限薪令下的银行薪酬大数据: 部分员工降薪10% 高管收入打8
阴道镜、LEEP用于宫颈癌前病变诊断及治疗效果

636中国计划生育学杂志2024年3月第32卷第3期㊀C h i nJF a m P l a n n,V o l.32,N o.3,M a r c h2024临床分析 阴道镜、L E E P用于宫颈癌前病变诊断及治疗效果朱学英㊀聂静雅∗㊀商秀明㊀杨晓莉浙江省湖州市南浔区人民医院(313009)摘㊀要㊀目的:观察阴道镜㊁利普刀(L E E P)用于宫颈癌前病变诊断㊁治疗效果.方法:回顾性分析2016年10月-2021年6月本院收治的120例初诊为宫颈癌前病变患者临床资料,经宫颈液基细胞学检查(T C T)㊁人乳头瘤病毒(H P V)检查㊁阴道镜下宫颈组织活检异常,L E E P术后病理学明确诊断,统计阴道镜宫颈组织活检与L E E P术后病理符合情况,统计L E E P术手术及术后2周并发症㊁术后3个月复查转归情况.结果:120例L E E P术后病理检查,C I NⅠ62例(51.7%)㊁C I NⅡ36例(30.0%)㊁C I NⅢ22例(18.3%),阴道镜宫颈组织活检与L E E P术后病理结果符合率为75.0%.L E E P术时间12.60ʃ1.61m i n㊁术中出血量21.00ʃ3.07m l㊁住院时间3.66ʃ1.05d.L E E P术后2周并发症发生率为29.2%.术后3个月复查转阴率为94.2%.结论:宫颈癌前病变诊断与治疗中,阴道镜㊁L E E P术应用效果良好,安全性较高,能有效根治宫颈癌前病变.关键词㊀宫颈癌前病变;阴道镜;利普刀;治疗;转归E f f e c t o f t h e a p p l i c a t i o no f t h e c o l p o s c o p y a n d l o o p e l e c t r o s u r g i c a l e x c i s i o n p r o c e d u r e f o r d i a g n o s i n g a n d t r e a t i n g t h e p r eGc a n c e r o u s c e r v i c a l l e s i o n sZ HU X u e y i n g,N I EJ i n g y a,S HA N G X i u m i n g,Y A N G X i a o l iN a n x u nP e o p l e'sH o s p i t a l,H u z h o u,Z h e j i a n g P r o v i n c e,313009A b s t r a c t㊀O b j e c t i v e:T oo b s e r v e t h e e f f e c t o f t h e a p p l i c a t i o no f t h e c o l p o s c o p y a n d l o o p e l e c t r o s u r g i c a l e x c i s i o n p r o c eGd u r e(L E E P)f o rd i a g n o s i n g a n dt re a t i n g t h e p r e c a n c e r o u sc e r v i c a l l e s i o n sof p a t i e n t s.M e t h o d s:T h ec l i n i c a l d a t ao f120p a t i e n t sw i t ht h e p r e c a n c e r o u sc e r v i c a l l e s i o n sd i a g n o s e d i nt h e f i r s tv i s i t t oh o s p i t a lb e t w e e n O c t o b e r2016a n dJ u n e2021A w e r e a n a l y z e d r e t r o s p e c t i v e l y.T h e a b n o r m a l r e s u l t so f t h i n p r e p c y t o l o g y t e s t(T C T),h u m a n p a p i l l o m aGv i r u s(H P V)e x a m i n a t i o n a n d c e r v i c a l t i s s u e b i o p s y u n d e r c o l p o s c o p y o f t h e p a t i e n t sw e r e u s e d t o s c r e e n i n g t h e i r p r eGc a n c e r o u s c e r v i c a l l e s i o n s,a nd t he p a t i e n t sw e r ed ef i n i t ed i ag n o s i so f p r e c a n c e r o u s c e r v i c a l l e s i o n sb yp a th o l o g y a f t e rL E E Ps u r g e r y.T h e c o i n c i d e n c e s i t u a t i o nb e t w e e nt h ed e t e c t i o nr e s u l t so f t h e p r e c a n c e r o u sc e r v i c a l l e s i o n so f t h e p aGt i e n t sb y c o l p o s c o p i c c e r v i c a l t i s s u eb i o p s y a n d t h e i r p o s t o p e r a t i v e p a t h o l o g y o fL E E Pw e r e a n a l y z e ds t a t i s t i c a l l y.T h ec o m p l i c a t i o n sd u r i n g L E E Pa n dw i t h i n2we e k s af t e rL E E P,a n d t h e p r og n o s i sw i thi n3m o n t h s a f t e rL E E Po f t h e p aGt i e n t sw e r e c o u n t e d.R e s u l t s:I n t h e p a t i e n t sw i t h p r e c a n c e r o u s c e r v i c a l l e s i o n s a c c o r d i n g t o t h e p a t h o l o g i c r e s u l t s a f t e rL E E Ps u r g e r y,t h e r ew e r e62(51.7%)c a s e sw i t hc e r v i c a l i n t r a e p i t h e l i a l n e o p l a s i a(C I N)Ⅰ,36(30.0%)c a s e sw i t hC I NⅡa n d22(18.3%)c a s e sw i t hC I NⅢ.T h ec o i n c i d e n c e r a t eb e t w e e nt h ec o l p o s c o p y b i o p s y o f t h e p a t i e n t sa n dt h e i r p a t h o l o g i c r e s u l t s a f t e r L E E P s u r g e r y w a s75.0%.T h e d u r a t i o n o f L E E P s u r g e r y o f t h e p a t i e n t sw a s12.60ʃ1.61m i n,t h e i n t r a o p e r a t i v eb l o o d l o s sw a s21.00ʃ3.07m l,a n d t h eh o s p i t a l s t a y o f t h e p a t i e n t sw a s3.66ʃ1.05d a y s.T h ei n c i d e n c e o f c o m p l i c a t i o n s o f t h e p a t i e n t sw i t h i n2w e e k s a f t e rL E E Ps u r g e r y w a s29.2%.T h e n e g a t i v e c o n v e r s i o n r a t eo f t h e p r e c a n c e r o u s c e r v i c a l l e s i o n s o f t h e p a t i e n t sw i t h i n3m o n t h s a f t e rL E E Ps u r g e r y w a s94.2%.C o n c l u s i o n:I n t h ed i a g n o s i s a n d t re a t m e n t of t h e p a t i e n t sw i t h p r e c a n c e r o u s c e r v i c a l l e s i o n s,t h e c o m b i n e da p p l i c a t i o no f c o l p o s c o p y a n dL E E Ps u r g e r y f o r t h e p a t i e n t s h a s g o o d e f f e c t a n d h i g h s a f e t y,a n dw h i c h c a n c u r e t h e p a t i e n t sw i t h p r e c a n c e r o u s c e r v iGc a l l e s i o n s.K e y w o r d s㊀P r e c a n c e r o u s c e r v i c a l l e s i o n s;C o l p o s c o p y;L o o p e l e c t r o s u r g i c a l e x c i s i o n p r o c e d u r e;T r e a t m e n t;P r o g n o s i sD O I:10.3969/j.i s s n.1004 8189.2024.03.031收稿日期:2023 12 25㊀修回日期:2024 01 02∗通信作者:8505603@q q.c o m㊀㊀宫颈癌前病变是妇科常见疾病[1].对有性生活的女性,需定期做宫颈液基细胞学检查(T C T)和人乳头瘤病毒(H P V)检查,进行宫颈防癌筛查,若存在异常临床多采用阴道镜进一步检查,准确的病理诊断及分级对临床治疗及处理有重要意义[2].阴道镜能准确提供宫颈视图,观察宫颈癌前病变情况,常联合宫颈组织活检来提高诊断准确率,但检测结果仍未达到预期[3].利普刀(L E E P)作为微创性诊断和治疗宫颈疾病的专业技术,利于宫颈疾病病理检查和治疗[4].对此,本研究对阴道镜㊁L E E P在宫颈癌前病变诊断与治疗价值进行探讨.1资料与方法1.1一般资料回顾性分析2016年10月-2021年6月本院收治的初诊为宫颈癌前病变患者120例临床资料.纳入标准:阴道细胞异生,高度怀疑宫颈癌前病变;接受宫颈T C T㊁H P V检查;临床资料完整.排除标准:哺乳期或妊娠期;患严重免疫抑制性疾病;凝血功能异常;病菌感染.1.2检查方法宫颈常规采样,使用A p t i m a H P V E6/EG7m R N A技术进行H P V的基因检测和基因分型,检查14种高危险型H P V,其中16型㊁18/45型单独检查阴阳性,16型单独报告,18/45型一起报告,余11型H P V综合报告.余做T C T,病理科医生读片.阴道镜检查观察患者宫颈是否光滑㊁有无肿块㊁出血㊁糜烂㊁囊肿等情况,放大倍镜,观察宫颈血管㊁白斑等较微小部位,同时涂抹醋酸和碘酒,确定可疑病变区.阴道镜下取异常区组织活检,术后常规止血及抗感染治疗.L E E P术后病理检查:采用L E E P刀,经期后3~7d进行手术,选择局部浸润麻醉或静脉麻醉,根据宫颈癌前病变深度㊁范围选择合适的切割深度,以超过病变边缘3mm为宜,总深度ȡ10mm,将切除组织送病理检查,电凝针止血.1.3观察指标①统计阴道镜宫颈组织活检与L E E P术后病理对宫颈癌前病变的检测结果,T C T检查结果包括未见上皮内病变和恶性细胞(N I L M)㊁不能明确意义的非典型鳞状细胞(A S C U S)㊁低度鳞状上皮内病变(L I S L)和高度鳞状上皮内病变(H I S L),其中L I S L㊁H I S L提示T C T结果异常行阴道镜检查.当H P V检查结果为H P V16㊁H P V18㊁H P V45阳性时,患者需行阴道镜检查,而当H P V检查结果为除H P V16㊁18㊁45外其他阳性以及T C T检查结果为N I L M或A S C U S时,可根据患者H P V感染持续时间及年龄等选择择期复查或阴道镜检查.阴道镜宫颈组织活检㊁L E E P术后病理学结果包括宫颈鳞状上皮内病变Ⅰ级(C I NⅠ)㊁宫颈鳞状上皮内病变Ⅱ级(C I NⅡ)㊁宫颈鳞状上皮内病变Ⅲ级(C I NⅢ),其中C I NⅢ是指高级别宫颈上皮内瘤变,包括原位癌.②统计L E E P术手术基本情况.③统计L E E P术患者术后2周并发症发生率以及术后3个月复查转阴率,术后并发症包括阴道出血㊁阴道感染等,术后3个月复查转阴率即为细胞学检查为阴性或阴道镜下组织活检为阴性,且无C I N病变残留.1.4数据分析使用软件S P S S20.0进行数据分析.计量资料使用( xʃs)表示,采用t检验;计数资料使用(%)表示,采用χ2检验.P<0.05表示有显著差异.2结果2.1不同检测方式结果纳入患者年龄(45.0ʃ8.6)岁(24~74岁),有分娩史101例㊁无分娩史19例.L E E P术后病理学检查,C I NⅠ62例(51.7%)㊁C I NⅡ36例(30.0%)㊁C I NⅢ22例(18.3%),与L E E P术后病理结果比较,阴道镜宫颈组织活检准确率为75.0%.见表1.表1㊀阴道镜检查结果与L E E P术后病理结果对比(例)阴道镜宫颈活检合计L E E P术后组织病理学检查C I NⅠ㊀㊀C I NⅡ㊀㊀C I NⅢC I NⅠ6241201C I NⅡ363303C I NⅢ220319合计1204453232.2围术期基本情况L E E P术手术时间12.60ʃ1.61m i n,术中出血量21.00ʃ3.07m l,住院时间3.66ʃ1.05d.2.3并发症及复查转阴率进行L E E P患者术后2周出现阴道出血23例(19.2%)㊁阴道感染12例(10.0%),并发症发生率29.2%.术后3个月仍有7例检测出阳性,采用其他治疗手段加以根治,复查转阴率为94.2%.736中国计划生育学杂志2024年3月第32卷第3期㊀C h i n JF a m P l a n n,V o l.32,N o.3,M a r c h20243讨论宫颈癌前病变一般轻度病变患者能自然好转,但未好转患者以及中重度病变患者会由于病情持续发展,导致患癌率增加[5].因此,早期诊断与治疗对宫颈癌前病变患者具有重要意义.阴道镜检查通过局部组织形态改变所形成的异常图像,明确病变范围[6],阴道镜检查可将病灶放大10~40倍,借以观察肉眼看不到较微小病变,协助临床及早发现癌前病变[7].然而阴道镜检查虽能清楚显示宫颈血管状态,对病情评估有利,但辨识度较低,极易发生误诊与漏诊[8].L E E P术切除病理组织,具有不影响切口边缘组织活检和可连续切除的优点,广泛用于宫颈疾病检查中,能有效降低宫颈癌的误诊率和漏诊率[9 10].本文中与L E E P术后病理结果比较,阴道镜宫颈组织活检符合为75.0%,其原因可能是阴道镜宫颈组织活检检查中,取材较局限且表浅,取出的细胞病变组织较少,或没有取到宫颈癌前病变最严重部位组织,从而造成误诊与漏诊[11].然而由于电环切刀面积受限,不可能切除过多宫颈组织,L E E P术一般适用于宫颈癌前病变面积较小的患者[12].针对C I NⅢ患者一般不建议患者做L E E P术切除[13].本次研究中,L E E P术手术时间㊁术中出血量㊁住院时间均较少,说明L E E P术具有操作便捷㊁安全㊁创伤小㊁手术时间短等优势,并可确保治疗后宫颈能恢复至以前的光滑程度和柔韧程度,还能有效免除常规治疗切除子宫对患者造成的身心伤害[14].实施L E E P治疗使病变组织细胞变形与彻底坏死,不需手术缝合,节省手术时间,对周围组织损伤较小,局部血液仍能处于流通状态,能有效促进伤口愈合,对患者术后恢复有利[15].本研究中行L E E P患者术后2周并发症发生率为29.2%,与喻华英等[16]研究结果相类似.本研究术后3个月复查转阴率为94.2%,说明阴道镜下L E E P术具有较高疗效,能有效治愈宫颈癌前病变[17],部分病变患者出现复发情况需采用其他治疗手段根治[18].综上所述,阴道镜㊁L E E P术用于宫颈癌前病变诊断㊁治疗安全有效,L E E P术手术治疗创伤小㊁出血量少,术后并发症低,安全性较高,复发率较低.参考文献[1]㊀陈静,陈煜岊,弋文娟.阴道镜子宫颈活组织检查对子宫颈癌前病变诊断的准确性及子宫颈癌漏诊影响因素分析[J].肿瘤研究与临床,2020,32(8):579 583.[2]㊀孙蓉,杨鹰.分析电子阴道镜对宫颈疾病的诊断价值[J].实用心脑肺血管病杂志,2020,28(S1):81 83.[3]㊀赵晓,张伟珍,王泽华,等.阴道镜下宫颈活检诊断宫颈上皮内瘤变的准确性及其漏诊宫颈癌的相关因素研究[J].中国妇幼保健,2020,35(10):1937 1940.[4]㊀王春宝,雷婷,南鹏飞,等.一种子宫颈锥切(L E E P)标本病理取材新方法[J].临床与实验病理学杂志,2019,35(6):738 739.[5]㊀于淑莉.阴道镜活检与L E E P术在诊治宫颈病变中的价值[J].河北医学,2017,23(10):1705 1708.[6]㊀张玲,叶梅青.L E E P在治疗宫颈良性疾病中的应用[J].皖南医学院学报,2018,37(4):350 352.[7]㊀朱俊美,靳东芳,白小英.不同方法诊断宫颈病变的价值探讨[J].现代中西医结合杂志,2015,24(2):163 165.[8]㊀包家梅.宫颈环形电切术治疗宫颈疾病的临床分析[J].检验医学与临床,2012,9(22):2857 2858.[9]㊀初慧君,孙业武,焦今文,等.L E E P对阴道镜检查不充分患者的诊断价值[J].现代妇产科进展,2018,27(11):848 850.[10]㊀林红娣,余幼芬,沈军英,等.L E E P治疗宫颈病变的护理配合和疗效观察[J].护士进修杂志,2017,32(19):1812 1813.[11]㊀许艳茹,潘静,段莉华.宫颈L E E P环形电切治疗宫颈高级别癌前病变效果分析[J].中国肿瘤临床与康复,2017,24(8):932 934.[12]㊀康红.L E E P治疗宫颈癌前病变的临床疗效研究及追踪情况[J].中国妇幼保健,2016,31(15):3025 3027.[13]㊀汪红娟,张静.阴道镜㊁活检及宫颈管诊刮对宫颈病变妇女的诊断效果分析[J].贵州医药,2020,44(4):635 636.[14]㊀黄琼.L E E P术后病理检查与阴道镜宫颈活检对宫颈癌前病变的诊断准确性及宫颈癌漏诊相关因素分析[J].中国妇幼保健,2020,35(5):960 963.[15]㊀王艳,张泽莉,赵德华,等.阴道镜下L E E P治疗宫颈病变837例临床分析[J].中华全科医学,2011,9(12):1875 1876.[16]㊀喻华英,张宝霞.阴道镜联合利普(L E E P)刀治疗宫颈癌变的可行性及安全性分析[J].实用癌症杂志,2018,33(3):503506.[17]㊀刘鸣.阴道镜下宫颈活检联合宫颈环形电切术在宫颈病变诊治中的应用价值[J].中国医药导报,2019,16(8):102 105.[18]㊀陈丽梅,刘莉,陶祥,等.1005例子宫颈H S I L患者行L E E P术后24个月内的复发及其影响因素分析[J].中华妇产科杂志,2019,54(8):534 540.[责任编辑:董㊀琳]836中国计划生育学杂志2024年3月第32卷第3期㊀C h i nJF a m P l a n n,V o l.32,N o.3,M a r c h2024。
CSNP1GCR01-BOW 1Gb 嵌入式存储设备说明书

CSNP1GCR01-BOWVersion:V1.1JAN20, 2021Revision HistoryVersion Date DescriptionV1.0 16/03/2020 Origin DraftV1.1 20/01/2021 Updated operating temperatureContentsREVISION HISTORY (I)1. INTRODUCTION (1)1.1O VERVIEW (1)1.2F EATURES (1)1.3B LOCK D IAGRAM (1)2. PRODUCT SPECIFICATIONS (2)2.1P IN A SSIGNMENTS (T OP V IEW) (2)2.2P ACKAGE D IMENSIONS (2)3. PERFORMANCE (4)4. DC CHARACTERISTICS (5)5. AC CHARACTERISTICS (6)5.1B US T IMING (D EFAULT M ODE) (6)5.2B US T IMING (H IGH-SPEED M ODE) (6)6. REFERENCE DESIGN (8)1. Introduction1.1 OverviewCSNP1GCR01-BOW is an 1Gb density of embedded storage based on NAND Flash and SD controller. This product has many advantages comparing to raw NAND, it has embedded bad block management, and stronger embedded ECC.CSNP1GCR01-BOW is LGA-8 package. The size is 8mm x 6mm x0.75mm.1.2 Featuresl Interface: Standard SD Specification Version 2.0 with 1-I/O and 4-I/O.l Power supply: Vcc = 2.7V - 3.6Vl Default mode: Variable clock rate 0 - 25 MHz, up to 12.5 MB/sec interface speed (using 4 parallel data lines)l High-Speed mode: Variable clock rate 0 - 50 MHz, up to 25 MB/sec interface speed (using 4 parallel data lines)l Operating Temperature: -30°C to +85°Cl Storage Temperature: -40°C to +85°Cl Standby Current:< 200uA1.3 Block Diagram2. Product Specifications2.1 Pin Assignments (Top View)PIN# SD MODE SPI MODE NAME TYPE1DESCRIPTION NAME TYPE DESCRIPTION1 SDD2 I/O/PP Data Line [Bit2] RSV Reserved2 CD/SDD32I/O/PP3SDNAND Detect/CS I3Chip Select (Neg True)Data Line [Bit3]3 SCLK I Clock SCLK I Clock4 VSS S Supply V oltage Ground VSS S Supply V oltage Ground5 CMD PP Command/Response DI I Data In6 SDD0 I/O/PP Data Line [Bit0] DO O/PP Data Out7 SDD1 I/O/PP Data Line [Bit1] RSV Reserved8 VCC S Supply V oltage VCC S Supply V oltage1) S: power supply; I: input; O: output using push-pull drivers; PP: I/O using push-pull drivers;2) The extended SDD lines (SDD1-SDD3) are input on power up. They start to operate as SDD lines afterSET_BUS_WIDTH command. The Host shall keep its own SDD1-SDD3 lines in input mode, as well,while they are not used. It is defined so, in order to keep compatibility to SDNAND.2.2 Package DimensionsTOP VIEWSIDE VIEWCommon DimensionsSymbol Min Nom Max NoteA 0.65 0.75 0.85B 1.17 1.27 1.37C 6.90 7 7.10D 7.90 8 8.10E 5.90 6 6.10F 10.90 11 11.1H 0.75 0.85 0.95SDNAND Package Dimensions (unit: mm)3. PerformanceParameter RangeWork Model -30°~ 85℃TemperatureStorage Model - 40°~ 85℃Work Model 8% to 95%, Non-condensing HumidityStorage Model 8% to 95%, Non-condensing4. DC CharacteristicsSymbol PARAMETER CONDITIONS MIN TYP MAX UNITS V IL Input low voltage VSS-0.3 0.25VCC V V IH Input high voltage 0.625VCC VCC+0.3 VV OL Output low voltage IOL=100μA@VCC_min0.125VCC VV OH Output high voltage IOH=100μA@VCC_min0.75VCC VI IN Input leakage current VIN=VCC or 0 -10 +/-1 10 μAI OUT Tri-state output leakagecurrent-10 +/-1 10 μAI STBY Standby current 3.3V@clockstop150 200 μAI OP Operation current 3.3v@50MHz(Write)15 25 mA 3.3v@50MHz(Read)15 25 mA5. AC Characteristics5.1 Bus Timing (Default Mode)SYMBOL PARAMETER MIN MAX UNIT NOTEF SD SD clock frequency 0 25 MHzT WL Clock low time 10 nsT WH Clock high time 10 nsT TLH Clock rise time 10 nsT THL Clock fall time 10 nsT ISU Input setup time 5 nsT IH Input hold time 5 nsT ODLY Output delay time 0 14 ns5.2 Bus Timing (High-speed Mode)SYMBOL PARAMETER MIN MAX UNIT NOTEF SD SD clock frequency 0 25 MHzT WL Clock low time 10 nsT WH Clock high time 10 nsT TLH Clock rise time 10 nsT THL Clock fall time 10 nsT ISU Input setup time 5 nsT IH Input hold time 5 nsT ODLY Output delay time 0 14 nsT OH Output hold time 2.5 nsCSNP1GCR01-BOW8 6. Reference DesignNote :R DAT and R CMD (10K~100 kΩ) are pull-up resistors protecting the CMD and the DAT lines against bus floating when SDNAND is in a high-impedance mode.The host shall pull-up all DAT0-3 lines by R DAT , even if the host uses the SDNAND as 1-bit mode only in SD mode. It is recommended to have 2.2uF capacitance on VCC.R CLKreference 0~120 Ω.。
通信原理第4章(2014年北邮上课精简版)
η AM
边带功率 = AM总功率
调制指数a(调幅系数)
AM 信号表达式
S AM (t ) = [1 + m (t ) ] Ac cos ωc t
其中 1 + m(t ) 中的直流为 1,交流为 m(t ) 。为了包络解调 不失真恢复原始基带信号,要求 m ( t ) ≤ 1 。 AM 信号一般表示为 S AM (t ) = Ac 1+ amn (t ) cos ωc t ,
第4章 模拟调制系统
本章的主要内容
一、调制的目的、定义和分类 二、幅度调制(AM、DSB、SSB、VSB)
n n n
时域和频域表示、带宽 调制与解调方法
抗噪声性能 三、角度调制(FM、PM)
n n n n
基本概念 单频调制时:调频和调相信号的时域表示 宽带调频信号的带宽
抗噪性能 四、频分复用
《通信原理》
解:
(2) 基带信号为随机信号时已调信号的频谱特性 在一般情况下,基带信号是随机信号,如语音信号。此时
,已调信号的频谱特性用功率谱密度来表示。 AM已调信号是一个循环平稳的随机过程,其功率谱密度为 其自相关函数时间平均值的傅里叶变换。 分析可知,在调制信号为确知信号和随机信号两种情况下, 分别求出的已调信号功率表达式是相似的。 参见教材70页。
H(w)
-w c
形成单边带信号的滤波特性
H(w) 1 -w c 0 1 0 wc w wc w
H(w)
-w c
形成单边带信号的滤波特性
通过推导(参见教材 71-72 页),可得 SSB 信号的时域表达式
S SSB (t) = Ac m(t ) cos ωct m Ac m (t )sin ωct
参考文献标引格式的要求

