Naming Compounds
3 立体结构的表示方法
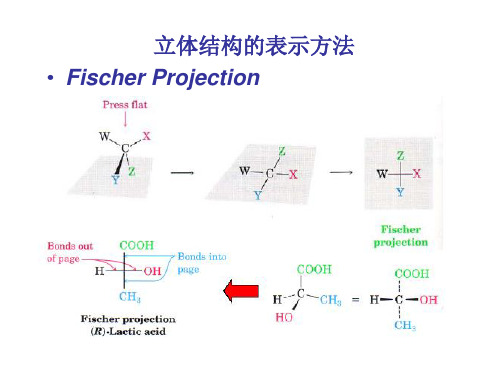
CHO H OH CH2OH
HgO
COOH H OH
d D型
(+)
氧化前后,中心 手性碳构型不变
l D型
(-)
CH2OH
• 具有相同DL构型的分子,不一定具有相 同的旋光方向。 • 相同的化合物在不同条件下(溶剂、波长 或温度),可能旋光方向相反。
• Assigning R, S configuration
H
2S
H
The plane of symmetry
H
2R Meso compounds
• 2个手性中心:两个对映体,4个立体异 构体。 当两个手性中心连有相同基团时:一对对 映体,一个立体异构体。 多于一个手性中心的分子异构体数:2n个 异构体。
(6)对手性醇采用手性非对映试剂的衍生 化处理,再用气相色谱测定差向异构体 的比率
Ph Me N Cl O Ph N N P O Cl
(7)MTPA (Mosher’s acid)方法:用 于手性仲醇的构型确定。 MTPA: -methoxy--trifluoromethyl phenylacetic acid
(2) 机理已明确的情况下,涉及手性中心的转化
SN2机理:产物与原反应物构型已发生反转, 即产物构型与原反应物构型相反。
HO
COOH C H CH3
NaOH
H
COOH C Br CH3
NaN3
N3
COOH C H CH3
H2N
COOH C H CH3
(3)生物化学方法 对于氨基酸和甾体化合物:某些酶具有 构型特异性催化化学反应。 8个L氨基酸反应,如第9个氨基酸也反应, 极可能为L氨基酸。
COOH OH H COOH HO H
chapter 5 nomenclature

As an introduction to the IUPAC nomenclature system, we shall first consider compounds that have no specific functional groups. Such compounds are composed only of carbon and hydrogen atoms bonded together by sigma bonds (all carbons are sp3 hybridized). 5-3 Alkanes Hydrocarbons having no double or triple bond functional groups are classified as alkanes or cycloalkanes, depending on whether the carbon atoms of the molecule are arranged only in chains or also in rings. Although these hydrocarbons have no functional groups, they constitute the framework of other classes of compounds, and provide an ideal starting point for studying and naming organic compounds. The alkanes and cycloalkanes are also members of a larger class of compounds referred to as aliphatic. Simply put, aliphatic compounds are compounds that do not incorporate any aromatic rings in their molecular structure.
化学结构命名英语作文

化学结构命名英语作文Chemical Structure Nomenclature。
Introduction。
Chemical structure nomenclature is a system for naming chemical compounds. It is used to identify and describe compounds, and to communicate information about their structure and properties. There are a number of different chemical structure nomenclature systems in use, but the most common is the International Union of Pure and Applied Chemistry (IUPAC) system.IUPAC Nomenclature。
The IUPAC nomenclature system is based on theprinciples of simplicity, clarity, and conciseness. It uses a set of prefixes and suffixes to indicate the number and type of atoms in a compound, and the way in which they are bonded together.The following are the basic rules of IUPAC nomenclature:The name of a compound is based on the name of the parent hydrocarbon.The prefixes "mono-", "di-", "tri-", etc., are used to indicate the number of substituents on the parent hydrocarbon.The suffixes "-ane", "-ene", and "-yne" are used to indicate the type of bonding in the parent hydrocarbon.The prefixes "chloro-", "bromo-", "fluoro-", etc., are used to indicate the presence of halogen atoms.The prefixes "hydroxy-", "amino-", "carboxy-", etc., are used to indicate the presence of functional groups.Example。
