群勃龙.庚酸睾酮.康力龙报价单模板(中英文)
报价单
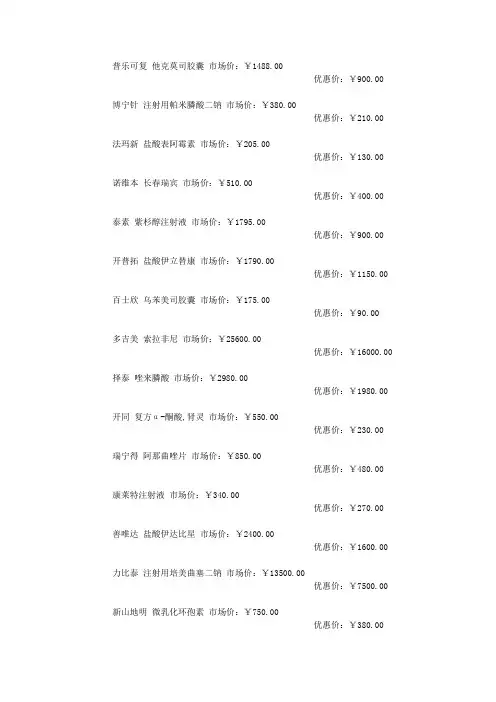
普乐可复他克莫司胶囊市场价:¥1488.00优惠价:¥900.00博宁针注射用帕米膦酸二钠市场价:¥380.00优惠价:¥210.00法玛新盐酸表阿霉素市场价:¥205.00优惠价:¥130.00诺维本长春瑞宾市场价:¥510.00优惠价:¥400.00泰素紫杉醇注射液市场价:¥1795.00优惠价:¥900.00开普拓盐酸伊立替康市场价:¥1790.00优惠价:¥1150.00百士欣乌苯美司胶囊市场价:¥175.00优惠价:¥90.00多吉美索拉非尼市场价:¥25600.00优惠价:¥16000.00择泰唑来膦酸市场价:¥2980.00优惠价:¥1980.00开同复方α-酮酸,肾灵市场价:¥550.00优惠价:¥230.00瑞宁得阿那曲唑片市场价:¥850.00优惠价:¥480.00康莱特注射液市场价:¥340.00优惠价:¥270.00善唯达盐酸伊达比星市场价:¥2400.00优惠价:¥1600.00力比泰注射用培美曲塞二钠市场价:¥13500.00优惠价:¥7500.00新山地明微乳化环孢素市场价:¥750.00优惠价:¥380.00爱必妥西妥昔单抗市场价:¥4698.00优惠价:¥2450.00泰索帝多西紫杉醇注射液市场价:¥2300.00优惠价:¥1400.00抑那通注射用缓释醋酸亮丙瑞林市场价:¥2550.00优惠价:¥1600.00赫赛汀注射用曲妥珠单抗市场价:¥25800.00优惠价:¥14500.00骁悉吗替麦考酚酯片市场价:¥770.00优惠价:¥500.00福至尔市场价:¥1680.00优惠价:¥1000.00华蟾素片市场价:¥290.00优惠价:¥100.00康士得(比卡鲁胺片)市场价:¥1596.00优惠价:¥1200.00紫龙金片市场价:¥231.00优惠价:¥120.00西黄丸北京同仁堂全国最低价格市场价:¥480.00优惠价:¥230.00参一胶囊市场价:¥248.00优惠价:¥120.00金龙胶囊市场价:¥207.00优惠价:¥110.00慈丹胶囊市场价:¥241.00优惠价:¥140.00艾瑞宁市场价:¥1200.00优惠价:¥650.00 日达仙市场价:¥1690.00优惠价:¥900.00健择市场价:¥549.00优惠价:¥400.00健脾益肾颗粒市场价:¥80.00优惠价:¥45.00金克槐耳颗粒市场价:¥160.00优惠价:¥90.00易瑞沙(英国)市场价:¥5500.00优惠价:¥4500.00易瑞沙(印度)全国最低价格市场价:¥2800.00优惠价:¥1500.00。
康复龙.康力龙.群勃龙报价单模板(中英文)

生产厂家-武汉欣欣佳丽生物科技有限公司报价 品名(Product Name)
1
2 3 4 5 6 7 8 9
备注(Remarks)
供应商(Supplier)
康复龙Oxymetholoe 群勃龙Trenbolone 替勃龙Tibolone 群勃龙庚酸酯trenbolone enanthate 屈他雄酮丙酸酯Drostanolone Propionate 康力龙stanozolol 苯丙酸睾酮Testosterone phenylpropionate 米勃龙 群勃龙醋酸酯Trenbolone Acetate
≥99.0%
数量 (Quantity)
价格 (Price)
NXINJIALI BIO-TECH CO.,LTD.)
