HY-P0017_Aprotinin_MCE
Sysmex CS-5100 - Application 说明书

Sysmex CS-5100 - Application guidePage 1 of 19 Application guide for the Platelet function test by automated aggregation withEpinephrine (AG002K)This application guide describes the parameters and the procedure for measuring epinephrine-induced platelet aggregation, using the Epinephrine kit (AG002K) for optimal performance on Sysmex CS-5100. This application guide is based on the Sysmex CS-5100 system with the software version 00-13. This document is the only valid source of information for the assay application in the Sysmex CS-5100 system. HYPHEN BioMed has validated the instructions provided for the combination of reagents, instrument, software and customizable features of this system in order to optimize the performance of the product and comply with specifications. The modification of these parameters may affect performance and results. If the application is modified, the user shall be responsible for the validation of the modifications and their impact on all the results.For further information, please refer to the analyzer and reagents instruction manual. A specific configuration is required to perform the test.1. Test Principle:When added to platelet-rich plasma (PRP), epinephrine binds to the α2-adrinergic receptors on the platelet surface, inducing an initial wave of reversible aggregation. This primary aggregation leads to exposure of fibrinogen receptors and inhibition of adenylate cyclase activity. A second wave of aggregation, clearly distinct from the first, is triggered by ADP release from δ granules and by thromboxane A2 synthesis. Epinephrine is considered to be a weak agonist as the effects it produces are partial and reversible.2. Test synopsis:3. Reagents and material required but not provided:∙Distilled water∙Saline solution (0.9% NaCl)∙4mL Cup (424-1160-8)∙SB Cuvette (064-1041-9) and SB Kit tool (063-4151-5)∙Laboratory materials and equipment4. Preparation and stability of reagents:HYPHEN BioMed reagents). [3] It is recommended to transfer to 4 mL cup. [4] For multiple concentrations. Do not use a non-validated concentration.5. Recommendations:Homogenize well the reagents prior to each use. To obtain a homogeneous reactivity, it is essential to allow the temperature of the reagents stabilize for at least 30 minutes on board the analyzer prior to any use. Do not inter-change reagent vials from different batches.Quality control tests must be performed regularly, and with each change of reagent batch, after any major maintenance work on the analyzer, and when the results do not comply with the expected values for the method.The performance may vary slightly depending on the analyzer used. Each laboratory may establish its own acceptance range.6. Results:∙ The results are expressed in % of maximum aggregation. ∙It is recommended to make duplicate measurements.7. Performance and characteristics:less than 20% and the abnormal samples less than 30% for the maximum aggregation measurement (%).Aggregation curve with Epinephrine (For information only)Expected valuesThe values obtained on healthy subjects vary from one laboratory to another, each laboratory must determine its own reference ranges.The reference range was established on health adult subjects (n=150) using Sysmex CS-5100, 2000i, 2100i, 2400, 2500 (Central 90%, 97.5th percentile).The reference range measured is 68.8 – 99.8 % of maximum aggregation (median: 87.2% of maximum aggregation). SpecificityThe mean value measured out of 150 normal platelet-rich plasmas is 87.0 % of the maximum aggregation.exhibit different behavior because Intralipids and Interference Check.A Plus are artificial materials.Interference Studies have been established using the CS-2000i and 5100 System.[2]Platelet Poor Plasma (PPP) samples with high intralipids levels may give an error code [7030.0000.0000 / Ana_PPP_High1]. Accordingly, the associated result may give an error code [7030.0000.0000 / Ana_PPP_High1] and [7050.0000.0000 / Ana_Tube_Position].The cause of the error code is excess turbidity.[3]Refer to ISTH recommendation (Cattaneo, et al, 2013), "hemolyzed samples may affect platelet aggregation and should be discarded."Comparison of Methods:The comparison of the methods was established using a CS-2000i system and confirmed for the CS-5100 system.8. Setup of test:The special position may contain a maximum of 48 (24x2) cuvettes with magnetic stir bar (SBC) to be inserted with the SBC tool.Place the PPP and PRP on the sample rack side by side in the order PPP and then PRP. Thus, the PPPs will be in odd numbered positions while the PRP will be in the even numbered positions. Touch the agonist button to start the measurement on the PRP- Once the PPP and PRP analysis has ended, the instruments calculates the percentage of maximum aggregation (% Max aggregation) automatically.Assay Group Setup-[PPP]▲ [Main Menu] - [Settings] - [Assay Group Settings] - [PPP]► [Basic][1][1] It is recommended to perform the tests in duplicate.►[Re-analysis]NOTE: Please adjust the parameters of the Re-analysis conditions, if necessary.► [Reflex]NOTE: This tab allows defining the Reflex options. Please adjust the parameters of the Reflex conditions, if necessary.