Applications in the pharmaceutical industry of ARTP and MMC
药品注册用英语

药品注册用英语现在做注册资料经常会涉及英语表达,为了使我们写注册资料时的英语更纯正,希望各位达人能积极勇跃提供经常涉及的英语表达,使我们的注册水平更上一层楼。
我先抛砖引玉CEP:欧洲药典适应性证书certificate of suitability to monograph of European Pharmacopoeia。
是欧洲药典所收载的原料药的一种认证程序,用以确定原料药的质量可以用欧洲药典的方法加以控制。
这一程序适用于生产的和提取的有机或无机物质以及发酵生产的非直接基因产品。
DMF:Drug master File美国药物主文件档案。
是指提交给FDA的用于提供关于人用药品的生产设备、工艺或生产、工艺处理、包装和储存中使用的物料的详细的和的信息。
分为五种类型:I:生产地点、设备、操作程序和人员II:原料药、原料药中间体、生产原料药和中间体使用的物料和药品III:包装材料IV:赋形剂、色素、调味剂、香料或生产这些物质所用的物料V: FDA接受的参考信息EDMF:European Drug Master File欧洲药物主文件档案。
是指欧洲制剂申请中有关原料药信息的文件,又称原料药主文件档案(ASMF)。
EDMF 只有在制剂申请的支持下才能提交。
EDMF 分为两部分:1.申请人部分(AP):供制剂申请人使用的非信息;2. 限制部分(RP):EDMF持有人认为是的信息。
EDMF的使用围:1. 新原料药2. 已知的但欧洲药典或其成员国药典没有收载的原料药3. 欧洲药典或成员国药典已收载的原料药ANDA:Abbreviated New Drug Application 美国简略新药申请。
是FDA规定的仿制药申请程序。
Generic:仿制的,非特殊的API:Active Pharmaceutical Ingredient 原料药Dossier:文档,档案。
TSE:Transmitting animal Spongiform Encephalopathy agent 传播性动物海绵状脑病体Q7A:ICH(国际协调会议)原料药GMP 指南。
fda_new_drug_application(新药申请指南)
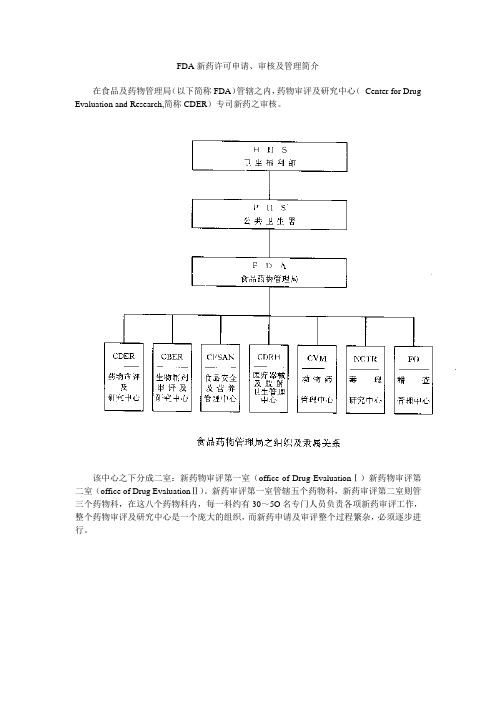
FDA新药许可申请、审核及管理简介在食品及药物管理局(以下简称FDA)管辖之内,药物审评及研究中心(Center for Drug Evaluation and Research,简称CDER)专司新药之审核。
该中心之下分成二室:新药物审评第一室(office of Drug EvaluationⅠ)新药物审评第二室(office of Drug EvaluationⅡ)。
新药审评第一室管辖五个药物科,新药审评第二室则管三个药物科,在这八个药物科内,每一科约有30~5O名专门人员负责各项新药审评工作,整个药物审评及研究中心是一个庞大的组织,而新药申请及审评整个过程繁杂,必须逐步进行。
药物审评及研究中心(Center for Drug Evalution and Research,CDER)之组织说明如下:1.秘书室(Executive Secretariant Staff)2.总务室(Office of Management)(l)药物资记中心(Drug Information Resource)(2)医学图书馆(Medical Library)(3)总务及王计(Management and Budget)(4)资讯系统设计(Information System Design)3.专业进修室(Professional Development)4.顾问团(Advisors and Consultants Staff)5·前导性新药审评(Pilot Drug Evaluation)6.非处方药审评室(office of OTC Drug Evaluation)(1)单篇非处方药审评室(MonograPh Review Staff)(2)非处方药政策性科(OTC Drug Policy Staff)(3)医学审评科(Medical Review Staff)7.非专利处方药室(office of Generic Drugs)(1)化学第一科(ChemistryⅠ)(2)化学第二科(ChemistryⅡ)(3)生体相等性科(Bioequivalence)8.研究发展室(Office of Research Resources)(1)研究及试验科(Research and Testing)(2)药品分析科(Drug Analysis)(3)生体药学科(Biopharmaceutics)(4)临床药理科(Clinical pharmacology)9.新药标准室(Office of Drug Standards)(l)新药市场广告及信息科(Drug Marketing,Advertising and Communications)10.新药合法性室(Office of Compliance)(1)新药信息科(Drug Labeling Compliance)(2)新药品质审核科(Drug Quality Evaluation)(3)新药产品及制造品质科(Mahufacturing and Product Quality)(4)科学性侦查科(Scientific Investigations)(5)新药管理科(Regulatory Affairs)11.新药流行学及统计生物学室(Office of Epidemiology and Biostatistics)(1)流行学及监视科(Epidemiology and Surveillances)(2)生物统计科(Biometrics)12.新药审评第一室(Office of Drug EvaluationⅠ,ODEI)(1)心脏药物科(Division of Cardio-Renal Drug Products)(2)神经药物科(Division of Neuropharmacological Drug Products)(3)肿瘤肺药物科(Division of Oncology and Pulmonary Drug Products)(4)影像、手术及牙药物科(Division of Medical Imaging Surgical and Dental Drug Products)(5)胃肠及凝血药物科(Division of Gastrointestinal and Coagulation Drug Products)13.新药审评第二室(Office of Drug Evaluation Ⅱ, ODE Ⅱ)(1)新陈代谢及内分泌药物科(Division of Metabolism and Endocrine Drug ProductS)(2)抗传染药物科(Division of Anti-Infective Drug Products )(3)抗病毒药物科(Division of Anti—Viral Drug ProductS)二、新药申请(一)药物的定义依据联邦食品药物及化妆品法第二章第201节,药物的定义如下:l.美国药典,同种治疗法药典,或者国家处方集(National Formulary)中所列的物质。
《化学药品创新药I期临床试验申请药学共性问题相关技术要求》

附件化学药品创新药I期临床试验申请药学共性问题相关技术要求为鼓励创新,加快新药创制,满足公众用药需求,国家局发布了《关于调整药物临床试验审评审批程序的公告》(2018年第50号,以下简称50号公告),实行临床试验默许制以及pre-IND沟通流制度。
自50号公告实施以来,符合要求的创新药I期临床试验申请均得到了快速审评。
对于I期临床试验申请,为了保障受试者的安全,药学审评通常重点关注与安全性相关的问题,例如杂质、稳定性、无菌制剂生产条件和除菌/灭菌方法、以及临床前动物安全性评价试验与后续人体临床试验所用样品的质量可比性等。
国家局发布的《新药I期临床试验申请技术指南》(2018年第16号)对相关药学研究内容和资料提交要求已经进行了阐述,但是审评中发现部分创新药I期临床试验申请仍然存在一些与上述安全性内容相关的药学问题。
为了更好地实施国家局50号公告,促进创新药的研究和开发,本技术要求对创新药I期临床试验申请药学共性问题进行总结,以供申请人参考。
一、关于样品试制共性问题:提供的样品试制信息非常有限,处方工艺信息(特别是涉及复杂原料药或者复杂制剂时)过于简单。
一般性要求:参照《新药I期临床试验申请技术指南》相关要求提供原料药和制剂的生产商、生产地址和处方工艺信息,汇总关键研究批次(包括用于安全性研究、稳定性研究、临床研究(如已制备)等批次)的试制信息、关键项目的批分析数据等。
对于复杂原料药(例如多肽、小分子核酸、聚合物产品、含多个手性中心、含发酵工艺或者天然来源等药物)、复杂制剂(例如微球/微乳/脂质体、胶束、透皮制剂、吸入制剂等)、复杂给药途径(例如制备成混悬液、乳液或者凝胶通过皮科、眼科和耳用等局部给药)以及复杂药械组合产品,应注意对重要的生产步骤、设备和工艺参数等进行较为详细的描述。
对于无菌制剂,应对无菌生产条件和除菌/灭菌方法等进行较为详细的描述,并且提供无菌保障措施。
鉴于国内目前临床试验申请为60天默许制,I期临床试验申请如果研究资料符合要求通常可快速开展临床试验,建议申报I期临床试验时(特别是涉及复杂原料药和制剂、复杂给药途径、药械组合产品时)在拟定的临床样品制备地点至少完成1批样品的制备,并且提供相关的试制信息、检验报告。
关于国际药品注册翻译说明

国际药品注册翻译医药翻译网的国际药品注册翻译译员多毕业于国内外著名医科大学,并在各自的国际药品注册翻译领域有过丰富翻译经验。
国际药品注册翻译人员都经过严格测试,大多有国外留学、工作经历,具有良好的国际药品注册翻译能力。
国际药品注册翻译网项目组成员对国际药品注册翻译的文化背景、语言习惯、专业术语等有深入的把握。
医药翻译网鼎力提供每位国际药品注册翻译客户质量最高、速度最快的国际药品注册翻译。
医药翻译网凭借严格的质量控制体系、规范化的运作流程和独特的审核标准已为各组织机构及来自全球的医药公司提供了高水准的国际药品注册翻译,不少的医药公司还跟我们签定了长期合作协议。
国际药品注册翻译的质量和速度质量是企业生存和发展的根本,为确保国际药品注册翻译的准确性,项目的全过程如下:一、庞大国际药品注册翻译团队保证各类国际药品注册翻译稿件均由专业人士担任。
二、规范化的国际药品注册翻译流程。
从获得资料的开始到交稿全过程进行质量的全面控制,并同时做到高效率,快速度的原则。
三、及时组建若干翻译小组,分析各项要求,统一专业词汇,确定语言风格,译文格式要求。
四、国际药品注册翻译均有严格的语言和专业技术双重校对。
从初稿的完成到统稿,从校对到最终审核定稿,甚至词汇间的细微差别也力求精确。
五、不间断的进行招聘,充足的人力资源不断汇集国际药品注册翻译界的精英和高手。
不断对内部及外聘国际药品注册翻译人员进行系统的再培训工程。
六、曾6 小时翻译4.5 万字的速度客户所需。
七、有效沟通。
国际药品注册翻译大项目组协调各方面工作:高级项目经理项目经理(Project Manager)翻译(Translation)编辑(Editing)校对(Profreading)质量控制(Quality Assurance)国际药品注册翻译技术配备一、制作部配备有先进的计算机处理设备,多台扫描仪、打印机、光盘刻录机、宽带网络接入、公司拥有独立的服务器,各项领先技术确保所有文件系统化处理和全球同步传输。
制药行业英语词汇总结

