Ivabradine hydrochloride_148849-67-6_DataSheet_MedChemExpress
钛酸异丙酯产品安全技术说明书(阿拉丁)

GHS02:易燃物; GHS06:急毒性物质钛酸异丙酯Titanium(IV) isopropoxide99.99%CAS No. 546-68-9EC-编号208-909-64急救措施4.1必要的急救措施描述一般的建议请教医生。
向到现场的医生出示此安全技术说明书。
如果吸入如果吸入,请将患者移到新鲜空气处。
如呼吸停止,进行人工呼吸。
请教医生。
在皮肤接触的情况下用肥皂和大量的水冲洗。
请教医生。
在眼睛接触的情况下用大量水彻底冲洗至少15分钟并请教医生。
如果误服禁止催吐。
切勿给失去知觉者喂食任何东西。
用水漱口。
请教医生。
4.2最重要的症状和影响,急性的和滞后的无数据资料4.3及时的医疗处理和所需的特殊处理的说明和指示无数据资料5消防措施5.1灭火介质火灾特征无数据资料灭火方法及灭火剂干粉 干砂不要用水喷射。
5.2源于此物质或混合物的特别的危害无数据资料5.3救火人员的预防如有必要,佩戴自给式呼吸器进行消防作业。
5.4进一步的信息喷水冷却未打开的容器。
6泄露应急处理6.1人员的预防,防护设备和紧急处理程序使用个人防护装备。
避免吸入蒸气、气雾或气体。
保证充分的通风。
消除所有火源。
注意蒸气积累达到可爆炸的浓度,蒸气可蓄积在地面低洼处。
6.2环境预防措施如能确保安全,可采取措施防止进一步的泄漏或溢出。
不要让产品进入下水道。
6.3抑制和清除溢出物的方法和材料围堵溢出物,用非可燃性材料(如砂子、泥土、硅藻土、蛭石)吸收溢出物,将其收集到容器中,根据当地的或国家的规定处理6.4参考其他部分丢弃处理请参阅第13节。
7安全操作与储存7.1安全操作的注意事项避免接触皮肤和眼睛。
避免吸入蒸气或雾滴。
火舌回闪有可能穿过相当长的距离。
容器遇火可能会爆炸切勿靠近火源。
-严禁烟火。
采取措施防止静电积聚。
7.2安全储存的条件,包括任何不兼容性在氩气下操作,避免潮湿。
储存于氩气中 使容器保持密闭,储存在干燥通风处。
67种内分泌干扰物质

2,4,52,4,5-三氯苯氧基乙 Trichlorophenoxyacetic acid 酸(2,4,5-滴) 2,4-Dichlorophenoxyacetic acid Amitrole Atrazine Alachlor Simazine(CAT) Hexachlorocyclohenane Ethyl Parathion Carbaryl Chlordane Oxychlordane Trans-Nonachlor 1,2-dibromo-3DDT DDE and DDD Kelthane(Dicofol) Aldrin Endrin Dieldrin Endosulfan (Benzoepin) Heptachlor Heptachlor epoxide Malathion Methomyl
2,4-二氯苯氧基乙酸 除草剂 (2,4-滴) 杀草强 莠去净 草不氯 西玛津 六氯环己烷 乙基对硫磷 西维因 氯丹 氧化氯丹 反式-九氯 1,2-二溴-3-氯丙烷 滴滴涕 滴滴伊和滴滴滴 三氯杀螨醇 艾氏剂 异狄氏剂 狄氏剂 硫丹 七氯 环氧七氯 马拉硫磷 灭索威 除草剂、树脂硬化剂 除草剂 除草剂 除草剂 杀虫剂 杀虫剂 杀虫剂 氯丹的代谢中间产物 杀虫剂 杀虫剂 杀虫剂 杀虫剂 (DDT的代谢中间产物) 杀虫剂 杀虫剂 杀虫剂 杀虫剂 杀虫剂 杀虫剂 七氯的代谢中间产物 杀虫剂 杀虫剂
化学物质名称中文名用途furans非产品polychlorinatedbiphenylpcbs多氯联苯polybromobiphenylppb多溴联苯防火材料hexachlorobenzenehcb六氯苯杀菌剂pentachlorophenolpcp五氯苯酚杀菌消毒剂除草剂防腐剂245trichlorophenoxyaceticacid除草剂24dichlorophenoxyaceticacid除草剂除草剂10alachlor除草剂11simazinecat除草剂12杀虫剂13carbaryl杀虫剂14chlordane杀虫剂15oxychlordane氧化氯丹氯丹的代谢中间产物16transnonachlor杀虫剂1712dibromo3chloropropane杀虫剂18ddt滴滴涕杀虫剂19ddeddd滴滴伊和滴滴滴20kelthanedicofol杀虫剂21aldrin杀虫剂22endrin杀虫剂23dieldrin杀虫剂24endosulfanbenzoepin杀虫剂25heptachlor杀虫剂26heptachlorepoxide七氯的代谢中间产物27malathion马拉硫磷杀虫剂28methomyl杀虫剂29methoxychlor甲氧滴滴涕杀虫剂30mirex杀虫剂31nitrofen除草醚除草剂32toxaphenecampechlor毒杀芬杀虫剂33tributyltin鱼网之防腐剂船上抗腐蚀油漆34triphenyltin鱼网之防腐剂船上抗腐蚀油漆35trifluralin除草剂36表面活性剂的降解产物67种内分泌干扰物质二恶英及其多氯代苯并呋热媒无碳复写纸电容器变压器绝缘245三氯苯氧基乙酸245滴24二氯苯氧基乙酸24hexachlorocyclohenaneethylparathion六氯环己烷乙基对硫反式九氯12二溴3氯丙烷杀虫剂ddt的代谢中间产物alkylphenolfromc5c9烷基酚从c5至c937bisphenol合成树脂原料38di2ethlhexylphthalate塑胶的塑化剂39butylbenzylphthalate塑胶的塑化剂40dicyclohexylphthalate塑胶的塑化剂41dicyclohexylphthalate塑胶的塑化剂42diethylphthalate塑胶的塑化剂43benzoapyrene苯并芘非产品44dichloropheno
Healthy Blue SC会员手册说明书
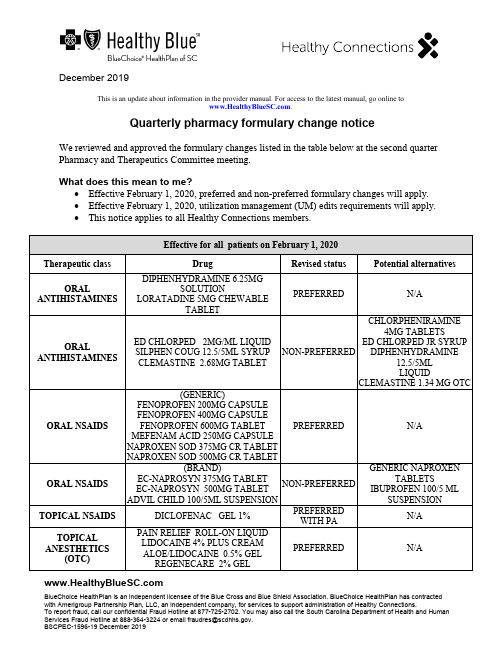
December 2019BlueChoice HealthPlan is an independent licensee of the Blue Cross and Blue Shield Association. BlueChoice HealthPlan has contracted with Amerigroup Partnership Plan, LLC, an independent company, for services to support administration of Healthy Connections.To report fraud, call our confidential Fraud Hotline at 877-725-2702. You may also call the South Carolina Department of Health and Human ************************************************************.BSCPEC-1596-19 December 2019This is an update about information in the provider manual. For access to the latest manual, go online to .Quarterly pharmacy formulary change noticeWe reviewed and approved the formulary changes listed in the table below at the second quarter Pharmacy and Therapeutics Committee meeting.What does this mean to me?