Kunishima教授讲课文章汇总
纳米金比色检测葡萄糖

/ac Naked Eye Detection of Glucose in Urine Using Glucose Oxidase Immobilized Gold NanoparticlesChangerath Radhakumary and Kunnatheeri Sreenivasan*Laboratory for Polymer Analysis,Biomedical Technology Wing,Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram695012,Indiab Supporting InformationC hemical sensors are miniaturized devices that can deliverreal-time and online information on the presence of specific compounds or ions in complex matrixes.1Metallic nanoparticle based sensing has emerged as an important colorimetric tool for the detection of biomolecules linked to the onset of diseases to aid in early diagnosis.Among them,colloidal gold is extensively used for molecular sensing due to the wide opportunities it offers in the design of easy to perform methods.2The synthesis of gold colloid with an organic monolayer in a one-step process is also exploited, and this monolayer provides the extraordinary stability to the nanoparticles along with the additional surface properties.3À8 Optical sensing based on the plasmon resonance absorption exhibited by nanoparticles has been used with a view to develop analytical tools in clinical diagnosis.9À11It is well-known that the gold nanoparticles display surface plasmon resonance(SPR) absorption bands at a specific wavelength(∼519nm).The frequency and width of the SPR absorption depend on the size and shape of the metal nanoparticle as well as on the dielectric constant of the metal itself and of the medium surrounding it.12 On aggregation of the gold nanoparticles,the absorption maxima shifts to longer wavelengths resulting in the color change of the colloid from wine red to blue due to mutually induced dipoles that depend on interparticle distance and aggregate size.13In the present study,we use functionalized gold nanoparticles for urine glucose sensing.We focus on urine because it is the most informativefluid that can be obtained noninvasively and is used routinely to diagnose and monitor a variety of medical conditions.14The glucose level in blood is used as a clinical indicator of diabetes.The presence of glucose in urine is a more dangerous condition,as it is an indication of worsening of diabetes. According to the World Health Organization,over150million people in the world were affected with diabetes in the year2004 and it is expected to climb further to366million in2030.The affected population has to be tested for blood glucose levels daily for an effective treatment.In order to avoid the inconveniences caused by drawing blood intravenously or by hand pricking,a preliminary screening of the patients with high level diabetes (having renal glycosuria)15,16can be done instantly by checking their urine glucose levels.The visible color change of the functionalized gold nanopar-ticles on interacting with urine glucose enable the patients to have a self-checking method at home and seek immediate medical attention.Recently,Malhotra et al.and Li et al.have studied in length the characteristics of glucose oxidase(GOD) immobilized GNPs.17,18These authors have shown enhanced stability of GOD by the process of immobilization.Ma and Ding have reported that,while free GOD in solution only retains about 22%of its relative activity at90°C,the immobilized GOD on gold nanorods retains about39.3%activity.19Li et al.also suggested that such GOD/GNPs bioconjugates can be consid-ered as a catalytic nanodevice to construct a nanoreactor basedReceived:December18,2010Accepted:February25,2011inaccessible to the bulk of the population.on a glucose oxidation reaction for a biotechnological purpose.18 Interestingly,the feasibility of the use of these entities in the sensing of glucose has not been attempted.Zhang et al.have reported the use of modified glassy carbon electrode for the application of glucose sensing.The GNPs was deposited on the electrode surface through the glucose oxidase catalyzed oxidation of glucose which in turn was measured by differential pulse stripping voltammetry.20Very recently,Jiang et al.have demon-strated the application of a AuNP based colorimetric assay for the simple but effective detection of glucose in the rat brain by taking advantage of the cascade reactions of GOD catalyzed oxidation of glucose and the Fenton reaction of H2O2,as well as the oxidative cleavage of ssDNA with OH radicals.21There has been an ever increasing demand for the develop-ment of simple,cost-effective methodologies in an easy to read out format for the detection of clinically relevant molecules to aid in diagnosis.Such approaches are extremely important,particu-larly in third world countries