FDA(Food and drug administration ):(美国国家)食品药品管理局IND(Investigation new drug):临床研究申请(指申报阶段,相对于NDA而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(New drug application):新药申请ANDA(Abbreviated New drug application):简化新药申请EP诉(Export application):出口药申请(申请出口不被批准在美国销售的药品)Treatment IND:研究中的新药用于治疗drug:Abbreviated New简化申请的新药DMF(Drug master file):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA 时才能参考其内容)Holder:DMF持有者CFR(Code of federal regulation):(美国)联邦法规Panel:专家小组Batch production:批量生产;分批生产Batch production records:生产批号记录Post-or Pre- market surveillance:销售前或销售后监督Informed consent:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)Prescription drug:处方药OTC drug(over— the— counter drug):非处方药U.S.Public Health Service:美国卫生福利部NIH(NATIONAL INSTITUTE OF HEALTH):(美国)全国卫生研究所Clinical trial:临床试验Animal trial:动物试验Accelerated approval:加速批准Standard drug:标准药物Investigator:研究人员;调研人员Preparing and Submitting:起草和申报Submission:申报;递交Benefit(S):受益Risk (S):受害Drug substance:原料药Established name:确定的名称Generic name:非专利名称Proprietary name:专有名称;INN(international nonproprietary name):国际非专有名称Narrative summary记叙体概要Adverse effect:副作用Adverse reaction:不良反应Archival copy:存档用副本Review copy:审查用副本Official compendium:法定药典(主要指USP、NF).USP(The united states Pharmacopeia):美国药典(现已和NF合并一起出版)NF(National formulary):(美国)国家药品集OFFICIAL=Pharmacopeia = COMPENDIAL:药典的;法定的;官方的Agency:审理部门(指FDA等)Sponsor:主办者(指负责并着手临床研究者)Identity:真伪;鉴别;特性Strength:规格;规格含量(每一剂量单位所含有效成分的量)Labeled amount:标示量Regulatory specification:质量管理规格标准(NDA 提供)Regulatory methodology:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)Regulatory methods validation:管理用分析方法的验证(FDA对NDA提供的方法进行验证)Dietary supplement:食用补充品COS/CEP 欧洲药典符合性认证ICH (International Conference on Harmonization of Technical Requirements for Registration ofPharmaceuticals forHuman Use)人用药物注册技术要求国际协调会议Acceptance Criteria: 接收标准(接收测试结果的数字限度、范围或其它合适的量度标准)Active Pharmaceutical Ingredient (API) (or Drug Substance): 活性药用成分(原料药) 旨在用于药品制造中的任何一种物质或物质的混合物,而且在用于制药时,成为药品的一种活性成分。
药剂专业的英语求职信

药剂专业的英语求职信Respected Hiring Manager,I am excited to express my strong interest in the [Position Title] role at your esteemed [Company Name]. As a passionate and dedicated professional with extensive experience in the pharmaceutical industry, I believe I possess the necessary skills and qualifications to make a valuable contribution to your organization.Throughout my academic and professional journey, I have developed a deep fascination for the pharmaceutical field. My undergraduate degree in [Relevant Degree] from [University Name] has provided me with a solid foundation in the core principles and concepts of pharmaceutical science. During this time, I had the opportunity to participate in various research projects, laboratory experiments, and clinical observerships, which further solidified my understanding of the industry and its crucial role in improving global healthcare.After completing my studies, I joined [Company Name] as a [Previous Position Title]. In this role, I honed my technical expertiseand practical skills in [Relevant Skills]. I took great pride in my ability to [Key Achievements/Responsibilities], consistently delivering high-quality results that exceeded the expectations of my superiors. My strong analytical thinking, attention to detail, and problem-solving capabilities enabled me to tackle complex challenges with ease, contributing to the overall success of the company.What sets me apart as a candidate for this position is my unwavering passion for the pharmaceutical industry and my commitment to staying at the forefront of its ever-evolving landscape. I am constantly seeking opportunities to expand my knowledge and stay up-to-date with the latest advancements in the field. This has led me to actively participate in industry conferences, workshops, and continuing education programs, where I have had the privilege of learning from renowned experts and exchanging ideas with my peers.Moreover, I possess excellent communication and interpersonal skills, which have been instrumental in my ability to collaborate effectively with cross-functional teams, liaise with healthcare professionals, and build lasting relationships with clients and stakeholders. I take pride in my ability to explain complex scientific concepts in a clear and concise manner, ensuring that all stakeholders have a comprehensive understanding of the information.In addition to my technical expertise and communication skills, I ama dedicated and highly organized individual with a strong work ethic.I thrive in fast-paced, dynamic environments and have a proven track record of prioritizing tasks, managing deadlines, and delivering results on time. My ability to work both independently and as part of a team has allowed me to contribute to the success of various projects and initiatives.I am particularly excited about the opportunity to join [Company Name] as the [Position Title]. Your organization's reputation for [Company Strengths/Achievements] aligns perfectly with my own passion and aspirations. I am confident that my skills, experience, and genuine interest in the pharmaceutical field will enable me to make a significant impact in this role and contribute to the continued growth and success of your organization.Thank you for considering my application. I welcome the opportunity to discuss my qualifications further and to learn more about the [Position Title] role and how I can contribute to your team. I look forward to hearing from you and the possibility of joining your esteemed organization.Sincerely,[Your Name]。
FDA药品批准程序简介课件
Drug Application 药品申请
NDA (IND) 新药Chemistry, Manufacturing, & Controls (CMC) 化学性,生产和控制(CMC)Animal Studies 动物试验Bioavailability 生物有效性Clinical Studies 临床试验
Type of ANDA 非专利药申请的分类
No.
Type of Application申请类型
