Pro71-02 SOP Checklist - Quality Management
DELL QSA中英
Remarks
SPP-002 品質管理之運作
ቤተ መጻሕፍቲ ባይዱ
口号 合适度 持续改进
SPP-002 品質管理之運作
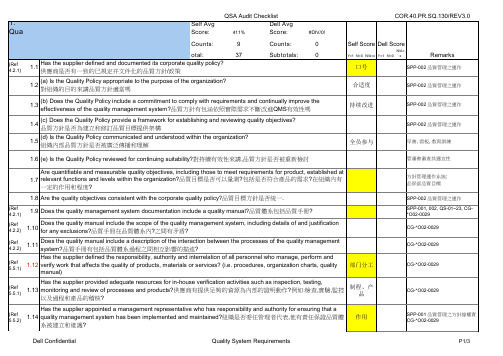
(b) Does the Quality Policy include a commitment to comply with requirements and continually improve the effectiveness of the quality management system?品質方針有包涵依照實際需求不斷改進QMS有效性嗎 (c) Does the Quality Policy provide a framework for establishing and reviewing quality objectives? 品質方針是否為建立和修訂品質目標提供架構 (d) Is the Quality Policy communicated and understood within the organization? 組織內部品質方針是否被廣泛傳播和理解
(Ref 4.2.1) (Ref 4.2.2) (Ref 4.2.2) (Ref 5.5.1)
管審會審查其適宜性
方針管理運作系統; 品保部品質目標 SPP-002 品質管理之運作 SPP-001, 002, QS-01~23, CG*O02-0029 CG-*O02-0029
1.9 Does the quality management system documentation include a quality manual?品質體系包括品質手冊? 1.10 1.11 Does the quality manual include the scope of the quality management system, including details of and justification for any exclusions?品質手冊在品質體系內?之間有矛盾?
SQM程序文件和主要操作流程

Nonconforming Control Handling, Pkg.
Overall Average (%)
Criteria: Approved Conditionally Approved Not Approved
100.0%
90 ~ 100% 80 ~ 89% < 80 %
91.7%
Quality Record EICC
Output Vendor Survey Report
QSA Report
QPA Report
Non Critical Parts
SQRC Report
MP IQC SI MRB ECN
SCAR
PPAP/Safe Launch EC Management & Batch Run AVL Maintain
3.0
進料檢驗規范
特採作業程序 CE:新零件承認
G-QRA-08
特採作業程序文件 .doc
新零件承認規范 環保材料承認規范
I-CE-03 I-CE-02
SQM Main Operation Flow
Input
1. New Vendor. 2. Annually Audit Plan. 3. Customer Request. 4. To be defined as Critical Parts. 5. Parts with New Tech and Process. 1. VS / QSA / QPA & NCR CLCA. 2. Project Based SQRC Score. 3. FAI / FDI / CPK / Gauge R&R / PFMEA / Process Parameter / SOP / PMP / Quality Report (Ramp Up) / Drawings / EC Updating Recort 1. Manufacturing site change 2. Material change 3. Process / Subcontractor Change 4. Tooling / Design Change 5. Requested by Hipro RD / PM 6. 2nd sources phase in 1. IQC DR / LRR / VLRR. 2. QA Hold Production. 3. Customer Complaint 1. Quality / Cost / Delivery / Service / Technology Ranking.