where high tech diagnostics aids are inaccessible to bulk of the population.14Simple procedures which could be performed at home without the need of sophisticated expensive instrumentation possibly bring radical changes in rural health care management.Visual detection of disease specific marker molecules in biologicalfluids such as urine,saliva,or blood is an attractive approach to address these issues.A color change observable by the naked eye in response to the concentration of an analyte can be an indication of a disease condition warranting further medical attention.Herein,we report conjugation of GOD,the enzyme specific toβ-D glucose onto thiol capped GNPs,using carbodiimide chemistry and its use in the colorimetric detection of glucose in urine.’EXPERIMENTAL SECTIONMaterials.Gold chloride,glucose oxidase(GOD),trisodium citrate,Tween20,16-mercaptohexadecanoic acid(16-MHDA), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide(EDC),and N-hydroxysuccinimide(NHS)were obtained from Sigma-Al-drich,Bangalore,India.The glucose standard used is from the glucose kit supplied by Enzyme Technologies Pvt.Ltd., Mumbai,India.Preparation of Gold Nanoparticles.GNPs were synthesized as reported by Turkevich et al.22Briefly,to a boiling solution of 20mL of1.0mM HAuCl4,2mL of a1%solution of trisodium citrate dihydrate was added under constant stirring.The contents were removed from the hot plate when the solution turned red. The cooled contents were kept under refrigeration until its usage.Preparation of Alkane Thiol Modified Gold Colloids.The surface modification of gold colloids using alkane thiols was done as per Aslan and Luna.2Briefly,equal volumes of gold colloid and Tween20(2mg/mL)in phosphate buffer at pH7.0were gently mixed and allowed to stand for a minimum of20min to allow for the physisorption of Tween20to the gold nanoparticles.Then,a solution of0.5mM16-MHDA in methanol was added to the above solution and allowed to stand for4h.Excess Tween20and thiols were removed from the surface modified gold colloids by centrifugation for20min at14000rpm with an ultracentrifuge (Sigma3À30K,Germany).The absorbance spectra of the colloidal gold and the modified colloids with Tween20,16-MHDA was taken with a UVÀvisible spectrophotometer (Varian,Cary-Win Bio,Melbourne,Australia)using1cm path length polystyrene cuvettes.Conjugation of GOD on Alkanethiol Modified Gold Col-loids.Immobilization of GOD with16-MHDA capped gold nanoparticles was done on the basis of the EDC/NHS chemistryvia the formation of an amide linkage between the carboxyl groups of the16-MHDA and the primary amine groups of the GOD.The thiolated gold colloids(3mL)were activated with 5mL of a mixture of EDC and NHS,both10mM in phosphate buffer at pH7.0by incubating the mixture at room temperature for30min.Then,2mL of GOD solution(1mg/mL in phosphate buffer)was added to the above mixture and incubated at room temperature for2h and then kept overnight at4°C. After centrifugation at14000rpm,the GOD immobilized gold nanoparticles were resuspended in3mL of phosphate buffer(pH 7.0)and kept refrigerated until use.Fourier Transform Infrared Spectroscopy(FT-IR)of the Nanoparticles.The FT-IR spectra of the citrate stabilized GNPs and GOD capped GNPs were recorded in the range of 600À4000cmÀ1on a Nicolet5700FT-IR spectrometer (Nicolet Inc.,Madison,USA)using a Diamond ATR accessory.Particle Size and Zeta Potential Determination.The tech-nique of dynamic light scattering(DLS;Malvern Instruments Ltd.,Malvern,UK)was used for the determination of the size of the nanoparticles.All measurements were performed at a fixed angle of90°at25°C.The zeta potential of a particle is the overall charge that the particle acquires in a particular medium and is also measured using the same equipment at25°C.Transmission Electron Microscopy.Transmission electron microscopy(TEM)images were obtained on a Hitachi,H7650 microscope(Tokyo,Japan).The gold colloid and the GOD capped gold colloid were deposited onto a200mesh copper grid coated with a Formvar film and dried overnight.Glucose Sensing Using GOD Functionalized Gold Col-loids.The shift in the SPR absorption maxima of the GOD functionalized GNPs on adding predetermined quantities of glucose standard is recorded using a UVÀvisible absorption spectrophotometer,and a calibration curve was plotted with the wavelength against the optical density.The urine samples,which did not contain glucose,were collected from a clinical laboratory. These fluids were spiked with glucose and used as the test samples.The corresponding shift in the absorption maxima was used to calculate the amount of glucose