opment Zone No. 118,HuBei Prov., China.
丽生物科技有限公司报价单(Quotation)
CAS编号(CAS NO.)
质量规格 161-33-8 5630-53-5 2322-77-2 521-17-5 10418-03-8 1255-49-8
10161-34-9
≥98.0%
≥98.0% ≥99.0% ≥99.0% ≥99.0% ≥99.0% ≥99.0%
生产厂家武汉欣欣佳丽生物科技有限公司报价单quotation品名productnamecas编号cas质量规格specifcations数量quantity价格price康复龙oxymetholoe群勃龙trenbolone替勃龙tibolone群勃龙庚酸酯trenboloneenanthate屈他雄酮丙酸酯drostanolonepropionate康力龙stanozolol苯丙酸睾酮testosteronephenylpropionate群勃龙醋酸酯trenboloneacetate10161349备注remarks供应商supplier武汉欣欣佳丽生物科技有限公司wuhanxinxinjialibiotechco
Testosterone Cypionate 环戊丙酸睾酮注射液

注射用绒促性素 2000iu Ghorionic gonadotrophine(hCG) 上海丽珠
注射用绒促性素 1000iu Ghorionic gonadotrophine(hCG) 上海丽珠
大力补 德国 10mg*100片 metandienone 德国
大力补 国产英文 10mg*100片 metandienone 国产
司坦唑醇片 2mg*100片 stanozolol 广西 百会
司坦唑醇片 20mg*100片 stanozolol 武汉 远大
司坦唑醇片 德国 20mg*100片 Stanozolol 德国
规格:油针剂:50mg/1ml、100mg/1ml、200mg/1ml。是否医保用药:非医保是否非处方药:处方
可以有效的增长肌肉块和力量,比Enanthate稍稍更有效。
Cypionate是一种比较难得到的长效睾酮,Cypionate可以有效的增长肌肉块和力量,比Enanthate稍稍更有效,在睾酮中很受欢迎,但它处水较多,且相对其它合成类药物比可能的反作用相对多些,临床一般有效用量200 - 1000mg每周,许多人更用到2000mg/周,当然这样不太安全。
CAS 编号:58-20-8
包装规格: 5kg听装规格
型号:98%Testosterone CypionateCAS#: 58-20-8
性状:油针剂。
功能主治:本品为人工合成的雄激素,临床上主要用于治疗无睾症或类无睾症、隐睾症、功能性子宫出血、月经过多、子宫内膜异位症、子宫肌瘤、更年期综合征、转移性乳腺癌和卵巢癌、垂体性侏儒症、老年性骨质疏松、再生障碍性贫血等。 用法及用量:肌注:50-200mg/次,1次/2周。
基本药物集中采购跟标产品品种价格表
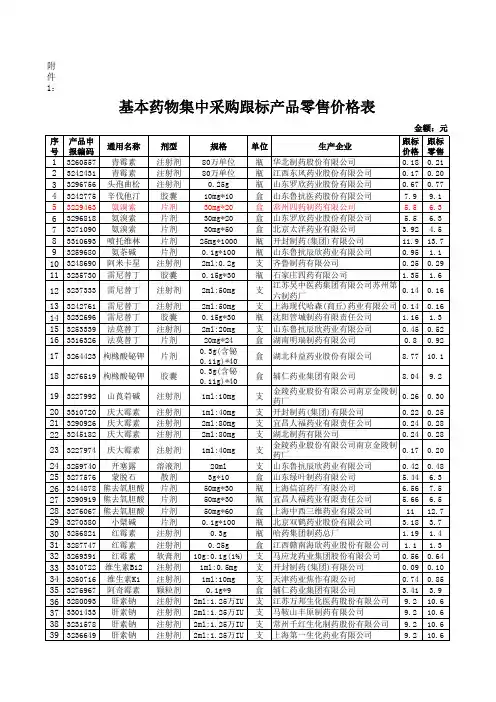
68 3237336 69 3280789 70 71 72 73 74 75 76 77 78 79 80 81 82 83
3234496 纳洛酮 3304012 纳洛酮 3310414 破伤风抗毒 素 3280645 破伤风抗毒 素 3236185 环丙沙星 3249488 环丙沙星 3250356 环丙沙星 3254767 环丙沙星 3228324 氯霉素 3257866 噻吗洛尔 3310758 苯唑西林 3237203 左氧氟沙星 3240654 氧氟沙星 3247374 氧氟沙星 氧氟沙星 氧氟沙星
59 3249258 乳酸钠林格 60 61 62 63 64 65 66 67
3293281 乳酸钠林格 3260083 乳酸钠林格 3247400 乳酸钠林格 3229539 碳酸氢钠 3283899 碳酸氢钠 3260119 碳酸氢钠 3250373 碳酸氢钠 3254792 碳酸氢钠 纳洛酮 纳洛酮
3259740 开塞露 3277576 蒙脱石 3244878 熊去氧胆酸 3290919 熊去氧胆酸 3276067 熊去氧胆酸 3270380 小檗碱 3256821 红霉素 3287747 红霉素 3269391 红霉素 3310722 维生素B12 3250716 维生素K1 3276967 阿奇霉素 3280093 肝素钠 3301433 肝素钠 3231578 肝素钠 3236649 肝素钠 3255393 肝素钠 3244906 肝素钠
氯化钾 氯化钾
0.18 0.21 4.23 1.74 2.7 1.57 2.7 4.85 1.55 1.55 1.55 1.55 1.38 6.28 1.4 1.36 2.06 2.06 13.4 1.31 0.91 0.8 0.59 0.95 0.4 7.38 4 4 1.28 2.24 4.9 2.0 3.1 1.8 3.1 5.6 1.8 1.8 1.8 1.8 1.6 7.2 1.6 1.6 2.4 2.4 15.4 1.5 1.0 0.92 0.68 1.1 0.46 8.5 4.6 4.6 1.5 2.6
difene_英文说明书

Package: Blister Pack (10 tablets per blister)Ingredients:- Active Ingredient: Ibuprofen (200 mg)- Inactive Ingredients: Magnesium stearate, cellulose, lactose monohydrate, pregelatinized starch, croscarmellose sodium, hypromellose, titanium dioxide, and iron oxide.Indications:Difene Tablets are a nonsteroidal anti-inflammatory drug (NSAID) used to relieve symptoms of various conditions, including:- Pain: such as headache, dental pain, menstrual cramps, arthritis, backache, and muscular pain.