Xuriden(uridine triacetate)处方使用说明书2015版
复方醋酸棉酚片说明书

复方醋酸棉酚片说明书导读:我根据大家的需要整理了一份关于《复方醋酸棉酚片说明书》的内容,具体内容:复方醋酸棉酚片(雷迪欣)用于功能性子宫出血、子宫肌瘤并月经过多及子宫内膜异位症,及由此引起的痛经、经血多、经期长、经期乳房胀痛、腹痛等症。
下面是我整理的,欢迎阅读。
复方醋酸棉酚...复方醋酸棉酚片(雷迪欣)用于功能性子宫出血、子宫肌瘤并月经过多及子宫内膜异位症,及由此引起的痛经、经血多、经期长、经期乳房胀痛、腹痛等症。
下面是我整理的,欢迎阅读。
复方醋酸棉酚片商品介绍通用名:复方醋酸棉酚片生产厂家: 西安北方药业有限公司批准文号:国药准字H61022557药品规格:20mg*5片药品价格:¥90元【通用名称】复方醋酸棉酚片【商品名称】复方醋酸棉酚片(雷迪欣)【英文名称】CompoundGosspolAcetateTablets【拼音全码】FuFangCuSuanMianFenPian(LeiDiXin)【主要成份】复方醋酸棉酚片(雷迪欣)为复方制剂,其组为:醋酸棉酚、氯化钾、维生素B1、维生素B6。
【性状】复方醋酸棉酚片(雷迪欣)为片剂。
【适应症/功能主治】用于功能性子宫出血、子宫肌瘤并月经过多及子宫内膜异位症,及由此引起的痛经、经血多、经期长、经期乳房胀痛、腹痛等症。
【规格型号】20mg*5s【用法用量】口服。
一次1片,一日1次。
晚饭后服用。
30天为一个疗程,常规为6程。
【不良反应】可有低钾血症、肌无力、食欲减退、恶心、呕吐等胃肠道反应以及心悸及肝功能轻度改变;可引起绝经的更年期症状出现,闭经、性欲减退、潮热、皮肤瘙痒、出汗等。
【禁忌】1.孕妇及哺乳期的妇女禁用。
2.老年患者禁用。
3.对复方醋酸棉酚片(雷迪欣)过敏者禁用。
【注意事项】1.心、肝、肾功能异常者慎用。
2.如发生低钾症,可口服或静脉补充钾盐,按医嘱。
3.长期服用复方醋酸棉酚片(雷迪欣)应注意检测血钾及心电图。
【儿童用药】3岁以下儿童因其肝、肾功能发育不全,应禁用。
兰迪降压药说明书

亲爱的朋友,很高兴能在此相遇!欢迎您阅读文档兰迪降压药说明书,这篇文档是由我们精心收集整理的新文档。
相信您通过阅读这篇文档,一定会有所收获。
假若亲能将此文档收藏或者转发,将是我们莫大的荣幸,更是我们继续前行的动力。
兰迪降压药说明书苯磺酸氨氯地平片(兰迪)1.高血压(单独或与其他药物合并使用)。
2.心绞痛:尤其自发性心绞痛(单独或与其他药物合并使用)。
下面是我们整理的兰迪说明书,希望对大家有所帮助。
兰迪降压药商品介绍苯磺酸氨氯地平片(兰迪)通用名:苯磺酸氨氯地平片生产厂家:扬子江药业集团上海海尼药业有限公司批准文号:国药准字Hxx0468药品规格:5mg*7s药品价格:¥15元【通用名称】苯磺酸氨氯地平片【商品名称】苯磺酸氨氯地平片(兰迪)【英文名称】Amlodipine Besylate Tablets【拼音全码】BenHuangSuanAnLvDiPingPian(LanDi)【主要成份】苯磺酸氨氯地平。
【性状】苯磺酸氨氯地平片(兰迪)为白色片。
【适应症/功能主治】1.高血压(单独或与其他药物合并使用)。
2.心绞痛:尤其自发性心绞痛(单独或与其他药物合并使用)。
【规格型号】5mg*7s【用法用量】通常口服起始剂量为5mg,每日一次,大不超过10mg,每日一次。
瘦小者、体质虚弱者、老年患者或肝功能受损者从 2.5mg,每日一次开始用药;合用其它抗高血压药者也从此剂量开始用药。
用药剂量根据个体需要进行调整,调整期应不少于7-14天,以便医生充分评估患者对该剂量的反应。
但在临床有保障的前提下,可以加快调整速度。
治疗心绞痛的推荐剂量是5-10mg,老年患者或肝功能受损者需减量。
【不良反应】患者对苯磺酸氨氯地平片(兰迪)能很好地耐受。
较常见的副反应是头痛、水肿、疲劳、失眠、恶心、腹痛、面红、心悸和头晕。
较为少见的副反应为瘙痒、皮疹、呼吸困难、无力、肌肉痉挛和消化不良。
与其他钙拮抗相似,极少有心肌梗塞和胸痛的不良反应报道,而且这些不良反应不能与病人本身的基础疾病明确区分,尚未发现与苯磺酸氨氯地平片(兰迪)有关的实验室检查参数异常。
雷米普利片说明书
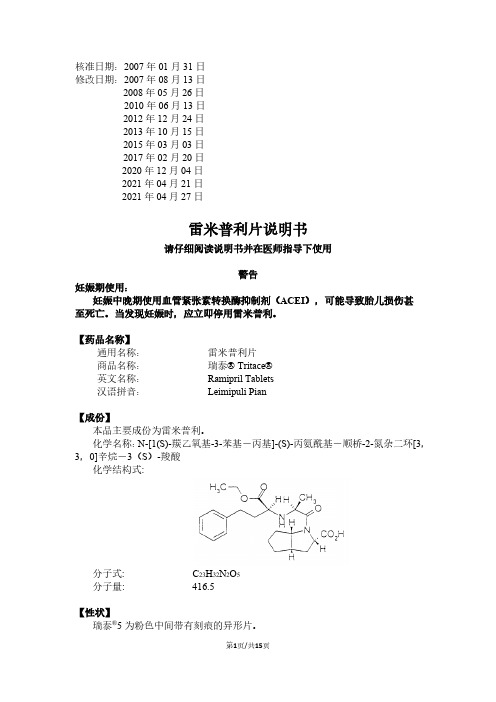
核准日期:2007年01月31日修改日期:2007年08月13日2008年05月26日2010年06月13日2012年12月24日2013年10月15日2015年03月03日2017年02月20日2020年12月04日2021年04月21日2021年04月27日雷米普利片说明书请仔细阅读说明书并在医师指导下使用警告妊娠期使用:妊娠中晚期使用血管紧张素转换酶抑制剂(ACEI),可能导致胎儿损伤甚至死亡。
当发现妊娠时,应立即停用雷米普利。
【药品名称】通用名称:雷米普利片商品名称:瑞泰 Tritace英文名称:Ramipril Tablets汉语拼音:Leimipuli Pian【成份】本品主要成份为雷米普利。
化学名称:N-[1(S)-羰乙氧基-3-苯基-丙基]-(S)-丙氨酰基-顺桥-2-氮杂二环[3,3,0]辛烷-3(S)-羧酸化学结构式:分子式: C23H32N2O5分子量: 416.5【性状】瑞泰®5为粉色中间带有刻痕的异形片。
【适应症】-原发性高血压-急性心肌梗死(2~9天)后出现的轻~中度心力衰竭(NYHA II和III)-非糖尿病肾病患者(肌酐清除率<70 ml/min/1.73m2, 尿蛋白>1 g/天),尤其是伴有动脉高血压的患者-降低心肌梗死、卒中和心血管原因死亡的风险用于55岁以上,因为冠状动脉疾病、卒中、外周血管病或糖尿病病史并伴有至少一个其他心血管危险因素导致发生重大心血管事件风险增高的患者,以降低心肌梗死、卒中和心血管因素所致死亡的风险。
注:本品不是原发性醛固酮增多症的治疗选择。
【规格】5.0 mg【用法用量】口服。
建议每天于同一时间服用本品。
由于进食不会改变本品的生物利用度,因此可以在餐前、餐时或餐后服用(见【药代动力学】)。
本品须用液体送服。
不得咀嚼或碾碎。
成人经利尿剂治疗的患者本品治疗开始后可能出现低血压,这在同时采用利尿剂进行治疗的患者中更有可能出现。
壹丽安(醋酸去氨加压素片)

壹丽安(醋酸去氨加压素片)【药品名称】商品名称:壹丽安通用名称:醋酸去氨加压素片英文名称:Desmopressin acetate tablets【成份】去氨加压素【适应症】弥凝用于治疗中枢性尿崩症。
服用弥凝后可减少尿液排出,增加尿渗透压,减低血浆渗透压,从而减少尿频和夜尿。
弥凝用于治疗夜间遗尿症(五岁或以上的患者。
)【用法用量】剂量因人而异,应区分调整。
治疗中枢性尿崩症:一般成人和儿童的初始适宜剂量为为每次0.1毫克,每日三次。
再根据患者的疗效调整剂量。
根据临床经验,每天的总量在0.2-1.2毫克之间。
对多数患者的适宜剂量为每次0.1-0.2毫克,每日三次。
治疗夜间遗尿症:初始适宜剂量为睡前服用0.2毫克,如疗效不显著可增至0.4毫克,连续使用三个月后停用此药至少一周,以便评估是否需要继续治疗。
治疗期间需限制饮水,详见""注意事项""。
【不良反应】常见(>1/100)的副作用一般反应:头痛;消化系统:胃痛及恶心;上呼吸道:鼻出血。
使用醋酸去氨加压素时若不限制饮水可能会引起水潴留/低钠血症及其并发症(头痛、恶心/呕吐、血清钠降低和体重增加,更严重者可引起抽搐。
)上市报道经验:儿童中有罕见情绪障碍的病例报道;有出现皮肤过敏和更为严重的全身过敏反应的病例报道。
【禁忌】习惯性或精神性烦渴症患者;心功能不全或其他疾患需服用利尿剂的患者。
【注意事项】在下列情况下,使用醋酸去氨加压素应特别谨慎。
1 年幼及老年患者;2 体液或电解质失衡患者;3 具有颅内压升高危险患者。
【药物相互作用】一些可引起释放抗利尿激素的药物,如三环类抗抑郁剂、氯丙嗪、卡马西平等,可增加抗利尿作用和水潴留的危险。
吲哚美辛(消炎痛)可增加醋酸去氨加压素的尿浓缩作用,但不会影响其药效的持续时间。
该作用可能没有任何临床意义。
【药理作用】本品所含去氨加压素,与天然激素精氨酸加压素的结构类似。
它与精氨酸加压素的区别,主要是对半胱氨酸作脱氨基处理和以D-精氨酸取代L-精氨酸。
瑞复美说明书

批准日期:2013年01月22日修订日期:来那度胺胶囊说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:来那度胺胶囊商品名称:瑞复美®(Revlimid®)英文名称:Lenalidomide Capsules汉语拼音:Lainadu’an Jiaonang【成份】本品主要成份为:来那度胺。
化学名称:3-(4’-氨基-1-氧-1,3-二氢-2H-异吲哚-2-基)哌啶-2,6-二酮化学结构式:分子式:C13H13N3O3分子量:259.3【性状】本品为硬胶囊,内容物为白色至类白色的粉末。
