MSCE_2014011515580646
XP正版序列号XP好用的正版序列号

XP正版序列号XP好⽤的正版序列号收集的XP正版序列号VOL版:DG8FV-B9TKY-FRT9J-6CRCC-XPQ4G(上海版)MRX3F-47B9T-2487J-KWKMF-RPWBY(⼯⾏版)QC986-27D34-6M3TY-JJXP9-TBGMD(⼴州版)QHYXK-JCJRX-XXY8Y-2KX2X-CCXGD(⼴州政府版)T72KM-6GWBP-GX7TD-CXFT2-7WT2B(上海版)2005年上海政府0686版谢谢⼤家的⽀持,帮我点下需要的⼴告谢谢我会更加努⼒整理我的百科服务⼤家 QC986-27D34-6M3TY-JJXP9-TBGMD(珆塆交⼤学⽣版)VMXC2-M9HKH-DRYGC-FHQ7H-BJY33(0408版)TDWGX-DMF97-BJYDQ-X9DJV-CYHWQ(不明)G6X78-XG4KV-3MXT7-FT8YM-F3YW3(不明)T8FMX-Q4HQJ-3JW77-JGPDC-FY9DG(不明)MFBF7-2CK8B-93MDB-8MR7T-4QRCQ(北京版)FCKGW-RHQQ2-YXRKT-8TG6W-2B7Q8(韩⽂版)DRXKM-94K47-38QVX-F8K7R-2H7CD(⽇⽂版)RFYPJ-BKXH2-26FWP-WB6MT-CYH2Y(英⽂版)7HPVP-8VHPV-G7CQ3-BTK2R-TDRF3(英⽂版)BCJTW-2M9JH-M8HHT-KWWWM-3444Y(英⽂版)CD87T-HFP4C-V7X7H-8VY68-W7D7M(英⽂版)OEM版:华硕:家庭版:KR63J-B34MB-CVP9K-T478G-8Y3XG联想:家庭版: PWBPT-6PGKF-TP6MY-299P4-CPXQG (XXXXX-119-0001544-XXXXX)专业版: FCDGH-QW3DJ-VBC6C-9BYTX-4GKQJ (XXXXX-119-0001553-XXXXX)VF4HT-MPWB8-TWV6R-K6QM4-W6JCMH3B8D-MQPF9-WQMFB-GV3R4-VTF7W(04年联想版)DELL:家庭版: RCBF6-6KDMK-GD6GR-K6DP3-4C8MT (XXXXX-119-0001024-XXXXX)专业版: XJM6Q-BQ8HW-T6DFB-Y934T-YD4YT (XXXXX-119-0001024-XXXXX)KG7G9-67KHV-4FQKV-4DYXK-BHQTJCOMPAQ: 家庭版: KG27H-JV9M6-2CXKV-GMP22-HF2BQ (XXXXX-119-0001015-XXXXX)专业版: KYKVX-86GQG-2MDY9-F6J9M-K42BQ (XXXXX-119-0001015-XXXXX)HP:家庭版: MK48G-CG8VJ-BRVBB-38MQ9-3PMFT (XXXXX-119-0001067-XXXXX)专业版: DMQBW-V8D4K-9BJ82-4PCJX-2WPB6 (XXXXX-119-0001067-XXXXX)P2BXT-D7Y8P-F6WF2-HYXYP-49TJDACER:家庭版: CXCY9-TTHBT-36J2P-HT3T3-QPMFB (XXXXX-119-0001006-XXXXX)专业版: BW2VG-XXDY6-VW3P7-YHQQ6-C7RYM (XXXXX-119-0001006-XXXXX)KDD3G-HGVGM-M24p4-6BMMY-9XHF8IBM:家庭版: DMY26-78CX9-Q89DP-Q8QK8-VF2B8 (XXXXX-119-0001076-XXXXX)专业版: HCBR8-FGC2K-RY7BM-HM3KT-BKVRW (XXXXX-119-0001076-XXXXX)清华同⽅:家庭版: KMHJF-9M82Y-YPFV7-YQHXH-F9JW8 (XXXXX-119-0001794-XXXXX)专业版: M68XC-TX2C9-PKK8H-GP8JH-RC8XB (XXXXX-119-0001805-XXXXX)TCL:家庭版: XPGYX-J7BF9-4YJVV-7MWK9-WQT3Y (XXXXX-119-0001607-XXXXX)七喜:家庭版: GJMY6-GMJHY-2VJ79-K67WT-KQHYT (XXXXX-119-0001661-XXXXX)Samsung:家庭版: XVX72-2WCXQ-48VWH-T66HT-C7R2B (XXXXX-119-0001085-XXXXX) TOSHIBA家庭版: WDHPC-6WQPF-W3R3K-J2VF4-JFP8W (XXXXX-119-0001114-XXXXX) SONY:专业版: K7RGC-CDXYJ-FTYH2-Y3VVV-KBYC7 (XXXXX-119-6385501-XXXXX)⽅正:家庭版:FK4VC-XP9C3-BD78M-68492-BP9BY (XXXXX-119-0002964-XXXXX)专业版:F4G2M-BH2JF-GTGJW-W82HY-VMRRQ (XXXXX-119-0002973-XXXXX)富⼠通:家庭版:JY6V8-QV6YB-BD3GX-67DC9-JT7WD (XXXXX-119-0001373-XXXXX) TOSHIBA:家庭版:WDHPC-6WQPF-W3R3K-J2VF4-JFP8W (XXXXX-119-0001114-XXXXX)惠普英⽂:KYKVX-86GQG-2MDY9-F6J9M-K42BQ(XXXXX-119-0001015-XXXXX)P2BXT-D7Y8P-F6WF2-HYXY9-49TJD序列号:6XKGD-PGHV3-D46CB-XQ8V3-V7FTJ法语专业版⼤量微软贵宾VIP序列号(1) H2HYJ-28PQM-6HGFG-CWMMD-V2C62M74XB-7K8Y4-YT6MY-B6XX4-PWBF4VVBQJ-VHP8J-7DHHP-FK68Y-YHY2VD2Q4M-M47JY-FVXFJ-JPRKR-6GG82RCB4W-27GPV-HKPF7-WH43D-V6XPDY88HJ-4K4VP-W6Y3H-BWH6M-43GCPM7D6F-3GY6B-PWXPF-JPPYD-8XD2JFWFX8-2VBWD-2K8T7-R26QW-93T3CPW2DM-BHPPK-YV8V3-VFYVY-VQFKD8W4DX-VDYKX-JYFYC-VY4Q3-YJ4PD93XDW-CQTX4-PJ2Y4-YHRW4-G4DBFD2H37-MCK77-8YW4V-7C3YQ-K98RJQ3MM2-PX8HT-DG34Y-MH7Q4-WDG3WTRHB3-TTDC6-QDTKW-8GRQY-G7H6M3VHPK-BYHDR-X63B6-8FFFM-P6TW2CB4F7-JHGCM-47K6G-PB7BY-BX2W3TMYYT-RYXXQ-BHJ78-F6TWC-97BXTYTTD2-BQHYG-8F4D2-WCVHC-64TTTGRPC7-FVKTR-MXVMJ-4TD33-JMDF8DXVWV-PDKBH-2PK6Y-YQWCP-GQ43TFWT4K-YXKYV-FTWJG-B6WJF-CB73X273M3-4KG6D-34MMY-XYFWF-2P4VYTC2PX-Y77HM-QJQ7X-BHVBT-QF9PC4MXCM-8R7Q8-V74VD-6PPWY-QQBPMF4K8K-K6XFX-K7M68-M6P4P-MVV6YWFMT2-DWMYG-JYHVK-DCXYD-7M84BBWWYK-6QF64-7KJPR-HJBJ7-JD9G9V3QXP-MBQPK-RQGB3-6XFJR-2P2BBQXYMP-G3WTH-DX3RT-VX8FR-7MDHH44V3B-JHRC3-T4PRP-C4GHK-FTT2F3TC47-R6GKX-KMM3V-37DR2-K3CGBX8CXT-B38P8-MR6CG-XGJ76-734BXMWXP3-28PMK-CQYD7-QV6VC-X7F66YTX4R-RPQJC-2FTT2-XGH23-KDPHDCJ78M-4DDKT-6CCQF-VFB7J-6HM9QGKH26-V6VWJ-3YYJY-QFF2P-PYXBHJP6M2-HJCFC-8KCJ6-M2KMW-69B9TW4DYV-RJ7VP-X78K7-7KF78-DT8JD3F276-7BYC6-WT46Q-JMR7W-KT9F6H7CJG-Y7HPQ-W3D2C-3H64M-6FVVCT3RMP-XXVR7-TGGMK-H74VG-Y3YQVPJCYY-P6TQ4-DMPY4-4WGXV-4DJRB74GFB-DDKVK-V3M28-73GYW-MTFJWDFHFT-4XQRX-4J76Q-PKPP8-72TT6G7HBY-RPXFX-JFTP2-YYVVX-8W9QD VJGX6-R7VQ8-P6GW8-J6RPK-4QCB9 KMG2X-6D8XP-M23XR-6FWKX-B8JRH CRTGH-B68P2-XB6JC-44GCD-X9JHMP6PMF-2YWVR-XTHRJ-H2RM6-3BCVD WKM33-X3KC3-GWRJ3-MF2RR-QPGW7 7H7MF-Y6H36-KQ2W3-3QWMP-MG4V9 BXGG6-3M3VH-2QV7R-M6XGV-34XMP FJPJ4-33W73-68XT3-YRYJX-JJ3P9 HFWXW-4MPRF-H7WJD-P6JQV-4X6RK W8Y84-JTWGP-PFWDQ-G4D37-DQWWK 6MH6Y-G82FC-73XR4-TMW7H-CRGC2 Y3K3R-G2R7W-WMM6G-YW2KY-TXKMD KXPM7-B64RQ-7DWJP-7DT8Y-VGK2H 22WXC-BDHBH-D6XTY-8V7FC-6YCW3 V3PB3-H6MMJ-7BDHD-P3BWJ-K9J9P BCVB6-PWK3H-GMXQB-Y84XF-JHTJ4 BTMT7-MJV82-MV4FV-2GYMB-7GWC9 FPBY6-J33HB-RDVTJ-HFPKT-4C7WY2DD2D-3Y7JY-B8P27-6XPD8-M9VQF DHWBP-RJBCX-GXDCV-7YBBQ-MW9VW 7RWJY-C4H4G-28R8J-67JKB-WBX3D DB43K-FY62D-8C8X7-KCW3M-RMX4G 7PKQG-Y244T-HJGKJ-WD6MM-86DX3 THT72-6RFVK-6R3MK-TV8H3-KJFP2P7YRP-DWGPY-G34BX-4R8MC-X4GGY QTHKF-XP37X-GGDDH-M7P8D-RBMHJ 2TBP7-VTJDW-HGVBC-M37CJ-72WBD TG47B-6QVYK-CWKKJ-FXPQY-283MG DYFK6-63K44-74QW4-D44WC-F7PDV TPC7B-MGV6W-H2GY6-YRX4Q-9PQ9G 3CWQB-8Q78J-YMVY3-DBMCW-K3HV3 WQGR2-RJWGW-2VCMV-B284Q-BGMXH 8QFJX-XVD6T-36JK8-YFJ8X-MY47RQY4F4-H7WDG-7D2BH-XJRWB-V9J28 CRMCF-6BG6R-8G7HT-MYHWV-WCHV7 VC7C8-26TCW-GPK7T-HHKG3-TWV8X FPDJW-CC3D3-VH4V6-WD8HM-GKCD3 WHFX8-8QFYV-73F6D-GRRH6-78RKM 3D4TG-RBYQP-HYGDJ-BPR6D-JXQX3 KDM48-RMWC8-Q8FRM-PDQBQ-VYCBC 2DWJ8-66CPC-CDTTK-DDVYH-B7KP2 JJRX2-32RK2-GJP3M-M427J-MBQWX QHVBK-BM8W3-7QQPQ-26H23-X4CD6 DT277-RP2QW-3G2X9-364RD-J8KTG HPJRB-W3RM3-WK4RH-KDCT4-HQTDP WKM33-X3KC3-GWRJ3-MF2RR-QPGW7 7H7MF-Y6H36-KQ2W3-3QWMP-MG4V9 RXG3Q-28G3V-MTWPV-4TPDR-MPDHF QHB43-KH2KY-6DH36-D3DFT-MDG64F8MJP-QFPDB-4YXVX-XVM64-4QP6P DRQKX-JXDFJ-K3Y8W-DGQPP-GV89H BXGG6-3M3VH-2QV7R-M6XGV-34XMP FJPJ4-33W73-68XT3-YRYJX-JJ3P9 HFWXW-4MPRF-H7WJD-P6JQV-4X6RK W8Y84-JTWGP-PFWDQ-G4D37-DQWWK FJV3W-T8QKF-BG34F-CBVT8-2D2P3 BWTHJ-6QFJ3-3PCTB-J4FC8-W4M6T24WPT-3PJ23-77HR7-3PRQH-M9GFD66PJG-TYFPY-FFK2D-P424G-78T2K YMG7X-8PH82-686XC-23T3R-FDXHW TV3F2-37DQ4-66FKR-8DQTP-X94PM6JHVK-CCQP3-VFCRH-RXHY3-RQPPT YHTCM-VY2R3-TRD3M-34PCG-GFFGH RYMMY-PMGBJ-RKDJD-X2V3Q-TWBHV H67RX-VDRDH-MCXMR-JT2JQ-B6Y3M B8FTF-YGHBH-DJPJP-BH6TM-QDFHR DRCRH-7HPH4-MPKR7-RGKR7-GFVKMGK2MH-MFXGX-KWQBT-RWK8J-QGR8G MF7CF-3XXMG-7PP4Y-PYV83-2X7D3P87VW-YQ8J6-Y4QGP-JMKQ4-Q72W2V34JQ-DM8PH-YTCV7-FB738-GPBRH MGPD7-GF6VT-83YQD-422F8-GF3TK8R4KM-JQTQM-H8WGX-8F3MV-FCBCB FT627-G4XDP-4KDG8-66B4C-KC3T3W6JR7-V2FKM-6K2YV-FYQBW-X327M WGK4X-GHKXG-FFFKY-XDXV7-84PTQ HFBMY-QXQF2-F8RVK-JBPMR-Q3G6D 233WJ-DKFJ6-XK772-KRDKX-MR3J68VMPV-6C3HM-6XDBJ-XKC63-4P9V887G2P-DPQ4F-GTBB7-PCVXW-MVDKY KQF36-BJM8P-6JPWW-6W6H8-Y46TX22DVC-GWQW7-7G228-D72Y7-QK8Q37PJCG-CXBBM-WPRP3-JC348-D8WMGF38JH-BGF8G-G7GMW-XM4T6-VK3CK