- Fever: to reduce feverish symptoms.- Inflammation: to reduce inflammation in conditions such as arthritis, ankylosing spondylitis, and tendinitis.Contraindications:Do not use Difene Tablets if you have any of the following conditions:- Hypersensitivity to ibuprofen or any other NSAID.- Severe kidney disease.- Active peptic ulcer or gastrointestinal bleeding.- Third trimester of pregnancy.- Severe liver disease.- Hemorrhagic diathesis.- Children under 12 years of age (unless directed by a physician).Warnings and Precautions:- Consult a healthcare professional before taking Difene Tablets if you have a history of stomach ulcers, gastrointestinal bleeding, heart disease, high blood pressure, liver or kidney disease, asthma, or if you are taking any other medications.- Do not exceed the recommended dose to avoid potential side effects.- Do not use Difene Tablets for more than 10 days unless directed by a healthcare professional.- Avoid alcohol consumption while taking Difene Tablets.- Use caution when driving or operating machinery as Difene Tablets may cause drowsiness or dizziness in some individuals.- Difene Tablets may interact with certain medications, including anticoagulants, corticosteroids, and diuretics. Consult a healthcare professional if you are taking any of these medications.Dosage:The recommended dosage of Difene Tablets is as follows:- Adults and children over 12 years of age: 1 tablet every 4 to 6 hours as needed, not to exceed 6 tablets in 24 hours.- Elderly patients: Adjust dosage based on renal function and healthcare professional advice.How to Use:- Swallow the tablet whole with a glass of water.- Do not chew or crush the tablet.- Take Difene Tablets with or without food.Side Effects:The following side effects may occur while taking Difene Tablets:- Gastrointestinal: Nausea, vomiting, stomach pain, heartburn, indigestion, diarrhea, constipation, and ulcers.- Hematologic: Increased risk of bleeding and bruising.- Dermatologic: Skin rash, itching, and hives.- Cardiovascular: Increased blood pressure, heart failure, and myocardial infarction.- Central nervous system: Dizziness, headache, and drowsiness.Overdose:If an overdose is suspected, contact a healthcare professional immediately. Symptoms of an overdose may include severe stomach pain, vomiting, bleeding, and kidney damage.Storage:- Store Difene Tablets at room temperature (15°C to 30°C or 59°F to 86°F).- Keep the blister pack tightly closed when not in use.- Protect from light and moisture.- Do not use after the expiration date.Manufactured by:[Manufacturing Company Name][Address][City, State, ZIP Code]Please read this leaflet carefully before taking Difene Tablets. If you have any questions or concerns, consult your healthcare professional.---Note: This is a fictional product and the information provided here is for illustrative purposes only. The actual dosage, contraindications, warnings, and side effects may vary based on the specific product andits labeling. Always consult the product's official labeling and a healthcare professional before use.。
