ce认证mdd分类导则
医疗器械CE认证标准清单
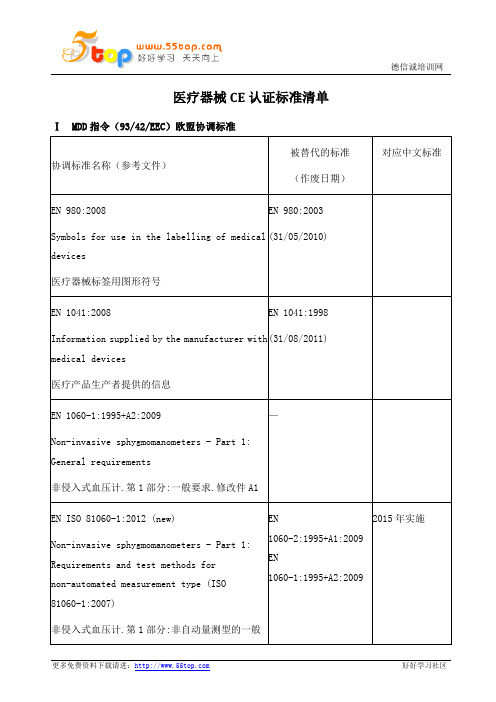
EN ISO 10993-3:2003
(21/03/2010)
GB/T16886.3-2008,IDT
EN 12470-4:2000+A1:2009
Clinical thermometers - Part 4: Performance of electrical thermometers for continuous measurement
医疗器械
ⅠMDD指令(93/42/EEC)欧盟协调标准
协调标准名称(参考文件)
被替代的标准
(作废日期)
对应中文标准
EN 980:2008
Symbols for use in the labelling of medical devices
医疗器械标签用图形符号
EN 980:2003
(31/05/2010)
医疗电气设备.基本安全和主要性能的一般要求
EN 60601-1:1990
+A1:1993+A2:1995+ A13:1996
EN 60601-1-1:2001
EN 60601-1-4:1996
+ A1:1999
(01/06/2012)
EN 60601-1-2:2007
Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests
欧盟对医疗器械的法规要求及分类标准

D
02 欧盟医疗器械法规概述
医疗器械指令(MDD)
01
MDD是欧盟针对医疗器械的基本法规,规定了医疗器 械的定义、分类、基本要求、评估程序和市场监管等内 容。
02
MDD要求医疗器械必须符合安全性和性能的基本要求 ,并通过相应的符合性评估程序获得CE标志,才能在欧 盟市场销售和使用。
03
MDD还规定了医疗器械制造商、进口商和经销商的责 任和义务,包括建立质量管理体系、进行临床评估、报 告不良事件等。
体外诊断医疗器械法规(IVDR)
IVDR对体外诊断医疗器械的分类更加详细,要求制 造商提供更多的临床数据和性能评估报告,证明产品 的准确性和可靠性。
IVDR是欧盟针对体外诊断医疗器械的法规,于2017 年发布,2022年5月起强制执行。
IVDR还要求制造商建立严格的质量管理体系和生产 过程控制,确保产品的稳定性和一致性。同时, IVDR还规定了更加严格的市场监管措施,包括加强 不良事件报告和召回制度等。
技术文档应包括医疗器械的设计 、制造、性能、安全性、有效性 等方面的详细信息,以及相关的
试验、验证和评估结果。
技术文档应随时可供欧盟监管机 构检查,并应随着医疗器械的更
新和改进而不断更新。
上市后监管义务
制造商应对其已上市的医疗器械进行持 续的监管,以确保其在使用过程中的安
全性和有效性。
制造商应建立上市后监管计划,包括定 期收集和分析医疗器械的使用数据、不 良事件报告等信息,以及采取必要的纠
临床试验要求
对于高风险医疗器械,欧盟要求 进行临床试验以验证其安全性和
有效性。
临床试验必须在欧盟境内进行, 并符合欧盟相关法规和标准的要
求。
申请临床试验需要提交试验方案 、研究者资质、伦理委员会批准 等文件,并接受监管机构的审核
mdd和mdr的条款

MDD和MDR的条款一、引言随着医疗技术的快速发展和全球化进程的加速,医疗器械在医疗保健领域中的作用日益突出。
医疗器械的监管要求也随之变得严格和复杂。
欧洲联盟(EU)对医疗器械实施了医疗器械指令(MDD)和医疗器械法规(MDR),以确保医疗器械的安全性和有效性。
本文将深入探讨MDD和MDR的主要条款、特点和差异,以帮助医疗器械制造商、供应商和用户更好地理解和遵守相关法规。
二、MDD和MDR概述MDD是欧盟关于医疗器械的基本法规,于1993年正式实施,并进行了多次修订。
MDD的主要目的是确保医疗器械在上市时具有足够的性能和安全性,为患者提供可靠的治疗。
MDR是欧盟最新的医疗器械法规,于2017年正式实施,取代了MDD。
MDR旨在确保医疗器械在整个生命周期内都符合高标准的安全性和有效性要求,并加强了对医疗器械的监管。
三、MDD的主要条款MDD的主要条款包括以下几个方面:1.医疗器械的定义和分类:MDD明确了医疗器械的定义,并将医疗器械分为不同类别,以便对不同类型的医疗器械实施不同的监管要求。
2.符合性评估:MDD要求医疗器械必须通过符合性评估程序才能上市销售。
符合性评估机构必须是欧盟授权的公告机构之一。
3.CE认证:为了满足MDD的要求,医疗器械必须获得CE认证标志,证明其符合相关指令的要求。
CE认证标志是欧盟产品安全性的象征。
4.上市后监督:MDD要求制造商对已上市的医疗器械进行持续监督,以确保其安全性和有效性。
制造商必须建立有效的追溯系统,以便对医疗器械进行召回和追溯。
5.临床数据和性能评估:MDD要求制造商提供充分的临床数据和性能评估报告,以证明医疗器械的有效性和安全性。
四、MDR的主要特点与变化与MDD相比,MDR的主要特点与变化包括以下几个方面:1.范围扩大:MDR扩大了监管范围,涵盖了所有类型的医疗器械,包括体外诊断医疗器械、有源植入式医疗器械等。
2.全生命周期监管:MDR要求对医疗器械进行全生命周期监管,从研发、生产、上市到退役等各个环节都必须符合相关要求。
欧盟CE认证的指令有哪些

欧盟CE认证,即只限于产品不危及人类、动物和货品的安全方面的基本安全要求,而不是一般质量要求,协调指令只规定主要要求,一般指令要求是标准的任务。
因此准确的含义是:CE标志是安全合格标志而非质量合格标志,是构成欧洲指令核心的"主要要求"。
欧盟CE认证机构港易质量认证公司,在欧洲认证咨询方面有多年的经验总结:“CE”标志是一种安全认证标志,被视为制造商打开并进入欧洲市场的护照。
欧盟CE认证指令要求有:1、机械指令(MD);2、建筑产品指令(CPD);3、低电压指令(LVD);4、医疗器械指令(MDD);5、无线电与通讯(RED);6、个人防护产品(PPE);7、娱乐游艇设备(RCD);8、燃具设备(90/396/EEC);9、电磁兼容性(EMC);10、电梯指令(Lift);11、防爆指令(ATEX);12、热水锅炉器具(92/42eec)。
在欧盟市场“CE”标志属强制性认证标志,不论是欧盟内部企业生产的产品,还是其他国家生产的产品,要想在欧盟市场上自由流通,就必须加贴“CE”标志,以表明产品符合欧盟《技术协调与标准化新方法》指令的基本要求。
这是欧盟法律对产品提出的一种强制性要求。
CE认证公告机构Notified Body指定机构NB,是由欧盟成员国提名,由欧盟委员会指定的机构。
进行CE合格评定,具有对产品安全性提供法律解释权,许多指令需要指定机构的参与,部分指令称指定机构为”能力机构“或”授权机构“。
港易质量认证公司专业办理,以最短的周期做最权威的CE认证,以确保您的产品出口欧盟能够顺利清关,被国外客户认可产品的质量。
港易质量认证公司是一家专业的从事于产品出口认证咨询,质量体系认证咨询,信用评级咨询,产品检测咨询的技术服务型公司,与ITS、SGS、CONTECNA、CCIC、CQC等国内外机构建立了良好的业务合作关系,为国内厂家的认证提供便利。
港易质量认证公司致力于为客户提供海关联盟CU-TR认证、俄罗斯GOST认证、尼日利亚SONCAP认证、沙特SASO认证、肯尼亚PVOC认证、乌干达PVOC认证、坦桑尼亚PVOC认证、南非LOA 认证,埃及COI认证、欧盟CE认证、CB认证、加纳CTN,科威特KUCAS认证、伊拉克COC认证、澳洲SAA认证、美国DOT认证、美国FDA认证、日本PSE认证、印尼SNI认证、泰国TISI认证、印度BIS认证、BSCI认证、加拿大CSA认证等等、提供安全、电磁兼容、化学、性能测试、环境测试等全方位的技术服务。
普通医疗器械产品CE认证分类

根据 MDD 附录九 [93/42/EEC]规则 1 非插入式器械属于I类器械,但适用以下其他规则的除外.规则 2 用于输送和储存血液、体液或人体组织和其他液体和气体为人体吸收、服用或注入的非插入式样器械,属于IIa类:如果他们可以同IIa或更高类别的有源器械连接使用;或如果他们的预定功能属于储存和输送血液和其他体液、储存人体器官或人体组织.其他的都属于I类。
规则 3插入式器械用于改变血液、体液和其他注入人体的液体的生物和化学成分属于III 类器械。
但处理方法属于过滤、气体和热能的分离或交换的该类非插入式器械属于IIa.除此以外的其他情况属于I类。
规则 4同受伤皮肤接触的非插入式器械:如果用于形成机械屏障,阻止或吸收渗出液体,属于I类;如果主要用于辅助治疗已经伤及真皮的创伤,属于IIb类;其他情况属于IIa,包括主要用于处理创伤周围环境的器械.规则 5插入式器械,除非属于外科手术插入式器械或同有源器械连接使用,如果属于暂时性的使用方式,属于I类;如果属于短期性的使用方式,属于IIa类;但不包括在口腔中仅至咽部、在耳道中仅至耳鼓、在鼻腔中使用但不被粘膜吸收的器械;如果属于长期性的使用方式,属于IIb类;但在口腔中仅至咽部、在耳道中仅至耳鼓、在鼻腔中使用但不被粘膜吸收的器械属于IIa类;其他需要与IIa或更高类别的器械连接使用的插入式器械,属于IIa类;但不包括外科手术插入式器械。
规则 6暂时性使用方式的外科手术插入式器械属于IIa类,但以下情况除外: 如果为了诊断、监测或矫正心脏或主血管系统疾病,器械直接触及这些器官,在属于III类;可重复使用的外科器械属于I类;以电离辐射的方式提供活力(energy)的器械属于IIb类;对人体生理发生作用或为人体全部或大部分所吸收的器械属于IIb类;通过发送装置给人体施用药物,如果对人体具有某种危险,则属于IIb类.规则 7以短期方式使用的外科插入式器械属于IIa,但以下情况除外:如果为了诊断、监测或矫正心脏或主血管系统疾病,直接触及这些器官的器械,属于III类;直接触及中枢神经系统的专用器械属于III类;以电离辐射的方式提供活力(energy)的器械属于IIb类;对人体生理发生作用或为人体全部或大部所吸收的器械属于III类;在人体内促使人体发生某种化学变化,但不属于安装在牙齿上或为人体给药的器械,属于IIb类。