S3-25品确-阶段检查表 Quality Approval--Phase Checklist (2)

NO:品确阶QuP作成Prepared by・工・・零件零件开发质量保证计划跟踪表Tracking Form of Quality Assurance Plan for Part Development明确顾客产品要求Understand Client Product Requirements检查基准书编制计划Inspection Standard Formulation Plan供应商自我评价计划Supplier Self-Assessment Plan审核日期Date of Audit 审核确认方式Audit Confirmation Method确认人 Confirmed by总结Conclusion56项目启动Project Lanuch过程开发准备Preparation of Process Development过程开发实施Implementati on of Process Development过程开发验证Verification of Process Development过程评价Process EvaluationPPAP 及初期管理PPAP &Initial ManagementPPAP 文件包批准计划PPAP Package Approval Plan 是否整合确认PPAP 三级文件包初版? Has the PPAP 3rd level package (initial ver.) been consolidated and confirmed?初始流动管理计划Initial Flow Management PlanNA供方SupplierNo.1234是否进行了品确自我审核?Has the quality approvel self-audit been completed?对审核发现问题点是否制定改善计划? Have the improvement plans for the problems discovered during the audit been formulated?改善计划是否满足大日程要求? Does the improvement plan correspond to the requirements of the master schedule?自审计划是否充分考虑改善时间? Has sufficient consideration been given to the improvement time in the self-audit plan?顾客评价计划Customer Evaluation PlanNA工程能力调查计划Survey Plan of Process Capability是否制定了工程能力初始调查计划? Has the initial survey plan of process capability been formulated?批组管理计划Lot Management Plan实施验证是否完了? Has the implementation verification been completed?不合格品反馈流程及对策制定Feedback Process & Countermeasures Forming for RejectedPartsNA标准样品是否已获客户批准返回?适用时,外观批准报告已获客户批准。
BYD供应商BSR审核表
环境检测报告及药箱
3
2
Whether all kinds of chemical MSDS could be available for the staff who will keep touch with chemical at working spot (in form of staff's mother tongue ) and whether there is personal protect goods was duly equiped 2.9 for the employee who may contact with poison chemical. 所有接触化学 品的员工是否能在工作现场及时地获到所有化学品的MSDS(并且以员工 母语的形式),是否有为接触有毒有害的化学品的员工配备适当的个人防 护用品? Whether the company have a clear and definite documented policy of career health and safe management system. 公司是否有建立应急预案 和应变程序来将影响降低到最低(如消防演练、火灾监控和灭火装置、恢 复计划等)? The supervisor and employee must follow business moral requirement, such as,probity management,without malfeasance incoming and so on. 公司管理者和员工都应遵循商业道德要求,如:廉洁经营,无不正当收益 等
ISO9000和ISO14000证书
3
3
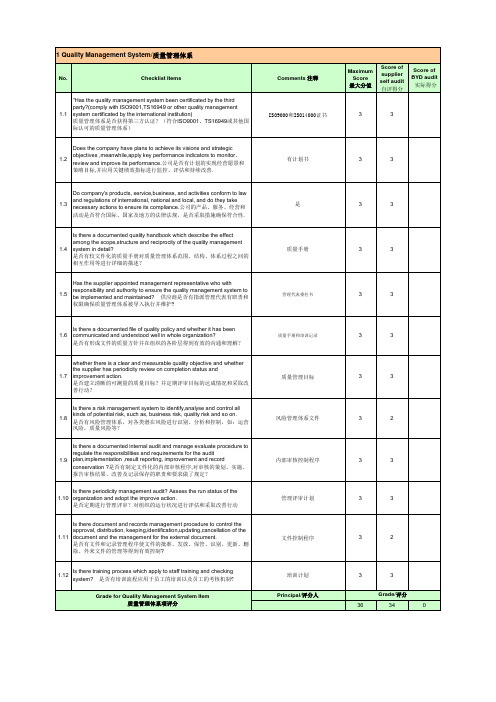
Does the company have plans to achieve its visions and strategic objectives ,meanwhile,apply key performance indicators to monitor、 1.2 review and improve its performance.公司是否有计划的实现经营愿景和 策略目标,并应用关键绩效指标进行监控、评估和持续改善.