►[QC]NOTE: This tab allows defining the QC options. Please adjust the parameters of the QC conditions, if necessary. ►[Test Protocol]Assay Parameter Setup- [PPP]▲ [Main Menu] - [Settings] - [Assay Group Settings] - [PPP] - [Basic] - [PPP]►► [Data Check]►[Evaluation Preset]▼▼ [Evaluation Check Parameter]▼Assay Group Setup-[Epi]▲ [Main Menu] - [Settings] - [Assay Group Settings] - [Epi]► [Basic][1][1] It is recommended to perform the tests in duplicate.►NOTE: Please adjust the parameters of the Re-analysis conditions, if necessary.► [Reflex]NOTE: This tab allows defining the Reflex options. Please adjust the parameters of the Reflex conditions, if necessary. ►[QC]NOTE: This tab allows defining the QC options. Please adjust the parameters of the QC conditions, if necessary.►Assay Parameter Setup- [Epi_min]▲ [Main Menu] - [Settings] - [Assay Group Settings] - [Epi] - [Basic] - [Epi_min]► [Calculation Method]►►[Evaluation Preset]▼ [Evaluation Parameter]▼▼ [Research Parameter]Assay Parameter Setup- [Epi_s]▲ [Main Menu] - [Settings] - [Assay Group Settings] - [Epi] - [Basic] - [Epi_s]►► [Data Check]►[Evaluation Preset]▼▼ [Evaluation Check Parameter]▼Assay Parameter Setup- [Epi_e]▲ [Main Menu] - [Settings] - [Assay Group Settings] - [Epi] - [Basic] - [Epi_e]► [Calculation Method]►►[Evaluation Preset]▼ [Evaluation Parameter]▼▼ [Research Parameter]Formula setting - [Epi%]FormulaManagement ID Assay Parameter Formula18720 Epi% (([Epi_s]-[ Epi_min])/([Epi_s]-[PPP]))*10018721 Epi2% (([Epi2_s]-[ Epi2_min])/([Epi2_s]-[PPP]))*10018722 Epi3% (([Epi3_s]-[ Epi3_min])/([Epi3_s]-[PPP]))*10018723 Epi4% (([Epi4_s]-[ Epi4_min])/([Epi4_s]-[PPP]))*10018724 Epi5% (([Epi5_s]-[ Epi5_min])/([Epi5_s]-[PPP]))*100。
抗-PL7抗体与其他阳性MSAs临床表现的比较

doi:10.3969/j.issn.1000⁃484X.2019.13.016抗⁃PL7抗体与其他阳性MSAs 临床表现的比较刘 轩 铁 宁 王 静 李鸿斌(内蒙古医科大学附属医院风湿科实验室,呼和浩特010050) 中图分类号 R593.26 文献标志码 A 文章编号 1000⁃484X (2019)13⁃1614⁃04作者简介:刘 轩,女,硕士,主管技师,主要从事自身免疫病的病因和自身抗体检测相关性方面的研究,E⁃mail:408404264@㊂通讯作者及指导教师:李鸿斌,男,博士,教授,主任医师,主要从事自身免疫病和自身抗体检测等相关性研究,E⁃mail:lhbwb73@㊂[摘 要] 目的:研究抗苏氨酰⁃tRNA 合成酶(anti⁃PL7)抗体与其他肌炎特异性自身抗体(MSAs)阳性患者临床特征的不同㊂方法:采用线性免疫印迹法(LIA)筛选出MSAs 阳性标本54例,分为anti⁃PL7抗体阳性组和其他MSAs 阳性组㊂比较两组患者临床资料㊂结果:anti⁃PL7组伴随抗Ro 抗体⁃52KD(anti⁃Ro52)抗体阳性的阳性率最高(70%);抗信号识别颗粒(anti⁃SRP)组外周血乳酸脱氢酶(LDH)水平明显高于anti⁃PL7组和甘氨酰⁃tRNA 合成酶(anti⁃EJ)组(P <0.05);抗组氨酰⁃tRNA 合成酶(anti⁃Jo1)组和anti⁃EJ 组外周血谷丙转氨酶(ALT)水平明显高于抗丙氨酰⁃tRNA 合成酶(anti⁃PL12)组(P <0.05);anti⁃PL7组最常见的临床表现为肺间质病变(70%),其次是发热㊁四肢肌痛肌无力㊁心脏受累,发生率均为60%;anti⁃PL12组与anti⁃EJ 组最常见的临床表现为皮疹(75%和80%);anti⁃Jo1组最常见的临床表现为关节炎(77%)㊂结论:不同的MSAs 与不同的临床表现相关㊂[关键词] anti⁃PL7抗体;MSAs;临床表现;诊断Comparison of clinical manifestations of anti⁃PL7antibody and other positive MSAsLIU Xuan ,TIE Ning ,WANG Jing ,LI Hong⁃Bin .Laboratory of Rheumatology and Immunology ,Affiliated Inner Mongolia Medical University ,Huhhot 010050,China[Abstract ] Objective :To investigate the difference of clinical characteristics between anti⁃threonyl⁃tRNA synthase(anti⁃PL7)antibody and other myositis specific autoantibodies (MSAs)positive patients.Methods :54cases of MSAs positive specimens were screened by(LIA)with linear Western blot,then they were divided into anti⁃PL7antibody positive group and other MSAs positive group.The clinical data of the two groups were compared.Results :The positive rate of anti⁃PL7group accompanied by anti⁃Ro antibody⁃52KD (anti⁃Ro52)antibody positive was the highest (70%).The level of lactate dehydrogenase (LDH)in peripheral bloodof anti⁃signal recognition granule(anti⁃SRP)group was significantly higher than that of anti⁃PL7group and glycosyl⁃tRNA synthase (anti⁃EJ)group (P <0.05).The level of alanine transaminase (ALT)in peripheral blood of anti⁃histidyl tRNA synthase (anti⁃Jo1)group and anti⁃EJ group was significantly higher than that of anti⁃alanyl⁃tRNA synthase (anti⁃PL12)group (P <0.05).The most common clinical manifestation in anti⁃PL7group was pulmonary interstitial disease(70%),followed by fever,myasthenia of extremities and cardiac involvement (60%).The most common clinical manifestations in anti⁃PL12group and anti⁃EJ group were skin rash(75%,80%)and in anti⁃Jo1group was arthritis (77%).Conclusion :Different MSAs autoantibodies are associated with different clinical manifestations.[Key words ] anti⁃PL7antibody;MSAs;Clinical manifestation;Diagnosis 在肌炎患者中发现的自身抗体被称为肌炎特异性自身抗体(Myositis specific autoantibodies,MSAs)或肌炎相关自身抗体(Myositis associated autoantibodies,MAAs)㊂每个MSAs 都与一种独特的疾病或表型有关[1],明确每一种MSAs 对应的疾病㊁临床表现和预后对日后的诊断和临床分型有重要作用㊂MSAs 包括抗氨基酰⁃tRNA 合成酶(Aminoacyl⁃transfer RNA synthetase,ARS )抗体㊁抗Mi⁃2(Helicase /histone deacetylase protein complex)抗体和抗信号识别颗粒(Signal recognition particle,SRP)抗体,其中,ARS 抗体包括抗组氨酰⁃tRNA 合成酶(Anti⁃histidyl⁃tRNA synthetase,Jo⁃1)㊁抗甘氨酰⁃tRNA 合成酶(Anti⁃glycyl⁃tRNA synthetase,EJ)㊁抗丙氨酰⁃tRNA 合成酶(Anti⁃alanyl⁃tRNA synthetase,PL12)和抗苏氨酰⁃tRNA合成酶(Anti⁃threonyl⁃tRNA synthetase,PL7)等抗体[2]㊂anti⁃PL7和anti⁃PL12患者有可能出现间质性肺疾病(Interstitial lung disease,ILD)和胃肠道并发症,而抗Ro抗体⁃52KD (抗Ro52)与更高的癌症风险㊁更严重的肌肉和关节受累有关㊂抗合成酶综合征(Anti⁃synthetase syndrome, ASS)是一种复杂的自身免疫性疾病,以出现一种抗ARS的自身抗体为特征㊂这种临床综合征,包括肌炎㊁ILD㊁多发性关节炎㊁技工手和/或发热等多种表现[3]㊂本研究我们比较了anti⁃PL7阳性抗体与其他阳性MSAs患者在病程㊁临床特征以及血清肌酶的不同,以期为临床诊断特发性炎性肌病的分型提供依据㊂1 资料与方法1.1 资料 2016年12月至2018年5月内蒙古医科大学附属医院住院申请抗肌炎自身抗体检测的标本235份,所有标本均采用免疫印迹法测定血清中抗Jo⁃1㊁EJ㊁PL⁃7㊁PL⁃12㊁Mi⁃2㊁SRP㊁Ro52抗体(试剂盒购自德国欧蒙医学实验诊断股份公司)㊂筛选出MSAs 