent determinations m/z122→58for chlormequat and m/z131→66for(N56).b21Contains incurred residues(122m g kg).J.Hau et al./J.Chromatogr.A878(2000)77–8681 procedure enables efficient extraction of chlormequat was additionally used to verify the presence of the 21at the m g kg level in a number of different food analyte.matrices.3.3.Selected reaction monitoring(SRM)vs.single 3.2.Mass spectrometry ion monitoring(SIM)3.2.1.Electrospray ionisation(ESI)and MS–MS Fig.4shows a comparison of chromatograms of chlormequat recorded on the TSQ700obtained using SRM and´Under ESI conditions,chlormequat shows an SIM on the same sample(pear puree).Although the 1abundant M ion without fragmentation(Fig.1).As absolute signal intensity in the SRM run is lower,the35tetraalkyl ammonium compounds are already signal-to-noise(S/N)ratio of the Cl trace was charged in solution,they are easily amenable to found to be at least three times higher than using the3537 ionisation directly from the liquid phase.The daugh-SIM mode.The S/N ratio of the Cl to the Cl ter ion spectrum obtained upon dissociation of the peaks follows this trend closely;we observed,for 1M ion of chlormequat is shown in Fig.2,both for example,S/N518for the transition m/z122→58, the labelled and non-labelled compound.As reported S/N57for m/z124→58,and S/N56and S/N51 previously[10,12],the main fragmentation of chlor-for SIM of m/z122and m/z124,respectively. mequat in positive ESIMS is the loss of the chloro-ethyl moiety,leading to a product ion with m/z58 3.4.Separation by LC(Fig.3).With respect to the two most abundantchlorine isotopes,this corresponds to a loss of64Under the conditions given here,chlormequat and66amu,respectively[Fig.2(a)and(b)].Analo-elutes at approximately11min.The overall LC rungously,the fragmentation of d-chlormequat leads to time was set at15min,so that96injections could be9a major fragment at m/z66by loss of65and67made per day.Retention times were very stable and amu,respectively[Fig.2(c)and(d)].An interesting varied less than0.1min within one batch of solvent, aspect is that the ratio of the ions with m/z58and59although a distinct dependency on solvent composi-is inverted compared to m/z66/68.This effect tion(ammonium acetate concentration)was noted. depends on the collision energy;however,we did not The chromatograms are free from interference(Fig. perform a detailed study of this phenomenon.4,left;Fig.5).The use of acetonitrile instead of For quantitative work,all four transitions from methanol did not improve separation or peak shape.1both isotope peaks of the M ion were recorded in The importance of sample preparation is apparent3537the SRM mode.The area ratio of the Cl and Cl when data from the present study are compared to isotope peaks,which should be approximately3:1,those reported by Startin et al.[10].The authorsFig.1.ESIMS of non-labelled chlormequat(a)and its d-cognate(b).982J.Hau et al./J.Chromatogr.A878(2000)77–863537Fig.2.ESIMS–MS daughter ion spectra of chlormequat,showing(a)the Cl isotope peak and(b)the Cl isotope peak of unlabelled 3537chlormequat;(c)the Cl isotope and(d)the Cl isotope peak of d-chlormequat.9chose direct analysis of a methanol–water pear ever,that a fair amount of solid matter precipitated extract without a prior clean-up step,with the result in the ion source region of both instruments,block-that the chromatographic behaviour degraded pro-ing the sampling orifices.Afirst approach to avoid portionally to the increasing number of samples blockage on the TSQ700was to use a home-made injected onto the column.