高中化学有机物命名Naming Organic Compounds

Naming Organic Compounds: Beyond saturated hydrocarbonsCyclic compoundsAdd cyclo- to beginning of name if the alkane is known to be arranged in a circle (C n H2n ). No position number is needed if only one alkyl group is attached.Aromatic hydrocarbon, or benzene (C6H6: Electron orbitals overlap to form continuous orbitals = delocalized electrons throughout the ring.)Assign position number 1 to the alkyl group that comes first in alphabetical order, then number in the direction to give the rest of alkyl groups the lowest number possible. Example: 1-ethyl-4-methylbenzeneUnsaturated hydrocarbonsSingle bond between carbons: -ane ethane H3-C—C-H3 C n H2n+1Double bond between carbons: -ene ethene H2-C==C-H2 C n H2nTriple bond between carbons: -yne ethyne H-C==C-H C n H n Locate the longest continuous chain containing a double bond. If there is only one double bond, add the suffix –ene to the proper prefix for this number of carbons.Add the names of alkyl groups as in single-bond compounds, EXCEPT: Number the carbons so that the 1st C in the double bond is nearest the end that has the lowest number.Place the position number of alkyl groups before proper group, and place position number for the double bond before the alkene name.Alkynes are named similarly, except all references to a double bond becomes a triple bond, and –ene becomes –yne.Example: 2-ethyl-3-methyl-1-butene CH3|CH3–CH--C==CH2|CH2--CH3Functional Groups (R = rest of molecule, usually C and H atoms) Compound Formula ExampleAlcohol R-OH hydroxyl group 1-propan olAlkyl Halide -X X = any halide 1,2-dibromopropaneEther R-O-R’one oxygen bonded to 2hydrocarbon groups diethyl etherCH3-CH2 –O--CH2--CH3Aldehyde O||R-C-H Carbonyl group attachedto end carbonEthan al O||CH3—C--HKetone O||R-C-R’Carbonyl group attachedto a middle carbon2-propan one O||CH3—C-- CH3Carboxylic Acid O||R-C-OH Carboxyl group ethanoic acid O||CH3—C—OHEster O||R-C-O-R’Carboxyl group withoutthe Hmethyl ethanoate O||CH3—C—O-- CH3CH3-CH2- -CH3 the parent hydrocarbon: Use theLONGEST STRAIGHT chain of carbon atoms.2.Add the name of the alkyl groups attached to thechain. If more than one group is attached, use theproper numerical prefix to indicate how manygroups are attached. (2=di, 3-tri,etc.)3.Assign numbers to the carbons in the parentchain. Assign so that attached groups are at thelowest number possible.4.Insert the numbers in front of the proper group.5.Separate position numbers from names withhyphens.Example: the parent hydrocarbon.CH3–CH –CH2–CH –CH –CH3hexaneCH3CH3CH32.Add the name of the alkyl groups attached to the chain.3 methyl groups: trimethylhexane3.Assign numbers to the carbons in the parent chain.6 5 4 3 2 1CH3–CH –CH2–CH –CH –CH3CH3CH3CH34-5. Insert position numbers and add hyphens.2,3,5-trimethylhexane。
化学化工专业英语 课文翻译 第八课8.2.