• Effective February 1, 2020, preferred and non-preferred formulary changes will apply. • Effective February 1, 2020, utilization management (UM) edits requirements will apply. • This notice applies to all Healthy Connections members.Effective for all patients on February 1, 2020Therapeutic class DrugRevised status Potential alternativesORALANTIHISTAMINESDIPHENHYDRAMINE 6.25MGSOLUTIONLORATADINE 5MG CHEWABLETABLETPREFERREDN/AORALANTIHISTAMINESED CHLORPED 2MG/ML LIQUID SILPHEN COUG 12.5/5ML SYRUP CLEMASTINE 2.68MG TABLET NON-PREFERRED CHLORPHENIRAMINE4MG TABLETSED CHLORPED JR SYRUPDIPHENHYDRAMINE12.5/5MLLIQUIDCLEMASTINE 1.34 MG OTCORAL NSAIDS(GENERIC)FENOPROFEN 200MG CAPSULE FENOPROFEN 400MG CAPSULE FENOPROFEN 600MG TABLET MEFENAM ACID 250MG CAPSULE NAPROXEN SOD 375MG CR TABLET NAPROXEN SOD 500MG CR TABLETPREFERRED N/A ORAL NSAIDS(BRAND) EC-NAPROSYN 375MG TABLET EC-NAPROSYN 500MG TABLET ADVIL CHILD 100/5ML SUSPENSION NON-PREFERRED GENERIC NAPROXENTABLETSIBUPROFEN 100/5 ML SUSPENSIONTOPICAL NSAIDSDICLOFENAC GEL 1% PREFERREDWITH PAN/A TOPICALANESTHETICS(OTC)PAIN RELIEF ROLL-ON LIQUIDLIDOCAINE 4% PLUS CREAMALOE/LIDOCAINE 0.5% GELREGENECARE 2% GELPREFERRED N/ALIDODOSE 3% GELREGENECARE SPRAYALOCANE 4% GELAFTERBURN 2.5% GELXOLIDO 2% CREAM BURN RELIEF 0.5% AEROSAL ASPERCREME 4% SPRAYLIDOCAINE 3% CREAMLIDOCAINE 4% CREAMLIDOCAINE 5% CREAMAFTERSUN 0.5% GELLIDOCAINE 4% PADTOPICAL ANESTHETICS(RX)LIDOCAINE 3% CREAMLIDOCAINE 5% OINTMENT NON-PREFERREDOTC LIDOCAINEPRODUCTSRX LIDOCAINE5% PATCH(PA REQUIRED)MISCELLANEOUS ANTICONVULSANTSPREGABALIN 25MG CAPSULEPREGABALIN 50MG CAPSULEPREGABALIN 75MG CAPSULEPREGABALIN 100MG CAPSULEPREGABALIN 150MG CAPSULEPREGABALIN 200MG CAPSULEPREGABALIN 225MG CAPSULEPREGABALIN 300MG CAPSULEPREGABALIN SOL 20MG/MLPREFERREDWITH NO PRIORAUTHORIZATION(PA)N/AATOPICDERMATITIS PIMECROLIMUS 1% CREAMPREFERREDWITH STEPTHERAPY (ST)N/AFIBRATESFENOFIBRATE 130MG CAPSULEFENOFIBRATE 145MG TABLETFENOFIBRIC 35MG TABLETFENOFIBRIC 105MG TABLETFENOFIBRIC 135MG DR CAPSULENON-PREFERREDWITH STFENOFIBRATE134MG, 160MG, 200MG,43MG, 48MG,54 MG,67 MGFENOFIBRIC ACID 45 MGALCOHOL SWABS (MANUFACTURERS) GLOBAL DIABETICRITE AID NON-PREFERREDMANUFACTURERSBD DIABETESDYNAREXHEALTH MARTULTIMEDALCOHOL SWABS (MANUFACTURERS) BD DIABETESDYNAREXHEALTH MARTULTIMEDPREFERRED N/AIRON SUPPLEMENTS (GENERIC OTC)IRON 45MG TABLETSLOW-RELEASE FE 45MG TABLETHEMAX TABLETGENTLE IRON 28MG CAPSULEHIGH POTENCY FE 27MG TABLETNU-IRON 150 150MG CAPSULEABATRON AF TABLETSLOW IRON 50MG TABLETPREFERRED N/AFERGON 27MG TABLETIRON SUPPLEMENTS(BRAND OTC)FOLITAB 500 TABLET IRON 28MG TABLETFERROUS GLUC 324MG TABLETEZFE 200MG CAPSULEFERROUS GLUC TAB 324MGFERROUS SULF 324MG EC TABLETFERRETTS 325MG TABLETFERREX 150MG CAPSULEFERREX 28 MIS FERREX 150 PLUS CAPSULE FERREX 150 FORTE PL CAPSULECHEWABLE IRONPEDIATRIC IRON CHEWABLEFERROUS SUL 220/5ML LIQUIDFERROUS SULF 300/5ML SYRUPFEOSOL 200MG TABLETSLOW RELEASE FE 143MG CRTABLETNON- PREFERRED OTC GENERIC IRONSUPPLEMENTSRX PRODUCTS:HEMATOGEN FA CAPSULEHEMETAB TABLETMULTIGEN TABLETMULTIGEN PLS TABLETMULTIGEN FOLICTABLETFERRAPLUS 