nadcap审核清单中英文对照

nadcap审核清单中英文对照Nadcap Audit Checklist - English and Chinese ComparisonIntroduction:Nadcap (National Aerospace and Defense Contractors Accreditation Program) is a global cooperative accreditation program for aerospace engineering, defense, and related industries. It aims to ensure the highest level of quality and consistency in manufacturing processes. As part of the Nadcap audit process, a checklist is used to evaluate compliance with industry standards. This article provides a comprehensive comparison of the Nadcap audit checklist in both English and Chinese.1. Quality Management System (QMS):质量管理体系(QMS):1.1 Quality Manual质量手册1.2 Control of Documents文件控制1.3 Control of Records记录控制2. Personnel Competence and Training:人员能力和培训:2.1 Competence, Training, and Awareness能力、培训和意识2.2 Training Records培训记录3. Product Realization:产品实现:3.1 Planning of Product Realization产品实现计划3.2 Customer-Related Processes与客户相关的过程3.3 Design and Development设计和开发3.4 Purchasing采购3.5 Production and Service Provision生产和服务提供3.6 Control of Monitoring and Measuring Equipment 监测和测量设备控制4. Measurement, Analysis, and Improvement:测量、分析和改进:4.1 Monitoring, Measurement, Analysis, and Improvement监测、测量、分析和改进4.2 Internal Audit内部审核4.3 Control of Nonconforming Product不符合产品控制4.4 Corrective Action纠正措施4.5 Preventive Action预防措施Conclusion:总结:The Nadcap audit checklist serves as a valuable tool for evaluating compliance with industry standards in the aerospace and defense sectors. This article has provided a comprehensive comparison of the checklist in both English and Chinese, covering various aspects of quality management, personnel competence and training, product realization, and measurement, analysis, and improvement. By adhering to the requirements outlined in thechecklist, organizations can ensure the highest level of quality and consistency in their manufacturing processes, ultimately contributing to the overall success of the aerospace and defense industries.注:以上内容仅供参考,具体的Nadcap审核清单中英文对照请参考相关官方文件。
SOP Quality Valve 质量阀模板

•
质量阀总结及决议/Quality Valve Summary and Decision
– 12.1, 12.2, 13.0
质量阀计划 QUALITY VALVE PLAN
SOP QUALITY VALVE MEETING
Feb 13 2004 (Chinese Time)
Resp.
PM / PA MYM PQM PQM ACE ACE ACE TVE ACE ACE ACE / TMIE ACE DSM EGM TCE PTSIE TVE TVE DSE / DVSE PPM PPM PPM PPM PPM / SLL PPM QSM QSM * * * * * * * * TMIE TMIE TMIE TMIE TMIE TMIE TMIE TMIE
9.0 Service Readiness 10.0 Module / Vehicle Plant Readiness
X X X X
05 年型 LNJ -- SOP质量阀
10.1 PFMEA on Dress Line & Marriage 10.2 DVT Readiness 10.3 Significant Build Issues / Open Concerns
12.0 Quality Valve Wrap-up
12.1 Other Significant Quality Concerns 12.2 Quality Valve Summary * * PQM/QSM PQM/QSM PM / CE / Plt. Mgr. X X X X X X
13.0 Quality Valve Decision
PILOT I QUALITY VALVE MEETING
QSAcheck list
(Ref 5.6.2)
1.22
Dell Confidential
Quality System Requirements
P2/3
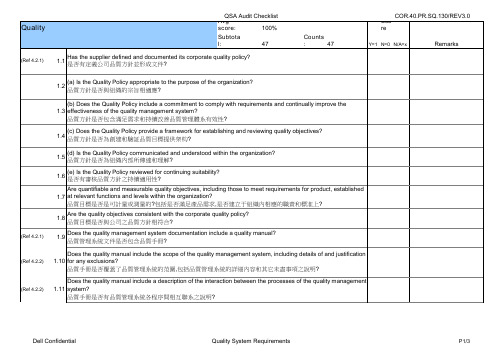
QSA Audit Checklist 1.23 (f) Follow-up actions from previous management reviews 與先前管理審核重復之狀況 (g) Changes that could affect the quality management system, and 可影響品質管理系統的變化 (h) Recommendations for improvement 改善建議 Does the output of management reviews include any decisions and actions related to: 管理審核輸出是否包括下列相關結果和改善: 1.26 1.27 1.28 1.29 1.30
(Ref 8.2.2)
COR.40.PR.SQ.130/REV3.0
1.24 1.25
(Ref.5.6.3)
(a) Improvement of the effectiveness of the quality management system and its process? 品質管理系統效力的改善及過程? (b) Improvement of product related to customer requirements, and 滿足客戶需求的產品改善 (c) Resource needs 需求措施 Are implementation of action items from management review tracked? 管理審核改善項目的執行是否有被追溯? Are records maintained of these management reviews? 是否保存有管理審查的記錄? Does the supplier conduct internal audits at planned intervals? 供應商是否按計划進行內審?