litron laboratories 价目表

litron laboratories 价目表
【原创版】
目录
1.介绍 Litron Laboratories
2.Litron Laboratories 的价目表概述
3.价目表中的具体项目和价格
4.对价目表的评价和建议
正文
Litron Laboratories 是一家致力于提供高质量实验室设备和服务
的公司。
他们的产品和服务涵盖了实验室的各个方面,包括实验设备、实验室家具、实验室消耗品等。
为了方便客户了解和选择他们的产品和服务,Litron Laboratories 提供了一份详细的价目表。
这份价目表概述了 Litron Laboratories 提供的所有产品和服务,包括实验设备、实验室家具和实验室消耗品。
在实验设备方面,他们提供了各种类型的实验室设备,如光学显微镜、电子显微镜、离心机等。
在实验室家具方面,他们提供了各种类型的实验室家具,如实验台、储物柜、通风柜等。
在实验室消耗品方面,他们提供了各种类型的实验室消耗品,如试剂、实验器材等。
在价目表中,每个项目都详细列出了其价格。
例如,他们的光学显微镜价格从 1000 美元到 5000 美元不等,电子显微镜价格从 5000 美元到 20000 美元不等,离心机价格从 500 美元到 2000 美元不等。
这些价格都是根据产品的质量和性能来设定的,旨在为客户提供最好的产品和服务。
总的来说,Litron Laboratories 的价目表提供了一份清晰、详细的产品和服务清单,方便客户了解和选择他们的产品和服务。
然而,这份价目表也有一些不足之处。
例如,有些项目的价格较高,可能会让一些客户
望而却步。
类固醇违法案例
任某辉、张葳生产、销售假药一审刑事判决书被告人任某辉被告人张葳被告人曾某深圳市龙华区人民检察院以深龙华检刑诉〔2019〕38号起诉书指控被告人任某辉、张葳、曾某犯生产、销售假药罪,于2019年1月8日向本院提起公诉。
本院适用普通程序,依法组成合议庭,于2020年5月6日作出(2019)粤0309刑初151号刑事判决书。
宣判后,被告人张葳不服,提出上诉。
广东省深圳市中级人民法院于2020年7月15日以(2020)粤03刑终1136号刑事裁定书裁定撤销(2019)粤0309刑初151号刑事判决,发回本院重审。
本院受理后,依法重新组成合议庭于2021年3月4日公开开庭审理了本案。
深圳市龙华区人民检察院指派检察员罗淑芳出庭支持公诉,被告人任某辉及其辩护人罗晓春、被告人张葳及其辩护人骆建军、韦敏杰、被告人曾某及其辩护人林塭丰、白峻到庭参加诉讼。
现已审理终结。
公诉机关指控:2014年11月至2018年6月,被告人任某辉在未取得营业执照和药品经营许可证的情况下,购进价值1920000元疑似假药康力龙、宝丹酮的材料、半成品、包装材料等,再进行配制、包装、分装,然后通过网络及微信朋友圈对外销售,收取货款共计2920000元。
其中任某辉于2017年4月至2017年10月24日,多次销售“伟哥”、“西力士”、“克伦特罗”、“康力龙”给被告人张葳,共计收取货款12500元。
2016年6月至2018年5月,被告人张葳在未取得营业执照和药品经营许可证的情况下,通过网络购进疑似假药的材料、半成品、包装材料、成品等,再进行配制、包装、分装,然后通过网络及微信朋友圈对外销售。
其中张葳在2017年4月至10月24日,通过微信向被告人任某辉购买12500元的“伟哥”、“西力士”、“克伦特罗”、“康力龙”,向被告人曾某购买11580元的疑似假药。
张葳向被告人曾某销售疑似假药HGH等共计187060元,另通过网络、微信朋友圈销售“克伦特罗”、“T3”、“康力龙”、“HGH”、“HCG”等共计376219元。
Zulresso(Brexanolone)商品说明书
UnitedHealthcare ® Community PlanZulresso ® (Brexanolone)Policy Number : CS2023D0080J Effective Date : November 1, 2023 Instructions for UseTable of Contents Page Application ..................................................................................... 1 Coverage Rationale ....................................................................... 1 Applicable Codes .......................................................................... 2 Background .................................................................................... 2 Clinical Evidence ........................................................................... 2 U.S. Food and Drug Administration ............................................. 