90 TABLETTARON FORTE CAPSULEFOLIVANE-F CAPSULEFOLIVANE-PLS CAPSULECENTRATEX CAPSULEIRON SUPPLEMENTS(PRESCRIPTIONSTRENGTH)IFEREX 150 FORTE CAPSULE HEMATOGEN CAPSULE HEMATOGEN FORTE CAPSULE TRICON CAPSULE MYFERON 150 FORTE CAPSULE FERROCITE PLUS TABLET FEROCON CAPSULE PUREVIT DUA FE PLUS CAPSULE HEMATINIC PL VIT/MIN TABLET HEMATINIC/FA TABLET POLY-IRON 150 FORT CAPSULE CORVITA 150 TABLET TRIGELS-F FORTE CAPSULE TL ICON CAPSULE SE-TAN PLUS CAPSULE NON- PREFERRED OTC GENERIC IRON SUPPLEMENTSRX PRODUCTS:HEMATOGEN FA CAPSULE HEMETAB TABLET MULTIGEN TABLET MULTIGEN PLS TABLET MULTIGEN FOLIC TABLET FERRAPLUS 90 TABLETTARON FORTE CAPSULE FOLIVANE-F CAPSULEFOLIVANE-PLS CAPSULE CENTRATEX CAPSULEUM edits — effective for all members no later than February 1, 2020 No changes in preferred/non-preferred status revision or addition to UM edit onlyANDROGENS*JATENZO CAPSULE ADD ST WITH QUANTITY LIMITS (QL)58 MG AND 198 MG QL: 4 PER DAY 237 MG QL: 2 PER DAY ANTICONVULSANTSNAYZILAM SPRAY 5MG ADD PA WITH QLQL: 50 MG PER 30 DAYS ANTICONVULSANTSOXTELLAR XR 150 MGOXTELLAR XR 600 MGREVISED QL LIMIT:150 MG: 3 TABLETS PER DAY 600 MG: 4 TABLETS PER DAYANTINEOPLASTICAGENTSPIQRAY 200 MG TABLETSPIQRAY 250 MG TABLETSPIQRAY 300 MG TABLETSADD PA WITH QL QL: 1 CARTON PER 28 DAYS ANTINEOPLASTICAGENTSXPOVIO PAK 60MGXPOVIO PAK 80MGXPOVIO PAK 100MGADD QL 1 CARTON PER 28 DAYSANTINEOPLASTICAGENTSNUBEQA 300MG TABLET ADD QL 4 TABLETS PER DAY ANTINEOPLASTICAGENTS TURALIO CAP 200MG ADD QL 4 TABLETS PER DAY ANTINEOPLASTICAGENTS PIQRAY 200MG TAB DOSE PIQRAY 300MG TAB DOSE PIQRAY 250MG TAB DOSE REVISE QL1 CARTON PER 28 DAYS CHOLESTEROLAGENTS EZALLOR SPRINKLE 5 MG CAP EZALLOR SPRINKLE 10 MG CAP EZALLOR SPRINKLE 20 MG CAP EZALLOR SPRINKLE 40 MG CAP ADD PA AND QLQL: 1 TABLET PER DAY COPD AGENTS DUAKLIR 400/12 INHALER ADD ST AND QLQL: 1 INHALER PER 30 DAYSCYSTIC FIBROSISAGENTSKALYDECO PAK 25MG ADD QL2 PACKETS PER DAYCYSTIC FIBROSISAGENTSORKAMBI GRANULES ADD QL2 PACKETS PER DAY HIVDOVATO TABLET EDURANT 25 MG TABLET DELSTRIGO TABLET COMPLERA TABLET ODEFSEY TABLET JULUCA TABLET ADD PA FOR NEW STARTS AND ADD QLQL: 1 PER DAY HIVINTELENCE TABLET ADD PA FOR NEW STARTS AND ADD QLQL:200 MG- 2 TABLETS PER DAY 400 MG- 4 TABLETS PER DAY 25 MG – 16 TABLETS PER DAYHIVATRIPLA TABLET BIKTARVY TABLET CIMDUO TABLET DESCOVY TABLETEMTRIVA 200 MG CAPSULE EPIVIR 300 MG TABLET EPZICOM TABLET EVOTAZ TABLET GENVOYA TABLET PIFELTRO 100 MG TABLET PREZCOBIX TABLET PREZISTA 800 MG TABLET REYATAZ 300 MG CAPSULESTRIBILD TABLET SUSTIVA 600 MG TABLETSYMFI TABLET SYMFI LO TABLET SYMTUZA TABLET TRIUMEQ TABLET TRUVADA TABLET TYBOST 150 MG TABLET VIDEX EC 400 MG CAPSULE VIDEX EC 250 MG CAPSULE VIRAMUNE XR 400 MG TABLETADD QL 1 PER DAYTEMIXYS TABLETHIVREYATAZ 200 MG CAPSULE REYATAZ 150 MG CAPSULE VIDEX EC 200 MG CAPSULE ZERIT 40 MG CAPSULE ZERIT 30 MG CAPSULE COMBIVIR TABLET DUTREBIS TABLET EPIVIR 150 MG TABLET ISENTRESS HD 600 MG TABLET PREZISTA 600 MG TABLET RETROVIR 300 MG TABLET SELZENTRY 75 MG TABLET TIVICAY 10 MG, 25 MG AND 50 MGTABLETTRIZIVIR TABLETVIRAMUNE 200 MG TABLET ZIAGEN 300 MG TABLET ADD QL 2 PER DAYHIV ISENTRESS 100 MG GRANULE PACKET FOR SUSPENSION ADD QL2 PACKETS PER DAYHIVVIDEX EC 125 MG CAPSULE VIRAMUNE XR 100MG TABLET ADD QL 3 PER DAYHIVAPTIVUS 250 MG