欧盟CE认证指令相关问题

欧盟CE认证指令相关问题目前,CE认证涉及20多个指令。
最常见的是LVD低压指令、EMC 指令、toy指令、MD机械指令、MDD医疗器械指令、PED压力设备指令、PPE个人防护指令、CPd施工指令、reach化工产品指令、ATEX 防爆指令,这是认证市场中最常见的指令,也是目前证书数量最多的指令。
两者都属于CE认证。
只有不同指令的CE证书使用不同的检测标准,例如玩具指令的CE认证标准,主要是EN71系列标准。
机械指令的常用标准是en60204和en isol2100,因此并非所有经CE认证的产品都使用相同的标准,这是不可能的。
不同的认证指令有不同的适用标准。
同时,对于不同性质的产品,欧盟CE认证体系也规定了相应的认证要求,如ped压力设备指令、PPE个人防护指令、MDD 医疗器械指令。
这些指令基本上是必须由第三方欧盟授权认证机构参与的认证指令,也就是说,这些指令必须要求欧盟公告编号机构颁发CE证书,它不是可以颁发证书的临时机构,否则它不能被欧盟海关认可。
那么,欧盟的通报机构是什么?现在让我解释一下,所谓的欧盟公告机构是指欧盟委员会,该委员会已发布认证实验室的批准号。
只要他们通过了监管框架下的审查,欧盟委员会就会给他们颁发一个批准号,我们称之为公告号。
获得这些公告编号的机构或实验室称为欧盟公告。
不同的公告机构可能不具备相同的资质。
一些实验室或机构可能仅具备LVD低压指令的产品测试能力。
可能他们只获得LVD低压指令的产品授权。
一些实验室具有压力设备的测试能力。
欧盟委员会授权的产品可能是压力设备指令的产品。
因此,对于这些公告机构,还存在产品资格。
并不是说一个公告机构可以为所有指令颁发CE证书。
几乎没有这样的实验室。
据估计,整个欧盟无法找到一个机构可以为所有指令颁发CE证书。
这是CE认证的一般情况。
ce认证指令与标准

ce认证指令与标准
CE 认证指令是指欧盟委员会发布的一系列指令,用于规范和管理
欧盟内部市场上的产品。
这些指令涵盖了各种产品领域,如电气设备、机械设备、玩具、医疗器械等。
CE 认证标准是指符合欧盟指令要求的产品标准。
这些标准通常由
欧洲标准化组织(CEN、CENELEC、ETSI 等)制定,并在欧盟范围内
得到广泛认可和应用。
在进行 CE 认证时,需要根据产品所属的指令和标准进行评估和测试。
评估和测试的内容包括产品的安全性、电磁兼容性、环保要求等
方面。
只有通过评估和测试,并符合指令和标准要求的产品,才能获
得 CE 认证标志。
以下是一些常见的 CE 认证指令
1.低电压指令(LVD):适用于电压在 50-1000V AC 或 75-
1500V DC 之间的电气设备。
2.机械指令(MD):适用于机械设备和机器。
3.电磁兼容指令(EMC):适用于可能产生电磁干扰或受到电磁
干扰的设备。
4.个人防护设备指令(PPE):适用于个人防护设备,如头盔、
手套、安全鞋等。
5.医疗器械指令(MDD):适用于医疗器械。
6.玩具安全指令(TSD):适用于玩具。
7.无线电设备指令(RED):适用于无线电设备,如手机、无线
电对讲机等。
8.压力设备指令(PED):适用于压力设备,如锅炉、压力容器
等。
不同的指令和标准可能适用于不同的产品领域和类型。
因此,在进行 CE 认证之前,需要仔细了解产品所属的指令和标准,并选择合适的认证机构进行评估和测试。
CE认证及标示

CE认证及标示欧盟为消除各成员国间的贸易壁垒,逐步建立成为一个统一的大市场,以确保人员、服务、资金和产品(如医疗器械)的自由流通。
在医疗器械领域,欧盟委员会制定了三个欧盟指令,以替代原来各成员的认可体系,使有关这类产品投放市场的规定协调一致。
这三个指令分别是:1.有源植入性医疗器械指令(AIMD,90/335/EEC),适用于心脏起搏器,可植入的胰岛素泵等有源植入性医疗器械。
AIMD于1993年1月1日生效。
过渡截止期为1994年12月31日,从1995年1月1日强制实施。
2.活体外诊断器械指令(IVD),适用于血细胞计数器,妊娠检测装置等活体外诊断用医疗器械。
该指令目前仍在起草阶段,可能于1998年末或1999年初正式实施。
3.医疗器械指令(Medical Devices Direc-tive,93/42/EEC),适用范围很广,包括除有源植入性和体外诊断器械之外的几乎所有的医疗器械,如无源性医疗器械(敷料、一次性使用产品、接触镜、血袋、导管等);以及有源性医疗器械,如核磁共振仪、超声诊断和治疗仪、输液泵等。
该指令已于1995年1月1日生效,过渡截止日期为1998年6月13日从1998年6月14日起强制执行。
上述指令规定,在指令正式实施后,只有带有CE标志的医疗器械产品才能在欧盟市场上销售。
医疗器械CE认证(Medical Devices Direc-tive,93/42/EEC)介绍MDD是目前欧洲可见到的最为全面的医疗器械方面的规定,在该指令中,共有23个条款和12个附录。
其重要部分包括在以下条款中:第1条款:本指令适用于医疗器械及其附件第2条款:成员国必须确保投放其市场和使用的医疗器械是安全的。
第3条款:所谓“安全”的器械应满足附录1中的基本要求。
第4条款:带有CE标志的医疗器械可在欧盟自由流通。
特殊条款(附录和X)允许使用无CE标志客户定制产品及临床研究的产品。
第5条款:符合协调标准的医疗器械被认为满足基本要求。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
MEDDEV 2.4/1 – rev. 8 PART 2: GUIDELINES FOR THE CLASSIFICATION OF MEDICAL DEVICES (July 2001) .................4.2GENERAL EXPLANATION OF RULES/PRACTICAL ISSUES/EXAMPLESRule 1 -Devices that either do not touch the patient or contact intact skin onlyGeneral explanation of the ruleThis is a fallback rule applying to all devices that are not covered by a more specific rule.This is a rule that applies in general to devices that come into contact only with intact skin or that do not touch the patient.RULE 1EXAMPLESAll non-invasive devices are in Class I, unless one of the rules set out hereinafter applies.- Body liquid collection devices intended to be used in such a way that a return flow is unlikely (e.g. to collect body wastes such as urine collection bottles, ostomy pouches, incontinence pads or collectors used with wound drainage devices). They may be connected to the patient by means of catheters and tubing.- Devices used to immobilize body parts and/or to apply force or compression on them (e.g. non-sterile dressings used to aid the healing of a sprain, plaster of Paris, cervical collars, gravity traction devices, compression hosiery).- Devices intended in general for external patient support (e.g. hospital beds, patient hoists, walking aids, wheelchairs, stretchers, dental patient chairs).- Corrective glasses, frames, stethoscopes for diagnosis, eye occlusion plasters, incision drapes, conductive gels, non-invasive electrodes (electrodes for EEG or ECG), image intensifying screens.