QCP品质控制计划模板
Regular Size 正常样本容量Regular Freq.正常频率Tighten Size 加严样本量Tighten Freq.加严频率1吸力测试RTD test ★-0.8 ~ -1.8KPa适配OK,有烟雾被吸出,两个LED 灯同时亮Normal mate & two Led light 吸烟治具RTD tester 重新制作及分析原因Readjust and analysis 2充电功能测试Charge function test★接上U020,两个LED 等呈呼吸灯,充电电流为0.35~0.4A two Led breathing light 直流电源 DC Power 重新制作及分析原因Readjust and analysis 3外观appearance★电池管外观无刮伤或生锈No scraped or get rusty目视Visual check重新制作及分析原因Readjust and analysis4实物组装assemble and install★依据SOP 定义作业手法以及BOM所用物料执行确认According to the requirements of SOP and the BOM for confirming.实物组装assemble and install 退料Return material to MRB1充电电压Charging voltage★ DC3.3V-4.2V充电板测试治具 Charging test fixture80pcs 2H 80pcs 1HIPQC 抽检IPQC Sampling退料Return material to MRB2充电电流Charging current★0.35A-0.4A充电板测试治具 Charging test fixture80pcs 2H 80pcs 1HIPQC 抽检IPQC Sampling退料Return material to MRB3静态电流Quiescent Current★<10μA主板测试治具PCBA test fixture80pcs 2H 80pcs 1HIPQC 抽检IPQC Sampling退不良物料Return defective materialto MRB4防静电环Anti-static ring★防静电阻大于106Ohn防静电环测试仪Anti-static ring tester所有防静电环All Anti-staticring4H所有防静电环All Anti-staticring2HIPQC 抽检IPQC Sampling返工Rework5充电PCBA 规格charge PCBA direction 闪红灯或依据BOM 定义Red light or According to BOM目视Visual check80pcs 2H 80pcs 1HIPQC 抽检IPQC Sampling退料Return material to MRB《产品制程巡检记录表》《IPQC process inspection record 》吸烟治具RTD TesterC078烟弹C078Cartridges U020充电器U020 charge 直流电源 DC Power《首检确认表》FAI checklist 充电板测试治具 Charging test fixture防静电环Anti-static ring 防静电环测试仪Anti-static ring tester充电测试/分板Charging test /Separate board 15PCS《首检确认表》FAI checklist《首检确认表》FAI checklist 《产品制程巡检记录表》《IPQC process inspection record 》《产品制程巡检记录表》《IPQC process inspection record 》《产品制程巡检记录表》《IPQC process inspection record 》《首检确认表》FAI checklist 电池首件品确认First Article Inspection (FAI)每天开拉时或订单变更时whenproduction linestart or order changedIPQC 抽检IPQC Sampling《产品制程巡检记录表》《IPQC process inspection record 》Methods 方法责任部门Responsible DepartmentsControl Method 控制方法Reaction plan 反应计划NO.编号Product 产品Process 过程Product/Process /Spec./Tolerance 产品/过程/规格/公差Evaluation/Measurement Tech.评价/测量技术Sampling plan 抽样计划每天开拉时或订单变更时when production line start or order changed10PCSCore Team 核心小组:Customer Engineering Approve/Date (If Req'd)客户工程批准/日期:(如需要)NA Part name/Description零件名称/描述:C086电池组装C086 Battery assembly Supplier/Plant approval/Date 供应商/工厂批准/日期:待定Customer Quality Approve/Date (If Req'd)客户质量批准/日期:(如需要)NA Supplier/Plant供应商/工厂名称:SMACO Supplier/Plant Code供方代码:C086 BatteryOther Approval/Date(If Req'd)其他批准/日期(如需要):NAOther Approval/Date(If Req'd)其他批准/日期:(如需要) NAPart Number/Latest Change Level 零件号/最新更改水平: NAPart/Process NO.零件/过程编号Process Name/Operation Description过程名称/操作描述Machine,Device,Jig,Tools for MFG.material制造用机器、装置、夹具、工具、物料Characteristics 特性Characteristicclass 特性分类Tighten Sampling Plan should be applied if 1. customer required 2. Product quality concern 3. major customer complaint4. Management and/or CFT DecisionRegular Size 正常样本容量Regular Freq.正常频率Tighten Size加严样本量Tighten Freq.加严频率Methods 方法责任部门ResponsibleDepartmentsControl Method控制方法Reaction plan反应计划NO.编号Product产品Process过程Product/Process/Spec./Tolerance产品/过程/规格/公差Evaluation/Measurement Tech.