Requirement要求
1
Paragraph I CertificationI类证书
无专利存在
2
Paragraph II CertificationII类证书
专利已过期
3
Paragraph III CertificationIII类证书
Worldwide Pharmaceutical Market
by Sectors ($ Billions)
世界药品市场分类(单位:十亿美元)
2000 2001 2002 2003 2008 增长* Ethical 317.1 363.4 401.0 437.6 677.8 9.1处方药Generic 24.0 27.0 30.5 37.0 64.0 11.6非专利药OTC 70.5 73.8 78.5 82.0 101.0 4.3 Biophar- 22.1 26.3 31.0 36.5 58.6 9.0Maceutical生物药 Total 433.7 490.5 541.0 593.1 901.4 8.7 Source: IMS *Estimated from 2003 to 2008
Generic Drug Requirement
非专利药要求
国际药物注册标准词汇
国际药物注册英语词汇互译FDA(food and drug adminisration):(美国)食品药品监督管理局NDA(new drug application):新药申请ANDA(abbreviated new drug application):简化新药申请EP(export application):出口药申请(申请出口不被批准在美国销售的药品)treatment IND:研究中的新药用于治疗abbreviated(new)drug:简化申请的新药DMF(drug master file):药物主文件(持有者为谨慎起见而准备的资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA时才能参考其容)holder:DMF持有者CFR(code of federal regulation):(美国)联邦法规PANEL:专家小组batch production:批量生产;分批生产batch production records:生产批号记录post or pre-market surveillance:销售前或销售后监督informed consent:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)prescription drug:处方药OTC drug(over—the—counter drug):非处方药U.S. public health service:美国卫生福利部NIH(national institute of health):(美国)全国卫生研究所animal trail:动物试验accelerated approval:加速批准standard drug:标准药物investigator :研究人员;调研人员preparing and submitting:起草和申报submission:申报;递交benefit(s):受益risk(s):受害drug product:药物产品drug substance:原料药established name:确定的名称generic name:非专利名称proprietary name:专有名称;INN(international nonproprietary name):国际非专有名称narrative summary: 记叙体概要adverse effect:副作用adverse reaction:不良反应protocol:方案archival copy:存档用副本review copy:审查用副本official pendium:法定药典(主要指USP、NF).USP(the united state pharmacopeia):美国药典(现已和NF合并一起出版)NF(national formulary):(美国)国家药品集official=pharmacopeial = pendial:药典的;法定的;官方的agency:审理部门(指FDA)sponsor:主办者(指负责并着手临床研究者)identity:真伪;鉴别;特性strength:规格;规格含量(每一剂量单位所含有效成分的量)labeled amount:标示量regulatory specification:质量管理规格标准(NDA提供)regulatory methodology:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)regulatory methods validation:管理用分析方法的验证(FDA对NDA提供的方法进行验证)Dietary supplement:食用补充品ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use)人用药物注册技术要求国际协调会议ICH:Quality-质量Q1A(R2): Stability Testing of New Drug Substances and Products (SecondRevision)新原料药和制剂的稳定性试验(第二版)Q1B: Photostability Testing of New Drug Substances and Products新原料药和制剂的光稳定性试验Q1C: Stability Testing for New Dosage Forms新制剂的稳定性试验Q1D: Bracketing and Matrixing Designs for Stability Testing of DrugSubstances and Drug Products原料药和制剂稳定性试验的交叉和矩阵设计Q1E: Evaluation of Stability Data对稳定性数据的评估处理Q1F: Stability Data Package for Registration Applications in ClimaticZones III and IV在气候带III和IV,药物注册申请所提供的稳定性数据Q2A: Text on Validation of Analytical Procedures分析程序的验证Q2B: Validation of Analytical Procedures: Methodology分析程序的验证:方法学Q3A(R): Impurities in New Drug Substances (Revised Guideline)新原料药中的杂质(修订版)Q3B(R): Impurities in New Drug Products (Revised Guideline)新制剂中的杂质(修订版)Q3C: Impurities: Guideline for Residual Solvents杂质:残留溶剂指南Q3C(M): Impurities: Guideline for Residual Solvents (Maintenance)杂质:残留溶剂指南(修改容)Q4: Pharmacopoeias药典Q4A: Pharmacopoeial Harmonisation 药典的协调Q4B: Regulatory Acceptance of Pharmacopoeial Interchangeability药典互替在法规上的可接受性Q5A: Viral Safety Evaluation of Biotechnology Products Derived from CellLines of Human or Animal Origin来源于人或者动物细胞系的生物技术产品的病毒安全性评估Q5B: Quality of Biotechnological Products: Analysis of the ExpressionConstruct in Cells Used for Production of r-DNA Derived Protein Products生物技术产品的质量:源于重组DNA的蛋白质产品的生产中所用的细胞中的表达构建分析Q5C: Quality of Biotechnological Products: Stability Testing ofBiotechnological/Biological Products生物技术产品的质量:生物技术/生物产品的稳定性试验Q5D: Derivation and Characterisation of Cell Substrates Used forProduction of Biotechnological/Biological Products用于生产生物技术/生物产品的细胞底物的起源和特征描述Q5E: parability of Biotechnological/Biological Products Subject toChanges in Their Manufacturing Process基于不同生产工艺的生物技术产品/生物产品的可比较性Q6: Specifications for New Drug Substances and Products新原料药和制剂的质量规格Q6A: Specifications: Test Procedures and Acceptance Criteria for New DrugSubstances and New Drug Products: Chemical Substances质量规格:新原料药和新制剂的检验程序和可接收标准:化学物质Q6B: Specifications: Test Procedures and Acceptance Criteria forBiotechnological/Biological Products质量规格:生物技术/生物产品的检验程序和可接收标准Q7: Good Manufacturing Practices for Pharmaceutical Ingredients活性药物成份的GMPQ7A: Good Manufacturing Practice Guide for Active PharmaceuticalIngredients活性药物成份的GMP指南Q8: Pharmaceutical Development药物研发Q9: Quality Risk Management质量风险管理ICH:Safety-安全S1A: Guideline on the Need for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究需要的指南S1B: Testing for Carcinogenicity of Pharmaceuticals药物致癌性的检验S1C: Dose Selection for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究之剂量选择S1C(R): Addendum: Addition of a Limit Dose and Related Notes附录:极限剂量和有关注释的的补充S2A: Guidance on Specific Aspects of Regulatory Genotoxicity Tests forPharmaceuticals受法规管辖的药物基因毒性检验的特定方面的指南S2B: Genotoxicity: A Standard Battery for Genotoxicity Testing forPharmaceuticals基因毒性:药物基因毒性检验的标准S3A: Note for Guidance on Toxicokinetics: The Assessment of SystemicExposure in Toxicity Studies毒物代动力学指南的注释:毒性研究中的全身性暴露量的评估S3B: Pharmacokinetics: Guidance for Repeated Dose Tissue DistributionStudies药物代动力学:重复剂量的组织分布研究指南S4: Single Dose Toxicity Tests单剂量毒性检验S4A: Duration of Chronic Toxicity Testing in Animals (Rodent andNon-Rodent Toxicity Testing)动物体慢性毒性持续时间的检验(啮齿动物和非啮齿动物毒性检验)S5A: Detection of Toxicity to Reproduction for Medicinal Products药物对生殖发育的毒性的检验S5B(M): Maintenance of the ICH Guideline on Toxicity to Male Fertility:An Addendum to the Guideline on Detection of Toxicity to Reproduction forMedicinal Products对男性生殖能力的毒性的指南的变动:药物对生殖发育的毒性的检验指南增加了一个附录S6: Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals生物技术生产的药物的临床前安全评价S7A: Safety Pharmacology Studies for Human Pharmaceuticals人用药的安全药理学研究S7B: The Nonclinical Evaluation of the Potential for Delayed VentricularRepolarization(QT Interval Prolongation) By Human Pharmaceuticals药物延迟心室复极化(QT间期)潜在作用的非临床评价S8: Immunotoxicology Studies for Human Pharmaceuticals人用药免疫毒理学研究M3(M): Maintenance of the ICH Guideline on Non-Clinical Safety Studies forthe Conduct of Human Clinical Trials for Pharmaceuticals药物的对人临床试验的非临床安全研究指南的变动E-Efficacy(有效)E1: The Extent of Population Exposure to Assess Clinical Safety for DrugsIntended for Long-Term Treatment of Non-Life-Threatening Conditions对用于无生命危险情况下长期治疗的药物进行临床安全评估的族群暴露量围E2A: Clinical Safety Data Management: Definitions and Standards forExpedited Reporting临床安全数据管理:速报制度的定义和标准E2B(R): Revision of the E2B(M) ICH Guideline on Clinical Safety DataManagement Data Elements for Transmission of Individual Case SafetyReports个案安全报告送交的临床安全数据管理的数据要素指南(E2B(M))的修订版E2B (M): Maintenance of the Clinical Safety Data Management including:Data Elements for Transmission of Individual Case Safety Reports临床安全数据管理的变动包括:个案安全报告送交的数据要素E2B(M): Maintenance of the Clinical Safety Data Management includingQuestions and Answers临床安全数据管理的变动,包括问答E2C: Clinical Safety Data Management: Periodic Safety Update Reports forMarketed Drugs临床安全数据管理:已上市药品的周期性安全数据更新报告Addendum to E2C: Periodic Safety Update Reports for Marketed DrugsE2C的附录:已上市药品的周期性安全数据更新报告E2D: Post-Approval Safety Data Management: Definitions and Standards forExpedited Reporting批准后的安全数据管理:速报制度的定义和标准E2E: Pharmacovigilance Planning药物警戒计划E3: Structure and Content of Clinical Study Reports临床研究报告的结构和容E4: Dose-Response Information to Support Drug Registration支持药品注册的剂量-效应资料E5: Ethnic Factors in the Acceptability of Foreign Clinical Data引入海外临床数据时要考虑的人种因素E6: Good Clinical Practice: Consolidated GuidelineGCP:良好的临床规:统一的指南E7: Studies in Support of Special Populations: Geriatrics对特定族群的支持的研究:老人病学E8: General Considerations for Clinical Trials对临床试验的总的考虑E9: Statistical Principles for Clinical Trials临床试验的统计原则E10: Choice of Control Group and Related Issues in Clinical Trials临床试验中控制组和有关课题的选择E11: Clinical Investigation of Medicinal Products in the PediatricPopulation小儿科药物的临床调查E12A: Principles for Clinical Evaluation of New Antihypertensive Drugs新抗高血压药物的临床评价原则E14: The Clinical Evaluation of QT/QTc Interval Prolongation andProarrhythmic Potential for Non-Antiarrhythmic Drugs非抗心率失常药物的QT/QTc 间期和致心率失常潜在作用的临床评价Multidisciplinary Guidelines 多学科兼容的指南M1: Medical Terminology医学术语M2: Electronic Standards for Transmission of Regulatory Information (ESTRI)药政信息传递之电子标准M3: Timing of Pre-clinical Studies in Relation to Clinical Trials (SeeSafety Topics)有关临床试验的临床前研究的时间安排M4: The mon Technical Document (See CTD section for plete Status ofthe guidelines)通用技术文件(见有关CTD章节)M5: Data Elements and Standards for Drug Dictionaries药物词典的数据要素和标准临床试验常用的英文缩略语TTP:time-to-progression 疾病进展时间SAE:severity Adverse Event 严重不良事件AE:Adverse Event 不良事件SOP:Standard Operating Procedure 标准操作规程CRF:Case Report form 病例报告表DLT:剂量限制毒性MTD:最大耐受剂量KPS:Karnofsky Performance Status行为状态评分CR:plete response完全缓解PR:partial response部分缓解SD:病情稳定PD:progressive disease病情进展CTC:常用药物毒性标准IEC:independent ethics mittee 独立伦理委员会IRB :institutional review board 伦理委员会CRA:临床研究助理CRO:Contract Research Organization 合同研究组织DFS:Disease Free Survival 无病生存期OS:(Overall Survival)总生存时间IC:Informed consent 知情同意ADR:Adverse Drug Reaction 不良反应GAP:Good Agricultural Practice 中药材种植管理规GCP:Good Clinical Practice 药物临床试验质量管理规GLP:Good Laboratory Practice 药品实验室管理规GMP:Good Manufacturing Practice 药品生产质量管理规GSP:Good Supply Practice 药品经营质量管理规GUP:Good Use Practice 药品使用质量管理规PI :Principal investigator 主要研究者CI:Co-inveatigator 合作研究者SI :Sub-investigator 助理研究者COI :Coordinating investigtor 协调研究者DGMP:医疗器械生产质量管理规ICF:Informed consent form 知情同意书RCT :randomized controlled trial, 随机对照试验NRCCT:non-randomized concurrent controlled trial, 非随机同期对照试验EBM:evidence-based medicine 循证医学RCD:randomized cross-over disgn 随机交叉对照试验HCT:historial control trial, 历史对照研究RECIST:Response Evaluation Criteria In Solid Tumors. 实体瘤疗效反应的评价标准QC:Quality Control质量控制UADR:Unexpected Adverse Drug Reaction,非预期药物不良反应。
医药行业专业英语词汇
医药行业专业英语词汇(非常有用)FDA和EDQM术语: CLINICAL?TRIAL:临床试验? ANIMAL?TRIAL:动物试验? ACCELERATED?APPROVAL:加速批准? STANDARD?DRUG:标准药物? INVESTIGATOR:研究人员;调研人员PREPARING?AND?SUBMITTING:起草和申报? SUBMISSION:申报;递交? BENIFIT (S):受益? RISK(S):受害? DRUG?PRODUCT:药物产品? DRUG?SUBSTANCE:原料药? ESTABLISHED?NAME:确定的名称? GENERIC?NAME:非专利名称? PROPRIETARY?NAME:专有名称;? INN(INTERNATIONAL?NONPROPRIETARY?NAME):国际非专有名称? ADVERSE?EFFECT:副作用? ADVERSE?REACTION:不良反应? PROTOCOL:方案? ARCHIVAL?COPY:存档用副本? REVIEW?COPY:审查用副本? OFFICIAL?COMPENDIUM:法定药典(主要指USP、?NF).? USP (THE?UNITED?STATES?PHARMACOPEIA):美国药典NF(NATIONAL?FORMULARY):(美国)国家处方集? OFFICIAL=PHARMACOPEIAL=?COMPENDIAL:药典的;法定的;官方的? AGENCY:审理部门(指FDA)? IDENTITY:真伪;鉴别;特性? STRENGTH:规格;规格含量(每一剂量单位所含有效成分的量)? LABELED?AMOUNT:标示量? REGULATORY?SPECIFICATION:质量管理规格标准(NDA提供)? REGULATORY?METHODOLOGY:质量管理方法? REGULATORY?METHODS?VALIDATION:管理用分析方法的验证COS/CEP?欧洲药典符合性认证ICH(International?Conference?on?Harmonization?of?Technical?Requirements?for?Registration?of PharmaceuticalsforHumanUse)人用药物注册技术要求国际协调会议ICH文件分为质量、安全性、有效性和综合学科4类。