5mg:白色胶囊,印有“REV 5 mg”字样。
10mg:蓝绿色/浅黄色胶囊,印有“REV 10 mg”字样。
15mg:浅蓝色/白色胶囊,印有“REV 15 mg”字样。
25mg:白色胶囊,印有“REV 25 mg”字样。
【适应症】本品与地塞米松合用,治疗曾接受过至少一种疗法的多发性骨髓瘤的成年患者。
【规格】(1) 5mg (2) 10mg (3) 15mg (4) 25mg【用法用量】必须在有多发性骨髓瘤治疗经验的医生监督下开始并提供治疗用药。
若患者的中性粒细胞绝对计数(ANC)<1.0 ×109/L,或患者的血小板计数<50 ×109/L,且其骨髓中浆细胞占有核细胞的比例<50%,或患者的血小板计数<30 ×109/L,且其骨髓中浆细胞占有核细胞的比例>50%,则不得开始本品的治疗。
推荐剂量本品的推荐起始剂量为25mg。
在每个重复28天周期里的第1~21天,每日口服本品25 mg,直至疾病进展。
地塞米松的推荐剂量为在每28天治疗周期的第1、8、15和22天口服40 mg地塞米松。
处方医生应根据患者的肾功能状况谨慎选择本品的起始剂量和随后的剂量调整(见表1),应根据患者的年龄选择地塞米松的起始剂量和随后的剂量调整(见表2)。
美国药品(Syprine,Trientine)中文说明书

【药品名称】英文药名:Syprine(Trientine)中文药名:盐酸曲恩汀【药理作用】本品为肝豆状核变性治疗药,系螯合剂,可用以除去体内过量的铜。
【临床应用】用于对青霉胺不能耐受或用青霉胺复发的肝豆状核变性病患者。
【制剂规格】(二盐酸盐)胶囊250mg。
【用法用量】口服,本品推荐的初始剂量为儿童每日500~750mg,成人750~1250mg,日剂量分2、3或4次服用。
剂量可增加至进最大,成人200mg/d,12岁或12岁以下儿童1500mg/d。
本品日剂量仅在临床疗效下充分或游离血清铜浓度持续维持在3.15μmol/L以上时方可增加。
每隔6~12个月应确定最适的长期维持剂量。
重要的是本品应空腹给药,至少在用餐前1h或用餐后2h,而与任何其他药物、食品或乳制品应至少相隔1h。
胶囊应当用水整颗吞下,不应打开或咀嚼。
【如何购买】美国是医药分开的国家,药房全部实行严格的处方药与非处方药分类管理。
对处方药的销售,必须凭美国医生(电子/纸质)处方。
如今国内患者可以依托科技,通过好医友国际医疗平台实现远程的病历交互,由美国医生根据患者病情开具电子处方,以正规渠道在好医友美国药房购买到处方药。
【禁忌症】对本品过敏者禁用。
【不良反应】可引起铁离子不足、全身红斑狼疮等,偶见胃灼热、腹部疼痛、贫血、急性胃炎、食欲减退、皮疹、肌痛等。
【注意事项】(1)应监测血清铜浓度。
(2)整个用药期间,应在医师监护下。
(3)如铁严重不足,可于短期内补给,在服铁剂后须间隔2小时再服本品。
(4)服用本品至少间隔1小时才能服用其他食物、药物或乳制品。
(5)孕妇、哺乳妇女用本品时,应考虑利弊慎用。
(6)本品和青霉胺不可互相交换使用。
(7)不适用于胱氨酸尿、风湿性关节炎和胆汁性肝硬变患者的治疗。
诺雷得说明书

诺雷得(醋酸戈舍瑞林缓释植入剂)说明书(共3页)-本页仅作为预览文档封面,使用时请删除本页-诺雷得(醋酸戈舍瑞林缓释植入剂)说明书【诺雷得药品名称】商品名:诺雷得通用名:醋酸戈舍瑞林缓释植入剂英文名:GoserelinAcetateSRDepot汉语拼音:ChusuangesheruilinHuanshizhiruji【诺雷得成份】诺雷得主要成份为醋酸戈舍瑞林。
【诺雷得性状】诺雷得为无菌、白色或乳白色柱形聚合物。
【诺雷得规格】支(以戈舍瑞林计)【诺雷得药理作用】本药是一种合成的、促黄体生成素释放激素的类似物,长期使用可抑制垂体的促黄体生成激素的分泌,从而引起男性血清睾酮和女性血清雌二醇的下降,停药后这一作用是可逆的。
男性病人在次用药之后21日左右,睾酮浓度可降低到去势后的水平,在每28天用药1次的治疗过程中,睾酮浓度一直保持在去势后的浓度范围内。
这种睾酮抑制作用可使大多数病人的前列腺肿瘤消退,症状改善。
女性患者在初次用药后21日左右,血清雌二醇浓度受到抑制,并在以后每28天的治疗中维持在绝经后水平。
这种抑制与激素依赖性的乳腺癌,子宫内膜异位症相关。
【诺雷得药代动力学】本药具有几乎完全的生物利用度。
每4周使用一注射埋植剂,可保持有效血药浓度,而无组织蓄积。
诺雷德的蛋白结合能力较差,在肾功能正常的情况下,血浆清除半衰期为2-4小时,肾功能不全病人的半衰期将会延长,但对于每月都使用埋植剂的患者来说,这影响非常小,故没有必要改变这些病人的用量。
在肝功能不全的病人中,药代动力学无明显的变化。
【诺雷得毒理研究】在长期重复使用诺雷德的雄性鼠中,曾观察到良性脑垂体肿瘤的发病率上升,这一事实与以前已观察到的行外科去势后的大鼠的情形相似,但尚未发现与人体使用的经验有任何关联。
在小鼠实验中,长期重复使用数倍于人类常用剂量的诺雷德后,可发现胰岛细胞及幽门部胃粘膜细胞增生。
这些事实与临床的关系尚不清楚。
【诺雷得适应症】可用激素治疗的前列腺癌及绝经前及围绝经期的乳腺癌。
曲前列尼尔注射液Treprostinil-详细说明书与重点

曲前列尼尔注射液Treprostinil 【商品名称】瑞莫杜林Remodulin成份:本品主要成份为曲前列尼尔。
化学名称:[[(1R, 2R, 3aS, 9aS)-2, 3, 3a, 4, 9, 9a-六氢-2-羟基-1-[(3S)-3-羟基辛烷基]-1 H -苯并[f]茚-5-基]氧基]乙酸,化学结构式:分子式:C23H34O5分子量:390.52辅料:枸橼酸钠二水合物﹑盐酸﹑间甲酚﹑氢氧化钠﹑氯化钠﹑注射用水。
适应症:本品用于治疗肺动脉高压(PAH, WHO分类1)﹐以减轻运动引起的相关症状。
在建立本品疗效的研究中﹐研究受试者包括NYHA功能分级II∼IV级的原发性和遗传性肺动脉高压(58%)﹑与先天性体肺循环分流相关的肺动脉高压(23%)以及与结缔组织疾病相关的肺动脉高压(19%)。
规格:(1)20ml : 20mg (2)20ml : 50mg (3)20ml : 100mg (4)20ml : 200mg用法用量:本品用20ml玻璃瓶包装﹐共有四个规格﹐分别含有20﹑50﹑100或200mg曲前列尼尔(1mg/ml ﹑2.5mg/ml﹑5mg/ml或10mg/ml)。
本品输注前需用注射用水或0.9%NaCl注射液稀释。
本品的给药方式为皮下或静脉注射给药。
首次接受前列环素输注治疗患者的初始剂量:本品只能连续皮下(SC)或静脉(IV)输注。
皮下输注是首选给药路径﹐但是﹐如果因为输注部位严重疼痛或反应而不能耐受皮下给药﹐也可经中心静脉导管给药。
初始输注速率为1.25ng/kg/min。
如果由于全身效应不能耐受初始剂量﹐应将注射速率降低至0.625ng/kg/min。
剂量调整:长期剂量调整的目标是确定曲前列尼尔的剂量﹐使其可改善肺动脉高压症状﹐同时减少本品的其他药理学效应(头痛﹑恶心﹑呕吐﹑坐立不安﹑焦虑以及输注部位疼痛或反应)。
根据临床疗效进行剂量调整。
在治疗的前四周﹐输注速率的增加值为每周1.25ng/kg/min﹐之后为每周2.5ng/kg/min。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
FDA批准时间:2015-09-04Xuriden (uridine triacetate)药品使用说明书用于罕见的遗传性乳清酸尿症的治疗提供:HAOEYOU ( 好医友 )HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use XURIDEN safely and effectively. See full prescribing information for XURIDEN.XURIDEN TM (uridine triacetate) oral granulesInitial U.S. Approval: 2015------------------INDICATIONS AND USAGE---------------- XURIDEN is a pyrimidine analog for uridine replacement indicated for the treatment of hereditary orotic aciduria. (1) ------------ DOSAGE AND ADMINISTRATION------------ Recommended Dosage (2.1):• The starting dosage is 60 mg/kg once daily; the dose may be increased to120 mg/kg (not to exceed 8 grams) once daily for insufficient efficacy.