z u r e ,2019,71:214G218.[28]M u c h a M ,O o iL ,L i n l e y J E ,e ta l .T r a n s c r i pt i o n a l c o n t r o lo f K C N Qc h a n n e l g e n e s a n d t h e r e gu l a t i o n o f n e u r o n a l e x c i t a b i l Gi t y[J ].JN e u r o s c i ,2010,30(40):13235G13245.[29]M i c e l i F ,S o l d o v i e r iMV ,A m b r o s i n oP ,e t a l .G e n o t y p e Gph e n o Gt y p e c o r r e l a t i o n s i n n e o n a t a l e p i l e p s i e s c a u s e d b y mu t a t i o n s i n t h ev o l t a g es e n s o ro fK (v )7 2p o t a s s i u m c h a n n e ls u b u n i t s [J ].P r o cN a t lA c a dS c iUSA ,2013,110(11):4386G4391.[30]Y uF ,X u J ,X i a oZ ,e t a l .E n d o pl a s m i c r e t i c u l u mr e t e n t i o no f K C N Q 2p o t a s s i u mc h a n n e lm u t a n t s f o l l o w i n g t e m p e r a t u r e e l Ge v a t i o n [J ].B i o m e d M a t e rE n g ,2017,28(s 1):S 243G253.[31]L iX ,G u oS ,L i uK ,e t a l .G A B R G 2d e l e t i o n l i n k e d t o g e n e t i ce p i l e p s y w i t hf e b r i l es e i z u r e s p l u sa f f e c t st h ee x p r e s s i o no f G A B A A r e c e pt o rs u b u n i t sa n do t h e r g e n e sa td i f f e r e n t t e m Gpe r a t u r e s [J ].N e u r o s c i e n c e ,2020,438:116G136.[32]H e r n a n d e z C C ,S h e nY ,H uN ,e t a l G A B R G 2V a r i a n t sA s s o c i a t e d w i t hF e b r i l e S e i z u r e s [J ].B i o m o l e c u l e s ,2023,13(3):414.[33]L iX ,G u oS ,X uS ,e t a l .N e o c o r t e x Ga n dh i p p o c a m p u s Gs pe c if i c d e l e t i o no fG a b rg 2c a u s e s t e m p e r a t u r e Gd e p e n d e n ts e i z u r e s i n m i c e [J ].C e l lD e a t hD i s ,2021,12(6):553.[34]S k o t t eL ,F a d i s t a J ,B y b j e r gGG r a u h o l mJ ,e t a l .G e n o m e Gw i d e a s s o Gc i a t i o n s t u d y o f f e b r i l e s e i z u r e s i m p l i c a t e s f e v e r r e s p o n s e a n d n e u Gr o n a l e x c i t a b i l i t y ge n e s [J ].B r a i n ,2022,145(2):555G568.[35]C h o i J ,C h o i S A ,K i mS Y ,e t a l .A s s o c i a t i o na n a l ys i so f i n t e r Gl e u k i n G1β,i n t e r l e u k i n G6,a n dh m g b 1v a r i a n t sw i t h p o s t i c t a l s e Gr u mc y t o k i n e l e v e l s i n c h i l d r e nw i t h f e b r i l e s e i z u r e a n d g e n e r Ga l i z e de p i l e p s y wi t hf e b r i l es e i z u r e p l u s [J ].JC l i n N e u r o l ,2019,15(4):555G563.[36]S h a h r o k h i A ,Z a r e GS h a h a b a d i A ,N a e i m i P o o rM ,e t a l .A s s o c i Ga t i o no f t h e s i n g l e n u c l e o t i d e p o l y m o r ph i s m s o f t h e g e n e s e n Gc o d i n g I L G2a n dI F N Gγw i t hf e b r i l es e i z u r e [J ].A c t a M e dI Gr a n ,2017,55(6):354G359.[37]M a n i u I ,C o s t e aR ,M a n i u G ,e ta l .I n f l a m m a t o r y bi o m a r k e r s i n f e b r i l e s e i z u r e :a c o m p r e h e n s i v e b i b l i o m e t r i c ,r e v i e wa n d v i Gs u a l i z a t i o na n a l ys i s [J ].B r a i nS c i ,2021,11(8):1077.[38]张玉松,房健,孙祺,等.热性惊厥大鼠海马I L G6表达及丙戊酸对其甲基化水平的干预研究[J ].中华行为医学与脑科学杂志,2018,27(7):598G603.k i n G6g e n e p o l y m o r ph i s m s a n d f e b r i l e s e i z u r e r i s k :A m e t a Ga Gn a l ys i s [J ].M e d i c i n e (B a l t i m o r e ),2019,98(39):e 17167.[40]余炜杰,陈俊国,张旭,等.新疆维吾尔族和汉族儿童热性惊厥与I L G6基因多态性的相关性[J ].中国妇幼保健,2021,36(3):572G575.[41]陶哲,申延丰,于毅,等.白细胞介素G6基因多态性与热性惊厥相关性研究[J ].中国小儿急救医学,2017,24(6):471G473[42]K u m a rK J ,K u r v a r iG ,K u m a rH C K ,e ta l .Ac o m pa r a t i v ea Gn a l y s i s o fs e r u mi n t e r l e u k i n G6l e v e l s i nc h i l d r e n w i t hf eb r i l e s e i z u r e s a n d f e b r i l ec o n t r o l s [J ].JN e u r o s c i R u r a l P r a c t ,2022,13(2):336G338.[43]Y a n g L ,Y o uC ,Q i u S ,e t a l .N o v e l a n d d e n o v o p o i n t a n d l a r ge m i c r o d e l e t i o nm u t a t i o ni nP R R T 2Gr e l a t e de p i l e p s y [J ].B r a i n B e h a v ,2020,10(5):e 01597.[44]杨小玲,张月华,许小菁,等.良性家族性婴儿癫痫家系临床表型和P R R T 2基因突变分析[J ].中华儿科杂志,2014,52(11):806G811.[45]冼慧文,高平明.儿童热敏感相关癫痫25例临床特征及分子遗传学分析[J ].中国临床医生杂志,2022,50(4):487G492.[46]M i s h i m aT ,F u j i w a r aT ,K o f u j iT ,e t a l .S y n t a x i n1Br e gu l a t e s s y n a p t i c G A B A r e l e a s ea n de x t r a c e l l u l a r G A B A c o n c e n t r a Gt i o n ,a n di sa s s o c i a t e d w i t ht e m p e r a t u r e Gd e p e n d e n ts e i z u r e s [J ].JN e u r o c h e m ,2021,156(5):604G613.[47]S c h u b e r tJ ,S i e k i e r s k a A ,L a n gl o i s M ,e ta l .M u t a t i o n si n S T X 1B ,e n c o d i n g a p r e s y n a pt i c p r o t e i n ,c a u s e f e v e r Ga s s o c i a t e d e p i l e p s y s y n d r o m e s [J ].N a tG e n e t ,2014,46(12):1327G1332 [48]田杨,侯池,王秀英,等.S T X 1B 基因突变致遗传性癫痫伴热性惊厥附加症一家系分析并文献复习[J ].中华儿科杂志,2019,57(3):206G210[49]P a v l i d o uE ,H a ge l C ,P a n t e l i a d i s C .F e b r i l e s e i z u r e s :r e c e n t d e Gv e l o p m e n t s a n du n a n s w e r e d q u e s t i o n s [J ].C h i l d sN e r vS ys t ,2013,29(11):2011G2017[50]S a r t o r i S ,N o s a d i n iM ,T e s s a r i nG ,e t a l .F i r s t Ge v e r c o n v u l s i v es e i z u r e s i n c h i l d r e n p r e s e n t i n g t o t h e e m e r g e n c y d e pa r t m e n t :r i s k f a c t o r sf o rs e i z u r er e c u r r e n c ea n dd i a g n o s i so fe p i l e p s y [J ].D e vM e dC h i l dN e u r o l ,2019,61(1):82G90.(收稿日期:20230323)㊀㊀㊀㊀本刊文献著录根据G B 7714G2005«文后参考文献著录规则»采用顺序编码制著录.依照其在文中出现的先后顺序用阿拉伯数字加方括号标出.正文中指明原始文献作者姓名时,角码应标注在作者姓名之右上角;正文中未指明作者或非原始文献作者时,角码应标注在句末末字的右上角;正文直接叙述其文献序号时不在右上角标注(如:操作按文献[1]所示).参考文献表按引用先后顺序用阿拉伯数字加角码标出排列于文末,并在题名或书名后,分别加用[J ]或[M ].参考文献表中的作者姓名:1~3名全部列出,3名以上只列前3名,后面加 等 或其他与之相应的文字,如e ta l.外文期刊名称用缩写,以«I n d e x M e d i c u s »中的格式为准;中文期刊用全名.每条参考文献均需著录起止页.参考文献与其全文必须核对无误.本刊编辑部813 中国中西医结合儿科学2023年8月第15卷第4期㊀C h i nP e d i a t r I n t e g rT r a d i tW e s tM e d ,A u g 2023,V o l 15,N o .4Copyright ©博看网. All Rights Reserved.。
酚类物质的研究进展

第41卷第6期2023年12月沈阳师范大学学报(自然科学版)J o u r n a l o f S h e n y a n g N o r m a lU n i v e r s i t y(N a t u r a l S c i e n c eE d i t i o n)V o l.41N o.6D e c.2023文章编号:16735862(2023)06054808酚类物质的研究进展姜忠丽,杜昭换,赵秀红,涂向辉,毛鹏(沈阳师范大学粮食学院,沈阳110034)摘要:酚类物质是广泛存在于植物组织中的一类植物化学物质,对植物的生长发育具有重要作用,其与人类健康也密切相关,如其具有抗感染㊁抗病毒㊁抗细菌㊁抗过敏㊁抗出血和增强免疫力等功效㊂目前,酚类物质主要应用于食品㊁医药㊁化工㊁畜牧养殖等多个领域,利用纳米㊁微胶囊等可提高其生物利用度,从而产生较好的生物学效应㊂随着生物学理论与技术的快速发展,近年来天然来源酚类物质的开发及其在食品中的应用成为研究热点,研究主要集中在多酚的提取㊁分离纯化㊁结构鉴定及生物活性等方面㊂在文献分析的基础上,对酚类物质的提取㊁来源及生理功能进行了综述,并对其未来的研究方向及难点问题进行了展望,以期为食品工业中酚类物质的开发和利用提供借鉴㊂关键词:酚类物质;来源;生理功能;提取方法;研究进展中图分类号:T S213.21文献标志码:Ad o i:10.3969/j.i s s n.16735862.2023.06.011R e s e a r c h p r o g r e s s o f p h e n o l i c s c o m p o u n d sJ I A N GZ h o n g l i,D UZ h a o h u a n,Z HA OX i u h o n g,T UX i a n g h u i,MA OP e n g(C o l l e g e o fG r a i nS c i e n c e a n dT e c h n o l o g y,S h e n y a n g N o r m a lU n i v e r s i t y,S h e n y a n g110034,C h i n a)A b s t r a c t:P h e n o l sa r eac l a s so f p h y t o c h e m i c a l s w h i c h w i d e l yp r e s e n t i n p l a n t t i s s u ea n d p l a yi m p o r t a n t r o l e s i n p l a n t g r o w t ha n dd e v e l o p m e n t.T h e y a r ea l s oc l o s e l y r e l a t e dt oh u m a nh e a l t h,s u c ha s a n t i-i n f e c t i o n,a n t i-v i r u s,a n t i-b a c t e r i a,a n t i-a l l e r g y,a n t i-b l e e d i n g a n de n h a n c e i mm u n i t y.A t p r e s e n t,p h e n o l i cc o m p o u n d sa r e m a i n l y u s e di nf o o d,m e d i c i n e,c h e m i c a l i n d u s t r y,a n i m a lh u s b a n d r y a n d o t h e rf i e l d s.T h e u s e o f n a n o p a r t i c l e s a n d m i c r o c a p s u l e s c a n i m p r o v e t h e i rb i o a v a i l a b i l i t y a n d p r o d uc e b e t t e r b i o l o g i c a le f f e c t s.W i t ht h er a p id de v e l o p m e n to fb i o l o g i c a lt h e o r i e sa n dt e c h n o l o g i e s,t h e d e v e l o p m e n to fn a t u r a l l y-d e r i v e d p h e n o l i cc o m p o u n d sa n dt h e i ra p p l i c a t i o n s i nf o o dh a v eb ec o m ear e s e a r c hh o t s p o t i nr e c e n t y e a r s.I t m a i n l y f o c u s i n g o nt h ee x t r a c t i o n,s e p a r a t i o n a n d p u r if i c a t i o n,s t r u c t u r e i d e n t i f i c a t i o n a n d b i o l og i c a l a c t i v i t y o fp o l y p h e n o l s.O n t h e b a s i s o f l i t e r a t u r e a n a l y s i s,t h e e x t r a c t i o n,s o u r c e a n d p h y s i o l o g i c a l f u n c t i o n o fp h e n o l i c c o m p o u n d sh a v eb e e nr e v i e w e d,a n dt h e f u t u r e r e s e a r c hd i r e c t i o n sa n dd i f f i c u l t p r o b l e m sh a v eb e e n p r o s p e c t e d,i n o r d e r t o p r o v i d e r e f e r e n c e f o r t h e d e v e l o p m e n t a n du t i l i z a t i o n o f p h e n o l s i nt h e f o o d i n d u s t r y.K e y w o r d s:p h e n o l i cc o m p o u n d s;s o u r c e;p h y s i o l o g i c a l f u n c t i o n;e x t r a c t i o n m e t h o d s;r e s e a r c hp r o g r e s s酚类(p h e n o l i c s)是指芳香烃苯环上一个-H被-O H取代后生成的含有酚羟基的一大类化合物,是植物的主要次生代谢物之一㊂根据酚羟基的数目,酚类化合物可划分为一元酚和多元酚㊂多元酚又收稿日期:20230322基金项目:辽宁省教育厅基本科研项目(J Y T M S2*******)㊂作者简介:姜忠丽(1967 ),女,辽宁盖州人,沈阳师范大学教授㊂称多酚,被称为 第七类营养素 [1],主要包括黄酮类㊁单宁类㊁酚酸类及花色苷类等㊂近20年来,在食品营养学和预防医学方面,大量研究证明,多酚类物质可以多方面促进人体健康[2]㊂1 酚类物质的种类酚类物质根据其结构特点,可分为类黄酮(b i o f l a v o n o i d s)和非类黄酮类化合物㊂类黄酮主要是指黄酮类化合物[3],泛指2个苯环(A 环与B 环)通过三碳链相互连接而形成的一系列化合物;而非类黄酮类即酚酸类,其往往具有一个苯环,多为对羟基苯甲酸和肉桂酸的衍生物[4]㊂其结构通式分别如图1㊁图2所示㊂图1 黄酮类化合物结构图F i g .1 S t r u c t u r eo f f l a v o n o i d s 图2 酚酸类化合物结构图F i g .2 S t r u c t u r eo f ph e n o l i ca c i d s 酚类物质通常并不以简单的形式存在,它们往往会与其他物质相结合[5],如:原花青素类常与木质素类物质结合而形成聚合物;黄酮苷在植物体中常以糖苷的形式与不同的糖结合而存在;酚酸也是以酯合或糖苷化的形式存在于植物体内㊂由此就形成了酚类物质在植物体的3种存在形式,即游离态㊁结合态和酯化态㊂游离态㊁酯化态的酚类物质通常是可溶的,能溶于水和极性溶剂;而结合态的酚类物质多不溶于水,常存在于共价结合体中㊂其中,游离态多酚在水果中的含量比结合态多酚高,特别是某些颜色较深或酸涩味较重的水果,其游离多酚含量占总多酚的90%以上[6]㊂而在粮谷类中,尤其是在玉米和小麦制品中,其结合态多酚含量大多比游离态多酚多得多[7]㊂目前,对酚类物质存在形式的研究多集中在游离酚类化合物的组成和生物学功能上,而对结合态酚类化合物的组成及生物学功能方面研究得较少㊂2 酚类物质的研究现状2.1 酚类物质的来源酚类化合物广泛存在于各种高等植物器官中[8],如蔬菜㊁水果㊁香辛料㊁谷物㊁豆类和果仁等,且多分布于植物的外皮即在接受阳光照射的部分㊂酚类物质最早被发现于茶叶中,约占茶叶干重的20%~30%,其决定了茶叶的色㊁香㊁味及功效㊂茶多酚是茶叶中多酚类物质的总称,按其主要化学成分可分为儿茶素类㊁黄酮类㊁花青素类㊁酚酸类四大类[9]㊂J a i t z 等[10]从红酒中鉴定出没食子酸㊁儿茶素㊁咖啡酸㊁表儿茶酸㊁顺式对香豆素㊁反式对香豆素㊁阿魏酸㊁杨梅酮㊁顺式白藜芦醇㊁反式白藜芦醇和槲皮素等11种酚类物质㊂胡建刚等[11]鉴定出黄酒中的多酚主要为香草酸㊁丁香酸㊁对香豆酸㊁阿魏酸㊁牡荆素㊁芦丁等,少数存在没食子酸㊁香豆酸㊁儿茶素㊁咖啡酸㊁原儿茶酸㊁山柰酚㊁槲皮素㊁金丝桃苷㊁鞣花酸㊁肉桂酸㊁芥子酸等㊂L a z a r o 等[12]对古巴果酒和米酒中总酚含量(t o t a l p h e n o l i c c o n t e n t ,T P C )进行了测定,其T P C 在200~12250m g G A EL -1之间㊂除了茶叶和酒之外,在果蔬及谷物等植物中也相继发现酚类物质㊂W a n g 等[13]从蓝莓中分离鉴定出花青素23种㊁黄酮醇32种㊁原花青素11种㊁其他黄酮类2种㊁酚酸13种等81种酚类化合物㊂R o n g 等[14]研究表明,在苹果皮和果肉中,多酚类物质以原花青素为主;而在果皮中的槲皮素和果肉中的羟基肉桂酸酯含量丰富㊂L e g u a 等[15]对血橙进行了生物活性化合物分析,发现血橙中酚酸和黄酮类化合物含量极为丰富;其中,对香豆素含量最多,其次是阿魏酸和芥子酸;而黄酮类化合物主要以橙皮苷(黄酮苷)为主㊂黄龙等[16]对不同品种苦瓜中的酚类物质进行定性定量分析后发现,苦瓜中的酚类物质主要是香草酸㊁表儿茶素㊁芦丁等㊂杨成峻等[17]在花椒果皮中分离鉴定出没食子酸㊁原儿茶酸㊁绿原酸㊁香草酸㊁咖啡酸㊁丁香酸㊁儿茶酸㊁阿魏酸㊁对香豆酸等9种酚酸及酚酸衍生物,其中绿原酸是花椒酚酸的主要成分㊂而含有阿魏酸等酚酸则是谷物类食品的一大特色[18]㊂Z h a n g 等[19]从黑藜麦中鉴定出6种酚酸945 第6期 姜忠丽,等:酚类物质的研究进展(没食子酸㊁3,4-二羟基苯甲酸㊁香草酸㊁绿原酸㊁对香豆素和阿魏酸),2种黄烷-3-醇(儿茶素和表儿茶素),1种黄酮类(槲皮素)和1种酚苷(阿魏酸4-葡萄糖苷)㊂翟小童等[20]在玉米籽粒的果皮㊁种皮㊁糊粉层等部位检测到酚类物质,其中含量较高的有香草酸㊁对羟基苯甲酸㊁阿魏酸等㊂W u等[21]首次发现核桃仁多酚中游离形式的胡桃醌㊁山柰酚㊁槲皮素-7-o-β-D-葡萄糖苷和二氢槲皮素㊂B e l s c a k等[22]在对可可产品生物活性成分的比较研究发现,可可豆中多酚的含量特别高,经测定,其黄烷醇类占37%,花色苷占4%,原花青素占58%㊂B u t s a t等[23]发现泰国米的谷壳㊁皮层㊁胚乳中主要存在3种酚酸,分别为阿魏酸㊁香草酸和对香豆酸,其中阿魏酸在皮层中最多,而香草酸㊁对香豆酸则多存在于谷壳中㊂2.2酚类物质的提取方法目前,酚类物质的提取分离方法多种多样㊂经典的提取方法是有机溶剂浸提法[24],其不需特殊的仪器,应用较普遍,但存在产品安全性低㊁耗时长㊁提取率低等缺点㊂随着科学的不断进步,人们更加注重高效㊁节能㊁环保,因而一些基于先进仪器的新型提取方法应运而生,其优缺点比较结果见表1㊂不同提取方法对酚类物质的提取率存在着差异,常见的提取方法[25]有超声辅助提取法㊁微波辅助萃取法和生物酶解法等㊂表1酚类物质常见提取方法优缺点比较T a b l e1A d v a n t a g e s a n dd i s a d v a n t a g e s o f c o m m o nm e t h o d s f o r e x t r a c t i n gp h e n o l s提取方法有机溶剂提取超声辅助提取微波辅助萃取生物酶解优点操作简单㊁溶剂易取得效率高㊁溶剂耗量小时间短㊁效率高产率高㊁多用于结合酚提取缺点提取率低对人体有害且某些多酚会发生降解不能用于提取结合酚酶的作用条件较温和,对其要求较为严格2.2.1有机溶剂提取法有机溶剂提取法是较为传统的经典多酚提取方法,主要是指用水㊁甲醇㊁乙醇和乙酸乙酯等有机溶剂利用相似相溶的原理从食品中提取酚类物质的过程,其具有操作简单㊁提取速度快㊁使用的溶剂易取得等优点[26]㊂T u r k m e n等[27]以80%甲醇提取红茶多酚,提取率最高可达到14.27m g㊃g-1㊂梁杏等[28]以50%乙醇提取核桃饼粕多酚,核桃饼粕多酚提取率为6.95%㊂L i等[29]以甲醇溶液为溶剂,使蓝莓多酚类化合物在40ħ的条件下被提取出来,经测定,其总酚含量在(154.7ʃ1.01)~(398.0ʃ5.8)m g/100g㊂姚永志等[3031]以水作溶剂提取花生红衣多酚物质时,其提取率为6.41%,而当用乙醇作溶剂时,则可达到7.858%㊂刘晚霞等[32]以70%乙醇为提取剂得到小米糠多酚提取液㊂O r o z c o等[33]以80%甲醇为提取剂,经过正己烷除脂和乙酸乙酯萃取后,获得糙米多酚提取液㊂2.2.2超声辅助提取法超声辅助提取法[34]是基于有机溶剂提取法的优化处理,在溶液提取的同时用超声波处理提取液,以达到提高提取率与加快提取时间的目的㊂S h e h a t a等[35]通过超声辅助提取法提取橙皮多酚,结果表明,在50ħ,57.7%乙醇浓度和44m i n 的提取时间下,其总酚含量T P C可达到292.158μg G A E/g㊂何志勇和夏文水[36]对橄榄多酚进行了提取,比较了传统有机溶剂和超声辅助提取法,结果显示超声辅助提取法比传统有机溶剂提取法的多酚提取率提高了2.2%㊂杨志刚等[37]研究超声波辅助提取常熟黑米类黄酮时发现,在超声波辅助提取条件下,其提取率比有机溶剂浸提法提取率高㊂D e m i r d o v e n等[38]比较了超声波和常规方法从红白菜中提取花青素,结果显示超声波比常规方法的花青素提取率提高了11.92%㊂但仍有文献报道高强度的超声处理会降低某些食品中的酚类物质含量㊂张清安等[39]研究了超声处理对黑米酒总酚含量的影响,随着超声波功率㊁频率和时间的增加,黑米酒中的总酚含量与未经超声处理黑米酒的总酚含量相比略有下降㊂Z h a n g等[40]研究了超声处理对葡萄酒中酚类化合物的影响,结果表明超声处理加速了葡萄酒中锦葵花素-3-O-葡萄糖苷的降解,同时处理时间越长,降解速度越快㊂到目前为止,超声对酚类物质影响的机制仍不明确㊂相信随着对超声波各特征参数与其食品中酚类物质相关性的进一步研究,未来该技术在食品酚类物质的提取中会有更好的应用㊂2.2.3微波辅助萃取法微波辅助萃取法同超声辅助提取法的原理几乎相同,其是在有机溶剂提取法的基础上加以微波辅055沈阳师范大学学报(自然科学版)第41卷助的方式将提取工艺进行优化㊂该方法具有提取时间短㊁溶剂要求低㊁提取纯度高㊁成本低等优点,而且在不破坏酚类化合物结构的情况下,还能提高提取液中酚类化合物的含量㊂P a n 等[41]采用微波辅助萃取法提取绿茶叶中的茶多酚与咖啡因,结果表明,微波辅助萃取法提取较超声波辅助提取法多酚得率提高了2%㊂L i 等[42]分别使用微波法㊁索氏法和有机溶剂法提取大豆中的酚类化合物,结果发现微波法较其他2种提取方法多酚得率分别提高了50.0%和55.6%㊂陈培栋[43]研究微波处理对糙米多酚的影响,发现微波处理后糙米多酚和总黄酮类含量均超过原始糙米的50%㊂陈秋娟等[44]在对荸荠皮中的多酚类物质进行微波辅助提取研究时发现,用微波辅助提取荸荠皮中的多酚类物质,其提取率比传统的有机溶剂提取率高㊂W a n g 等[45]研究发现,对苦荞种子进行适当的微波预处理,可以在一定程度上提高萌发率,同时对黄酮类化合物含量和抗氧化活性有明显的改善作用㊂2.2.4 生物酶解法生物酶解法是一种将酶引入混合物中提高综合效率的可持续技术,通过使用酶作为催化剂破坏食品材料的细胞壁以释放生物活性成分,使其更容易进入溶剂,从而达到提取的目的㊂R u s s o 等[46]研究从紫楚菊中提取多酚,对酶辅助提取和常规溶剂萃取法进行了比较,结果显示,酶辅助提取法的总酚得率提高了5%㊂崔春兰等[47]采用传统有机溶剂浸提法和酶辅助提取法提取苹果渣中的多酚类物质,相比于有机溶剂提取而言,酶辅助提取的提取物产量分别提高了1.6倍和12.9倍㊂付晓燕等[48]对发芽燕麦酚类物质的含量㊁成分及抗氧化活性进行了比较,发现酚类物质含量在燕麦发芽8d 的过程中显著提高,并且与传统溶剂萃取法相比,酶辅助萃取法提取的总酚含量更高㊂生物酶解法具有高效温和㊁环保㊁可持续发展等特点,避免了有机溶剂对人体的有害作用,多用于提取结合酚,但酶需要在特定条件下才能发挥作用,且该技术尚处于实验室阶段,实验费用较高,技术尚不成熟,因而在实际生产中尚未大规模投入㊂2.3 酚类物质的生理功能特性酚类物质作为一类储量丰富的可再生绿色资源,在人们的日常生活中发挥着巨大的作用,其抗氧化㊁抗菌㊁抗癌㊁抗肿瘤㊁降血糖㊁降血脂[49]㊁增强免疫功能等作用是发展含酚类保健食品的先决条件㊂近几年,在医药㊁食品[50]㊁保健品㊁化妆品等领域已经报道了酚类物质的抗氧化㊁抗菌㊁降血脂㊁降血糖㊁降低农药对机体毒性㊁吸收紫外线和结合金属离子等的作用㊂2.3.1 抗氧化酚类物质良好的抗氧化特性与其化学结构有着密切的关系:由于酚类物质中含有大量的酚羟基,使之具有很强的还原性,从而能与自由基发生化学反应,达到清除体内过剩自由基㊁延缓机体衰老的目的㊂邵佩等[51]对藤茶抗氧化能力的研究结果显示,藤茶总多酚对羟基自由基㊁D P P H 自由基和超氧阴离子自由基均有良好的清除作用㊂李晓静等[52]对香蕉皮单宁进行了提取并评价了其抗氧化活性,香蕉皮单宁对D P P H 自由基㊁超氧阴离子自由基和羟基自由基均具有明显的清除能力,且半数抑制质量浓度(I C 50)分别为0.300,1.185,0.730m g ㊃m L -1㊂另外,有研究报告对比了小麦粉㊁全麦粉㊁麸皮及糊粉层的抗氧化活性,发现其抗氧化活性依次增强,这可能与它们所含的酚类物质含量多少有关[19]㊂S a n g k i t o k o m o l 等[53]发现血糯米中的花色苷对人类离体红细胞的抗氧化活性有明显的改善作用㊂N e e l a m 等[54]发现多酚作为抗氧化剂也被证明可以保护蛋白质㊁脂质和核酸等关键细胞成分免受氧化伤害,从而降低患有与氧化应激相关的多种退行性疾病的可能性㊂2.3.2 抑菌㊁消炎及抗病毒研究表明,黄酮类化合物具有抑菌作用,可提高机体抵抗传染病的能力,如木椰草素㊁黄芩苷㊁黄芩素等,而槲皮素㊁桑色素㊁二氢槲皮素及山柰酚等有抗病毒作用㊂与传统的抗菌药物(如抗生素和磺胺类药物)相比,其无毒副作用的优点引起了人们的关注,因而其有被开发为新型抑菌剂的潜力㊂白传记等[55]的实验证明,茶多酚对金黄色葡萄球菌㊁大肠杆菌㊁沙门氏菌等的生长和繁殖有较强的抑制作用㊂李振等[56]的研究表明,茶多酚对金黄色葡萄球菌等致病菌有明显的抑制作用㊂A x e l l e 等[57]研究了姜黄多酚通过调节关键脂肪因子和抗氧化酶改善胰岛素介导的脂质积累并减弱氧化应激期间3T 3-L 1脂肪细胞的促炎反应㊂M e r i e m 等[58]在研究芸香的酚类含量及体外抗氧化㊁抗炎和抗菌评价时发现,酚类物质通过抑制蛋白变性来起到抗炎的作用,并且酚类物质含量越多其抗炎作用越155 第6期 姜忠丽,等:酚类物质的研究进展255沈阳师范大学学报(自然科学版)第41卷显著㊂此外,李丽等[59]还考察了香蕉皮单宁抑菌性能受温度㊁酸碱值㊁盐分等因素的影响,发现其抑菌能力不受高温处理的影响,但会随着p H的升高(2.0~8.0)逐渐减弱,随着盐质量分数的增加(1%~7%)显著增强㊂这可能是由于在碱性环境中香蕉皮单宁发生氧化反应而失去抑菌作用,而盐类的存在在一定程度上协同了单宁的抗菌能力㊂G i o v a a n等[60]考察了29种多酚物质在不同浓度水平下对小麦中镰刀菌所产的单端孢霉毒素的产毒情况,其中大部分多酚物质在1.5mm o l㊃L-1或1.0mm o l㊃L-1条件下对脱氧雪腐镰刀菌烯醇的抑制率达到70%㊂此外,花生红衣中的多酚类物质对黄曲霉毒素B1产毒也具有显著抑制作用㊂因此,酚类化合物的抑菌㊁消炎及抗病毒功能对人体而言具有重要贡献㊂2.3.3降血压人体肾脏之所以能够维持血压平衡是通过 血管紧张素 的分泌使血压增高,以及 舒缓激肽 的平衡使血压下降㊂当促进这2类物质转化酶活性过强时,血管紧张素Ⅱ会增高,血压升高㊂而茶多酚具有较强的抑制转化酶活性的作用[61],故可以起到降低或维持血压恒定的作用,绿原酸能通过改善血管内皮增生来起到降血压的作用㊂目前已报道的多酚对高血压的保护作用机制主要由动物实验数据支持,包括抑制氧化应激㊁提高一氧化氮(N O)生物利用度㊁改善内皮功能㊁抑制血管收缩素内皮素-1合成及调节肾素-血管紧张素-醛固酮系统㊂虽然酚类物质降血压数据不足,许多问题仍未解决,但整体而言,有关多酚可以降低或维持血压的证据[62],还是让人倍受鼓舞㊂2.3.4降血糖酚类物质的降糖活性与其影响参与葡萄糖代谢的相关基因表达和酶活性㊁干扰胃肠道葡萄糖的吸收㊁抑制蛋白质的非酶糖基化有关[63]㊂一些研究者通过动物试验或临床试验证实,酚类物质在有效预防及辅助治疗糖尿病和并发症方面是有效的[64]㊂目前,大多数降血糖药物具有毒性和副作用㊂而从天然资源中提取的酚类物质具有降血糖活性且无毒性或毒性低等优点,引起了研究者们日益浓厚的研究兴趣㊂Z h a o等[65]发现桑葚富含多种酚酸㊁类黄酮等酚类化合物,其中花青素类(主要是矢车菊3-葡萄糖苷)通过调控P I3K/A K T通路及降低肝脏氧化损伤的途径来降低胰岛素抵抗㊂除了矢车菊3-葡萄糖苷花青素以外,桑葚中其他酚类化合物是否也有助于降血糖活性的发挥也有待进一步研究㊂W a n g等[66]研究发现,诺丽果含有大量的酚类物质,临床试验和动物试验也表明诺丽果汁具有调节血糖水平的潜力㊂流行病学研究进一步证实了大量摄入富含酚类物质食品与T2D M治疗正相关,而诺丽果富含酚类物质提取物对肠道微生物的影响及对葡萄糖稳态调节作用的机制仍然需要深入研究㊂糖尿病是一种典型的代谢紊乱疾病,其发病机理复杂多样,除了已报道的调控途径以外,酚类化合物对其他与糖尿病有关的代谢通路的影响也有待进一步的研究㊂2.3.5其他功能研究表明,酚类化合物对神经退行性疾病㊁癌症㊁肥胖等疾病也有所改善[67]㊂其中,姜黄素和儿茶素可以通过免疫调节和抗氧化清除特性保护神经元,从而预防阿尔茨海默病㊂酚类物质也可以中和自由基并最大限度地降低患癌症的风险㊂此外,具有蛋白质结合活性的多酚也被证明可以通过与消化酶反应并抑制消化酶来防止脂质㊁碳水化合物和蛋白质在消化道中的消化㊂3结论和展望酚类物质来源丰富,生理功能众多,可挖掘利用的空间很大㊂目前酚类物质多应用于化妆品方面㊂例如,芦荟提取物㊁金缕梅提取物㊁银杏叶提取物常被广泛应用于清洁型化妆品中,以茶多酚为主的茶叶提取物和富含原花青素的葡萄籽提取物被广泛应用于护肤型化妆品中㊂此外,酚类物质的应用主要集中在天然多酚的生物材料的制备,其中包括金属多酚涂层㊁分层薄膜或胶囊㊁纳米微粒和多酚凝胶等㊂一方面,它改善了天然多酚水溶性差㊁稳定性差㊁生物利用率低等问题;另一方面,这些材料可以与多种药物结合用于治疗癌症㊁细菌感染㊁炎症等,由于其选用的材料均是食品级,且制备过程多利用分子间的互作,因而是一种安全㊁简便的技术手段[68]㊂除此之外,国内外学者利用栅栏效应将植物多酚和其他保鲜剂复配[69],或与其他保鲜手段相结合,充分发挥其协同作用,以达到综合保鲜的效果㊂但迄今为止,酚类物质的应用仍然受限㊂其主要原因:第一,原料方面,对酚类物质目前的研究及应用仍然不够全面,今后更应扩大其研究范围,使应用取材更加广泛灵活;第二,生理功能方面,对酚类物质的功能特性研究还不够深入,今后应加大酚类物质的成分㊁结构及与之相对应的生物活性结构的研究,探索其对人 三高 的影响机制,明确改性目标;第三,从未来发展趋势角度,应推动酚类物质在特殊医学用途配方食品中的应用,通过优化提取工艺和改性方法,使酚类物质的应用更加广泛和深入㊂随着酚类物质系统化研究的不断深入,富含酚类物质且对人体有益的食品㊁药品将会不断面市,对医药和保健食品等领域贡献更大㊂参考文献:[1]凌关庭.