present.Selectivity of the Method.The selectivity of the method was investigated by checking the shift in SPR absorption maxima of the GOD functionalized GNPs on interacting with normal urine samples and the same spiked with albumin/creatinine in the ratio of40mg/mM and cysteine200μM/L.To confirm the precision and recovery of the probe,each set of experiments was carried out in triplicate,and similar results within the maximum error of 2À3%were obtained.’RESULTS AND DISCUSSIONThe surface plasmon resonance makes the absorption cross section of the nanoparticles several orders of magnitude stronger than the most strongly absorbing molecules and the light scattering cross section several orders of magnitude stronger than the organic dyes.23Hence,most of the applications of gold nanoparticles as sensors are based on detecting the shift in surface plasmon peak either due to change in the local dielectric constant of the nanoparticles by adsorbed biomolecules or due to analyte induced aggregation of the nanoparticles.24À29Both these effects rely on the selectivity provided by the functionalized capping agents.Functionalization of GNPs.The typical plasmon absorptionpeak of the gold colloid was observed at 519nm (Supporting Information,Figure S1),indicating the formation of spherical GNPs.30,31This is further substantiated in Figure S2(Supporting Information)which shows the TEM images of the GNPs at a magnification of 500nm,and the nanoparticles displayed an average size of 11to 12nm.After adding Tween 20,the SPR absorption maximum of the gold colloid was shifted to 523(1nm due to the physical adsorption of the surfactant on the GNPs and was consistent with the reported shifts of the band upon formation of dielectric layers around colloidal metals.2The absorption maxima further shifted to 524(1nm upon chemisorption of 16-MHDA,indicating the formation of a thicker monolayer around the nan-oparticles,as reported by Aslan etal.2The presence of a physisorbed layer of Tween 20on the surface of colloidal gold could prevent irreversible aggregation of gold nanoparticles during and after chemisorption of alkane thiols.2On conjugating the ÀCOOH group of the 16-MHDA with the amino group of GOD,the SPR was further red-shifted to 534(1nm.The spectral shift is not accompanied by any broadening,confirming nonaggregation of the particles at this stage.Fourier Transform Infrared Spectroscopy (FT-IR).We re-corded FT-IR spectra of the nanoparticles to get further insight on the surface modification (Supporting Information,Figure S3).Citrate stabilized GNPs showed peaks at 1509and 1399cm À1,characteristic of citrate ions.GOD conjugated GNPs showed intense peaks at 1654and 1549cm À1,typical of amide I (ÀC d O)and amide II (N ÀH bending)bands of the enzyme and a strong ÀC ÀO Àstretching band at 1074cm À1.The peak at 3278cm À1was assigned the N ÀH/O ÀH stretching frequency of the GOD.Effect of Glucose on the SPR Absorption Maximum of the GOD Conjugated GNPs.On adding varied amount of glucose (from 10to 100μg/mL),the SPR absorption band was found to shift from 535to 569nm,reflecting the formation of aggregates having enhanced sizes.The reaction is instant,and no incubation time is required between glucose and GOD conjugated GNPs.The corresponding spectra are shown in Figure 1.The red shift in the plasmon peak was found to vary almost linearly with the concentration of glucose,suggesting the possi-bility of the use of this methodology for the quantitative estimation(inset in Figure 1).The magnitude of wavelength shift was con-comitantly increased with concentration of glucose,and beyond 90μg/mL,it is leveled o ff,indicating the saturation of the reactive sites.The lowest amount of glucose that can produce a red shift in the SPR absorption maxima was found to be 10À90μg/mL with a detection limit of 5μg/mL.Particle Size and Zeta Potential Determinations.The particle sizes and zeta potentials of the GNPs before and after the addition of glucose (50and 100μg/mL)are given in Table 1.The average particle size of GNPs was 22.5(0.7nm,and the same was increased to 158nm on conjugating with GOD.On adding 50μg/mL glucose,the particles size was increased to 231.7nm and the size was enlarged to 1202nm on the addition of 100μg/mL glucose,showing the tendency toward the formation of aggregates.The formation of aggregated assembly is reflected again in the color change of the solution from red to blue (Figure2).As shown in Table 1,the zeta potential of the GNPs was reduced from À48(0.41mV to À21.4mV,on conjugating with GOD.When 50μg/mL glucose was added to GOD functionalized GNPs,the charge was reduced to À14.1mV