GKM3J-M2FQF-7JFKT-TPB84-RBJXP DRPK6-BDCB3-G2WXG-VWJ4V-829YH8VWCB-QMTHX-FVK2R-C6YJV-6TW8G6CGHB-GPMPR-42MGV-6PKB8-FV3V4 MGB64-RPKCV-3GKBC-PH8T4-YTXQ9Q6DJY-7DKTV-KQTFX-8X7WF-DWRW6 VCTQM-VTQ8Q-Q4GQ8-H3X8M-CQWT26YHC2-WW82B-C64DR-XXPDJ-C4P7M CRMCF-6BG6R-8G7HT-MYHWV-WCHV7 TTJ8X-K7G7K-TMW4F-6FPXH-C677H JYM4H-TMPVF-QCDPQ-HFRX2-2HFDTV66VK-XWC7M-VH3KR-JY6CV-HMYPQB6DBQ-MKKVX-VBYXJ-M373C-773HTRX2K6-XQDQT-FYXWY-8XWK8-9XTYD XWGPK-JRKGF-TGWKC-3T7M4-BGCBC YF8PG-GH747-DMTC3-2797D-6WBWM VTB7G-JDRHV-JRV7K-3BX4B-3TW6TFF7BH-G3TQF-GRQFX-PPF38-MH6MX CCYFQ-CYK72-HTB3Y-MF37F-DHXQK 4876D-XRHK2-PHG2T-JVJWY-72D6VPV77H-MCP3H-VBBJD-WQF8M-XK8DW⼤量微软VIP序列号(2)QYRY6-4Y3WR-QVR86-MDJMY-4C694 VGPT2-PV626-2X6T7-D7R6Q-7GRQJWF47Q-DGPDW-FMJBY-3RWY2-K3Y6D CWWQT-VQ3RH-TPWPQ-HRJRP-9P8FV 3WFVR-J3R6R-3TFVX-Y2GC8-VMP9Q47PQQ-D2F6R-FJ3QV-RV2GH-3JQBGGK2MH-MFXGX-KWQBT-RWK8J-QGR8G MPH32-4B3G8-2MVHQ-6V4QK-4VWG974GFB-DDKVK-V3M28-73GYW-MTFJW DFHFT-4XQRX-4J76Q-PKPP8-72TT6G7HBY-RPXFX-JFTP2-YYVVX-8W9QD VJGX6-R7VQ8-P6GW8-J6RPK-4QCB9 KMG2X-6D8XP-M23XR-6FWKX-B8JRH CRTGH-B68P2-XB6JC-44GCD-X9JHMP6PMF-2YWVR-XTHRJ-H2RM6-3BCVD VJGX6-R7VQ8-P6GW8-J6RPK-4QCB98Y32G-27HKV-TGGGM-4YDKG-DGKH2 CGXJJ-DXCMJ-7CD7D-7BMJT-PCCPH8GMVK-JGX6P-T36GY-P7K2Q-GD2MPT8WT3-VHMDT-JDHP2-KKGYH-7R4467VDYJ-3HYPJ-63CBQ-Y22QH-GM2B2 7777W-HB76V-68FBQ-PTHT6-PBHCH Windows XP PRO build 2600 or XP pro 2600 evaluation:7VF4T-D7QMK-CC87K-P2468-3M4PQX3BTD-7P44K-2FWW2-3DGB8-B88DJT3GXK-WDT8Y-XMKD3-Q6MQD-DT862 VG2PR-HTKCV-TC3D8-XVTDR-D2WHD PQQRK-GFPHC-QVJC8-6MTJQ-R8DKV J84TF-7CTBW-JH3TB-YXR3W-BJBRQJ84TF-7CTBW-JH3TB-YXR3W-BJBRQ 23QFT-DVY67-6G2VV-7JM76-BWQR3 68YG6-DCFRM-B7KYT-TJRMF-CXJKX 68YG6-DCFRM-B7KYT-TJRMF-CXJKX 87H3J-HD7TM-V66FW-PBDBX-HVH4R YG4WP-9YXKX-QM2YV-PR4X7-KDJTC CHXHP-PB2FG-B68BB-H8XGF-7V2PY K74GF-Y232R-PH3RR-YKB4H-PJCV3 BRJX8-FWBHG-2RXWK-H84W7-628J4 4V2YV-HHHX4-CKVDY-4R486-498V2G87BW-MYGY7-4FWGY-CCHQB-8FJ3B 6KM4D-2Q76R-278RX-QMXJD-88D7V4JRPY-6XGY7-2MY2V-BWRX7-YCHGP WMMJQ-8TKHC-MC7TJ-7WCF2-D8BRY RJQ6X-R4C2W-FFMT7-TGXVQ-9GTVG VM3QK-HYQ8B-VHVFB-DBQWP-XW83Y GYBRP-VG8CH-JRHTV-X8C7J-6GYMB F3RJW-XFQWM-4FH6Q-3CTMR-R82GR 8J8K6-GPMBM-RWVFV-2Y8VX-B66JY8J8K6-GPMBM-RWVFV-2Y8VX-B66JY 88F24-PGH68-DTMQM-8VV47-DG7283JJQX-H6YQB-CCX7K-6WXCK-Q6BYR XBB6Y-G6QWG-WPCFQ-F8B6B-HMCPW WYTDP-6YFYF-4KFGJ-GHRYY-9KPHH 6WF3Q-TRX4M-47PRY-MFQXV-86PJG F2R8R-QJJ3V-KJKMT-64T4T-B78YT BTHFD-Y6FCP-7KMCT-7JYXH-CBPFR DGBY2-QVG8W-RTYFT-23R4B-RP7KC F6FRH-PXF8H-YKTBF-37MKG-TYCFC R67CQ-27X24-FY4B8-Q6WTF-647QR46KGC-3TXQD-H7KPC-FT6T6-TRB733MTDQ-JRMKW-V7K8D-KBFFK-GFHK6 PJVMC-QG747-HR2FF-V3QTD-YMX3V MJYQX-C266W-P2C8C-PYPYW-QGMFQ YKX8H-CT4G3-GX3CJ-KC7JX-33M46G4H23-RY2C3-C3G9M-K8MQW-C82P7 6MQDH-VM2CX-7J8B2-XVT4K-MCH6R JGMVY-4RDFG-338KG-J2RBG-RCV7Y KJCT3-VHD4Q-TFGKV-X2YCX-7WD74 WF2W8-7JP2F-RT82Q-YP2GM-J739G DKVTJ-62RY2-6VHXT-TG84W-PWQ97 QTMHK-64FMF-6HV7X-MPJP7-43HT3 RH2BY-MJKG7-36VKJ-TVQBX-BCGR6 MB7V3-QC28J-DCR34-4HVQC-J8PQD FKJBB-T4M84-43XHP-X728H-2HQT33QMDV-PHB2Q-D44QQ-4XPGC-QTXX2 K4TFW-VDP6W-YDBFY-YRYBD-3QPRB KXDBM-Q3TXY-Q32XB-TGHBX-DW7F4 7W4TC-XM6GX-8B84Q-M4VPV-GQ4MX KG7P2-3PT7H-QKPX2-Y242R-HHM99 HKF34-VF4T6-KCBPF-PBFX8-BBV82 RJFGM-DQ88P-R7M2M-PB37G-4B32M GPX86-TRJ6X-XJM6Y-P7MPJ-DHCVR JMHVM-BDPV3-PVCW8-48RWR-BKKDG 3P8QD-V2H78-VV7VB-KB2XY-7QYX8Y27TX-MB326-77Q84-G3R3M-MGG8R4V2YV-HHHX4-CKVDY-4R486-498V2G87BW-MYGY7-4FWGY-CCHQB-8FJ3B 6KM4D-2Q76R-278RX-QMXJD-88D7V4JRPY-6XGY7-2MY2V-BWRX7-YCHGP WMMJQ-8TKHC-MC7TJ-7WCF2-D8BRYRJQ6X-R4C2W-FFMT7-TGXVQ-9GTVG VM3QK-HYQ8B-VHVFB-DBQWP-XW83Y GYBRP-VG8CH-JRHTV-X8C7J-6GYMB F3RJW-XFQWM-4FH6Q-3CTMR-R82GR 8J8K6-GPMBM-RWVFV-2Y8VX-B66JY8J8K6-GPMBM-RWVFV-2Y8VX-B66JY 88F24-PGH68-DTMQM-8VV47-DG7283JJQX-H6YQB-CCX7K-6WXCK-Q6BYR XBB6Y-G6QWG-WPCFQ-F8B6B-HMCPW WYTDP-6YFYF-4KFGJ-GHRYY-9KPHH 6WF3Q-TRX4M-47PRY-MFQXV-86PJGF2R8R-QJJ3V-KJKMT-64T4T-B78YT BTHFD-Y6FCP-7KMCT-7JYXH-CBPFR DGBY2-QVG8W-RTYFT-23R4B-RP7KC F6FRH-PXF8H-YKTBF-37MKG-TYCFCR67CQ-27X24-FY4B8-Q6WTF-647QR46KGC-3TXQD-H7KPC-FT6T6-TRB733MTDQ-JRMKW-V7K8D-KBFFK-GFHK6 PJVMC-QG747-HR2FF-V3QTD-YMX3V MJYQX-C266W-P2C8C-PYPYW-QGMFQ YKX8H-CT4G3-GX3CJ-KC7JX-33M46G4H23-RY2C3-C3G9M-K8MQW-C82P76MQDH-VM2CX-7J8B2-XVT4K-MCH6R JGMVY-4RDFG-338KG-J2RBG-RCV7Y KJCT3-VHD4Q-TFGKV-X2YCX-7WD74 WF2W8-7JP2F-RT82Q-YP2GM-J739G DKVTJ-62RY2-6VHXT-TG84W-PWQ97 QTMHK-64FMF-6HV7X-MPJP7-43HT3 RH2BY-MJKG7-36VKJ-TVQBX-BCGR6 MB7V3-QC28J-DCR34-4HVQC-J8PQD FKJBB-T4M84-43XHP-X728H-2HQT33QMDV-PHB2Q-D44QQ-4XPGC-QTXX2 K4TFW-VDP6W-YDBFY-YRYBD-3QPRB KXDBM-Q3TXY-Q32XB-TGHBX-DW7F4 7W4TC-XM6GX-8B84Q-M4VPV-GQ4MX KG7P2-3PT7H-QKPX2-Y242R-HHM99 HKF34-VF4T6-KCBPF-PBFX8-BBV82 RJFGM-DQ88P-R7M2M-PB37G-4B32M GPX86-TRJ6X-XJM6Y-P7MPJ-DHCVR JMHVM-BDPV3-PVCW8-48RWR-BKKDG 3P8QD-V2H78-VV7VB-KB2XY-7QYX8Y27TX-MB326-77Q84-G3R3M-MGG8R 887VY-FKDX7-2DJ6P-Y44RY-94KXJ TMJQF-RWQC3-X32TP-2T27X-FY38W VD8CH-JF88R-Q66F7-TWH8H-36KX3 JBK6F-3F8HK-HKCMW-XJQWV-BWH24 RH32K-GHGVX-BF7HF-H7MTD-7PYBJ DJ8CJ-CMDJK-YK4V8-FK76G-X3T6Q7MXWG-DHHBX-6VJR4-3HKQF-KGDY2 DXMHT-B4X42-XT7HG-X2JRK-XHXK9 FJ2CP-PW3F4-YDRWJ-GHCPQ-TBGW3 2FYMH-V7PW7-2J682-2HF3K-42X6T4F77P-4VRM2-GM44G-3GXJQ-MMC7M GP66R-4M7F3-FV2VV-VH4VW-BBGF8 DYQMF-MCV2X-D2CTV-QVDRQ-WWM9Y BTJ6H-HKD6W-HK7FH-XB764-QHVKC MDFT6-B748H-DWXGF-FPJPG-HX4MM QKMHW-6YDKC-HRMQK-JF3VF-XHRGQ 6MD6G-KKFXD-VJKC3-B8286-GRC3D 462WC-8RPYB-H6RTX-MKG32-TB29P PWBFG-XMKMF-MPTV8-XP87K-BQ6TD X2Y9C-KKQX3-VF8WK-37VQ3-GMQYM VY4VR-X7XGY-7Y2FV-T4VJ3-PPP3F WVJKC-PWV74-6QRCW-2RCPW-3QPGC 4K32D-H2GKK-B3VB6-RCHBG-462TQ WDDBP-6HFQC-J7RD4-MXY8H-TM3XD TRGW4-DDRG3-K4DTW-TY6QP-K74P3 F883H-8KFWR-FH3M2-MPJ7F-2C68W3QCVY-GQMGJ-G6G22-6CBY7-P44QP BBBH7-43CMD-BWFXY-MPVG4-8FQ33 XWTC3-Y8G6Y-YH7X8-D673M-WFBDX CGT6V-WC2GW-R3JBH-MV7PF-CDH82 W3BRM-CWPPD-DR26B-V6V3W-3V78F P3QXG-YGDJG-7XKTV-C6BC8-2F83H FHTMG-G7FQX-BQ6G7-HG2V7-QKRFP CDVCW-H7RQP-M2M3G-7GC7R-P4B3T F2KV6-RJ36T-P3XB2-B2KDC-Y9RRJ KMVXP-JTTVF-R46WY-WDKQP-WC9RC Q6K2Y-4DQ6T-PPXKB-M4GTC-VWCW2 QX8HH-FPTYD-MRP8W-BCQGR-M3VJV VH63Q-D7WRD-J4WVK-RVKT7-R2866 T7T62-Q64RK-QD3QY-3CR7G-28VHD MMRMD-QQWK4-2T6RH-W8GKV-2MFVY 2XVY8-JF8WX-R4MFY-3J7D2-DKQQM G2JVQ-V4T28-T36WB-72C2R-THM4J QMVDJ-RQ8TV-8KGJJ-BQHMR-YKBGJ KMQ2C-9H7KR-KYBW7-CBCVP-XPJXF G7FFB-8DPKP-W722F-K2JRJ-K4X39 MTTRH-YDMC8-JBYX4-TDRWD-C967Q XYX3Q-T4B24-XRCQB-XFRQT-H8KK4 4M2D3-8QFVX-WW244-7M4QT-G476TR6F88-TBRW8-8MTRW-8VBJB-HQKCJ F6FG2-TPMGK-JW347-YBQC2-PTRY37WMMM-Q7VJR-TJ4Q3-RMJTC-66C6H P3KCD-YKDCJ-TJCH4-RYPTP-8VDTP P283V-RFRHW-KVPWQ-WB46M-D8VHG 3Y8RD-RV2HF-DCH66-PYQ62-3RXYR FPVWB-VD7PH-G2WH6-TPWJF-H7RQT B8PJ4-277CH-6WH3F-PW6Y7-RDGVH