• See the full prescribing information for 60 mg/kg and120 mg/kg weight-based dosing tables.Preparation and Administration (2.2)• Measure the dose using either a scale accurate to at least 0.1 gram, or a graduated teaspoon, accurate to the fraction of the dose to be administered.• Administer the dose with food (applesauce, pudding or yogurt) or in milk or infant formula. See full prescribing information for preparation and administration instructions. -----------DOSAGE FORMS AND STRENGTHS--------- Oral granules: 2 gram packets. (3)------------------CONTRAINDICATIONS------------------- None (4)-------------WARNINGS AND PRECAUTIONS----------- None (5)-----------------ADVERSE REACTIONS-------------------- No adverse reactions were reported with XURIDEN in patients with hereditary orotic aciduria (6.1).To report SUSPECTED ADVERSE REACTIONS, contact Wellstat Therapeutics Corporation at(1-800-914-0071) or FDA at 1-800-FDA-1088 or/medwatch.See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labelingRevised: 09/ 2015FULL PRESCRIBING INFORMATION: CONTENTS*1INDICATIONS AND USAGE2DOSAGE AND ADMINISTRATION2.1Recommended Dosage2.2Preparation and Administration 3DOSAGE FORMS AND STRENGTHS 4CONTRAINDICATIONS5WARNINGS AND PRECAUTIONS6ADVERSE REACTIONS6.1Clinical Trials Experience8USE IN SPECIFIC POPULATIONS8.1Pregnancy8.2Lactation8.4Pediatric Use11DESCRIPTION 12CLINICAL PHARMACOLOGY12.1Mechanism of Action12.2Pharmacodynamics12.3Pharmacokinetics13NONCLINICAL TOXICOLOGY13.1Carcinogenesis, Mutagenesis, Impairment ofFertility14CLINICAL STUDIES16HOW SUPPLIED/STORAGE AND HANDLING 17PATIENT COUNSELING INFORMATION*Sections or subsections omitted from the full prescribing information are not listed.FULL PRESCRIBING INFORMATION1INDICATIONS AND USAGEXURIDEN TM is indicated for the treatment of hereditary orotic aciduria.2DOSAGE AND ADMINISTRATION2.1Recommended DosageThe recommended starting dosage of oral XURIDEN is 60 mg/kg once daily. Increase the dosage of XURIDEN to 120 mg/kg (not to exceed 8 grams) once daily for insufficient efficacy, such as occurrence of one of the following:•Levels of orotic acid in urine remain above normal or increase above the usual or expected range for the patient•Laboratory values (e.g., red blood cell or white blood cell indices) affected by hereditary orotic aciduria show evidence of worsening•Worsening of other signs or symptoms of the diseaseThe XURIDEN dose to be administered at the 60 mg/kg and 120 mg/kg dose levels by body-weight is presented in Tables 1 and 2.A 2 gram packet of XURIDEN contains approximately ¾ teaspoon of XURIDEN. Therefore, in the tables below for patients requiring doses in multiples of 2 grams (¾ teaspoon) an entire packet(s) may be administered without weighing or measuring.XURIDEN Daily Dose Based on Body Weight (kg)Patient Weight Table 1: XURIDEN 60 mg/kg§ Dose LevelKilograms Dose to be Administered Using aScale (grams)Dose in Teaspoonsup to 5 0.4 1/86-10 0.4 to 0.6 ¼11-15 0.7 to 0.9½16-20 1 to 1.221-25 1.3 to 1.526-30 1.6 to 1.8¾ *31-35 1.9 to 2.1*36-40 2.2 to 2.4141-45 2.5 to 2.746-50 2.8 to 351-55 3.1 to 3.31 ¼56-60 3.4 to 3.661-65 3.7 to 3.9**1 ½ **66-70 4 to 4.2**71-75 4.3 to 4.5Above 75 6*** 2 ***§ total daily dose by weight category in the tables was rounded to achieve the approximate dose level * may use 1 entire 2 gram packet without weighing or measuring** may use 2 entire 2 gram packets without weighing or measuring*** may use 3 entire 2 gram packets without weighing or measuringPatient Weight Table 2: XURIDEN 120 mg/kg§ Dose LevelKilograms Dose to be Administered Using aScale (grams)Dose in Teaspoonsup to 5 0.8 ¼6-10 0.8 to 1.2 ½11-15 1.4 to 1.8 ¾16-20 2 to 2.4121-25 2.6 to 326-30 3.2 to 3.6 1 ¼31-35 3.8 to 4.2* 1 ½ **36-40 4.4 