3 References ..................................................................................... 3 Policy History/Revision Information ............................................. 3 Instructions for Use ....................................................................... 3 This Medical Benefit Drug Policy does not apply to the states listed below; refer to the state-specific policy/guideline, if noted: StatePolicy/GuidelineFloridaRefer to the state’s Medicaid clinical policyIndiana Zulresso ® (Brexanolone) (for Indiana Only)Kansas None LouisianaRefer to the state’s Medicaid clinical policy North CarolinaNone OhioZulresso ® (Brexanolone) (for Ohio Only)TexasRefer to the drug specific criteria found within the Texas Medicaid Provider Procedures ManualZulresso (brexanolone) is proven and medically necessary for the treatment of postpartum depression in patients who meet all of the following criteria :Diagnosis of major depressive disorder (MDD) according to the current DSM (i.e., DSM-5), by a mental health professional; andOnset of current depressive episode was during the third trimester or within 4 weeks postpartum; andCurrent depressive episode is considered moderate to severe based on a standardized, validated tool; andPatient has not previously received Zulresso (brexanolone) for the current postpartum depressive episode from the most recent pregnancy (within 6 months); andPatient has not previously received Zurzuvae (zuranolone) for the current postpartum depressive episode from the most recent pregnancy (within 6 months); andThe provider and/or the provider’s healthcare setting is certified in the Zulresso REMS program, with ability to support onsite continuous monitoring; andBrexanolone is dosed in accordance with the United States Food and Drug Administration (FDA)-approved labeling; and Commercial Policy • Zulresso ® (Brexanolone)Approval is for a single 60-hour infusionThe following list(s) of procedure and/or diagnosis codes is provided for reference purposes only and may not be all inclusive. Listing of a code in this policy does not imply that the service described by the code is a covered or non-covered health service. Benefit coverage for health services is determined by federal, state, or contractual requirements and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claim payment. Other Policies and Guidelines may apply.HCPCS Code DescriptionJ1632 Injection, brexanolone, 1 mgDiagnosis Code DescriptionF53.0 Postpartum depressionBrexanolone is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive modulator, that is chemically identical to endogenous allopregnanolone.Meltzer-Brody et al. assessed brexanolone as a treatment for moderate to