‘‘orthogonalflow’’device at the heated capillary, In contrast to this,using the conditions described which consisted essentially of a small metal bar of herein,column performance did not deteriorate even1-mm width that wasfixed ca.1mm in front of the after more than1200injections.We observed,how-sampling orifice and blocked the line-of-sight pene-Fig.3.Fragmentation of chlormequat(see also Fig.2).Given are the m/z of the fragments for the non-labelled(left column)and the d-compound(right column).9J.Hau et al./J.Chromatogr.A878(2000)77–8683´Fig.4.LC–ESIMS–MS selected reaction monitoring chromatogram traces(left)and selected ion monitoring traces(right)of a pear puree 21sample spiked with7.8m g kg chlormequat.Data were recorded on the TSQ700and are shown without smoothing.tration of particles into the sampling system.Al-samples to compensate for matrix influences[15,16]. though this device prolonged the uptime of the Linear calibration curves were obtained from the21 instrument,the loss of sensitivity was too important limit of detection(LOD)to780m g kg,in all cases2for this study.Therefore,a column switching valve with coefficients of determination r.0.999.Cali-was used which was controlled by the mass spec-brations were found to have slopes between0.6and trometer,so that the LCflow was directed to waste 1.2,depending on the food matrix(data not shown). during the initial phase of a run and switched to the The goodness offit(accuracy)of the measured MS before the expected elution of the analyte peak.values with the theoretical data was verified by The device proved to operate reliably and allowed plotting the measured deuterium enrichment against continuous trouble-free operation overnight and over the calculated values as described in[17],achieving weekends.Furthermore,the use of an LC column correlation coefficients of0.998or better,and slopes with2-mm I.D.was fully compatible with the high in the range0.93–1.04.This is close to the theoret-matrix loads experienced in this study.ical value of 1.00and indicates good agreementbetween measured and calculated values.3.5.Calibration and method performance The variabilities within and between series were characteristics calculated from sets of results of triplicate(Quattro-LC)orfive to eight replicate(TSQ700)analyses of21All calibration curves were prepared in the same,a sample with incurred residues(203m g kg),and or at least a very similar,matrix as the actual are depicted in Table 2.The limit of detection84J.Hau et al./J.Chromatogr.A878(2000)77–86Fig.5.LC–ESIMS–MS chromatograms of a naturally incurred cereal sample.Data were acquired using the TSQ700(left traces)and the2121Quattro-LC(right traces)and are shown with afive-point smooth.The amounts calculated were3.6m g kg and4.3m g kg,respectively.21(LOD)was estimated to be2and5m g kg for the more‘‘sensitive’’than the TSQ700and provides Quattro-LC and TSQ700,respectively,using quanti-better intra-and inter-assay precision.This differ-3537tation on both the Cl and Cl peaks.This calcula-ence is not surprising as the TSQ700is a relatively tion is based on a signal-to-noise ratio of3:1and is old instrument that was originally developed for valid for the principal food commodities that were GC–MS–MS applications and later retrofitted to35analysed(Table2).If only the Cl transition is LC–MS operation,while the Quattro-LC is a recent considered for positive identification,then an LOD design,developed specifically for LC–MS–MS 21below1m g kg is achieved for the Quattro-LC.It work.In spite of the instrumental differences,the is evident that the Quattro-LC is about three-fold performance of both instruments is clearly within thesame order of magnitude and is sufficient for the21Table2detection of chlormequat at low m g kg levels in all Key analytical parameters for the present method,given with the food commodities investigated here.transition m/z122→m/z58Parameter Instrument 3.6.Analysis of various food samples forchlormequatTSQ700Quattro-LC21LOD(m g kg) 1.50.521A number of food commodities