CAPSULE INVIRASE 500 MG TABLET ISENTRESS 400 MG TABLET KALETRA 200 MG-50 MG TABLETLEXIVA 700 MG TABLET SELZENTRY 300 MG TABLET SELZENTRY 150 MG TABLET SUSTIVA 200 MG CAPSULE VIRACEPT 625 MG TABLET ZERIT 20 MG CAPSULE ZERIT 15 MG CAPSULEADD QL 4 PER DAYHIVREYATAZ 50 MG POWDER FORSUSPENSIONADD QL5 PACKETS PER DAYHIVCRIXIVAN 400 MG CAPSULE PREZISTA 150 MG TABLET RESCRIPTOR 200 MG TABLET RETROVIR 100 MG CAPSULE ISENTRESS 100 MG CHEWABLEADD QL 6 PER DAY HIV SELZENTRY 25 MG TABLET ADD QL 8 PER DAY HIV TROGARZO 150MG/ML VIAL ADD QL8 VIALS PER 28 DAYSHIVINVIRASE 200 MG CAPSULE KALETRA 100 MG-25 MG TABLETPREZISTA 75 MG TABLET VIRACEPT 250 MG TABLET ADD QL 10 PER DAY HIVCRIXIVAN 200 MG CAPSULE NORVIR 100 MG TABLET NORVIR 100 MG CAPSULEADD QL 12 PER DAYNORVIR 100 MG ORAL POWDERPACKETRESCRIPTOR 100 MG TABLET SUSTIVA 50 MG CAPSULEHIV APTIVUS 100 MG/ML SOLUTION ADD QL 13 ML PER DAYHIV PREZISTA 100 MG/ML SUSPENSION ADD QL 14 ML PER DAY HIV KALETRA 400 MG-100 MG/5 MLORAL SOLUTIONNORVIR 80 MG/ML ORAL SOLUTION ADD QL 16 ML PER DAY HIV ISENTRESS 25 MG CHEWABLE ADD QL24 TABLETS PER DAYHIV EMTRIVA 10 MG/ML SOLUTION ADD QL 29 ML PER DAYHIVEPIVIR 10 MG/ML ORAL SOLUTION ZIAGEN 20 MG/ML SOLUTION ADD QL 32 ML PER DAY HIVVIDEX 4 GM PEDIATRIC ORALSOLUTIONVIDEX 2 GM PEDIATRIC ORALSOLUTIONVIRAMUNE 50 MG/5 MLSUSPENSION ADD QL 40 ML PER DAY HIV VIRACEPT 50 MG/G POWDERADD QL 53 GM PER DAYHIV FUZEON 90 MG VIAL ADD QL60 VIALS PER 30 DAYSHIV LEXIVA 50 MG/ML SUSPENSION ADD QL 60 ML PER DAYHIV SELZENTRY 20 MG/ML ORALSOLUTION ADD QL 62 ML PER DAYHIV RETROVIR 10 MG/ML SYRUP ADD QL 64 ML PER DAYHIVZERIT 1 MG/ML SOLUTION ADD QL 80 ML PER DAY IRRITABLE BOWEL SYNDROME (IBS)AGENTSZELNORM 6MG TABLET ADD PA AND QL QL 2 TABLETS PER DAY LAMBERT-EATON MYASTHENIC SYNDROME AGENTSRUZURGI 10MG TABLET ADD PA AND QL QL 10 TABLETS PER DAYNARCOTIC ANTAGONISTS SUBLOCADE 100/0.5 INJECTION SUBLOCADE 300/1.5 INJECTION REMOVE PANARCOTIC ANTAGONISTS VIVITROL 380MG INEJCTION REMOVE PA AND ADD QL QL 1 VIAL PER 28 DAYSNARCOTIC ANTAGONISTS ZUBSOLV 2.9-0.71 SUB REVISE QL QL 5 PER DAY ORAL DIABETICAGENTS*QTERNMET XR TABLETADD ST AND QLQL:5 MG/5 MG/1000 MG, 10 MG/5 MG/1000 MG:1 TABLET PER DAY2.5 MG/2.5 MG/1000 MG, 5 MG/2.5 MG/10000MG: 2 TABLETS PER DAYORAL DIABETICAGENTS QTERN 5-5MG TABLET ADD QL1 TABLET 28 DAYSINJECTABLE DIABETIC AGENTSOZEMPIC 2/1.5ML INJECTION ADD QL 1 PER 28 DAYSPRENATAL VITAMINS DUET DHADUET DHA BALANCEDNESTABS ABC NESTABS DHA OBTREX DHA SELECT-OB+DHATHERANATAL COMPLETEVITAFOL FE+ VITAFOL-OB+DHABAL-CARE DHA ESSENTIAL ADD QL 2 PER DAYPRENATAL VITAMINS CITRANATAL B-CALMADD QL 3 PER DAYTOPICAL ANTIPRURITICS DOXEPIN HCL 5% CREAM,ZONALON 5% CREAM, PRUDOXIN5% CREAM ADD PA AND QLQL 1 TUBE PER FILL; 1 FILL PER 3 MONTHSTOPICAL ANESTHETIC COMBINATIONSLIDOCAINE/PRILOCAINE CREAMREVISE QL30 GM PER 30 DAYS* Clinical edits will be put in place as these new drugs to come market.What action do I need to take?Please review these changes and work with your Healthy Connections members to transition them to formulary alternatives. If you determine preferred formulary alternatives are notclinically appropriate for specific members, you will need to obtain prior authorization (PA) to continue coverage beyond the applicable effective date.What if I need assistance?We recognize the unique aspects of member cases. If your Healthy Connections member cannot be converted to a formulary alternative for medical reasons, call our Pharmacy department at 866-902-1689 and follow the voice prompts for pharmacy PA.You can find the Preferred Drug List on our website at > Providers > Pharmacy Information. If you need assistance with any other item, contact the Customer Care Center at 866-757-8286.。