severe postpartum depression (PPD) in two double-blind, randomized, placebo-controlled, phase 3 trials.2 Women in the trial were 18-45 years of age, 6 months post-partum or less at screening, and diagnosed with PPD with a Hamilton Rating Scale for Depression (HAM-D) score of ≥ 26 and 20-25 for study 1 and study 2, respectively. Study participants were randomly assigned to receive either brexanolone 90 μg/kg per hr. (BRX90), brexanolone 60 μg/kg per hr. (BRX60), or matching placebo for a single 60-hour infusion in study 1. In study 2, BRX90 or placebo was infused as a single 60-hour infusion. The primary efficacy endpoint was the change from baseline in the 17-item HAM-D total score at 60 hours. This was assessed in all patients who started infusion of brexanolone or placebo, had a valid HAM-D baseline assessment, and had at least one post-baseline HAM-D assessment. The trials are NCT02942004 (study 1) and NCT02942017 (study 2). In study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (SE 1.2) in the BRX60 group and 17.7 points (1.2) in the BRX90 group compared with 14.0 points (1.1) in the placebo group [difference -5.5 (95% CI -8.8 to -2.2), p = 0.0013 for the BRX60 group; -3.7 (95% CI -6.9 to -0.5), p = 0.0252 for the BRX90 group]. In study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group [difference -2.5 (95% CI -4.5 to -0.5), p = 0·0160]. The authors conclude that brexanolone for PPD resulted in significant and clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with rapid onset of action and durable treatment response during the study period. The authors conclude that results suggest that brexanolone injection is a novel therapeutic drug for PPD that has the potential to improve treatment options for women with this disorder.Brexanolone safety, tolerability, and pharmacokinetics were evaluated in a multicenter, open-label study in 20 patients aged 15 to 17 years diagnosed with PPD and were comparable to those in adult patients with PPD.1Professional SocietiesThe American College of Obstetricians and Gynecologists (ACOG) has published a clinical practice guideline with recommendations on treatment and management of perinatal mental health conditions including depression. ACOG recommends consideration of brexanolone administration in the postpartum period for moderate-to-severe perinatal depression with onset in the third trimester or within 4 weeks postpartum. The decision to use brexanolone should balance the benefits (e.g., rapid onset of action) with the risks and challenges (e.g., limited access, high cost, lack of data supporting safety with breastfeeding, requirement for inpatient monitoring during the infusion, lack of efficacy data beyond 30 days).