B6JM8-H7FCY-J4K6B-KMFV4-3MKDC2D6W4-YQFDF-F3Q4C-RXKKF-7248F WJJW8-6TWPW-CG4CD-WMYC2-2X67V JM3J3-HRCGD-YQ8KY-7776M-RQ22G GB736-KDM4R-HXTPG-6XMFT-HMDR8 QQ336-MHWKF-366C2-J3B4X-Y2HYB VBY36-T4DXF-FDJ88-J7HM8-6846MQJ3TB-PJF7Y-PPTX4-YW2J2-HJJ7JH7HXR-DVD76-2JXK3-D3M7R-7FW4K XVHFR-JTXDC-K8GYQ-KWM8V-DX8TY 2KCP2-M6TCM-XHPRX-DJGBT-VDVV9 BPDBV-T3Q4Y-YWKJX-HJ8BT-CPPY8 GC6HB-RG7DM-V273V-J72KT-FCTF62RR6T-7MDYP-3VMBH-G6CYT-KT9TJ3PTKV-KM2WH-T4DKH-MWDCT-WKBD3 3VV6P-2YH38-4VWTB-FP2PH-CT68KY7H78-6KPY7-Y7VVR-FMD3F-67Y9K YFHVY-WCY4H-27YRG-DKD2V-FVC6F RCBFF-3YTPT-4MWF2-H6KHK-MBJHR TQWDC-6CBC7-VJ63R-6J8V4-72W9T DYPBB-B3GMP-6T3KM-6K47D-CVQ64 QY6WC-VY8DX-QYBYY-6W2F7-R3F32 QW2C8-2MQY3-Q82QC-GF88V-48CCF HP6M2-WTTFW-MQRW4-CKTQB-K694F 7K83H-68YRX-QCDC4-4CVQQ-CPP2R CTY8M-VGYX2-PVDWM-2PQCY-X3THW 2V8BJ-6D3GC-XJ3HC-HR2G6-7VVH8 QQGVB-P2TBD-QGYQF-8GT4P-T6BTB PRF4K-GV7FJ-JM3P3-F27DR-7FP4T62FBT-8F48M-37JYY-FVDPK-9DVG4 PWPHK-X3MX6-HB2JF-XHTM4-MGWTV J2KX8-MWFRG-V3HPH-PW8DV-77KVH 4H3J7-FD7GW-B443K-DTRJG-3WWMV FPCJD-MQDB2-PRYTP-W84KF-3Q32V TP44X-8KGRV-TWM22-TYVXQ-MQ4J7 JMPGH-RHYDQ-KW237-JTG4Y-M9DXQ 76P4H-8GQ44-GYJXK-YHWFY-W7J42FMJH3-88BJ7-6TKR3-8CXJY-YJY6BY33HQ-DD4GK-PF6KF-CMCCR-J66JM X47CH-7TCQJ-GGQW4-YPCR7-GMCWR BFWDV-XG6JC-DGGT3-PCJD6-CB7W6 BFWDV-XG6JC-DGGT3-PCJD6-CB7W6 HQXQM-GJ84Q-2M8JM-RTWQ2-X6WCQ PTWG3-HMJCR-Y3D3M-HG7VQ-47GQW QQJGQ-J27JR-MMQRT-7T7T2-C2TRR DCKMH-CC8PW-GWJR4-8HG2T-XFJ22 X6WRT-MDVQ8-4B3DF-7FD2G-2K2MK GWH82-PHBMF-862PK-XKYMF-XM76Q 64YKW-Y7QGF-DC7TJ-K2BW4-36HCK GH3K3-XGB7D-W8J28-VJ3JV-QGXQKT7VFH-RWB6R-DWW7K-DX8WQ-V9RKG 7B3YD-KPVBV-YFV88-HF8FV-G4V39 RXTWJ-TDG2G-RTQG8-6DPH8-G2KGX D7GGR-Y3BPP-TXCJV-FRDPR-TC724 8JMHP-73QVX-VKWQV-VQ6M4-3VW74 KWD3D-7QH4H-FXHJQ-DW2MF-W4F3B VKTR6-7P7JX-V6PDD-DTB2M-DDWT7 Q42T7-CY43D-X4KMX-2GR6H-R3W8KF4J7P-PG6CD-GHVFF-J2D4T-Q8F42 GG37K-Q4P3P-QGKFF-W67P8-WBPJP MB8MJ-P48YD-KQHVH-32FF7-BYRBQ MB8MJ-P48YD-KQHVH-32FF7-BYRBQ P3C64-CV3YJ-6BHRV-22KRG-3TXJF QW68W-8MRCG-3FMY4-VP3W2-73T88 Y8BQP-R6VC7-XRFXW-WMWYC-4JVBV C4XQJ-BDGGQ-TTXBB-PDHCR-H6937 CTHVC-HCYXX-3XBMR-7W7TV-C2W3K 74YRJ-PX72R-Q6Y4Q-TQ3MK-PFK8X VF7FV-3QTFX-MQYJQ-RYBPK-6KPYM G77TW-4C6J3-DJ3H3-PXYFH-MCGW4 2CGXM-3X8XD-HRKJB-C2DVF-BP7D7 2CGXM-3X8XD-HRKJB-C2DVF-BP7D7 4WTPK-R7X3G-XKWPR-VR8D6-TVHC2 DXHG8-B2KTY-8X3Q8-G2FHK-8J2JRD3JQG-63P4G-3DX8P-QVH3D-4PDG8 XJR6B-X8XC3-RC2GQ-BGKBM-FK3VW RCBFF-3YTPT-4MWF2-H6KHK-MBJHR TQWDC-6CBC7-VJ63R-6J8V4-72W9T DYPBB-B3GMP-6T3KM-6K47D-CVQ64 QW2C8-2MQY3-Q82QC-GF88V-48CCF HP6M2-WTTFW-MQRW4-CKTQB-K694F 7K83H-68YRX-QCDC4-4CVQQ-CPP2R CTY8M-VGYX2-PVDWM-2PQCY-X3THW 2V8BJ-6D3GC-XJ3HC-HR2G6-7VVH8 QQGVB-P2TBD-QGYQF-8GT4P-T6BTB PRF4K-GV7FJ-JM3P3-F27DR-7FP4T62FBT-8F48M-37JYY-FVDPK-9DVG4 PWPHK-X3MX6-HB2JF-XHTM4-MGWTV J2KX8-MWFRG-V3HPH-PW8DV-77KVH 4H3J7-FD7GW-B443K-DTRJG-3WWMV FPCJD-MQDB2-PRYTP-W84KF-3Q32V TP44X-8KGRV-TWM22-TYVXQ-MQ4J7 JMPGH-RHYDQ-KW237-JTG4Y-M9DXQ 76P4H-8GQ44-GYJXK-YHWFY-W7J42 FMJH3-88BJ7-6TKR3-8CXJY-YJY6BY33HQ-DD4GK-PF6KF-CMCCR-J66JM X47CH-7TCQJ-GGQW4-YPCR7-GMCWR 3BC2T-KYYWD-J4H2D-FG4PR-8CFY8F4Y8C-B2FRK-7T24W-BDCGC-FRKPT RH2DQ-QQBX6-PY6WT-6C6WT-YCJC3 TFC77-R427R-GP8MJ-QVKPP-W627Y XTG6D-HJHV7-X83KM-XJ2F8-V988P BFWDV-XG6JC-DGGT3-PCJD6-CB7W6 BFWDV-XG6JC-DGGT3-PCJD6-CB7W6 HQXQM-GJ84Q-2M8JM-RTWQ2-X6WCQPTWG3-HMJCR-Y3D3M-HG7VQ-47GQW QQJGQ-J27JR-MMQRT-7T7T2-C2TRR DCKMH-CC8PW-GWJR4-8HG2T-XFJ22 X6WRT-MDVQ8-4B3DF-7FD2G-2K2MK GWH82-PHBMF-862PK-XKYMF-XM76Q 64YKW-Y7QGF-DC7TJ-K2BW4-36HCK GH3K3-XGB7D-W8J28-VJ3JV-QGXQKT7VFH-RWB6R-DWW7K-DX8WQ-V9RKG 7B3YD-KPVBV-YFV88-HF8FV-G4V39 RXTWJ-TDG2G-RTQG8-6DPH8-G2KGX D7GGR-Y3BPP-TXCJV-FRDPR-TC724 8JMHP-73QVX-VKWQV-VQ6M4-3VW74 KWD3D-7QH4H-FXHJQ-DW2MF-W4F3B VKTR6-7P7JX-V6PDD-DTB2M-DDWT7 Q42T7-CY43D-X4KMX-2GR6H-R3W8KF4J7P-PG6CD-GHVFF-J2D4T-Q8F42 GG37K-Q4P3P-QGKFF-W67P8-WBPJP 2B662-DDVTM-RYDPW-WH42D-PFR98 RBBYW-V22PQ-C6TT4-3BP7T-72Y6BC6VCJ-FYRBJ-8WHFW-MB473-38DVJ PH2TJ-KHHG3-CBFW4-JD4WT-HTDRG GWPJ4-TVK2W-FFPT3-FT4M3-WWKCX KXYY8-266JT-7HY8F-PKD4X-GYHG9X8W8K-YHX4P-TJDYK-JHBCF-3KBRK K3VVB-T27BH-RBQXP-YM3BC-DTG6B RRQBK-VTGBY-HBR2D-YGB8K-M6CJW CG8WG-MCKK7-C6DBY-GF4QJ-37Y6J 76FXB-2R3PP-7WX6V-6JF6M-7TRHC7CBM3-V3YXF-WHWRK-GTTBR-FVYMJ FGGDB-HM8RD-W6GRX-MMFJD-H2C9J F6JQQ-GQ34M-27T3P-KFF8Y-42F4X2MMRP-HKTYX-3GFFR-TRW2G-KGX9H XDMDM-QPH6T-R4KYV-8BJJR-8CCG2 PKK8J-DPQ6P-H4HC3-TRPK8-CKRDV PKK8J-DPQ6P-H4HC3-TRPK8-CKRDV J6PTP-8WMQ3-BRD2T-88HJC-3TDK9 DFHXP-B7FPB-T4H44-BYXXG-7K2Y4Y462T-PV76H-V23HB-J8348-2MB32⼤量微软VIP序列号(3)WWGF6-VBQGC-QF7X8-H2TB2-3M98Y WCV7T-Y7V6F-HBBY3-YMK8T-XW9BV JV482-V3RMG-6CHB3-Q7CJX-QJKXV MB8MJ-P48YD-KQHVH-32FF7-BYRBQ MB8MJ-P48YD-KQHVH-32FF7-BYRBQ P3C64-CV3YJ-6BHRV-22KRG-3TXJF QW68W-8MRCG-3FMY4-VP3W2-73T88 Y8BQP-R6VC7-XRFXW-WMWYC-4JVBV C4XQJ-BDGGQ-TTXBB-PDHCR-H6937 CTHVC-HCYXX-3XBMR-7W7TV-C2W3K 74YRJ-PX72R-Q6Y4Q-TQ3MK-PFK8X VF7FV-3QTFX-MQYJQ-RYBPK-6KPYM G77TW-4C6J3-DJ3H3-PXYFH-MCGW4 2CGXM-3X8XD-HRKJB-C2DVF-BP7D7 2CGXM-3X8XD-HRKJB-C2DVF-BP7D7 4WTPK-R7X3G-XKWPR-VR8D6-TVHC2 DXHG8-B2KTY-8X3Q8-G2FHK-8J2JRD3JQG-63P4G-3DX8P-QVH3D-4PDG8 XJR6B-X8XC3-RC2GQ-BGKBM-FK3VW VHBH2-K4TTQ-WXH3R-MVGCK-7CYG4 G4K6V-XKD72-FYCTK-4HHRC-FKVJ8D3MJF-YM7V4-K2VP3-H76QY-WHC3V XJRC4-C8GRD-WV2XT-BDPWM-3KHK3 3KFWW-RC64T-YFMCH-RP7FG-D3Y8Q QJFHQ-34VQK-PX22V-72H2B-XMJ7K VVV8G-J3R27-Y2Q8Y-PPB8T-C6WDQ JC428-44FPJ-F7JR4-6JVHC-D2HBF6232K-TW8F2-7RV7C-4VQV8-3JDJX26P6G-CG2YT-BDXGY-F7DMX-6MMBR WW6PX-8V2G4-F6VXK-RCMTR-WBMTG PYWR7-KCG7X-KXCPV-2MHJV-WKH9R PG24F-MFVBD-WTTHF-RVBCD-WRBQM 7TB6J-CTWDD-YMTCK-4KMR8-YKQH8 4CPV8-46HVG-CVRRG-QJFVM-4RXMP BF3HV-Y7MH8-PCCCF-YBJ4D-HPWX3 JYCGQ-D2YB3-HQ2PY-7YDKX-JK83B XMWFT-THTJB-7XPJT-DVJHK-VCTTH TXCY6-XDBK8-PFJH2-C2GX3-GFWY7 QJ3YV-8HDJV-J8X2K-TXYV3-DJMQCW468R-W73H2-XW7M3-PYT2X-TBKCM P4GFV-3WHYH-J8BBH-XKY4P-WDCX7 QBD6P-D44PQ-822D4-P82PT-HYYCG CVB8P-QXQ3X-DD3JF-2YXC6-7HR3HV7GT4-2TDWM-4GG8B-8F26M-8VG8Q RJ88X-BKDJV-4CB6D-FQD3B-2WRQH RYBYW-G38RB-V78J8-T46CY-M7X9Y HDX4C-2F7CF-QBG6H-MKKD8-JM9494WPC6-TQ4BQ-2XTFQ-CP2VR-FB77P M7HCD-F8BCW-R3B7K-QYMYB-YPV7R DMXDT-WP2YF-K6Q2H-8CVXM-2MGHH PM3KK-KC2DV-PJFM4-PV8HT-MV6WJ VGFYJ-W7MFJ-FGH63-VFY3K-7CQ6K4KXKX-JJKFJ-4T7DY-7P2YJ-FR639 FVG6W-74R8V-43KTH-H6MF3-BRWMD FVG6W-74R8V-43KTH-H6MF3-BRWMD 8HBG3-X3CVW-Y88C4-V84PJ-YD99P DG2M4-D2R7C-6FVBK-Y3TD2-28J4BX42KY-W8CX3-RQGCR-QH2R2-Y327J 7BQC2-3W364-VXX6W-GPM67-XYDD6 MXYGB-8Q3XF-YXRJH-6J3M2-332TGV4JPF-VQT84-JMQVC-2P2K6-MQY2F7WT4C-J7TTF-78FHX-WFWVX-C6VYR X3RBM-VDVBC-R2KB7-4TB67-MP7YW Q7JX4-3KTBG-6V6VP-VRBDY-JHHCJX7T28-W4VXG-DFD4P-JP8B7-VXBMJ FP6W7-2TRY4-2VG7R-KDRDF-FDQ4K RCRMJ-WRC7F-TFT36-KYK3J-4JM49 WHK2R-F6FMQ-MTQB7-4D8HC-8XFXH 67CQV-V8XT4-RXPXH-WD72F-VDTFH