阳性的自身免疫病患者55例,其中抗Mi⁃2抗体阳性患者1例,因例数太少,未入组,入组54例MSAs阳性患者(汉族47例,蒙古族7例),分为anti⁃PL7组,其中男性3例;年龄52~63岁,平均年龄(57±5.5)岁;女性7例,年龄46~67岁,平均年龄(56.3±7.7)岁㊂其他MSAs阳性组,分为anti⁃PL12组㊁anti⁃Jo1组㊁anti⁃EJ组和anti⁃SRP组,详细信息见表1㊂1.2 方法1.2.1 采用线性免疫印迹法检测肌炎自身抗体 检验各组患者血清中的抗Jo⁃1㊁EJ㊁PL7㊁PL12㊁Mi⁃2㊁SRP㊁Ro52抗体㊂EUROBlotMasterⅡ免疫印迹仪㊁抗肌炎自身抗体谱(IgG)检测试剂盒均购自德国欧蒙医学实验诊断股份公司㊂操作步骤均按说明书进行,结果用EUROLineScan软件进行判断㊂结果判断:系统自动判读出各条带的显色灰度值,灰度值≤5为免疫印迹法(LIA)阴性(-);6~10为LIA临界(±);11~25为LIA弱阳性(+);26~50为LIA阳性(2+);≥51为LIA强阳性(3+)㊂同一膜条上有多个靶抗原阳性,则以灰度值高的为最终阳性强度结果㊂1.2.2 收集患者的相关资料 54例MSAs患者流行病学资料(性别㊁发病年龄㊁病程㊁民族㊁死亡率)㊁临床资料(咳嗽㊁吞咽困难㊁四肢近端肌无力/肌痛㊁关节痛或关节炎㊁皮疹㊁技工手㊁雷诺现象㊁发热㊁胃食管反流㊁心脏受累㊁ILD)和实验室资料[血清肌酸激酶(Creatine kinase,CK)㊁乳酸脱氢酶(Lactate de⁃hydrogenase,LDH)㊁天冬氨酸转氨酶(Aspartate ami⁃notransferase,AST)㊁谷丙转氨酶(Alanine transaminase,ALT)和α⁃羟基丁酸脱氢酶(Alpha hydroxybutyric acid dehydrogenase,HBDH)]㊂1.3 统计学处理 应用SPSS16.0软件进行统计分析,计数资料采用率(%)表示㊂计数资料组间比较采用χ2检验,样本量<5时,用Fisher的精确χ2检验㊂计量资料采用x±s表示,组间比较用t检验,以P<0.05为差异有统计学意义㊂2 结果2.1 anti⁃PL7组与其他MSAs阳性组患者的一般特征 anti⁃PL7组与anti⁃EJ组发病年龄差异有统计学意义(P<0.05),anti⁃PL7组同时伴有anti⁃Ro52抗体阳性的阳性率最高(70%),见表1㊂2.2 anti⁃PL7组与其他MSAs阳性组外周血肌酶水平比较 anti⁃SRP组外周血LDH水平明显高于anti⁃PL7组和anti⁃EJ组(P<0.05),anti⁃Jo1组和anti⁃EJ组外周血ALT水平明显高于anti⁃PL12组,差异有统计学意义,P<0.05,见表2㊂表1 anti⁃PL7组与其他MSAs阳性组患者的一般特征[n(%)]Tab.1 General characteristics of anti⁃PL7and other MSAs positive patients[n(%)]Index anti⁃PL7(n=10)anti⁃PL12(n=8)anti⁃Jo1(n=26)anti⁃EJ(n=5)anti⁃SRP(n=6)Total(n=54) Age of onset,(x±s)years54.8±6.448.8±20.648.0±11.739.6±13.51)52.2±12.349.0±13.2 Sex,female[n(%)]7(70%)5(62.5%)20(76.9%)5(100%)6(100%)42(77%) Course after disease(month)(x±s)65±8230±3939±4742±3736±3443±52 Anti⁃Ro52[n(%)]7(70%)5(62.5%)17(65.3%)2(40%)3(50%)34(63%) Nation[n(%)]Mongolian nationality2(20%)1(12.5%)2(0.07%)1(20%)1(16.7%)7(13%) The Han nationality8(80%)7(87.5%)24(92.3%)4(80%)5(83.3%)47(87%) Note:Compared with anti⁃PL7group,1)P<0.05.表2 MSAs阳性患者血清肌酶的比较(U/L)Tab.2 Comparison of serum myoenzymes in patients with MSAs positive(U/L)Serum myoenzymeslevels(normal range)anti⁃PL7anti⁃PL12anti⁃Jo1anti⁃EJ anti⁃SRP CK(0-250)2543±12972541±23502456.2±16671467.5±1693 3863±2632.3 LDH(110-240) 553±269.61)613±261.7537.9±310.2 289.5±108.61)848±273.2 AST(13-35)105.7±59.257.8±51.9111.2±106.7 86.5±74.6212.5±163.3 ALT(7-40)71.4±33.438±32.581.6±292) 89.5±65.52)79.4±56.4 HBDH(72-182) 459±235.3548.5±544 510.7±364.4 270±105.5641±382.9 Note:Compared with anti⁃SRP group,1)P<0.05;Compared with anti⁃PL12group,2)P<0.05.表3 anti⁃PL7组与其他MSAs阳性组患者的症状和体征[n(%)]Tab.3 Symptoms and signs of anti⁃PL7and other MSAs positive patients[n(%)]anti⁃PL7(n=10)anti⁃PL12(n=8)anti⁃Jo1(n=26)anti⁃EJ(n=5)anti⁃SRP(n=6)Total(n=54) Cough5(50%)2(25%)12(46%)1(17%)20(37%)Dysphagia3(30%)2(8%)5(9%) Muscle weakness6(60%)1)4(50%)12(46%)22(41%)Arthritis4(40%)4(50%)20(77%)2(40%)2(33%)34(63%)Skin rashes4(40%)6(75%)3)4(15%)4(80%)3)2(33%)20(37%) Mechanic′s hands2(25%)2(4%) Raynaud′s phenomenon3(30%)4(50%)2(8%)9(17%) Fever6(60%)5(63%)16(62%)3(60%)2(33%)31(57%) Gastro⁃oesophageal reflux disease2(20%)2(4%) Heart involvement6(60%)5(63%)14(54%)25(46%) ILD7(70%)2)5(63%)17(65%)2)2(33%)31(57%) Note:Compared with anti⁃EJ group and anti⁃SRP group,1)P<0.05;compared with anti⁃EJ group,2)P<0.01;compared with anti⁃Jo1group,3)P<0.05.2.3 anti⁃PL7组与其他MSAs阳性组患者临床表现的相关性 anti⁃PL7阳性组最常见的临床表现为肺间质病变(70%),其次是发热㊁四肢肌痛肌无力㊁心脏受累,发生率均为60%,半数以上患者均以咳嗽为首发症状,关节炎㊁皮疹发生率为40%;anti⁃PL12阳性组最常见的临床表现为皮疹(75%),其次是发热㊁ILD㊁心脏受累,发生率均为63%;anti⁃Jo1阳性组最常见的临床表现为关节炎(77%),其次是发热㊁ILD;anti⁃EJ组最常见的临床表现为皮疹(80%),其次为发热和关节炎㊂anti⁃PL7组肌无力/肌痛与anti⁃EJ㊁anti⁃SRP组比较,差异有统计学意义(P<0.05);anti⁃PL7组和anti⁃Jo1组ILD与anti⁃EJ 组比较,差异有统计学意义(P<0.05);anti⁃PL12组和anti⁃EJ组皮疹与anti⁃Jo1组比较,差异有统计学意义(P<0.05),见表3㊂3 讨论本研究发现,MSAs阳性的发病人群存在民族的差异,少数民族中蒙古族也有一定的阳性率㊂不同的MSAs抗体与不同的临床表现相关㊂anti⁃PL7组同时伴随anti⁃Ro52抗体阳性的阳性率最高(70%),其次是anti⁃Jo1组(65.3%)和anti⁃PL12组(62.5%),而国外文献报道[3,4]:anti⁃Ro52在anti⁃Jo1患者中尤为普遍,anti⁃Ro52与anti⁃Jo1的相关性(74%)高于anti⁃PL12(43%)或anti⁃PL7(44%)的相关性,与本实验结果相同㊂anti⁃Ro52抗体与技工手的早期形成㊁皮肌炎特征性的皮肤改变和关节炎相关[3]㊂血清肌酶主要与肌肉损伤相关,1975年Bohan等[5]制定的多发性肌炎/皮肌炎的诊断标准,其中一项为血清肌酶升高:如CK㊁ALT㊁AST和LDH 等升高,其中以CK最敏感,其升高程度与不同的特发性炎性肌病有关[6]㊂SRP是一种与蛋白质合成相关的核蛋白,与免疫性坏死性肌病相关[7],有研究表明anti⁃SRP抗体与更严重疾病㊁更高水平的肌肉坏死和肌酸激酶水平有关[8⁃10]㊂我们的研究发现,anti⁃SRP组外周血LDH水平明显高于anti⁃PL7组和anti⁃EJ组(P<0.05),表明anti⁃SRP组患者肌肉损伤更为严重㊂anti⁃Jo1组和anti⁃EJ组外周血ALT水平明显高于anti⁃PL12组,差异有统计学意义(P<0.05),提示anti⁃Jo1和anti⁃EJ可能与较严重的肌肉受累有关㊂半数以上的anti⁃PL7阳性的患者首发症状为咳嗽,该抗体阳性的30%患者还伴有吞咽困难㊂anti⁃PL7㊁anti⁃PL12㊁anti⁃Jo1抗体阳性的患者多伴有肌无力/肌痛㊂关节炎在anti⁃Jo1组患者中最常见(77%),此结论与国外文献报道一致[11,12],即肌肉疾病在抗Jo1㊁抗PL7或抗EJ抗体的患者中很常见,关节炎在抗Jo1抗体的患者中更常见㊂有文献报道,anti⁃PL12和anti⁃PL7患者出现胃食管反流性疾病的发生率均高于anti⁃Jo1患者[3]㊂在我们的研究中,筛选出的54例MSAs阳性的患者中,仅有2例有胃食管返流,均发生在anti⁃PL7组,anti⁃PL12组未见胃食管返流性疾病的患者,这可能与样本基数不够大有关㊂本研究中,anti⁃PL7组ILD发生率最高(70%),其次为anti⁃Jo1组(65%)和anti⁃PL12组(63%),这与国外文献报道相似,anti⁃PL7和anti⁃PL12以更严重的肺部受累为特点,而anti⁃Jo1则与较严重的肌肉受累有关相一致[13,14]㊂Labirua⁃Iturburu等[15]对18例欧洲anti⁃PL7阳性患者的研究发现,该抗体还可能和心包炎有关,本研究中心脏受累的各组患者阳性率分别为anti⁃PL7组(60%)㊁anti⁃PL12组(63%)和anti⁃Jo1组(54%)㊂本研究样本量相对较小,且患者均来自内蒙古地区,阳性患者中有少部分蒙古族,不能代表全部的蒙古族和汉族患者,因此应对结果进行谨慎解释㊂综上所述,anti⁃PL7抗体阳性的患者与其他MSAs患者相比,半数以上以咳嗽为首发症状,70%的患者会发展为ILD,并伴随心脏受累㊁胃食道症状;anti⁃Jo1阳性与患者关节炎和肌肉㊁心脏受累㊁ILD相关;anti⁃SRP与肌肉严重受损㊁LDH明显增高有关㊂不同的MSAs与不同的临床表现相关,为临床筛查㊁诊断疾病和指导临床分型提供重要依据㊂参考文献:[1] Neil J McHugh,Sarah L Tansley.Autoantibodies in myositis[J].Nat Rev Rheumatol,2018,14:290⁃302.[2] Hozumi,Fujisawa T,Nakashima K,et prehensiveassessment of myositis⁃specific autoantibodies in polymyositis/der⁃matomyositis⁃associated interstitial lung disease[J].Respir Med, 2016,121:91⁃99.