有 第七类营养素 之称的多酚类物质[J ].中国食品添加剂,2000(1):2837.[2]颜才植,叶发银,赵国华.食品中多酚形态的研究进展[J ].食品科学,2015,36(15):249254.[3]牛帅科,赵艳卓,牛早柱,等.葡萄果实中酚类物质研究进展[J ].保鲜与加工,2022,22(2):107112.[4]T S A O R.C h e m i s t r y a n db i o c h e m i s t r y o f d i e t a r yp o l y p h e n o l s [J ].N u t r i e n t s ,2010,2(12):12311246.[5]王玲平,周生茂,戴丹丽,等.植物酚类物质研究进展[J ].浙江农业学报,2010,22(5):696701.[6]V I N S O NJA ,S U X H ,Z U B I K L ,e t a l .P h e n o l a n t i o x i d a n t q u a n t i t y a n d q u a l i t y i n f o o d s :F r u i t s [J ].JA gr i cF o o d C h e m ,2001,49(11):53155321.[7]R O C H I NS M ,U R I B EJG ,S A L D O V A RSS ,e t a l .P h e n o l i cc o n t e n t a n da n t i o x i d a n t a c t i v i t y of t o r t i l l a s p r o d u c e d f r o m p ig m e n t e dm a i z e p r o c e s s e db y c o n v e n t i o n a l n i x t a m a l i z a t i o n o r e x t r u s i o n c o o k i n g[J ].JC e r e a l S c i ,2010,52(3):502508.[8]金莹,孙爱东.多酚的食物来源及生物有效性[J ].食品与发酵工业,2006,32(9):101106.[9]韦铮,贺燕,黄先智,等.茶多糖-茶多酚对小鼠肠道氧化应激的改善与作用机制[J ].食品科学,2022,43(11):149155. [10]J A I T Z L ,S I E G L K ,E D E R R ,e ta l .L C -M S /M Sa n a l y s i so f p h e n o l sf o rc l a s s i f i c a t i o no fr e d w i n ea c c o r d i n g t o g e o g r a p h i c o r i g i n ,g r a p e v a r i e t y a n dv i n t a g e [J ].F o o dC h e m ,2010,122(1):366372.[11]胡建刚,刘镇,周建弟,等.液相色谱-串联质谱法同时测定黄酒中20种多酚含量[J ].食品安全质量检测学报,2023,14(2):217225.[12]L A Z A R O N ,MA R I A PS ,A N A G ,e t a l .C o m p a r i s o n o f p h y s i c o c h e m i c a l p r o p e r t i e s ,a m i n o a c i d s ,m i n e r a l e l e m e n t s ,t o t a l p h e n o l i c c o m p o u n d s ,a n da n t i o x i d a n t c a p a c i t y o fC u b a nf r u i t a n dr i c ew i n e s [J ].F o o dS c iN u t r ,2021,9(7):36733682.[13]WA N GC ,Z HA N G M ,WU L M ,e t a l .Q u a l i t a t i v e a n d q u a n t i t a t i v e a n a l y s i s o f p h e n o l i c c o m po u n d s i nb l u e b e r r i e s a n d p r o t e c t i v e e f f e c t s o nh y d r o g e n p e r o x i d e -i n d u c e d c e l l i n j u r y [J ].JS e p S c i ,2021,44(14):28372855.[14]R O N G T ,Y A N G R ,Y O U N GCJ ,e t a l .P o l y p h e n o l i c p r o f i l e s i n e i g h t a p p l e c u l t i v a r s u s i n g h i g h -p e r f o r m a n c e l i q u i d c h r o m a t o g r a p h y (H P L C )[J ].JA g r i cF o o dC h e m ,2003,51(21),63476353.[15]L E G U AP ,MO D I C A G ,P O R R A S I ,e t a l .B i o a c t i v e c o m p o u n d s ,a n t i o x i d a n t a c t i v i t y a n d f r u i t q u a l i t y ev a l u a t i o no f e l e v e nb l o o do r a n g e c u l t i v a r s [J ].JS c i F o o dA g r i c ,2022,102(7):29602971.[16]黄龙,邓媛元,张名位,等.不同苦瓜品种果肉中酚类物质含量及抗氧化能力比较[J ].中国农业科学,2011,44(22):46604668.[17]杨成峻,陈明舜,刘成梅,等.花椒果皮多酚类成分鉴定及降血糖活性[J ].食品科学,2023,44(2):271278.[18]龚二生.糙米多酚组分及其抗氧化活性研究[D ].南昌:南昌大学,2018.[19]Z HA N G Y ,Y A N Y ,L I W Q ,e ta l .M i c r o w a v i n g r e l e a s e d m o r e p o l y p h e n o l sf r o m b l a c k q u i n o a g r a i n s w i t h h y p o g l y c e m i c e f f e c t s c o m p a r e dw i t h t r a d i t i o n a l c o o k i n g m e t h o d s [J ].JS c i F o o dA g r i c ,2022,102(13):59485956.[20]翟小童,韩林,乔聪聪,等.玉米籽粒次生代谢物质分布及其抗氧化活性[J ].食品科学,2023,44(2):296303.[21]WUST ,S H E N D Y ,WA N G R H ,e t a l .P h e n o l i c p r o f i l e sa n da n t i o x i d a n t a c t i v i t i e so f f r e e ,e s t e r i f i e da n db o u n d p h e n o l i c c o m p o u n d s i nw a l n u t k e r n e l [J ].F o o dC h e m ,2021,350(18):129217.[22]B E L S C A K A ,K OM E SD ,HO R Z I CDJ ,e t a l .C o m p a r a t i v e s t u d y o f c o mm e r c i a l l y av a i l a b l e c o c o a p r o d u c t s i n t e r m s o f t h e i r b i o a c t i v e c o m po s i t i o n [J ].F o o dR e s I n t ,2009,42(56):707716.[23]B U T S A T S N ,S I R I AMO R N P U N S .A n t i o x i d a n tc a p a c i t i e sa n d p h e n o l i cc o m po u n d s o ft h e h u s k ,b r a n a n d e n d o s p e r mo fT h a i r i c e [J ].F o o dC h e m ,2010,119(2):606613.[24]夏婷,赵超亚,杜鹏,等.食品中多酚类化合物种类㊁提取方法和检测技术研究进展[J ].食品与发酵工业,2019,45(5):231238.355 第6期 姜忠丽,等:酚类物质的研究进展455沈阳师范大学学报(自然科学版)第41卷[25]杨新,陈莉,卢红梅,等.茶多酚提取与纯化方法及其功能活性研究进展[J].食品工业科技,2019,40(5):322328.[26]田富林,黄文晶,王展,等.植物多酚提取研究进展[J].食品与机械,2020,36(9):211216.[27]T U R KM E N N,V E L I O G L U YS.D e t e r m i n a t i o no f a l k a l o i d s a n d p h e n o l i c c o m p o u n d s i nb l a c k t e a p r o c e s s e db y t w od i f fe r e n tm e t h o d s i nd if f e r e n t p l u c k i ng s e a s o n s[J].JS c i F o o dA g r,2007,87(7):14081416.[28]梁杏,陈朝银,赵声兰,等.响应面法优化核桃饼粕多酚提取工艺[J].食品科技,2015(6):241246.[29]L ID N,L IB,MA Y,e ta l.P o l y p h e n o l s,a n t h o c y a n i n s,a n df l a v o n o i d sc o n t e n t sa n dt h ea n t i o x i d a n tc a p a c i t y o f v a r i o u s c u l t i v a r s o f h i g h b u s ha n dh a l f-h i g hb l u e b e r r i e s[J].JF o o dC o m p o sA n a l,2017,62(11):8493.[30]姚永志,王子涵,左锦静.水作溶剂提取花生红衣多酚物质的研究[J].现代食品科技,2006(4):110112.[31]姚永志,左锦静,王子涵.乙醇提取花生红衣多酚物质的研究[J].中国油脂,2007(3):5153.[32]刘晚霞,刁静静,贾鹏禹,等.小米糠多酚提取液组成成分分析[J].饲料研究,2019,42(11):8386.[33]O R O Z C O RF,L IL,HA R F L E T T C,e t a l.E f f e c t so f e n v i r o n m e n t a n d g e n o t y p eo n p h e n o l i ca c i d s i nw h e a t i nt h eh e a l t h g r a i nd i v e r s i t y s c r e e n[J].JA g r i cF o o dC h e m,2010,58(17):93419352.[34]吴建华,吴志瑰,裴建国,等.多酚类化合物的研究进展[J].中国现代中药,2015,17(6):630636.[35]S H E HA T A M G,A B D E I A Z I Z N M,Y O U S S E F M M,e ta l.O p t i m i z a t i o n c o n d i t i o n s o fu l t r a s o u n d-a s s i s t e de x t r a c t i o nof p h e n o l i c c o m p o u n d s f r o mo r a ng e p e e l su s i n g r e s p o n s e s u r f a c em e th o d o l o g y[J].JF o o dP r o c e s sP r e s, 2021,45(10):15870.[36]何志勇,夏文水.橄榄多酚的提取研究[J].林产化学与工业,2007,27(1):7780.[37]杨志刚,张燕萍,杨海定.超声波辅助提取常熟黑米类黄酮及其抗氧化活性分析[J].食品科学,2013,34(18): 118122.[38]D E M I R D O V E N A H,O Z D O G A N K N,T O K A T L IKE.E x t r a c t i o no f a n t h o c y a n i n s f r o mr e d c a b b a g e b y u l t r a s o n i ca n d c o n v e n t i o n a lm e t h o d s:O p t i m i z a t i o na n de v a l u a t i o n[J].JF o o dB i o c h e m,2015,39(5):491500.[39]张清安,史芳芳,王袭,等.超声波处理对黑米酒中酚类物质㊁颜色及抗氧化性的影响[J].食品与机械,2016, 32(12):16.[40]Z HA N G Q A,WA N G T T.E f f e c t o f u l t r a s o u n d i r r a d i a t i o no n t h e e v o l u t i o no f c o l o r p r o p e r t i e s a n d m a j o r p h e n o l i cc o m p o u nd s i nw i ne d u r i n g s t o r a g e[J].F o o dC h e m,2017,234:372380.[41]P A N XJ,N I U G G,L I U H Z.M i c r o w a v e-a s s i s t e de x t r a c t i o no f t e a p o l y p h e n o l sa n dt e ac a f f e i n ef r o m g r e e nt e a l e a v e s[J].C h e m E n g P r o c e s s,2003,42(2):129133.[42]L IY,L I S,L I N SJ,e t a l.M i c r o w a v e-a s s i s t e de x t r a c t i o no fn a t u r a l a n t i o x i d a n t s f r o mt h ee x o t i cG o r d o n i aa x i l l a r i sf r u i t:O p t i m i z a t i o na n d i d e n t i f i c a t i o no f p h e n o l i c c o m p o u n d s[J].M o l e c u l e s,2017,22(9):14811497.[43]陈培栋.微波处理对糙米理化性质影响及作用机理研究[D].南京:南京财经大学,2018.[44]陈秋娟,罗杨合,张志,等.微波辅助提取荸荠皮中多酚类物质的工艺研究[J].安徽农业科学,2013,41(24): 1014410146.[45]WA N GJ F,B I A NZX,WA N GSM,e t a l.E f f e c t s o f u l t r a s o n i cw a v e s,m i c r o w a v e s,a n d t h e r m a l s t r e s s t r e a t m e n t o n t h e g e r m i n a t i o no fT a r t a r y b u c k w h e a t s e e d s[J].JF o o dP r o c e s sE n g,2020,43(10):13494.[46]R U S S O D,F A R A U N E I,L A B A N C AF,e t a l.C o m p a r i s o n o f d i f f e r e n t g r e e n-e x t r a c t i o n t e c h n i q u e s a n d d e t e r m i n a t i o n o f t h e p h y t o c h e m i c a l p r o f i l ea n da n t i o x i d a n ta c t i v i t y o fE c h i n a c e aa n g u s t i f o l i aL.e x t r a c t s[J].P h y t o c h e m A n a l, 2019,30(5):547555.[47]崔春兰,郑虎哲,顾立众,等.响应曲面分析法优化苹果渣中多酚类物质的果胶酶辅助提取工艺[J].现代食品科技, 2013,29(9):22352240.[48]付晓燕,隋勇,谢笔钧,等.不同方法提取发芽燕麦酚类物质的含量㊁组成和抗氧化活性比较[J].食品工业科技, 2014,35(15):5457.[49]伊娟娟,左丽丽,王振宇.植物多酚的分离纯化及抗氧化㊁降脂降糖功能研究[J].食品工业科技,2013,34(19): 391395,399.[50]苏绍辉,蒋磊.茶多酚的生理功能及其在食品和饲料中的应用[J].现代食品,2022(24):6164.[51]邵佩,蹇顺华,庄虎,等.不同藤茶提取物的理化指标㊁成分分析及抗氧化活性评价[J].中国食品添加剂,2022, 33(10):251257.[52]李晓静,韩宗元,穆雪姣,等.超声波法提取香蕉皮单宁及抗氧化活性研究[J].食品工业科技,2019,40(24): 120124.[53]S A N G K I T O K OMO L W,T E N C OMN A OT,R O C E J A N A S A R O JA.A n t i o x i d a n t e f f e c t s o f a n t h o c y a n i n s r i c h e x t r a c tf r o mb l a c ks t i c k y r i c e o nh u m a ne r y t h r o c y t e s a n d m o n o n u c l e a r l e u k o c y t e s[J].A f r JB i o t e c h n o l,2010,9(48):82228229.。
2010最新SCI期刊影响因子
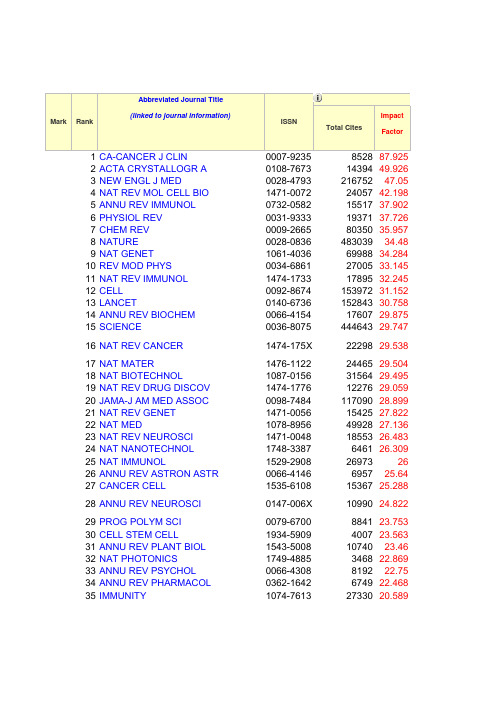
Abbreviated Journal Title(linked to journal information)ImpactFactor2ACTA CRYSTALLOGR A 0108-76731439449.9263NEW ENGL J MED0028-479321675247.054NAT REV MOL CELL BIO 1471-00722405742.1985ANNU REV IMMUNOL 0732-05821551737.9026PHYSIOL REV 0031-93331937137.7267CHEM REV 0009-26658035035.9578NATURE 0028-0836********.489NAT GENET 1061-40366998834.28410REV MOD PHYS 0034-68612700533.14511NAT REV IMMUNOL 1474-173********.24512CELL 0092-867415397231.15213LANCET0140-673615284330.75814ANNU REV BIOCHEM 0066-41541760729.87515SCIENCE0036-807544464329.74716NAT REV CANCER 1474-175X 2229829.53817NAT MATER1476-11222446529.50418NAT BIOTECHNOL1087-01563156429.49519NAT REV DRUG DISCOV 1474-177********.05920JAMA-J AM MED ASSOC 0098-748411709028.89921NAT REV GENET 1471-00561542527.82222NAT MED1078-89564992827.13623NAT REV NEUROSCI 1471-00481855326.48324NAT NANOTECHNOL 1748-3387646126.30925NAT IMMUNOL1529-2908269732626ANNU REV ASTRON ASTR 0066-4146695725.6427CANCER CELL1535-61081536725.28828ANNU REV NEUROSCI 0147-006X 1099024.82229PROG POLYM SCI 0079-6700884123.75330CELL STEM CELL1934-5909400723.56331ANNU REV PLANT BIOL 1543-50081074023.4632NAT PHOTONICS1749-4885346822.86933ANNU REV PSYCHOL 0066-4308819222.7534ANNU REV PHARMACOL 0362-1642674922.46835IMMUNITY1074-76132733020.58936CHEM SOC REV 0306-00121760120.08637ENDOCR REV 0163-769X 1272719.761Total CitesMarkRankISSN38ADV PHYS0001-8732422719.632 39ANNU REV CELL DEV BI1081-0706832819.571 40NAT CELL BIOL1465-73922555719.527 41ANNU REV BIOPHYS1936-122X49019.304 42BEHAV BRAIN SCI0140-525X629019.045 43ANNU REV BIOPH BIOM1056-8700458518.955 44ALDRICHIM ACTA0002-510099918.688 45ACCOUNTS CHEM RES0001-48423072718.203 46ANNU REV PHYSIOL0066-4278698018.17 47LANCET NEUROL1474-4422843218.126 48J CLIN ONCOL0732-183X10425317.793 49PHYS REP0370-157********.752 50NAT REV MICROBIOL1740-1526839817.644 51EPIDEMIOL REV0193-936X225917.5 52ANNU REV PHYS CHEM0066-426X598117.464 53CELL METAB1550-4131646217.35 54PHARMACOL REV0031-6997893717 55NAT METHODS1548-7091869716.874 56ANN INTERN MED0003-48194518416.225 57NAT CHEM BIOL1552-4450510116.058 58PROG MATER SCI0079-6425380815.769 59LANCET INFECT DIS1473-3099636215.583 60NAT PHYS1745-2473769315.491 61J CLIN INVEST0021-97388713315.387 62MOL PSYCHIATR1359-41841018815.049 63CIRCULATION0009-732214596114.816 64CLIN MICROBIOL REV0893-85121003414.691 65MOL CELL1097-27653898714.608 66J EXP MED0022-10076586514.505 67LANCET ONCOL1470-2045825114.47 68NAT NEUROSCI1097-62563267714.345 69CURR OPIN CELL BIOL0955-06741355914.153 70J NATL CANCER I0027-88743579514.069 71BRIT MED J0959-535X7117513.66 72ANNU REV PATHOL-MECH1553-400696213.5 73SURF SCI REP0167-5729341113.462 74DEV CELL1534-58071478513.363 75NEURON0896-62735616113.26 76NANO TODAY1748-013273413.237 77ANNU REV GENET0066-4197543713.235 78PLOS MED1549-1277842513.05 79CELL HOST MICROBE1931-3128213513.02180PLOS BIOL1544-91731569912.916 81GASTROENTEROLOGY0016-50855527612.899 82PSYCHOL BULL0033-29092614912.854 83ANNU REV MICROBIOL0066-4227714412.804 84TRENDS NEUROSCI0166-22361666312.794 85ASTROPHYS J SUPPL S0067-00492150712.771 86MICROBIOL MOL BIOL R1092-2172741712.585 87DRUG RESIST UPDATE1368-7646165412.581 88J AM COLL CARDIOL0735-10976235012.535 89AM J PSYCHIAT0002-953X4219312.522 90AM J HUM GENET0002-92973465412.303 91NAT STRUCT MOL BIOL1545-99851945812.273 92ARCH GEN PSYCHIAT0003-990X3396312.257 93MAT SCI ENG R0927-796X393812.217 94MOL SYST BIOL1744-4292281212.125 95TRENDS CELL BIOL0962-89241022212.115 96GENE DEV0890-93695404412.075 97FRONT NEUROENDOCRIN0091-3022200312.048 98ANNU REV NUCL PART S0163-8998185911.964 99ADV DRUG DELIVER REV0169-409X1455511.957 100ASTRON ASTROPHYS REV0935-495660511.857 101ANGEW CHEM INT EDIT1433-785116462611.829 102BBA-REV CANCER0304-419X227111.685 103TRENDS COGN SCI1364-66131162611.664 104TRENDS BIOCHEM SCI0968-00041496011.572 105ANNU REV GENOM HUM G1527-8204171811.568 106TRENDS