F2XRK-DXTKX-FGGTW-W3HB3-QDV36 YG8TT-GFT2G-YC328-KBFWT-TMJH7 MBFRF-4PWW3-CXWMQ-7XDQV-7XP8T M7XDR-G4438-QGH3D-7HC3V-DMK3V CMX3K-J2PX2-2H2MT-JFYQW-693T9 HKY2H-HVV6B-VJ72T-DK22F-MTCCFQ7Q3F-7VPFF-WPWY2-CGWXX-PGF6Q 28QMD-7V6GK-8P2DX-TBWGC-FCPFC 72KCR-PTJHK-7VR3V-JXFTV-DGMXD V6MQ7-CPB8F-2K2QX-3BQM4-CM74X 3PXGR-DDF4K-K4KHP-Q7PBC-R9YVT QWCK3-K47KC-VP4HV-X4KPV-CDXRJ MFGG8-J4K42-KXJ8M-4GJJ4-7GQDVX8YK4-YW6FH-MGC8H-FXGJC-HR64V RMJ64-887KG-C6FF7-BBRRR-RHWPR TFTQM-XRPW6-HJMQG-JVMHD-T7RMG RVXXD-WYDCP-24M2T-22W2K-CP2CJ RKXJY-RHHGP-FY7T8-8GM48-TCJJH FVX3D-6JDD2-VRP28-MVWD8-JRR63 TBMVR-KKQ2V-H28YT-3JMBC-JGWVP QQRMX-784BF-KT26J-FBV38-6HT6C RKQ4D-MCPTW-7R8VX-F7XY6-687G6 HBMY4-XBFMR-8MGH3-QPRW7-78T6R MQKHH-JFGBH-PQKHF-3PTVM-BMYFV MG8KT-R4PCY-HCM8B-PPM7W-47DBG YYKPR-Q3HX7-4RHQG-YB7VC-YQPT984H67-MRR4X-R8GWP-BKRVX-B23H484H67-MRR4X-R8GWP-BKRVX-B23H4M8MGP-3763H-KRKDP-7QJVK-WBPFKC8MG6-TB8QY-XP2WD-DKC4D-TP7CR2QY33-C2YH4-BM7RT-CVDVT-FXC9XB4QBB-2Q7MT-M2XGG-QHDJT-GWH4G VKF4H-W2KFT-HW28K-6K2KK-WF32F WPY6P-MWTRM-DJ2B4-G22TC-VRWF8 TPB84-QWHKJ-7668V-RV7D3-WYFJH8H6G8-XWQ6R-PPC6H-G3C8K-XJ9WX VFMRJ-RG86J-GBHC6-RKTH4-6T43Q4DCVM-2FGMK-WG4JG-CQJYF-VJDHC W3B8K-QJCVH-CWT6W-GGPBF-4P83GV7WTH-6JXX2-H3KMB-FR4BP-VV7CH DWFJ6-JR8HB-BPJ2D-Q3BHW-C6MKD BJQTQ-6B84V-Q24WD-XV7BB-GDPFM XXVRG-GYWTF-CYVP7-JX2VX-P3H3RJ2DCD-2FHRF-DK68Q-J7RRB-DF4DY QKRX4-WP7HT-YQMBW-GTDQD-7MY42 6BMR4-VKP67-37PVB-BJ3BJ-8HCFP4PDGH-Y3GMV-JKFGG-G4T4T-D69QR BVDHG-3K8YX-QMYTT-QHXPF-JX4HB VH8VJ-HM6RT-VRHVX-37GDB-D78R38W7VD-6DHQW-6TP8D-BGVVY-F2BRW CH66Q-QGVBP-JDV3Y-722GM-MCJJM DYH3W-RJY6J-P2KFR-PFKCG-8BTDH4VK7K-337CP-6DJQX-XWDPH-87HWP8VMTQ-XCY23-6T3JT-HVHR2-CYD8B3WQHH-VC64P-4JVW3-GQFWP-RM8C6 MPTBC-38V42-FG8MR-X4C32-FRMR48YF4F-Y2VKT-48VCF-8FGB4-XWYXGPJ3BB-BJDBM-KK43K-M7GR6-GV6P3 GWMFW-BVTCH-MQDVC-TBPRY-HXWHQ BTFR4-MPHKT-46X2T-GC24C-3PD7H3B3BW-KD3Q2-6JXVQ-DMGBM-CVGMV KF64T-J22Q4-44QP8-6DRD6-RJ27X3Y424-C64BJ-F46X4-FTJBR-7HM7G HVTMG-QXD7W-MMCJM-QBR3R-JPHXC F3V7P-D7YRF-JCR3C-TT7FV-HF2FC FMRD8-HBXDP-TYDTY-77QWX-MJYF3 63KJD-C4T3W-JPYKP-QK4WD-HGW3Y7G347-33XHH-MQF3F-TB2Q2-Q697QM826F-FJ2PY-YGFYX-44VFJ-6FKKCJ6W3H-8YWWC-JFXF2-W2WJ4-DMQHV C4WPQ-DY888-HGC6M-CHHPY-Q47HJ JC428-44FPJ-F7JR4-6JVHC-D2HBFV2JCD-YV2KR-J2863-MH7GH-HK472M4VJ6-KVBMB-KRRVH-DHP3K-82DR88KRK6-W8HJM-83C8J-FPJFV-6X9J8 DXVDC-6TWCC-K3FDH-7MMG4-HVQCR KCJXT-QW6KK-GK4XR-6TTR2-QT4XRX6F83-H3KDD-76846-YPPYV-PRW82 YVQC8-WRM24-HPMR2-FJ72Q-3TX3K FMDWP-JJP6M-JFDXF-BW4FY-JC4KX RR4KB-RHCT8-XXFQG-H7T86-Q87DV YGV4W-HTGQ4-JGKRB-XXV2X-CK7BV BPYTJ-TKMWJ-R6M3Q-4PC8Q-B3VTM BRVCH-CK3GP-RMC68-BRT2P-GGPDK YRRCQ-QJJDX-Q8446-2HJ4M-VCX4B DYQVP-VXGWT-6WGWP-847KG-VX6CH HRJ3B-8HM6G-RHJKW-7GFKF-JV4QB KXKJD-MP3MD-VVDCY-FW4WH-PX8X4 4VXBR-RB8YJ-GYQMK-XCCWY-PVCY4 MWGM4-XJRCY-PCT2D-TWVGM-YQCXG TPPQQ-4HCRP-7X2BM-8M4WB-D67MD 87FV3-GBCTH-X4PQV-HVFPG-9GWFP HP3BF-CVPFT-2BMCF-DR7YM-GVHTF PP7FF-F7DMG-3YKVG-3YK2V-K6F2G6R6P3-TCM6Q-JKVCQ-G7QY4-7W28M KFVXF-G7F24-CK7HC-JXMYF-F4C3C2VWTK-R8T7V-C8PTF-MKY87-HGWQ7 YKC42-KC63F-YFPY4-K3YGM-B86C9 QTQCG-7YMCF-WRTJW-8GWQ2-MHPTJ 8YGWK-DHRJ6-YVC7K-MHHR3-R8PRJ KWTY7-8MXJD-KKYMR-GHCXC-VPRVT WVWP3-QH837-FD3YP-X2XFG-DXGYB QGT66-X3GVF-W764T-38K8Y-WGTF7 FWDQR-QWMFR-FT2JF-WDQRP-CF2Y3 3V8BT-73P8F-8J8YM-T2VJ8-YDTYRC646W-6FHJQ-4FBTR-GYQFG-TF8GW D3FVV-MDJJP-P83VR-88MKC-6C7QQG66TD-777MB-Y2MQJ-Y3KYB-QP7HR 333X2-VTW2X-MMKHH-DPTDF-MVVC4 KRVRR-6CQWH-Y6XGY-6YVGG-VB8GR RB2TT-XJVGR-GXXB2-D6MQC-FKKRG RFC68-HVXRD-WRXYJ-2P4QP-M4GVV JVBHH-PC7YC-H87CK-R6R6H-PKVX8 RKK4D-4H3QK-FJCFC-6XCMV-V47PF8XCHD-F6DHJ-78DG2-YHDQ2-9FT7T HH3M4-P6KWG-KW4J7-FWDTB-PCWWY HVJYR-43RDY-8KBK2-YCF64-XCK82 QKRF2-JQ33R-WDP6J-86DYP-TGWBB TH83F-Y2CW8-3FFYX-MBRH6-QGPXJ GH63D-JW6CD-7M86V-3R368-QGBMT WCKRW-DBGVH-HG87C-XW33V-Q64BW 3BD68-28BDK-3XWDK-TQGT4-DXDFG Q8HPX-F47TK-WDKGM-774TF-4WYMY 2BC48-3WC87-62F4W-YF8D7-JRGJ2 YYGXQ-HB4QJ-RG6DD-6F3BV-2DJHW 8XYX7-8V4GG-PQW6J-JWT3V-Q9GWC H483F-3Y8WH-32BMT-WHMBF-DPHD8 CGKQP-K7PQV-7BHGH-QK8PT-P7GRK TB8GV-JFV3G-V4QDH-DDFVX-HGQ6D 6WWHG-JBPB4-MHDF3-HV3FG-C4H6C 8JDD4-JBXF4-H6QYK-6QYRP-2GRTDY3RYY-44PXY-2TT3K-JDRXR-XJWHV KFBPG-WRHG7-6XVVD-RFXCW-WF6RC T3HCP-2XMTX-VTXHH-4JHMF-CTJW4 TGWKF-87R34-MDJMX-DB8C2-BW8K7 TGWKF-87R34-MDJMX-DB8C2-BW8K7 WDBTM-M2GC2-G6VKH-TWK4G-VRF23 VT3WX-FDKTC-PRPP7-W6QXR-93X93 74MJT-WBV78-M276R-PKRH8-BXDYJ JPT4K-VDDRG-JK4WQ-BTX7G-GQ6DQ 4KXVY-B6WKW-J8BW8-37KWK-J22RH 44YKG-XVWKX-V6HD6-QRWGV-62Y7V R6GMJ-GT6R3-74B3M-2HYGF-Y97PW YF38K-8J7WH-M2VRP-QG2FM-CBVDC 742JC-WWTQK-GWHY8-7CRW7-6PWWF 3VQ27-GXFCW-JR8K6-46BWV-4PHTR VPVJQ-HPCT2-WX38H-Y7Y7Y-B4K2C2KQYW-YHW3P-CH3X4-F86JM-RK23D FR7B3-PWBFH-V2X3Y-H3DC6-FMM4V 2JJ7P-6QBDD-YDMGK-28RVB-CTBJBD68MH-8V4KG-VVCHQ-B24BV-4DP3TJ7D8H-FW3Y2-JWT2R-CW4T3-T6RYY HQJDP-PFYXK-YKWWC-RX3T4-HT7JF KFBR8-J7QGD-D343R-XM7M6-9DQ6H BQ4YX-PDV6V-K3YRD-6RJJ7-DPRHPH7H7R-C2VRC-XK87X-DMTHB-7M4PC 6GG63-PHRVQ-BG3X7-48WXT-2XVDH RFRK8-HHGMY-BBMHQ-RCQF3-2BDM2 B728J-3P7FY-TRMRV-F7FCR-QC648 VV4JV-3QF73-PCFPC-HVWFX-DVR6T DBDVQ-PDYWM-P87B3-DXC3P-PWD3H 34VTV-BGTFP-BMWDT-XV4WK-32VCH6P6W6-HB3B3-6MQ3X-Y8DJP-TT7J2P64RC-836BM-VMGMF-P3YRP-2BHQ7 RVDM7-RJP4X-3JQRT-QP86V-FDCRR BC6JY-484YM-6878W-GCM7V-CVRTR TPH4R-XFQ3K-TD26T-KPKYC-GVFC2 RQT2K-RRX8J-46HKR-W6TCG-WC34D 47HQ8-DFKXM-JQHPD-4482X-3V62G JT8R3-CQXYV-DP2MV-JQKPX-CTB7G MKY4F-4HHDQ-MYXPG-JVJ4P-FXJXX F6Q88-2V6YQ-JC8CB-82TP7-JMHJY KVQQ4-BX72V-7HPVQ-VFPJT-G4F6R R7XKD-VTGX8-CD4CP-J6DQ4-9G8WT XHGBK-6GW3M-QJPRV-Y4GKQ-K2GC7。
自动进口许可证(加工贸易)代码

自动进口许可证(加工贸易)代码
摘要:
一、自动进口许可证(加工贸易) 代码的概述
二、自动进口许可证(加工贸易) 代码的具体内容
三、自动进口许可证(加工贸易) 代码的作用和意义
四、自动进口许可证(加工贸易) 代码与其他相关代码的区分
五、自动进口许可证(加工贸易) 代码在实际应用中的案例分析
正文:
自动进口许可证(加工贸易) 代码,是指在我国进行加工贸易业务时,所需的进口商品所需的许可证代码。
它是我国对外贸易的管理手段之一,通过对进口商品进行管理和控制,保障国家经济安全,促进加工贸易业务的有序进行。
自动进口许可证(加工贸易) 代码的具体内容包括:代码的编号、名称、适用范围、有效期限等。
这些信息都是根据我国的相关法律法规和政策规定,由商务部进行统一管理和公布的。
自动进口许可证(加工贸易) 代码的作用和意义主要体现在以下几个方面:一是对进口商品进行有效管理,防止不合格商品进入我国市场;二是保障国家经济安全,防止非法贸易和走私活动;三是促进加工贸易业务的有序进行,保障企业的合法权益。
自动进口许可证(加工贸易) 代码与其他相关代码的区分主要在于其适用范围和业务场景。
例如,一般贸易进口许可证代码适用于一般贸易进口业务,
而自动进口许可证(加工贸易) 代码则适用于加工贸易进口业务。
在实际应用中,自动进口许可证(加工贸易) 代码的案例分析可以帮助我们更好地理解和掌握这一代码的使用方法。
例如,一家从事加工贸易业务的企业,在进口原材料时,需要提供自动进口许可证(加工贸易) 代码,以便顺利完成进口手续。
英文物品报关类别

Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Cameras & Photo Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial Business & Industrial
MSC 订舱状态查询流程
点击 3. 订
。 舱 状 态 回 执 显 示
“预配标志”显示“N”代表船公司未确认放箱预配,并可将鼠标点在备注内容上,会显示 船公司未确认放箱预配原因 “预配标志”显示“Y”代表船公司已确认放箱预配 *由于 MSC 船公司确认放箱预配后, 需经由联东船代信息处理后才能至联东现场发放设备交 接单,所以可能产生我司网上显示“预配标志”为“Y”,联东网上未有放箱预配信息的 情况。如遇此情况,请客户稍后再至联东网上查询。