to 4.8 1 ¾41-45 5 to 5.42***46-50 5.6 to 651-55 6.2 to 6.6 2 ¼56-60 6.8 to 7.22 ½61-65 7.4 to 7.866-70 8****2 ¾ ****71-75 8****Above 75 8****§ total daily dose by weight category in the tables was rounded to achieve the approximate dose level** may use 2 entire 2 gram packets without weighing or measuring*** may use 3 entire 2 gram packets without weighing or measuring**** may use 4 entire 2 gram packets without weighing or measuring2.2Preparation and AdministrationPreparationMeasure the dose using either a scale accurate to at least 0.1 gram, or a graduated teaspoon, accurate to the fraction of the dose to be administered.Once the measured dose has been removed from the XURIDEN packet, discard the unused portion of granules. Do not use any granules left in the open packet.Administration with Food1.Place 3 to 4 ounces of applesauce, pudding or yogurt in a small clean container.2.Mix the measured amount of granules in the applesauce, pudding or yogurt3.Swallow applesauce/pudding/yogurt immediately. Do not chew the granules. Do not save theapplesauce/pudding/yogurt for later use.4.Drink at least 4 ounces of water.Administration in Milk or Infant FormulaXURIDEN can be mixed with milk or infant formula instead of the soft foods described above for patients receiving up to 3/4 teaspoon (2 grams) of XURIDEN. After weighing the dose of XURIDEN:1.Pour 5 mL of milk or infant formula into a 30 mL medicine cup.2.Insert the tip of the oral syringe into the medicine cup and draw up 5 mL of milk/infant formulainto the syringe.3.Hold the syringe with the tip pointing upward. Pull down on the plunger until the plungerreaches 10 mL. This will add air to the syringe.4.Place the cap over the tip of the syringe. Then invert the syringe so the syringe tip is pointingdown, and remove the plunger.5.Pour the measured amount of XURIDEN granules into the syringe barrel and reinsert the syringeplunger. Do not push up on the plunger.6.Gently swirl the syringe to mix the XURIDEN granules with the liquid.7.Turn the syringe so the syringe tip is pointing up. Then remove the syringe cap and push up onthe plunger until the plunger reaches the 5 mL mark. This will remove air from the syringe.8.Place the tip of the syringe in the patient’s mouth between the cheek and gum at the back of themouth. Gently push the plunger all the way down.9.Refill the syringe with another 5 mL of milk/infant formula.10.Gently swirl the syringe to rinse any remaining XURIDEN granules from the syringe barrel.11.Place the tip of the syringe in the patient’s mouth between the cheek and gum at the back of themouth. Gently push the plunger all the way down.12.Follow with a bottle of milk or infant formula, if desired.3DOSAGE FORMS AND STRENGTHSOral granules: 2 grams of orange-flavored oral granules (95% w/w) in single-use packets4CONTRAINDICATIONSNone5WARNINGS AND PRECAUTIONSNone6ADVERSE REACTIONS6.1Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions and using a wide range of doses, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.The safety of XURIDEN was assessed in 4 patients with hereditary orotic aciduria ranging in age from 3 to 19 years (3 male, 1 female) who received 60 mg/kg of XURIDEN once daily for six weeks. The patients continued to receive XURIDEN for at least 9 months at dosages of up to 120 mg/kg once daily. No adverse reactions were reported with XURIDEN.8USE IN SPECIFIC POPULATIONS8.1PregnancyRisk SummaryThere are no available data on XURIDEN use in pregnant women to inform a drug-associated risk. When administered orally to pregnant rats during the period of organogenesis, uridine triacetate at doses similar to the maximum recommended human dose (MRHD) of 120 mg/kg per day was not teratogenic and did not produce adverse effects on embryo-fetal