[3] Iago Pinal⁃Fernandez,Maria Casal⁃Dominguez,Julio A Huapaya,etal.A longitudinal cohort study of the anti⁃synthetase in black patients and patients with anti⁃PL7and anti⁃PL12autoantibodies [J].Rheumatology,2017,56:999⁃1007.[4] Marie I,Hatron PY,Dominique S,et al.Short⁃term and long⁃termoutcome of anti⁃Jo1⁃positive patients with anti⁃Ro52antibody[J].Semin Arthritis Rheum,2012,41:890⁃899.[5] Bohan A,Peter JB.Polymyositis and dermatomyositis(first of twoparts)[J].N Engl J Med,1975,292(7):344⁃347. [6] Lundberg IE,Miller FW,Tjärnlund A,et al.Diagnosis andclassification of idiopathic inflammatory myopathies[J].Intern Med,2016,280(1):39⁃51.[7] Dalakas MC.Myositis:Are autoantibodies pathogenic in necrotizingmyopathy?[J].Nat Rev Rheumatol,2018,14(5):251⁃252.[8] Watanabe Y,Uruha A,Suzuki S,et al.Clinical features andprognosis in anti⁃SRP and anti⁃HMGCR necrotizing myopathy[J].Neurol Neurosurg Psychiatry,2016,87(10):1038⁃1044. [9] Basharat P,Christopher⁃Stine L.Immune⁃mediated necrotizingmyopathy:update on diagnosis and management[J].Curr Rheumatol Rep,2015,17(12):72⁃84.[10] Wang L,Liu L,Hao H,et al.Myopathy with anti⁃signalrecognition particle antibodies:clinical and histopathologicalfeatures in Chinese patients[J].Neuromuscul Disord,2014,24(4):335⁃341.[11] Hamaguchi Y,Fujimoto M,Matsushita T,et mon anddistinct clinical features in adult patients with anti⁃aminoacyl⁃tRNA synthetase antibodies:heterogeneity within the syndrome[J].PLoS One,2013,8(4):e60442.[12] Klein M,Mann H,Ple sˇtilováL,et al.Arthritis in idiopathicinflammatory myopathy:clinical features and autoantibodyassociations[J].J Rheumatol,2014,41(6):1133⁃1139. [13] Hervier B,Devilliers H,Stanciu R,et al.Hierarchical cluster andsurvival analyses of antisynthetase syndrome:phenotype andoutcome are correlated with anti⁃tRNA synthetase antibodyspecificity[J].Autoimmun Rev,2012,12:210⁃217. [14] Marie I,Josse S,Decaux O,et parison of long⁃termoutcome between anti⁃Jo1⁃and anti⁃PL7/PL12positive patientswith antisynthetase syndrome[J].Autoimmun Rev,2012,11:739⁃745.[15] Labirua⁃Iturburu A,Selva⁃O′Callaghan A,Vincze M,et al.Anti⁃PL⁃7(anti⁃threonyl⁃tRNA synthetase)antisynthetase syndrome:clinical manifestations in a series of patients from a Europeanmulticenter study(EUMYONET)and review of the literature[J].Medicine(Baltimore),2012,91:206⁃211.[收稿2018⁃07⁃27 修回2018⁃09⁃12](编辑 倪 鹏)。
hss-p-5.75.09 - hyaluronic acid derivatives说明书

5.75.09Section:Prescription DrugsEffective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject:Hyaluronic Acid DerivativesPage:1 of 7Last Review Date:March 13, 2020Hyaluronic Acid DerivativesDescriptionDurolane, Euflexxa, GelSyn-3, GenVisc 850, Hyalgan , SodiumHyaluronate, Supartz , Synojoynt*, Triluron, TriVisc, Visco-3 (sodium hyaluronate)Gel-ONE , Hymovis, Monovisc, Orthovisc (hyaluronan)Synvisc, Synvisc-One (hylan G-F 20)Bolded medications are the preferred products*These medications are included in this policy but are not available in the market as of yetBackgroundOsteoarthritis of the knee is a disease in which the elastoviscous property of the synovial fluid in the knee joint becomes diminished, resulting in less protection and shock absorption. Durolane, Euflexxa, Gel-One, GelSyn-3, GenVisc 850, Hyalgan, Hymovis, Monovisc, Orthovisc, Sodium Hyaluronate, Synvisc, Synvisc-One, Supartz, Synojoynt, Triluron, TriVisc, Visco-3 are hyaluronan derivatives that are injected into the knee joints to increase the elastoviscous properties of arthritic joint fluid and slow its outflow from the joint . The goal of therapy is torestore the viscoelasticity in the affected joints, thereby decreasing pain, improving mobility, and restoring the natural protective functions (1).The American College of Rheumatology (ACR) updated its guidelines for the treatment of osteoarthritis (OA) of the knee in 2012. In mild symptomatic OA, treatment may be limited toFederal Employee Program® 1310 G Street, N.W.Washington, D.C. 20005 202.942.1000Fax 202.942.1125Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 2 of 7patient education, physical and occupational therapy and other non-pharmacologic modalities. Nonpharmacologic modalities strongly recommended for the management of knee OA were aerobic, aquatic, and/or resistance exercises as well as weight loss for overweight patients. Nonpharmacologic modalities conditionally recommended for knee OA included medial wedge insoles for valgus knee OA, subtalar strapped lateral insoles for varus knee OA, medially directed patellar taping, manual therapy, walking aids, thermal agents, tai chi, self-management programs, and psychosocial interventions. Pharmacologic modalities conditionally recommended for the initial management of patients with knee OA included acetaminophen, oral and topical NSAIDs, tramadol, and intraarticular corticosteroid injections (1).Regulatory StatusFDA-approved indication: Hyaluronic acid derivatives are indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy, simple analgesics (e.g., acetaminophen), NSAIDs, tramadol, or intra-articular steroid injections (2-18).The hyaluronic acid derivatives are contraindicated for use in patients with known hypersensitivity to hyaluronan (sodium hyaluronate) preparations. Orthovisc lists hypersensitivity to gram positive bacterial