盐酸拉维达韦片说明书
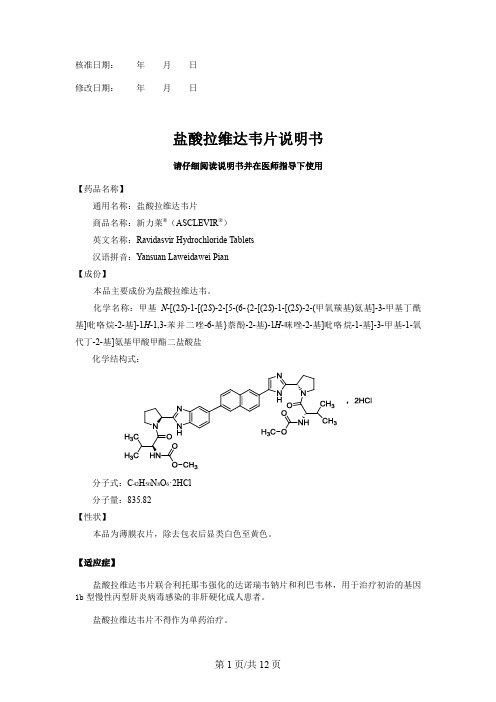
核准日期:年月日修改日期:年月日盐酸拉维达韦片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:盐酸拉维达韦片商品名称:新力莱®(ASCLEVIR®)英文名称:Ravidasvir Hydrochloride Tablets汉语拼音:Yansuan Laweidawei Pian【成份】本品主要成份为盐酸拉维达韦。
化学名称:甲基N-[(2S)-1-[(2S)-2-[5-(6-{2-[(2S)-1-[(2S)-2-(甲氧羰基)氨基]-3-甲基丁酰基]吡咯烷-2-基]-1H-1,3-苯并二唑-6-基}萘酚-2-基)-1H-咪唑-2-基]吡咯烷-1-基]-3-甲基-1-氧代丁-2-基]氨基甲酸甲酯二盐酸盐化学结构式:分子式:C42H50N8O6·2HCl分子量:835.82【性状】本品为薄膜衣片,除去包衣后显类白色至黄色。
【适应症】盐酸拉维达韦片联合利托那韦强化的达诺瑞韦钠片和利巴韦林,用于治疗初治的基因1b型慢性丙型肝炎病毒感染的非肝硬化成人患者。
盐酸拉维达韦片不得作为单药治疗。
【规格】0.2g(以C42H50N8O6计)【用法用量】推荐剂量本品口服,可空腹或与食物同服。
本品用法用量:每次200mg,每日1次,连续12周。
服用本品时须同时应用达诺瑞韦钠片、利托那韦和利巴韦林。
推荐达诺瑞韦钠片用法用量:口服,每次100mg,每日2次;连续12周(详见达诺瑞韦钠片说明书)。
推荐利托那韦(RTV)用法用量:口服,每次100mg,每日2次,连续12周(详见利托那韦片说明书)。
推荐利巴韦林用法用量:利巴韦林的剂量根据体重确定。
如体重<75kg,每次500mg,每日2次;如体重≥75kg,每次600mg,每日2次;连续12周(详见利巴韦林制剂说明书)。
剂量调整、暂停给药和停止治疗不建议调整盐酸拉维达韦片的剂量,并应避免暂停给药。
但如果因不良反应需要暂停给药联合治疗方案中的任何一种药物,则不得单独应用盐酸拉维达韦片治疗。
雅D-苯甘氨酸甲酯盐酸盐制备及纯化工艺研究-化学工程与技术专业毕业论文

D-苯甘氨酸甲酯盐酸盐制备及纯化工艺研究-化学工程与技术专业毕业论文河北科技大学硕士学位论文IIAbstract=========;暑=;昌昌============;==≈==;穹皇=========;号皇;=兰===昌==暑=======置==宣号=== =:AbstractD。
phenylglycine methyl ester hydrochloride,a type of white crystalline powder and important p harmaceutical i ntermediate,iS a n e ssential active side chain f or t he production of cephalexin,cefaclor,and other IMactam antibiotics.In this paper,the synthesis and crystallization purification technique of D-phenylglycine methyl ester hydrochloride is systematically studied.The study on the synthesis of D—phenylglycine methyl ester hydrochloride.A preliminary synthetic route of D·phenylglycine methyl ester hydrochloride was designed,and sulfoxide chloride method was determinedexperimentally as a preferred syntheticroute.In the experiment,the synthesis technique conditions such as thesequence of adding reagents,the ratio of reagents,the reaction temperature,the reflux time,and the adding rate of sulfoxide chloride were investigated and optimized.After the completion of the reaction,the vacuum azeotropic distillation and cooling crystallization with the temperature controlling should be done to the mother liquor.Finally through the post‘pmcessing,we can get.t11e crystal product of D—phenylglycine methyl ester hydrochloride.The purity of the product detected by using HPLC is more than 98.5%.with the yield more than 96%.The study on the thermodynamics of D-phenylglycine methyl ester hydrochloridecrystallization.Thesolubility and supersolubility of D—phenylglycine methylesterhydrochloride in six pure solvents(water,methanol,ethanol,acetone,ethyl acetate,and toluene)as well as in two kinds of binary mixed solvents with different compositions (methanol—ethyl acetate and methanol—toluene)were measured under the conditions of atmospheric pmssure and the temperature range of 283.1 5K-333.1 5K by using Laser