- Permanent magnets for removal of ocular debrisPractical issues of classificationSome non-invasive devices are indirectly in contact with the body and can influence internal physiological processes by storing, channeling or treating blood, other body liquids or liquids which are returned or infused into the body or by generating energy that is delivered to the body. These must be excluded from the application of this rule and be handled by another rule because of the hazards inherent in such indirect influence on the body.Rule 2 - Channeling or storing for eventual administrationGeneral explanation of the ruleThese types of devices must be considered separately from the non-contact devices of rule 1 because they may be indirectly invasive. They channel or store substances that will be eventually delivered into the body. Typically these devices are used in transfusion, infusion, extracorporeal circulation, delivery of anaesthetic gases and oxygen.In some cases devices covered under this rule are very simple gravity activated delivery devices.RULE 2EXAMPLESAll non-invasive devices intended for channeling or storing blood, body liquids or tissues, liquids or gases for the purpose of eventual infusion, administration or introduction into the body are in Class IIa:- if they may be connected1 to an active medical device in Class IIa or a higher class,- Devices intended to be used as channels in active drug delivery systems, e.g. tubing intended for use with an infusion pump.- Devices used for channeling, e.g. antistatic tubing for anesthesia, anesthesia breathing circuits and pressure indicator, pressure limiting devices.- Syringes for infusion pumps.- if they are intended for use for storing or channelling blood or other body liquids or for storing organs, parts of organs or body tissues (are in Class II a)- Devices intended to channel blood (e.g. in transfusion, extracorporeal circulation).- Devices intended for temporary storage and transport of organs for transplantation.- Devices intended for long term storage of biological substances and tissues such as corneas, sperm, human embryos, etc.in all other cases they are in Class I.- Devices that provide a simple channeling function, with gravity providing theforce to transport the liquid, e.g. administration sets for infusion.- Devices intended to be used for a temporary containment or storage functionsuch as cups and spoons specifically intended for administering medicines2.- Syringes without needlesPractical issues of classificationBlood bags are covered as an exception under a separate rule (see rule 18).If a device, e.g. tubing, can be used for a purpose that would cause it to be connected to an active device such a device will be automatically in Class II A, unless the manufacturer clearly state that it should not be connected to an active device of Class II A or higher.Explanation of special conceptsNote 1: "May be connected to an active device". Such connection is deemed to exist between a non-active device and an active device where a non-active device forms a link in the transfer of the substance between the patient and the active device and the safety and performance of one of the devices is influenced by the other device. For instance, this applies to tubing in an extracorporeal circulation system which is downstream from a blood pump and in the same blood flow circuit, but not directly in contact with the pump.Note 2: Solutions intended for preservation of organs during storage and transport are not medical devices.Rule 3 - Devices that modify biological or chemical composition of blood, body liquids or other liquidsGeneral explanation of the ruleThese types of devices must be considered separately from the non-contact devices of rule 1 because they are indirectly invasive. They treat or modify substances that will be eventually delivered into the body. This rule covers mostly the more sophisticated elements of extracorporeal circulation sets, dialysis systems and autotransfusion systems as well as devices for extracorporeal treatment of body fluids which may or may not be reintroduced immediately into the body, including, where the patient is not in a closed loop with the device..RULE 3EXAMPLESAll non-invasive devices intended for modifying the biological or chemical composition of blood, other body liquids or other liquids intended for infusion into the body are in Class IIb,- Devices intended to remove undesirable substances out of the blood by exchange of solutes such as hemodyalizers.