医药行业专业英语词汇
医药行业专业英语词汇(非常有用)FDA和EDQM术语: CLINICAL?TRIAL:临床试验? ANIMAL?TRIAL:动物试验? ACCELERATED?APPROVAL:加速批准? STANDARD?DRUG:标准药物? INVESTIGATOR:研究人员;调研人员PREPARING?AND?SUBMITTING:起草和申报? SUBMISSION:申报;递交? BENIFIT (S):受益? RISK(S):受害? DRUG?PRODUCT:药物产品? DRUG?SUBSTANCE:原料药? ESTABLISHED?NAME:确定的名称? GENERIC?NAME:非专利名称? PROPRIETARY?NAME:专有名称;? INN(INTERNATIONAL?NONPROPRIETARY?NAME):国际非专有名称? ADVERSE?EFFECT:副作用? ADVERSE?REACTION:不良反应? PROTOCOL:方案? ARCHIVAL?COPY:存档用副本? REVIEW?COPY:审查用副本? OFFICIAL?COMPENDIUM:法定药典(主要指USP、?NF).? USP (THE?UNITED?STATES?PHARMACOPEIA):美国药典NF(NATIONAL?FORMULARY):(美国)国家处方集? OFFICIAL=PHARMACOPEIAL=?COMPENDIAL:药典的;法定的;官方的? AGENCY:审理部门(指FDA)? IDENTITY:真伪;鉴别;特性? STRENGTH:规格;规格含量(每一剂量单位所含有效成分的量)? LABELED?AMOUNT:标示量? REGULATORY?SPECIFICATION:质量管理规格标准(NDA提供)? REGULATORY?METHODOLOGY:质量管理方法? REGULATORY?METHODS?VALIDATION:管理用分析方法的验证COS/CEP?欧洲药典符合性认证ICH(International?Conference?on?Harmonization?of?Technical?Requirements?for?Registration?of ?Pharmaceuticals?for?Human?Use)人用药物注册技术要求国际协调会议ICH文件分为质量、安全性、有效性和综合学科4类。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Applications in the pharmaceutical industry of ARTP and MMC1.ARTPIn recent years,ARTP(Atmospheric Room Temperature Plasma)has developed rapidly.ARTP is a new glow discharge technology.It uses inert gas discharge to produce a large number of high-energy active particles at Room Temperature and Atmospheric pressure.The technology can generate the plasma including a large amount of chemically reactive species causing intensive damage of genetic material. These species can permeate into the cell,and cause the DNA damage,then induce SOS repair mechanism of microbial cells.SOS is a high fault rate repair process,which can cause varied mismatch sites,and thus form the final stable genetic mutants,which target strains can be screened.Compared with the traditional mutagenesis methods,ARTP is not only discharge safe and pollution-free,but also has a variety of damage mechanisms to genetic materials,so the diversity of acquired mutagenesis also increases.The characteristics of ARTP mutagenesis show unique advantages in dealing with microbial mutagenesis breeding.At present,ARTP has been widely used in the breeding of bacteria,actinomycetes,fungi,yeast and other microorganisms,and has been more and more widely used in the fields of antibiotics,drug intermediates,raw materials and health care products.2.MMCMicrofluidic chip is used to generate droplets and exchange liquid of microbial culture in MMC (Microbial microdroplet culture system).The volume of liquid droplets is2microns,and200independent microbial droplets can be cultured and continuously passed in parallel.In particular,because of the excellent performance of dissolved oxygen and mixing in the droplets in the water-in-oil micro-system,the microbial growth performance is close to or better than that of shaker.In addition,based on the advantage of chip system,MMC can also meet various experimental requirements,such as fluorescence,visible light,dissolved oxygen and pH online detection,microbial colony count,high-throughput screening,adaptive evolution and other different functions,showing great application potential in antibiotics,drug intermediates,raw materials,health care products and other fields.Fig.1Microbial microdroplet culture(MMC)system.(A)The outer design of MMC.(B)The inner design of MMC.The millifluidic chip is indicated with red rectangle.(C)Millifluidic chip for droplet generation,droplet coalescence,droplet segregation and detection.3.Application of ARTP and MMCThe following table shows partial application of ARTP and MMC in medicine and other related fields.Tab1Applications of ARTP and MMC in medicine and other related fields(partial)NO.Title Author Institution Journal Strain Target Product Result1Inhibition and trendanalysis of carotenoidmetabolic flux ofRhodosporidiumtoruloideWANGYafang,LIUYan,LIXianzhen,YANG FanSchool of BiologicalEngineering,DalianPolytechnic UniversityJournal of DalianPolytechnicUniversitRhodosporidum toruloidesCarotenoidDetermined.The results showed that the defectof the carotenoid synthesis pathway couldreduce the contents of Carotenoid,biomass andlipid by70%,48%and40%,spectively2Enhancement ofascomycin productionvia a combination ofatmospheric and roomtemperature plasmamutagenesis inStreptomyceshygroscopicus andmedium optimizationZhituo Yu,XiaofangShen,Yuanjie Wu,SongbaiYang,Dianwen Ju,Shaoxin ChenDepartment ofMicrobiological andBiochemical Pharmacy,The Key Laboratory ofSmart Drug Delivery,Ministry of Education,School of Pharmacy,Fudan University,Shanghai Institute ofPharmaceutical Industry,China State Institute ofPharmaceutical Industry,ShanghaiAMB ExpressStreptomyceshygroscopicus AscomycinIn order to improve the strain production,theoriginal S.hygroscopicus ATCC14891strainwas treated here with atmospheric and roomtemperature plasma to obtain a stablehigh-producing S.hygroscopicus SFK-36strainwhich produced495.3mg/L ascomycin,a32.5%increase in ascomycin compared tothe ATCC14891.3Mutagenesis ofRhodobactersphaeroides usingatmospheric and roomtemperature plasmatreatment for efficientRong-SongZou,SuyanLi,Le-LeZhang,Yi-JunHan,GeGao,XiangyanCollege of Forestry,Beijing ForestryUniversity,Center,Shenzhou Biology&Technology,BiobreedingResearch Center,WuxiJournal