Dynamics Method.Then the Apelblat equation,CNIBS/Redlich.Kister equation.and NRTL equation were used to correlate the data of solubility,and it showeda satisfactoryresult.Finally,thermodynamic properties in the dissolution process wereanalyzed systematically,and changes of the free energy of standard molar enthalpy,Standard molar entropy and Standard Gibbs of D-phenylglycine methyl ester hydrochloride in the dissolution process in different solvents were calculated.The study on thermodynamics of crystallization of D—phenylglycine methyl ester hydrochloride provides a theoretical reference and data base forthe development of its crystallizationtechnique.IIlThe crystallization kinetics of D.phenylglycine methyl ester hydrochloride was studied by batch dynamic methods.Samples of different times were measuredunderdifferent processing conditions.Then the CSD Was analyzedby laserCrystallization dynamic models of D.phenylglycine methyl ester granulometer.hydrochloride were established based on the mass balance equation and population balance equation.ARertransformation of moments on theexperimental data,the mathematical models for crystal growth rate and secondary nucleation rate were obtained,and the operating parameterswhich affected the crystallographicprogress were analyzed.The study on the crystallizationtechnique of D-phenylglycine methyl esterhydrochloride.The crystallization technique of D..phenylglycinemethyl ester hydrochloride Was studied on the basis of crystallization thermodynamics and dynamics.The influence of crystallization method and process conditions on the quality and vield of product Wasstudied.A new crystallization and purification technique ofD-phenylglycinemethyl ester hydrochloride Was developed.The crystals produced by this new technique is of high purity,high yield,and good color level(1evel 1),and their particle size increaSed anduniformed obviously.With stablequality of the product,low production COst aS wellas easy operation control,this technique has applied for China National invention patent.Andthousand tons has been realized.the industrialization of the annualoutput ofKey words D-phenylglycine methyl ester hydrochloride;Synthesis technique;Crystallization thermodynamics;Crystallization kinetics;The crystallizationtechniqueIV物理量名称及符号表物理量名称及符号表4一Apelblat方程参数;尸一压强;彳一晶体外表积:尸一系统搅拌强度量;40一指数因子;Qi-粒数密度;B—Apelblat方程参数:B一为生函数;Qi-引入结晶器的晶浆流量;矿一总成核速率;Q一引出结晶器的晶浆流量;啷一均相成核速率;,.一晶核半径;召s一二次成核速率;R一气体常量;C--Apelblat方程参数;R2一相关指数;C一溶液主体浓度;仅,,一与溶剂有关的非随机参数;Ci一溶液界面浓度;Gl,一NRTL模型参数;C。
世界卫生组织癌症研究机构三类致癌物清单

2013 2010 2013 2010 2010 2010 2010 2010 2013 2010 2010 2010 2010 2010 1999 1999 1987 1999 1999 1999 1999 1999 1999 1992 1987 1987 1999 1999 1999 2006 1999 1987 1991 2010 1987 1987 1987 1987 2017 2017 1987
3类致癌物清单(共502种)