8 . 2 introductionMastering chemical nomenclature is little different from learning a new language, such as German . In order to understand the German scientific literature , you must , e . g , learn that the compound H2 is called Wasserstoff .English-speaking chemists call it hydrogen . Your task now is to memorize the names of enough compounds and become sufficiently familiar with the several systems of naming compounds that chemistry ceases to be a " foreign language .The first thing to learn about naming chemical compounds is that there is usually more than one way to do it . We begin with the simplest system , in which a trivial name , 1 . e . , one that has no sensible origin , is assigned to a compound .Some examples areSome names , such as quicklime for CaO , derive from the origin of the compoundin this case , limestone , CaCO3 Such word origins are often remembered only by etymologists , but the names have persisted for so long that they are an established part of the language . Can you imagine anyone seriously asking for a drink of dihydrogen oxide ? The word water serves the purpose much better .As we come to less common and more complex compounds , the use of trivial names gives way to a more systematic approach. If there are only two elements in the compound , it is customary to name the more metallic element first and the less metallic , or more electronegative , element second , with the suffix “- ide , , . Some examples areFor compounds containing still only two elements but more than two atoms , the pre fixes " mono -, , , " di -” , " tri 一, , etc , become necessary . Some examples of such compounds are the oxides of nitrogen . Another such series is that of the oxide of chlorine .Because chlorine , like nitrogen , is slightly less electronegative than oxygen , the word chlorine cornes firstif no confusion can result , the prefixes " mono , , and “di -, are sometimes dropped .A class of compounds in which such prefixes are seldom used is that in which the metal atom usually exhibits only one oxidation state Depending on the oxidation state of the other element , the number of anions per cation is then fixed . Some examples areThe next level of complexity in naming Inorganic compounds arises when there are three elements present . Very often , one of these elements is oxygen . Such compounds are named by combining the suffix “ ate “ , with the name of the less electronegative of the two nonmetallic elements. For example , NaNo3 is sodium nitrate . The problem with this is that there is a similar compound with nitrogen in the + 3 oxidation state , NaNO2. such compounds with the element in a lower oxidation state use the suffix “-ite , , , so NaNO2 is sodium nitrite . But the number of chemical compounds is not bounded by the chemists ' vocabulary , and there are several such examples entailing more than two oxidation states .To solve this problem , the prefix ' ' hy - po 一, , ( meaning " below , , is used in the name of the compound In which the less electronegative element is in the lowest oxidation state , and the prefix " per-, , ( meaning " highest , , is usedwhen it is in the highest oxidation state Some examples of the use of this system are shown In the following table ( Table 8 . 2In the inorganic acids , the suffixes “-ous , , and "- ic , , are used to denote the lower and higher oxidation states , respectively These same suffixes are also used with the names of a number of metals , namely , those that usually exhibit more than one oxidation state Some examples are cobaltous and cobaltic , and mercurous and mercuric . The nomenclature is complicated slightly by the fact that , for a few such metals , these terms are derived from the Latin name of the element rather than the English name .All but eleven of the elements are given a symbol corresponding to one or two letters In the English name of the compound ( The first letter is always capitalized and the second letter 15 never capitalized One of these exceptions is tungsten , whose symbol ( W is derived from the German name of the element , Wolfram The other ten havesymbols derived from their Latin names . These are , stibium ( Sb for antimony , cuprum ( Cu )for copper , aurum ( Au for gold ,ferrum ( Fe for iron , plumbum ( Pb for lead , hydrargyrum ( Hg for mercury , kalium ( K for potassium , argentum ( Ag for silver , natrium ( Na for sodium , and stannum ( Sn for tin. The use of the suffixes ' ’- ous , , and “-ic , , with three of these metals 15 illustrated belowThe system works well as long as there are only two major oxidation states of the metal atom , as in those examples .The most rational and selfconsistent system of nomenclature of inorganic compounds is that adopted in 1957 by the ultimate authority in such matters , the International Union of Pure and Applied Chemistry. these rules , popularly called the IUPAC Rules , are the model for chemists throughout the world to follow , and are becoming ever more dominant In the chemical literature . Note that the oxidation state of the metal atom is specified by a Roman numeral whenever there could be some doubt about it , but not otherwise Let us see how the examples shown above are named according to this system掌握化学命名是学习新的语言,如德国略有不同。
化学与化工专业英语2-化学基础知识

3)smell
odourless
pungent
penetrating
choking
offensive
sour sweet bitter
4)solubility
soluble
insoluble
slightly soluble
very soluble
5)observations
brisk effervescence
75 45 37 44 62 21
镭 氡
铼 铑 铷 钌 钐 钪
Selenium* Silicon* Silver* Sodium* Strontium* Sulfur* Tantalum* Technetium* Tellurium* Terbium* Thallium* Thorium Thulium Tin* Titanium* Tungsten* Uranium Vanadium* Xenon* Ytterbium Yttrium* Zinc* Zirconium*
Exercise
试用英语描述氧气、氮气和金属铁的物理 性质。
Part 2 Chemical Equations
化 学 方 程 式 1.反应名称:
disproportionation neutralization; hydrolysis exothermic reaction endothermic reaction reversible reaction forward reaction reverse reaction spontaneous reaction nonspontaneous reaction
over quotient
化学专业英语-命名
O F
Oxygen Fluorine
Cl Ar
Chlorine Argon
Common transition elements
Fe: iron
Mn: manganese Hg: mercury Ag: silver Au: gold Ni: nickel
Cu: copper
Zn: zinc
2. The rules of nomenclature
3.2.2 Polyatomic ions containing oxygen
(1) Acid radicals for normal salt (正酸根-ate )
Anion’s name = Central Element’s root -ate
For example:
ClO3- Chlorate
3.2 Naming metal oxide (金属氧化物) Metal oxide = Metal cation + oxide
For example:
FeO Iron(II) oxide (Ferrous oxide)
Fe2O3
Na2O
Iron(III) oxide (Ferric oxide)
For example:
PH3: phosphine或phosphane AsH3: arsine或arsane
SiH4: silane
5. Nonoxide acid (无氧酸)
命名规则:hydro-词根-ic acid
For example:HCl : hydrochloric acid H2S : hydrosulfuric acid HBr : hydrobromic acid HI : hydroiodic acid
NamingandCovalentCompounds:命名和共价化合物
Period:_____________________Naming and Covalent CompoundsMaking Ionic CompoundsIon NotationNa 1+How many protons and electrons does S 2– have?Give the ion notation for Calcium that lost 2 electrons.How many electrons does K 1+ have?Give the ion notation for an atom with 8 protons and 10 electrons.Give the ion notation for an atom with 34 protons and 36 electrons.Fe 3+ :did it gain or lose electrons and how many?Find from number of protonsFind from p-e=charge ORNumber of electrons: (+) lost or (-) gained.Tells you: sodium (11 protons) and 1 electron lost (+), so only 10 electrons.Li 1+O 2-Li 2O123Li 1+ O 2- 21Write the chemical symbols with theoxidation numbers.Cross the numbers notthe signs.Reduce numbers ordrop ones and put the symbols together.Way 1Mg 2+Cl 1-MgCl 21.2.3.Mg 2+Cl 1- Write the chemicalsymbols with theoxidation numbers.Add enough ionstogether so that thecharges equal zero.Add up the ions and write the compound as a formula.Way 2Cl 1-You know it is a balanced compound because2(1) + 1(-2) = 0. Balanced ionic compounds have a neutral charge.Again, you know it is a balanced compound because 1(2) + 2(-1) = 0. Balanced ionic compounds have a neutral charge.Make the ionic compound of magnesium oxide.Combine Fe(II) and O.Make lithium chloride. Combine Iron(III) and Fluorine.Make potassium sulfate (SO 42-).Combine sodium and carbonate (CO 3)2-.Period:_____________________Covalent BondingYou must fulfill two criteria when making covalent bonds:1) the individual atoms must have the proper number of valence electrons; 2) when bonded each atom must have 8 electrons through sharing.Each has 6 valence electrons by itself and 8 by sharing.OPut the number you need in the middle to share . O OA double covalent bond.Read each oxygen as 6 v.e. plus 2 