and subsequently to À5.85mV on the addition of 100μg/mL glucose.The considerable reduction in zeta potential also indicates the tendency for the formation of aggregates.GOD oxidizes glucose to gluconic acid and H 2O 2.H þions resulted from the formation of gluconic acid could reduce anionic character of the enzyme,bringing down the negative zeta potential.At acidic pH,nanoparticles have shown less zeta potential due to the increased chemical potential of H þions.32The magnitude of the measured zeta potential is an indication of the net charge over the nanoparticles and can be used toFigure 1.UV Àvisible absorption spectra of GOD conjugated GNP showing red shift on reacting with di fferent quantities of glucose standard.(Inset is the graphical relationship of wavelength shift against increasing quantities of glucose.)Table 1.Size and Charge of the Nanoparticlessampleparticle size (nm)zeta potential (mV)gold nanoparticles 22.5(0.7À48(0.41Au-GOD158(2À21.4(0.1Au-GOD-glucose 50μg/mL 231.7(9À14.1(0.3Au-GOD-glucose 100μg/mL1202(26À5.9(0.5Figure 2.Color of (A)GNPs and (B)GOD-GNP on reacting with g 100μg/mL glucose.predict the long-term stability of the particles.If all the particles in suspension have either a large negative or a positive zetapotential,there will not be any tendency for them to come closer due to strong repulsion.33However,if the particles have low zeta potential values,the probability of them coming together to form an aggregate is higher.The GOD functionalized GNPs synthe-sized in our laboratory did not show any sign of aggregation even after 2months under refrigerated conditions.Transmission Electron Microscopic (TEM)Analysis.We measured the size of the particles using TEM.Figure 3A shows the size of the GOD conjugated GNPs as 44À47nm.Figure 3B ÀD displays the sizes of the GOD functionalized GNPs on interaction with 5,100,and 150μg/mL glucose,respectively.The size of the nanoparticles increased on adding increasing quantities of glucose.When 50μg/mL glucose was added,the size was increased to 73À75nm;with 100μg/mL glucose,it became 102to 145nm,and a total aggregate was formed on adding 150μg/mL glucose.TEM,however,showed lower values for the size of the nanoparticles compared to DLS measurements (Table 1).DLS measurement records higher values since the light is scattered by the core particle and the layers formed on the surface of the particles.TEM,on the other hand,shows the size of metallic core only.He et al.have also made similar observations earlier.34Glucose induced aggregation of GOD conjugated GNPs leading to color change is well supported by the absorption,particle size measurements,and TEM investigations.Glucose Sensing in Urine Samples.We spiked a urine sample with glucose (50μg/mL),and the absorption peaks are shown in Figure 4.The peak maximum showed a shift of 21nmexactly as in the case of the aqueous standard sample (see Figure 1),demonstrating the feasibility of extending the method for the estimation of glucose in real samples.Rather than quantitative estimation,it seems that the method provides a quick qualitative screening of samples.It is apparent from Figure 2that a concentration of 100μg/mL glucose showed a visible color change of the solution to conclude that the glucose content of the test solution is g 100μg/rmation of this kind could be used for further assessment of the health status of the individual.The optical detection method discussed here may be useful for rural populations where clinical laboratory facilities are limited.Figure 3.TEM images of (A)GOD conjugated GNP,(B)50μg/mL glucose added,(C)100μg/mL glucose added,and (D)150μg/mL glucose added.Figure 4.Wavelength shift with a urine sample spiked with 50μg/mL glucose.导致纳米金聚集的原因Selectivity of the Method.To check the cross reactivity of the GOD functionalized GNPs,we conducted the following studies.Diabetic patients are at increased risk of renal diseases. The principal feature of diabetic nephropathy is proteinuria which is defined as the albumin/creatinine ratio being g30 mg/mmol.35We spiked the urine sample with100μL of aqueous solution containing albumin/creatinine in the ratio40mg/mM to mimic the urine samples of patients with diabetic nephro-pathy.It is also reported that the concentration of cysteine in urine is200μM/L.36A urine sample(100μL)spiked with cysteine is also added to Au-GOD to check its selectivity toward glucose.The SPR absorption maximum of the Au-GOD shifted only on adding50μL of100mg/dL glucose standard.No red shift for albumin/creatinine or cysteine was found,confirming that the GOD functionalized GNPs were highly specific to glucose and