3类致癌物:对人类致癌性可疑,尚无充分的人体或动物数据。 序号 英文名称 中文名称 1 Acenaphthene 二氢苊 Acepyrene (3,4Acepyrene (3,4-二氢环戊二烯并[cd] 2 dihydrocyclopenta[cd]pyre 芘]) ne) 3 Aciclovir 阿昔洛韦 4 Acridine orange 吖啶橙 5 Acriflavinium chloride 吖啶黄 6 Acrolein 丙烯醛 7 Acrylic acid 丙烯酸 8 Acrylic fibres 丙烯酸(类)纤维 Acrylonitrile-butadiene9 丙烯腈-丁二烯-苯乙烯共聚物 styrene copolymers 10 Actinomycin D 放线菌素D 11 Agaritine 伞菌氨酸(蘑菇氨酸) 12 Aldicarb 涕灭威 13 Allyl chloride 烯丙基氯 14 Allyl isothiocyanate 异硫氰酸烯丙酯 15 Allyl isovalerate 异戊酸烯丙酯 16 Amaranth 苋菜红 1-Amino-217 1-氨基-2-甲基蒽醌 methylanthraquinone 18 4-Amino-2-nitrophenol 4-氨基-2-硝基苯酚 19 2-Amino-4-nitrophenol 2-氨基-4-硝基苯酚 20 2-Amino-5-nitrophenol 2-氨基-5-硝基苯酚 21 2-Amino-5-nitrothiazole 2-氨基-5-硝基噻唑 22 5-Aminoacenaphthene 5-氨基二氢苊 23 2-Aminoanthraquinone 2-氨基蒽醌 24 11-Aminoundecanoic acid 11-氨基十一酸 25 Amitrole 杀草强 26 Ampicillin 氨苄青霉素 27 Anaesthetics, volatile 有挥发性的麻醉药 Angelicin plus ultraviolet 28 白芷素伴紫外线A辐射 A radiation 29 Aniline 苯胺 30 Anthanthrene 二苯并[cd,jk]芘 31 Anthracene 蒽 32 Anthranilic acid 邻氨基苯甲酸 33 Antimony trisulfide 三硫化二锑 34 Apholate 环磷氮丙啶 Arsenobetaine and other organic arsenic compounds 砷甜菜碱和人不能代谢的其它有机 35 that are not metabolized in 砷化合物 humans 36 Atrazine 阿特拉津 37 Aurothioglucose 金硫葡糖 38 2-(1-Aziridinyl)ethanol 2-1-吖丙啶乙醇 39 Aziridyl benzoquinone 氮丙啶基苯醌 40 Azobenzene 偶氮苯 时间(年) 2010 2010 2000 1987 1987 1995 1999 1987 1987 1987 1987 1991 1999 1999 1999 1987 1987 1987 1993 1993 1987 1987 1987 1987 2001 1990 1987 1987 1987 2010 2010 1987 1989 1987 2012 1999 1987 1987 1987 1987
泛昔洛韦

密度:1.4g/cm3 熔点:102-104°C 沸 点 : 5 5 0 . 2 ºC 闪 点 : 2 8 6 . 6 ºC 折射率:1.628 外观:灰白色粉末 溶解性:易溶于丙酮或甲醇,难溶于乙醇或异丙醇
摩尔折射率:82.66 摩尔体积(cm3/mol):239.8 等张比容(90.2K):639.3 表面张力(dyne/cm):50.4 极化率(10-24cm3):32.77
进入人体内后迅速转变成喷昔洛韦,喷昔洛韦可被病毒编码的胸苷激酶磷酸化成OCV单磷酸,再经宿主的磷 酸化成为喷昔洛韦三磷酸盐,三磷酸盐在病毒感染的细胞内迅速形成,缓慢代谢,致半衰期延长,参与HBV DNAp的三磷酸鸟苷(Pgtp)竞争,并进入DNA,作用于DNA合成的起始和延伸步骤,抑制DNA的合成,对水痘-带状疱疹 病毒、单纯疱疹病毒1型和2型和HBV均有较强的抑制作用。
泛昔洛韦
药品
目录
01 化合物简介
03 药典信息
02 药品简介 04 安全信息
泛昔洛韦,是一种有机化合物,化学式为C14H19N5O4,是第二代开环核苷类抗病毒药,主要用于疱疹病毒感 染,尤其是带状疱疹。
化合物简介
基本信息 理化性质
分子数据 化学数据
化学式:C14H19N5O4 分子量:321.332 CAS号:-87-4
疏水参数计算参考值(XlogP):无 氢键供体数量:1 氢键受体数量:8 可旋转化学键数量:9 互变异构体数量:3 拓扑分子极性表面积:122 重原子数量:23 表面电荷:0 复杂度:404 同位素原子数量:0 确定原子立构中心数量:0
药品简介
作用机理
药代动力学
不良反应
泛昔洛韦口服迅速吸收,生物利用度77%,在体内很快转达变为喷昔洛韦,t1/2约为2h,约60-65%经肾排出。 在水痘-带状疱疹病毒感染的细胞内有一个较长的半衰期(9-10小时),单纯疱疹病毒1型和2型感染的细胞内半 衰期分别为10小时和20小时。
重组贻贝粘蛋白的表征及功效评价

生物技术进展 2023 年 第 13 卷 第 4 期 596 ~ 603Current Biotechnology ISSN 2095‑2341研究论文Articles重组贻贝粘蛋白的表征及功效评价李敏 , 魏文培 , 乔莎 , 郝东 , 周浩 , 赵硕文 , 张立峰 , 侯增淼 *西安德诺海思医疗科技有限公司,西安 710000摘要:为了推进重组贻贝粘蛋白在医疗、化妆品领域的应用,对大肠杆菌规模化发酵及纯化生产获得的重组贻贝粘蛋白进行了表征及功效评价。
经Edman 降解法、基质辅助激光解吸电离飞行时间质谱、PITC 法、非还原型SDS -聚丙烯酰胺凝胶电泳法、凝胶法、改良的Arnow 法对重组贻贝粘蛋白进行氨基酸N 端测序、相对分子量分析、氨基酸组成分析、蛋白纯度分析、内毒素含量测定、多巴含量测定;通过细胞迁移、斑马鱼尾鳍修复效果对重组贻贝粘蛋白进行功效评价。
结果显示,获得的重组贻贝粘蛋白与理论的一级结构一致,蛋白纯度达95%以上,内毒素<10 EU ·mg -1,多巴含量大于5%;重组贻贝粘蛋白浓度为60 μg ·mL -1时能够显著促进细胞增殖的活性(P <0.01);斑马鱼尾鳍面积样品组与模型对照组相比极显著增加(P <0.001)。
研究结果表明,重组贻贝粘蛋白具有显著的促细胞迁移和修复愈合的功效,具备作为生物医学材料的潜质。
关键词:贻贝粘蛋白;基因重组;生物材料;表征;功效评价DOI :10.19586/j.20952341.2023.0021 中图分类号:S985.3+1 文献标志码:ACharacterization and Efficacy Evaluation of Recombinant Mussel Adhesive ProteinLI Min , WEI Wenpei , QIAO Sha , HAO Dong , ZHOU Hao , ZHAO Shuowen , ZHANG Lifeng ,HOU Zengmiao *Xi'an DeNovo Hith Medical Technology Co., Ltd , Xi'an 710000, ChinaAbstract :In order to promote the application of recombinant mussel adhesive protein in the medical and cosmetics field , the recombi⁃nant mussel adhesive protein obtained from scale fermentation and purification of Escherichia coli was characterized and its efficacy was evaluated. Amino acid N -terminal sequencing , relative molecular weight analysis , amino acid composition analysis , protein purityanalysis , endotoxin content , dihydroxyphenylalanine (DOPA ) content of recombinant mussel