development [see Data].The background risk of major birth defects and miscarriage for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.DataAnimal DataIn an embryo-fetal development study, uridine triacetate was administered orally to pregnant rats during the period of organogenesis at doses up to 2000 mg/kg per day (about 2.7 times the maximum recommend human dose (MRHD) of 120 mg/kg per day on a body surface area basis). There was no evidence of teratogenicity or harm to the fetus and no effect on maternal body weight and overall health.8.2 LactationRisk SummaryThere are no data on the presence of uridine triacetate in human milk, the effect on the breastfed infant or the effect on milk production. The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for XURIDEN and any potential adverse effects on the breastfed infant from XURIDEN or from the underlying maternal condition.8.4Pediatric UseThe safety and effectiveness of XURIDEN have been established in pediatric patients. Use of XURIDEN is supported by a single open-label clinical trial of uridine triacetate in 4 patients and a retrospective review of the clinical course of 18 patients with hereditary orotic aciduria who were treated with uridine beginning at ages 2 months to 12 years. There are no apparent differences in clinical response between adults and pediatric patients with hereditary orotic aciduria treated with uridine, however, data are limited. [see Clinical Studies (14)]11DESCRIPTIONXURIDEN (uridine triacetate) oral granules is a pyrimidine analog indicated for uridine replacement therapy. Uridine triacetate has the chemical designation (2',3',5'-tri-O-acetyl-ß-D-ribofuranosyl)-2,4(1H,3H)-pyrimidinedione. The molecular weight is 370.3 and it has an empirical formula of C15H18N2O9. The structural formula is:Each single-use 2 gram packet of XURIDEN orange-flavored oral granules (95% w/w) contains 2 grams of uridine triacetate and the following inactive ingredients: ethylcellulose (0.062 grams), Opadry Clear [proprietary dispersion of hydroxypropylmethylcellulose and Macrogol] (0.015 grams), and natural orange juice flavor (0.026 grams).12CLINICAL PHARMACOLOGY12.1Mechanism of ActionUridine triacetate is an acetylated form of uridine. Following oral administration, uridine triacetate is deacetylated by nonspecific esterases present throughout the body, yielding uridine in the circulation (Figure 1).Figure 1: Uridine Triacetate Conversion to UridineXURIDEN provides uridine in the systemic circulation of patients with hereditary orotic aciduria who cannot synthesize adequate quantities of uridine due to a genetic defect in uridine nucleotide synthesis.12.2PharmacodynamicsHereditary orotic aciduria (uridine monophosphate synthase deficiency) is a rare congenital autosomal recessive disorder of pyrimidine metabolism caused by a defect in uridine monophosphate synthase (UMPS). The UMPS gene encodes uridine 5′monophosphate synthase, a bifunctional enzyme that catalyzes the final two steps of the de novo pyrimidine biosynthetic pathway in mammalian cells.The defect in UMP synthase in hereditary orotic aciduria has two primary biochemical consequences. First, the blockade of de novo UMP synthesis results in a systemic deficiency of pyrimidine nucleotides, accounting for most clinical consequences of the disease. Second, orotic acid from the de novo pyrimidine pathway that cannot be converted to UMP is excreted in the urine, accounting for the common name of the disorder, orotic aciduria. Orotic acid crystals in the urine can cause episodes of obstructive uropathy.XURIDEN delivers uridine into the circulation, where it can be used by essentially all cells to make uridine nucleotides, compensating for the genetic deficiency in synthesis in patients with hereditary