had no interference with any other molecules present in the urine.The SPR absorption pattern and the corresponding color images in the inset are shown in Figure S4 (Supporting Information).’CONCLUSIONSWe devised a simple method for the detection of glucose in fluids like urine using GOD immobilized gold nanoparticles.The color of the solution was found to change from red to blue in the presence of∼100μg/mL glucose.Plasmon absorption peak was red-shifted proportionally up to100μg/mL glucose,and good correlation was obtained between the wavelength shift and concentration pointing out the feasibility of the use of the method for the quantitative measurement of glucose in urine. The color change observable by the naked eye can advanta-geously be used for a preliminary screening at home itself and may be referred for more focused investigations.The method seems to be a potential one,particularly for the rural population in third world countries.’ASSOCIATED CONTENTb Supporting Information.Additional information as noted in text.This material is available free of charge via the Internet at .’AUTHOR INFORMATIONCorresponding Author*E-mail:sreeni@sctimst.ac.in.Phone:091À471À2520248.Fax: 091À471À2341814.’ACKNOWLEDGMENTWe are grateful to the Director of SCTIMST and Head of the Biomedical Technology Wing,SCTIMST,for providing all the facilities for conducting this study.We are also thankful to Ms.S. Many and Mr.W.Paul for their technical assistance in getting the TEM images and DLS data.’REFERENCES(1)Wolfbeis,O.S.;Weidgans,B.M.In Fiber Optic Chemical sensors and biosensors-A view back in Optical Sensors;Baldin,F.,Chester,A.N., Homola,J.,Martellucci,S.,Eds.;Springer:The Netherlands,2006;pp 17À44.(2)Aslan,K.;Prez-Luna,ngmuir2002,18,6059–6065.(3)Templeton,A.C.;Wuelfing,W.P.;Murray,R.W.Acc.Chem.Res. 2000,33,27–36.(4)Shon,Y.S.;Mazzitelli,C.;Murray,ngmuir2001, 17,7735–7741.(5)Cliffel,D.E.;Zamborini,F.P.;Gross,S.M.;Murray,R.W. Langmuir2000,16,9699–9702.(6)Aguila,A.;Murray,ngmuir2000,16,5949–5954.(7)Templeton,A.C.;Pietron,J.J.;Murray,R.W.;Mulvaney,P.J. Phys.Chem.B2000,104,564–570.(8)Kometani,N.;Tsubonishi,M.;Fujita,T.;Asami,K.;Yonezawa, ngmuir2001,17,578–580.(9)Perez-Luna,V.H.;Aslan,K.;Betala,P.In Encyclopedia of Nanoscience and Nanotechnolgy;Schwarz,J.A.,Contescu,C.I.,Putyera, K.,Eds.;Marcel Dekker:New York,USA,2004;Vol2,pp27À49.(10)Daniel,M.C.;Astruc,D.Chem.Rev.2004,104,293–346.(11)Durne,J.Angew.Chem.,Int.Ed.2009,48,2–28.(12)Link,S.;Sayed,M.A. E.Annu.Rev.Phys.Chem.2003, 54,331–366.(13)Aslan,K.;Zhang,J.;Lakowicz,J.R.;Geddes,C.D.J.Fluoresc. 2004,14,391–400.(14)Martinez,A.W.;Phillips,S.T.;Whitesides,G.M.Anal.Chem. 2010,82,3–10.(15)Rennke,H.G.;Rose,B.D.Renal Pathophysiology-the essentials, 1st ed.;Lippincott Williams:Philadelphia,p194.(16)Corazon,D.Conjecture Corporation;Wilborn,C.,Ed.;09 September,2010c;.(17)Pandey,P.;Singh,S.P.;Arya,S.K.;Gupta,V.;Datta,M.;Singh, S.;Malhotra,ngmuir2007,23,3333–3337.(18)Li, D.;He,Q.;Cui,Y.;Duan,L.;Li,J.BBRC2007, 355,488–493.(19)Ma,Z.;Ding,T.Nanoscale Res Lett.2009,4,1236–1240.(20)Zhang,H.;Liu,R.;Sheng,Q.;Zheng,J.Colloids Surf.,B2011, 82,532–535.(21)Jiang,Y.;Zhao,H.;Lin,Y.;Zhu,N.;Ma,Y.;Mao,L.Angew. Chem.,Int.Ed.2010,49,4800–4804.(22)Turkevich,J.;Stevenson,P.C.;Hillier,J.Discuss.Faraday Soc. 1951,11,55–75.(23)Huang,X.;Sayed,I.H.E.;Qian,W.;Sayed,M.A.E.J.Am.Chem. Soc.2006,128,2115–2120.(24)Nath,N.;Chilkoti,A.J.Fluoresc.2004,14,377–389.(25)McFarland,C.L.;Haynes,C.A.;Mirkin,R.P.;Duyne,V.; Godwin,c.2004,81,544A.(26)Travan,A.;Pelillo,C.;Donati,I.;Marsich,E.;Benincasa,M.; Scarpa,T.;Semeraro,S.;Turco,G.;Gennaro,R.;Paoletti,S.Biomacro-molecules2009,10,1429–1435.(27)Durr,N.J.;Larson,T.;Smith,D.K.;Korgel,B.A.;Sokolov,K.; Yakar,A.B.Nano Lett.2007,7,941–945.(28)Huang,C.C.;Chang,H.T.Anal.Chem.2006,78,8332–8338.(29)Rex,M.;Hernandez,F.E.;Campiglia,A.D.Anal.Chem.2006, 78,445–451.(30)Ai,K.;Liu,Y.;Lu,L.J.Am.Chem.Soc.2009,131,9496–9497.(31)Liu,J.;Lu,Y.Anal.Chem.2004,76,1627–1632.(32)Sun,L.;Zhang,Z.;Wang,S.;Zhang,J.;Li,H.;Ren,L.;Weng,J.; Zhang,Q.Nanoscale Res Lett.2009,4,216–220.(33)Application Note by Malvern Instruments;Malvern Instruments Ltd.:Worcestershire,U.K.,May2,2005.(34)He,Y.Q.;Liu,S.P.;Kong,L.;Liu,Z.F.Spectrochim.Acta,Part A 2005,61,2861–2866.(35)Andersen,S.;Blouch,K.;Bialek,J.;Deckert,M.;Parving,H.H.; Myers,B.D.Kidney Int.2000,58,2129–2137.(36)Ku s mierek,K.;G z owacki,R.;Bald,E.Anal.Bioanal.Chem. 2006,385,855–860.。
诺贝尔奖得主山中伸弥论文翻译

摘要已分化的细胞能通过将核物质转移入卵母细胞或与胚胎干细胞融合而重编程至胚胎细胞样状态。
我们对诱导这种重编程过程的因子知之甚少。
在这里,我阐述了通过四个因子,Oct3/4, Sox2, c-Myc,and Klf4,在胚胎干细胞培养条件下将鼠胚胎或成体纤维母细胞向多能干细胞的诱导。
出乎意料的是,Nanog不是必须的。
这些细胞被我们称为诱导多能干细胞(inducedpluripotent stem cell,iPS cell),它们表现出胚胎干细胞(embryonicstem cell,ES cell)的形态和属性,并且表达胚胎干细胞的标记基因。