for the 2 bonds = 8!O8 66Oxygen dichloride: OCl 26 v.e.8 shared7 v.e. 8 sharedMake F 2. Make N 2.Make S 2. Make oxygen difluoride: OF 2 Make methane: CH 4.Make carbon dioxide: CO 2 Naming CompoundsIonic compounds(metals and non-metals): Name the metal and non-metal and change the ending to “-ide”.BeO: Beryllium oxideMgCl 2: Magnesium chloride. Covalent compounds (2 non-metals):Use the prefixes to show how many atoms are there.CO: Carbon monoxide CO 2: Carbon dioxide. Polyatomic compounds (3 or more elements): Use the names on the polyatomic ion chart.Al(PO 4): Aluminum phosphate Be(CrO 4): Beryllium chromate.1. NF 3 ___________________________________2. FeO___________________________________3. Na 2SO 3 ___________________________________4. LiBr 2 ___________________________________5. O 2Cl 4 ___________________________________6. CS 2 ___________________________________7. Ca 3P 2 ___________________________________8. NaCl ___________________________________ 9. LiOH ___________________________________ 10. N 2F 3 ___________________________________O Cl ClShort hand。
化学物质命名规律1
5. penta-;
7. hept(a)- (sept(a)-); 10. dec(a)-
9. non(a)-;
常用数字前缀 :
词首:mono-, di-, 一, hexa-, 二, tri-, 三, tetra-, 四, penta五 deca-
sodium hypochlorite sodium hypochlorite
K2Cr2O7
Cu3(AsO4)2 Cr(C2H3O2)3
potassium dichromate
potassium dichromate
copper(II) arsenate cupric arsenate chromium(II) acetate chromic acetate
Table 1 Some Common Ions
1acetate bromide chlorate chloride chlorite cyanide C2H3O2BrClO3ClClO2CN-
1hydrogen sulfite hydride hydroxide hypochlorite iodate nitrate HSO2HOHClOIO3NO3-
methethpropbutpenthex甲乙丙丁戊已heptoctnondeccyclopoly庚辛壬葵环聚twosystemsofnamingcompoundstwosystemsofnamingcompounds?h2owatercaoquicklime?nh3ammoniacaco3limestone?hg2cl2calomelcuicuprousiodidepcopperiiodideppcui2febr2febr3sncl2sncl4cupriciodideferrousbromideferricbromidestannouschloridestannicchloridecopperiiiodideironiibromideironiiibromidetiniichoridetinivchloridechapter1chapter1nomenclatureofinorganiccompoundsnomenclatureofinorganiccompoundstable1somecommonions12ammoniumnh4coppericuhydrogenhpotassiumksilveragsodiumnabariumba2calciumca2chromiumiicr2copperiicu2ironiife2leadiipb2table1somecommonions23magnesiummg2manganeseiimn2mercuryiihg2mercuryihg22tiniisn2strontiumstr?nti?msr2zinczn2aluminumal3chromiumiiicr3ironiiife3table1somecommonions32arsenateaso43aso33po43po33arsenitecarbonateco32cro42cr2o72c2o42chromatephosphatephosphitedichromateoxalateoxideo2sulfides2sulfateso42so32sulfitetable1somecommonions11acetatec2h3o2brclo3clclo2cnbromidehydrogensulfitehso2hohcloio3no3hydrideychloratechloridechloritecyanidehydroxideh
中学化学物质的英文命名
中学化学物质的英⽂命名化合物的英⽂命名(Nomenclature of compounds)I ⽆机物的命名(Inorganic compounds)1 元素与单质的命名“元素”和“单质”的英⽂意思都是“element”,有时为了区别,在强调“单质”时可⽤“free element”。
因此,单质的英⽂名称与元素的英⽂名称是⼀样的。
下⾯给出的既是元素的名称,同时⼜是单质的名称。
S-block ElementIA IIAH Hydrogen Be BerylliumLi Lithium Mg MagnesiumNa Sodium Ca CalciumK Potassium Sr StrontiumRb Rubidium Ba BariumCs CesiumFr Francium Ra RadiumP-block ElementIIIA IV A V AB BoronC Carbon N NitrogenAl Aluminium Si Silicon P PhosphorusGa Gallium Ge Germanium As ArsenicIn Indium Sn Tin Sb AntimonyTl Thallium Pb Lead Bi BismuthVIA VIIA 0He Helium O Oxygen F Fluorine Ne NeonS Sulfur Cl Chlorine Ar ArgonSe Selenium Br Bromine Kr KryptonTe Tellurium I Iodine Xe XenonPo Polonium At Astatine Rn Radon Common Transition ElememtFe : iron Mn : manganeseCu: copper Zn: zincHg: mercury Ag: silverAu: gold2化合物的命名(4)tetra –,(5)penta- (6)hexa-,(7)hepta-,(8)octa-,(9)nona-,(10)deca-,但是在不会引起歧义时,这些前缀都尽可能被省去。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Sections (Zumdahl 6th Edition) 2.8-2.9 Outline: The Foundation of Stoichiometry
The Periodic Table helps organize types Binary Compounds: Metal and Non-Metal Binary Compounds: Two Non-Metals Acids (with and without oxygen)
• Binary – only two elements (as ions) combined • Can’t combine two metals (e.g. Cu & Zn –Brass) • The metal ion (cation, or positive) is always named first and the anion second. • A monatomic cation takes its name from the name of the element, e.g. Na+ is called sodium in the names of compounds containing this ion. • The non-metal ion , anion,(or negative ion) is named by taking the first part of the element and adding –ide, e.g. Cl- is chloride.