评价/测量技术Sampling plan抽样计划Core Team 核心小组:Customer Engineering Approve/Date(If Req'd)客户工程批准/日期:(如需要)NAPart name/Description零件名称/描述:C086电池组装C086 Battery assembly Supplier/Plant approval/Date供应商/工厂批准/日期:待定Customer Quality Approve/Date(If Req'd)客户质量批准/日期:(如需要)NASupplier/Plant供应商/工厂名称:SMACO Supplier/Plant Code供方代码:C086 BatteryOther Approval/Date(If Req'd)其他批准/日期(如需要):NAOther Approval/Date(If Req'd)其他批准/日期:(如需要) NAPart Number/Latest Change Level 零件号/最新更改水平: NAPart/ Process NO.零件/过程编号Process Name/OperationDescription过程名称/操作描述Machine,Device,Jig,Tools forMFG.material制造用机器、装置、夹具、工具、物料Characteristics 特性Characteristicclass特性分类Tighten Sampling Plan should be applied if1. customer required2. Product quality concern3. major customer complaint4. Management and/or CFT DecisionPCBA test fixtureRegular Size 正常样本容量Regular Freq.正常频率Tighten Size加严样本量Tighten Freq.加严频率Methods 方法责任部门ResponsibleDepartmentsControl Method控制方法Reaction plan反应计划NO.编号Product产品Process过程Product/Process/Spec./Tolerance产品/过程/规格/公差Evaluation/Measurement Tech.评价/测量技术Sampling plan抽样计划Core Team 核心小组:Customer Engineering Approve/Date(If Req'd)客户工程批准/日期:(如需要)NAPart name/Description零件名称/描述:C086电池组装C086 Battery assembly Supplier/Plant approval/Date供应商/工厂批准/日期:待定Customer Quality Approve/Date(If Req'd)客户质量批准/日期:(如需要)NASupplier/Plant供应商/工厂名称:SMACO Supplier/Plant Code供方代码:C086 BatteryOther Approval/Date(If Req'd)其他批准/日期(如需要):NAOther Approval/Date(If Req'd)其他批准/日期:(如需要) NAPart Number/Latest Change Level 零件号/最新更改水平: NAPart/ Process NO.零件/过程编号Process Name/OperationDescription过程名称/操作描述Machine,Device,Jig,Tools forMFG.material制造用机器、装置、夹具、工具、物料Characteristics 特性Characteristicclass特性分类Tighten Sampling Plan should be applied if1. customer required2. Product quality concern3. major customer complaint4. Management and/or CFT Decision主板测试治具Anti-static ring testerRegular Size 正常样本容量Regular Freq.正常频率Tighten Size加严样本量Tighten Freq.加严频率Methods 方法责任部门ResponsibleDepartmentsControl Method控制方法Reaction plan反应计划NO.编号Product产品Process过程Product/Process/Spec./Tolerance产品/过程/规格/公差Evaluation/Measurement Tech.评价/测量技术Sampling plan抽样计划Core Team 核心小组:Customer Engineering Approve/Date(If Req'd)客户工程批准/日期:(如需要)NAPart name/Description零件名称/描述:C086电池组装C086 Battery assembly Supplier/Plant approval/Date供应商/工厂批准/日期:待定Customer Quality Approve/Date(If Req'd)客户质量批准/日期:(如需要)NASupplier/Plant供应商/工厂名称:SMACO Supplier/Plant Code供方代码:C086 BatteryOther Approval/Date(If Req'd)其他批准/日期(如需要):NAOther Approval/Date(If Req'd)其他批准/日期:(如需要) NAPart Number/Latest Change Level 零件号/最新更改水平: NAPart/ Process NO.零件/过程编号Process Name/OperationDescription过程名称/操作描述Machine,Device,Jig,Tools forMFG.material制造用机器、装置、夹具、工具、物料Characteristics 特性Characteristicclass特性分类Tighten Sampling Plan should be applied if1. customer required2. Product quality concern3. major customer complaint4. Management and/or CFT DecisionPCBA test fixture防静电环Anti-static ring防静电环测试仪/分板PCBA test /Separate boardRegular Size 正常样本容量Regular Freq.正常频率Tighten Size加严样本量Tighten Freq.加严频率Methods 方法责任部门ResponsibleDepartmentsControl Method控制方法Reaction plan反应计划NO.编号Product产品Process过程Product/Process/Spec./Tolerance产品/过程/规格/公差Evaluation/Measurement Tech.