将iPS cells皮下注射转移至裸鼠导致包含所有三个胚层的一系列组织的肿瘤的形成。
接下来将其注射进入鼠囊胚,iPS cells导致了鼠胚胎的发育。
这些数据证明,多潜能干细胞能通过仅仅添加一些确定的因子而从纤维母细胞培养物中直接生成。
简介起源于哺乳动物囊胚内细胞团的胚胎干细胞维持在多潜能性时具有无限生长的能力,并且能分化成三个胚层内的的所有细胞( Evans and Kaufman, 1981; Martin, 1981)。
人类胚胎干细胞被认为能治疗许多疾病,例如Parkinson’s disease,spinal cordinjury, and diabetes (Thomson et al., 1998 )。
尽管如此,就像病人移植后组织排斥反应的问题一样,人类胚胎的使用面临着巨大的伦理困难。
避免这些问题的唯一途径是从病人自体细胞直接生成多潜能细胞。
体细胞能够通过将核物质转移进卵母细胞( Wilmut et al., 1997)或与胚胎干细胞融合(Cowanet al., 2005; Tada et al., 2001 )而重新编程。
这表明未受精的卵子和胚胎干细胞含有能授予体细胞生物全能性或多潜能性的因子。
我们假设这些因子在维持胚胎干细胞的特性方面起重要作用,在体细胞多潜能性的诱导中也起关键作用。
化身: 译本质再思考 翻

第21卷 第1期 宁 波 大 学 学 报(人 文 科 学 版) Vol. 21 No.1 2008年1月 JOURNAL OF NINGBO UNIVERSITY(LIBERAL ARTS EDITION) Jan. 2008—————————————— 收稿日期:2007 - 06 - 07作者简介:钱志富(1966 -),男,四川武胜人,宁波大学外语学院副教授,博士。
化身:翻译本质再思考钱志富(宁波大学 外语学院,浙江 宁波 315211)摘要:从化身这一全新的视角对翻译本质进行认识,翻译就是为源语言在目的语中寻找化身。
翻译家在翻译的过程中要首先弄懂原身、真身、化身等概念,翻译的过程就是将真身从原身转移到化身的过程,至于传统译学理论中所关心的直译、意译、归化、异化等都是为获得化身而采取的一系列策略而已。
关键词:翻译;化身;本质中图分类号:H059 文献标识码:A 文章编号:1001 - 5124(2008)01 - 0063 - 04关于翻译本质的思考,自从有翻译实践以来,一直都没有停止过。
笔者曾在《诗歌的可译与不可译新解》一文中这样说到诗歌翻译的本质:“好的译诗应该是原诗在另一种语言中的化身。
”[1]文章引起了比较大的反响。
当然,笔者的这篇文章并不主要在谈诗歌的化身理论,而是谈诗歌的可译和不可译。
经过差不多四五年的研究和思考,笔者想在此正式提出诗歌翻译的化身理论,同时觉得几乎一切的翻译都是化身。
这里先解释一下“化身”。
化身原本是一个佛学概念,这里笔者借用,觉得能够比较好地把握住翻译的本质。
所谓化身是指某一事物虽然发生了某种形式的变化,但它并没有发生本质性的变化,变来变去还是他自己,而不是另外一种事物。
比如孙悟空有72般变化,但变来变去还是他孙悟空,而不会变成猪八戒或者沙和尚。
释迦牟尼有三十二相,八十种好,但无论如何他一定是释迦牟尼佛而不会是其他佛。
翻译也是如此。
翻译本身有许多种策略和方法,然而一个作品无论怎样翻译,无论将它从何种源语言翻译成何种目的语,也无论翻译的过程中发生过何种变异以及文化上的变迁,但它仍然是它原来的那个作品,而不会变成别的作品。
奇妙的光盘

奇妙的光盘
谈锋
【期刊名称】《浙江档案》
【年(卷),期】1984(0)Z2
【摘要】从加利福尼亚大学伯克莱分校大门往右一拐,有一座毫不起眼的米黄色临街楼房,这就是专门研制电子计算机存储尖端技术的“联合自动化公司”。
就在这里,我们看到了现代信息存储技术的骄子——光盘和光盘机。
【总页数】2页(P57-58)
【关键词】光盘技术;信息存储技术;计算机存储;自动化公司;伯克莱;存储容量;微电子工业;图书馆行业;技术信息;报纸版面
【作者】谈锋
【作者单位】<人民日报>记者
【正文语种】中文
【中图分类】TP333;TP333
【相关文献】
1.园本课程真奇妙——坩‘奇妙的壳”主题看园本课程的“奇妙”实施 [J], 束腊娣
2.园本课程真奇妙——坩‘奇妙的壳”主题看园本课程的“奇妙”实施 [J], 束腊娣
3.光盘上的秘密——奇妙的"0"和"1" [J], 智磊
4.光盘积分制每日光盘打卡食材搭配精准化在北京住总集团,光盘行动这样做! [J],
5.河南卫视"奇妙游"系列晚会成功原因探析——以《端午奇妙游》《七夕奇妙游》《中秋奇妙游》为例 [J], 郭锐;韦裕杰
因版权原因,仅展示原文概要,查看原文内容请购买。
台湾中历善果林丁

弟子规种子教师研习营二—新世纪健康饮食周泳杉老师主讲(第三集)2007/7/11 台湾中坜善果林档名:52-229-03上一节课,我们提到癌症的三个阶段,这三个阶段让我们体认到西方现代的科学,跟我们中国文化里面所讲到的因、缘、果,事实上是不谋而合的,因此真理是没有时间跟空间的限制。
我们看到因缘果这三项因素,在整个疾病发病的过程当中,扮演的角色都不一样。
坎贝尔教授在这里做了一个简单的结论,他告诉我们动物性的食物,它的营养成分会促进肿瘤的发展,就是它是缘,植物性食物的营养则能够抑制肿瘤的发展。
这个缘到底怎样来运用,能够用得很恰当,这里坎贝尔教授讲了一个指导性的原则。
再来他告诉我们大量的实验证实,因为坎贝尔教授有很多的实验,我们今天没有办法一一的来跟大家做介绍。
但是他的这些实验告诉我们一个很重要的观念,就是癌症的启动阶段远不如促进阶段那麽关键。
也就是让我们认识到,促进癌症的发展这一条是可以掌握的。
因此身体的健康绝对是掌握在自己的手上,而不是把它推给基因,或者是推给环境。
另外坎贝尔教授讲,尽管实验室的证据非常的充分,可是还是有很多人不相信,很多人讲:你那个都是用老鼠来做实验,你那个都是用细胞来做实验,请问人体的真实状况是怎麽样?有很多人不相信,他即使有这麽多的证据,他还是存疑,新世纪健康饮食(第三集)2007/7/11 台湾中坜善果林档名:52-229-031新世纪健康饮食(第三集)2007/7/11 台湾中坜善果林档名:52-229-032因此被迫坎贝尔教授,他一定要拿出人体试验的数据。
後来坎贝尔教授,在他的研究成果到了某个程度的时候,他就开始思考必须要找到真正人体健康跟饮食关系的一些证据,才能够让整个实验更为完整,让更多的人得到利益。
他就开始来思考,怎样来做人体的研究、大量健康膳食的研究,因此才促成後来坎贝尔教授跟中国一些学者合作,然後去做一个「中国健康饮食调查报告」,这个报告後来也顺利的发表出来。
实体结构(连续体)分析

接求解
Rn = fnext − f int(σn) = 0
任晓丹
实体结构(连续体)分析
时域离散
一般结构初边值问题有限元法 应变局部化问题
材料非局部本构关系
初边值问题的控制方程 初边值问题的有限元列式及求解
动力方程的完全求解一般基于时域动力积分算法,其中 Newmark 法应用最为广泛,基于考虑如下三段式 Newmark 法
初边值问题的控制方程 初边值问题的有限元列式及求解
如下控制方程的系数均已求得,需进一步考虑其时域离散求 解
Md¨ + Cd˙ + f int = f ext
将时间步 [0, T] 离散为 M 个时间增量步
∪M [0, T] = [tn, tn+1]
n=1
若不考虑动力效应,则静力平衡方程可以在每个时间点上直
∑ np ϵ(x, t) ≈ BA(x)dA(t) = B(x)d(t)
A=1
任晓丹
实体结构(连续体)分析
一般结构初边值问题有限元法 应变局部化问题
材料非局部本构关系
初边值问题的控制方程 初边值问题的有限元列式及求解
有限元控制方程
将上述插值格式代入平衡方程弱形式,考虑单元节点虚位移 δd 的任意性,可以得到如下固体结构的有限元控制方程:
ρNTb∗ dΩ +
NTt∗ dS
e=1 Ωe
∂t Ωe
任晓丹
实体结构(连续体)分析
单元积分
一般结构初边值问题有限元法 应变局部化问题
材料非局部本构关系
初边值问题的控制方程 初边值问题的有限元列式及求解
等参元换元积分∫
∫
f(x) dΩ = f(ξ) J dΩ¯e
纳米坡缕石的表面修饰及摩擦学性能研究

稳 定性 ;经表 面 修饰 的 纳 米坡 缕 石具 有 优 异 的减 摩 作 用 和 良好 的抗 磨 效 果 ,与 基 础 油 相 比 ,摩 擦 因数 降低 4 . % ,磨 31
? 损 失重 减 少 1 . % 。 01
关 键 词 :纳 米坡 缕 石 ;表 面 修饰 ;摩擦 磨 损 性能
中图分 类 号 :T 1 文 献标 识 码 :A 文章 编 号 :0 5 0 5 ( 0 0 1 0 9— HI7 2 4— 10 2 1 ) 0— 3 3
纳 米 坡缕 石 的表 面 修 饰 及摩 擦 学性 能研 究
丁 旭 陈建海 杨 绿 李 屹 周 元 康
( 州大学机械工程学院 贵
贵 州 贵 阳 5 00 ) 50 3
摘 要 :通 过球 磨 改 性 的方 法 ,使 用 油 酸 、硅烷 、季 铵盐 、钛 酸 酯 4种 修 饰 剂 对 纳 米 坡 缕 石 颗 粒 进 行 表 面 修 饰 ,使
mo i e a o p l g r k t a i l ip a s a g o e o ma c s o rc i n r d e i g a d a twe r t e fi t n c e c e t d f d n n — ay o s i p r c e d s l y o d p r r n e ffi to e u t n n i a ,h rc i o f i n i e t f n o i
21 O 0年 l j 0 第3 5卷 第 l 0期
润滑与密封
L UBRI CAT1 0N ENGI NEE NG RI
0c . 2 0 t 01 Vo . 5 No 1 13 . 0
DOI 1 . 9 9 jis. 2 4—0 5 . 01 . 0 01 : 0 3 6 /.sn 0 5 10 2 0 1. 0
AB_8树脂吸附和分离桑葚红色素的新工艺
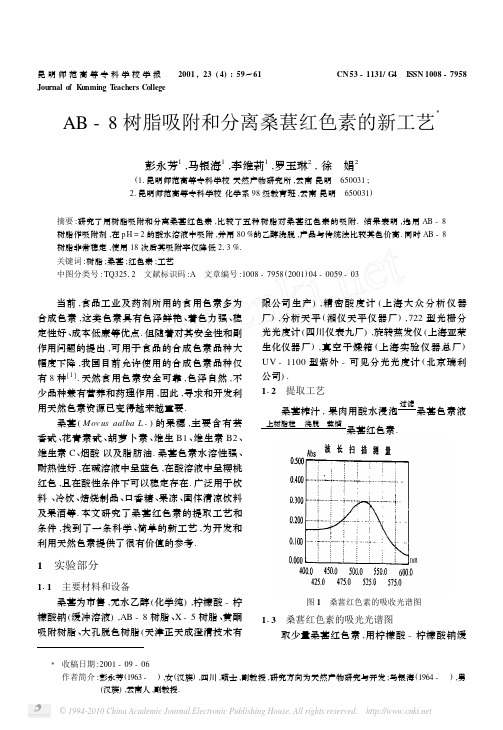
桑葚 ( M ov us aalba L 1) 的果穗 ,主要含有芸 香甙 、花青素甙 、胡萝卜素 、维生 B1 、维生素 B2 、 维生素 C、烟酸 以及脂肪油. 桑葚色素水溶性强 、 耐热性好 ,在碱溶液中呈蓝色 ,在酸溶液中呈樱桃 红色 ,且在酸性条件下可以稳定存在. 广泛用于饮 料 、冷饮 、焙烧制品 、口香糖 、果冻 、固体清凉饮料 及果酒等. 本文研究了桑葚红色素的提取工艺和 条件 ,找到了一条科学 、简单的新工艺 ,为开发和 利用天然色素提供了很有价值的参考.