Different types (Sub-Classes) of Metals
• Alkali metals - (lithium, sodium, potassium, rubidium and cesium) - soft, low melting points, react with water to liberate hydrogen, form 1:1 compounds with chlorine. • Alkaline earths - (beryllium, magnesium, calcium, strontium, barium and radium) - react in a 1:2 ratio with chlorine. (Type I in text) • Transition metals - (e.g. iron, copper, silver, gold, tungsten and cobalt) - structural metals. Multi-valence; (Type II in text) • Actinides and Lantanides: Often multi-valence • Metalloids - (antimony, arsenic, boron, silicon and tellurium) - intermediate between metals and nonmetals. Acts as metals with non-metals; Act as non-metals with metals.
Problem: Give the Name and Chemical Formulas of the Compounds formed from the following pairs of Elements (Notice: Some are not simple one-to-one, although they are binary)
B C N 2 ⋅ 5 → Al Si P V Cr Mn Fe Co Ni Cu Zn Ga Ge As NbMo Tc Ru Rh Pd Ag Cd In Sn Sb Ta W Re Os Ir Pt Au Hg Tl Pb Bi Du Sg Bo HaMe
Ce Pr Nd PmSm Eu Gd Tb Dy Ho Er TmYb Lu Th Pa U Np PuAmCmBk Cf Es FmMd No Lr
←
2
⋅
7
→
The Alkali Metals The Alkaline Earth Metals
The Halogens The Noble Gases
Groups in the Periodic Table
Main Group Elements (Vertical Groups) Group IA - Alkali Metals Group IIA - Alkaline Earth Metals Group IIIA - Boron Family Group IVA - Carbon Family Group VA - Nitrogen Family Group VIA - Oxygen Family (Calcogens) Group VIIA - Halogens Group VIIIA - Noble Gases Other Groups ( Vertical and Horizontal Groups) Group IB - 8B - Transition Metals Period 6 Group - Lanthanides (Rare Earth Elements) Period 7 Group - Actinides
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np PuAmCm Bk Cf Es FmMd No Lr Boron family Carbon Family Nitrogen family Oxygen Family
The Periodic Table of the Elements H Li Be Na Mg ← K Ca Sc Ti Rb Sr Y Zr Cs Ba La Hf Fr Ra Ac Rf B C N O 2 ⋅ 5 → Al Si P S V CrMn Fe Co Ni Cu Zn Ga Ge As Se NbMo Tc Ru Rh PdAg Cd In Sn Sb Te Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po Du Sg Bo Ha Me The Transition Metals He F Ne Cl Ar Br Kr I Xe At Rn
ቤተ መጻሕፍቲ ባይዱ
Classification of the Elements
• Most of the elements are metals - metallic luster, ability to conduct electricity and heat, and malleability. • The remaining elements are classified as nonmetals - no luster, poor conductors of electricity and heat and brittleness. • There are only 11 non-metals and they are grouped together in the periodic table
CaF2
calcium fluoride
HI
hydrogen iodide
How do we know what charge to make each ion? • The periodic table is our best source of information (a great “cheat sheet”) • Metals in column 1 give up one electron, etc. • Non-metals is column 7 take one electron. • Notice the ions have the number of electrons that the nearest inert gas has.
a) sodium and oxygen b) zinc and chlorine c) calcium and fluorine d) strontium and nitrogen e) hydrogen and iodine f) scandium and sulfur
Na2O
sodium oxide
Non-Metals • Chalcogens - (oxygen, sulfur, selenium and tellurium) - form 1:1 compounds with alkaline-earths, but 2:1 compounds with alkali-metals. • Halogens - (fluorine, chlorine, bromine and iodine) - highly reactive and form 1:1 compounds with alkali-metals. • Noble gases - (helium, neon, argon, krypton, radon) - virtually inert to chemical reactions. • Nitrogen (Phosphorous), Carbon and Boron.
Ce Pr Nd PmSm Eu Gd Tb DyHo Er TmYb Lu Th Pa U Np PuAmCmBk Cf Es FmMd No Lr Lanthanides: The Rare Earth Elements The Actinides
Naming Binary Compounds (Type I; Ionic)
← 2 ⋅1 →
The Periodic Table of the Elements
H Li Be NaMg ← K Ca Sc Ti Rb Sr Y Zr Cs Ba La Hf Fr Ra Ac Rf