PDF created with pdfFactory Pro trial version
1. 客户在我司网站上成功提交了电子订舱信息,并在 下 , 按 或
界面 者 查
询,该票状态显示为
,才可查询 MSC 订舱回执
PDF created with pdfFactory Pro trial version
2. 客 户 需 点 击
, 在Байду номын сангаас界 面下可录 入单 票 或 者
MSC 订舱状态查询
一. 网站登入 1. 登入查询网址 /dczx/dczx.jsp,录入用户名、密码,然后登陆
PDF created with pdfFactory Pro trial version
2. 点击“网上营业厅” 二. MSC 订舱状态查询
www56solutioncomdczxdczxjsp录入用户名密码然后登陆pdfcreatedpdffactoryprotrialversionwwwpdffactorycommsc订舱状态查询客户在我司网站上成功提交了电子订舱信息并在界面询该票状态显示为才可查询msc订舱回执pdfcreatedpdffactoryprotrialversionwwwpdffactorycom预配标志显示n代表船公司未确认放箱预配并可将鼠标点在备注内容上会显示船公司未确认放箱预配原因预配标志显示y代表船公司已确认放箱预配由于msc船公司确认放箱预配后需经由联东船代信息处理后才能至联东现场发放设备交接单所以可能产生我司网上显示预配标志为y联东网上未有放箱预配信息的情况
法国erp号格式

法国erp号格式
法国ERP号,也被称为法国税号或VAT税号,是法国税务系统中的重要识别码,用于识别在法国境内进行合法经营的商户或个人。
ERP号的格式如下:
1、长度:ERP号通常为13位数字。
2、结构:前3位代表省份编码,接下来的5位是商户注册号,最后5位是校验码。
让我们更详细地了解ERP号的各个部分:
1省份编码(3位):这3位数字代表了商户所在法国的省份。
例如,巴黎的省份编码是75,而奥尔良的省份编码是80。
2商户注册号(5位):这5位数字代表了商户在法国的注册号。
每个商户在同一个省份内都有唯一的注册号。
3校验码(5位):这5位数字用于验证ERP号的真实性。
校验码的计算涉及到了前8位数字(省份编码和商户注册号),通过特定的算法得出。
要验证ERP号的真实性,可以使用以下步骤:
1、将前8位数字从左到右顺序相加。
2、将上一步得到的和的个位数记作加数A(如果和为0,则A为0;如果和为10,则A为1)。
3、将上一步得到的和的十位数记作加数B。
4、将加数A和加数B相加,再加上ERP号的第9位数字(即校验码)。
5、如果得出的结果能被10整除,那么这个ERP号就是真实的。
否则,ERP 号是无效的。
请注意,为了确保ERP号的唯一性,法国税务机构会定期检查并更新数据库中的信息。
因此,任何试图使用无效ERP号或不正当手段获得税号的商户都可能面临法律责任。
此外,由于ERP号涉及到税务问题,因此只有被授权的第三方或法国税务机构才能进行相关验证。
基于AIS数据的预抵船舶联系信息查询系统

基于AIS数据的预抵船舶联系信息查询系统
申慧超;胡勤友;杨春
【期刊名称】《上海海事大学学报》
【年(卷),期】2010(031)004
【摘要】为获取指定时间内到达指定区域或港口的船舶联系信息,开发基于自动识别系统(Automatic Identification System, AIS)数据的预抵船舶联系信息查询系统.该系统运用直接匹配、别名匹配和模板匹配等,结合预先建立的地理层次树,筛选出指定时间段内到达指定区域或港口的船舶;根据AIS数据与船舶资料数据库的关联,建立预抵船舶联系信息查询系统.实验表明,该系统可为船舶服务公司提供方便的查询途径,提高工作效率.
【总页数】4页(P13-16)
【作者】申慧超;胡勤友;杨春
【作者单位】上海海事大学,商船学院,上海,201306;上海海事大学,商船学院,上海,201306;上海海事大学,商船学院,上海,201306
【正文语种】中文
【中图分类】U675.79
【相关文献】
1.基于AIS信息的船舶抵离港频数的统计 [J], 刘茹茹;洪锋;刘传洋
2.基于AIS数据的船舶轨迹修复方法研究 [J], 张黎翔;朱怡安;陆伟;文捷;崔俊云
3.基于AIS数据的渔业船舶碰撞风险度评估模型 [J], 邵承谱;朱浩纲;温小飞;平弘
4.基于AIS数据的交汇水域船舶会遇态势辨识 [J], 马杰;李文楷;张春玮;张煜
5.基于AIS数据的预抵船舶联系信息快速查询方法 [J], 谭成兵;詹林
因版权原因,仅展示原文概要,查看原文内容请购买。
世界各国安规证书查询网站

推荐] 世界各国安规证书查询网站/forum/view_7_1.html1.CB查询2. UL查询C产品认证公众服务平台1 2009/06/30更新4.CSA查询5.VDE查询6.TCO 查询7.PSE(JQA)查询8. SEMKO, GS (Intetek ETL-SEMKO)9.ETL, Entela(Intetek ETL-Entela)10.电信终端设备、无线电通信设备进网许可证查詢C产品信息查询212. 英国ASTA 查询13.美国UL查询14.巴西认证资料的网址15意大利IMQ查询C产品信息查询317.TUV 莱茵证书查询18.TUV PS证书查询19.KEMA 证书查询20.ITS 证书查询21.BSI 证书查询20070823更正链接22.CB certificate查询23.中国电磁兼容认证中心查询24.瑞士ETSI查询25.瑞士SEV查询26.BSEN查询27.EN查询28.IHS查询29.IS查询30.AUSTRIA – OVE License check查询31.BELGIUM – CEBEC License check查询32.CANADA – CSA License check查询33.CETL License check查询34.FINLAND – FIMK License check查询35.FRANCE – LCIE License check查询36.GERMANY – VDE License check查询37.GERMANY-TUVRheinland License check查询38.GERMANY-TUV PS License check查询39.ITALY – IMQ查询HERLANDS – KEMA查询41.SINGAPORE查询42.SWEDEN – SEMK 查询43.TAIWAN查询44.United Kingdom– BSI查询45.United Kingdom– ASTA/BEAB查询46.JAPAN – JET查询47.JPA(PSE)48.[url=/searchresult1.asp]英。
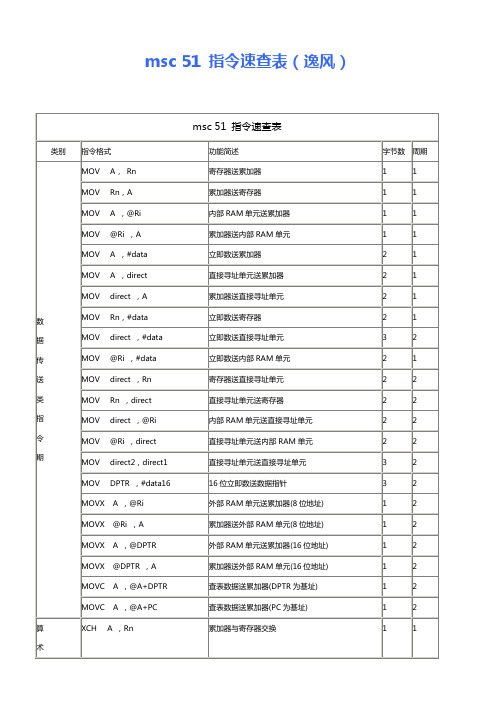
msc51指令速查表
1
DEC Rn
寄存器减1
1
1
DEC @Ri
内部RAM单元减1
1
1
DEC direct
直接寻址单元减1
2
1
MUL AB
累加器乘寄存器B
1
4
DIV AB
累加器除以寄存器B
1
4
逻
ANL A, Rn
累加器与寄存器
1
1
辑
运
算
类
指
令
ANL A, @Ri
累加器与内部RAM单元
1
1
ANL A, #data
累加器与立即数
msc 51 指令速查表(逸风)
msc 51 指令速查表
类别
指令格式
功能简述
字节数
周期
数
据
传
送
类
指
令
期
MOV A, Rn
寄存器送累加器
1
1
MOV Rn,A
累加器送寄存器
1
1
MOV A ,@Ri
内部RAM单元送累加器
1
1
MOV @Ri ,A
累加器送内部RAM单元
1
1
MOV A ,#data
立即数送累加器
1
1
POP direct
栈顶弹出指令直接寻址单元
2
2
PUSH direct
直接寻址单元压入栈顶
2
2
ADD A, Rn
累加器加寄存器
1
1
ADD A, @Ri
累加器加内部RAM单元
1
1
ADD A, direct
累加器加直接寻址单元
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Journal of Materials Science and Chemical Engineering,2014, 2, 57-62Published Online January 2014 (/journal/msce)/10.4236/msce.2014.210010Electrochemical Synthesis of CeB6 NanotubesH. B. Kushkhov, M. K. Vindizheva, R. A. Mukozheva, A. H. Abazova, M. R. TlenkopachevKabardino-Balkar State University, Nalchik, RussiaEmail: karashaeva@mail.ru, karashaeva@Received November 2013ABSTRACTThis work presents the results of joint electroreduction of tetrafluorborate and cerium-ions, and determines the conditions of electrochemical synthesis of cerium borides in KCl-NaCl melts at the 973 K on tungsten electrode by the linear and cyclic voltammetry. Based on the current-voltage studies the optimal modes of cerium boride electrodeposition were found.KEYWORDSMolten Chloride; Linear and Cyclic Voltammetry; Cerium Borides; High Temperature Electrosynthesis; Nanotubes1. IntroductionBorides of rare earth metals (REM) are widely used in various fields of modern technology. The electrochemi- cal synthesis of rare-earth borides at moderate tempera- tures (973 - 1023 K) is a cost-effective alternative to the direct solution-phase synthesis. The increased interest in the development of new efficient methods of producing rare earth borides are due to remarkable properties of these materials, such as chemical inertness, heat resis-tance, a wide range of electrochemical and magnetic pro- perties, etc. There is an indication of the using possibility cerium hexaboride for refractory production for use in neutral or reducing atmosphere and in vacuum at tem-peratures of 2000˚C or higher [1].Electroreduction from the molten salts is a specific method for the preparation of compounds of elements such as refractory metals, actinides and rare earth metals [2]. Manifold variations of electrolytic production of me- tals and compounds based on them—this is a great selec- tion of solvent, a variety of chemical and electrochemical characteristics of process and a temperature range, which is suitable for the process.Of the various methods for the synthesis of cerium borides is the closest way to get them through the elec- trolysis of molten media [3]. Electrolysis was carried out in graphite crucibles, serving both the anode and a cath- ode made of graphite or molybdenum. The composition of the bath electrolysis includes oxides of rare earth met- als and boric anhydride with additives of fluorides of alkali and alkaline earth metals to reduce the temperature and viscosity of the bath. Temperature electrolysis mix-ture was 1223 K - 1273 K, the voltage on the bath was 3.0 - 15.0 V, current density was 0.3 - 3.0 A/cm2. The composition of the bath for cerium hexaboride obtaining was: CeO2 + 2B2O3 + CeF3.As noted in work [3], the obtaining of the individual boride phase is practically impossible or very