表 5 桑葚红色素的不同提取工艺与产量、色价的关系
提取工艺
传统提取法
pH = 2 酸水
80 % 乙醇
AB - 8 树脂提取法
pH = 2 酸水
80 % 乙醇
产量 (g) 产率 ( %)
6169 13138
6153 13106
1103 2106
0197 1194
A520 色价
01113 5165
01107 5135
交换与吸附 ,1997113 (1) :83 —87. [ 4 ] 周小华等. D61 树脂吸附和分离萝卜红色素 [J ] . 离
子交换与吸附 ,1995111 (5) :455 —459.
Absorbing and Separating Movus alba L1 Red pigment by AB - 8 resin
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
文章一Kunishima教授讲课的主要内容Kunishima教授主要介绍了日本建筑业的七大特点:1.有限制的竞争●参加投标的承包商要取得建设省颁发的相应级别的承包资质;●承包资质根据工程的类型,大小划分不同级别;●参加中央政府项目投标要有建设省的建设承包资质许可,参加地方(县)项目投标还要取得地方建设承包资质许可。
每个县都有地方建设许可证制度;●参加一个项目投标的承包商个数有限制,招标方一般在有投标资格的申请者中选5~10家承包商参与投标;●通过对承包资质的管理和对投标者数量的限制,达到控制竞争的目的。
2.通过协商共享项目利益●为了避免投标者之间的恶性竞争,鼓励参加投标的各承包商之间在投标报价之前进行内部协商;●参加投标的多家承包商可以通过内部合作共享项目承包的利益;●一个大项目往往分割为许多部分,然后分割承包给不同的承包商,以实现项目承包利益在承包商之间的合理分配。
3.最高价格●每个项目(公款项目)都由业主(政府)制度一个最高限价,这个最高限价只有少数几个人知道,是保密的,但投标者一般总有办法摘到这个限价;●只有低于这个最高限价的投标价有效;●多家投标者的报价一般是近态分布的,最高限价代表了多家家投标单位的平均水平。
4.由参与投标竞争的其它承包商为中标者担保●中标者要在参与投标竞争的承包商中找两家为自己作履约担保;●如果中标者由于破产等原因不能完成建设任务,由担保者继续完成承包合同。
5.30%~40%的工程预付款●地方政府项目在签定承包合同后,一次性须付30%的工程合同款;●中央政府项目在签订承包合同后,一次性预付40%的工程合同款;●剩余的工程款等工程全部完成后再付;●有携预付款潜逃的承包商,但很少;●工程建设过程中的资金缺口,由承包商向银行贷款或自筹解决。
6.返聘政府职员●在政府工作的职员离职后,一般由承包商或半官方的建设机构返聘;●因为在政府工作的职员一般都是大学毕业的优秀生,又有建筑业工作的经验,所以很受承包商的欢迎。
7.工程咨询的理论与实践的分离●工程建设过程中的设计、规划、计划等咨询性工作,一般由政府业主自己完成,或由承包商自己完成,没有独立的咨询方;●日本工程咨询的理论与实践存在较大的差距,有关的法规正在改进这方面的规定。
文章二上周末,日本东京大学著名的Kunishima教授专程来监理所向我们作了精彩的报告。
报告主要介绍了日本建筑业的七个特点,大意如下:1.Controlled Competition with respect to Type and Size of Works and Regions与中国类似,在日本招揽工程首先要取得建设省(相当于我国的建设部)的承包资质,然后才允许承包相应类型和规模的工程项目。
如果想在日本其它县(相当于我国的省)招揽工程,还必须取得该地区的承包资格。
但与我国不同的是,日本还有一些半官方机构给承包商评级。
通过资质的管理,建筑市场的竞争就得到了有序的控制。
2.Fair Sharing by Negotiation在日本,大中型工程项目通常要分割为若干小部分然后再分别发包出去。
所以承包商们一般事先经过内部协商,根据各自的实力进行合理的利润分配,再作投标。
3.Ceiling Price在招投标之前,日本的官方机构会根据以前类似工程的数据给出一个投标最高价。
如果承包商的报价超过这个最高价,则作废标处理。
这一点与我国不同,我们是在标底的一定上下限之内均有效。
由于最高价的重要性,有些承包商可能会想方设法通过贿赂等手段把它搞到手。
pletion Guarantee by Competitors为了避免承包商在建设过程中由于破产等等原因致使工程中断,业主会要求参与投标的其它两、三位承包商为中标的承包商提供完工担保。
如果出现中标承包商不能继续工程建设的情况,则由担保的承包商顶替承担其工程任务。
5.Advanced Payment 30~40%在日本的建设项目中,工程开工之前业主支付30~40%的工程款,其余款项至工程结算时再予支付。
工程建设期间,承包商如果资金缺乏,可以向银行申请贷款。
6.Amakudari这是个日本词汇的译音,意思是建设主管部门的工作人员退休离职后返聘到有关的建筑公司中工作。
在日本,最优秀的大学毕业生都很愿意到政府部门工作,所以政府部门的离职人员一般也具有相当高的素质。
另外,还由于这些人员特殊的工作背景,他们通常都具有良好的社会渠道与经验,所以很受承包商等单位的欢迎。
7.Gaps between Principles and Practices of Consulting Service Works关于日本的工程咨询,理论上有一系列的规定和做法,但在工程实践中并不是严格按照规定来做,即实际与理论之间存在着较大的差距。
文章三对日本建筑业的几点认识与思考根据Kunishima教授所介绍的日本建筑业的七个Key Systems,我们对日本建筑业有如下几点认识:1.关于日本建筑业的管理体制日本建筑市场的准入制度日本的各级政府依据承包商的资质等级和项目所在地点对参加项目投标的承包商进行严格的资质管理【1】。