ofBioscience andBioengineeringRhodobactersphaeroidesCoenzyme Q10In the first round of screening in24-well plates,three mutants were obtained,with theproduction of CoQ10at311mg/L,307mg/L,and309mg/L,which were increasedfrom the parent’s production at265mg/L.Furthermore,a second round of mutation and3NO.Title Author Institution Journal Strain Target Product Resultproduction of coenzyme Q10g Sun,andXiao qiangGongResearch Institute ofApplied Technologies,Tsinghua Universityscreening was performed based on the mutantstrain with the highest production in the firstround,leading to the identification of a mutantAR01with the production of CoQ10at w330mg/L.Finally,590mg/L CoQ10was obtainedfor AR01after100h fermentation,whichwas w25.5%higher than that of the originalparent strain.4Astaxanthinoverproduction in yeastby strain engineeringand new gene targetuncoveringJin Jin,YingWang,MingdongYao,XiaoliGu,Bo Li,Hong Liu,MingzhuDing,WenhaiXiao,YingjinYuanKey Laboratory ofSystems Bioengineering(Ministry of Education),School of Chemical&EngineeringTianjinUniversity2.SynBioResearch Platform,Collaborative InnovationCenter of ChemicalScience and Engineering(Tianjin)TianjinUniversityBiotechnology forBiofuelsSaccharomycescerevisiaeAstaxanthinAstaxanthin yield was further increased by0.83-fold(to10.1mg/g DCW)via ARTPmutagen esis,which is the highest reportedyield at shake-fask level in yeast so far.5Enhanced doxorubicinproduction byStreptomyces peucetiususing a combination ofclassical strainmutation and mediumWang X,TianX,Wu Y,ShenX,YangS,Chen Sa Shanghai Institute ofPharmaceutical IndustryPreparativeBiochemistry andBiotechnologyStreptomycespeucetiusdoxorubicinI n this study,a DXR-resistance screeningmethod was developed to screen for DXRhigh-producing mutants.Then,S.peucetiusSIPI-11was treated several times with UV andARTP(atmospheric and room tempera tureplasma)to induce mutations.Treated strains4NO.Title Author Institution Journal Strain Target Product Result optimization.were screened by spreading on aDXR-containing plate,isolating a mutant(S.peucetius33-24)with enhanced DXR yield(570mg/L vs.119mg/L for the original strain).6Breeding andfermentationoptimization ofL-arginineproducing strainsCHENGGong XUJian-ZhongGUOYan-Feng XUKai ZHANGWei-Guo*The Key Laboratory ofIndustrialBiotechnology,Ministryof Education,School ofBiotechnology,JiangnanUniversityMicrobiologyChinaCorynebacterium.glutamicumL-arginineAfter several rounds of screening,a strain wasselected resistant to15g/L L-homoarginine and0.7g/L8-azaguaine,and designated asC.glutamicum ARG3-16(L-HAr,8-AZr,SGr,L-His−).L-arginine production of ARG3-16was49.79%higher than that of the originalstrain.7Mutation Breeding ofγ-Polyglutamic AcidProducing Strain byAtmospheric and RoomTemperature PlasmaCHENShuangxi,ZHANGErchao,ZHANG Lele,XIA QihaoInstitute ofBioengineering,Collegeof Life Science,HenanUniversityChinese Journal ofPharmaceuticalsBacillussubtilisγ-polyglutamicacidOne mutant named HNCL1266was obtainedwith the yield ofγ-polyglutamic acid in shakingflask of26g/L,which was30%higher thanthat of HD11.The mutant strain showed highhereditary stability.8The Screening andCharacterization ofHigh-yield-carotenoidStrain fromDeinococcuswulumuqiensis R12byARTPJin WeiyueSong MingkaiJiao WenhaoJiang LingLi ShuangXu XianState key laboratory ofmaterials-orientedchemical engineering,College ofBiotechnology andPharmaceuticalengineering,NanjingTech University:StateBiotechnologyBulletinD.wulumuqiensiscarotenoidThe strain M1produced612μg/g dry cellweight(DCW)of carotenoid after fermenting72h,2.8times of carotenoid by original D.wulumuqiensis R12(212μg/g DCW),andheredity was stable.The carotenoid of the strainM1showed stronger activity in high ironion reducing power(OD700=0.52)than that(OD700=0.34)of original strain,and higher5NO.Title Author Institution Journal Strain Target Product Resultkey laboratory of materials-oriented chemical engineering, College of food science and light industry Nanjing Tech University DPPH scavenging rate(33.33%)than23.09%, indicating the mutant strain M1possessed better anti-oxidative capacity.9Screening of BacillusLincheniformis withhigh-yieldtetramethylpyrazine byatmospheric roomtemperature plasmamutationMeng Wu,Ding Xuemei,WangRuiming,XiaoDongguangCollege of biologicalEngineering,TianjinUniversity of Scienceand TechnologyFaculty of light industry,Qilu University ofTechnology,JinanChina BrewingBaclicuslincheniformistetramethylpyrazine TTMPCompared to the original stain,the maximumTTMP yield increased from37.89g/L to43.16g/L,which incrcased by13.91%.10Mutation Breeding ofUridine-ProducingStrains by MethodcombiningAtmospheric and RoomTemperature PlasmaandHigh-throughputScreeningHeyun Wu,HongchaoZhang,HuiYuan,Guoliang Li,XiaoguangFan,NingChen,XixianXieNational and LocalUnited Engineering Labof Metabolic ControlFermentationTechnology,TianjinCollege ofBiotechnology,TianjinUniversity of Scienceand Technology,TianjinBacillussubtilisUridineAs a result,four mutant strains,B.subtilisA219,B.subtilis A260,B.subtilis A566and B.subtilis F126,were isolated.Compared with the original strain,B.subtilis TD131,which accumulated3.5g/L of uridine in a5L fermentor,the fourmutants had significant improvements inuridine productivity,amounted to12g/L,14.5g/L,16g/L and18g/L respectively.6NO.Title Author Institution Journal Strain Target Product Result11ARTP mutagenesisbreeding