proteins as an additional contraindication (4). Caution should be exercised when Gel-One, Hyalgan, Visco-3, Synvisc, Synvisc-One, Supartz, and Triluron are administered to patients with allergies to avian proteins, feathers, and egg products (3-8, 18).Hyaluronic acid derivatives are contraindicated to treat patients with knee joint infections, infections or skin diseases in the area of the injection site (2-17).A treatment cycle for most of the hyaluronan derivatives typically involves multiple weekly injections. Euflexxa, GelSyn-3, Sodium Hyaluronate, Synvisc, Triluron, TriVisc, and Visco-3 are given for a total of three injections. Orthovisc is given for three or four injections. GenVisc 850, Supartz and Hyalgan are given for a total of three or five injections. Durolane, Gel-One, Synojoynt, and Synvisc-One differ from the other hyaluronan derivatives in that it only requires one injection. Repeat courses of hyaluronan derivatives may be administered if symptoms return (2-18).Upon the basis of high quality supporting evidence, the American Academy of Orthopedic Surgeons cannot recommend using hyaluronic acid for patients with symptomatic osteoarthritis of the knee (19).Related policiesSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 3 of 7Hyaluronate PowderPolicyThis policy statement applies to clinical review performed for pre-service (Prior Approval, Precertification, Advanced Benefit Determination, etc.) and/or post-service claims.Hyaluronic acid derivatives may be considered medically necessary for the treatment of osteoarthritis of the knee and if the conditions indicated below are met.Hyaluronic acid derivatives may be considered investigational for all other indications.Prior-Approval RequirementsAge18 years or older (22 or older for Synvisc, Synvisc-One, and TriVisc)DiagnosisPatient must have the following:Osteoarthritis of the kneeAND ALL of the following:1. Inadequate response to TWO or more of the following conservative non-pharmacologic therapy:a. Cardiovascular (aerobic) activity, such as: walking, biking, stationarybike, aquatic exerciseb. Resistance exercisec. Weight reduction (for persons who are overweight)d. Participation in self-management programse. Wear of medially directed patellar tapingf. Wear of wedged insolesg. Thermal agentsh. Walking aidsi. Physical therapyj. Occupational therapy2. Inadequate response, intolerance, or contraindication to TWO or more of thefollowing:Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 4 of 7a. Acetaminophenb. Oral NSAIDsc. Topical NSAIDs3. Inadequate response, intolerance, or contraindication to intra-articularsteroid injections in which efficacy lasted less than 8 weeks4. Radiologic confirmation of Kellgren-Lawrence Scale score of grade 2 orgreater5. NO dual therapy with another hyaluronic acid injectable6. Non-preferred medications only: Patient MUST have tried at least TWO ofthe preferred products unless the patient has a valid medical exception (e.g.inadequate treatment response, intolerance, contraindication)Prior – Approval Renewal RequirementsAge18 years or older (22 or older for Synvisc, Synvisc-One, and TriVisc)DiagnosisPatient must have the following:Osteoarthritis of the kneeAND ALL of the following:1. Documentation of improvement in pain with previous course of treatment2. At least 12 months has elapsed since last injection of the prior treatmentcycle3. Documentation of reduction of dosing of NSAIDs or other analgesicsduring the 12 month period following the last injection of the prior treatmentcycle4. NO dual therapy with another hyaluronic acid injectable5. Non-preferred medications only: Patient MUST have tried at least TWOof the preferred products unless the patient has a valid medical exception(e.g. inadequate treatment response, intolerance, contraindication) Policy GuidelinesPre - PA AllowanceNoneSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 5 of 7Prior - Approval LimitsDuration12 monthsQuantity One course of therapy for each kneePrior – Approval Renewal LimitsSame as aboveRationaleSummaryOsteoarthritis of the knee is a disease in which the elastoviscous property of the synovial fluid in the knee joint becomes diminished, resulting in less protection and shock absorption. Durolane, Euflexxa, Gel-One, GelSyn-3, GenVisc 850, Hyalgan, Hymovis, Monovisc, Orthovisc, Sodium Hyaluronate, Synvisc, Synvisc-One, Supartz, Synojoynt, Triluron, TriVisc, Visco-3 are hyaluronan derivatives that are injected into the knee joints to increase the elastoviscous properties of arthritic joint fluid and slow its outflow from the joint. The goal of therapy is to restore the viscoelasticity in the affected joints, thereby decreasing pain, improving mobility, and restoring the natural protective functions (1-18).Prior approval is required to ensure the safe, clinically appropriate and cost effective use of the hyaluronic acid derivatives while maintaining optimal therapeutic outcomes.References1. American College of Rheumatology, Subcommittee on Osteoarthritis Guidelines.Recommendations for the medical management of osteoarthritis of the hip and knee:2012 update. Arthritis Care & Research 2012; 64(4):465-474.2. Euflexxa [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc.; July 2016.3. Hyalgan [package insert]. Parsippany, NJ: Fidia Pharma USA Inc.; May 2014.4. Orthovisc [package insert]. Woburn, MA: Anika Therapeutics; June 2005.5. Supartz [package insert]. Durham, NC: Bioventus LLC; April 2015.6. Synvisc [package insert]. Ridgefield, NJ: Genzyme Corp.; December 2014.7. Synvisc-One [package insert]. Ridgefield, NJ: Genzyme Corp.; September 2014;8. Gel-One [package insert]. Warsaw, IN: Zimmer Inc.; May 2011.9. Monovisc [package insert]. Bedford, MA: Anika Therapeutics; December 2013.10. Hymovis [package insert]. Parsippany, NJ: O Fidia Pharma USA Inc.; October 2015.Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 6 of 711. GenVisc 850 [package insert]. Doylestown, PA: OrthogenRx Inc.; January 2015.12. GelSyn-3 [package insert]. Durham, NC: Bioventus LLC; January 2016.13. Durolane [package insert]. Durham, NC: Bioventus LLC; November 2017.14. Visco-3 [package insert]. Warsaw, IN: Zimmer, Inc.; May 2017.15. Sodium Hyaluronate [package insert]. North Wales, PA: Teva Pharmaceuticals USA,Inc.; March 2019.16. Synojoynt [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc.;September 2019.17. TriVisc [package insert]. Doylestown, PA: OrthogenRx, Inc.; September 2018.18. Triluron [package insert]. Florham Park, NJ: Fidia Pharma USA Inc.; March 2019.19. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee.Evidence-based guideline 2nd edition. May 2013.Policy HistoryDate Action ReasonJanuary 2012 Added minimum age - only approved for adultsDecember 2012 Annual editorial review and reference updateDecember 2013 Annual editorial review and reference updateMarch 2014 Annual editorial reviewAddition of examples of non-pharmacological agents and agents of priorfailure medications.April 2014 Line-Addition of Monovisc to PAMarch 2015 Annual criteria review and reference updateMarch 2016 Change from one tried and failed to two tried and failed non-pharmacologic and pharmacologic therapies and addition of the tried and failed of intra-articular steroid and radiologic confirmation of Kellgren-Lawrence Scalescore of grade 2 or greaterAddition of HymovisPolicy # change from 5.11.04 to 5.75.09May 2016 Addition of GelSyn-3 and GenVisc 850December 2016 Annual editorial review and reference updateAdded: no dual therapy with another hyaluronic acid injectableMarch 2017 Bolded preferred products in the title pageJuly 2017 GelSyn-3 has been changed to preferredSeptember 2017 Annual reviewDecember 2017 Addition of Durolane and Visco-3March 2018 Annual editorial reviewRemoval of Tramadol from the T/F listSeptember 2019 Annual review and reference update. Addition of Sodium Hyaluronate,Synojoynt, and TriViscSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 7 of 7December 2019 Annual review. Addition of requirement to trial preferred products January 2020 Addition of TriluronMarch 2020 Annual reviewKeywordsThis policy was approved by the FEP® Pharmacy and Medical Policy Committee on March 13, 2020 and is effective on April 1, 2020.。
mce铁死亡脂质过氧化实验步骤

mce铁死亡脂质过氧化实验步骤MCE铁死亡脂质过氧化实验步骤:MCE铁死亡脂质过氧化实验是一种用于研究细胞脂质过氧化损伤的常用实验方法。
以下是MCE铁死亡脂质过氧化实验的基本步骤:1. 细胞处理:-准备细胞:选择需要研究的细胞系,将其培养至适当的生长状态。
-细胞处理:将细胞分为试验组和对照组。
试验组接受处理,包括暴露于一定浓度的铁离子(Fe2+)和添加氧化剂,如过氧化氢(H2O2)。
-对照组设定:设置一个对照组,不接受任何处理,以用于对比和分析实验结果。
2. 实验处理:-铁离子处理:将试验组的培养基中添加一定浓度的铁离子(Fe2+),使其与细胞产生接触。
-氧化剂处理:在铁离子处理后,添加所需浓度的氧化剂,如过氧化氢(H2O2)。
-控制组处理:对照组只添加培养基,不加入铁离子和氧化剂。
3. 孵育条件:-孵育时间:将细胞培养在适宜的孵育温度下,根据实验要求确定孵育时间,通常为数小时至数天。
-适宜条件:提供适当的气体组成、湿度和培养基成分来维持细胞的正常生长。
4. 检测脂质过氧化:- TBARS测定:采用thiobarbituric acid reactive substances (TBARS)法检测脂质过氧化产物。
将细胞产生的上清液和合适的试剂混合反应,形成染色物质,并用光谱仪或分光光度计测定其吸光度,从而定量脂质过氧化水平。
-细胞活性:使用细胞存活检测试剂盒如MTT(3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide)或CCK-8 (Cell Counting Kit-8)来测定细胞存活率,以评估细胞的受损程度。
5. 数据分析:-实验重复:进行足够的实验重复,以获得可靠的结果。
-数据统计分析:对实验结果进行统计学分析,如平均值和标准差计算,t检验或方差分析等。
MCE铁死亡脂质过氧化实验通过模拟细胞内的脂质过氧化反应,提供一种可靠的方法来研究细胞脂质损伤的机制和影响因素。
mce铁死亡脂质过氧化实验步骤

mce铁死亡脂质过氧化实验步骤引言:mce(Malondialdehyde and Cholesterol Ester)铁死亡脂质过氧化实验是一种常用的体外实验方法,用于评估细胞膜脂质过氧化程度。
本文将详细介绍该实验的步骤及操作流程。
一、实验前准备1. 准备所需试剂和设备,包括mce试剂盒、离心机、超声波清洗器等。
2. 温度控制:将实验室温度控制在37摄氏度。
二、样品准备1. 收集需要进行实验的样品,如细胞培养物或动物组织。
2. 将样品放入离心管中,并加入PBS缓冲液悬浮均匀。
3. 离心样品,去掉上清液。
三、实验操作1. 向样品中加入mce试剂,按比例加入适量的试剂。
2. 轻轻振摇样品,使试剂充分混合。
3. 将样品置于37摄氏度的恒温水浴中孵育一定时间,一般为30分钟。
4. 取出样品,离心使沉淀物沉积在离心管底部。
5. 将上清液转移到新的离心管中。
6. 加入适量的氯仿,并轻轻振摇混合。
7. 离心样品,使沉淀物和氯仿分离。
8. 取上清液转移到新的离心管中。
9. 加入适量的PBS缓冲液,并轻轻振摇混合。
10. 离心样品,使沉淀物和PBS缓冲液分离。
11. 取上清液转移到新的离心管中。
四、测量结果1. 取出上清液,加入相应试剂。
2. 按照试剂盒说明书的要求,进行相应的检测,如比色法、荧光法等。
3. 根据试剂盒提供的标准曲线,计算样品中的mce含量。
五、数据分析1. 对测量结果进行统计学分析,计算平均值和标准差。
2. 根据实验设计和目的,对数据进行图表展示和解释。
3. 进一步分析结果,探讨实验的意义和可能的影响因素。
六、实验注意事项1. 实验操作过程中需严格遵守无菌操作规范,避免污染。
2. 每一步操作都要准确无误,避免操作失误对实验结果的影响。
3. 在实验过程中,注意安全操作,避免对人身及环境造成危害。
4. 仪器设备的使用和维护要规范,确保实验的准确性和可重复性。
结论:mce铁死亡脂质过氧化实验是一种简单有效的体外实验方法,用于评估细胞膜脂质过氧化程度。
Immunoway YS0016-YS0017 荧光 phalloidin 用户手册说明书