adhesive protein were determined by the following methods : Edman degradation , matrix -assisted laser desorption ionization time -of -flight mass spectrometry (MALDI -TOF -MS ), phenyl -isothiocyanate (PITC ), nonreductive SDS -polyacrylamide gel electrophoresis (SDS -PAGE ), gel method , modified Ar⁃now. The efficacy of recombinant mussel adhesive protein was evaluated by cell migration and repairing effect of zebrafish tail fin. Re⁃sults showed that the obtained recombinant mussel adhesive protein was confirmed to be consistent with the theoretical primary structure , protein purity of more than 95%, endotoxin <10 EU ·mg -1, DOPA content above 5%. When the recombinant mussel adhesive protein concentration was 60 μg ·mL -1, the effect of promoting cell proliferation was the most obvious , and it had very significant activity (P <0.01). The caudal fin area of zebrafish in sample group was significantly increased compared with model control group (P <0.001). The results indicated that recombinant mussel adhesive protein can promote cell migration and repair healing and has the potential to be used as biomedical materials.Key words :mussel adhesive protein ; gene recombination ; biological materials ; representation ; efficacy evaluation贻贝粘蛋白(mussel adhesive protein , MAP )也称作贻贝足丝蛋白(mussel foot protein ,Mfps ),收稿日期:2023⁃02⁃24; 接受日期:2023⁃03⁃31联系方式:李敏 E -mail:*******************;*通信作者 侯增淼 E -mail:***********************.cn李敏,等:重组贻贝粘蛋白的表征及功效评价是海洋贝类——紫贻贝(Mytilus galloprovincalis)、厚壳贻贝(Mytilus coruscus)、翡翠贻贝(Perna viri⁃dis)等分泌的一种特殊的蛋白质,贻贝中含有多种贻贝粘蛋白,包括贻贝粘蛋白(Mfp 1~6)、前胶原蛋白(precollagens)和基质蛋白(matrix proteins)等[1]。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
Ivabradine hydrochloride CAS No.:
148849-67-6
Product Data Sheet
Cat. No.:
HY-B0162A MWt:
505.05Formula:
C27H37ClN2O5Purity :
>98%
Solubility:Mechanisms:
Biological Activity:
Pathways:GPCR/G protein; Target:Adrenergic Receptor DMSO 100 mg/mL; Water 82mg/mL
g y Ivabradine hydrochloride, a new If inhibitor with IC 50 of 2.9 μM, which is a pure heart rate lowering
agent.
IC50 Value: 2.9 μM Ivabradine acts on the If (f is for "funny", so called because it had unusual properties compared with other current systems known at the time of its discovery) ion current, which is highly expressed in the sinoatrial node. If is a mixed Na+–K+ inward current activated by hyperpolarization and modulated by the autonomic nervous system. It is one of the most important ionic currents for regulating pacemaker activity in the sinoatrial (SA) node. Ivabradine selectively inhibits the References:
[1]. Fang, Y., et al., Heart rate reduction induced by the if current inhibitor ivabradine improves diastolic function and attenuates cardiac tissue hypoxia. J Cardiovasc Pharmacol, 2012. 59(3): p.
260-7.[2]. Kroller-Schon, S., et al., Differential effects of heart rate reduction with ivabradine in two models g g p y ()y
pacemaker If current in a dose-dependent manner. Blocking this channel reduces cardiac pacemaker activity, slowing the heart rate and allowing more time for blood to flow to the
myocardium From wikipedia []
of endothelial dysfunction and oxidative stress. Basic Res Cardiol, 2011. 106(6): p. 1147-58.Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