- Devices intended to separate cells by physical means,e.g. gradient medium for sperm separation.- Haemodialysis concentrates.unless the treatment consists of filtration, centrifugation or exchange of gas or heat, in which case they are in Class IIa.- Particulate filtration of blood in an extracorporeal circulation system. These are used to remove particles and emboli from the blood.- Centrifugation of blood to prepare it for transfusion or autotransfusion.- Removal of carbon dioxide from the blood and/or adding oxygen.- Warming or cooling the blood in an extracorporeal circulation system.Practical issues of classificationThese devices are normally used in conjunction with an active medical device covered under rule 9 or rule 11. Filtration and centrifugation should be understood in the context of this rule as exclusively mechanical methods.Rule 4 -Devices in contact with injured skinGeneral explanation of the ruleThis rule is intended to cover primarily wound dressings independently of the depth of the wound. The traditional types of products (e.g. used as a mechanical barrier) are well understood and do not result in any great hazard. There have also been rapid technological developments in this area, with the emergence of new types of wound dressings for which non-traditional claims are made, e.g. management of the micro-environment of a wound to enhance its natural healing mechanism. More ambitious claims relate to the mechanism of healing by secondary intent, such as influencing the underlying mechanisms of granulation or epithelial formation or preventing contraction of the wound. Some devices used on breached dermis may even have a life-sustaining or life-saving purpose, e.g. when there is full thickness destruction of the skin over a large area and/or systemic effect. Dressings containing medicinal products acting as ancillary to the dressing fall within Class III under Rule 13.RULE 4EXAMPLESAll non-invasive devices which come into contact with injured skin:- are in Class I if they are intended to be used as a mechanical barrier, for compression or for absorption of exudates,- Wound dressings, such as absorbent pads, island dressings, cotton wool, wound strips and gauze dressings to act as a barrier or to maintain the wound positionally or to absorb exudates from the wound.- are in Class IIb if they are intended to be used principally with wounds which have breached the dermis and can only heal by secondary intent - Are principally intended to be used with severe wounds that have substantially and extensively breached the dermis, and where the healing process can only be by secondary intent such as:- dressings for chronic extensive ulcerated wounds- dressings for severe burns having breached the dermis and covering an extensive area- dressings for severe decubitis wounds- dressings incorporating means of augmenting tissue and providing a temporary skin substitute- are in Class IIa in all other cases, including devices principally intended to manage the micro-environment of a wound.- Have specific properties intended to assist the healing process by controlling the level of moisture at the wound during the healing process and to generally regulate the environment in terms of humidity and temperature, levels of oxygen and other gases and ph values or by influencing the process by other physical means.- These devices may specify particular additional healing properties whilst not being intended for extensive wounds requiring healing by secondary intent.- Adhesives for topical use.- Polymer film dressings, hydrogel dressings and non-medicated impregnated gauze dressings.Practical issues of classificationProducts covered under this rule are extremely claim sensitive, e.g. a polymeric film dressing would be in Class II A if the intended use is to manage the micro-environment of the wound and in Class I if its intended use is limited to retaining an invasive cannula at the wound site. Consequently it is impossible to say a priori that a particular type of dressing is in a given class without knowing its intended use as defined by the manufacturer. However, a claim that the device is interactive or active with respect to the wound healing process usually implies that the device is in Class II B.Most dressings that are intended for a use that is in Class II A or II B, also perform functions that are in Class I, e.g. that of a mechanical barrier. Such devices are nevertheless classed according to the intended use in the higher class.For such devices incorporating medicines see rule 13 or animal tissues see rule 17.Explanation of special concepts- Breached dermis: the wound exposes at least partly the subcutaneous tissue.