******************* 400-8787-807Fuorescent Phalloidin User ManualI. INTRODUCTIONPhalloidin is a bicyclic peptide that belongs to a family of toxins isolated from the deadly Amanita phalloides mushroom. Fluorescent phalloidins bind F-actin with nanomolar affinity and are water soluble, thus providing convenient probes for labeling, identifying, and quantifying F-actin in cryopreserved tissue sections, cell cultures, or cell-free experiments. Phalloidin contains an unusual thioether bridge between cysteine and tryptophan residues that forms an inner ring structure. At elevated pH, this thioether is cleaved and the toxin loses its affinity for actin.Fluorescently labeled phalloidins stain F-actin at nanomolar concentrations. Labeled phalloidins have similar affinity for both large and small filaments, binding in a stoichiometric ratio of about one phalloidin molecule per actin subunit in muscle and non-muscle cells from various species of plants and animals. Different from antibodies, the binding affinity of phalloidin does not change significantly with actin among different species. Non-specific staining is negligible, and the contrast between stained and unstained areas is extremely large. Phalloidin shifts the monomer/polymer equilibrium toward the polymer, lowering the critical concentration for polymerization up to 30-fold. Phallotoxins also stabilize F-actin, inhibiting depolymerization by cytochalasin, potassium iodide and elevated temperatures. Because the phalloidin conjugates are small, with an approximate diameter of 12-15Å and molecular weight of <2000 Daltons, a variety of actin-binding proteins including myosin, tropomyosin and troponin can still bind to actin after treatment with phalloidin. Even more significantly, phalloidin-labeled actin filaments remain functional; labeled glycerinated muscle fibers still contract, and labeled actin filaments still move on solid-phase myosin substrates. Fluorescent phalloidin can also be used to quantify the amount of F-actin in cells.II. MATERIALS REQUIRED BUT NOT PROVIDED1. Methanol2. PBS (1X)3. 4% Formaldehyde4. 0.5% Triton X-1001 / 4For research use only. Not for diagnostic or therapeutic procedures.III. PROCEDURAL GUIDELINESHandle fluorescent, biotinylated, and unlabeled phalloidins with care although the amount of toxin present in a vial could be lethal only to a mosquito (LD50 of phalloidin = 2 mg/kg).IV. WORKING SOLUTION PREPARATIONStock Solution: Dissolve the lyophilized powder in 300 μl methanol for the 300 Assays size or 50 μl methanol for the 50 Assays size.Dilute 1 μl fluorescent phalloidin stock solution in 200 μl PBS before use.(For fluorescent phalloidins, the recommended dilution ratio is 1:40 - 1:200, one time experiment is equivalent to 1-5 μl stock solution in a total staining volume of 200 μl.) Note: The dilution ratio can be adjusted appropriately according to the experimental effect.V. ASSAY PROCEDUREStaining fixed cellsThe following protocol describes the staining procedure for adherent cells grown on glass coverslips or 8-well chamber slides. Phalloidins also can be used to stain fixed frozen or paraffin tissue sections, as well as yeast and fungi.1. Wash cells 3 times with PBS.2. Fix cells on ice with 4% formaldehyde solution in PBS for 15 minutes.Note: Methanol can disrupt actin during the fixation process. Therefore, it is best to avoid any methanol containing fixatives or other solvent-based fixatives. The preferred fixative is methanol-free formaldehyde.3. Wash cells 3 times with PBS.4. Permeabilize cells with 0.5% Triton X-100 in PBS at room temperature for 10 minutes.5. Wash cells 3 times with PBS.6. Dilute 1-5 μl fluorescent phalloidin stock solution in 200 μl PBS for each cover slip or chamber to be stained. Place the staining solution on the coverslip for 20 minutes at room temperature.Note:Staining volume can be adjusted according to the sample. To avoid evaporation, keep the coverslips inside a covered container and the chamber slides covered during the incubation.7. Wash 2-3 times with PBS.8. Image using fluorescence microscopy. Fluorescent phalloidins are photostable enough to image in PBS, but for best results we recommend mounting with antifade mounting medium.Staining living cellsFluorescently-labeled phalloidin is not cell-permeant and has therefore has not been used extensively with living cells. However, living cells have been labeled by pinocytosis orunknown mechanism. In general, a larger amount of stain will be needed for staining living cells. Alternatively, fluorescent phalloidins have also been injected into cells for monitoring actin distribution and cell motility.Actin filamentswere stained with Phalloidin - 488(green) and 594 (red)鬼笔环肽荧光系列说明书一、引言鬼笔环肽是从伞形毒蕈蘑菇中分离出来的一种毒素。
碧云天 EMSA突变探针-SP1 说明书
碧云天生物技术/Beyotime Biotechnology 订货热线:400-168-3301或800-8283301 订货e-mail :****************** 技术咨询:***************** 网址:碧云天网站 微信公众号EMSA 突变探针-SP1 (1.75μM)产品编号 产品名称包装 GS078MEMSA 突变探针-SP1 (1.75µM)60µl产品简介:EMSA 突变探针-SP1是用于EMSA(也称gel shift)研究的SP1 consensus oligonucleotide 的突变体。
可以作为EMSA 探针-SP1的阴性对照,用于EMSA 结合反应中突变探针的冷竞争反应等。
EMSA 突变探针-SP1的序列如下:5' -ATT CGA TCG GTT CGG GGC GAG C-3' 3' -TAA GCT AGC CAA GCC CCG CTC G-5'EMSA 突变探针-SP1中SP1的公认的结合位点发生了突变,从而使SP1无法和该突变探针结合。
在探针冷竞争反应中,正常的标记探针和SP1的结合的条带会被抑制;而在突变探针冷竞争反应(cold competition)中,正常的标记探针和SP1的结合的条带不会被抑制。
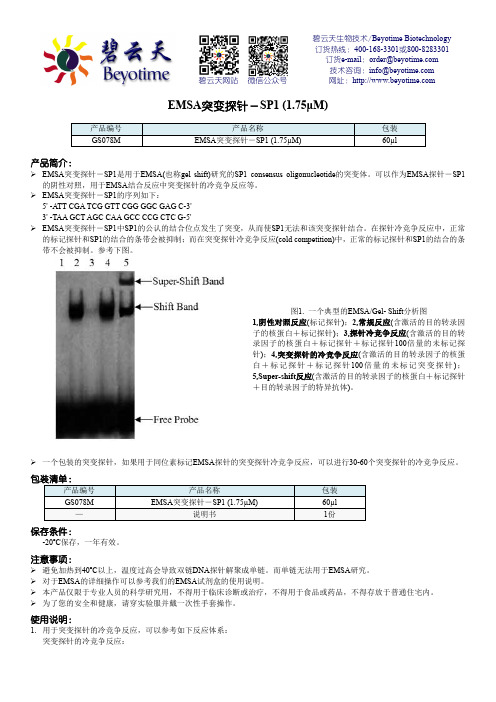
参考下图。
图1. 一个典型的EMSA/Gel- Shift 分析图1,阴性对照反应(标记探针);2,常规反应(含激活的目的转录因子的核蛋白+标记探针);3,探针冷竞争反应(含激活的目的转录因子的核蛋白+标记探针+标记探针100倍量的未标记探针);4,突变探针的冷竞争反应(含激活的目的转录因子的核蛋白+标记探针+标记探针100倍量的未标记突变探针);5,Super-shift 反应(含激活的目的转录因子的核蛋白+标记探针+目的转录因子的特异抗体)。
一个包装的突变探针,如果用于同位素标记EMSA 探针的突变探针冷竞争反应,可以进行30-60个突变探针的冷竞争反应。
伊红美蓝琼脂(平板)说明书

广东环凯微生物科技有限公司网址:地址:广州市黄埔区科学城神舟路788号邮编:510663传真:860288778876产品说明书Product Manual【产品名称】通用名称:伊红美蓝琼脂(平板)英文名称:Eosin Methlene Bule Agar Plate【产品编号与包装规格】产品编号产品类型包装规格024087即用型成品平板20个(90mm)/盒【产品用途】用于分离革兰氏阴性肠道菌特别是大肠菌群和粪大肠菌群。