评价/测量技术Sampling plan抽样计划Core Team 核心小组:Customer Engineering Approve/Date(If Req'd)客户工程批准/日期:(如需要)NAPart name/Description零件名称/描述:C086电池组装C086 Battery assembly Supplier/Plant approval/Date供应商/工厂批准/日期:待定Customer Quality Approve/Date(If Req'd)客户质量批准/日期:(如需要)NASupplier/Plant供应商/工厂名称:SMACO Supplier/Plant Code供方代码:C086 BatteryOther Approval/Date(If Req'd)其他批准/日期(如需要):NAOther Approval/Date(If Req'd)其他批准/日期:(如需要) NAPart Number/Latest Change Level 零件号/最新更改水平: NAPart/ Process NO.零件/过程编号Process Name/OperationDescription过程名称/操作描述Machine,Device,Jig,Tools forMFG.material制造用机器、装置、夹具、工具、物料Characteristics 特性Characteristicclass特性分类Tighten Sampling Plan should be applied if1. customer required2. Product quality concern3. major customer complaint4. Management and/or CFT DecisionRegular Size 正常样本容量Regular Freq.正常频率Tighten Size加严样本量Tighten Freq.加严频率Methods 方法责任部门ResponsibleDepartmentsControl Method控制方法Reaction plan反应计划NO.编号Product产品Process过程Product/Process/Spec./Tolerance产品/过程/规格/公差Evaluation/Measurement Tech.评价/测量技术Sampling plan抽样计划Core Team 核心小组:Customer Engineering Approve/Date(If Req'd)客户工程批准/日期:(如需要)NAPart name/Description零件名称/描述:C086电池组装C086 Battery assembly Supplier/Plant approval/Date供应商/工厂批准/日期:待定Customer Quality Approve/Date(If Req'd)客户质量批准/日期:(如需要)NASupplier/Plant供应商/工厂名称:SMACO Supplier/Plant Code供方代码:C086 BatteryOther Approval/Date(If Req'd)其他批准/日期(如需要):NAOther Approval/Date(If Req'd)其他批准/日期:(如需要) NAPart Number/Latest Change Level 零件号/最新更改水平: NAPart/ Process NO.零件/过程编号Process Name/OperationDescription过程名称/操作描述Machine,Device,Jig,Tools forMFG.material制造用机器、装置、夹具、工具、物料Characteristics 特性Characteristicclass特性分类Tighten Sampling Plan should be applied if1. customer required2. Product quality concern3. major customer complaint4. Management and/or CFT DecisionRegular Size 正常样本容量Regular Freq.正常频率Tighten Size加严样本量Tighten Freq.加严频率Methods 方法责任部门ResponsibleDepartmentsControl Method控制方法Reaction plan反应计划NO.编号Product产品Process过程Product/Process/Spec./Tolerance产品/过程/规格/公差Evaluation/Measurement Tech.评价/测量技术Sampling plan抽样计划Core Team 核心小组:Customer Engineering Approve/Date(If Req'd)客户工程批准/日期:(如需要)NAPart name/Description零件名称/描述:C086电池组装C086 Battery assembly Supplier/Plant approval/Date供应商/工厂批准/日期:待定Customer Quality Approve/Date(If Req'd)客户质量批准/日期:(如需要)NASupplier/Plant供应商/工厂名称:SMACO Supplier/Plant Code供方代码:C086 BatteryOther Approval/Date(If Req'd)其他批准/日期(如需要):NAOther Approval/Date(If Req'd)其他批准/日期:(如需要) NAPart Number/Latest Change Level 零件号/最新更改水平: NAPart/ Process NO.零件/过程编号Process Name/OperationDescription过程名称/操作描述Machine,Device,Jig,Tools forMFG.material制造用机器、装置、夹具、工具、物料Characteristics 特性Characteristicclass特性分类Tighten Sampling Plan should be applied if1. customer required2. Product quality concern3. major customer complaint4. Management and/or CFT DecisionRegular Size 正常样本容量Regular Freq.正常频率Tighten Size加严样本量Tighten Freq.加严频率Methods 方法责任部门ResponsibleDepartmentsControl Method控制方法Reaction plan反应计划NO.编号Product产品Process过程Product/Process/Spec./Tolerance产品/过程/规格/公差Evaluation/Measurement Tech.评价/测量技术Sampling plan抽样计划Core Team 核心小组:Customer Engineering Approve/Date(If Req'd)客户工程批准/日期:(如需要)NAPart name/Description零件名称/描述:C086电池组装C086 Battery assembly Supplier/Plant approval/Date供应商/工厂批准/日期:待定Customer Quality Approve/Date(If Req'd)客户质量批准/日期:(如需要)NASupplier/Plant供应商/工厂名称:SMACO Supplier/Plant Code供方代码:C086 BatteryOther Approval/Date(If Req'd)其他批准/日期(如需要):NAOther Approval/Date(If Req'd)其他批准/日期:(如需要) NAPart Number/Latest Change Level 零件号/最新更改水平: NAPart/ Process NO.