ECOL EVOL0169-53471907311.564 107MATER TODAY1369-7021276611.452 108REP PROG PHYS0034-4885825611.444 109GENOME RES1088-90512209411.342 110ANNU REV ENTOMOL0066-4170693911.271 111ANNU REV BIOMED ENG1523-9829244111.235 112COORDIN CHEM REV0010-85452197111.225 113ANNU REV PHYTOPATHOL0066-4286445411.212 114TRENDS MOL MED1471-4914478011.049 115PROG ENERG COMBUST0360-1285313511.024 116CURR BIOL0960-98223723710.992 117CURR OPIN IMMUNOL0952-7915825210.881 118HEPATOLOGY0270-91394164010.84 119AM J RESP CRIT CARE1073-449X4520310.689 120MASS SPECTROM REV0277-7037306810.623 121LIVING REV RELATIV1433-8351118910.6122BLOOD0006-497112659710.555 123ADV INSECT PHYSIOL0065-280638010.5 124CURR OPIN PLANT BIOL1369-5266768810.333 125ECOL LETT1461-023X1041310.318 126CRIT REV BIOCHEM MOL1040-9238232810.216 127Q REV BIOPHYS0033-5835213810.2 128IMMUNOL REV0105-28961069310.05 129NANO LETT1530-6984462389.991 130ANNU REV MED0066-421942579.94 131TRENDS PLANT SCI1360-138597569.883 132MOL BIOL EVOL0737-4038237849.872 133ARCH INTERN MED0003-9926359779.813 134EUR HEART J0195-668X236119.8 135FEMS MICROBIOL REV0168-644560259.783 136ANNU REV CLIN PSYCHO1548-59438379.613 137J CELL BIOL0021-9525717529.575 138PLOS GENET1553-739088839.532 139BRAIN0006-8950342829.49 140P NATL ACAD SCI USA0027-84244513869.432 141GUT0017-5749284559.357 142ANNU REV FLUID MECH0066-418956399.353 143CANCER METAST REV0167-765935379.345 144CURR OPIN STRUC BIOL0959-440X96069.344 145ANN NEUROL0364-5134296699.317 146PLANT CELL1040-4651316269.293 147PROG CRYST GROWTH CH0960-89745699.25 148CIRC RES0009-7330399039.214 149NAT PROD REP0265-056844799.202 150J ALLERGY CLIN IMMUN0091-6749303639.165 151SEMIN IMMUNOL1044-532330769.155 152PROG NEUROBIOL0301-008290149.14 153PSYCHOL REV0033-295X201069.082 154TRENDS PHARMACOL SCI0165-614792979.064 155EMBO J0261-4189747828.993 156CURR OPIN GENET DEV0959-437X75988.987 157PLOS PATHOG1553-736665278.978 158BIOL PSYCHIAT0006-3223319098.926 159PHARMACOL THERAPEUT0163-725889228.897 160MOL CELL PROTEOMICS1535-947697088.791 161ANNU REV NUTR0199-988541008.783 162TRENDS IMMUNOL1471-490666768.768 163TRENDS GENET0168-9525107218.689164MED RES REV0198-632527378.656 165J AM CHEM SOC0002-78633518138.58 166DIABETES0012-1797452828.505 167SYST BIOL1063-515784658.48 168ADV MATER0935-9648574568.379 169LEUKEMIA0887-6924152108.296 170CURR OPIN CHEM BIOL1367-593172008.295 171BIOTECHNOL ADV0734-975027888.25 172CELL DEATH DIFFER1350-9047116558.24 173CLIN INFECT DIS1058-4838385618.195 174ANNU REV ECOL EVOL S1543-592X121548.19 175NEUROLOGY0028-3878713028.172 176PROG LIPID RES0163-782731418.167 177CELL RES1001-060232608.151 178ANN RHEUM DIS0003-4967204068.111 179NAT GEOSCI1752-089413408.108 180NAT CLIN PRACT ONCOL1743-425417308.075 181REV GEOPHYS8755-120958008.021 182WHO TECH REP SER0512-305423288 183J PHOTOCH PHOTOBIO C1389-556712347.952 184ANNU REV PUBL HEALTH0163-752528287.915 185PROG SURF SCI0079-681617307.913 186ANNU REV MATER RES1531-733141737.911 187ANN SURG0003-4932323377.9 188CURR OPIN MICROBIOL1369-527462617.866 189CURR OPIN BIOTECH0958-166971267.82 190J HEPATOL0168-8278174137.818 191NEUROSCI BIOBEHAV R0149-763490457.791 192CATAL REV0161-494026947.765 193PROG RETIN EYE RES1350-946226617.755 194STEM CELLS1066-5099129697.747 195ADV IMMUNOL0065-277621627.725 196J AM SOC NEPHROL1046-6673272697.689 197ACM COMPUT SURV0360-030036407.667 198EUR UROL0302-2838129017.667 199ANNU REV EARTH PL SC0084-659738607.581 200ANTIOXID REDOX SIGN1523-086466497.581 201AGING CELL1474-971825647.554 202CURR PROB CANCER0147-02722627.545 203CANCER RES0008-54721316627.543 204ACS NANO1936-085133137.493 205MOL INTERV1534-038410957.48206NUCLEIC ACIDS RES0305-1048957997.479 207SCHIZOPHRENIA BULL0586-761475787.467 208ACTA PHYS SLOVACA0323-04653097.455 209REV MED VIROL1052-927614467.449 210BRAIN RES REV0165-017378057.39 211HUM MOL GENET0964-6906295677.386 212BIOMATERIALS0142-9612468357.365 213ASTROPHYS J0004-637X2035967.364 214ARTHRITIS RHEUM-US0004-3591411567.332 215BRIEF BIOINFORM1467-546328987.329 216PHYS REV LETT0031-90073321307.328 217OCEANOGR MAR BIOL0078-321820307.312 218CAN MED ASSOC J0820-3946100247.271 219CURR OPIN PHARMACOL1471-489240127.259 220ARTERIOSCL THROM VAS1079-5642296517.235 221J AUTOIMMUN0896-841132397.231 222CURR OPIN NEUROBIOL0959-438897847.211 223DEVELOPMENT0950-1991492047.194 224BLOOD REV0268-960X14237.185 225J NEUROSCI0270-64741273667.178 226ADV ORGANOMET CHEM0065-305510177.154 227ONCOGENE0950-9232573667.135 228MUTAT RES-REV MUTAT1383-574220307.097 229CRIT REV ENV SCI TEC1064-338913037.091 230HUM REPROD UPDATE1355-478641217.042 231STROKE0039-2499466377.041 232THORAX0040-6376155217.041 233NEUROPSYCHOPHARMACOL0893-133X15516 6.993 234ADV FUNCT MATER1616-301X16763 6.99 235CEREB CORTEX1047-321115940 6.979 236CLIN PHARMACOL THER0009-923612686 6.961 237PLANT J0960-741225981 6.946 238PHYSIOLOGY1548-92131611 6.945 239EARTH-SCI REV0012-82524397 6.942 240FRONT ECOL ENVIRON1540-92952211 6.922 241SEMIN CANCER BIOL1044-579X3708 6.918 242HEALTH TECHNOL ASSES1366-52783284 6.91 243TRENDS BIOTECHNOL0167-77998118 6.909 244EMBO REP1469-221X8743 6.907 245TRENDS MICROBIOL0966-842X6906 6.894 246HUM MUTAT1059-77948765 6.887 247AUTOPHAGY1554-86273197 6.829248EMERG INFECT DIS1080-604018017 6.794 249CLIN CANCER RES1078-043252244 6.747 250PROG NUCL MAG RES SP0079-65651772 6.742 251DIABETES CARE0149-599243007 6.718 252GASTROINTEST ENDOSC0016-510717856 6.713 253ONCOLOGIST1083-71595337 6.701 254NAT CLIN PRACT ENDOC1745-83661125 6.688 255DRUG DISCOV TODAY1359-64466723 6.63 256GENOME BIOL1474-760X12688 6.626 257BIOL REV1464-79314449 6.625 258HYPERTENSION0194-911X28849 6.614 259TRENDS ENDOCRIN MET1043-27604220 6.562 260DIABETOLOGIA0012-186X21339 6.551 261TRAC-TREND ANAL CHEM0165-99365401 6.546 262CHEM BIOL1074-55218863 6.523 263CHEM BIOL1074-55218863 6.523 264J COSMOL ASTROPART P1475-75168871 6.502 265MOL ASPECTS MED0098-29971674 6.492 266CYTOKINE GROWTH F R1359-61013990 6.489 267J PATHOL0022-341712210 6.466 268AM J TRANSPLANT1600-613512638 6.433 269J NUCL MED0161-550519201 6.424 270HAEMATOL-HEMATOL J0390-60788299 6.416 271FASEB J0892-663835849 6.401 272ACTA NEUROPATHOL0001-63228563 6.397 273ISME J1751-73621270 6.397 274ALLERGY0105-453810370 6.38 275CRIT CARE MED0090-349328896 6.373 276AUTOIMMUN REV1568-99722970 6.368 277NAT CLIN PRACT NEURO1745-834X1105 6.362 278CHEST0012-369241623 6.36 279STUD MYCOL0166-06161107 6.349 280LAB CHIP1473-01978339 6.342 281SEMIN CELL DEV BIOL1084-95213824 6.342 282RADIOLOGY0033-841946643 6.341 283NAT PROTOC1754-21896411 6.335 284MRS BULL0883-******** 6.33 285ARCH NEUROL-CHICAGO0003-994220691 6.312 286AM J CLIN NUTR0002-916546005 6.307 287CLIN CHEM0009-914724297 6.263 288HUM BRAIN MAPP1065-94718443 6.256 289TRAFFIC1398-92195857 6.255290REV PHYSIOL BIOCH P0303-4240999 6.25 291MOL THER1525-00169418 6.239 292PLANT PHYSIOL0032-088952576 6.235 293ADV PARASIT0065-308X1316 6.231 294J CLIN ENDOCR METAB0021-972X65847 6.202 295KIDNEY INT0085-253836441 6.193 296ENVIRON HEALTH PERSP0091-676521856 6.191 297HUM-COMPUT INTERACT0737-******** 6.19 298SMALL1613-68107450 6.171 299J CELL SCI0021-953336789 6.144 300CURR OPIN LIPIDOL0957-96723404 6.133 301B AM METEOROL SOC0003-00079074 6.123 302CELL MOL LIFE SCI1420-682X13428 6.09 303INT REV CYTOL0074-76963528 6.088 304FREE RADICAL BIO MED0891-584926148 6.081 305NEUROSCIENTIST1073-85842497 6.079 306J THROMB HAEMOST1538-79339876 6.069 307MOL CELL BIOL0270-730670185 6.057 308J BONE MINER RES0884-043119632 6.043 309NEW PHYTOL0028-646X20398 6.033 310J HIGH ENERGY PHYS1126-670837887 6.019 311AM J GASTROENTEROL0002-927026199 6.012 312NANOMEDICINE-UK1743-5889931 5.982 313MOL BIOL CELL1059-152428605 5.979 314BASIC RES CARDIOL0300-84282379 5.973 315SLEEP MED REV1087-07921961 5.967 316MOL ECOL0962-108322557 5.96 317J CONTROL RELEASE0168-365920423 5.949 318J INTERN MED0954-******** 5.942 319NEUROBIOL AGING0197-458010728 5.937 320GLOBAL ECOL BIOGEOGR1466-822X3191 5.913 321STRUCTURE0969-212610927 5.904 322BRAIN PATHOL1015-63053260 5.903 323NAT CLIN PRACT CARD1743-42971484 5.902 324PROG HISTOCHEM CYTO0079-6336240 5.9 325HEART FAIL REV1382-41471076 5.865 326J INFECT DIS0022-189938500 5.865 327AGE0161-9152699 5.839 328GREEN CHEM1463-92627435 5.836 329ORPHANET J RARE DIS1750-1172684 5.825 330LASER PHOTONICS REV1863-8880328 5.814 331CARDIOVASC RES0008-636317024 5.801332BRIT J PSYCHIAT0007-125019155 5.777 333PLOS COMPUT BIOL1553-734X4674 5.759 334J MED GENET0022-259310710 5.751 335ADV MICROB PHYSIOL0065-2911845 5.75 336NEUROSIGNALS1424-862X557 5.75 337NANOTOXICOLOGY1743-5390258 5.744 338NEUROIMAGE1053-811940455 5.739 339CELL MICROBIOL1462-58146620 5.725 340ADV COLLOID INTERFAC0001-86865220 5.675 341AM J PATHOL0002-944035130 5.673 342COCHRANE DB SYST REV1469-493X23102 5.653 343ANN ONCOL0923-753416964 5.647 344J IMMUNOL0022-1767124712 5.646 345CLIN GASTROENTEROL H1542-35655503 5.642 346BMC BIOL1741-70071152 5.636 347AGEING RES REV1568-16371170 5.622 348POLYM REV1558-3724422 5.612 349AM J EPIDEMIOL0002-926230208 5.589 350WORLD J BIOL PSYCHIA1562-2975782 5.564 351GLOBAL CHANGE BIOL1354-101310842 5.561 352RNA BIOL1547-6286491 5.559 353ENDOSCOPY0013-726X7323 5.545 354J INVEST DERMATOL0022-202X20245 5.543 355NAT CLIN PRACT RHEUM1745-83821168 5.533 356EUR RESPIR J0903-193621199 5.527 357ADV VIRUS RES0065-35271328 5.522 358EPIDEMIOLOGY1044-39837426 5.511 359CHEM COMMUN1359-734584236 5.504 360BIPOLAR DISORD1398-56473717 5.502 361LASER PHYS LETT1612-20112423 5.502 362ADV APPL MECH0065-2156966 5.5 363OPHTHALMOLOGY0161-642022568 5.491 364CURR OPIN COLLOID IN1359-02943575 5.488 365SEMIN IMMUNOPATHOL1863-2297407 5.479 366J CEREBR BLOOD F MET0271-678X11951 5.457 367EXP ASTRON0922-6435321 5.444 368NANOMED-NANOTECHNOL1549-9634741 5.44 369DRUG METAB REV0360-25322058 5.439 370CURR OPIN NEUROL1350-75403865 5.43 371BIOSENS BIOELECTRON0956-566314196 5.429 372EVOLUTION0014-382027799 5.429 373ORG LETT1523-706054155 5.42374CANCER-AM CANCER SOC0008-543X60723 5.418 375MOL PHARMACEUT1543-83842117 5.408 376SLEEP0161-810511511 5.402 377NEUROREHAB NEURAL RE1545-96831460 5.398 378HEART1355-603711674 5.385 379CHEM-EUR J0947-653938981 5.382 380J COGNITIVE Neuroscience0898-929X11863 5.382 381NEUROTHERAPEUTICS1933-7213826 5.381 382COMPUT INTELL0824-79351223 5.378 383EUR CELLS MATER1473-2262727 5.378 384PAIN0304-395925665 5.371 385CHEM MATER0897-475657387 5.368 386CHEM MATER0897-475657387 5.368 387PSYCHOTHER PSYCHOSOM0033-31902305 5.368 388REV ENDOCR METAB DIS1389-9155969 5.365 389MOL MICROBIOL0950-382X34568 5.361 390ANESTHESIOLOGY0003-302221357 5.354 391J BIOL CHEM0021-9258406606 5.328 392BREAST CANCER RES1465-54114644 5.326 393B WORLD HEALTH ORGAN0042-96868463 5.302 394CANCER TREAT REV0305-73722546 5.295 395J CATAL0021-951731744 5.288 396INT J NONLIN SCI NUM1565-13391629 5.276 397CRIT REV ONCOL HEMAT1040-84283690 5.269 398INT J EPIDEMIOL0300-577111744 5.262 399MOL ENDOCRINOL0888-880914504 5.257 400APPL CATAL B-ENVIRON0926-337315299 5.252 401NEUROPSYCHOL REV1040-7308862 5.231 402J CELL MOL MED1582-18383733 5.228 403J CLIN PSYCHIAT0160-668916807 5.218 404ANAL CHEM0003-270082246 5.214 405J PINEAL RES0742-30983693 5.209 406CARDIOVASC DRUG REV0897-5957520 5.208 407BRIT J PHARMACOL0007-118824221 5.204 408RNA1355-83829573 5.198 409CURR OPIN HEMATOL1065-62512298 5.193 410ADV SYNTH CATAL1615-415010194 5.187 411EUR J IMMUNOL0014-298020139 5.179 412SEMIN LIVER DIS0272-80873049 5.171 413INTENS CARE MED0342-464212420 5.168 414ADV STUD BEHAV0065-3454979 5.167 415CRIT REV SOLID STATE1040-8436571 5.167416BIOCHEM J0264-602150632 5.155 417AM J KIDNEY DIS0272-638618420 5.152 418J VIROL0022-538X88215 5.15 419J PROTEOME RES1535-389310352 5.132 420CURR CANCER DRUG TAR1568-00961812 5.129 421CELL TRANSPLANT0963-68972943 5.126 422BIOESSAYS0265-92478656 5.125 423PHILOS T R SOC B0962-843618674 5.117 424ACS CHEM BIOL1554-89291360 5.108 425MON NOT R ASTRON SOC0035-871170989 5.103 426CURR MOL MED1566-52402398 5.096 427J CLIN PSYCHOPHARM0271-07494683 5.092 428MOL NEURODEGENER1750-1326379 5.091 429OBES REV1467-78812844 5.086 430PHYS LETT B0370-269357638 5.083 431STEM CELL REV1550-8943583 5.083 432PLANT CELL ENVIRON0140-779111291 5.081 433BRAIN BEHAV IMMUN0889-15913403 5.061 434MEDICINE0025-79745246 5.054 435NEOPLASIA1522-80024232 5.025 436PUBL ASTRON SOC JPN0004-62645033 5.022 437MOL MED1076-15512785 5.02 438PSYCHOL MED0033-291713045 5.012 439J MOL MED-JMM0946-27164958 5.004 440INT REV PHYS CHEM0144-235X13995 441OSTEOPOROSIS INT0937-941X9728 4.997 442J HYPERTENS0263-635213845 4.988 443NEURO-ONCOLOGY1522-85171867 4.984 444J AM ACAD CHILD PSY0890-856715806 4.983 445J CHILD PSYCHOL PSYC0021-963010594 4.983 446CHROMOSOMA0009-59153428 4.979 447MAYO CLIN PROC0025-61969060 4.973 448CURR ALZHEIMER RES1567-20501280 4.971 449J MOL CELL CARDIOL0022-28289263 4.965 450MOL CANCER THER1535-71639861 4.953 451NAT CLIN PRACT NEPHR1745-8323864 4.938 452GLIA0894-14918207 4.932 453CRIT CARE1466-609X5771 4.931 454CLADISTICS0748-30072999 4.929 455BIOINFORMATICS1367-480336932 4.926 456CNS DRUG REV1080-563X670 4.923 457PHYS REV D1550-7998113952 4.922458DNA RES1340-28381627 4.917 459J LIPID RES0022-227517618 4.917 460IEEE SIGNAL PROC MAG1053-58883176 4.914 461AIDS0269-937019911 4.909 462ENVIRON MICROBIOL1462-29128268 4.909 463CURR OPIN DRUG DISC1367-67331916 4.904 464INT J BIOCHEM CELL B1357-27259576 4.887 465BIOFUEL BIOPROD BIOR1932-104X327 4.885 466J SEX MED1743-60954088 4.884 467ATMOS CHEM PHYS1680-731610675 4.881 468P IEEE0018-921917919 4.878 469INT J NEUROPSYCHOPH1461-14572392 4.874 470J NEUROL NEUROSUR PS0022-305022316 4.869 471SOFT MATTER1744-683X3898 4.869 472ECOL MONOGR0012-96157461 4.862 473INT MATER REV0950-66081911 4.857 474CURR GENE THER1566-52321159 4.852 475INVEST RADIOL0020-99964866 4.85 476CLIN J AM SOC NEPHRO1555-90413285 4.844 477RENEW SUST ENERG REV1364-03212580 4.842 478Q REV BIOL0033-57702999 4.818 479TOXICOL SCI1096-608011199 4.814 480J CHEM THEORY COMPUT1549-96183898 4.804 481ANTIMICROB AGENTS CH0066-480441312 4.802 482J MED CHEM0022-262349474 4.802 483AM NAT0003-014723373 4.796 484CARCINOGENESIS0143-333419181 4.795 485J MATER CHEM0959-942829162 4.795 486INT J PLASTICITY0749-64194179 4.791 487ENVIRON INT0160-41205746 4.786 488CRIT REV PLANT SCI0735-******** 4.769 489CHEMSUSCHEM1864-5631665 4.767 490J PHYSIOL-LONDON0022-375145237 4.764 491ARCH DERMATOL0003-987X11875 4.76 492ENDOCRINOLOGY0013-722745870 4.752 493GENE THER0969-71289279 4.745 494EVOL APPL1752-4571290 