日本各级政府按照所承包工程的大小、类型确定承包商的的资质等级,其中参加中央政府管辖项目的投标必须获得中央政府的资质认定,参加地方政府项目的投标则需得到地方政府主管部门的资质认定。
日本政府的资质管理以颁发许可证的形式完成。
日本的招投标制度招标项目由业主根据投标单位的平均水平确定最高价(Ceiling Price),超过最高价的为废标【3】。
参加投标的承包商可以通过协商和谈判确定中标单位【2】,政府出于避免恶性竞争的考虑也允许承包商之间的这种类似于串标的行为。
作为中标条件,政府要求中标的承包商必须从参加投标的承包商中找出两家作为履约担保【4】.作为担保的承包商必须保证在中标者由于特殊原因(如破产)无法继续施工时,按原定中标价完成承包合同——这一规定也强化了承包商之间的利益共享机制。
我们认为,日本政府关于招投标的这些规定除了避免承包商之间的恶性竞争,也有排斥外国竞争者的考虑,这与日本政府对其他行业(如汽车)的保护政策如出一辙。
关于预付工程款的规定政府项目在签订合同后一次性向承包商支付30-40%的工程款,剩余的款项待工程完工后再付,不足的工程款由承包商自行解决。
【5】2.日本建设管理的法制日本的建筑业管理法规虽然完善,但其执法过程也不是无懈可击。
其突出特点就是建筑企业与政府主管部门之间有着千丝万缕的利益联系,根据Kunishima 教授课上所述,日本的建筑企业返聘退休的政府官员到企业任职是十分普遍的现象【6】,这一方面是因为政府官员有着较高的职业素质(日本政府官员多为名校高材生),另一方面也是出于自身利益的考虑,如许多承包商凭借与政府部门的良好关系,可以事先知道Ceiling Price【3】。
日本建筑业的管理法规与工程界的实际情况存在较大的差距【7】,如政府要求设计与施工分离,但实际工程实施中,往往是设计和施工由业主交给一家承包商完成。
针对这种情况,日本政府正在修订有关法规。
3.关于信息技术在日本建筑业中的应用根据Kunishima教授对我关于Construction CALS提问的回答,我感觉Kunishima教授在日本虽然有着较高的学术地位,但由于长期从事工程技术管理工作的缘故,对IT应用的巨大意义并不十分认同(如认为计算机辅助进度控制并非十分必要),对Construction CALS也只是表示了有限度的关注,这不能不说是一个遗憾。
文章四日本KUNISHIMA PREFESSIONOR 讲课内容包括三部分:1.Safety construction,2. 7keys about construction,3.construction management , 其中主要介绍了日本建筑业的七大特点:一.日本建筑承包商整体规模。
建筑队伍人数在整个人口中的比例,建筑业的output在整个GDP的比例为18%。
二.建筑业的7 KEYS1.Controlled competition with resptct to type and size of works and region.1).根据工程的规模,类型来评定承包资质,划分承包等级。
而承包商对工程的承包要依其资质而定,对中央政府和地方的项目投标,根据省及县的有关制度,中央项目投标要有建设省的资质许可,地方项目投标要有地方建设建设资质许可。
2).承包商往往根据自己所取得的资质对项目进行投标,根据规定,每个承包商都可以根据条件对项目进行投标,但实际并非如此,因为日本对每个项目参加投标的承包商都有数量上的限制,业主一般都是从具备条件的申请承包中选出适量数量的承包商参加投标。
2.Admission education,Fair sharing by negotiation.承包商可以对项目的投标在投标报价进行投标前的内部协调,进行内部合作,以达到共同承包项目,共同分享利益。
这样是避免了恶性竞争,可是每个项目就往往被分成若干个小项目。
3.Ceiling price.业主对项目制定一个最高价,对承包商来说,这个最高价是保密的,投标价只有适当低于这个最高价才有效,。
通常情况下,投标报价都成正态分布,而最高价往往接近平均水平。
4.Competition guarantee by competitors中标承包商必须找两家承包商作为自己的担保单位,这样,中标承包商因其他原因不能完成合同要求时,担保商就有义务履行未完成合同,从而能确保合同的预期实现。
5.Advanced payment 30%-40%在承包合同签定时,业主必须一次性预付30%——40%的合同条款,剩下的部分等到合同完成后付清,在履行合同期间,如有资金短缺情况,由承包商通过贷款或其他方式自行解决。
6..AMAKUDARI: Re-employmeny of former government officials in private and quasi-public institutions建设机构或承包商经常聘请离职的政府官员,这是因为这些离职官员以前往往是优秀的大学毕业生又加上他们经验丰富。
7..gaps between principles and practices or consulting service works 在日本没有专门性的咨询机构,这个工作往往由业主或承包商自己完成,故经常存在理论和实际相脱节。
目前日本政府已经认识到了这个问题,并在制定相关法规改进这个问题。
三.1.Associations for technologics.2. Small siz construction Project3 public owner ;fearfulconstruciton managementprinciplespracticestechniques文章五听Kunishima(国岛正彦)教授讲课心得3月24日日本东京大学Kunishima教授来监理所讲课,介绍了General Construction in Japan 和Development And Trend of Construction Project Management in Asian. 这次讲课非常成功,国岛教授绘声绘色和充满激情的演讲迎来在场的学生的热烈掌声。