orotic aciduria. When intracellular uridine nucleotides are restored into the normal range, overproduction of orotic acid is reduced by feedback inhibition, so that urinary excretion of orotic acid is also reduced.12.3PharmacokineticsAbsorptionXURIDEN delivers 4- to 6-fold more uridine into the systemic circulation compared to equimolar doses of uridine itself. Maximum concentrations of uridine in plasma following oral XURIDEN are generally achieved within 2 to 3 hours, and the half-life ranges from approximately 2 to 2.5 hours.A study in patients with hereditary orotic aciduria included an assessment of plasma uridine pharmacokinetics in 4 patients. Three of the patients were previously treated with oral uridine. OnDay 0 (baseline), these three patients received their usual daily dose of oral uridine as a single dose (150 to 200 mg/kg once daily) and on Day 1, initiated oral XURIDEN treatment (60 mg/kg once daily). A fourth patient was enrolled who was naïve to uridine replacement therapy. The dose of XURIDEN was increased on Day 116 to 120 mg/kg once daily in two patients (Patients 3 and 4) and plasma uridine concentrations were assessed on Day 160 (44 days after the dose increase).Plasma uridine levels in all four patients are depicted in Figure 2. Pharmacokinetic parameters are summarized in Table 3. Mean exposure to plasma uridine as assessed by C max and AUC was greater after oral XURIDEN than after oral uridine (approximately 4-fold on an equiweight basis, and 6-fold on an equimolar basis), although individual differences in relative bioavailability were noted. Plasma concentrations of the uridine catabolite uracil were generally below the limit of quantitation in all patients.Table 3: Pharmacokinetic Parameters for Plasma UridinePharmacokinetic Parameters (Plasma Uridine) Day 0 (Baseline)(Oral Uridine,150 to 200 mg/kgonce daily)N = 3 aDay 1(OralXURIDEN,60 mg/kg oncedaily)N = 4Day 28(Oral XURIDEN,60 mg/kg oncedaily)N = 4Day 160(Oral XURIDEN,120 mg/kg once daily)N = 2 bC max (µM)mean ±SD56.0 ± 16.6 91.3 ± 32.2 88.7 ± 43.2 80.9 ± 20.0T max (hours)median (range c) 2.0 (1.0, 4.0) 2.0 (1.2, 2.1) 1.3 (1.0, 2.5) 3.0 (2.0, 4.0)t1/2 (hours)mean ±SD 1.6 ± 0.7 1.6 ± 0.6 2.3 ± 1.6 8.2 ± 6.8AUC(0-8) (µM•hr)mean ±SD238.0 ± 163.2 311.2 ± 153.3 278.7 ± 148.5 465.6 ± 95.3a Data shown are from patients previously treated with oral uridineb The dose of XURIDEN was increased on Day 116 to 120 mg/kg per day. Serial plasma samples were taken on Day 160 (44 days after the dose increase) for plasma uridine levels.c Tmaxrange is expressed as the minimum and maximum values obtainedFigure 2 Plasma Uridine Following Oral Administration of Uridine (Day 0) or XURIDEN (Days 1, 28 and 160) in Patients with Hereditary Orotic AciduriaFood Effect on Uridine PK:A study in healthy adult subjects receiving a slightly different formulation of uridine triacetate granules (6 gram dose) under fed and fasted conditions showed no difference in the overall rate and extent of uridine exposure.DistributionCirculating uridine is taken up into mammalian cells via specific nucleoside transporters, and also crosses the blood brain barrier.ExcretionUridine can be excreted via the kidneys, but is also metabolized by normal pyrimidine catabolic pathways present in most tissues.Drug Interaction StudiesIn vitro enzyme inhibition data did not reveal meaningful inhibitory effects of uridine triacetate or uridine on CYP3A4, CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP2E1. In vitro enzyme induction data did not reveal an inducing effect of uridine triacetate or uridine on CYP1A2, CYP2B6, or CYP3A4.In vitro data showed that uridine triacetate was a weak substrate for P-glycoprotein. Uridine triacetate inhibited the transport of a known P-glycoprotein substrate, digoxin, with