4.744 495MOL NEUROBIOL0893-******** 4.735 496DRUGS0012-66678608 4.732 497PLANT BIOTECHNOL J1467-76441617 4.732 498ADDICT BIOL1355-62151055 4.728 499ARCH PEDIAT ADOL MED1072-47107883 4.726500METAB ENG1096-71761425 4.725 501SEMIN ARTHRITIS RHEU0049-01722922 4.724 502CURR OPIN INFECT DIS0951-******** 4.723 503FRONT HORM RES0301-3073270 4.722 504INT J CANCER0020-713637606 4.722 505CURR MED CHEM0929-86739224 4.708 506BREAST CANCER RES TR0167-68069695 4.696 507PLOS NEGLECT TROP D1935-2735992 4.693 508J ECOL0022-047711390 4.69 509PEDIATRICS0031-400549012 4.687 510PERSPECT PLANT ECOL1433-8319770 4.684 511CANCER PREV RES1940-6207385 4.678 512IEEE T IND ELECTRON0278-004610306 4.678 513J NEUROINFLAMM1742-2094787 4.675 514CONSERV BIOL0888-889214167 4.666 515INORG CHEM0020-166979450 4.657 516INFLAMM BOWEL DIS1078-09984625 4.643 517ENVIRON SCI TECHNOL0013-936X68301 4.63 518GONDWANA RES1342-937X2189 4.605 519LAB INVEST0023-68379928 4.602 520ADV POLYM SCI0065-31954067 4.6 521CURR OPIN RHEUMATOL1040-87113393 4.6 522BRIT J HAEMATOL0007-104820427 4.597 523INT J RADIAT ONCOL0360-301635394 4.592 524IEEE T EVOLUT COMPUT1089-778X5038 4.589 525SPACE SCI REV0038-63086078 4.589 526CURR ISSUES MOL BIOL1467-3037382 4.588 527J CELL PHYSIOL0021-954113857 4.586 528EPIGENETICS1559-2294545 4.584 529J NEUROPATH EXP NEUR0022-30698087 4.564 530AM J MED GENET C1552-4868924 4.562 531CLIN PHARMACOKINET0312-******** 4.56 532HEART RHYTHM1547-52713936 4.559 533ALZHEIMERS DEMENT1552-5260799 4.553 534J THORAC ONCOL1556-08642585 4.547 535FUNCT ECOL0269-84637240 4.546 536MACROMOLECULES0024-929788896 4.539 537EUR J NUCL MED MOL I1619-70708793 4.531 538MOL PHARMACOL0026-895X21568 4.531 539HUM GENET0340-67178572 4.523 540ATHEROSCLEROSIS0021-915015753 4.522 541NAT CLIN PRACT GASTR1743-43781024 4.52542NEUROBIOL DIS0969-99617175 4.518 543VLDB J1066-88882247 4.517 544CARBON0008-622319880 4.504 545BIOMACROMOLECULES1525-779715346 4.502 546FISH FISH1467-29601068 4.489 547MIS QUART0276-77836186 4.485 548ASTRON J0004-625633695 4.481 549CRIT REV CL LAB SCI1040-8363910 4.48 550CURR TOP MED CHEM1568-02663872 4.473 551AM J MED0002-934323323 4.466 552SCHIZOPHR RES0920-996412453 4.458 553ADV CANCER RES0065-230X1815 4.456 554THROMB HAEMOSTASIS0340-624515722 4.451 555PHYS TODAY0031-92283501 4.437 556PROTEOMICS1615-985314334 4.426 557J BIOL RHYTHM0748-73042299 4.418 558BIOFOULING0892-******** 4.415 559BRAIN STRUCT FUNCT1863-2653238 4.415 560CURR PHARM DESIGN1381-61288781 4.414 561ECOLOGY0012-965842832 4.411 562MOL PLANT MICROBE IN0894-02827767 4.407 563MODERN PATHOL0893-******** 4.406 564J LEUKOCYTE BIOL0741-540013946 4.403 565PHARMACOGENOMICS J1470-269X1531 4.398 566AM J PHYSIOL-ENDOC M0193-184918615 4.395 567BIOPHYS J0006-349549705 4.39 568ECOGRAPHY0906-******** 4.385 569GEOCHIM COSMOCHIM AC0016-703738529 4.385 570DEV BIOL0012-160629930 4.379 571IEEE T PATTERN ANAL0162-882822851 4.378 572WORLD PSYCHIATRY1723-8617550 4.375 573BBA-MOL CELL RES0167-48897018 4.374 574CHEM-ASIAN J1861-47282088 4.373 575AM J PUBLIC HEALTH0090-003623364 4.371 576NANO RES1998-0124294 4.37 577GEOLOGY0091-761325639 4.368 578TRENDS CARDIOVAS MED1050-17382181 4.367 579BIOL PSYCHOL0301-05113983 4.363 580ALIMENT PHARM THER0269-281311831 4.357 581AM HEART J0002-870318708 4.357 582BBA-MOL CELL BIOL L1388-19814858 4.357 583OBSTET GYNECOL0029-784423769 4.357584MOL NUTR FOOD RES1613-41252662 4.356 585WATER RES0043-135433139 4.355 586J ANTIMICROB CHEMOTH0305-745316521 4.352 587PLOS ONE1932-620320466 4.351 588BIOCONJUGATE CHEM1043-180210370 4.35 589BRIT J CANCER0007-092031646 4.346 590NEUROPSYCHOLOGIA0028-393216540 4.345 591PIGM CELL MELANOMA R1755-14712362 4.344 592INT J OBESITY0307-056516258 4.343 593RADIOTHER ONCOL0167-81408896 4.343 594NUCL PHYS B0550-321341039 4.341 595CURR OPIN GASTROEN0267-13791547 4.331 596ARCH SURG-CHICAGO0004-001013619 4.323 597ANTIVIR THER1359-65352956 4.322 598P NUTR SOC0029-66513754 4.321 599AM J RESP CELL MOL1044-15499930 4.319 600SEMIN RADIAT ONCOL1053-42961563 4.318 601CANCER EPIDEM BIOMAR1055-996516984 4.31 602TRENDS PARASITOL1471-49224074 4.298 603BMC EVOL BIOL1471-21484236 4.294 604GLOBAL BIOGEOCHEM CY0886-******** 4.294 605CURR OPIN CLIN NUTR1363-19502606 4.291 606TOP CURR CHEM0340-10224636 4.291 607CELL CALCIUM0143-41604288 4.288 608J NUTR BIOCHEM0955-28633876 4.288 609ENDOCR-RELAT CANCER1351-00883434 4.282 610TELLUS B0280-65092907 4.278 611ARTHRITIS RES THER1478-63625513 4.271 612J EXP BOT0022-095717681 4.271 613NUCL FUSION0029-55156839 4.27 614MACROMOL RAPID COMM1022-133610015 4.263 615BRIT J DERMATOL0007-096317207 4.26 616J GEN PHYSIOL0022-12957746 4.26 617BIOCHEM PHARMACOL0006-295223088 4.254 618BIORESOURCE TECHNOL0960-852420537 4.253 619J NEUROTRAUM0897-71515958 4.252 620ANN MED0785-38903260 4.246 621PROG CARDIOVASC DIS0033-06201688 4.246 622QUATERNARY SCI REV0277-379110031 4.245 623ELECTROCHEM COMMUN1388-248110935 4.243 624J R SOC INTERFACE1742-56891946 4.241 625PSYCHOSOM MED0033-317410073 4.236626RHEUMATOLOGY1462-032410005 4.236 627AM J PREV MED0749-37979457 4.235 628ANN EMERG MED0196-06448293 4.232 629CRIT REV TOXICOL1040-84442399 4.232 630DIVERS DISTRIB1366-95162409 4.224 631J PHYS CHEM C1932-744725129 4.224 632GENES IMMUN1466-48793115 4.222 633OCUL SURF1542-0124363 4.222 634J ORG CHEM0022-326392992 4.219 635EXPERT OPIN INV DRUG1354-37843515 4.218 636EXPERT REV VACCINES1476-05841643 4.214 637JAIDS-J ACQ IMM DEF1525-413510719 4.207 638INFECT IMMUN0019-956752874 4.205 639ORGANOMETALLICS0276-733340038 4.204 640SOC COGN AFFECT NEUR1749-5016512 4.203 641HUM GENE THER1043-03426286 4.202 642IMMUNOL CELL BIOL0818-******** 4.2 643DNA REPAIR1568-78644298 4.199 644J APPL ECOL0021-89019571 4.197 645PSYCHONEUROENDOCRINO0306-45306284 4.194 646MOL PAIN1744-8069789 4.187 647CRYSTENGCOMM1466-80334407 4.183 648ASTRON ASTROPHYS0004-636188679 4.179 649MECH AGEING DEV0047-63744839 4.179 650ANN MATH0003-486X9238 4.174 651CELL ONCOL1570-5870460 4.169 652ADV BIOCHEM ENG BIOT0724-******** 4.165 653CRYST GROWTH DES1528-748311344 4.162 654J CLIN MICROBIOL0095-113749301 4.162 655MOL CANCER RES1541-77863244 4.162 656CURR TOP MICROBIOL0070-217X4451 4.16 657MOL CANCER1476-45981766 4.16 658J METAMORPH GEOL0263-49294231 4.157 659REGION ANESTH PAIN M1098-73392507 4.157 660ARTHRIT RHEUM-ARTHR0004-35914988 4.152 661STRUCT BOND0081-59931884 4.152 662STEM CELLS DEV1547-32871749 4.146 663MITOCHONDRION1567-72491123 4.145 664J HAZARD MATER0304-389421180 4.144 665BBA-MOL BASIS DIS0925-44394273 4.139 666REJUV RES1549-1684916 4.138 667ASTROPART PHYS0927-65053219 4.136668B AM MUS NAT HIST0003-00902256 4.133 669ANN FAM MED1544-17091887 4.13 670ANN SURG ONCOL1068-92659632 4.13 671DIABETES OBES METAB1462-89022617 4.126 672COGNITIVE PSYCHOL0010-02855397 4.122 673HEREDITY0018-067X5497 4.122 674EUR J CANCER0959-804917414 4.121 675BIOTECHNOL BIOFUELS1754-683483 4.118 676PHYS CHEM CHEM PHYS1463-907620798 4.116 677PROG INORG CHEM0079-63791666 4.111 678J AM ACAD DERMATOL0190-962217472 4.105 679RETROVIROLOGY1742-46901642 4.105 680PSYCHOPHARMACOLOGY0033-315822332 4.103 681J CHROMATOGR A0021-967357286 4.101 682CELL SIGNAL0898-******** 4.094 683J PHARMACOL EXP THER0022-356535121 4.093 684J PEDIATR-US0022-347622057 4.092 685J NUTR0022-316631695 4.091 686PROG QUANT ELECTRON0079-6727619 4.091 687BONE8756-328213402 4.089 688CURR OPIN ONCOL1040-87462389 4.088 689CELL CYCLE1538-41018938 4.087 690J BIOGEOGR0305-02707909 4.087 691CLIN EXP ALLERGY0954-******** 4.084 692DALTON T1477-922633871 4.081 693LEARN MEMORY1072-05024127 4.079 694BRIT J SURG0007-132318455 4.077 695J MAMMARY GLAND BIOL1083-30211637 4.074 696APOPTOSIS1360-81853862 4.066 697BIOSCIENCE0006-35688783 4.064 698BMC SYST BIOL1752-0509716 4.064 699AM J SURG PATHOL0147-518514848 4.062 700EARTH PLANET SC LETT0012-821X35555 4.062 701CORTEX0010-94524205 4.058 702EPILEPSIA0013-958018410 4.052 703COGN AFFECT BEHAV NE1530-70261608 4.051 704TRENDS FOOD SCI TECH0924-22444287 4.051 705AM J PHYSIOL-LUNG C1040-060512359 4.043 706GEOTEXT GEOMEMBRANES0266-11441024 4.039 707PLASMA PROCESS POLYM1612-88501206 4.037 708INT REV NEUROBIOL0074-77421561 4.017 709J UROLOGY0022-534747386 4.016710J BIOENERG BIOMEMBR0145-479X2769 4.015 711MOL BIOSYST1742-206X1393 4.015 712CLIN MICROBIOL INFEC1198-743X5566 4.014 713MOVEMENT DISORD0885-318512863 4.014 714AM J PHYSIOL-CELL PH0363-614316835 4.013 715AM J BIOETHICS1526-516111914 716CURR OPIN SOLID ST M1359-028622874 717ECONOMETRICA0012-9682206434 718J NEUROCHEM0022-304236694 3.999 719BBA-BIOMEMBRANES0005-273610220 3.998 720PROG BIOPHYS MOL BIO0079-61073092 3.992 721PHARMACOGENET GENOM1744-68722007 3.991 722CURR DRUG METAB1389-20022136 3.989 723CHRONOBIOL INT0742-05282142 3.987 724BMC MED1741-7015864 3.985 725CLIN SCI0143-52217410 3.982 726PLANT MOL BIOL0167-441212187 3.978 727ACTA BIOMATER1742-70612320 3.975 728BIOL CELL0248-49002396 3.974 729J AM MED INFORM ASSN1067-50274183 3.974 730J POLYM SCI POL CHEM0887-624X22907 3.971 731FERTIL STERIL0015-028224036 3.97 732SEMIN NUCL MED0001-29981666 3.962 733CURR OPIN NEPHROL HY1062-48212420 3.961 734CURR PROB CARDIOLOGY0146-2806378 3.957 735INT J HYDROGEN ENERG0360-319915069 3.945 736J BACTERIOL0021-919363692 3.94 737CURR DRUG TARGETS1389-45012576 3.932 738PHYSIOL GENOMICS1094-83413893 3.931 739GLYCOBIOLOGY0959-66585577 3.929 740PHARMACOL RES1043-66184154 3.929 741PSYCHOPHYSIOLOGY0048-57728686 3.926 742J MED INTERNET RES1438-88711319 3.924 743GENET MED1098-36002417 3.922 744CLIM DYNAM0930-******** 3.917 745CLIN NUCL MED0363-97622343 3.915 746EXP NEUROL0014-488615395 3.914 747HIPPOCAMPUS1050-96315550 3.913 748CLIN EXP METASTAS0262-08982607 3.91 749NEUROPHARMACOLOGY0028-390811509 3.909 750ADV MAR BIOL0065-28811007 3.9 751LANGMUIR0743-746384792 3.898752BIOCHIMIE0300-90846039 3.897 753BEST PRACT RES CL EN1521-690X1515 3.893 754PHARMACOGENOMICS1462-24162225 3.893 755GENETICS0016-673141385 3.889 756OSTEOARTHR CARTILAGE1063-45846425 3.888 757J CHEM INF MODEL1549-95968973 3.882 758CNS DRUGS1172-70472459 3.879 759AMINO ACIDS0939-44513036 3.877 760MILBANK Q0887-378X1917 3.872 761J MOL BIOL0022-283669710 3.871 762METABOLOMICS1573-3882552 3.871 763CLIN IMMUNOL1521-66166011 3.863 764CHEM REC1527-89991029 3.862 765SURG OBES RELAT DIS1550-72891210 3.862 766ARCH OPHTHALMOL-CHIC0003-995017277 3.859 767HUM REPROD0268-116123668 3.859 768GENE CHROMOSOME CANC1045-22575025 3.858 769SOL ENERG MAT SOL C0927-02488327 3.858 770J TISSUE ENG REGEN M1932-6254464 3.857 771HISTOPATHOLOGY0309-01676874 3.855 772CURR PROTEIN PEPT SC1389-20371322 3.854 773J RHEUMATOL0315-162X20874 3.854 774PROTIST1434-46101039 3.853 775TOB CONTROL0964-45633703 3.852 776ADDICTION0965-214010432 3.842 777J COMPUT AID MOL DES0920-654X3170 3.835 778AM J OPHTHALMOL0002-939418083 3.833 779J ALZHEIMERS DIS1387-28772679 3.832 780BRIT J ANAESTH0007-091211136 3.827 781CLIM PAST1814-9324401 3.826 782FUNCT INTEGR GENOMIC1438-793X890 3.825 783CHEMBIOCHEM1439-42277221 3.824 784INT J PARASITOL0020-75197986 3.819 785J EVOLUTION BIOL1010-061X6529 3.816 786EUKARYOT CELL1535-97785055 3.806 787HUM HERED0001-56521847 3.806 788FUNGAL DIVERS1560-2745893 3.803 789ADV AGRON0065-21132334 3.8 790MENT RETARD DEV D R1080-40131487 3.8 791GENES BRAIN BEHAV1601-18481760 3.795 792J POWER SOURCES0378-775333580 3.792 793CANCER IMMUNOL IMMUN0340-70044218 3.791。
OSHA现场作业手册说明书

DIRECTIVE NUMBER: CPL 02-00-150 EFFECTIVE DATE: April 22, 2011 SUBJECT: Field Operations Manual (FOM)ABSTRACTPurpose: This instruction cancels and replaces OSHA Instruction CPL 02-00-148,Field Operations Manual (FOM), issued November 9, 2009, whichreplaced the September 26, 1994 Instruction that implemented the FieldInspection Reference Manual (FIRM). The FOM is a revision of OSHA’senforcement policies and procedures manual that provides the field officesa reference document for identifying the responsibilities associated withthe majority of their inspection duties. This Instruction also cancels OSHAInstruction FAP 01-00-003 Federal Agency Safety and Health Programs,May 17, 1996 and Chapter 13 of OSHA Instruction CPL 02-00-045,Revised Field Operations Manual, June 15, 1989.Scope: OSHA-wide.References: Title 29 Code of Federal Regulations §1903.6, Advance Notice ofInspections; 29 Code of Federal Regulations §1903.14, Policy RegardingEmployee Rescue Activities; 29 Code of Federal Regulations §1903.19,Abatement Verification; 29 Code of Federal Regulations §1904.39,Reporting Fatalities and Multiple Hospitalizations to OSHA; and Housingfor Agricultural Workers: Final Rule, Federal Register, March 4, 1980 (45FR 14180).Cancellations: OSHA Instruction CPL 02-00-148, Field Operations Manual, November9, 2009.OSHA Instruction FAP 01-00-003, Federal Agency Safety and HealthPrograms, May 17, 1996.Chapter 13 of OSHA Instruction CPL 02-00-045, Revised FieldOperations Manual, June 15, 1989.State Impact: Notice of Intent and Adoption required. See paragraph VI.Action Offices: National, Regional, and Area OfficesOriginating Office: Directorate of Enforcement Programs Contact: Directorate of Enforcement ProgramsOffice of General Industry Enforcement200 Constitution Avenue, NW, N3 119Washington, DC 20210202-693-1850By and Under the Authority ofDavid Michaels, PhD, MPHAssistant SecretaryExecutive SummaryThis instruction cancels and replaces OSHA Instruction CPL 02-00-148, Field Operations Manual (FOM), issued November 9, 2009. The one remaining part of the prior Field Operations Manual, the chapter on Disclosure, will be added at a later date. This Instruction also cancels OSHA Instruction FAP 01-00-003 Federal Agency Safety and Health Programs, May 17, 1996 and Chapter 13 of OSHA Instruction CPL 02-00-045, Revised Field Operations Manual, June 15, 1989. This Instruction constitutes OSHA’s general enforcement policies and procedures manual for use by the field offices in conducting inspections, issuing citations and proposing penalties.Significant Changes∙A new Table of Contents for the entire FOM is added.∙ A new References section for the entire FOM is added∙ A new Cancellations section for the entire FOM is added.∙Adds a Maritime Industry Sector to Section III of Chapter 10, Industry Sectors.∙Revises sections referring to the Enhanced Enforcement Program (EEP) replacing the information with the Severe Violator Enforcement Program (SVEP).∙Adds Chapter 13, Federal Agency Field Activities.