【检验原理】蛋白胨提供碳源和氮源;乳糖是大肠菌群可发酵的糖类;磷酸氢二钾是缓冲剂;琼脂是培养基凝固剂;伊红和美蓝是抑菌剂和pH 指示剂,可抑制革兰氏阳性菌,在酸性条件下产生沉淀,形成紫黑色菌落或具黑色中心的外围无色透明的菌落。
75%酒精消毒外包装,在洁净环境下拆开包装袋,使用。
【质量控制】贮存于2-25℃,避光、干燥处;贮存期见产品标签。
【注意事项】1、注意无菌操作,避免外源污染。
2、质检报告可以登录环凯网站 ,打开“质检报告”页面,输入产品批号下载。
【废物处理】检测之后带菌物品置于121℃下高压灭菌30分钟后处理。
【执行标准】Q/HKSJ 03广东环凯微生物科技有限公司企业标准普通微生物培养基【说明版本】2019年11月23日【参考文献】1、GB4789.6-2016食品安全国家标准食品微生物学检验致泻大肠埃希氏菌检验2、GB4789.38-2012食品安全国家标准食品微生物检验大肠埃希氏菌计数3、GB/T5750.12-2006中华人民共和国国家标准食品安全国家标准生活饮用水标准检验方法微生物指标广东环凯微生物科技有限公司网址:地址:广州市黄埔区科学城神舟路788号邮编:510663传真:************-8619 ************8602************88778876。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening LibrariesData SheetBIOLOGICAL ACTIVITY:Aprotinin is a serine protease inhibitor isolated from bovine lung which inhibits trypsin and chymotrypsin with K i values of 0.06 pM and 9 nM, respectively.IC50 & Target: Ki: 0.06 pM (Trypsin), 9 nM (Chymotrypsin)[1]In Vitro: Aprotinin, a serine protease inhibitor isolated from bovine lung, is a complex protease inhibitor that is an antifibrinolytic,inhibits contact activation, and decreases the inflammatory response to cardiopulmonary bypass [2]. Aprotinin inhibits trypsin (bovine, K i = 0.06 pM), chymotrypsin (bovine, K i = 9 nM), plasmin (human, 0.23 nM)[1]. Aprotinin is also a competitive protein inhibitor of NOS activity. It inhibits NOS⁻I and NOS⁻II with K i values of 50 μM and 78 μM, respectively [3]. Aprotinin significantly inhibits fibrinolysis with an IC 50 of 0.16±0.05 μM [4].In Vivo: High dose aprotinin can reduce blood loss and transfusion requirements associated with primary cardiac procedures suchas coronary artery bypass graft (CABG) or heart valve replacement surgery [5]. Aprotinin inhibits thrombus formation in a dose⁻dependent manner. Aprotinin at a dose of 1.5 mg kg ⁻1 (bolus) and 3 mg kg ⁻1 h ⁻1 infusion (maintenance infusion) causes a tendency towards a reduction in bleeding time. Aprotinin significantly reduces the bleeding time starting at a dose of 3 mg kg ⁻1bolus plus 6 mg kg ⁻1 h ⁻1 showing a reduction of approximately 84%±2.9%. At the highest dose of 5 mg kg ⁻1 and 10 mg kg ⁻1 h ⁻1,the strongest effects are observed [4]. Aprotinin may affect tumor necrosis factor⁻alpha (TNF) levels. Soluble TNFRI levels are significantly increased following I/R in the aprotinin treated wild type mice and not detected in all TNFRInull mice [6].PROTOCOL (Extracted from published papers and Only for reference)Animal Administration:[4][6]Rat: Male Wistar rats (180⁻220 g) are used in the study. Aprotinin is dissolved in physiological saline.Aprotinin is administered by bolus injection followed by a maintenance infusion. The doses given are 1.5 mg kg ⁻1 and 3 mg kg ⁻1 h ⁻1,3mg kg ⁻1 and 6 mg kg ⁻1 h ⁻1 up to 5 mg kg ⁻1 and 10 mg kg ⁻1 h ⁻1. Plasma concentrations for the two agents are assessed by pharmacokinetic studies in rats [4].Mouse: An intact mouse model of ischemia/reperfusion (30 min⁻I/60 min⁻R) is used and left ventricular peak + dP/dt is measured in wild type mice (WT, C57BL/6; n=10), WT mice with aprotinin (4mL/kg; n=10), transgenic mice devoid of the TNFRI (TNFRInull;n=10), and TNFRInull with aprotinin (n=10)[6].References:[1]. Fritz H, et al. Biochemistry and applications of aprotinin, the kallikrein inhibitor from bovine organs. Arzneimittelforschung. 1983;33(4):479⁻94.[2]. Levy JH, et al. Efficacy and safety of aprotinin in cardiac surgery. Orthopedics. 2004 Jun;27(6 Suppl):s659⁻62.[3]. Venturini G, et al. Aprotinin, the first competitive protein inhibitor of NOS activity. Biochem Biophys Res Commun. 1998 Aug 10;249(1):263⁻5.Product Name:Aprotinin Cat. No.:HY-P0017CAS No.:9087-70-1Molecular Formula:C 284H 432N 84O 79S 7Molecular Weight:6511.44Target:Influenza Virus Pathway:Anti⁻infection Solubility:H 2O[4]. Sperzel M, et al. Evaluation of aprotinin and tranexamic acid in different in vitro and in vivo models of fibrinolysis, coagulation and thrombus formation. J Thromb Haemost. 2007 Oct;5(10):2113⁻8. Epub 2007 Jul 31.[5]. Davis R, et al. Aprotinin. A review of its pharmacology and therapeutic efficacy in reducing blood loss associated withcardiac surgery. Drugs. 1995 Jun; 49(6):954⁻83.[6]. Sabbagh MJ, et al. Aprotinin exacerbates left ventricular dysfunction after ischemia/reperfusion in mice lacking tumor necrosis factor receptor I. J Cardiovasc Pharmacol. 2008 Oct;52(4):355⁻62.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USACertificate of AnalysisPHYSICAL AND CHEMICAL PROPERTIESMolecular Formula:C 284H 432N 84O 79S 7Molecular Weight:6511.44Storage:Powder -20°C 3 years 4°C 2 years In solvent-80°C 6 months -20°C1 monthChemical Structure:ANALYTICAL DATAAppearance:White to off-white (Solid)Purity (LCMS):99.56%Conclusion:The product has been tested and complies with the given specifications.Product Name:Aprotinin Cat. No.:HY-P0017CAS No.:9087-70-1Batch No.:24242Chemical Name:Trypsin inhibitor, pancreatic basicCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USASafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :AprotininCatalog No. :HY-P0017CAS No. :9087-70-11.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C284H432N84O79S7Molecular Weight:6511.44CAS No. :9087-70-14. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