an IC50 of 344 µM. Due to the potential for high local (gut) concentrations of the drug after dosing, the interaction of XURIDEN with orally administered P-gp substrate drugs cannot be ruled out.In vivo data in humans are not available.13 NONCLINICAL TOXICOLOGY13.1Carcinogenesis, Mutagenesis, Impairment of FertilityLong-term studies in animals have not been performed to evaluate the carcinogenic potential of uridine triacetate.Uridine triacetate was not genotoxic in the Ames test, the mouse lymphoma assay and the mouse micronucleus test.Orally administered uridine triacetate did not affect fertility or general reproductive performance in male and female rats at doses up to 2000 mg/kg per day (about 2.7 times the maximum recommend human dose (MRHD) of 120 mg/kg per day on a body surface area basis).14CLINICAL STUDIESThe efficacy of XURIDEN was evaluated in an open-label study in 4 patients with hereditary orotic aciduria (3 male, 1 female; age range from 3 to 19 years). Three patients were previously treated with uridine and were switched at study entry to XURIDEN. All patients were administered XURIDEN orally at a daily dosage of 60 mg/kg once daily. The study duration was 6 weeks.The study assessed changes in the patients’ pre-specified hematologic parameters during the 6-week trial period. The pre-specified hematologic parameters were: neutrophil count and percent neutrophils (Patient 1), white blood cell count (Patient 2), and mean corpuscular volume (Patients 3 and 4).For patients switched from oral uridine to oral XURIDEN (Patients 1, 2, and 3), the primary endpoint was stability of the hematologic parameter; for the treatment-naïve patient (Patient 4), the primaryendpoint was improvement of the hematologic parameter. Secondary endpoints were urine orotic acid and orotidine levels, and growth (height and weight) for all patients.After six weeks of treatment, Patients 1 and 3 met the pre-specified criteria for stability of the hematologic parameter. When Patient 2 was switched from uridine to XURIDEN treatment, the pre-specified criteria for white blood cell count remained stable; however documentation of a low white blood cell count prior to uridine initiation was not available. Patient 4 did not meet the pre-specified endpoint of improvement of the hematologic parameter.Table 4 summarizes the primary efficacy results.Table 4: Primary Efficacy Results for Study 1Patient Pre-specifiedhematologicparameter(Age-specificreference range) Primary Endpoint Baseline(Day 0)Week 6(Day 42)%ChangefromBaselinePatient #1 Neutrophil count(1.5 to 8.0x103/mm3)Stable hematologic value 0.95 0.81 -15%Neutrophil %(26 to 48%)Stable hematologic value 21 23 10%Patient #2 White Blood CellCount(3.8 to 10.6 x109/L)Stable hematologic value 7.8 7.4 -5%Patient #3 Mean CorpuscularVolume(75 to 91 fL)Stable hematologic value 109.9 108.5 -1%Patient #4 Mean CorpuscularVolume(72 to 90 fL)Improved hematologic value 114.6 113.4 -2%At baseline, three patients had normal urine orotic acid levels and all four patients had normal urine orotidine levels. Three patients who had achieved normal urine orotic acid levels when they were treated with uridine maintained normal levels 6 weeks after transitioning to XURIDEN. All four patients had normal urine orotidine levels at baseline which remained stable after 6 weeks of treatment with XURIDEN.During an extension phase of the trial, patients continued to receive XURIDEN. Dosing during the extension phase ranged from 60 mg/kg to 120 mg/kg once daily. After 6 months of treatment, Patient #1’s neutrophil count and neutrophil percent values normalized; hematologic parameters for theother three patients remained stable. Orotic acid and orotidine levels also remained stable for all four patients.The treatment