∙Cancels OSHA Instruction FAP 01-00-003, Federal Agency Safety and Health Programs, May 17, 1996.DisclaimerThis manual is intended to provide instruction regarding some of the internal operations of the Occupational Safety and Health Administration (OSHA), and is solely for the benefit of the Government. No duties, rights, or benefits, substantive or procedural, are created or implied by this manual. The contents of this manual are not enforceable by any person or entity against the Department of Labor or the United States. Statements which reflect current Occupational Safety and Health Review Commission or court precedents do not necessarily indicate acquiescence with those precedents.Table of ContentsCHAPTER 1INTRODUCTIONI.PURPOSE. ........................................................................................................... 1-1 II.SCOPE. ................................................................................................................ 1-1 III.REFERENCES .................................................................................................... 1-1 IV.CANCELLATIONS............................................................................................. 1-8 V. ACTION INFORMATION ................................................................................. 1-8A.R ESPONSIBLE O FFICE.......................................................................................................................................... 1-8B.A CTION O FFICES. .................................................................................................................... 1-8C. I NFORMATION O FFICES............................................................................................................ 1-8 VI. STATE IMPACT. ................................................................................................ 1-8 VII.SIGNIFICANT CHANGES. ............................................................................... 1-9 VIII.BACKGROUND. ................................................................................................. 1-9 IX. DEFINITIONS AND TERMINOLOGY. ........................................................ 1-10A.T HE A CT................................................................................................................................................................. 1-10B. C OMPLIANCE S AFETY AND H EALTH O FFICER (CSHO). ...........................................................1-10B.H E/S HE AND H IS/H ERS ..................................................................................................................................... 1-10C.P ROFESSIONAL J UDGMENT............................................................................................................................... 1-10E. W ORKPLACE AND W ORKSITE ......................................................................................................................... 1-10CHAPTER 2PROGRAM PLANNINGI.INTRODUCTION ............................................................................................... 2-1 II.AREA OFFICE RESPONSIBILITIES. .............................................................. 2-1A.P ROVIDING A SSISTANCE TO S MALL E MPLOYERS. ...................................................................................... 2-1B.A REA O FFICE O UTREACH P ROGRAM. ............................................................................................................. 2-1C. R ESPONDING TO R EQUESTS FOR A SSISTANCE. ............................................................................................ 2-2 III. OSHA COOPERATIVE PROGRAMS OVERVIEW. ...................................... 2-2A.V OLUNTARY P ROTECTION P ROGRAM (VPP). ........................................................................... 2-2B.O NSITE C ONSULTATION P ROGRAM. ................................................................................................................ 2-2C.S TRATEGIC P ARTNERSHIPS................................................................................................................................. 2-3D.A LLIANCE P ROGRAM ........................................................................................................................................... 2-3 IV. ENFORCEMENT PROGRAM SCHEDULING. ................................................ 2-4A.G ENERAL ................................................................................................................................................................. 2-4B.I NSPECTION P RIORITY C RITERIA. ..................................................................................................................... 2-4C.E FFECT OF C ONTEST ............................................................................................................................................ 2-5D.E NFORCEMENT E XEMPTIONS AND L IMITATIONS. ....................................................................................... 2-6E.P REEMPTION BY A NOTHER F EDERAL A GENCY ........................................................................................... 2-6F.U NITED S TATES P OSTAL S ERVICE. .................................................................................................................. 2-7G.H OME-B ASED W ORKSITES. ................................................................................................................................ 2-8H.I NSPECTION/I NVESTIGATION T YPES. ............................................................................................................... 2-8 V.UNPROGRAMMED ACTIVITY – HAZARD EVALUATION AND INSPECTION SCHEDULING ............................................................................ 2-9 VI.PROGRAMMED INSPECTIONS. ................................................................... 2-10A.S ITE-S PECIFIC T ARGETING (SST) P ROGRAM. ............................................................................................. 2-10B.S CHEDULING FOR C ONSTRUCTION I NSPECTIONS. ..................................................................................... 2-10C.S CHEDULING FOR M ARITIME I NSPECTIONS. ............................................................................. 2-11D.S PECIAL E MPHASIS P ROGRAMS (SEP S). ................................................................................... 2-12E.N ATIONAL E MPHASIS P ROGRAMS (NEP S) ............................................................................... 2-13F.L OCAL E MPHASIS P ROGRAMS (LEP S) AND R EGIONAL E MPHASIS P ROGRAMS (REP S) ............ 2-13G.O THER S PECIAL P ROGRAMS. ............................................................................................................................ 2-13H.I NSPECTION S CHEDULING AND I NTERFACE WITH C OOPERATIVE P ROGRAM P ARTICIPANTS ....... 2-13CHAPTER 3INSPECTION PROCEDURESI.INSPECTION PREPARATION. .......................................................................... 3-1 II.INSPECTION PLANNING. .................................................................................. 3-1A.R EVIEW OF I NSPECTION H ISTORY .................................................................................................................... 3-1B.R EVIEW OF C OOPERATIVE P ROGRAM P ARTICIPATION .............................................................................. 3-1C.OSHA D ATA I NITIATIVE (ODI) D ATA R EVIEW .......................................................................................... 3-2D.S AFETY AND H EALTH I SSUES R ELATING TO CSHO S.................................................................. 3-2E.A DVANCE N OTICE. ................................................................................................................................................ 3-3F.P RE-I NSPECTION C OMPULSORY P ROCESS ...................................................................................................... 3-5G.P ERSONAL S ECURITY C LEARANCE. ................................................................................................................. 3-5H.E XPERT A SSISTANCE. ........................................................................................................................................... 3-5 III. INSPECTION SCOPE. ......................................................................................... 3-6A.C OMPREHENSIVE ................................................................................................................................................... 3-6B.P ARTIAL. ................................................................................................................................................................... 3-6 IV. CONDUCT OF INSPECTION .............................................................................. 3-6A.T IME OF I NSPECTION............................................................................................................................................. 3-6B.P RESENTING C REDENTIALS. ............................................................................................................................... 3-6C.R EFUSAL TO P ERMIT I NSPECTION AND I NTERFERENCE ............................................................................. 3-7D.E MPLOYEE P ARTICIPATION. ............................................................................................................................... 3-9E.R ELEASE FOR E NTRY ............................................................................................................................................ 3-9F.B ANKRUPT OR O UT OF B USINESS. .................................................................................................................... 3-9G.E MPLOYEE R ESPONSIBILITIES. ................................................................................................. 3-10H.S TRIKE OR L ABOR D ISPUTE ............................................................................................................................. 3-10I. V ARIANCES. .......................................................................................................................................................... 3-11 V. OPENING CONFERENCE. ................................................................................ 3-11A.G ENERAL ................................................................................................................................................................ 3-11B.R EVIEW OF A PPROPRIATION A CT E XEMPTIONS AND L IMITATION. ..................................................... 3-13C.R EVIEW S CREENING FOR P ROCESS S AFETY M ANAGEMENT (PSM) C OVERAGE............................. 3-13D.R EVIEW OF V OLUNTARY C OMPLIANCE P ROGRAMS. ................................................................................ 3-14E.D ISRUPTIVE C ONDUCT. ...................................................................................................................................... 3-15F.C LASSIFIED A REAS ............................................................................................................................................. 3-16VI. REVIEW OF RECORDS. ................................................................................... 3-16A.I NJURY AND I LLNESS R ECORDS...................................................................................................................... 3-16B.R ECORDING C RITERIA. ...................................................................................................................................... 3-18C. R ECORDKEEPING D EFICIENCIES. .................................................................................................................. 3-18 VII. WALKAROUND INSPECTION. ....................................................................... 3-19A.W ALKAROUND R EPRESENTATIVES ............................................................................................................... 3-19B.E VALUATION OF S AFETY AND H EALTH M ANAGEMENT S YSTEM. ....................................................... 3-20C.R ECORD A LL F ACTS P ERTINENT TO A V IOLATION. ................................................................................. 3-20D.T ESTIFYING IN H EARINGS ................................................................................................................................ 3-21E.T RADE S ECRETS. ................................................................................................................................................. 3-21F.C OLLECTING S AMPLES. ..................................................................................................................................... 3-22G.P HOTOGRAPHS AND V IDEOTAPES.................................................................................................................. 3-22H.V IOLATIONS OF O THER L AWS. ....................................................................................................................... 3-23I.I NTERVIEWS OF N ON-M ANAGERIAL E MPLOYEES .................................................................................... 3-23J.M ULTI-E MPLOYER W ORKSITES ..................................................................................................................... 3-27 K.A DMINISTRATIVE S UBPOENA.......................................................................................................................... 3-27 L.E MPLOYER A BATEMENT A SSISTANCE. ........................................................................................................ 3-27 VIII. CLOSING CONFERENCE. .............................................................................. 3-28A.P ARTICIPANTS. ..................................................................................................................................................... 3-28B.D ISCUSSION I TEMS. ............................................................................................................................................ 3-28C.A DVICE TO A TTENDEES .................................................................................................................................... 