difficult. The disadvantages are also high temperature of synthesis and complexity of the product separation from the mol- ten electrolyte due to the low solubility of borates and fluoride, contamination by-products, such as borates. Thus, in view of the increasing use of rare earth metals and various materials on their basis and with the addition of rare earth metals in the various fields of science and technology, it is becoming an urgent task of obtaining these materials. A promising way to obtain rare earth, their alloys with other metals is the electrolysis of molten salts REM, as well as mixtures thereof.For effective use of the electrolytic method of produc- ing of metallic cerium and their alloys and compounds are necessary to have reliable information about electro- chemical behavior of complexes formed by cerium ions in molten salts, and joint electroreduction with compo- nents connections.Products quality of rare earth borides is determined by the purity and dispersion, namely original powder grain size, from which it is made. The product quality is higher when the grain size of compounds powder is smaller. Previously, we have investigated the processes of joint electroreduction of rare-earth metals with boron ions in KCl-NaCl and KCl-NaCl-CsCl melts at different elec-H. B. KUSHKHOV ET AL. 58trodes. It is shown that the electroreduction of fluorobo-rate ion occurs at more positive potentials than deposi-tion potential of metallic cerium [4-6].In the works [7-10] the electrochemical behavior ofboron, the laws of electrode processes at its refining, thesolubility of boron compounds in molten alkali metalchlorides are studied. In literature the processes of joint electroreduction cerium and boron ions in halide meltson a tungsten electrode are poorly understood.The aim of this work is to study the process of joint electroreduction of cerium ions with fluoroborate ions inmolten equimolar KCl-NaCl on the tungsten electrodeand the electrochemical synthesis of cerium borides 973K.2. ExperimentalChemicals and ApparatusExperiments were carried out in a sealed quartz cell(Figure 1) in the argon atmosphere, purified from tracesof moisture and oxygen, which is necessary in order to obtain reliable results.In three-electrode cell, the working electrode was the tungsten (d = 1.0 mm, purity > 99.95%) needle electrode. Tungsten is chosen as the material for the working elec-trode, since the boron and cerium insoluble therein [11]. As the reference electrode we used quasi-reversible glassy-carbon (SU-2000, d = 2.0 mm) rod electrode. The using of glassy-carbon quasi electrode help us to avoid the using of oxygen-diaphragms. Oxide ceramics are not compatible with the halide melts containing rare earth ions. Glassy-carbon quasi-stationary reference electrode, apparently, is a compromise electrode, and is determined by the redox potentials which are established with the participation of the various components of the molten medium. Therefore, its value depends on the melt com- position and temperature. Glassy-carbon quasi-stationary reference electrode was used in our studies [4], and pre-viously by the authors [11] in chloride and chloride- fluoride melts [12-14]. The auxiliary electrode was the glassy carbon crucible, which was the container for melt at the same time.Electroreduction of cerium and tetrafluorborate ions was investigated by cyclic voltammetry. The current- voltage dependence was obtained by the electrochemical complex Autolab PGST 30 (Ecochemic, Holland), which was paired with computer. It has been estimated value of ohmic IR drop in the electrolyte at a time-dependent po-larization mode. The specific conductivity of molten po-tassium, sodium and cesium chlorides is 0.5 ohm−1cm−1. At the maximum distance of 0.5 cm between the refer- ence electrode and the working electrode and a current of 10 mA, scanning rate of 10 V/s the ohmic drop is about 10 - 15 mV. In addition, the electrochemical complex Figure 1. Scheme of high temperature electrochemical quartz cell.Autolab PGST 30 allows a survey of current-voltage curves with the IR-compensation.Potentiostatic electrolysis was carried out using a power supply with a current load of up to 5A.The salts preparation method was following. Sodium and potassium chlorides qualification «analytical grade» were recrystallized, calcined in a muffle furnace, mixed in the desired ratio (an equimolar mixture), and placed in an alundum crucible into glass. A glass cell was evacu-ated to a residual pressure of 0.7 Pa, first at room tem-perature and then heated at progressively stepped up to 473˚C, 673˚C, 873˚C. Then it was filled with inert gas (argon) and melted.Cerium ion added to the melt in the form of anhydrous cerium trichloride (99.9%, ultra- dry, Ltd. “Lanchi”). To avoid the formation of oxychlorides, experiments were performed under purified argon and dried in a sealed cell. Potassium tetrafluoroborate KBF4qualification “reagent grade” was washed in HF, than in ethanol, after than it was dried. All operations with anhydrous salts were car-ried out in glovebox mBraun Labstar 50 in the argon atmosphere.Products of electrolysis were identified by DRON-6 and observed by scanning electron microscope (SEM) Vega 3 TESCAN. Particle size was measured by laser diffractive analyzer Fritsch Analysette-22 Nanotech (Germany).3. Results and DiscussionCyclic current-voltage curves in the KCl-NaCl chlorideH. B. KUSHKHOV ET AL. 59 melt by adding cerium trichloride and potassium fluoro-borate are shown in Figure 2. Curve 1 in this figurerepresents the voltammogram of background electrolyte—equimolar molten KCl-NaCl. The absence of anywaves in it, and low leakage current at relatively highnegative potentials allows us to draw conclusions aboutthe cleanliness of the background electrolyte. When weadd in the background melt cerium trichloride C(CeCl3)= 4,3 × 10−4 mol/cm3 (Figure 2(a), curve 2) at potentials−(2.2 ÷ 2.3) V relative glassy-carbon quasi-stationaryreference electrode on voltammogram appears well re-producible reduction wave of cerium ions. The fluorobo-rate ions reduction wave observed at potentials −(1.3 ÷1.5) V (Figure 2(b), curve 2).To determine the sequence of the process of joint elec- troreduction fluoroborate ion and cerium complex ions tungsten