effect of XURIDEN on growth was assessed in the three pediatric patients (Patients 1, 3, and 4). At baseline, weight and height measurements were at or below the lower limit of normal for age (below 5th percentile for age) for Patients 1 and 4; height and weight measurements were within the normal range for age for Patient 3.After 6 months of treatment, Patients 1 and 3 experienced improved weight growth, as reflected in increases in their weight-for-age percentiles and weight velocity percentiles; Patient 4’s weight growth remained stable (i.e., weight percentile for age and weight velocity percentile for age was unchanged). Height growth remained stable in all three patients (i.e., height percentiles for age and height velocity percentiles for age were unchanged).Case reportsNineteen (19) case reports of patients with hereditary orotic aciduria have been documented in published literature. Eighteen (18) patients were diagnosed as infants or children between the ages of 2 months and 12 years and were treated with exogenous sources of uridine. One patient, diagnosed at age 28, was not treated with exogenous uridine.All 19 patients presented with significantly elevated levels of urinary orotic acid. Fifteen of 19 had abnormal hematologic parameters at presentation, including 15 with megaloblastic anemia, 8 with leukopenia and at least 2 with neutropenia. Oral administration of exogenous sources of uridine was reported to significantly improve hematologic abnormalities (megaloblastic anemia, leukopenia and neutropenia) within 2 to 3 weeks in almost all documented cases when administered in sufficient amounts. Concentrations of urinary orotic acid were significantly reduced within 1 to 2 weeks of initiating uridine replacement therapy. Some fluctuation in levels of urinary orotic acid were observed, but always at much lower levels than those reported prior to treatment. Improvements in body weight were also documented over time with continued uridine replacement therapy.The effects of exogenous uridine were maintained over months and years, as long as treatment continued at sufficient doses (with appropriate dose increases based on body weight increases). Most hematologic abnormalities and orotic aciduria reappeared within days up to 2 or 3 weeks when administration of uridine was stopped or the dose was reduced. If treatment was interrupted for longer periods, body weight growth receded. If absolute dosages were not adjusted adequately to compensate for body weight gains, signs and symptoms of hereditary orotic aciduria recurred.16HOW SUPPLIED/STORAGE AND HANDLINGXURIDEN orange-flavored oral granules (95% w/w) are available in single-use packets (NDC 69468-152-02) containing 2 grams of uridine triacetate in cartons of 30 packets each (NDC 69468-152-30). Store at controlled room temperature, 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). 17PATIENT COUNSELING INFORMATIONAdvise the patient or caregiver to read the FDA-approved patient labeling (Instructions for Use) AdministrationAdvise the patient or caregiver:•To measure the prescribed dose using either a scale accurate to at least 0.1 gram, or a graduated teaspoon, accurate to the fraction of the dose to be administered.•To discard the unused portion of granules in a packet after measuring out the dose.•That XURIDEN can be taken mixed in food (applesauce, pudding or yogurt) or mixed in milk or infant formula.•That the XURIDEN granules should not be chewed.___________________________________________________________________________Manufactured and distributed by:Wellstat Therapeutics CorporationGaithersburg, MD 20878XURIDEN TM is a trademark of Wellstat Therapeutics Corporation. The Wellstat logo is a registered trademark of Wellstat Therapeutics Corporation.。