containing pears, LOQ(m g kg) 4.0 1.5a and some cereals and cerealflours(wheat,rye,rice), Within series C.V. 6.8% 1.9%bBetween series C.V.10.6% 2.1%as well as cereal pre-mixes containing.50%wheat a were analysed on both LC–MS instruments withindf58.b df54.the framework of a limited survey,the results ofJ.Hau et al./J.Chromatogr.A878(2000)77–868521Fig.6.Results of a limited survey expressed as concentration ranges of chlormequat residues(m g kg)found in various food commodities. Each individual sample was determined in duplicate,with at least triplicate injections on both mass spectrometers.which are summarised in Fig.6.It can be seen that two LC–MS instruments with different ion source most of the samples investigated—particularly design,both achieving comparable quality parame-cereals—contain detectable levels of the residue.Of ters.the food samples analysed,the highest level of21chlormequat(5.5mg kg)was found in pears ofSouth African origin,above the Codex MRL of3Acknowledgements21mg kg[4];it was particularly striking that thelevels of chlormequat detected in pear were either The authors thank the team of the mechanical 21very low(,5m g kg)or very high.This extensive´workshop of the Nestle Research Center for help usage and presence of chlormequat is well supported with the‘‘orthogonal spray’’device on the TSQ700, and further illustrated by data published in national and Dr.Jean-Marc Aeschlimann for assistance with pesticide surveys[2,18].the statistical data evaluation.Finally,we also thankurent Fay for valuable discussions and com-ments.4.ConclusionWe have developed and validated a sensitive,Referencesquantitative analytical method for the detection and21monitoring of chlormequat at low m g kg levels.In[1]C.D.S.Tomlin(Ed.),The Pesticide Manual,11th ed.,British contrast to existing methods,it is applicable to aCrop Protection Council,UK,1997,p.220.wide variety of different food commodities and[2]R.K.Julhler,M.Vahl,J.AOAC Int.82(1999)331. incorporates a single SPE clean-up step followed by[3]Joint Meeting of the FAO Panel of Experts,PesticideResidues in Food,Report on Pesticide Residues in Food and isotope dilution LC–ESIMS–MS to quantify chlor-the Environment and the WHO Expert Group on Pesticide mequat.This method is suitable as a routine enforce-Residues,FAO Plant Production and Protection Paper No. ment method with detection limits well below10127,FAO,Rome,1994,p.50.21m g kg in a wide range of complex food matrices.[4]Codex Alimentarius Commission,Joint FAO/WHO Food The ruggedness of the method is demonstrated on Standards Programme,Report of the31st Session of the86J.Hau et al./J.Chromatogr.A878(2000)77–86Codex Committee on Pesticide Residues,The Hague,12–17[12]E.Moyano,D.E.Games,M.T.Galceran,Rapid Commun.April1999,ALINORM99/24A.Mass Spectrom.10(1996)1379.[5]T.Stijve,Dt.Lebensm.-Rundsch.76(1980)234.[13]K.Danzer,Fresenius Z.Anal.Chem.335(1989)869.[6]Ministry of Welfare,Health and Cultural Affairs,in:Ana-[14]F.Tafuri,M.Businelli,P.L.Giusquani,Analyst95(1970)lytical Methods for Residues of Pesticides,5th ed.,Rijswijk,675.The Netherlands,1988,p.43.[15]P.Haefelfinger,J.Chromatogr.218(1981)73.[7]F.Tafuri,M.Businelli,P.L.Giusquani,Analyst95(1970)[16]A.R.C.Hill,Quality Control Procedures for Pesticide Res-675.idue Analysis—Guidelines for Residues Monitoring in the[8]J.Sachse,Z.Lebensm.Unters.Forsch.163(1977)274.European Union,European Commission,Brussels,Docu-[9]M.Vahl,A.Graven,R.K.Juhler,Fresenius J.Anal.Chem.ment7826/VI/97,1997.¨361(1998)817.[17]A.A.Stampfli,I.Blank,R.Fumeaux,L.B.Fay,Biol.Mass [10]J.R.Startin,S.J.Hird,M.D.Sykes,J.C.Taylor,A.R.C.Hill,Spectrom.23(1994)642.Analyst124(1999)1011.[18]J.Brueggemann,H.-D.Ocker,Chem.Mikrobiol.Technol.[11]D.Wycherly,M.E.Rose,K.Giles,T.M.Hutton, D.A.Lebensm.10(1986)113.Rimmer,J.Chromatogr.A734(1996)339.。