- Secondary intent: the wound heals by first being filled with granulation tissue, subsequently the epithelium grows back over the granulation tissue and the wound contracts. In contrast primary intent implies that the edges of the wound are close enough or pulled together, e.g. by suturing, to allow the wound to heal.Rule 5Devices invasive in body orificesGeneral explanation of the ruleInvasiveness with respect to the body orifices (ear, mouth, nose, eye, anus, urethra and vagina) must be considered separately from invasiveness that penetrates through a cut in the body surfaces ( surgical invasiveness). For short term use, a further distinction must be made between invasiveness with respect to the less vulnerable anterior parts of the ear, mouth and nose and the other anatomical sites that can be accessed through natural body orifices.The surgically created stoma, which for example allows the evacuation of urine or faeces, should also be considered as a body orifice.Devices covered by this rule tend to be diagnostic and therapeutic instruments used in particular specialities (ENT, ophthalmology, dentistry, proctology, urology and gynecology).RULE 5EXAMPLESAll invasive devices with respect to body orifices, other thansurgically invasive devices and which are not intended forconnection to an active medical device:- are in Class I if they are intended for transient use,- Handheld mirrors used in dentistry to aid in dental diagnosis and surgery, dentalimpression materials, tubes used for pumping the stomach, impression tray, enemadevices, examination gloves and prostatic balloon dilation catheters.- are in Class IIa if they are intended for short term use,- Contact lenses, urinary catheters, tracheal tubes, stents,vaginal pessaries andperineal reeducation devices.except if they are used in the oral cavity as far as the pharynx, in- Dressings for nose bleeds, dentures intended to be removed by the patient.an ear canal up to the ear drum or in a nasal cavity , in which casethey are in Class I,- Urethral stents.- are in Class IIb if they are intended for long term use,except if they are used in the oral cavity as far as the pharynx, inan ear canal up to the ear drum or in a nasal cavity and are notliable to be absorbed by the mucous membrane, in which case theyare in Class IIa.- Orthodontic wire, fixed dental prostheses, fissures sealants.All invasive devices with respect to body orifices, other than surgically invasive devices, intended for connection to an active medical device in Class IIa or a higher class, are in Class IIa.- Tracheostomy or tracheal tubes connected to a ventilator, blood oxygen analysers placed under the eye-lid, powered nasal irrigators, nasopharyngeal airways, some enteral feeding tubes, fibreoptics in endoscopes connected to surgical lasers, suction catheters or tubes for stomach drainage, dental aspirator tips.Rule 6 - Surgically invasive devices for transient useGeneral explanation of the ruleThis rule covers principally three major groups of devices: devices that are used to create a conduit through the skin (needles, cannulae, etc.), surgical instruments (scalpels, saws, etc.) and various types of catheters, suckers, etc.RULE 6EXAMPLESAll surgically invasive1 devices intended for transient use are in Class IIa unless they are:- Needles used for suturing, needles of syringes, lancets, suckers, single use scalpels,single use scalpel blades,support devices in ophthalmic surgery, staplers, surgical swabs, drill bits connected to active devices, surgical gloves, etchants, tester of artificial heart valves, heart valve occluder, heart valve sizers and holders, trial hipprosthesis heads or stems, swabs to sample exudates, single use aortic punches (seenote 2)- intended specifically to diagnose, monitor or correct a defect 2 of the heart or of the central circulatory system1 through direct contact with these parts of the body, in which case they are in Class III 3- Cardiovascular catheters (e.g. angioplasty balloon catheters), including related guidewires and dedicated4 disposable cardiovascular surgical instruments e.g. electrophysiological catheters, electrodes for electrophysiological diagnosis and ablation.