high yieldbacteria of bacillus.ZhaoGuozhong,Wang Fengya,Xu Tingting,Li LixiaNorth ChinaPharmaceutical GroupCorporation;Science andtechnologyresearchBaclicuslincheniformisbacitracinThe mutant strain B-78was obtained byatmospheric pressure room temperature plasma(ARTP)mutagenesis,and its shake flask titerwas increased by38.5%compared with theoriginal strain.After5passages,it still hasstable antibiotic production characteristics.12Screening of HighL-AsparaginaseActivity Mutants ofRecombinant BacteriaWB600byAtmospheric and RoomTemperature PlasmasMutation SystemCHENXuan,LIUSong,FENGYue,DUGuocheng,CHEN JianKey Laboratory ofIndustrialBiotechnology,Ministryof Education,JiangnanJournal of foodand biotechnologyBacillussubtilisL-asparaginasea mutant of B.subtilis WB600(pMA5-wapA-ansZ)with a activity of48.4U/mL wasobtained,the activity value was increased by30%.13Isolation,Identificationand Mutation Breedingof Strain Producingα-Glucosidase InhibitorfromMulberry LeafZHUMengfeng,XU Wei,SHAO Rong,WEI PingCollege of Pharmacy,Nanjing Tech UniversityFood ScienceBacillusatrophaeusα-glucosidaseinhibitorCombining ARTP mutation breeding withhigh-throughput screening,a mutant strainnamed T-690was obtained from880mutantstrains,whose inhibition ratio(73.25%)wasincreased by40.61%compared with the originalstrain.7NO.Title Author Institution Journal Strain Target Product Result14Mutation breeding ofhigh9α-hydroxy-androst-4-ene-3,17-dionetransforming strainsfrom phytosterols andtheir conversionprocess optimizationYang Ma,XiangdongWang,MenghuiWang,Hui Li,Jinsong Shi,andZhenghongXuPharmaceutical Science,Jiangnan UniversityChinese Journal ofBiotechnologyMycobacterium9α-OH-ADThe high production strain named C33with agood genetic stability was selected and theproduct molar yield achieved to15.5%,whichwas34.8%higher than that of original strainwith15g/L phytosterols.Furthermore,thefermentation medium was optimized throughthe design of orthogonal experiment.Besides,oil-water bidirectional transformation systemwas set up to improve the9α-OH-AD molaryield of mutant strain C33.With adding12mLsoybean oil to each1g phytosterols,the molaryield of9α-OH-AD reached47.0%,whichincreased by2times than that of control(15.5%).15Screening of anacid-resistantSaccharomycesboulardii andoptimization of its highdensity cultivationconditionsLi Feilong,ChenXiaohua,Zheng Xin,XieYongzhen,Tan Zhilei,JiaShiruKey Laboratory ofIndustrial FermentationMicrobiology,Ministryof Education,College ofBiotechnology,TianjinUniversity of Science&Technology,TianjinChina brewingSaromycesboulardiiAcid-resistantThe results of stability of the mutant strainresistant to low pH showed that after20timegenemtion,mutant YB-3had good geneticstability and its survival rate was51.79%.In5Lfermentor,the high density cultvation of mutantYB-3were optimized.The dry cell weight ofS.boulardii obtained was58.79%,which wasincreased by55.52%than that of the originalstrain.8NO.Title Author Institution Journal Strain Target Product Result16Mutation screening ofKluyveromyces lactisusing ARTP andcatalysis characteristicsof the whole cell ofmutant strainCao Gang,LiuYun,GuoJinling,LvYucai,YuHuashun,Gong DachunLaboratory,China ThreeGorgesUniversity;2.Departmentof EnzymeProduction,Angel YeastCo.,LtdChinaBrewing,05,2018Kluyveromyceslactisketo-reducetaseThe conversion rate of mutant strain K15toacetophenone was up to91.8%,which was2.5time higher than that of the starting strain.Thestrain had a wide application prospect inbiocatalysis field.17Screening andBreeding of HighProducing Strain for[γ-HyMeLeu4]CyA byARTP-UV CompositeMutagenesisZHANG Li,DAI Meng,ZHENGGui-zhen,ZHAO Ying,LIU Jing,ZHANG Jia,WANGFu-qiangNew Drug R&DCo.Ltd.of N.ChinaPharm.Group(NCPG),Nat'lEngin.Res.Ctr.ofMicrob.Med.JOURNAL OFMICROBIOLOGY Feb,,2014,34(1):68-71NonomuraeadietziaecyclosporinderivativesThe results showed that a high yielding strainpossessing stable genetic performance wasobtained using the mutagenesis withtransformation efficiency increased by32%overthe starting strain.18Application of PlasmaTechnology in theMutation Breeding ofStreptomyces fradiaeand Pleurotus sp.WANG Zhong-zhong,ZHANG Ping,SHIYan-pengNingxiatairuiPharmaceuticalCo.,Ltd.Strains InstituteChineseVeterinary DrugJournaltreptomycesfradiae,Pleurotus sp.antibioticBesides,one strain of Streptomyces fradiae andthat had10.4%higher ability and one strainof Pleurotus sp.that had9.6%higher ability ofproducing antibiotic than original strains wereselected.9NO.Title Author Institution Journal Strain Target Product Result19Mutation ofStreptomycesaureofaciens with HighYieldDemethylchlortetracycline by Atmospheric andRoom TemperaturePlasmaLIN GuizhenYE RuifangCHENGLintongMAOQuanguiNational key laboratoryof bioreactor engineeringof east China universityof science andtechnology;Henan tianfangpharmaceutical co.LTD.Chinese Journal ofPharmaceuticalsStreptomycesaureofaciensdemethylchlortetracyclineproductivity.A mutant A6-6-9was screenedand showed high genetic stability after fourpared with the original strainunder the same fl ask fermentation process,themutant A6-6-9showed Faster aminonitrogenconsumption,slower growth rate and glucoseconsumption rate,and enhanced productivityof1with an increase of22.5%..20A Mutant strain withhigh Erythromucinyield obtained by usingnovel ARTP and UVMutationShenXiaojing,Zhang Ping,Shi YanpengNingxia TairuiPharmaceutical