3-29D.P ENALTIES............................................................................................................................................................. 3-30E.F EASIBLE A DMINISTRATIVE, W ORK P RACTICE AND E NGINEERING C ONTROLS. ............................ 3-30F.R EDUCING E MPLOYEE E XPOSURE. ................................................................................................................ 3-32G.A BATEMENT V ERIFICATION. ........................................................................................................................... 3-32H.E MPLOYEE D ISCRIMINATION .......................................................................................................................... 3-33 IX. SPECIAL INSPECTION PROCEDURES. ...................................................... 3-33A.F OLLOW-UP AND M ONITORING I NSPECTIONS............................................................................................ 3-33B.C ONSTRUCTION I NSPECTIONS ......................................................................................................................... 3-34C. F EDERAL A GENCY I NSPECTIONS. ................................................................................................................. 3-35CHAPTER 4VIOLATIONSI. BASIS OF VIOLATIONS ..................................................................................... 4-1A.S TANDARDS AND R EGULATIONS. .................................................................................................................... 4-1B.E MPLOYEE E XPOSURE. ........................................................................................................................................ 4-3C.R EGULATORY R EQUIREMENTS. ........................................................................................................................ 4-6D.H AZARD C OMMUNICATION. .............................................................................................................................. 4-6E. E MPLOYER/E MPLOYEE R ESPONSIBILITIES ................................................................................................... 4-6 II. SERIOUS VIOLATIONS. .................................................................................... 4-8A.S ECTION 17(K). ......................................................................................................................... 4-8B.E STABLISHING S ERIOUS V IOLATIONS ............................................................................................................ 4-8C. F OUR S TEPS TO BE D OCUMENTED. ................................................................................................................... 4-8 III. GENERAL DUTY REQUIREMENTS ............................................................. 4-14A.E VALUATION OF G ENERAL D UTY R EQUIREMENTS ................................................................................. 4-14B.E LEMENTS OF A G ENERAL D UTY R EQUIREMENT V IOLATION.............................................................. 4-14C. U SE OF THE G ENERAL D UTY C LAUSE ........................................................................................................ 4-23D.L IMITATIONS OF U SE OF THE G ENERAL D UTY C LAUSE. ..............................................................E.C LASSIFICATION OF V IOLATIONS C ITED U NDER THE G ENERAL D UTY C LAUSE. ..................F. P ROCEDURES FOR I MPLEMENTATION OF S ECTION 5(A)(1) E NFORCEMENT ............................ 4-25 4-27 4-27IV.OTHER-THAN-SERIOUS VIOLATIONS ............................................... 4-28 V.WILLFUL VIOLATIONS. ......................................................................... 4-28A.I NTENTIONAL D ISREGARD V IOLATIONS. ..........................................................................................4-28B.P LAIN I NDIFFERENCE V IOLATIONS. ...................................................................................................4-29 VI. CRIMINAL/WILLFUL VIOLATIONS. ................................................... 4-30A.A REA D IRECTOR C OORDINATION ....................................................................................................... 4-31B.C RITERIA FOR I NVESTIGATING P OSSIBLE C RIMINAL/W ILLFUL V IOLATIONS ........................ 4-31C. W ILLFUL V IOLATIONS R ELATED TO A F ATALITY .......................................................................... 4-32 VII. REPEATED VIOLATIONS. ...................................................................... 4-32A.F EDERAL AND S TATE P LAN V IOLATIONS. ........................................................................................4-32B.I DENTICAL S TANDARDS. .......................................................................................................................4-32C.D IFFERENT S TANDARDS. .......................................................................................................................4-33D.O BTAINING I NSPECTION H ISTORY. .....................................................................................................4-33E.T IME L IMITATIONS..................................................................................................................................4-34F.R EPEATED V. F AILURE TO A BATE....................................................................................................... 4-34G. A REA D IRECTOR R ESPONSIBILITIES. .............................................................................. 4-35 VIII. DE MINIMIS CONDITIONS. ................................................................... 4-36A.C RITERIA ................................................................................................................................................... 4-36B.P ROFESSIONAL J UDGMENT. ..................................................................................................................4-37C. A REA D IRECTOR R ESPONSIBILITIES. .............................................................................. 4-37 IX. CITING IN THE ALTERNATIVE ............................................................ 4-37 X. COMBINING AND GROUPING VIOLATIONS. ................................... 4-37A.C OMBINING. ..............................................................................................................................................4-37B.G ROUPING. ................................................................................................................................................4-38C. W HEN N OT TO G ROUP OR C OMBINE. ................................................................................................4-38 XI. HEALTH STANDARD VIOLATIONS ....................................................... 4-39A.C ITATION OF V ENTILATION S TANDARDS ......................................................................................... 4-39B.V IOLATIONS OF THE N OISE S TANDARD. ...........................................................................................4-40 XII. VIOLATIONS OF THE RESPIRATORY PROTECTION STANDARD(§1910.134). ....................................................................................................... XIII. VIOLATIONS OF AIR CONTAMINANT STANDARDS (§1910.1000) ... 4-43 4-43A.R EQUIREMENTS UNDER THE STANDARD: .................................................................................................. 4-43B.C LASSIFICATION OF V IOLATIONS OF A IR C ONTAMINANT S TANDARDS. ......................................... 4-43 XIV. CITING IMPROPER PERSONAL HYGIENE PRACTICES. ................... 4-45A.I NGESTION H AZARDS. .................................................................................................................................... 4-45B.A BSORPTION H AZARDS. ................................................................................................................................ 4-46C.W IPE S AMPLING. ............................................................................................................................................. 4-46D.C ITATION P OLICY ............................................................................................................................................ 4-46 XV. BIOLOGICAL MONITORING. ...................................................................... 4-47CHAPTER 5CASE FILE PREPARATION AND DOCUMENTATIONI.INTRODUCTION ............................................................................................... 5-1 II.INSPECTION CONDUCTED, CITATIONS BEING ISSUED. .................... 5-1A.OSHA-1 ................................................................................................................................... 5-1B.OSHA-1A. ............................................................................................................................... 5-1C. OSHA-1B. ................................................................................................................................ 5-2 III.INSPECTION CONDUCTED BUT NO CITATIONS ISSUED .................... 5-5 IV.NO INSPECTION ............................................................................................... 5-5 V. HEALTH INSPECTIONS. ................................................................................. 5-6A.D OCUMENT P OTENTIAL E XPOSURE. ............................................................................................................... 5-6B.E MPLOYER’S O CCUPATIONAL S AFETY AND H EALTH S YSTEM. ............................................................. 5-6 VI. AFFIRMATIVE DEFENSES............................................................................. 5-8A.B URDEN OF P ROOF. .............................................................................................................................................. 5-8B.E XPLANATIONS. ..................................................................................................................................................... 5-8 VII. INTERVIEW STATEMENTS. ........................................................................ 5-10A.G ENERALLY. ......................................................................................................................................................... 5-10B.CSHO S SHALL OBTAIN WRITTEN STATEMENTS WHEN: .......................................................................... 5-10C.L ANGUAGE AND W ORDING OF S TATEMENT. ............................................................................................. 5-11D.R EFUSAL TO S IGN S TATEMENT ...................................................................................................................... 5-11E.V IDEO AND A UDIOTAPED S TATEMENTS. ..................................................................................................... 5-11F.A DMINISTRATIVE D EPOSITIONS. .............................................................................................5-11 VIII. PAPERWORK AND WRITTEN PROGRAM REQUIREMENTS. .......... 5-12 IX.GUIDELINES FOR CASE FILE DOCUMENTATION FOR USE WITH VIDEOTAPES AND AUDIOTAPES .............................................................. 5-12 X.CASE FILE ACTIVITY DIARY SHEET. ..................................................... 5-12 XI. CITATIONS. ..................................................................................................... 5-12A.S TATUTE OF L IMITATIONS. .............................................................................................................................. 5-13B.I SSUING C ITATIONS. ........................................................................................................................................... 5-13C.A MENDING/W ITHDRAWING C ITATIONS AND N OTIFICATION OF P ENALTIES. .................................. 5-13D.P ROCEDURES FOR A MENDING OR W ITHDRAWING C ITATIONS ............................................................ 5-14 XII. INSPECTION RECORDS. ............................................................................... 5-15A.G ENERALLY. ......................................................................................................................................................... 5-15B.R ELEASE OF I NSPECTION I NFORMATION ..................................................................................................... 5-15C. C LASSIFIED AND T RADE S ECRET I NFORMATION ...................................................................................... 5-16。
Epic PHI Audit User Guide