(STRONG RECOMMENDATION, MODERATE-QUALITY EVIDENCE)This section is to be used for informational purposes only. FDA approval alone is not a basis for coverage.Zulresso is indicated for the treatment of postpartum depression (PPD) in patients 15 years and older.Zulresso is only available through a restricted program under a REMS called the Zulresso REMS due to the risk of excessive sedation or sudden loss of consciousness that can result in serious harm.Important requirements of the Zulresso REMS include the following:Healthcare facilities must enroll in the program and ensure that Zulresso is only administered to patients who are enrolled in the Zulresso REMSPharmacies must be certified with the program and must only dispense Zulresso to healthcare facilities who are certified inthe Zulresso REMSPatients must be enrolled in the Zulresso REMS prior to administration of ZulressoWholesalers and distributors must be registered with the program and must only distribute to certified healthcare facilities and pharmacies1.Zulresso [package insert]. Cambridge, MA: Sage Therapeutics; June 2022.2.Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, Li H, Sankoh AJ, Clemson C,Schacterle A, Jonas J, Kanes S. Brexanolone injection in post-partum depression: two multicentre, double-blind,randomised, placebo-controlled, phase 3 trials. Lancet. 2018 Sep 22;392(10152):1058-1070.3.AAmerican Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. 2013. Washington,DC. Pages 451-459.4.Treatment and Management of Mental Health Conditions During Pregnancy and Postpartum: ACOG Clinical PracticeGuideline No. 5. Obstet Gynecol. 2023;141(6):1262-1288. doi:10.1097/AOG.0000000000005202.Date Summary of Changes11/01/2023 Coverage Rationale•Replaced references to “brexanolone” with “Zulresso (brexanolone)”•Revised coverage criteria:o Added criterion requiring the patient has not previously received Zurzuvae (zuranolone) for thecurrent postpartum depressive episode from the most recent pregnancy (within 6 months)o Replaced criterion requiring “onset of current depressive episode was during the third trimester and 4 weeks postpartum” with “onset of current depressive episode was during the thirdtrimester or within 4 weeks postpartum”Supporting Information•Updated Clinical Evidence and References sections to reflect the most current information•Archived previous policy version CS2022D0080IThis Medical Benefit Drug Policy provides assistance in interpreting UnitedHealthcare standard benefit plans. When deciding coverage, the federal, state, or contractual requirements for benefit plan coverage must be referenced as the terms of the federal, state, or contractual requirements for benefit plan coverage may