Co.LtdStrains Institute,YinchuanChineseveterinarymedicinemagazine.Saccharopolyspora erythraeaErythromycinAtmospheric and room temperatureplasma(ARTP)-UV composite mutagenesiswere adopted to treat witherythromycin-producing strain,fourhigh-efficient producing strains were obtainedby screening,which was increased infermentation potency by25.2%over the originalstrain.The mutant12#with genetic stability wasObtained.Its yield of erythromycin was10029U/mL,28.6%higher than the original strain.ARTP-UV composite Mutation was a simpleand efficient method for screening.10NO.Title Author Institution Journal Strain Target Product Result21Production ofOritavancinIntermediate A82846Bby Strain Improvementand MediumOptimizationZHENGLinghui,HONG Yun,CHENXiaojing,TENG Yun,BAI HuaZhejiang HisunPharmaceutical Co.,Ltd.,TaizhouChinese Journal ofPharmaceuticalsNocardiaorientalisOllie'sintermediateNocardia orientalis HS807-N-1287was treatedwith atmospheric and room temperature plasma(ARTP)mutation for production of oritavancinintermediate A82846B(1),and screened in theselective plates containing1and tyrosine.Amutant HS807-A-639with high productivity of1was obtained.22Rapid MutationBreedingSchizochytrium StrainsProducing High-yieldDocosahexenoic Acidby Atmospheric andRoom TemperaturePlasmasYuan JunZhao Ben SunMengyuWang WuYang HailinThe Key Laboratory ofIndustrial Biotechnologyof Ministry ofEducation,JiangnanUniversityBIOTECHNOLOGY BULLETINSchizochytriumsp.DHAThe DHA-yield of mutant D32increasedsignificantly up to7.31g/L,29.8%higher thanthose by the original pared with theoriginal strain,the main saturated fatty acid(C14∶0and C16∶0)by D32decreasedsignificantly,while the unsaturated fatty acidcontent increased significantly.23Breeding Strains withCompound Mutationfor High ConversionRate of HydrocortisoneXUEWei-ying,SHENYan-bing,HUANG Wei,WANG Min,DENGMing-qianLaboratory of IndustrialFermentationMicrobiology,Ministryof Education,TianjinKey Laboratory ofIndustrial Microbiology,College ofBiotechnology/TianjinUniversity of Science&Journal ofShandongAgriculturalUniversity(Natural ScienceEdition)AbsidiacoeruleaHydrocortisoneThe primary screening method was establishedon the basis of colony morphology.After6daysof culture,the colony color was darker,therewas cricoid markings on the back,HCconversion was rescreened in the bacterial strainwith dense mycelium,and the mutant AL-172with high genetic stability was obtained.Whenthe RSA concentration was3.5g/L,theconversion rate of HC reached72.52%.NO.Title Author Institution Journal Strain Target Product ResultTechnology Substrate feed concentration and HC conversionrate increased by40%and16.20%respectivelycompared with laboratory preservation strains.Ithas a good prospect of application.24Mutation by UsingAtmospheric Pressureand Room TemperaturePlasmas andHigh-throughputScreening Method forImproving VitaminB12ProductionCAI-Yingying,XIA-Miaomiao,DONG-Huina,ZHANGTongcun,ZHANG DaweiCollege ofBiotechnology,TianjinUniversity of Science&Technology;Tianjin Institute ofIndustrialBiotechnology,ChineseAcademy of SciencesJournal of TianjinUniversity ofScience&TechnologyPseudomonasdenitrificansVitamin B12Through four rounds of ARTP mutation andhigh-throughput screening,PA320-M4-1B1mutants(103.2±2.1)mg/L were obtained,whichincreased the contents of VB12by43.8%,compared with the control strainPA320(71.9±1.8)mg/L,when the strains werecultivated on a rotary shaker at30℃for6,d.25High throughputscreening of high-yieldcephalosporin Cproducing stain bymutagenesis usingatmospheric and roomtemperatureplasma(ARTP)Ying-ying,HangHai-feng,ZhuangYing-ping andChu JuState Key Laboratory ofBioreactor Engineering,East China University ofScience and TechnologyChinese journal ofantibioticsCephalosporium Acremoniumcephalosporin C,CPCThe high-yield mixture strain G6wassuccessfully screened out and the CPC titer wasnearly19.75%,higher than that of the parentalstrain W-6in the shake flask.Conclusion Thiswork provides an appropriate strategy forobtaining high CPC-yield strains by using ARTPrandom mutation methods with an efficientscreening assay.The ARTP random mutationmethod has a high positive mutation rate and theyield of cephalosporin C is significantlyincreased.NO.Title Author Institution Journal Strain Target Product Result26Screening of highechinocandin Bdeacylase-producingstrain and optimizationof biotransformationconditionsQie Li-ping,WangXiao-lian,MaJie,QianSi-yu,DaiMing-wei,Zhang WeiNCPC New DrugResearch andDevelopmentCo.,Ltd.,NationalEngineering ResearchCenter of MicrobialMedicine,HebeiIndustry MicrobialMetabolic Engineering&Technology ResearchCenterChinese Journal ofAntibioticsStreptomyces EchinocandinA stable mutant strain ZH-6-78was obtainedand its conversion efficiency was26.7%higherthan that of the original strain.The optimumconversion conditions were determined asfollows:the conversion temperature was35℃,and the conversion time was24h.27Breeding of high yieldStreptomyceslinconinensisproducing strainHuangWen-fu,Zhang Ping,Niu Chun andBi Guo-dongNingxiatairuiPhamaceutical Co LtdStrains InstituteChinese journal ofantibiotics.StreptomyceslinconinensislinconmycinThe results showed that the average yield oflincomycin was increased by30.0%via flasksfermentation and by21.4%via180-tonindustrial fermenters.Passage experimentsshowed that the mutant strain of high yieldperformance was genetically stable.。