- Catheters containing or incorporating sealed radioisotopes, where the radioactive isotope as such is not intended to be released into the body, if used in the central circulatory system- reusable surgical instruments1, in which case they are in Class I3,- Scalpels, scalpel handles, drill bits, saws, that are not intended for connection to anactive device, and retractors forceps, excavators and chisels.- intended to supply energy in the form of ionizing radiation in which case they are in Class IIb,- Catheters containing or incorporating sealed radioisotopes, where the radioactive isotope as such is not intended to be released into the body, if used in the circulatory system, excluding the central circulatory system- intended to have a biological5effect or to be wholly or mainly absorbed4 in which case they are in Class IIb,- intended to administer medicines by means of a delivery system, if this is done in a manner that is potentially hazardous6 taking into account of the mode of application, in which case they are Class IIb.- Devices for repeated self-application where dosage levels and the nature of the medicinal product are critical, e.g. insulin pens.Explanations of special conceptsNote 1: Terms such as "surgically invasive device", "central circulatory system" and "reusable surgical instruments" are defined in Section I of Annex IX of the Directive. In particular surgical instruments connected to an active device are not considered to be “reusable surgical instruments”.Note 2: The expression "correct a defect" does not cover devices that are used accessorily in heart surgery, e.g. clamps. The first indent of this rule does not apply to aortic punches and similar cutting instruments which perform a similar function to a scalpel.Note 3: Surgical instruments which are not specifically intended for purposes described in the first indent, and irrespective of the site of application, are in class IIA, if they are intended for single use and in class I if they are reusable.Note 4: Dedicated means that the intended purpose of the device is to diagnose, monitor or correct a defect of the heart or of the central circulatory system.Note 5:Biological effect: All materials and devices have the potential to affect tissues following use in a surgically invasive procedure.A material is considered to have a biological effect if it actively and intentionally induces, alters or prevents a response from the tissues that is mediated by specific reactions at a molecular level. Such a device may be described as bioactive.Wholly or mainly absorbed: The term absorption refers to the degradation of a material within the body and the metabolic elimination of the resulting degradation products from the body.Note 6: The concept of "potentially hazardous manner" is related to the characteristics of the device and not the competence of the user.Rule 7 - Surgically invasive devices for short-term useGeneral explanation of the ruleThese are mostly devices used in the context of surgery or post-operative care (e.g. clamps, drains), infusion devices (cannulae, needles) and catheters of various types.RULE 7EXAMPLESAll surgically invasive devices intended for short term use are in Class IIa unless they are intended:- Clamps, infusion cannulae, skin closure devices, temporary filling materials. - Tissue stabilisers2 used in cardiac surgery- either specifically to diagnose, monitor or correct a defect2 of the heart or of the central circulatory system through direct contact with these parts of the body, in which case they are in Class III,- Cardiovascular catheters, cardiac output probes and temporary pacemaker leads. - Thoracic catheters intended to drain the heart, including the pericardium- Carotid artery shunts- or specifically for use in direct contact with the central nervoussystem, in which case they are in Class III,- Neurological catheters, cortical electrodes and connonoid paddles.