electrode polarization at different potentials of return was held (Figure 3), corresponding to a reduction potential of boron, potential of joint electroreduction and potential recovery of pure cerium. This shooting is possi- ble to correlate the waves observed on the anode and ca- thode regions of the voltammograms in cyclic polariza-tion.This picture can be assumed that the shift of reduction potential of complex halide cerium ions to the region of more positive values of the potential was not only due to the changing nature of the substrate, but also the inte- raction of cerium with the deposited boron observed. Pre-wave, which was observed on the voltammograms before a wave of pure cerium reduction corresponds to the reducing of cerium on deposited boron.With increasing concentration of fluoroborate ion with respect to the initial concentration of cerium chloride com- plexes in the cyclic voltammogram (Figure 4) are merged wave electroreduction fluoroborate ion and chlo- ride complexes of cerium in the stretched along the axis(a) (b)Figure 2. Cyclic voltammograms of NaCl-KCl melt on tungsten electrode (vs SU) adding (a) cerium trichloride, C(CeCl3) = 4.30 × 10−4mol/cm3 (curve 2). V = 0.1 V/s. S = 0.21 cm2; (b) potassium fluoroborate, C(KBF4) = 3.1 × 10−4 mol/cm3 (curve 2). V = 0.2 V/s. S = 1.6 cm2. Curve 1, back-ground electrolyte. T = 973 K. Figure 3. Cyclic voltammograms of NaCl-KCl-СеCl3 (3.1 × 10−4mol/cm3) KBF4 (3.1 × 10−4mol/cm3) melt at different return potentials, V: 1, (−2, 5); 2, (−2.2); 3, (−2. 0); 4, (−1.6), 5, 1.0. Т = 973 К. V = 0.05 В/c. S = 1.6 cm2.(a) (b) (c)Figure 4. Cyclic voltammograms at different return poten-tials, V: 1, 3.0; 2, 2.6; 3, 2.2; 4, 2.0; 5, 1.5. Т = 973 К. V = 0.1 V/s. S = 1.6 cm2: a) NaCl-KCl-СеCl3 (3.1 × 10−4mol/cm3) KBF4 (3.1 × 10−4mol/cm3); (b) NaCl-KCl-СеCl3 (3.1 × 10−4 mol/cm3) KBF4 (6.0 × 10−4mol/cm3);(c) NaCl-KCl-СеCl3 (3.1 × 10−4 mol/cm3) KBF4 (15 × 10−4 mol/cm3).of the wave potentials of reduction, which we attribute to the formation alloys cerium with boron.Further inc- reasing the concentration of fluoroborate ion n the melt leads to the formation only of boride phases.Our investigations can be concluded that the electro- synthesis of cerium borides is conducted only in the ki-netic mode. Consequently, the electrochemical synthesis process can be represented like successive stages: •reducing of more electropositive component (boron), •reducing of more electronegative component (cerium) on pre-selected boron,•mutual diffusion of cerium and boron to form the different boride phases up to the higher boride CeB6. The electrochemical processes that occurring during the formation of cerium borides can be represented by the following equations:()4x xBF Cl3e B4x F xCl−−−−−+→+−+(1)H. B. KUSHKHOV ET AL .60 ()6y 3y CeCl F 3e Ce 6y Cl yF −−−−−+→+−+ (2)p q qB pCe Ce B += (3)Results obtained at investigation of the joint electro-reduction of cerium halide ions and tetrafluoroborate ions were taken as a basis for the practical implementation of electrochemical synthesis of cerium hexaborides CeB 6.4. Electrochemical Synthesis of Cerium BorideThe electrosynthesis of cerium borides nanotubes was performed in a molten mixture of NaCl-KCl-CeCl 3-KBF 4 at 973 K on tungsten electrode in the range up −2.4 to −2.8 V to relative a quasi-stationary glassy carbon elec- trode.The select of electrolytic bath components was done on the basis of thermodynamic analysis and kinetic mea- surements of joint electrowinning of cerium and boron from halide melts. From the compounds of boron and cerium, which do not contain oxygen, cerium chloride and potassium tetrafluoroborate are fairly low melting point and good solubility in KCl-NaCl melt. This solvent was chosen because the decomposition voltage of the molten mixture KCl-NaCl more stress decomposition melts CeCl 3 and KBF 4, and because the alkali metal chlorides are highly soluble in water. These properties are necessary to the washing of cerium borides (Figure 5).The individual phase of boron, higher boride CeB 6 and the mixture of phases, including CeB 4 (Figure 6) were obtained in depending on the composition and the syn- thesis parameters. The purpose of electrosynthesis opti-mization was to obtain higher boride CeB 6 with most valuable properties.When we chose the concentration ratios of CeCl 3 and KBF 4, we take into account the first stage of electro- synthesis, during which the reducing of more electro-positive boron was done. Electroreduction of the cerium was started when KBF 4 concentration was ended. In these temperature conditions the optimum concentration of KBF4 is about (1.0 ÷ 1.5) × 10−3 mol/cm 3. According to our study, at higher concentrations of KBF 4 the cerium borides getting were complicated by instability of cath-ode deposit.(а) (b) (c)Figure 5. “Cathode-salt pear” (a), the product of electroly-sis before washing (b), and the resulting powder after washing (c).Figure 6. Radiographs of cerium boride powder obtained in KCl-NaCl-CeCl 3 (3.1 × 10−4 mol/cm 3) KBF4 (6.0 × 10−4 mol/cm 3) melt on tungsten electrode. U = −2.5 B: a) the line 1, CeB 6; 2, CeB 4; b) 1, CeB 6; 2, CeB 4; 3, B.The ceruim borides electrosynthesis was held in po-tentio and galvanostatic modes. It was observed that these modes are not equal. At galvanostatic electrolysis the true value of the current density is known only in the initial period of time, because during electrolysis varies significantly in cathode area. In most cases we used the potentiostatic electrolysis because the voltage (potential) determines the course of the reactions and monitors the reaction of deposition. If the anode material is glassy carbon and the voltage in the bath U < −1.8 V, the cath-ode deposit consists mainly is boron. Provided the volt-age U = −(1.8 - 2.5) V the mixture of different phases (B and CeB 4) was obtained. If the voltage U = −(2.5 - 2.8) V, the cathode deposit consists from higher boride CeB 6. The duration of the electrosynthesis was affected to the composition of the cathode deposits. The data in Ta-ble 1 show the dependence of the phase composition of the cathode deposits from the duration of electrolysis in the electrolyte of optimal composition, as well as tem-perature and voltage.The optimal duration of the high-temperature electro-chemical synthesis for prepare of CeB 6 is 90 - 120 min-utes. Thus, the synthesis of cerium borides was deter-mined by the following interrelated parameters: the composition of the electrolytic bath, the voltage and the temperature. The optimal values of these parameters was as follows: the composition of the melt, wt. %: CeCl 3 (3.5 ÷ 7.0), KBF 4 (4.5 ÷ 10.0), the rest—mixture of NaCl-KCl; voltage bath −(2.6 ÷ 2.8) V, time electrolysisH. B. KUSHKHOV ET AL . 