P HI Audit Us er G uideTable Of ContentsAuditable Actions (1)Navigating the PHI Audit Dashboard (2)Only community users with selected roles can access PHI Audit. For questionsabout PHI Audit access, contact your community administrator.The PHI Audit application enables you to audit:•Access to operations on a selected patient's data• A selected user's access to, and operations on, patient dataThe purpose of the audit tool is to review users’ accesses of Protected Health Information to verify accesses are in line with HIPAA requirements.PHI Audit allows you to:•Audit a specific patient's data to retrieve information about auditable actions that have been performed on the data.•Audit a specific user's actions to determine whose data they have accessed, and which auditable actions they performed on the data.Auditable ActionsPHI Audit retrieves information associated with actions described in the following table. You can select one or more actions when you create an audit.Patient PatientSearchResults Includes the search results from which the patient was selected.Patient PatientConsent Displays the consent selection that was selected for access to patient data.Patient Patient Opt In Indicates if the Opt In setting was changed. Patient Patient OptOutIndicates if the Opt Out setting was changed.Patient Patient PHIAccessDisplays the viewed patient data.User PatientConsent Displays the consent selection the user selected to access patient data.User PatientSearchCriteriaAudits the criteria used to search for a patient.User PatientSearchResults Audits the search results from which the user selected a patient.PHI Audit User GuideUser Patient Opt In Indicates if the user changed the Opt In setting. User Patient OptOutIndicates if the user changed the Opt Out setting.User Patient PHIAccess Indicates which tabs of patient data the user accessed.Navigating the PHI Audit DashboardImportant: As you perform tasks with the PHI Audit Dashboard, use theapplication's buttons to navigate. Do not use your browser's back button. Ifyou use your browser's back button for navigation, you may be returned to yourhealthcare community home page.Examples of application buttons:You can quickly initiate audit tasks from the Audit Dashboard window. To access tasks, scroll up or down the window as required to reach the section that supports the tasks you want to perform.In sections that display information in columns, you can sort the information by clicking column headings. Multiple clicks on a heading toggle the sort order between ascending and descending sequence.PHI Audit OverviewThe Start a new audit section provides access to two wizards used to create patient or user audits. For details, refer to:•Create a Patient Audit•Create a User AuditThe Scheduled audits section allows you to view or cancel audits that have not been submitted for system processing. For details, refer to View or Cancel Scheduled Audits.The Processed audits section allows you to view and delete processed audits. For details, refer to:•View a Processed Audit•Download Audit Data as CSV•Delete Processed AuditsOnly community users with selected roles can access PHI Audit. For questionsabout PHI Audit access, contact your community administrator.The method used to access PHI Audit can vary depending on your system configuration. If you are not sure how to navigate to PHI Audit, contact your community administrator.Perform the following steps to access PHI Audit:1. Access your health care community using the URL provided by your organization. Yourhealthcare community login page displays.2. Enter your User ID and Password, then click Login. Your health care community homepage displays.3. Navigate to the PHI Audit Dashboard screen. For example, click Audit on the communityhome page navigation bar. The PHI Audit Dashboard window displays.This step can vary, depending upon your system configuration. If you are notsure how to navigate to the PHI Audit Dashboard window, contact yourcommunity administrator.Access PHI AuditYou have successfully accessed PHI Audit.Create a Patient audit when you want to audit PHI data actions for a selected patient.This procedure assumes you have logged in to your healthcare community and navigated to the PHI Audit Dashboard window. For details, refer to Access PHI Audit.Perform the following steps to create a patient PHI Audit:1. On the PHI Audit Dashboard window, click Patient audit. Step 1 of the Patient AuditWizard popup displays.Create a Patient Audit2. Enter your search criteria. Your search criteria may vary, depending on your systemconfiguration.If you enter search criteria in an invalid format, the error indicationdisplays. Hold your cursor over the indicator for details.3. When you are Finished entering search criteria, click Search for patients. If your searchreturns no results, or unexpected results, modify your search criteria and try again.Otherwise, the search results display in the Patient Audit Wizard Step 1 popup.4. Select the patient whose PHI data you want to audit.5. Click Select and continue. Step 2 of the Patient Audit Wizard popup displays.PHI Audit User Guide6. Specify a date range for the audit.Note: The date range must be no more than 30 days.•Enter dates in the required format, or• Click the drop down arrow to display a calendar from which you can select a date.7. Select the Audit actions you want to include in the PHI patient data audit. For moreinformation, refer to PHI Audit Overview.8. Click Select and review. Step 3 of the Patient Audit Wizard popup displays.Create a Patient Audit9. Review your audit's date range and Audit actions criteria, and modify as required.10. Optional - modify the Email Me When Processed field.•If you want to use the default email address for notification emails, leave the field as-is.•If you want the notification email to go to a different email address, click Clear, then enter the alternate email address.11. Click Schedule audit. The audit is scheduled, and a success window, similar to thefollowing displays.12. Click close this window. The audit wizard window closes and you are returned to thePHI Audit Dashboard window.You have successfully created and scheduled a PHI patient data audit.Create a User audit when you want to audit PHI data actions performed by a selected user. This procedure assumes you have logged in to your healthcare community and navigated to the PHI Audit Dashboard window. For details, refer to Access PHI Audit.Perform the following steps to create a user PHI Audit:1. On the PHI Audit Dashboard window, click User audit. Step 1 of the User Audit Wizardpopup displays the list of available users within your organization.2. Select the user you want to audit.Create a User Audit 3. Click Select admin and continue. Step 2 of the User Audit Wizard popup displays.4. Specify a date range for the audit. Note: The date range must be no more than 30 days.•Enter dates in the required format, or• Click the drop down arrow to display a calendar from which you can select a date.5. Select the Audit actions you want to include in the PHI user data audit. For moreinformation, refer to PHI Audit Overview.6. Click Select and review. Step 3 of the User Audit Wizard popup displays.7. Review your audit's date range and Audit actions criteria, and modify as required.PHI Audit User Guide8. Optional - modify the Email Me When Processed field.•If you want to use the default email address for notification emails, leave the field as-is.•If you want the notification email to go to a different email address, click Clear, then enter the alternate email address.9. Click Schedule audit. The audit is scheduled, and a success window, similar to thefollowing displays.10. Click close this window. The audit wizard window closes and you are returned to thePHI Audit Dashboard window.You have successfully created and scheduled a PHI user data audit.Audits display in the Scheduled audits section of the Audit Dashboard windowonly until they are processed. Audits are processed as soon as possible.You may not be able to view or cancel scheduled audits, because they havealready been moved into the processing phase.View Scheduled audits when you want to:•Review the list of audits that have not been submitted for system processing.•Cancel scheduled audits so they are not submitted for system processing.This procedure assumes you have logged in to your healthcare community and navigated to the PHI Audit Dashboard window. For details, refer to Access PHI Audit.Perform the following steps to view Scheduled audits:1. On the PHI Audit Dashboard window, scroll down the screen as required to view theScheduled audits section.•The list of scheduled audits displays, or•The "There are no scheduled audits as this time" message displays,2. Review the list of scheduled audits as required. If you want to cancel scheduled audits,continue to the next step.3. Check the Cancel check box of each audit you want to cancel.4. Click Cancel checked. A confirmation dialog displays.PHI Audit User Guide5. Click Cancel requests. The checked audits are cancelled, and will not be submitted forsystem processing.You have successfully cancelled a scheduled PHI Audit.View Processed audits when you want to:•Review the list of audits that have been processed.•View audit details.This procedure assumes you have logged in to your healthcare community and navigated to the PHI Audit Dashboard window. For details, refer to Access PHI Audit.Perform the following steps to view Processed audits:1. On the PHI Audit Dashboard window, scroll down the screen as required to view theProcessed audits section.2. Sort the audit list as required by clicking column headings. For details, refer toNavigating. the PHI Audit Dashboard.3. Click the Audit name link of the audit whose information you want to view. The AuditDetails view displays in the Audit Dashboard window. The fields available in the AuditDetails view are dependent upon the audit type:•Patient audit view•User audit viewAn example Patient Audit excerpt is shown below.4. [Optional] If you want to see more information about an audit action, click its Detailsbutton. The action's details display in a new window.[Optional] Click Create PDF file to save the audit action details as a PDF.PHI Audit User Guide5. When you are finished viewing audit action details, click Back to the audit details page.The list of audit details displays in the Audit Dashboard window.6. When you are finished viewing audit details, click Back to the main audit page. The PHIAudit Dashboard page displays.You have successfully viewed a PHI audit.The Patient Audit Details screen is organized in two sections:1. The patient section displays a summary that includes the patient's name and other keyinformation.2. The auditable actions section displays a row for each included action.•Date on which the action occurred.•The user who performed the action.•The action name.•[Optional] A link to additional details about the action.From the screen, you can:•Download the audit data in CSV (Comma Separated Values) format.•Delete the audit.•View action details by clicking a Details button.•Return to the main audit page by clicking the button.The User Audit Details screen is organized in two sections:1. The user section displays a summary that includes the user's name and other keyinformation.2. The auditable actions section displays a row for each included action. The row includes:•The date on which the action occurred.•The patient on whose data the action was performed.•The action name.•[Optional] A link to additional details about the action.From the screen, you can:•Download the audit data in CSV (Comma Separated Values) format.•Delete the audit.•View action details by clicking a Details button.•Return to the main audit page by clicking the button.You can download an audit in CSV format. CSV format files can be opened using spreadsheet software such as Microsoft Excel. There is more information in the CSV format files than can be viewed from the Audit Dashboard user interface.Perform the following steps to download audit data in *.CSV format:1. In the Processed audits section, click the Audit name link of the audit you want todownload. The patient or user Audit details display. For details, refer to View a Processed Audit.2. Click Download .csv of audit. A File Download dialog displays.3. In the File Download dialog, click Save. A Save As dialog, similar to the followingdisplays.PHI Audit User Guide4. In the Save As dialog:•Navigate to the folder in which you want to save the *.csv file.•Enter a meaningful *.csv file name.•Click Save. The file is saved to the location you selected.5. To view the saved file, open it with a spreadsheet program such as Microsoft Excel.You have successfully downloaded and viewed a PHI audit in *.csv format.Audits are automatically deleted after a configurable amount of time. Perform this procedure when you want to delete audits before they are automatically deleted.This procedure assumes you have logged in to your healthcare community and navigated to the PHI Audit Dashboard window. For details, refer to Access PHI Audit.Perform the following steps to delete audits before they are automatically deleted:1. Scroll down through the Audit Dashboard window to the Processed audits section.Note: The screen shot indicates the example system is configured to delete audits after30 days.2. Check the Delete check box of each audit you want to delete..3. Click Delete checked. A confirmation dialog, similar to the following, displays.4. Click Delete reports. The audits are deleted. The Processed audits section refreshes.RESULTYou have successfully deleted processed audits.CC S V:HComma Separated Values. A plain text format for storing tabular data. Files in CSV format can be opened by spreadsheet software such as Microsoft Excel.HIP AA:PThe Health Insurance Portability and Accountability Act.P HI:Protected Health Information - information that must be secured to meet HIPAA requirements.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
限薪令下的银行薪酬大数据:部分员工降
薪10% 高管收入打8
限薪令下的银行薪重酬年夜数据:
部分员工降薪10% 高管收入打八折
银行业因其绝对较高的薪重酬一直被外界所关注,自限薪令颁布后,年夜部分银行都曾在2014年年报中表现,“本行董事长、行长、监事长以涉及其他副职担任人的薪重酬将按照国家对中心管理企业担任人薪重酬轨制改造意见实行,最终标准在肯定中”。
时隔半年,随着各家银行中报的宣布,银行业的薪重酬变幻化无穷也全部曝光。
依据曾经颁布的5家银行中报数据表现,部分银行高管薪重酬同比降幅到达了20%。
而同样被关注的员工薪重酬,从曾经表露的银行中报数据来看,部分银行员工匀称薪重酬确实有了明显的降落。
正常员工匀称工资降落
银行正常员工的薪重酬变化就像一个谜,员工以为钱变少了,银行以为没降,步调不合。
一家国有年夜行乃至为此停滞辟谣表现,上半年该行团体工资总额与去年同期基础持平,往年以来该行依据中心有关标准央企担任人薪重酬管理的相干政策,对总行党委成员薪重酬实现了调减。
但这种调减仅限于总行高管职员,并不涉涉及其他员工,更不所谓层
层降薪。
然而,从曾经颁布的股份制银行中报数据来看中,员工方所以为的薪重酬有所降落似乎更有依据。
正常来说,银行支付的薪重酬包孕,包孕短期薪重酬、卸任后福利、解雇福利跟其他临时职工福利等,其中短期薪重酬包孕工资、奖金、补助跟补助、职工福利费、医疗保险费、工伤保险费、生养保险费、记取房公积金、工会跟教导经费、短期带薪出勤等。
以总部位于北方大陆区的某上市银行动例,该行往年上半年应付职工薪重酬为亿元,其中员工工资、奖金跟补助为亿元,占业务管理费的%,同比增长5000万元,福利费有所增长为亿元,同比增长了3300万元。
按照该行43645的员工总数计算算,上半年薪重酬的匀称数为万元。
而上半年该行实现业务收入亿元,比上年同期增长亿元,增长%,每位员工匀称贡献业务收入162亿元。
经由进程对照前两年纪据不争脸出,上述银行的薪重酬有所降落,虽然幅度较小,其2013年应付职工薪重酬总计算亿元,员工总数为38976人,整年匀称薪重酬为万元,2014年的整年匀称薪重酬为万元,而按照往年上半年的薪重酬,整年的薪重酬水平将在32万元阁下,保守估计算降幅也在10%阁下。
另一家总部位于北方的上市银行中报则表现,共有各种
员工53,943人,其中,条约制员工46,192人,召还涉及聘请协定员工7,751人,离退休职员共677人。
上半年该行薪重酬支付共亿元,同比增长19亿元,以此计算算,上半年该行员工匀称薪重酬为亿元。
而自得其乐以员工本钱亿元来计算算,每一名员工上半年风景本为万元。
而该行2014年年报表现,该行去年员工本钱亿元,以该行50735名总员工数计算算,该行员工去年匀称本钱为万元,半年风景本为万元,比往年超过近2万元/人。
不过,也有银行人士表现,薪重酬不只是员工收入的一部分,员工持股、年金计划等也是一些隐性福利。
部分高管降薪20%
依据此前表露的各家银行年报数据,银行高管薪重酬基础都在年薪百万元以上。
工商银行行长易会满税前薪重酬总计算万元,增长了%;中国银行行长陈四清税前薪重酬总计算万元,增长了%;农业银行行长张云去年税前薪重酬总计算万元,较2013年增长了%。
与国有年夜行比较,股份制银行高管薪重酬更高。
安然银行行长邵平税前报酬总额为万元,招商银行行长田惠宇税前报酬总额为万元。
从往年1月1日起,被称为“限薪令”的《中心管理企业担任人薪重酬轨制改造计划》正式实施,首修改造涉涉及72家央企的担任人,包孕中石油、中石幻化无穷、中国移动等
结构部分录用担任人的53家央企,以涉及其他金融、铁路等19家企业。
改造后,央企担任人的薪重酬由基础年薪跟绩效年薪两部分造成,额定部分将由“任期鼓励收入”补弥补。
也许是受此影响,上半年银行高管频仍跳槽,中行在上半年就有多名高管卸任,信贷危险总监詹伟坚3月份分开中行,此前其税前总计算薪重酬为万元,是中行薪重酬最高的高管,同月,岳毅辞去中行副行长一职,加盟中银喷鼻港,担负中银喷鼻港副董事长、实行董事跟总裁之职。
而从中报下去看,多家银行的高管薪重酬有所降落。
中信银行数据表现,往年上半年董事、监事跟高等管理职员获取的薪重酬为国平易近币1159万元,2014年同期则为1239万元,一共增长80万元,依据《证券日报》记者统计算,中信银行相干董事、监事跟高等管理职员一共24人,以此匀称计算算,每人薪重酬降落尚缺乏4万元。
绝对而言,浦发银行高管薪重酬降幅较年夜,其布告表现,往年上半年为关键管理职员支付薪重酬共800万元,比去年同期的1000万元支付增长了200万元,降幅高达20%,能够说打了八折。