- or to supply energy in the form of ionizing radiation in whichcase they are in Class IIb,- Brachytherapy devices.- intended to have a biological effect or to be wholly or mainlyabsorbed in which case they are in Class III,- Absorbable sutures and biological adhesives.- or to undergo chemical change in the body, except if the devicesare placed in the teeth, or to administer medicines1, in which casethey are Class IIb.- AdhesivesPractical issues of classificationNote 1:Administration of medicines is more than just channeling, it implies also storage and/or influencing the volume and rate of the medicine delivered. Implanted capsules for the slow release of medicines are medicines and not medical devices. Note 2: The expression “correct a defect” does not cover devices that are used accessorily in heart surgery, e.g. tissue stabilisers.Rule 8 - Surgically invasive devices for long-term use and implantable devicesGeneral explanation of the ruleThese are mostly implants in the orthopaedic, dental, ophthalmic and cardiovascular fields as well as soft tissue implants such as implants used in plastic surgery.RULE 8EXAMPLESAll implantable devices and long-term surgically invasive devices are in Class IIb unless they are intended:- Prosthetic joint replacements, ligaments, shunts, stents, nails, plates, intra-ocular lenses, internal closure devices, tissue augmentation implants, infusion ports, peripheral vascular grafts, penile implants, non-absorbable sutures, bone cements and maxillo-facial implants, visco-elastic surgical devices intended specifically for ophthalmic anterior segment surgery 1.- to be placed in the teeth2, in which case they are in Class IIa,- Bridges, crowns, dental filling materials and pins, dental alloys, ceramics andpolymers.- to be used in direct contact with the heart, the central circulatory system or the central nervous system, in which case they are Class III,- Prosthetic heart valves,aneurysm clips, vascular prostheses, spinal stents, vascular stents, CNS electrodes and cardiovascular sutures.- Permanent vena cava filters- to have a biological effect or to be wholly or mainly absorbed, in which case they are in Class III,- Absorbable sutures,adhesives and implantable devices claimed to be bioactive through the attachment of surface coatings such as phosphorylcholine.- or to undergo chemical change3 in the body, except if the devicesare placed in the teeth, or to administer medicines, in which casethey are in Class III.- Rechargeable non-active drug delivery systems.Practical issues of classificationNote 1 :these products are implants because in normal conditions a significant amount of the substance remains at the surgical site after the procedure. If these devices contain animal tissues or derivatives of animal tissues, they are covered by rule 17.Note 2:Implants without bioactive coatings intended to secure teeth or prostheses to the maxillary or mandibular bones are in Class II B following the general rule. Hydroxy-apatite is considered as having biological effect only if so claimed and demonstrated by the manufacturer.Note 3:The clause about chemical change under this rule does not apply to products such as bone cements where the chemical change takes place during the placement and does not continue in long term.Rule 9 - Active therapeutic devices intended to administer or exchange energyGeneral explanation of the ruleDevices classified by this rul e are mostly electrical equipment used in surgery such as lasers and surgical generators. In addition there are devices for specialised treatment such as radiation treatment. Another category consists of stimulation devices, although not all of them can be considered as delivering dangerous levels of energy considering the tissue involved.RULE 9EXAMPLESAll active therapeutic devices intended to administer or exchange energy are in Class IIa Electrical and/or magnetic and electromagnetic energy- Muscle stimulators and external bone growth stimulators, TENS devices and eye electromagnets, electrical acupunctureThermal energy- Cryosurgery equipment, heat exchangers, except the types described