61is 90 ÷ 120 min, the temperature is 973 K.Phase composition of the “cathode-salt pears” identi-fied by X-ray analysis using a DRON-6 (Figure 7).Particle size was measured by laser diffraction ana- lyzer Fritsch Analysette-22 (Figure 7), and the order of 50 - 100 nm. The surface of the resulting powders have also examined using the digital scanning electron micro-scope Vega 3 TESCAN (Figure 8). The yield of the single-phase CeB 6 was 0.20 - 0.30 g/А × hour. Specific surface area of ultra-dispersive pow- ders of CeB 6 was 5 - 10 m 2/g.Our work was focused on the cathode deposit treat- ment. The comparative radiographs were made before and after different options of the cathode deposit wash-ing.The experiments showed that the best option of powdersTable 1. Electrochemical synthesis parameters, T = 973 K, cathode—W.Electrolyte composition, wt.% Voltage E, VTime τ, minPhaseParticle sizeNaCl—40.86; KCl—52.01; CeCl 3—4.66; KBF 4—2.48 −2.550 - 100CeB 490 - 110 HM2) Molar ratio CeCl 3:KBF 4 = 1: 2NaCl—39.88; KCl—50.75; CeCl 3—4.53; KBF 4—4.83 −2.680 - 100CeB 650 - 70 HM3) Molar ratio CeCl 3:KBF 4 = 1:5NaCl—37.39; KCl—47.59; CeCl 3—4.25; KBF 4—10.76−2.790 - 100CeB 670 - 90 HMFigure 7. Particle size distribution obtained by the electrochemical synthesis of 973K melt composition, wt.%: KCl (39.92)- NaCl (50.8)-KBF 4(4.57)-CeCl3(4.68); i = 0.3 A/cm2.Figure 8. SEM images of the CeB 6.H. B. KUSHKHOV ET AL. 62washing was the washing in distilled water, post-treat- ment with ammonium hydroxide solution and washing by KF than distilled water by decantation and centrifuga-tion then by washing with double-distilled water. Thus, to obtain reliable information on the phase composition of the synthesized compounds by electroly-sis and the possibility of direct electrochemical synthesis CeB6 nanotubes in halide melts.5. ConclusionThe joint electroreduction of tetrafluorborate and ce-rium-ions was conducted in equmolar NaCl-KCl melt on tungsten electrode at 973 K by cyclic voltammetry. The analysis of voltammograms was shown that the electro-synthesis in studied systems proceeds in the kinetic mode because reducing potentials of boron and cerium is very different. The results of this research found that under certain conditions, the concentrations of cerium and bo-ron and certain anionic composition of the melt are pos-sible for their joint electroreduction.Synthesis of cerium borides nanotubes was carried out by potentiostatic electrolysis of molten KCl-NaCl, con-taining CeCl3 and KBF4. Electrolysis performed on tung-sten electrode in the range of −2.4 to −2.8 V relatively of the quasi-stationary glassy-carbon electrode. The influ-ence of the electrolyte composition, temperature, current density, voltage and the duration of electrolysis on the synthesis products was studied. An optimal parameter for getting cerium boride CeB6 nanotubes was found. AcknowledgementsThe work was done using equipment of Access Center “X-ray diagnosis of materials” with the financial support of the Ministry of Education and Science of Russian Fed- eration, the state contract No. 16552.11.7074.REFERENCES[1]G. V. Samsonov and Y. B. Paderno, “Borides of Rare-Earth Metals,” Kiev. Publishing House “SA USSR”, 1961.[2]P. Taxil, P. Chamelot, L. Massot and C. Hamel, “Elec-trodeposition of Alloys or Compounds in Molten Salts and Applications,” Journal of Mining and Metallurgy,Vol. 39, No. 1-2B, 2003, pp. 177-200.[3]G. V. Samsonov, “Refractory Compounds of Rare-EarthMetals and Non-Metals,”Publishing House Metallurgie,Moscow, 1964, pp. 53-55.[4]M. K. Vindizheva, R. A. Karashaeva, S. A. Shermetova,et al., “The Investigation of Mechanism of Joint Elec-troreduction Cerium-Ionsand Fluoroborate-Ions in NaCl-КCl-CsCl Melt,” Scientific Works of Young Scientists,Nalchik, 2006, pp. 293-295.[5]Kh. B. Kushkhov, M. K. Vindizheva, R. A. Mukozhevaand M. R. Tlenkopachev, “The High Temperature Elec-trochemical Syntheses of Refractory Compounds of Sa-marium and Boron in Halide Melts,” Izvestiya KBSU, Vol.1, No. 2, 2011, pp. 29-34.[6]Kh. Kushkhov, M. Vindizheva, R. Mukozheva, M. Na-fonova and M. Tlencopachev, “The ElectrochemicalSyntheses of Lanthanum Borides in Halide Melts,” Mate-rials of XV Russian Conference of Physical Chemistry and Electrochemistry Molten Salts and Solid Electrolytes,Nalchik, 2010 , pp. 181-182[7]O. V. Chemezov, “Electrochimiskoe Povedenie Bora vKhloridnih I Khloridno-Ftoridnih Rasplavah,” Avtoref.diss. kand. chim. Nauk, Sverdlovsk, 1987, 17 p.[8]V. Danek, L. Votava and B. Matisovsky, “Reactions ofPotassium tetrafluorocborate in Molten Alkali Chlorides,”Chem. Izvestiya, Vol. 30, 1976, pp. 377-383.[9]L. P. Polyakova, G. A. Bukatova, E. G. Polyakov, E.Christensen, J. H. von Barer and N. J. Bjerrum, “Electro-chemical Behaviour of Boron in LiF-NaF-KF-Melts,”Journal of The Electrochemical Society, Vol. 143, No. 10.1996, pp. 3178-3186./10.1149/1.1837184[10]P. Fellner, M. Makita, K. Matishovski and A. Silny, “TheMechanism of Kathodic Process at Electrodeposition ofBoron from Ionic Melts,” V Conference of SocialistCountries in Chemistry of Ionic Melts, Kiev, 1984, p. 43.[11] F. A. Shank, “Structure of Double Alloys,” MetallurgyLtd., Moscow, 1973, p. 45.[12]S. A. Kuznetsov, H. Hayashi, K. Minato and M. Gaune-Escard, “Electrochemical Behavior and Some Thermo-dynamic Properties of UCl4and UCl3Dissolved in a LiCl-KCl Eutectic Melt,” Journal of The ElectrochemicalSociety, Vol. 152, 2005, p. 203./10.1149/1.1864532[13]S. A. Kuznetsov and M. Gaune-Escard, “Kinetics ofElectrode Processes and Thermodynamic Properties of Europium Chlorides Dissolved in Alkali Chloride Melts,”Journal of Electroanalytical Chemistry, Vol. 595, 2006, p.11. /10.1016/j.jelechem.2006.02.036 [14]S. A. Kuznetsov, “Molten Salts: From Fundamentals toApplications,” M. Kluwer Acad. Publ., Norwell, Vol. 52,2002, p. 283.。