零件/过程编号Process Name/OperationDescription过程名称/操作描述Machine,Device,Jig,Tools forMFG.material制造用机器、装置、夹具、工具、物料Characteristics 特性Characteristicclass特性分类Tighten Sampling Plan should be applied if1. customer required2. Product quality concern3. major customer complaint4. Management and/or CFT Decision1.螺套压到位(不可有明显缝Regular Size 正常样本容量Regular Freq.正常频率Tighten Size加严样本量Tighten Freq.加严频率Methods 方法责任部门ResponsibleDepartmentsControl Method控制方法Reaction plan反应计划NO.编号Product产品Process过程Product/Process/Spec./Tolerance产品/过程/规格/公差Evaluation/Measurement Tech.评价/测量技术Sampling plan抽样计划Core Team 核心小组:Customer Engineering Approve/Date(If Req'd)客户工程批准/日期:(如需要)NAPart name/Description零件名称/描述:C086电池组装C086 Battery assembly Supplier/Plant approval/Date供应商/工厂批准/日期:待定Customer Quality Approve/Date(If Req'd)客户质量批准/日期:(如需要)NASupplier/Plant供应商/工厂名称:SMACO Supplier/Plant Code供方代码:C086 BatteryOther Approval/Date(If Req'd)其他批准/日期(如需要):NAOther Approval/Date(If Req'd)其他批准/日期:(如需要) NAPart Number/Latest Change Level 零件号/最新更改水平: NAPart/ Process NO.零件/过程编号Process Name/OperationDescription过程名称/操作描述Machine,Device,Jig,Tools forMFG.material制造用机器、装置、夹具、工具、物料Characteristics 特性Characteristicclass特性分类Tighten Sampling Plan should be applied if1. customer required2. Product quality concern3. major customer complaint4. Management and/or CFT DecisionNo defects of no light or flash,Smoke is sucked outRegular Size 正常样本容量Regular Freq.正常频率Tighten Size加严样本量Tighten Freq.加严频率Methods 方法责任部门ResponsibleDepartmentsControl Method控制方法Reaction plan反应计划NO.编号Product产品Process过程Product/Process/Spec./Tolerance产品/过程/规格/公差Evaluation/Measurement Tech.评价/测量技术Sampling plan抽样计划Core Team 核心小组:Customer Engineering Approve/Date(If Req'd)客户工程批准/日期:(如需要)NAPart name/Description零件名称/描述:C086电池组装C086 Battery assembly Supplier/Plant approval/Date供应商/工厂批准/日期:待定Customer Quality Approve/Date(If Req'd)客户质量批准/日期:(如需要)NASupplier/Plant供应商/工厂名称:SMACO Supplier/Plant Code供方代码:C086 BatteryOther Approval/Date(If Req'd)其他批准/日期(如需要):NAOther Approval/Date(If Req'd)其他批准/日期:(如需要) NAPart Number/Latest Change Level 零件号/最新更改水平: NAPart/ Process NO.零件/过程编号Process Name/OperationDescription过程名称/操作描述Machine,Device,Jig,Tools forMFG.material制造用机器、装置、夹具、工具、物料Characteristics 特性Characteristicclass特性分类Tighten Sampling Plan should be applied if1. customer required2. Product quality concern3. major customer complaint4. Management and/or CFT Decision号:SMC-R-06-0059Revision 版次:A/吸力测试/Torsional withcigarette holder /RTD test /RTD TesterC078烟弹C078Cartridges返工Rework 3《产品制程巡检记录表》《IPQC process inspection record》功能特性灯色正常与标识一致2.工作时不允许出现无灯,闪灯,有烟雾被吸出Led color is right.目视Visual check。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
SMILE Johns Hopkins University Baltimore, MD USA
245421132 V1.0 Page 1 of 14 Author: Jaclyn Madden Document Number: Pro71-02 Effective Date: 23 September 2008 Review History Date of last review: 18-Jan-13 Reviewed by: Heidi Hanes SMILE Comments: This document is provided as an example only. It must be revised to accurately reflect your lab’s specific processes and/or specific protocol requirements. Users are directed to countercheck facts when considering their use in other applications. If you have any questions contact SMILE.