below Mechanical energy- Powered dermatomes, powered drills and dental hand pieces.Light- Phototherapy for skin treatment and for neonatal careSound- Hearing aidsUltrasound- Equipment for physiotherapyunless their characteristics are such that they may administer or exchange energy to and from the human body in a potentially hazardous way1, taking account of the nature, the density and the site of application of the energy, in which case they are in Class IIb.Kinetic energy- Lung ventilatorsThermal energy- Incubators for babies, warming blankets, blood warmers, electrically powered heat exchangers, for instance those used with patients incapable of reacting, communicating and/or who are without a sense of feelingElectrical energy- High-frequency electrosurgical generators,andelectrocautery equipment, including their electrodes, external pacemakers, external defibrillators, electroconvulsive therapy equipmentCoherent light- Surgical lasersUltrasound- Lithotriptors, surgical ultrasound devicesIonizing radiation- Radioactive sources for afterloading therapy, therapeutic cyclotrons, linear accelerators, therapeutic X-ray sources.All active devices intended to control and monitor the performance of active therapeutical devices in Class IIb or intended to influence directly the performance of such devices are in Class IIb.- External feedback systems for active therapeutic devices, afterloading control devices.Explanation of special conceptsNote 1:The decision as to whether a medical device administers or exchanges energy to and from the human body in a potentially hazardous way should take into account the following factors. The concept of "potentially hazardous" is dependent on the type of technology involved and the intended application of the device to the patient and not on the measures adopted by the manufacturer in view of good design management (e.g. use of technical standards, risk analysis). For instance all devices intended to emit ionizing radiation, all lung ventilators and lithotriptors should be in Class IIB. However, the manufacturer's obligation to comply with design requirements and solutions adopted, such as use of standards, exist independently from the classification system.Rule 10 -Active devices for diagnosis.General explanation of the ruleThis covers principally a whole range of widely used equipment in the fields ultrasound diagnosis and capture of physiological signals as well as therapeutic and diagnostic radiology.RULE 10EXAMPLESActive devices intended for diagnosis are in Class IIa:- if they are intended to supply energy which will be absorbed by the human body, except for devices used to illuminate the patient's body, in the visible spectrum,- Magnetic resonance equipment, pulp testers, evoked response stimulators, diagnostic ultrasound.- if they are intended to image in vivo distribution of radiopharmaceuticals,- Gamma cameras, positron emission tomography and single photon emission computer tomography.- if they are intended to allow direct diagnosis or monitoring of vital physiological processes1,- Electrocardiographs, electroencephalographs, cardioscopes with or without pacing pulse indicators2- Electronic thermometers- Electronic stethoscopes- Electronic blood pressure measuring equipmentunless they are specifically intended for monitoring of vital physiological parameters, where the nature of variations is such that it could result in immediate danger to the patient, for instance variations in cardiac performance, respiration, activity of CNS in which case they are in Class IIb.- Intensive care monitoring and alarm devices (for e.g. blood pressure, temperature, oxygen saturation), biological sensors, blood gas analysers used in open heart surgery, cardioscopes and apnea monitors, including apnea monitors in home careActive devices intended to emit ionizing radiation and intendedfor diagnostic and therapeutic interventional radiology3including devices which control or monitor4 such devices, orwhich directly influence their performance, are in Class II B.- Diagnostic X-ray sources.Examples of special concepts:Note 1:Vital physiological processes and parameters, include for example respiration, heart rate, cerebral functions, blood。