differ from the standard benefit plan. In the event of a conflict, the federal, state, or contractual requirements for benefit plan coverage govern. Before using this policy, please check the federal, state, or contractual requirements for benefit plan coverage. UnitedHealthcare reserves the right to modify itsPolicies and Guidelines as necessary. This Medical Benefit Drug Policy is provided for informational purposes. It does not constitute medical advice.UnitedHealthcare may also use tools developed by third parties, such as the InterQual® criteria, to assist us in administering health benefits. The UnitedHealthcare Medical Benefit Drug Policies are intended to be used in connection with the independent professional medical judgment of a qualified health care provider and do not constitute the practice of medicine or medical advice.。
中美康奈尔动物药业有限公司产品价格表
165元/袋 99元/袋 20元/瓶 154元/袋 121元/袋 22元/瓶 21元/瓶 19元/瓶 154元/袋 121元/袋 14元/瓶 99元/袋 18元/瓶 14元/瓶 88元/桶 18元/袋 14元/袋 22元/袋 17元/袋 14元/袋 18元/袋 11元/袋 13元/袋 15元/袋 14元/袋 187元/桶 143元/桶 209元/桶 121元/桶 198元/桶 154元/桶 143元/桶 154元/桶 165元/桶 209元/桶 132元/桶 5元/袋 99元/桶 176元/桶
阿普拉霉素、头孢喹诺、氟比洛芬、增效因子、粘膜修复因子等 乳酸诺氟沙星、盐酸左旋咪唑、黄连素、螯合乳酸钙、活性酶等 硫酸安普霉素、病毒唑、二甲硝唑、葡聚糖苷泰等 硫酸粘菌素、巴龙霉素、6542、白头翁、肠粘膜修复剂等 硫酸新霉素、金银花、败酱草、黄柏、仙鹤草等提取物 泰妙菌素、泰利霉素、米诺环素、紫花杜鹃甲素、粘膜修复剂等 硫氰酸红霉素、喘美莱斯、紫花杜鹃甲素等 金刚乙胺、奥司他韦、卡美丁、金丝桃素、贝诺酯等 酒石酸泰乐菌素、盐酸多西环素、纳米包被美他环素等 板蓝根、蟾酥、合成牛黄、阿昔洛韦、替洛隆、紫花杜鹃甲素等 甲磺酸培氟沙星、盐酸环丙沙星、替硝唑、左旋咪唑葡聚糖 阿莫西林、硫酸阿米卡星、地美硝唑、生育酚、粘膜修复因子等 磺胺氯吡嗪钠、妥曲珠利、、三磺钠盐、乙氧太胺苯甲脂等 妥曲珠利、氨丙林、衣巴索、VK3、虫卵破壁因子、止血敏等 地克珠利、安洛血、VK3、黄连素、肠毒素吸附因子 石膏、地黄、水牛角、黄连、栀子、吗啉胍、乙酰甲喹等 蟾酥、斑蝥、西佐糖、冰片、雄黄、金银花、黄芪、增噬力酸等 蟾酥、紫雏菊、金银花、连翘、生地黄、黄芪、金刚烷胺等 荆芥、防风、八角茴香、紫雏菊、蟾皮、板蓝根、金刚乙胺等 青黛、黄连、黄柏、薄荷、桔梗、万乃洛韦等 蟾酥、射干、板蓝根、苍术、金银花、冰片、止咳平喘因子等 黄芪、党参、白术、甘草、当归、黄柏、益母草、栀子等 党参、黄芪、当归、丁香、白勺、麦芽、虎杖、丹参、氨基酸等 穿心莲、苦参、黄芩、白头翁、乙酰甲喹等中西复方药 党参 、山楂(炒)、复合氨基酸、复合维生素、微生态制剂等 头孢噻呋纳、舒巴坦钠、甲砜霉素、多西霉素、肠粘膜修复剂 盐酸沙拉沙星、克林沙星、小檗碱、金霉素、止泻因子 氟苯尼考、丁胺卡那、哈喹诺、靶向因子、抗炎剂等 甲磺酸培氟沙星、尼奥霉素、曲安西龙、舒巴坦钠等 扶正祛邪、清热解毒、补气、补阳类等中药提取物、牛磺酸等 林可霉素、大观霉素、克林霉素、米诺环素、色甘酸钠等 磺胺间甲氧嘧啶钠、咪唑苯尿、多西环素、金霉素、乙胺嘧啶等 安普霉素、小檗碱、阿昔洛韦、地芬诺酯、肠粘膜修复因子 发散风寒类、清热解毒类、补气、补阳类等中药提取物、干扰素 延胡索酸泰妙菌素、紫花杜鹃甲素、药物分布改善剂等 阿莫西林、克林霉素、舒巴坦钠、纳米ek5、鱼腥草素钠等 生物素、叶酸、甘氨酸、赖氨酸、蛋氨酸、免疫增效剂等 五味子、蛇床子、益母草、当归、免疫球蛋白、益肽酶等 氟苯尼考、地美环素、泰妙菌素、粘膜修复剂、功能增效剂等
政府定价药品价格表
德国礼达大药厂
17
长春西汀
注射剂
10mg(冻干粉)
支
54.9
皖(0002799)
至2008.04.02
安徽威尔曼振星药业有限公司
18
阿司匹林
片剂
50mg×100片
瓶
12.5
计办价格[2002]625号
至2008.04.02
江苏恩华药业集团有限公司
19
纳洛酮
注射剂
1ml:0.4mg
支
支
16.0
发改价格[2005]1762号
至2008.04.02
11
奥美拉唑
肠溶胶囊
20mg×14粒
盒
68.0
计价格[2002]2822号
至2008.04.02
江苏飞马药业有限公司
12
小柴胡颗粒
颗粒剂
10g×6袋
盒
9.0
计价格[2001]1193号
至2008.04.02
江苏飞马药业有限公司
13
罗红霉素
胶囊
150mg×6粒
盒
12.1
发改价格[2004]881号
至2008.04.02
盒
8.8
发改价格[2006]1542号
至2008.04.02
长春万德制药有限公司
57
氧氟沙星
栓剂
100mg×7枚
盒
22.8
粤价[2005]207号
至2008.04.02
广东庆发药业有限公司
58
复方氨基酸18AA
注射剂
250ml:12.5g
瓶
10.5
发改价格[2006]2989号
至2008.04.02