CHECKLIST FOR SITE SOP REQUIRED ELEMENTS: Quality Management Plan
Element Present 12 Quality System Essentials of a Quality Management Plan
QSE 1 - Documents and Records Some of this information is also contained in the Document Control SOP Checklist. Refer to the
laboratory’s Document Control SOP as needed to prevent duplication. See section 7.4 for record modification requirements. 1.1 Document Control
1. 2. 3.
4. 5. 6. 7. 8.
9. 10.
All documents, to include policies, processes, procedures, and forms, are maintained in a controlled manner. The Quality Management SOP describes the following: 1. The methods for identifying and documenting the need for new documents or the need for changes to existing documents. 2. The processes for writing or revising documents. 3. The review and approval process for new or revised documents. The new or revised document requires laboratory director approval prior to implementation. 4. The process for implementing new or revised documents. There is a method for documenting staff’s knowledge of document content. Staff are to be notified of any new or revised documents and trained as necessary. They are required to review and sign the documents before using them. 5. The review process (initial, annual, and as needed with changes to policies, processes, procedures, etc.) is described. 6. Documents are uniquely identified and listed on a document master list. 7. The use of the document master list is described. 8. All working copies of a document are made from the master copy of the document. Distribution and destruction of working copies is tracked. 9. Documents are to be retired/archived according to set procedures. A copy of the retired document is maintained. Retired documents are clearly marked to prevent inadvertent use. Retirement/archiving are noted on the document master list. 10. Documents are stored in a way that limits access to authorized personnel and maintains document integrity. Methods for labeling stored documents, location of storage, and retention times are described.
Comments: 1.2 Reviewing, Retaining, Storing, Retrieving, and Destroying Records All laboratory records (requisitions, patient results, QC logs, maintenance logs, QA logs, etc.) are maintained in a controlled manner. The SOP describes how the following are managed: SMILE Johns Hopkins University Baltimore, MD USA
245421132 V1.0 Page 2 of 14 1. 2. 3. 4. 1. Records are created and include the name of the creator and date of creation. 2. Records are reviewed and signed by the laboratory director or designee at least monthly. 3. Records are labeled and stored so as to maintain patient confidentiality, limited access, and the physical integrity of the record. Records are stored such that they can be retrieved within 24 hours. They may be listed in a Records Index. 4. Retention times are established and destruction of records is documented. Records are not to be destroyed except as advised in writing by the applicable network(s). In the event that record destruction is requested, the lab should document and retain a record of what was destroyed indefinitely. Comments:
QSE 2 – Organizational Structure 2.1 Implementing a Laboratory Quality Management System
1. 2. 3. The SOP describes 1. The role and responsibilities of the Quality Manager. 2. Requirements for laboratory director or designee review of the Quality Management System, Quality Manual, and other policies, processes, and procedures prior to implementation and at least annually thereafter. 3. How information in the Quality Manual is communicated to all personnel. Comments:
2.2 Organizational Chart 1. 2. 3. The chart visually depicts the following 1. Laboratory management and administration. 2. Levels of authority and responsibility for all laboratory personnel are identified. 3. The laboratory reporting chain of command is identified.
